Abstract
The voltage-dependent block of N-methyl-D-aspartate (NMDA) receptor channels by extracellular Mg2+ is a critical determinant of its contribution to CNS synaptic physiology. The function of the narrow constriction of the channel in determining the block was investigated by analysing the effects of a set of different amino acid substitutions at exposed residues positioned at or near this region. NMDA receptor channels, composed of wild-type and mutant NR1- and NR2A-subunits, were expressed in Xenopus oocytes or human embryonic kidney (HEK) 293 cells.
In wild-type channels, the voltage dependence (δ) of the block by Mg2+ was concentration dependent with values of δ of ∼0.58 in 0.01 mm and ∼0.82 in 0.07 mm and higher concentrations. Under biionic conditions with high extracellular Mg2+ and K+ as the reference ion, Mg2+ weakly permeated the channel. Over intermediate potentials (∼-60 to -10 mV), this weak permeability had no apparent effect on the block but at potentials negative to ∼-60 mV, it attenuated the extent and voltage dependence of the block.
Substitutions of glycine, serine, glutamine or aspartate for the N-site asparagine in the NR1-subunit enhanced the extent of block over intermediate potentials but left the voltage dependence of the block unchanged indicating that structural determinants of the block remained. These same substitutions either attenuated or left unchanged the apparent Mg2+ permeability.
In channels containing substitutions of glycine, serine or glutamine for the N-site asparagine in the NR2A-subunit, the block by Mg2+ was reduced at negative potentials. Over intermediate potentials, the block was not strongly attenuated except for the glutamine substitution which reduced the voltage dependence of the block to ∼0.57 in 0.7 mm Mg2+.
Equivalent substitutions for the N + 1 site asparagine in the NR2A-subunit strongly attenuated the block over the entire voltage range. In 0.7 mm Mg2+, the voltage dependence of the block was reduced to 0.50 (glycine), 0.53 (serine) and 0.46 (glutamine).
Channels containing substitutions of the N-site or N + 1 site asparagines in the NR2A-subunit showed an increased Mg2+ permeability suggesting that these adjacent asparagines form a barrier for inward Mg2+ flux. Changes in this barrier contribute, at least in part, to the mechanism underlying disruption of the block following substitution of these residues.
The adjacent NR2A-subunit asparagines are positioned at or near the narrow constriction of the channel. Pore size, however, did not determine how effectively Mg2+ blocks mutant channels.
It is concluded that, at the narrow constriction in the NMDA receptor channel, the adjacent NR2A-subunit asparagines, the N-site and N + 1 site, but not the N-site asparagine of the NR1-subunit, form a critical blocking site for extracellular Mg2+. The contribution to the blocking site, in contrast to the prevailing view, is stronger for the N + 1 site than for the N-site asparagine. The block may involve binding of Mg2+ to these residues.
Many biological functions of the N-methyl-D-aspartate (NMDA) receptor are mediated by the flux of Ca2+ through its open channel (MacDermott, Mayer, Westbrook, Smith & Barker, 1986; Mayer & Miller, 1988; Bliss & Collingridge, 1993). The pore of NMDA receptor channels is blocked by extracellular Mg2+ preventing Ca2+ as well as monovalent cation influx (Nowak, Bregestovsky, Ascher, Herbet & Prochiantz, 1984; Mayer, Westbrook & Guthrie, 1984). The strong voltage dependence of this block, which is relieved with membrane depolarization, makes the Ca2+ signalling mediated by NMDA receptors conditional: Ca2+ influx occurs only with presynaptic release of glutamate and coincident post-synaptic depolarization (Bliss & Collingridge, 1993).
In physiological solutions, the voltage dependence of the block by extracellular Mg2+ gives an apparent site of interaction between 0.8 and 1 across the transmembrane electric field (Ascher & Nowak, 1988; Jahr & Stevens, 1990a). One interpretation of this is that a single Mg2+ ion blocks the channel almost entirely across the electric field. However, the voltage dependence of the block by intracellular Mg2+ argues against extracellular Mg2+ blocking so deep in the channel (Johnson & Ascher, 1990; Li-Smerin & Johnson, 1996). The strong voltage dependence could therefore reflect the combined electrical distances of multiple Mg2+ ions (or one Mg2+ ion and one or more Ca2+/K+/Na+ ions) entering the pore in a multi-ion process (but see Zarei & Dani, 1994). Alternatively, it could arise by other mechanisms such as ion-ion interactions within the pore (Ruppersberg, von Kitzing & Schoepfer, 1994; Zarei & Dani, 1995). Given that the deepest blocking point for extracellular Mg2+ is probably at or near the narrowest part of the channel, defining the contribution to the block of residues forming this region is essential for the development of a molecular picture of the block.
Native NMDA receptor channels are heteromers composed of the constitutive NR1-subunit and one or more of four different NR2-subunits (A, B, C, D) (Sheng, Cummings, Roldan, Jan & Jan, 1994; for review, see Hollmann & Heinemann, 1994). Based on sequence alignment, asparagine (N) residues in the pore-lining M2 segment of the NR1- and NR2-subunits occupy an identical position (Fig. 1A), homologous to the functionally critical Q/R-site in α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors (Hollmann & Heinemann, 1994). For both subunits, the N-site asparagines are exposed at the tip of a loop formed by the M2 segment (Fig. 1B), but these N-site asparagines are otherwise neither structurally nor functionally equivalent (Burnashev et al. 1992; Wollmuth, Kuner, Seeburg & Sakmann, 1996; Kuner, Wollmuth, Karlin, Seeburg & Sakmann, 1996). The narrowest part of the channel, termed the narrow constriction or selectivity filter (Hille, 1992), is formed primarily by the NR1-subunit N-site asparagine and an asparagine adjacent to the NR2A-subunit N-site, the N + 1 site (Fig. 1B; Wollmuth et al. 1996). In this working model, the NR2A-subunit N-site asparagine is positioned on the extracellular side of the narrow constriction (Fig. 1B; Kuner et al. 1996).
Figure 1. Amino acid sequence of the wild-type NR1 and NR2A M2-loop segments.
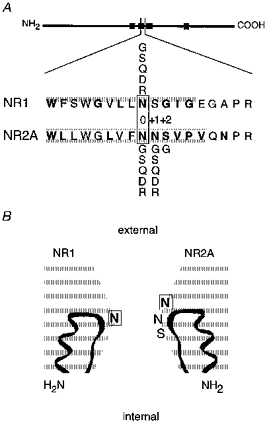
A, schematic drawing of the NMDA receptor subunits with the four hydrophobic segments (M1 to M4) indicated as filled boxes and the enlarged region showing a sequence alignment of amino acid residues forming the core region of the loop; this core region includes residues from the M2 segment (shaded) and the short linker connecting the M2 and M3 segments. The N-site is designated position ‘0′ with positions on the C-terminal side given a positive number. In the mature protein, the N-site asparagines correspond to position 598 (NR1) and 595 (NR2A). Residues that are exposed to the lumen of the channel are emboldened (Kuner et al. 1996). Five amino acids, glycine (G), serine (S), glutamine (Q), aspartate (D), and arginine (R), were substituted at positions NR1(N0), NR2A(N0) and NR2A(N + 1), whereas at the NR2A(S + 2) position, only glycine was substituted. B, positioning of exposed and polar residues near the narrow constriction. The two homologous asparagines (position 0; emboldened and boxed) are positioned in a staggered fashion with the N-site in the NR2A-subunit being more external than the NR2A-subunit N + 1 site and NR1-subunit N-site.
We examined how substitutions of residues positioned at or near the narrow constriction and exposed to the channel lumen (Fig. 1) contribute to extracellular Mg2+ block. Our goals were threefold. First, to determine which residues positioned near this region contribute to the block. Substitutions of these residues can have profound effects on Ca2+ permeability (Burnashev et al. 1992) as well as single channel properties (e.g. Premkumar & Auerbach, 1996; Schneggenburger & Ascher, 1997). We therefore recorded the block in the absence of Ca2+ (EGTA externally) and examined multiple substitutions at a single site, anticipating that if a side chain contributes to the block, a side chain-dependent pattern of block should be observed. The second goal was to determine whether the size of the narrow constriction contributes to the block. The size of hydrated Mg2+, at least 0.7 nm, is larger than the estimated 0.55 nm pore size of NMDA receptor channels (Villarroel, Burnashev & Sakmann, 1995; Zarei & Dani, 1995; Wollmuth et al. 1996), and Mg2+ block at the narrow constriction could arise by steric occlusion. Since the substitutions change the pore size to a known degree (Wollmuth et al. 1996; see Methods), the contribution of the size of this region to the block can be determined. Finally, we wanted to determine, at least in part, how residues positioned at the narrow constriction interact with Mg2+. We report that substitutions of two adjacent asparagines in the NR2A-subunit, especially that of the N + 1 site asparagine, but not of the N-site asparagine in the NR1-subunit, strongly reduce the block. These effects show little dependence on pore size suggesting that the block does not arise by steric occlusion. The adjacent NR2A-subunit asparagines contribute to the block mechanism in part by forming a barrier for inward Mg2+ flux as well as to some other process, possibly binding of Mg2+.
METHODS
Heterologous expression of NMDA receptor channels
All experiments were performed with previously described expression constructs for wild-type and mutant NMDA receptor subunits (Wollmuth et al. 1996). As described, NR1 mutants were co-expressed with wild-type NR2A or vice versa in Xenopus oocytes and human embryonic kidney 293 cells (HEK 293; reference no., CRL 1573). Xenopus oocytes were taken from frogs anaesthetized in an ice bath containing 0.3 % MS-222 for 40 min. For ease of description, mutated positions are identified by defining the N-sites as position ‘0′ (Fig. 1A; Kuner et al. 1996).
Solutions
Substitutions of residues positioned near the narrow constriction may change monovalent cation as well as Ca2+ permeability. To compare extracellular Mg2+ block in wild-type and mutant channels, we therefore used symmetrical [K+] on both sides of the membrane forcing the reversal potential to 0 mV and included an extracellular Ca2+ buffer. In all instances, the intracellular solution consisted of (mm): 100 KCl, 10 Hepes and 10 BAPTA; pH adjusted to 7.2 with KOH. For the extracellular solutions, the 10 mm BAPTA was replaced by 8 mm EGTA and 2 mm EDTA (0 Mg2+ control solution) or 10 mm EGTA to which MgCl2 was added to yield the free Mg2+ concentration indicated in the text. Free Mg2+ concentrations were determined from apparent affinity constants for EGTA, measured with the potentiometric method, with software kindly provided by R. Thieleczek (Moisescu & Thieleczek, 1978; Stephenson & Thieleczek, 1986). In all instances block was measured using whole-cell recordings of HEK 293 cells. Mg2+ influx was quantified using HEK 293 cells and oocyte outside-out patches. The high-K+ solution used as a reference to quantify inward Mg2+ permeability consisted of (mm): 100 KCl and 10 Hepes; pH adjusted to 7.2 with KOH. For outside-out oocyte patches, the same solution contained 0.18 mm CaCl2. The high-Mg2+ solution consisted of (mm): 78 MgCl2, 2 Mg(OH)2 and 10 Hepes, with the final pH 7.2. The Mg2+ salts were of ultrapure grade (Merck, Darmstadt, Germany).
Current recordings and data analysis
Currents were recorded at room temperature (19-23°C) using an EPC-9 amplifier with PULSE software (HEKA electronics GmbH, Lambrecht, Germany), low-pass filtered at 500 Hz, and digitized at 2 kHz. Pipettes were pulled from borosilicate glass and had resistances of 0.8-3 MΩ when filled with the pipette solution and measured in the K+ solution. To ensure that voltage drops due to series resistance were less than 2 mV, we often used series resistance compensation (70-85 %) with a lag time of 100 μs. Extracellular solutions were applied using a Piezo-driven double-barrel application pipette with one barrel containing the extracellular solution plus 50 μM glycine and the other barrel the same solution but with added 200 μM glutamate. The NMDA receptor current was defined as the difference between current recorded in the presence and absence of glutamate. The liquid-junction potential between the 103.5 mm K+ solution and the KCl pipette solution was -2 mV (pipette negative). The 80 mm Mg2+ solution generated a junction potential between the ground electrode and the high-KCl solution of -10.2 mV (ground electrode, 0 mV). All curve fitting was done using Igor Pro (WaveMetrics, Inc., Lake Oswego, OR, USA). Results are reported in the text as means ±s.e.m. and shown graphically as means ± 2 s.e.m.
Mg2+ permeability
Two measures of Mg2+ permeability in NMDA receptor channels with K+ as a reference were quantified: changes in zero-current (reversal) potentials and relative chord conductances. Changes in the reversal potential (ΔEr) were used as an approximate index of the driving force for Mg2+ relative to that for K+. They were determined by measuring the change of the reversal potential for glutamate-activated currents on replacing the 103.5 mm K+, 0.18 mm Ca2+ solution (for HEK 293 cells the solution contained no added Ca2+) with a similar solution except that the K+ was replaced by 80 mm Mg2+ and there was no added Ca2+. Relative chord conductances (GMg/GK) were used as an approximate index of the rate at which channels carry inward Mg2+ relative to K+. Current amplitudes were measured at -120 mV (high Mg2+) or -40 mV (high K+) and corrected for their respective reversal potentials. For mutant channels, both indexes of Mg2+ permeability were of limited quantitative value since they require the assumption that the substitutions have no effect on K+ permeation, a situation that probably does not hold. We therefore refer to these parameters as apparent permeability.
Voltage-dependent block
To model the relative location of a blocking site for Mg2+, we assumed that Mg2+ acts within the transmembrane electric field. With a Woodhull model (Woodhull, 1973), the current amplitude in the presence (IB) and absence (I0) of Mg2+ are related according to the relationship:
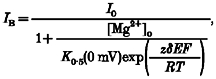 |
(1) |
where K0.5(0 mV) is the half-maximal block at 0 mV, δ is the portion of the membrane electric field sensed by the blocking site and z is the valence of the blocking ion. R, T and F have their normal thermodynamic meanings and the quantity RT/F was 25.4 mV (21°C). To control for run-down and to increase the accuracy of the measurement of the block over the entire voltage range, for each individual recording I0 was an average of the current amplitude recorded before and after exposure to the Mg2+ concentration. We used two approaches to determine K0.5(0 mV) and δ. First, a linear relationship was fitted to the linear region of ln(IB/(I0 - IB)), referred to as ln(r), plotted against voltage (DiFrancesco, 1982) where ln(IB/(I0 - IB)) is defined as:
| (2) |
The linear region was determined for average plots of ln(r) against voltage (using a maximal ln(r) of 2.5, i.e. where the block was at least 10 %) and then the same voltage range was used to fit individual records. The second approach involved fitting the Boltzmann equation to the fraction blocked:
| (3) |
where B is the fraction blocked (1 - IB/I0), Bmax is the maximal fraction blocked, and E0.5 is the voltage for half-maximal block. Assuming the blocking particle is impermeant:
A more complex relationship arises when permeation is explicitly included in the derivation. When Bmax is set equal to 1, this equation is identical to the Woodhull model (i.e. at extreme potentials all channels are blocked), and when fitted over the same voltage range as the ln(r) plots, it yielded indistinguishable parameters (data not shown). We therefore show values derived from this approach only when Bmax was a free parameter.
The use of macroscopic currents to quantify the voltage dependence of the block assumes that the only effect Mg2+ has at the single channel level is to occlude the channel rather than, for example, to alter its open probability. For wild-type channels such an assumption seems warranted (Jahr & Stevens, 1990b). We assumed that the predominant effect the mutant channels have on the block process is to alter this occlusion process; detailed single channel studies will be necessary to verify this assumption. In addition, for wild-type, the macroscopic current-voltage relationship in the absence of Mg2+ was linear (see Fig. 2A) yet the underlying single channel amplitudes showed a significant inward rectification (Wollmuth, Kuner & Sakmann, 1998). The basis for this difference is an increase in open probability at positive potentials (Nowak & Wright, 1992). In the case of extracellular Mg2+, this change in open probability presumably does not influence the block; at the concentrations of Mg2+ we tested (typically < 1 mm), the amplitudes of currents at positive potentials with or without Mg2+ present were identical in wild-type and all mutant channels indicating that block or potentiation at these potentials is negligible and that a constant number of channels are opened during the step.
Figure 2. Voltage-dependent block of wild-type NR1-NR2A channels by extracellular Mg2+.
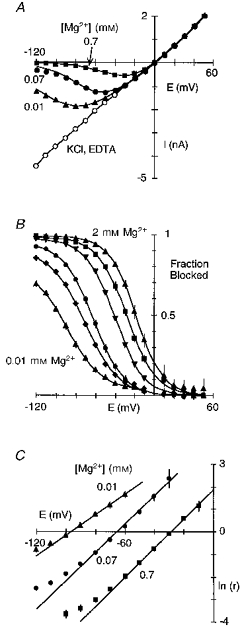
A, peak glutamate-activated currents plotted against membrane voltage in the absence (2 mm EDTA; KCl, EDTA) or presence of extracellular Mg2+ (0.01, 0.07 or 0.7 mm). Currents were generated by voltage steps, in 10 mV increments, and recorded in HEK 293 cells bathed in symmetrical [KCl] with 0 extracellular Ca2+. The 0 Mg2+ recording is an average of the currents recorded before and after exposure to each Mg2+ concentration. The continuous lines through the points in Mg2+ are from eqn (1) with the parameters δ and K0.5(0 mV) derived from eqn (2) (see C). Series resistance (Rs), 2.4 MΩ; Rs compensation, 80 %. B, mean fraction blocked (1 - IB/I0), where I0 and IB are the current amplitude in the absence and presence of different Mg2+ concentrations, respectively. In all instances, I0 was the average of currents recorded before and after application of the individual Mg2+ concentration. Each point is shown as the mean ± 2 s.e.m., and corresponds to Mg2+ concentrations of 0.01 mm (triangles), 0.03 mm (diamonds), 0.07 mm (circles), 0.3 mm (inverted triangles), 0.7 mm (squares) and 2 mm (triangles). Continuous curves are fitted Boltzmann equations (eqn (3)). In all instances, a simple Boltzmann equation readily fitted the results except for the block by 0.01 mm where an additional component existed between -50 and -10 mV. C, mean ln(IB/(I0 - IB)) (eqn (2)), referred to as ln(r), plotted against voltage for three different Mg2+ concentrations. Lines were fitted over the apparent linear region from -100 to -60 mV (0.01 mm), -80 to -30 mV (0.07 mm) and -70 to -10 mV (0.7 mm). The analysis was restricted to ln(r) values less than 2.5 where the current amplitude was reduced by at least 10 %.
Estimation of pore diameter
The size of the narrow constriction for wild-type and mutant channels was estimated using the relative permeability of differently sized organic cations (see Wollmuth et al. 1996). Usually, this estimate was based on at least four different organics. In some instances, we estimated pore diameter using two organics. Certain mutant channels were classified as having the same pore diameter as wild-type; alternatively, for substitutions which changed pore diameter we used the glycine (G) substitution of the same subunit (NR1(N0G) or NR2A(N + 1G)) and wild-type as calibration lines (see Fig. 8; Wollmuth et al. 1996).
Figure 8. Voltage dependence of the block in glutamine-substituted channels.
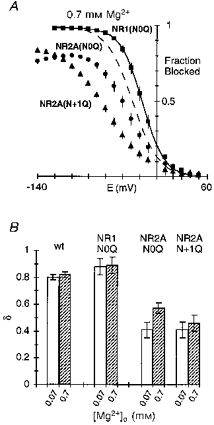
A, comparison of the mean fraction blocked by 0.7 mm Mg2+ in NR1(N0Q)-NR2A (▪), NR1-NR2A(N0Q) (•), and NR1-NR2A(N + 1Q) (▴). Dashed line is wild-type (Fig. 2B). B, mean voltage dependence of the block, δ, in glutamine-substituted channels. Values are shown as means ± 2 s.e.m.
RESULTS
Block of wild-type NR1-NR2A channels by extracellular Mg2+
Figure 2A illustrates extracellular Mg2+ block of NR1- NR2A channels in symmetrical [KCl] with 0 extracellular Ca2+. In the absence of Mg2+, the current-voltage relationship was linear. When Mg2+ (0.01-0.7 mm) was added to the extracellular solution, the inward current was attenuated in a strongly concentration- and voltage-dependent manner. Figure 2B shows plots of the fraction of channels blocked (1 - IB/I0), where the strong voltage dependence of the block appears as a steep region extending from -120 to -50 mV (0.01 mm) or -60 to -10 mV (2 mm). Using a Woodhull model (Woodhull, 1973), we quantified the voltage dependence of the block by transforming the blocked current into a linear form (eqn (2); Fig. 2C). The slope and the y-axis intercept of the fitted line to the linear region of these plots indicate the voltage dependence of the block, δ, and the voltage-independent affinity of Mg2+ for the channel, K0.5(0 mV), respectively. For wild-type channels, the voltage dependence as well as K0.5(0 mV) was dependent on the concentration, being 0.58 and 0.8 mm in 0.01 mm Mg2+ and around 0.82 and 4.0 mm at 0.07 mm Mg2+ and higher concentrations (Table 1).
Table 1.
Woodhull parameters for block of wild-type NR1-NR2A channels by extracellular Mg2+ in symmetrical [KCl]
| [Mg2+] (mm) | δ | K0·5(0 mV) (mm) | n |
|---|---|---|---|
| 0·01 | 0·58 ± 0·02 | 0·8 ± 0·1 | 8 |
| 0·03 | 0·71 ± 0·01 | 2·0 ± 0·2 | 8 |
| 0·07 | 0·80 ± 0·01 | 4·3 ± 0·3 | 10 |
| 0·3 | 0·82 ± 0·01 | 4·5 ± 0·2 | 8 |
| 0·7 | 0·82 ± 0·01 | 4·4 ± 0·2 | 6 |
| 2 | 0·86 ± 0·01 | 7·2 ± 0·2 | 4 |
Solutions: intracellular (mm): 123·5 KCl, 10 Hepes, 10 Bapta; control (0 Mg2+) extracellular (mm): 123·5 KCl, 10 Hepes, 8 EGTA, 2 EDTA. The composition of the test extracellular solution was the same except that EGTA replaced EDTA (10 mm EGTA) and MgCl2 was added to obtain the free Mg2+ concentration. Parameters were derived by fitting the linear part of plots of ln(IB/(I0 - IB)) against voltage (eqn (2); see Fig. 2C). Fitted ranges were: -100 to -60 mV (0·01 mm), -90 to -40 mV (0·03 mm), -80 to -30 mV (0·07 mm), -70 to -10 mV (0·3 and 0·7 mm) and -60 to -10 mV (2 mm). Values are means ± s.e.m.
Since the extent of block depended strongly on concentration, different voltage ranges were used to quantify the voltage dependence of the block. For a simple Woodhull model, the parameters should be independent of membrane potential. The Woodhull block parameters are assumed to reflect a specific binding site in a channel, indicating its position in the transmembrane electric field (δ) and its intrinsic voltage-independent affinity. Nevertheless, given that they are concentration dependent, they must be viewed more cautiously at present.
A Woodhull model describes the block by 0.7 mm Mg2+ quite well over intermediate or physiological membrane potentials (∼-70 to -10 mV; continuous line in Fig. 2A). However, at more negative potentials the block was weaker than that predicted, an effect seen in Fig. 2C where the block starting around -80 mV deviated upward from the line fitted over intermediate potentials. Hence, two regions of the block can be defined: over intermediate potentials (∼-70 to -10 mV), the block followed a simple Woodhull model whereas at more negative potentials it deviated from this model. This deviation indicates that in addition to simple block other mechanism(s) are occurring which may be present over the entire voltage range but are dominant at negative potentials. One such mechanism is that the blocking particle passes through the channel.
Mg2+ permeation in wild-type NR1-NR2A channels
Mg2+ poorly permeates NMDA receptor channels (Mayer & Westbrook, 1987; Iino, Ozawa & Tsuzuki, 1990) indicating a large energy barrier to the inward transport of Mg2+. Reversal potentials and relative chord conductances are indexes of this barrier. Figure 3A and B shows currents in wild-type channels in high K+ or Mg2+. In K+, the currents were large and reversed near -5 mV. When Mg2+ replaced K+, the currents were reduced at all potentials but a detectable inward current was observed negative to -52 mV (Fig. 3A, right panel), indicating that Mg2+ is less permeant than K+. On average, replacing K+ with Mg2+ produced a shift in the reversal potential of -52 ± 0.4 mV (n = 7). As an additional index of Mg2+ permeability in NMDA receptor channels, we compared the relative chord conductance in high Mg2+ (at -120 mV) to that in K+ (at -40 mV); the percentage relative chord conductance, GMg/GK, in wild-type was 0.4 ± 0.1 %. Thus, extracellular Mg2+ traverses the NMDA receptor channel but much less readily than K+.
Figure 3. Mg2+ permeates wild-type NR1-NR2A channels.
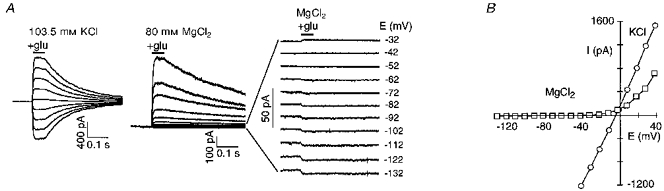
A, glutamate-activated currents at different membrane potentials in oocyte outside-out patches. Currents were generated by voltage steps, in 10 mV increments, and were recorded in patches bathed in high K+ (from -42 to +38 mV) or high Mg2+ (-132 to +38 mV) with the pipette containing 123.5 mm K+. Right panel, expanded traces recorded in Mg2+. Glutamate (200 μM; glu) was added for the periods indicated by the horizontal bars. B, peak current-voltage relationship for records shown in A. The K+ record is an average of the currents recorded before and after exposure to Mg2+.
Given that Mg2+ carries a definable inward current (see also Stout, Li-Smerin, Johnson & Reynolds, 1996), Mg2+ must be viewed as a permeant blocker in wild-type channels where the extent of block by Mg2+ is a balance between its rate of entry and exit from the extracellular side (simple block) and passage through the channel (permeation) (French & Shoukimas, 1985). Consider the simplified blocking scheme:
where the k values are the voltage-dependent rate constants, dependent on membrane potential (E), for Mg2+ entering (kon) and exiting (koff) a blocking site from the extracellular side or passing to the intracellular side (). (We have assumed that is negligible and does not contribute to the block under our recording conditions.) Channel block arises when Mg2+ occupies a blocking site. A simple voltage-dependent block process is defined in terms of kon(E) and koff(E) with the apparent affinity at any potential: KD(E) =koff(E)/kon(E), which is quantitatively described by a simple Woodhull model. However, permeation of Mg2+ adds another route by which Mg2+ can leave its blocking site, , and any measure of binding affinity will include this term: . Since we measured macroscopic currents in this study, we cannot define the individual rate constants. Nevertheless, three general points are noteworthy. First, inward Mg2+ permeation will quantitatively attenuate the extent of block by effectively reducing the dwell time of Mg2+ at its blocking site. Second, since all of the rate constants are voltage dependent, the effect of permeation on the block will be strongly dependent on membrane potential, becoming more prominent with negative potentials. Hence, at moderate potentials the situation may occur where , namely, that permeation does occur but does not significantly attenuate the block process at these potentials. Finally, we assume that Mg2+ permeation, as measured in Fig. 3, is an approximate index of but cannot quantitatively describe it. Since Mg2+ permeates wild-type channels poorly (Fig. 3), this process may not attenuate the block significantly, at least over physiological potentials, but additional information will be required to verify this point.
Extracellular Mg2+ block in channels containing glycine substituted for exposed residues at the narrow constriction
In considering how residues contribute to a blocking site, we initially examined the substitution of the small and non-polar glycine; this substitution removes any structural and energetic contribution a polar side chain would make to a blocking site and therefore would presumably attenuate the block. Figure 4A–C illustrates the block by extracellular Mg2+ in channels containing glycine substituted for any one of three asparagine residues positioned at (NR1(N0), NR2A(N + 1)) or external to (NR2A(N0)) the narrow constriction. For all three mutant channels, the current- voltage relationship in the absence of Mg2+ was linear, like that in wild-type. Similarly, the addition of Mg2+ reduced the inwardly directed current but the extent of this attenuation differed from that in wild-type as well as between the mutant channels, being weakest in NR2A(N + 1G) (Fig. 4C).
Figure 4. Mg2+ block in glycine-substituted channels.
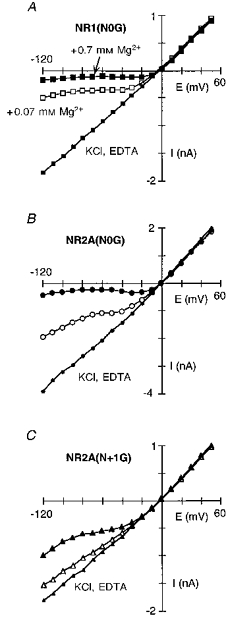
Extracellular Mg2+ block in NR1(N0G)-NR2A (A), NR1-NR2A(N0G) (B) and NR1-NR2A(N + 1G) (C) channels. Currents recorded and displayed as in Fig. 2A. Lines through the points in Mg2+ have no theoretical significance.
A direct comparison of the fraction blocked by 0.07 or 0.7 mm Mg2+ in these mutant channels with that in wild-type (dashed lines) is shown in Fig. 5A and B. The extent of block relative to that in wild-type, whether it was enhanced or attenuated, and the magnitude of this difference, depended strongly on membrane potential. At negative potentials the block in all three mutant channels was weaker to varying degrees. On the other hand, at intermediate potentials (∼-50 to -10 mV), the pattern of block was different. In 0.07 mm Mg2+, for example, the extent of block was enhanced in NR1(N0G) (open squares) as indicated by the rightward shift in the fraction blocked and remained steeply voltage dependent, in NR2A(N0G) (open circles) the block was also enhanced but to a lesser degree, and in NR2A(N + 1G) (open triangles) the block was nearly abolished. For all three mutant channels, the block over intermediate potentials was voltage dependent as quantified in Fig. 5C (0.07 mm) and Fig. 5D (0.7 mm). In NR1(N0G), the linear region was shifted to more positive potentials compared with wild-type (dashed line), but the voltage dependence of the block, around 0.80 in 0.07 or 0.7 mm Mg2+, was unchanged (Table 2). In contrast, for NR2A(N0G) and NR2A(N + 1G), the voltage dependence was attenuated. However, the extent of the attenuation depended on the Mg2+ concentration, being more strongly reduced in 0.07 mm Mg2+. In NR2A(N + 1G), for example, the voltage dependence was reduced to 0.50 in 0.7 mm Mg2+ but to nearly 0.12 in 0.07 mm Mg2+.
Figure 5. Voltage dependence of the block in glycine-substituted channels.
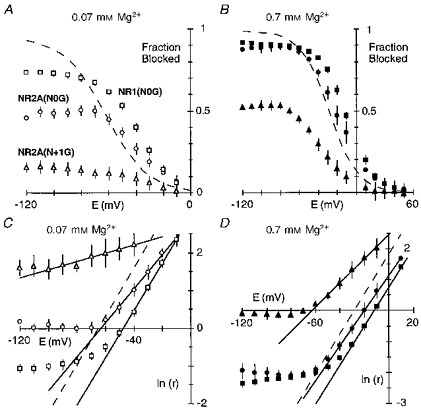
A and B, comparison of the mean fraction blocked by 0.07 mm Mg2+ (A) or 0.7 mm Mg2+ (B) in NR1(N0G)-NR2A (squares), NR1-NR2A(N0G) (circles), and NR1-NR2A(N + 1G) (triangles) channels. Dashed lines are Boltzmann fits for wild-type (Fig. 2B). C and D, mean ln(r) in 0.07 mm Mg2+ (C) or 0.7 mm Mg2+ (D) for glycine-substituted channels (same symbols as A and B), compared with wild-type (dashed lines). Lines were fitted over the apparent linear region from C: NR1(N0G), -50 to -10 mV; NR2A(N0G), -60 to -20 mV; NR2A(N + 1G), -100 to -40 mV; and D: NR1(N0G), -30 to +10 mV; NR2A(N0G), -40 to +10 mV; NR2A(N + 1G), -50 to -10 mV.
Table 2.
Woodhull parameters for extracellular Mg2+ block of NMDA receptor channels containing mutant NR1-subunits
| Subunit composition | [Mg2+] (mm) | δ | K0·5(0 mV) (mm) |
|---|---|---|---|
| NR1(N0G)-NR2A | 0·07 | 0·81 ± 0·03 | 1·5 ± 0·2 |
| 0·7 | 0·82 ± 0·03 | 1·6 ± 0·1 | |
| NR1(N0S)-NR2A | 0·07 | 0·80 ± 0·02 | 1·3 ± 0·1 |
| 0·7 | 0·80 ± 0·01 | 1·6 ± 0·1 | |
| NR1(N0Q)-NR2A | 0·07 | 0·88 ± 0·04 | 1·6 ± 0·2 |
| 0·7 | 0·89 ± 0·02 | 2·8 ± 0·5 | |
| NR1(N0D)-NR2A | 0·07 | 0·87 ± 0·04 | 0·29 ± 0·03 |
| 0·7 | — | — | |
| NR1(N0R)-NR2A | 0·07 | — | — |
| 0·7 | —* | —* |
Six to eight experiments were performed for each set of parameters. See Table 1 for details. Except for the N0G (see Fig. 5C and D), fitted ranges were comparable to wild-type.
The current was blocked by less than 10% at all potentials. Values are means ± s.e.m.
The voltage dependence of the block by 0.07 and 0.7 mm Mg2+ for the NR1(N0G), NR2A(N0G) and NR2A(N + 1G) channels as well as NR2A(S + 2G) compared with that in wild-type is summarized in Fig. 6. In NR1(N0G) and NR2A(S + 2G), the voltage dependence of the block was similar at both concentrations and comparable to that in wild-type. In contrast, for the two adjacent NR2A-subunit asparagines the voltage dependence was attenuated, with this attenuation being more prominent at 0.07 mm Mg2+ and in NR2A(N + 1G) channels. Thus, removing the side chain at either NR2A(N0) or NR2A(N + 1) disrupts the block over physiological potentials but much more strongly at N + 1 suggesting that the two asparagines contribute but not equally to a blocking site.
Figure 6. Attenuation of the voltage dependence of block in channels containing glycine substituted at NR2A(N0) or NR2A(N + 1) is concentration dependent.
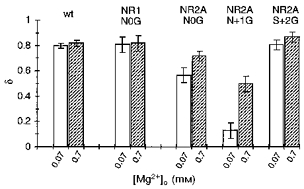
Mean voltage dependence of the block, δ, in glycine-substituted channels. Values are shown as means ± 2 s.e.m. wt, wild-type.
Substitution of glutamine for the adjacent NR2A-subunit asparagines but not the NR1-subunit N-site attenuates the block by Mg2+
Glycine is an extreme substitution, completely removing the side chain at the substituted position. Glutamine (Q) differs from the native asparagine only in an added methylene group, and substitution of it would primarily alter the geometric relationship of side chains leaving the chemically important amide group intact. A comparison of the block in channels containing glutamine substituted for any one of the three asparagines is shown in Fig. 7A–C. In NR1(N0Q) (Fig. 7A), the block by Mg2+ was comparable to that in wild-type. In contrast, the block in channels containing glutamine substituted in the NR2A-subunit at N0 (Fig. 7B) or N + 1 (Fig. 7C) was strongly attenuated. Figure 8A shows the fraction blocked by 0.7 mm Mg2+ in these glutamine-substituted channels. In the case of NR1(N0Q) (squares), the extent of block at any one potential was enhanced relative to that in wild-type (dashed line) but the voltage dependence of the block, around 0.88, was comparable (Fig. 8B; Table 2). On the other hand, in NR2A(N0Q) (circles) and NR2A(N + 1Q) (triangles), the block was reduced over the entire voltage range. At intermediate potentials, this attenuation was stronger in NR2A(N + 1Q) than in NR2A(N0Q). At very negative potentials this pattern was reversed. The voltage dependence of the block in these NR2A mutant channels was also reduced (Fig. 8B). Like the glycine substitutions, this attenuation was concentration dependent but the difference was greater in NR2A(N0Q) than in NR2A(N + 1Q). Indeed, in both mutant channels, the voltage dependence of the block was reduced to around 0.41 at 0.07 mm, whereas at 0.7 mm it was reduced to about the same extent for NR2A(N + 1Q) but only to 0.57 in NR2A(N0Q) (Table 3).
Figure 7. Mg2+ block in glutamine-substituted channels.
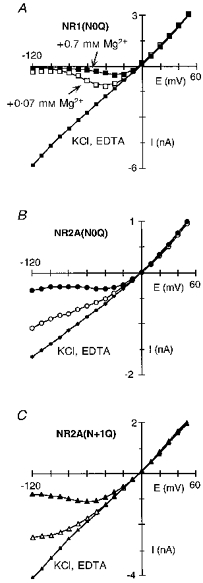
Extracellular Mg2+ block in NR1(N0Q)-NR2A (A), NR1-NR2A(N0Q) (B) and NR1-NR2A(N + 1Q) (C) channels. Currents recorded and displayed as in Fig. 2A. The continuous lines through the points in Mg2+ for NR1(N0Q) are from eqn (1) with the parameters δ and K0.5(0 mV) derived from eqn (2). For NR2A(N0Q) and NR2A(N + 1Q), the lines through the Mg2+ points have no theoretical significance.
Table 3.
Woodhull parameters for extracellular Mg2+ block of NMDA receptor channels containing mutant NR2A-subunits
| Subunit composition | [Mg2+] (mm) | δ | K0·5(0 mV) (mm) |
|---|---|---|---|
| NR1-NR2A(N0G) | 0·07 | 0·57 ± 0·03 | 1·2 ± 0·2 |
| 0·7 | 0·72 ± 0·02 | 2·2 ± 0·2 | |
| NR1-NR2A(N0S) | 0·07 | 0·62 ± 0·03 | 1·8 ± 0·3 |
| 0·7 | 0·78 ± 0·02 | 3·5 ± 0·3 | |
| NR1-NR2A(N0Q) | 0·07 | 0·41 ± 0·03 | 2·1 ± 0·1 |
| 0·7 | 0·57 ± 0·02 | 4·5 ± 0·4 | |
| NR1-NR2A(N0D) | 0·07 | — | — |
| 0·7 | —† | —† | |
| NR1-NR2A(N0R) | 0·07 | — | — |
| 0·7 | —* | —* | |
| NR1-NR2A(N + 1G) | 0·07 | 0·12 ± 0·03 | 1·0 ± 0·4 |
| 0·7 | 0·50 ± 0·03 | 8·7 ± 1 | |
| NR1-NR2A(N + 1S) | 0·07 | 0·32 ± 0·03 | 2·4 ± 0·2 |
| 0·7 | 0·53 ± 0·04 | 9·1 ± 1·5 | |
| NR1-NR2A(N + 1Q) | 0·07 | 0·42 ± 0·03 | 5·9 ± 1·5 |
| 0·7 | 0·46 ± 0·02 | 7·8 ± 1·0 | |
| NR1-NR2A(N + 1D) | 0·07 | 0·71 ± 0·02 | 1·5 ± 0·2 |
| 0·7 | 0·80 ± 0·02 | 3·7 ± 0·4 | |
| NR1-NR2A(N +1R) | 0·07 | — | — |
| 0·7 | —* | —* | |
| NR1-NR2A(S + 2G) | 0·07 | 0·80 ± 0·02 | 1·1 ± 0·2 |
| 0·7 | 0·87 ± 0·02 | 1·8 ± 0·2 |
Four to seven experiments were performed for each set of parameters. See Table 1 for details. Fitted ranges were typically from -70 to -30 mV (0·07 mm) and -50 to -10 mV (0·7 mm).
Mg2+ block could not be quantified (see text for details).
The current was blocked by less than 10% at all potentials. Values are means ± s.e.m.
In summary, the glutamine substitution further distinguishes the contribution of the asparagines to extracellular Mg2+ block. In the case of NR1(N0Q), the extent of block was enhanced but its voltage dependence was essentially unchanged. Indeed, as summarized in Table 2, all NR1(N0) substitutions, except for the positively charged arginine (R) which essentially abolishes the block, produced comparable effects on the block enhancing it over intermediate potentials but leaving the voltage dependence intact. On the other hand, substitution of glutamine at NR2A(N0) or NR2A(N + 1) strongly attenuated the extent as well as the voltage dependence of the block. This result is consistent with the amino acid residues at these positions contributing to the mechanism for Mg2+ block and also suggest that the geometric relationship of exposed side chains in the lumen is important.
In the NR2A-subunit, the N + 1 site asparagine is a more important determinant of Mg2+ block than the N-site asparagine
Table 3 compares the Woodhull parameters for the block of channels containing identical substitutions of the adjacent NR2A-subunit asparagines including glycine and glutamine as well as the polar serine (S) and the negatively charged aspartate (D). Substitution of serine at either NR2A(N0) or NR2A(N + 1) produced similar effects on the block as the glycine and glutamine substitutions; the voltage dependence was attenuated in a concentration-dependent manner with a much stronger attenuation in NR2A(N + 1S). Indeed, substitution of glycine, serine and glutamine at the N + 1 site consistently produced a stronger disruption of the block than the equivalent substitution at the N-site indicating that the N + 1 site represents a more important structural determinant of the block than the N-site. The block in channels containing aspartate at NR2A(N + 1) was also concentration dependent; surprisingly, however, the extent of block was not strongly enhanced, an issue we return to in the Discussion.
Substitution of aspartate at NR2A(N0) produced channels which yielded small currents. Also, in the absence of Mg2+ these channels showed a strong inward rectification and a time-dependent increase in the current amplitude with voltage steps to negative potentials; a more complex current pattern occurred in the presence of Mg2+ making quantification of the block difficult. We therefore did not explore Mg2+ block in this mutant channel.
The narrow constriction is not a steric barrier for Mg2+
The NR2A(N + 1) site, in contrast to the NR2A(N0) site, contributes to the size of the narrow constriction (Wollmuth et al. 1996). Since substitutions at NR2A(N + 1) alter pore size, the stronger effects of substitutions at this position on the block could reflect the possibility that the underlying mechanism of the block by Mg2+ is steric occlusion. If Mg2+ block is determined by steric occlusion of the channel lumen rather than by energetic factors, the extent of block especially at very negative potentials should be inversely related to pore diameter. However, as shown in Fig. 9, any such relationship was limited. Clearly, channels containing the NR2A(N + 1G) or NR2A(N + 1S) substitutions (open triangles) had an increased pore diameter as well as an attenuated block. However, the block was also attenuated in channels where pore diameter was reduced, NR2A(N + 1Q) (open triangles), or unchanged, NR2A(N0Q) and NR2A(N0S) (open circles). Further, pore diameter was greatly increased in NR2A(S + 2G) (open diamonds) and in NR1(N0G) (open squares) yet block was weakly affected. Hence, pore diameter per se does not determine how strongly Mg2+ blocks mutant NMDA receptor channels suggesting that the energetics of the side chains positioned at the narrow constriction contribute to the block. This energetic contribution could arise by a direct interaction of Mg2+ with the adjacent asparagines (binding) and/or the adjacent asparagines could act as an energy barrier for Mg2+ influx.
Figure 9. Mg2+ blocks NMDA receptor channels independent of pore size.
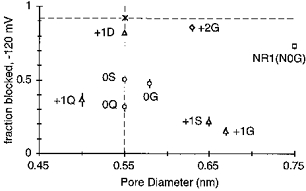
Fraction blocked by 0.07 mm Mg2+ at -120 mV plotted against pore diameter for wild-type (asterisk) and mutant NR2A channels (except for NR1(N0G)). The dashed lines are shown to indicate the values that would be observed if only one property, pore size (horizontal line) or Mg2+ block (vertical line), was changed.
Adjacent asparagines in the NR2A-subunit form an energy barrier for Mg2+ influx
In channels containing glycine substituted at NR1(N0), NR2A(N0) or NR2A(N + 1), the block by Mg2+ was weaker at negative potentials than in wild-type (Fig. 4). Such behaviour could reflect the possibility that Mg2+ acts as a stronger permeant blocker in these mutant channels than in wild-type. To test this possibility, we measured the apparent changes in reversal potential and relative chord conductance in high Mg2+ for these glycine-substituted channels. Quantitatively, any relationship between these indexes of Mg2+ permeability and block cannot be made since the substitutions may affect K+ as well as Mg2+ flux, and these indexes measured in 80 mm Mg2+ may overestimate the magnitude of the Mg2+ influx at low Mg2+ concentrations (Stout et al. 1996). Nevertheless, we anticipate that if an increased influx contributes to the attenuated block, a qualitative relationship between Mg2+ influx and block should exist.
Figure 10 shows current-voltage relationships in high K+ or Mg2+ for the glycine-substituted channels. In NR1(N0G) (Fig. 10A), the reversal potential in high Mg2+ was shifted to more negative potentials than in wild-type (arrow), suggesting that Mg2+ crosses this mutant channel less readily. Hence, the attenuated block at very negative potentials in NR1(N0G) apparently does not reflect an increased Mg2+ influx. Evidence consistent with this idea is that the fraction blocked, though attenuated relative to wild-type at negative potentials, became stronger with more negative potentials (see Fig. 5A and B). We have not explored further the basis of this attenuated block in NR1(N0G) but note that the breakthrough of current in Mg2+ at negative potentials (see Fig. 4A) cannot be explicitly ascribed to permeation.
Figure 10.
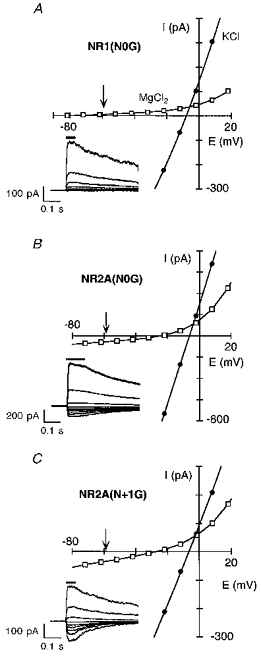
Inward Mg2+ permeability in glycine-substituted channelsGlutamate-activated currents recorded as in Fig. 3A and B except that outside-out patches were isolated from oocytes expressing NR1(N0G)-NR2A (A), NR1-NR2A(N0G) (B), or NR1-NR2A(N + 1G) (C) channels. The arrow in each plot indicates the expected reversal potential in Mg2+ given the observed reversal potential in the K+ solution and the average change in reversal potential for wild-type channels, ∼-52 mV. For NR1(N0G)-NR2A channels, currents in Mg2+ reversed around -77 mV. Insets, glutamate-activated currents in the presence of 80 mm MgCl2 shown in 20 mV increments (from -82 to +18 mV).
An alternative explanation for the lack of effect on Mg2+ influx is that the NR1(N0G) substitution enhanced K+ flux, shifting the reversal of the net current in high Mg2+ in a negative direction. If this is true, a similar reduced Mg2+ efflux should be seen from the internal face of the channel (the reversal should be shifted positive relative to wild-type). However, the opposite occurred (see Fig. 14A of Wollmuth et al. 1998), consistent with the idea that Mg2+ influx is not strongly enhanced in NR1(N0G).
In contrast to NR1(N0G), both NR2A(N0G) (Fig. 10B) and NR2A(N + 1G) (Fig. 10C) have a reversal potential in Mg2+ relative to wild-type which was shifted towards zero suggesting that Mg2+ permeates more readily. GMg/GK was also increased in both mutant channels compared with wild-type (Table 4). The rightward shift in the reversal potential and the increase in GMg/GK are consistent with Mg2+ acting as a stronger permeant blocker in these mutant channels than in wild-type. However, the shift in the reversal potential was more positive in NR2A(N0G) (∼-18 mV) than in NR2A(N + 1G) (∼-22 mV), yet the block was more strongly attenuated in NR2A(N + 1G) (see Fig. 5A and B). Nevertheless, the increase in GMg/GK, nearly twentyfold in NR2A(N + 1G) but only fourfold in NR2A(N0G), inversely paralleled the reduction in the extent of block. Thus, GMg/GK appears to reflect, at least qualitatively, how permeation influences the extent of block.
Table 4.
ΔEr and relative chord conductance for Mg2+ measured in wild-type and mutant NR1-NR2A NMDA receptor channels
| Subunit composition | ΔEr (mV) | GMg/GK (%) | n |
|---|---|---|---|
| NR1-NR2A | -51·4 ± 0·4 | 0·4 ± 0·1 | 7 |
| NR1(N0G)-NR2A | -70·9 ± 1 | 0·6 ± 0·1 | 6 |
| NR1(N0S)-NR2A | < -127 | — | 5 |
| NR1(N0D)-NR2A | -61·6 ± 1·5 | 0·7 ± 0·2 | 5 |
| NR1(N0Q)-NR2A | -50·1 ± 1·4 | 0·6 ± 0·2 | 3 |
| NR1-NR2A(N0G) | -18·0 ± 0·3 | 1·6 ± 0·2 | 5 |
| NR1-NR2A(N0S) | -30·0 ± 0·7 | 2·3 ± 0·2 | 5 |
| NR1-NR2A(N0Q) | -23·5 ± 1 | 4·0 ± 0·5 | 7 |
| NR1-NR2A(N + 1G) | -21·9 ± 0·8 | 8·9 ± 1 | 8 |
| NR1-NR2A(N + 1S) | -13·9 ± 0·4 | 14·6 ± 3 | 8 |
| NR1-NR2A(N + 1D) | -43·3 ± 0·5 | 2·1 ± 0·1 | 4 |
| NR1-NR2A(N + 1Q) | -58·1 ± 0·4 | 3·0 ± 0·3 | 5 |
| NR1-NR2A(S + 2G) | -43·3 ± 0·6 | 0·6 ± 0·1 | 7 |
Reference solution (mm): 103·5 KCl, 10 Hepes, 0·18 CaCl2 (oocyte macropatches) or 103·5 KCl, 10 Hepes (HEK 293 cells). Test solution (mm): 78 MgCl2, 2 Mg(OH)2, 10 Hepes. Results from oocyte outside-out patches and HEK 293 whole-cell recordings were indistinguishable and were combined. Current amplitudes used to calculate relative chord conductances were measured at -120 mV (high Mg2+) or -40 mV (high K+). Values are means ± s.e.m.
Table 4 summarizes ΔEr and GMg/GK for all substitutions tested. In NR1(N0)-substituted channels, ΔEr in high Mg2+, like that in N0G, tended to be reduced with the serine substitution producing channels where no inward current in high Mg2+ could be detected up to -127 mV. In contrast, substitutions of either adjacent NR2A-subunit asparagine but not the NR2A(S + 2) serine enhanced the apparent driving force for Mg2+, relative chord conductance or both. Thus, the adjacent NR2A-subunit asparagines, but not the NR1-subunit N-site asparagine or the NR2A(S + 2) serine, contribute to a barrier for inward Mg2+ permeability. In this regard, therefore, a function of the adjacent asparagines is to maintain a low (Scheme 1).
Scheme 1.
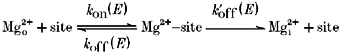
Substitutions of the adjacent asparagines in the NR2A-subunit produced the strongest effect on the increase in inward Mg2+ permeability and attenuation of the block. Since Mg2+ influx attenuates the extent of block, the function of the adjacent NR2A-subunit asparagines in the block mechanism could be to act solely as a barrier for Mg2+ influx (i.e. they contribute to the maintainance of a low in Scheme 1 without contributing to koff or kon). However, in mutant NR2A channels, the relationship between the extent of block and ΔEr which can be interpreted as an index of the barrier for Mg2+ influx, was poor (data not shown). In addition, the extent of block at -120 mV showed a good correlation with GMg/GK, indicating that this index of Mg2+ influx reflects elements of the block process. However, over intermediate potentials (∼-70 to -10 mV), no correlation existed suggesting that processes in addition to changes in Mg2+ influx were being altered in these mutant channels. Quantitatively, we cannot state the relative contribution of these different processes at different potentials, but additional evidence is presented below which suggests that, over intermediate potentials (∼-40 to -10 mV), the contribution of inward Mg2+ permeability to the block in mutant NR2A channels is small.
Mg2+ influx contributes to the attenuation of δ in wild-type at low Mg2+ concentrations
In wild-type, the voltage dependence of the block by 0.01 mm Mg2+, which could be quantified only over negative potentials (Fig. 2C), was attenuated relative to that at higher concentrations. Permeation of a blocking ion reduces the apparent voltage dependence of the block (French & Shoukimas, 1985). Substitution of serine at NR1(N0) produced channels where no inward current in high extracellular Mg2+ could be detected (Table 4). To test the idea that Mg2+ influx attenuates the voltage dependence of the block by 0.01 mm Mg2+, we quantified the block in NR1(N0S)-NR2A channels at this concentration (Fig. 11). The fraction blocked by 0.01 mm Mg2+ in NR1(N0S) is compared with that in wild-type in Fig. 11A. Up to -60 mV the block was indistinguishable in the two channel types, but starting at -70 mV the extent of block diverged, becoming stronger in NR1(N0S). In addition, the block was more steeply voltage dependent in NR1(N0S) yielding a δ of 0.82 ± 0.02 (n = 5). A comparison of δ as a function of concentration in NR1(N0S) and wild-type channels is shown in Fig. 11B. In contrast to wild-type, δ showed no concentration dependence in NR1(N0S), being independent of membrane potential. This result is consistent with the idea that in wild-type Mg2+ influx attenuates the voltage dependence of the block at negative potentials.
Figure 11. Mg2+ influx contributes to the attenuation of δ at low concentrations in wild-type.
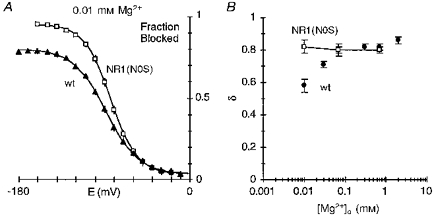
A, comparison of the mean fraction blocked by 0.01 mm Mg2+ in NR1(N0S)-NR2A (□) and wild-type (▴). The continuous lines are the Boltzmann equation (eqn (3)) fitted over the entire voltage range. Parameters: wild-type: Bmax, 76.6; E0.5, -88.7 mV; zδ, 1.48; and NR1(N0S): Bmax, 91.9; E0.5, -83.8 mV; zδ, 1.83. In ln(r) plots for NR1(N0S), the line was fitted over -100 to -60 mV as in wild-type. B, mean voltage dependence of the block, δ, at different Mg2+ concentrations in NR1(N0S)-NR2A (□) or wild-type (•) channels.
The adjacent asparagines in the NR2A-subunit contribute to the mechanism controlling the strong voltage dependence of the block
Substitutions of the adjacent asparagines in the NR2A-subunit strongly reduced the voltage dependence of the block as well as increased the apparent Mg2+ influx. To test whether an increased Mg2+ influx is the only mechanism underlying the reduced voltage dependence of the block in mutant NR2A channels, we examined the block in these channels in two additional ways. First, we measured the block by a wider concentration range of Mg2+ in NR2A(N0Q) and NR2A(N + 1G). These substitutions were selected for this analysis since at the respective positions they showed the most strongly reduced block. We anticipated that if Mg2+ influx is the sole mechanism attenuating the voltage dependence then there would be a continuous increase in δ at higher concentrations. However, as shown in Fig. 12A, this was not the case. In particular, for NR2A(N0Q) (open circles) the voltage dependence of the block reached a plateau starting at 0.3 mm Mg2+ (δ= 0.54 ± 0.04, n = 5) without becoming any stronger in 0.7 Mg2+ (δ≡0.57) or 2 mm Mg2+ (δ= 0.50 ± 0.01, n = 5). Similarly, for NR2A(N + 1G) (triangles), the voltage dependence again reached a plateau at higher Mg2+ concentrations though this effect occurred at 0.7 mm (δ≡0.50) and 2 mm Mg2+ (δ= 0.53 ± 0.03, n = 7).
Figure 12. Voltage dependence of block in mutant NR2A channels.
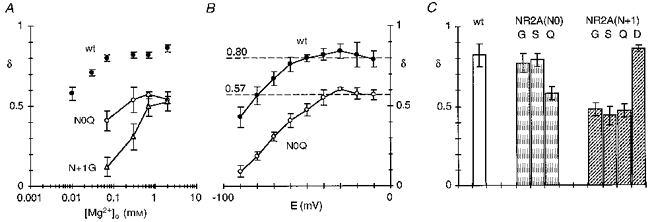
A, mean voltage dependence of the block, δ, at different Mg2+ concentrations in NR1-NR2A(N0Q) (^), NR1-NR2A(N + 1G) (▵) or wild-type (•) channels. B, mean voltage dependence of the block, δ, measured at single potentials (i.e. the derivative of ln(r) plots) in NR1-NR2A(N0Q) (^) or wild-type (•) channels. C, mean voltage dependence of the block, δ, at -20 mV in 0.7 mm Mg2+ for channels containing NR2A(N0) or NR2A(N + 1) substitutions.
The second approach we used was to quantify the derivative of ln(r) plots in 0.7 mm Mg2+ (Fig. 12B). This analysis is identical to that shown in Fig. 2C but δ was now based on the slope at a single voltage rather than a range of voltages. In wild-type (filled circles), the derivative of ln(r) was around 0.8 between -60 and -10 mV and was strongly reduced at more negative potentials. A constant δ could reflect that between -60 and -10 mV either Mg2+ influx influences the block to an equivalent extent or that it contributes little to the block. Other results (Fig. 11) are consistent with the latter alternative. In all mutant NR2A channels, a constant δ was found but this occurred over a more limited voltage range than expected for channels where Mg2+ is more permeant. In NR2A(N0Q), for example, δ was around 0.57 between -40 and -10 mV but at potentials negative to -40 mV it was strongly reduced (Fig. 12B, open circles). Hence we assume that between ∼-40 and -10 mV the primary escape route for Mg2+ from its blocking site is to the extracellular side (simple block, i.e. in Scheme 1), and that measured values of δ over this voltage range are an index of the block process (KD(E) rather than . A comparison of δ based on the derivative at -20 mV for substitutions at NR2A(N0) and NR2A(N + 1) to that in wild-type is shown in Fig. 12C. The values are comparable to those shown in Table 2 and indicate that the N + 1 site asparagine is a more important determinant of the mechanism underlying the voltage dependence of the block than the N-site asparagine.
In summary, the adjacent NR2A-subunit asparagines contribute to a barrier for Mg2+ influx. They also contribute to some other process in the block mechanism, possibly binding and/or the mechanism controlling the strong voltage dependence of the block. In mutant NR2A channels, this other process disrupts the block over the entire voltage range whereas at negative potentials, the block is further reduced by an increased permeation.
DISCUSSION
Our results demonstrate that the narrow constriction of NMDA receptor channels forms a structural determinant of the block by extracellular Mg2+. However, residues forming the narrow constriction contribute differentially to the block. The NR1-subunit N-site asparagine and the NR2A-subunit S + 2 serine, while determining the dimensions of the narrow constriction (Wollmuth et al. 1996), appear to contribute little. In contrast, the N-site and the N + 1 site asparagines in the NR2A-subunit represent critical determinants of a blocking site. Previous work has shown that the N-site in the NR2-subunit is one determinant of the block (Burnashev et al. 1992; Mori, Masaki, Yamakura & Mishina, 1992; Sakurada, Masu & Nakanishi, 1993). We found that the two adjacent asparagines in the NR2A-subunit do not contribute equally to the blocking site, with the N + 1 site asparagine representing the more important determinant.
Mg2+ block and permeation in wild-type channels
Physiological concentrations of Mg2+ are around 1 mm. In 0.7 mm Mg2+, the Woodhull model described the block in wild-type quite well over physiological potentials (∼-70 to -10 mV; Fig. 2A and C). Starting around -80 mV, however, the block was weaker than that expected. At very low concentrations (0.01 and 0.03 mm) where the block could be quantified only at potentials negative to -40 mV, the voltage dependence of the block was attenuated relative to that at higher concentrations (Table 1). One possible explanation for the deviation from the Woodhull model and the reduced voltage dependence at low concentrations is that at negative potentials Mg2+ passes through the channel more readily, reducing the effective dwell time of Mg2+ at its blocking site. Consistent with this idea, we found an inward current in high extracellular Mg2+ in wild-type (Fig. 3), and native NMDA receptor channels have been shown to transport Mg2+ inwardly (Stout et al. 1996). Further, in NR1(N0S)-NR2A channels, where no inward current in the presence of high Mg2+ could be detected, the voltage dependence of the block by 0.01 mm Mg2+ was independent of Mg2+ concentration (Fig. 11B). Comparison of the block by 0.01 mm Mg2+ in wild-type and NR1(N0S)-NR2A channels (Fig. 11A) suggests that up to -60 mV permeation does not significantly attenuate the block. Hence, two regions of the block in wild-type can be defined. Over the physiological range (∼-60 to -10 mV), the predominant escape route for Mg2+ from its blocking site is to the extracellular side (simple block). Consistent with this idea is that the Woodhull block parameters showed little concentration dependence between 0.07 and 0.7 mm Mg2+ where the block could be quantified over this potential range. At potentials negative to -60 mV, Mg2+ can now leave its blocking site and translocate to the cytoplasmic side of the channel. The attenuated current at these negative potentials in Mg2+ is therefore a combination of simple block by Mg2+ and weak permeation which reduces the block.
The estimated voltage-independent affinity for the channel, K0.5(0 mV), is also influenced by permeation. In 0.01 mm Mg2+, for example, permeation reduces the extent of block at negative but not intermediate potentials. This flattens out the fitted curve leading to a reduced voltage dependence and a lower y-axis intercept (i.e. a higher apparent affinity). We therefore compared K0.5(0 mV) in mutant channels only in 0.7 mm Mg2+ where the block could be quantified between -60 and -10 mV.
Asparagines forming the narrow constriction contribute differently to Mg2+ block
The homologous N-site asparagines in the two subunits are positioned at the tip of the loops formed by the M2 segments. The narrowest part of the channel is, however, determined primarily by the non-homologous NR1-subunit N-site and NR2A-subunit N + 1 site asparagines (Kuner et al. 1996; Wollmuth et al. 1996). The strong effects of substitutions of the adjacent asparagines in the NR2A-subunit on the alteration of both the extent and voltage dependence of the block suggest that they form a structure critical for the block. In addition, based on identical substitutions, the N + 1 site asparagine is a more critical determinant of the block than the N-site asparagine. On the other hand, substitutions at the NR1-subunit N-site asparagine or the NR2A-subunit S + 2 serine had no effect on the voltage dependence of the block, altering only the extent of block at any one potential.
Mechanism of interaction of Mg2+ with the adjacent asparagines in the NR2A-subunit
One issue that remains to be resolved for the development of a molecular description of the block mechanism is the method by which Mg2+ interacts with the two adjacent asparagines in the NR2A-subunit. Three extreme possibilities will be considered. (a) The adjacent NR2A-subunit asparagines could represent simply an energy barrier for Mg2+ inflow. Here, Mg2+ would bind to residues at a distance from the narrow constriction with the adjacent asparagines acting as a translocation barrier for Mg2+ inflow. Alternatively (b), the polar asparagine side chains could act as water substitutes specifically binding to Mg2+. Finally (c), the narrow constriction could mediate the unknown mechanism controlling the strong voltage dependence of the block.
The adjacent asparagines represent an energy barrier for Mg2+ influx
At least part of the contribution of the adjacent asparagines to the block mechanism is to act as an energy barrier for Mg2+ influx since substitutions of them increased the apparent inward Mg2+ permeability (Table 4). Thus, a function of the adjacent asparagines is to maintain a small in Scheme 1, with the result that extracellular Mg2+ acts as a simple blocker over physiological potentials. This contribution is critical for the function of NMDA receptor channels. If was high in wild-type channels, at the resting membrane potential, typically -60 to -70 mV, NMDA receptor channels would carry a significant amount of inward current during a single EPSP (e.g. Figs 4C and 7B). This would prevent the NMDA receptor from acting as a detector of post-synaptic depolarization.
How do the adjacent asparagines prevent Mg2+ from crossing the channel? One possibility is that they form a steric barrier for Mg2+. Since Mg2+ is a small ion (diameter, ∼0.13 nm; Frausto da Silva & Williams, 1991), giving it a large charge to surface area ratio, the removal of its inner hydration shell would require considerable free energy and its effective size would be considerably larger, at least 0.7 nm. Since NMDA receptor channels have a pore size of about 0.55 nm (Villarroel et al. 1995; Zarei & Dani, 1995; Wollmuth et al. 1996), hydrated Mg2+ could block NMDA receptor channels at the narrow constriction by steric occlusion. However, the relationship between how substitutions change pore size and block by Mg2+ is poor (Fig. 9). In addition, the strong increase in the apparent Mg2+ permeability in channels where no change in pore size occurred (e.g. NR2A(N0Q), NR2A(N0S)) suggests that Mg2+ crosses the narrow constriction in a partially dehydrated state. Hence, the energetics of the interaction between the narrow constriction and Mg2+ rather than its dimensions determines its function in Mg2+ block.
The adjacent asparagines form an energy barrier for Mg2+ influx. Various lines of evidence suggest, however, that they participate in the block mechanism in additional ways. First, no correlation between the extent of block at intermediate potentials and Mg2+ influx was found. Second, many of the substitutions, especially those of the N + 1 site, strongly altered the apparent voltage-independent affinity (Table 3). Finally, analysis of the block over intermediate potentials suggested that processes in addition to changes in permeation were occurring (Fig. 12).
We cannot quantitatively state the relative contribution of permeation without single channel analysis which will be required to define the effect of substitutions on the microscopic rate constants. However, analysis of how substitutions affect the block must explicitly consider permeation, which adds another route by which Mg2+ can leave its blocking site, (see Scheme 1), an effect that is especially strong at negative potentials (< -60 mV). Potentials at which to study the block at the single channel level must be selected carefully since a decrease in the dwell time does not necessarily reflect a change in binding but rather a change in inward permeability.
The adjacent asparagines represent a binding site for extracellular Mg2+
Given the polar nature of the asparagine side chain, an ion-dipole co-ordination would underlie this interaction as illustrated in Fig. 13. Such a conclusion for the N + 1 site seems reasonable since substitution of glycine, serine or glutamine at this position attenuated K0.5(0 mV) in 0.7 mm Mg2+ (Table 3). Nevertheless, the addition of a negative charge to a co-ordinating ligand typically enhances the strength of a co-ordination complex (Frausto da Silva & Williams, 1991; Falke, Drake, Hazard & Peersen, 1994). Substitution of the negatively charged aspartate at the N + 1 site, however, did not strongly enhance the block (Table 3). One possible explanation for this is that a negatively charged side chain at N + 1 forms a salt bridge with other side chains in the vicinity removing it from any interaction with Mg2+. Alternatively, binding of Mg2+ to residues at the narrow constriction could depend both on the ligand type as well as on their spatial configuration. Consistent with this idea is that substitution of glutamine, which alters the geometry but not the ligand type of the native asparagine side chain, at NR2A(N + 1) strongly disrupts the block. Hence, the carboxylate side chain at N + 1 could alter this ‘optimal’ structural relationship. The weakly enhanced block in NR2A(N + 1D) channels therefore would be the net effect of increased binding due to the negative charge and decreased binding due to changes in the spatial relationship between ligands.
Figure 13. The adjacent asparagines in the NR2A-subunit underlie the block of NMDA receptor channels by extracellular Mg2+.
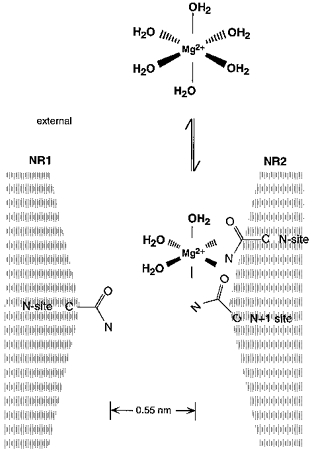
Schematic drawing of amino acid residues positioned at or near the narrow constriction. Mg2+ prefers an octahedral (sixfold) co-ordination. In solution, the co-ordination spheres are occupied by water. In the pore, bound Mg2+ loses anywhere from 1 to 6 waters of hydration with the free electron pairs in the carbonyl oxygen and/or amide nitrogen of the asparagine side chain substituting for the water molecules. The effective diameter of hydrated Mg2+, which is not drawn to scale, is at least 0.7 nm considering only the primary hydration shell and assigning a diameter of 0.28 nm to a water molecule and 0.13 nm to Mg2+ (Frausto da Silva & Williams, 1991).
Binding of Mg2+ to the less critical NR2A-subunit N-site is also not clear cut. Substitution of glycine or serine at this site actually weakly enhanced the extent of block over intermediate potentials (Table 3). Nevertheless, others have concluded that the N-site in the NR2A-subunit does contribute to Mg2+ binding (Sharma & Stevens, 1996), based in part on a parallel reduction in the block over the entire voltage range in NR2A(N0Q) channels. In addition, these authors suggested that other structural elements must also participate in binding Mg2+; this additional element is most probably the N + 1 site asparagine.
The parallel reduction in the block in NR2A(N0Q) seen by Sharma & Stevens (1996) differs from our observations in that for our comparable parameter, the fraction blocked, the deviation from wild-type depended strongly on membrane potential (Fig. 8A, circles). The basis for this divergent result is unