Abstract
Excitotoxicity has been implicated in the pathogenesis of amyotrophic lateral sclerosis (ALS). More recently, glial involvement has been shown to be essential for ALS-related motoneuronal death. Here, we identified an N-methyl-D-aspartate (NMDA) receptor co-agonist, D-serine (D-Ser), as a glia-derived enhancer of glutamate (Glu) toxicity to ALS motoneurons. Cell death assay indicated that primary spinal cord neurons from ALS mice were more vulnerable to NMDA toxicity than those from control mice, in a D-Ser-dependent manner. Levels of D-Ser and its producing enzyme, serine racemase, in spinal cords of ALS mice were progressively elevated, dominantly in glia, with disease progression. In vitro, expression of serine racemase was induced not only by an extracellular pro-inflammatory factor, but also by transiently expressed G93A-superoxide dismutase1 in microglial cells. Furthermore, increases of D-Ser levels were also observed in spinal cords of both familial and sporadic ALS patients. Collectively, Glu toxicity enhanced by D-Ser overproduced in glia is proposed as a novel mechanism underlying ALS motoneuronal death, and this mechanism may be regarded as a potential therapeutic target for ALS.
Keywords: ALS, D-serine, excitotoxicity, glia, NMDA
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common motor neuron disease, pathologically characterized by massive selective motoneuronal loss, inclusion bodies in remaining neurons and astrocytes, and gliosis around dying neurons in the ventral horns of spinal cords (Bruijn et al, 2004). Approximately 90% of ALS cases are sporadic, while 10% are inherited. About 20% of inherited cases have mutations in the gene encoding superoxide dismutase 1 (SOD1) (Rosen et al, 1993). Although the discovery of mutations in SOD1 has resulted in a considerable number of studies, the mechanism of selective motoneuronal death is still unclear. Among several proposed hypotheses on the pathogenesis of ALS, excitotoxicity mediated by ionotropic glutamate (Glu) receptors has been regarded as a principal cause of motoneuronal death (Bruijn et al, 2004; Van Damme et al, 2005). This notion was supported by the fact that a three-fold increase in the Glu level was detected in the cerebrospinal fluid of patients with ALS due to loss of Glu uptake by astrocytes (Rothstein et al, 1990, 1995) and that an inhibitor of Glu release, Riluzole, is the sole drug clinically effective against ALS. The former observation was confirmed by a cohort study indicating that the Glu level increased in the cerebrospinal fluid of 40% of sporadic ALS patients (Spreux-Varoquaux et al, 2002). Together with the finding that the loss of Glu transporters was detected in ALS model rats with a SOD1 mutant (Howland et al, 2002), it seems most likely that Glu toxicity has some important roles in the pathogenesis of both inherited and sporadic ALS. However, the detailed mechanisms underlying Glu toxicity on motoneurons still remain elusive.
There are three classes of ionotropic Glu receptors: N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and kainate receptors (Van Damme et al, 2005). The AMPA receptors have been assumed to play a central role in ALS-relevant Glu toxicity, because a defect in the editing of mRNA encoding GluR2, which resulted in increased Ca2+ influx, was found in patients with sporadic ALS (Kawahara et al, 2004). At the same time, the NMDARs have been shown in vitro to be as important as the AMPA receptors for Glu toxicity in neurons (Prehn et al, 1995). A more recent finding shed light on the relevance of the NMDARs in ALS pathogenesis: an NMDAR antagonist, memantine, has been reported to prolong survival in ALS model mice (Wang and Zhang, 2005). Activation of the NMDARs essentially requires the binding of a co-agonist to its glycine site. D-Serine (D-Ser) is a physiologically dominant excitotoxic co-agonist to the glycine site compared with glycine (Shleper et al, 2005; Panatier et al, 2006), and is endogenously converted from L-serine (L-Ser) by serine racemase (SRR). SRR is mainly expressed in glia (Wolosker et al, 1999a) and upregulated by glial activation (Wu and Barger, 2004; Wu et al, 2004). Considering that insults generated from activated glia are assumed to be essential for the induction of motoneuronal death (Pramatarova et al, 2001; Clement et al, 2003; Boillee et al, 2006), we hypothesized that in ALS, the excessive amounts of D-Ser generated from the activated glia may contribute to the development of Glu toxicity. In the present study, we demonstrate evidence supporting this hypothesis.
Results
Elevated levels of D-serine in ALS mice
To investigate the potential roles of D-Ser in ALS, we first performed immunofluorescence (IF) analysis with anti-D-Ser polyclonal antibody on frozen spinal cord sections, prepared from G93A-SOD1 transgenic mice (ALS mice) at 9 , 16 , and 21 weeks, corresponding to early (presymptomatic), middle (onset), and end stages, respectively. We found that D-Ser immunoreactivity increased with disease progression starting from the presymptomatic early stage, whereas no distinct change in it was observed among non-transgenic littermates (non-Tg) at the corresponding ages (Figure 1B). The specificity of the antibody was verified by specific disappearance of the immunoreactivity by preabsorption with D-Ser (Supplementary Figure S1). Chemiluminescence assays for the measurement of D-Ser levels using a D-Ser degradation enzyme, D-amino acid oxidase (DAO), also confirmed the elevation of D-Ser levels in spinal cords of ALS mice at 16 weeks (onset stage; Figure 1C). These results suggest that the progressive elevation of D-Ser levels is positively correlated with disease progression.
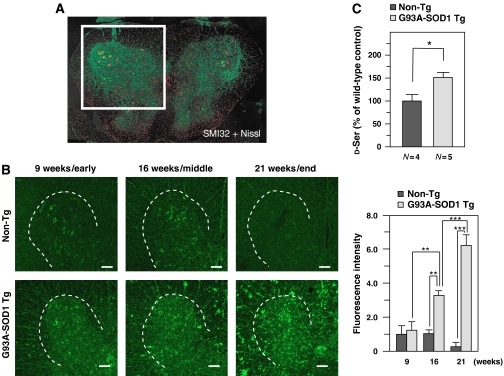
Figure 1.
Progressive elevation of D-Ser levels in spinal cords from mutant SOD1 transgenic mice. (A) A lumbar spinal cord from a non-Tg mouse at 16 weeks was labeled with neurofilament marker SMI32 (green) and a Nissl reagent (red). The indicated squared region including a spinal ventral horn was analyzed in most IF experiments in this study unless otherwise mentioned. (B) IF analysis with rabbit anti-D-Ser antibody was performed on lumbar spinal cord sections from non-Tg and G93A-SOD1 Tg mice (ALS mice) at 9, 16, and 21 weeks. Shown are typical results of stained images and surrounded areas with dotted lines indicate ventral horns (left). Scale bars=100 μm. Total accumulated D-Ser levels were measured as relative total fluorescence intensities in spinal ventral horns of sections from mice at each stage (N=4 for each) (right). (C) Tissue D-Ser levels of spinal cords from non-Tg and ALS mice at 16 weeks were measured by chemiluminescence assay. Values are presented as percentages of levels in spinal cords of non-Tg (100%)±s.e.m. The average chemiluminescence intensity of spinal cords extracts from ALS mice showed 51.4% increase compared with that of non-Tg. (*P<0.05, **P<0.01, ***P<0.001; error bars represent s.e.m.).
D-Serine in damaged motoneurons and activated glia
To study the possible involvement of D-Ser in the ALS pathogenesis and to identify D-Ser-producing cells in vivo, we examined D-Ser distribution in spinal cords from ALS mice. Double IF analysis with the anti-D-Ser antibody and neuronal markers showed that D-Ser accumulated around vacuolated motoneurons in the spinal ventral horns even at the presymptomatic early stage of the disease (Figure 2A), suggesting a correlation between the D-Ser accumulation and the neuronal damage. The elevation in the D-Ser level was also observed in non-neuronal cells in both gray and white matters (Figure 1B). We further performed double IF analysis of the spinal ventral horns using the anti-D-Ser antibody in association with an anti-glial fibrillary acidic protein (GFAP) antibody as a marker of astrocytes or a specific adhesion molecule to microglia (isolectin B4 from Griffonia simplicifolia (IB4) (Streit, 1990)). D-Ser was localized both in activated microglia and astrocytes at the end stage (Figure 2B and C) as well as at the earlier stages (data not shown), suggesting that D-Ser increase was also associated with glial activation in ALS.
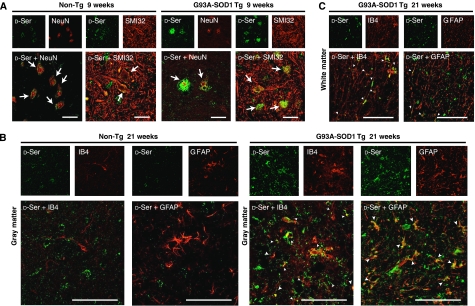
Figure 2.
D-Ser accumulation on the surfaces of damaged motoneurons at earlier stages and D-Ser distribution in activated microglia and astrocytes at an end stage in spinal cords from ALS mice. (A) Double IF analysis showed that D-Ser accumulated on the NeuN- or SMI32-positive vacuolated motoneurons at the lumbar spinal cords from ALS mice at an age of 9 weeks. (B, C) D-Ser was colocalized with both IB4- and GFAP-positive cells, in lumbar spinal cords of ALS mice at 21 weeks. Areas shown are magnified spinal ventral horns (A, B), and spinal white matter (C). For the double immunostaining, the rabbit polyclonal anti-D-Ser antibody was used together with mouse monoclonal anti-NeuN antibody (A), SMI32 (A), IB4 (B, C), or mouse monoclonal anti-GFAP antibody (B, C). Arrows indicated merged neurons and arrowheads showed merged glia. Scale bars=50 μm.
D-Serine exacerbates NMDA toxicity
We next examined D-Ser contribution to Glu toxicity through NMDARs. First, we investigated the toxicity of NMDA using primary cultured spinal cord cells (PSCs) derived from E14 mouse embryos. PSCs on the fifth day after being plated were exposed to NMDA and IF analysis was performed with antibodies against specific markers for neurons, astrocytes, and microglia. NMDA treatment caused 58.0% decrease in the number of NeuN-positive cells, most of which were also choline acetyl transferase (ChAT)-positive, whereas no significant changes were observed in numbers of either GFAP-positive cells or ionized calcium-binding adapter molecule 1 (Iba1)-positive cells (Figure 3A–F). This indicates that NMDA is predominantly toxic to neurons in PSCs.
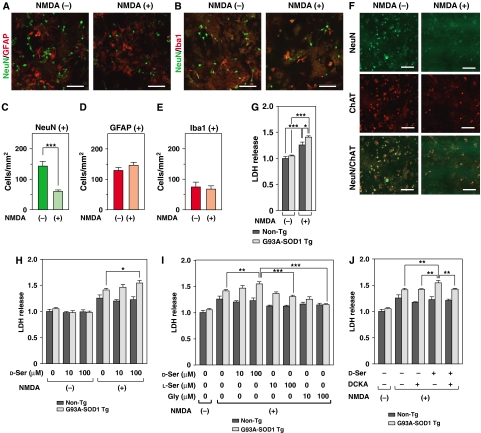
Figure 3.
Elevated vulnerability of PSCs from ALS mice to toxicities of D-Ser/NMDA. (A–F) NMDA treatment on PSCs triggers neuronal death, but not glial death. Double IF analysis with mouse monoclonal anti-NeuN (green) antibody in association with rabbit anti-GFAP (red (A)) or rabbit anti-Iba1 (red (B)) antibody was performed at 24 h after the beginning of treatment with 500 μM NMDA on PSCs from non-Tg mice. (C–E) The numbers of NeuN- (C), GFAP- (D), and Iba1- (E)-positive cells were counted in five random visual fields and averaged (***P<0.001; error bars represent s.e.m.). (F) NeuN-positive cells were characterized by double IF analyses with anti-ChAT antibody at 24 h after treatment with NMDA. Most NeuN-positive cells were ChAT-positive. Few PSCs were NeuN-, GFAP-, and Iba-1-negative. Scale bars=100 μm (A, B, F). (G–J) Cell mortality of PSCs from non-Tg and ALS mice was determined with LDH assays. The extent of LDH release was shown as folds of values measured in cells from non-Tg without NMDA treatment. (G) Treatment with 500 μM NMDA caused a 1.41-fold increase of LDH release from PSCs of ALS mice, whereas a 1.26-fold elevation was observed in those from non-Tg (N=3). (H) Treatment with 0, 10, and 100 μM of D-Ser under 500 μM NMDA on PSCs from ALS mice exhibited 1.41, 1.46, and 1.55-fold increase, respectively (N=3). (I) The effects of L-Ser or Gly on 500 μM NMDA-induced toxicity were measured and compared with that of D-Ser treatment (N=3). (J) The effect of treatment with an antagonist to the glycine sites of the NMDARs, DCKA (30 μM), on D-Ser (100 μM)/NMDA (500 μM)-induced toxicity was examined (N=3). (*P<0.05, **P<0.01, ***P<0.001; error bars represent s.e.m.). Shown is a typical result of an experiment repeated three times.
Next, cell death was estimated with measurement of lactate dehydrogenase (LDH) release that rises proportionally to the extent of cell death. Treatment with NMDA caused greater release of LDH from PSCs of ALS mice than that from PSCs of non-Tg mice, indicating the increased vulnerability of neurons derived from ALS mice to NMDA toxicity (Figure 3G). LDH release was further augmented in a D-Ser-dose-dependent manner in PSCs from ALS mice (Figure 3H), whereas L-Ser or glycine did not increase LDH release caused by NMDA treatment (Figure 3I), excluding the possibility that some amino acids nonspecifically increase NMDA toxicity. To confirm the contribution of D-Ser to the augmentation of NMDA toxicity, we co-administered 5,7-dichloro-kynurenic acid (DCKA), one of the most potent antagonist to the glycine-binding site of NMDARs, with D-Ser and NMDA (Figure 3J). DCKA inhibited the D-Ser-induced enhancement of NMDA toxicity, indicating that D-Ser potentiates NMDA toxicity through binding to the glycine site of NMDARs.
Upregulated SRR in activated microglia
How was excessive D-Ser produced? D-Ser is primarily generated by conversion of L-Ser by SRR that is mainly localized in glia. The specificity of an anti-SRR antibody was confirmed by immunoblot and IF analyses with the NSC34 cells transiently overexpressing mouse SRR (Supplementary Figure S2A and B). To estimate levels of the D-Ser-producing enzyme SRR in glia (Wolosker et al, 1999a), we performed IF analysis with the anti-SRR antibody using non-Tg and ALS mice at early, middle, and end stages. In a manner similar to the D-Ser levels in ALS mice, the number of SRR-positive cells increased at the early stage and further upregulated with disease progression (Figure 4A). Immunoblot analysis with anti-SRR antibody of spinal cord tissues from non-Tg and ALS mice at the end stage showed a 1.4-fold increase in the expression of SRR (Figure 4C). These data suggest that increase in both the number of D-Ser-producing cells and the expression level of SRR in each cell contributed to the elevation in the D-Ser level in ALS mice.
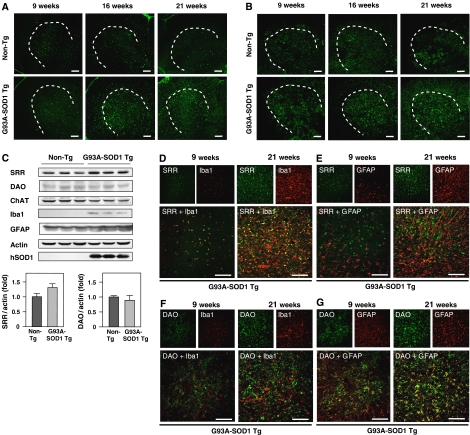
Figure 4.
Upregulation of SRR levels in activated microglia. (A, B) We performed IF analysis with mouse monoclonal anti-SRR antibody (A) or sheep polyclonal anti-DAO antibody (B) on lumbar spinal cord sections from non-Tg and ALS mice at 9, 16, and 21 weeks. (C) Immunoblot analysis of lysates from spinal cords from non-Tg and ALS mice at 21 weeks was performed with antibodies specific to SRR, DAO, ChAT, Iba1, GFAP, actin, and human SOD1 (hSOD1) (N=3) (upper panel). Densitometric calculations of SRR- or DAO-immunoreactive bands were standardized with those of actin immunoreactive bands (SRR/actin or DAO/actin ratio) (lower panel). Error bars represent s.e.m. (D–G) The cells labeled with the anti-SRR (D, E) or anti-DAO (F, G) antibody were characterized by double IF analyses with anti-Iba1 (D, F) or anti-GFAP (E, G) antibody at indicated ages of ALS mice. Areas surrounded with dotted lines represent spinal ventral horns and pictures without lines indicate their magnifications. Scale bars=100 μm (A, B, D–G).
D-Ser levels are also regulated by the degradation of D-Ser. D-Ser can be selectively metabolized by the peroxisomal flavoprotein DAO, an enzyme present in astrocytes (Urai et al, 2002), though the detailed mechanism responsible for its degradation remains elusive. We performed IF and immunoblot analyses with anti-DAO antibody whose specific recognition of DAO had been confirmed by IF analysis with the antibody preincubated with porcine DAO (Supplementary Figure S3). IF analysis revealed that the number of DAO-positive cells increased with disease progression in ALS mice (Figure 4B). In contrast, immunoblot analysis indicated that the expression levels of DAO remained unchanged between non-Tg and ALS mice (Figure 4C). These findings suggest that elevation of DAO levels was relatively low as a whole compared with those of SRR, and also that the degradation of D-Ser by DAO could not compensate for excessive synthesis of D-Ser. Thus, we think that the increase of D-Ser levels in spinal cords from ALS mice was primarily caused by the upregulation of SRR.
Double IF analysis with anti-GFAP antibody or anti-Iba1 antibody as a marker of microglia (Ito et al, 1998), in association with anti-SRR or anti-DAO antibody, indicated that the majority of SRR-positive cells were composed of amoeboid-shaped activated microglia (Figure 4D and E); and cells labeled with anti-DAO antibody merged with astrocytes in ALS mice at the end stage (Figure 4F and G). The latter was consistent with the fact that a D-Ser transporter alanine-serine-cysteine transporter 1 (Asc-1) is distributed to astrocytes (Ribeiro et al, 2002; Rutter et al, 2007). Accordingly, the elevation of the D-Ser levels in ALS mice might be caused by an imbalance between the production in activated microglia and the uptake/degradation in astrocytes.
SRR induction by extra- and intracellular factors
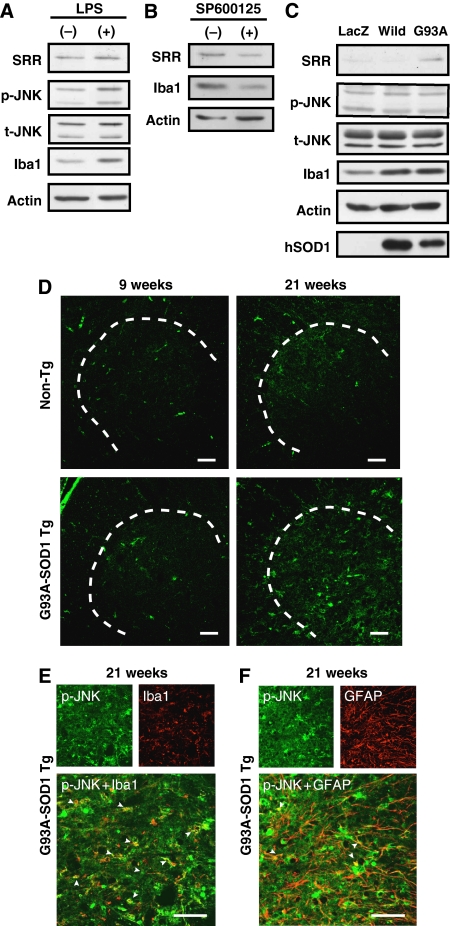
Expression of SRR is thought to be induced in microglia in inflammatory conditions through the activation of the c-Jun N-terminal kinase (JNK) pathway (Wu and Barger, 2004), although it is also localized to astrocytes and subsets of neurons in disease-free conditions (Schell et al, 1995; Williams et al, 2006). Immunoblot analysis showed that SRR expression was upregulated by lipopolysaccharide (LPS) treatment, possibly through activation of JNK, in MG5 microglial cells (Figure 5A). Conversely, co-incubation of primary cultured microglia with a JNK inhibitor, SP600125, downregulated the expression of SRR (Figure 5B). The expression level of Iba1 is assumed to represent the activation level of microglia (Figure 5A–C), because Iba1 expression is upregulated in accordance with microglial activation under several pathological conditions (Imai and Kohsaka, 2002). To examine the contribution of mutant SOD1 to the upregulation of SRR, we infected adenoviruses encoding wild-type SOD1 and G93A-SOD1 into MG5 cells. Immunoblot analysis showed that enforced expression of G93A-SOD1, but not wild-type SOD1, enhanced expression of SRR along with upregulation of Iba1 in MG5 cells (Figure 5C). This enhancement was not accompanied by the activation of JNK at 72 h after the infection.
Figure 5.
Activation of JNK in microglial cells in accordance with SRR upregulation. (A) MG5 microglial cells were incubated with 300 ng/ml of LPS for 72 h and harvested for immunoblot analysis using antibodies specific to SRR, phospho-JNK, total-JNK, Iba1, and actin. (B) Lysates of primary microglia, incubated with a JNK inhibitor (SP600125; 30 μM) for 96 h, were analyzed by immunoblot analysis with antibodies to SRR, Iba1, and actin. (C) G93A-SOD1-induced expression of SRR. MG5 cells were infected with adenoviruses encoding lacZ for control, wild-type SOD1 (wild) and G93A-SOD1 (G93A), at a multiplicity of infection of 200. At 72 h after infection, cells were harvested for immunoblot analysis with antibodies specific to SRR, phospho-JNK, total-JNK, Iba1, actin, and human SOD1. (D) IF analysis with mouse monoclonal anti-phospho-JNK antibody was performed on lumbar spinal cord sections of non-Tg and ALS mice at 9 and 21 weeks. (E, F) Those cells were characterized by double IF analyses with rabbit anti-Iba1 (E) or rabbit anti-GFAP (F) antibody at indicated ages of ALS mice. Arrowheads indicate merged microglia (Iba1) or astrocytes (GFAP). Areas surrounded with dotted lines represent spinal ventral horns and pictures without lines indicate their magnifications. Scale bars=100 μm (D–F).
We further studied the levels of phosphorylated JNK in spinal cords from non-Tg and ALS mice by IF analysis. The number of phosphorylated JNK-positive cells increased at the end stage in ALS mice, but not in non-Tg mice (Figure 5D), and phosphorylated JNK-positivity was overlapped with both activated microglia and astrocytes (Figure 5E and F). These results suggest that inflammation-induced phosphorylation of JNK was also related to the promoted transcription of SRR as well as JNK-independent mutant SOD1.
Inhibition of endogenous SRR activity reduces NMDA toxicity
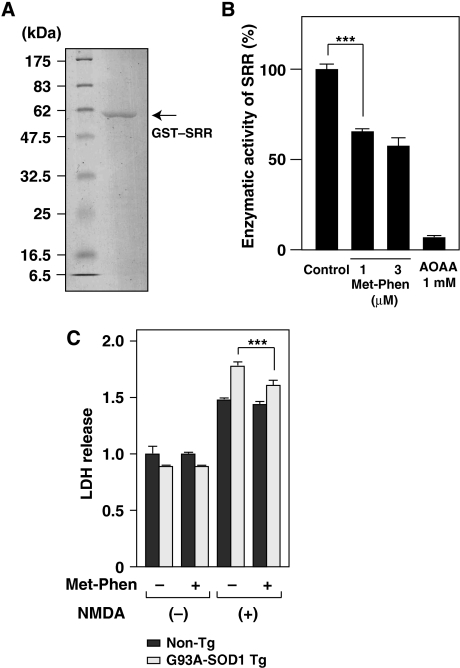
Next, we examined the contribution of endogenous SRR activity to NMDA toxicity in ALS. Figure 3G and H shows that PSCs from ALS mice were more vulnerable to NMDA than control mice and the exogenous addition of D-Ser enhanced NMDA toxicity. To ascertain a role of endogenous SRR in vulnerability of ALS motoneurons to NMDA toxicity, we treated PSCs with NMDA and a SRR inhibitor, phenazine methosulfate (Met-Phen) (Kim et al, 2005), and performed LDH assays. Because Met-Phen by itself is toxic to PSCs at higher concentrations (Supplementary Figure S4A), we were only able to examine the effect of Met-Phen on SRR activity at a concentration of 1 μM. Using enzyme activity assay with GST-fused recombinant SRR (Figure 6A and Supplementary Figure S4B and C), we recognized that Met-Phen reduced the SRR activity to 64.5% of the control at a concentration of 1 μM (Figure 6B). Replacement of cultured media by fresh media and preincubation of PSCs with 1 μM of Met-Phen partially but significantly alleviated NMDA toxicity to PSCs from ALS mice (Figure 6C). Considering that PSCs contained a substantial number of glia (Figure 3D and E), this result indicates that D-Ser, excessively synthesized by endogenous SRR, potentiated NMDA toxicity to ALS motoneurons.
Figure 6.
Reduced NMDA toxicity by inhibition of SRR activity in PSCs from ALS mice. (A) Purified GST–SRR fusion protein was subjected to SDS–PAGE and stained with Coomasie blue. (B) Enzymatic activity of GST–SRR was determined by monitoring levels of pyruvate generated from L-Ser in vitro. Activity of recombinant SRR was reduced by treatment with 1 μM of a SRR inhibitor, Met-Phen, and abolished by 1 mM of aminooxyacetic acid (AOAA), an inhibitor of PLP-dependent enzymes (N=3). (***P<0.001; error bars represent s.e.m.). (C) Cell mortality of PSCs was evaluated with LDH assays. The extent of LDH release was shown as folds of values measured in cultures from non-Tg without NMDA treatment. The effect of 1 μM Met-Phen with 500 μM of NMDA was examined in PSCs from non-Tg and ALS mice (N=3).
MAPKERK1/2, p38 activation by NMDA/D-serine
To characterize the signaling pathway underlying D-Ser-enhanced NMDA toxicity, we examined the involvement of MAPK family proteins, which have been suggested to be closely linked to both motoneuronal apoptosis in ALS (Raoul et al, 2002) and NMDA-mediated toxicity (Haddad, 2005). It was reported that prolonged exposure of primary cortical neurons (PCNs) to Glu resulted in sustained activation of extracellular signal-regulated kinase (ERK)1/2, which was completely prevented by blockage of NMDAR, but not those of any other Glu receptors, and such Glu-induced apoptosis-like cell death was largely prevented by inhibition of the ERK1/2 activity (Jiang et al, 2000). Further, ERK1/2 involvement has recently been demonstrated in multiple in vitro and in vivo models of chronic neuronal death (Colucci-D'Amato et al, 2003), although ERK1/2 was originally regarded as a signaling molecule transmitting pro-survival effects.
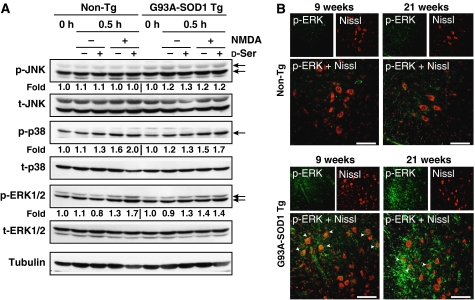
PCNs from both non-Tg and ALS mice treated with NMDA exhibited elevated phosphorylation of ERK 1/2 and p38, but not that of JNK (Figure 7A). These elevations were further enhanced by co-treatment with D-Ser. Performing IF analysis with anti-phospho-ERK1/2 antibody, we confirmed significant elevation of ERK1/2 phosphorylation in motor neuron-like cells and glia-like cells in spinal cords of ALS mice at both early and end stages, but not in those of non-Tg mice (Figure 7B). These results suggest that NMDAR stimulation with D-Ser may induce death of motoneurons in ALS mice by chronically activating ERK1/2-mediated pathways, in addition to the more widely known p38-mediated apoptotic pathways.
Figure 7.
Involvement of MAPKs in D-Ser-/NMDA-activated signals. (A) The expression levels of endogenous phospho-MAPKs (JNK, p38, and ERK1/2) in lysates of PCNs from non-Tg and ALS mice exposed to 500 μM NMDA±100 μM D-Ser for 0.5 h were measured by immunoblot analysis with antibodies to phospho-MAPKs and total-MAPKs, followed by densitometric analysis. Values under the columns of immunoblotted phospho-MAPKs were calculated as ratios of levels of phospho-MAPKs in cells treated with NMDA and/or D-Ser adjusted with levels of total-MAPKs. Shown is a typical result of the experiment repeated three times. (B) Sustained activation of ERK1/2 was observed. Double IF analysis with mouse monoclonal anti-phospho-ERK1/2 antibody and Nissl reagent was performed on spinal cord sections from non-Tg and ALS mice at ages of 9 and 21 weeks. Arrowheads indicate merged large motor neuron-like cells. Areas shown are spinal ventral horns. Scale bars=100 μm (B, C).
Increased D-serine in sporadic and familial ALS
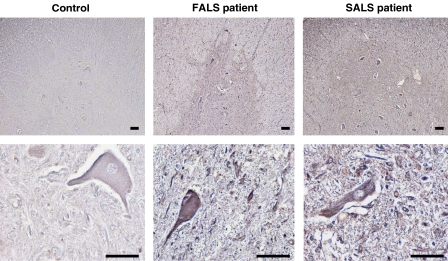
To examine the possible elevation of D-Ser levels in spinal cords of human ALS cases, we performed immunohistochemical analysis of spinal cords from postmortem human ALS cases with the anti-D-Ser antibody. A familial ALS (with A4V-SOD1) spinal cord and two of three sporadic ALS spinal cords showed notable elevations of the D-Ser levels in both motor neurons and non-neuronal cells, whereas no sample from the controls did so (Figure 8), indicating that D-Ser levels are also elevated in human ALS cases. These results support the idea that Glu toxicity enhanced by D-Ser may play a pathological role not only in ALS mice models, but also in at least one case of familial ALS and some forms of sporadic ALS.
Figure 8.
Increased D-Ser levels in spinal cords from patients with familial and sporadic ALS. Lumbar spinal cord sections from a familial ALS (FALS) patient with A4V-SOD1 mutation (N=1), sporadic ALS (SALS) patients (N=3), and controls (N=4) were immunostained with antibody to D-Ser. Shown are typical results of stained images of spinal ventral horns. The lower panels are magnified versions of upper ones. Scale bars=200 μm.
Immunohistochemical analysis of human ALS cases was further performed with the mouse monoclonal anti-mouse SRR or goat polyclonal anti-human DAO antibody. The antigen for SRR antibody was 93% identical to human SRR in the amino-acid sequence. Most SRR-positive cells appear to be non-neuronal. The number of the SRR-positive cells increased in two out of three sporadic ALS patients, whose spinal cord sections also showed increased immunoreactivity to D-Ser, but not in any of the controls nor in a familial ALS patient (Supplementary Figure S5A). On the other hand, spinal cord sections from sporadic ALS patients and a familial ALS patient showed a slight increase in the number of DAO-positive non-neuronal cells compared with controls (Supplementary Figure S5B). These immunostaining patterns of human ALS cases suggest that there is a similar mechanism of D-Ser-overproduction between human and mouse-model ALS.
Discussion
We would like to propose a novel hypothesis to explain the mechanism underlying motoneuronal death involving glial activation in the ALS pathogenesis. In this hypothesis, we think that the elevation of SRR expression, initially induced by glial activators and/or mutant SOD1, subsequently increases D-Ser levels in activated glia. The elevated levels of D-Ser in the extracellular space in turn augment Glu toxicity to motoneurons through NMDARs, which may be intracellularly mediated by both ERK1/2 and p38 MAPK.
D-Amino acids had been detected only in bacteria and invertebrates. Studies in the 1990s, however, pointed out the existence of D-Ser at micromolar levels and its producing enzyme SRR in the central nervous system (CNS) of higher species, including humans (Hashimoto et al, 1993; De Miranda et al, 2000). The physiological and pathological roles of D-Ser remain unclear except for its roles in regulation of the NMDAR activation and possibly the development of schizophrenia by NMDAR hypofunction (Chumakov et al, 2002). We have here provided evidence that elevated levels of D-Ser caused by glial activation may enhance Glu toxicity in the ALS pathology.
D-Ser, compared with L-Ser, is presumably prone to accumulate in CNS when its production is excessive, as suggested by the fact that the metabolic inactivation of D-Ser is much slower than that of L-Ser in CNS when D-Ser or L-Ser was systemically administered in vivo (Takahashi et al, 1997). Two major mechanisms underlying increased glial production of D-Ser have been reported: pro-inflammatory stimuli induce release of D-Ser from microglia by elevating transcription of SRR through the JNK-mediated activation of a transcription factor activator protein-1 (Wu and Barger, 2004); and Glu stimulation of AMPA receptors induces elevation of the enzymatic activity of SRR and release of D-Ser from astrocytes (Kim et al, 2005). In ALS, the transcriptional upregulation of SRR in microglia is speculated to be the main cause for the progressive increase in the overall D-Ser levels for two reasons: the expression levels of SRR are much higher in microglia than in astrocytes in spinal cords of ALS mice (Figure 4); and both inflammation-induced phosphorylation of JNK and the expression of mutant SOD1 seemed to elevate the SRR levels in ALS microglia (Figure 5). Yet at the same time, astrocytes may also contribute to the increase of the D-Ser level, because excessive amounts of Glu in the spinal cords of ALS can activate the enzymatic activity of SRR in astrocytes. This speculation is supported by the observation that immunoreactivity to D-Ser also elevated in astrocytes (Figure 2). Currently, however, it remains unclear whether D-Ser upregulation in astrocytes was caused by activated synthesis of D-Ser by SRR or only the result of uptake via D-Ser transporters such as Asc-1. Slight elevation of D-Ser levels in the spinal cords of ALS mice appears to occur at the age of 9 weeks (Figure 1) when significant glial activation does not occur (data not shown). This finding may support a possibility that neuronal SRR also contributed to the overproduction of D-Ser even though the expression level of neuronal SRR was less than that detectable with an IF analysis (data not shown). It is further conjectured that failure in D-Ser metabolism, caused by an inactivation of the degradation enzyme (e.g. DAO) and/or decrease in glial uptake, may be relevant to the development of the ALS pathogenesis. However, the whole molecular mechanism underlying the metabolic inactivation of D-Ser remains elusive.
D-Ser, a dominant co-agonist at the glycine site of the NR1 subunit in NMDARs, is thought to be toxic by exacerbating Glu toxicity when its extracellular levels are elevated. Ca2+ entry through NMDARs has the power to determine neuronal fate in many neuropathological conditions and intense or chronic activation of NMDARs can cause neuronal death (Hardingham and Bading, 2003). To be functional, NMDARs essentially require the binding of a co-agonist to the glycine site (Johnson and Ascher, 1987). The finding in this study that NMDA by itself caused cell death in PSCs (Figure 3G) indicates that glycine or D-Ser was excreted endogenously by PSCs. Considering that exogenous addition of D-Ser augmented NMDA toxicity (Figure 3H) and inhibition of endogenous SRR activity reduced the NMDA toxicity in PSCs from ALS mice (Figure 6), we speculate that D-Ser plays a major role in NMDA toxicity in ALS motoneurons. This speculation is also supported by the finding that exogenous addition of glycine, a competitive inhibitor for SRR (Dunlop and Neidle, 2005) as well as a co-agonist for NMDARs, significantly reduced the NMDA toxicity in PSCs from ALS mice (Figure 3I). In contrast, NMDA toxicity in control PSCs did not seem to be affected by D-Ser or by treatment with the SRR inhibitor (Figure 3H and 6), indicating that D-Ser/NMDA toxicity to ALS motoneurons is augmented by motoneuronal and glial alterations caused by expression of mutant SOD1.
How is D-Ser/NMDA toxicity augmented in ALS? Alteration in expression of NMDARs and regulation of NMDARs by its associated proteins may contribute to the increase in NMDA toxicity. To test this possibility, we examined the expression of NMDARs in ALS mice. No significant difference was observed in the expressions of NR2 subunits to which Glu binds between non-Tg and ALS mice at 3 weeks, when no pathological motoneuronal death occurred (Supplementary Figure 6A). In contrast, NR1 subunit to which D-Ser binds decreased in the expression level at 3 weeks and remained low even at 7 weeks when some of motoneurons begin to die (Supplementary Figure S6A and B). This result suggests that expression of NR1 was downregulated by a physiological negative-feedback mechanism in the presence of excessive concentrations of D-Ser. Thus, alteration in the expression levels of NMDARs does not appear to be responsible for the increasing vulnerability to D-Ser/NMDA toxicity in ALS. Putative contribution of an alteration in the receptor localization or in the expression of receptor-associated proteins to the development of NMDA toxicity in ALS remains to be clarified. Furthermore, some sort of ALS-related intracellular signaling or extracellular toxic factors such as inflammatory cytokines, some oxides, or excreted mutant SOD1 may also enhance D-Ser/NMDA-mediated toxicity. These issues should be addressed in the future investigation.
Based on the findings that the elevated level of Glu remains unchanged with the disease progression of ALS (Rothstein et al, 1990), whereas the D-Ser level progressively increases during the course of ALS (Figure 1), it is speculated that the D-Ser level has a positive correlation with disease progression of ALS. Moreover, the findings that the vulnerability of ALS motoneurons to NMDA was augmented by D-Ser (Figure 3) and reduced by SRR inhibition (Figure 6) support our hypothesis that the D-Ser level becomes a key determinant of Glu toxicity in ALS. Furthermore, in the light of histological findings in this study, D-Ser overproduction may be a common feature among ALS model mice, human familial ALS, and some forms of sporadic ALS. Given the relevance of this hypothesis, therapeutic strategies decreasing D-Ser levels by modulating enzymatic activity of D-Ser-producing SRR and D-Ser-degrading DAO and/or those blocking the association between D-Ser and the glycine site of NMDAR may be beneficial to ALS patients.
In conclusion, a novel mechanism for neuronal excitotoxicity regulated by glia-derived D-Ser has been proposed for ALS pathogenesis in this study. Considering that chronic activation of NMDARs was assumed to be involved in cell death in many neuropathological conditions, a similar hypothesis can be also relevant to other neurological diseases with comparable abnormalities.
Materials and methods
Mice
Transgenic mice expressing a high copy number of mutant human SOD1 with a Gly-93-Ala substitution (G93A-SOD1) were obtained from Jackson Laboratories. They were maintained in a specific pathogen-free environment as described previously (Chiba et al, 2005) and killed humanely. All animal experimental procedures were approved by the Institutional Animal Experiment Committee at KEIO University.
Immunofluorescence analysis
Cryostat sections from non-Tg and G93A-SOD1 Tg mice, NSC34 cells, and primary spinal cord cultures derived from embryonic day 14 (E14) embryos of non-Tg mice were processed for IF analysis (described in detail in Supplementary Methods).
D-Serine assay
Spinal cords were homogenized in three-fold volumes of 5% trichloroacetic acid (TCA) (3 μl 5% TCA for 1 mg spinal cord). Precipitated protein was removed by centrifugation. The supernatants were extracted five times with 1 ml of water-saturated diethyl ether to remove TCA and incubated with DAO (Sigma) (Scannone et al, 1964; Wolosker et al, 1999b), which degrades D-amino acids into an α-keto acid, NH3 and hydrogen peroxide. The amounts of D-Ser in samples were measured by quantification of generated hydrogen peroxide. Actually, 10-μl sample aliquots were added to 100 μl of media containing 100 mM Tris–HCl, pH 8.8, 10 U/ml peroxidase, and 8 μM luminol. After a 10-min delay, 10 μl of DAO (75 U/ml) was added and mixed gently with a pipette tip. Chemiluminescence was measured after 10 min at room temperature using a luminometer.
Primary cell preparation and cell-death assays
PCNs and PSCs at E14 were prepared as described previously (Chiba et al, 2005) and by the method developed by Urushitani et al (2006). Primary cultured microglial cells were separated from PCNs (Suzumura et al, 1987). Purity of primary microglia was estimated to be 95% by staining with FITC-conjugated isolectin B4 from G. simplicifolia (10 μg/ml, Sigma). On day 5 of culture, PSCs were treated with 500 μM NMDA (Wako), 100 μM D-Ser (Wako), or 30 μM DCKA (Sigma). To inhibit SRR activity and biosynthesis of D-Ser, media of PSCs were replaced by Dulbecco's modified Eagle's medium (DMEM, Sigma) with N2 supplement (Invitrogen) to remove D-Ser. PSCs were then preincubated with 1 μM phenazine methosulfate (Met-Phen, Sigma) for 1 h before NMDA treatment. At 24 h after the treatment, numbers of immunostained PSCs in random five visual fields of 450-μm2 (=0.2 mm2) were counted and then counted numbers were multiplied by 5 to estimate cell numbers in 1 mm2. LDH releases from PSCs in death assays were estimated with an LDH assay kit (Wako) at 24 h after treatment. Absorbance of the mixtures at the 560 nm wavelength was measured by a Plate Reader (Bio-Rad). Because LDH is constantly excreted into media from viable cells as well, the basal levels of LDH are relatively high. When LDH release is increased to the 1.3 × level, about 60% of neurons in PSCs die, as shown in Figure 3C and G.
Cell cultures, transfection, and adenoviral infection
A mouse SRR cDNA was PCR-amplified from total mouse brain cDNAs. The system of a replication-deficient adenoviral vector was purchased from TaKaRa (Japan). Adenoviral vectors encoding wild-type SOD1 and G93A-SOD1 were generated by inserting full-length cDNAs into pAxCAwt. NSC34 cells (Cashman et al, 1992), the hybrids of motor neuron-enriched, embryonic mouse spinal cord cells with mouse neuroblastoma N18TG2, were maintained in DMEM with 10% FBS (Hyclone, Logan, UT). The mouse microglial MG5 cells (Ohsawa et al, 1997) were cultured in a 5:2 (v/v) cocktail of the A1 astrocytic glial cells conditioned medium and DMEM containing 10% FBS. Transfection by lipofection (1 μg of DNA, 2 μl of Lipofectamine, 4 μl of PLUS Reagent) was performed as reported previously (Hashimoto et al, 2001). MG5 cells were infected using adenoviral vectors as described previously (Tsuji et al, 2002).
Immunoblot analysis
Spinal cords, removed from mice, were lysed in a buffer (20 mM HEPES/NaOH (pH7.4), 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1 mM DTT, protease inhibitor cocktail (Complete, Roche)). Cells were lysed in a buffer (20 mM Tris-HCl (pH7.5), 150 mM NaCl, 5 mM EGTA, 12 mM β-glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 1% deoxycholate, 1 mM DTT, protease inhibitor cocktail (Complete, Roche), phosphatase inhibitor cocktails 1 and 2 (Sigma)). PCNs, incubated with or without NMDA in the absence or presence of D-Ser (100 μM) for 0.5 h; MG5 cells, treated with or without 300 ng/ml of LPS for 72 h, or infected with adenoviruses carrying LacZ, wild-type SOD1, or G93A-SOD1 for 72 h; and primary microglia, treated with or without 30 μM SP600125 (Calbiochem-Novabiochem, San Diego, CA) for 96 h. Immunoblot analysis was performed as described in the Supplementary Methods. For the quantification of protein levels, the density of each band was measured by NIH Image Version 1.62.
Measurement of SRR activity
Recombinant GST–SRR fusion protein was purified as described in the Supplementary Methods. SRR activity was determined by measuring pyruvate generated from L-Ser (De Miranda et al, 2002). Pyruvate was produced in 50 μl of a reaction buffer containing 50 mM Tris–HCl (pH 8.3), approximately 5 μg of purified GST-fusion enzyme immobilized onto glutathione-sepharose beads, 20 μM pyridoxal 5′-phosphate, 1 mM MgCl2, and 20 mM L-Ser. Reaction mixtures were incubated with or without 1 μM Met-Phen or 1 mM aminooxyacetic acid under constant rotation at 37°C for 6 h. Then 10 μl of the supernatant was incubated with NADH (50 μM, Sigma) and LDH (10 μg/ml, Roche) in a total volume of 50 μl at 25°C for 1 h. Amounts of generated pyruvate were measured by monitoring the decrease in NADH absorbance at 340 nm. Full activity of recombinant SRR was defined as differences in the absorbance between reaction products incubated with and without GST–SRR.
Human samples
Patients were diagnosed as ALS by clinical and neuropathological criteria. At autopsy, spinal cords were removed; blocks of each level of spinal cords were immediately placed in 4% paraformaldehyde in PBS (pH 7.4), embedded in paraffin, and then subjected to neuropathological examination. The lumbar spinal cords used in this study originated from samples of a FALS patient with A4V-SOD1 mutation, three patients with SALS, and four non-ALS patients with no pathologic findings in spinal cords. The mean ages of FALS, SALS, and control groups were 39 years, 63.3±5.8 years, and 59.0±9.2 years, respectively. Immunostaining procedures were described in the Supplementary Methods.
Statistics
All values in the figures of this study indicate means±s.e.m. Statistical analyses for the experiments were performed with one-way ANOVA followed by a Fisher's PLSD, in which P<0.05 was assessed as significant.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary information
Acknowledgments
We deeply appreciate Dr A Hirano for the samples of a human familial ALS case. We thank Dr Y Ikeda for essential help. We are grateful to Dr K Terashita for scientific support; H Suzuki for adenoviral infection; T Hiraki for essential assistance; T Yoshida-Nishimoto for indispensable cooperation; Dr D Wylie for expert comments on the manuscript; R Kato, S Uchida, Y Kamei, and K Negishi for animal husbandry; and members of the Department of Anatomy for advice and encouragement. We are indebted to the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO). This work is supported by NOEVIR Co. LTD, the Japan Society for the Promotion of Science, the Nakabayashi Trust for ALS Research (TC), and Keio University Grant-in-Aid for Encouragement of Young Medical Scientists (JS). MY is a Japan Society for the Promotion of Science Research Fellow.
References
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW (2006) Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312: 1389–1392 [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27: 723–749 [DOI] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP (1992) Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn 194: 209–221 [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamada M, Hashimoto Y, Sato M, Sasabe J, Kita Y, Terashita K, Aiso S, Nishimoto I, Matsuoka M (2005) Development of a femtomolar-acting humanin derivative named colivelin by attaching activity-dependent neurotrophic factor to its N terminus: characterization of colivelin-mediated neuroprotection against Alzheimer's disease-relevant insults in vitro and in vivo. J Neurosci 25: 10252–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P et al. (2002) Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99: 13675–13680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH Jr, Julien JP, Goldstein LS, Cleveland DW (2003) Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302: 113–117 [DOI] [PubMed] [Google Scholar]
- Colucci-D'Amato L, Perrone-Capano C, di Porzio U (2003) Chronic activation of ERK and neurodegenerative diseases. BioEssays 25: 1085–1095 [DOI] [PubMed] [Google Scholar]
- De Miranda J, Panizzutti R, Foltyn VN, Wolosker H (2002) Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc Natl Acad Sci USA 99: 14542–14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miranda J, Santoro A, Engelender S, Wolosker H (2000) Human serine racemase: moleular cloning, genomic organization and functional analysis. Gene 256: 183–188 [DOI] [PubMed] [Google Scholar]
- Dunlop DS, Neidle A (2005) Regulation of serine racemase activity by amino acids. Brain Res Mol Brain Res 133: 208–214 [DOI] [PubMed] [Google Scholar]
- Haddad JJ (2005) N-methyl-D-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: a revolving neurochemical axis for therapeutic intervention? Prog Neurobiol 77: 252–282 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H (2003) The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26: 81–89 [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Kumashiro S, Nishikawa T, Oka T, Takahashi K, Mito T, Takashima S, Doi N, Mizutani Y, Yamazaki T, Kaneko T, Otomo E (1993) Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J Neurochem 61: 348–351 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I (2001) A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA 98: 6336–6341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD (2002) Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci USA 99: 1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S (2002) Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia 40: 164–174 [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57: 1–9 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Gu Z, Zhang G, Jing G (2000) N-methyl-D-aspartate receptor activation results in regulation of extracellular signal-regulated kinases by protein kinases and phosphatases in glutamate-induced neuronal apototic-like death. Brain Res 887: 285–292 [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529–531 [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S (2004) Glutamate receptors: RNA editing and death of motor neurons. Nature 427: 801. [DOI] [PubMed] [Google Scholar]
- Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH (2005) Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA 102: 2105–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Nakajima K, Kohsaka S (1997) Generation and characterization of a microglial cell line, MG5, derived from a p53-deficient mouse. Glia 21: 285–298 [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH (2006) Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125: 775–784 [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA (2001) Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci 21: 3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn JH, Lippert K, Krieglstein J (1995) Are NMDA or AMPA/kainate receptor antagonists more efficacious in the delayed treatment of excitotoxic neuronal injury? Eur J Pharmacol 292: 179–189 [DOI] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B (2002) Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron 35: 1067–1083 [DOI] [PubMed] [Google Scholar]
- Ribeiro CS, Reis M, Panizzutti R, de Miranda J, Wolosker H (2002) Glial transport of the neuromodulator D-serine. Brain Res 929: 202–209 [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, Mckennayasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Bergh RVD et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62 [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT (1990) Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol 28: 18–25 [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW (1995) Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol 38: 73–84 [DOI] [PubMed] [Google Scholar]
- Rutter AR, Fradley RL, Garrett EM, Chapman KL, Lawrence JM, Rosahl TW, Patel S (2007) Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating D-serine reuptake in the mouse CNS. Eur J Neurosci 25: 1757–1766 [DOI] [PubMed] [Google Scholar]
- Scannone H, Wellner D, Novogrodsky A (1964) A study of amino acid oxidase specificity, using a new sensitive assay. Biochemistry 3: 1742–1745 [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH (1995) D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA 92: 3948–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shleper M, Kartvelishvily E, Wolosker H (2005) D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci 25: 9413–9417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreux-Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Le Forestier N, Marouan A, Dib M, Meininger V (2002) Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J Neurol Sci 193: 73–78 [DOI] [PubMed] [Google Scholar]
- Streit WJ (1990) An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4). J Histochem Cytochem 38: 1683–1686 [DOI] [PubMed] [Google Scholar]
- Suzumura A, Mezitis SG, Gonatas NK, Silberberg DH (1987) MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol 15: 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Hayashi F, Nishikawa T (1997) In vivo evidence for the link between L- and D-serine metabolism in rat cerebral cortex. J Neurochem 69: 1286–1290 [DOI] [PubMed] [Google Scholar]
- Tsuji K, Mizumoto K, Yamochi T, Nishimoto I, Matsuoka M (2002) Differential effect of ik3-1/cables on p53- and p73-induced cell death. J Biol Chem 277: 2951–2957 [DOI] [PubMed] [Google Scholar]
- Urai Y, Jinnouchi O, Kwak KT, Suzue A, Nagahiro S, Fukui K (2002) Gene expression of D-amino acid oxidase in cultured rat astrocytes: regional and cell type specific expression. Neurosci Lett 324: 101–104 [DOI] [PubMed] [Google Scholar]
- Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP (2006) Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci 9: 108–118 [DOI] [PubMed] [Google Scholar]
- Van Damme P, Dewil M, Robberecht W, Van Den Bosch L (2005) Excitotoxicity and amyotrophic lateral sclerosis. Neurodegener Dis 2: 147–159 [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang D (2005) Memantine prolongs survival in an amyotrophic lateral sclerosis mouse model. Eur J Neurosci 22: 2376–2380 [DOI] [PubMed] [Google Scholar]
- Williams SM, Diaz CM, Macnab LT, Sullivan RK, Pow DV (2006) Immunocytochemical analysis of D-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia 53: 401–411 [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH (1999a) Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA 96: 13409–13414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO Jr, Ferris CD, Snyder SH (1999b) Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci USA 96: 721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Barger SW (2004) Induction of serine racemase by inflammatory stimuli is dependent on AP-1. Ann NY Acad Sci 1035: 133–146 [DOI] [PubMed] [Google Scholar]
- Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW (2004) Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J Neuroinflammation 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary information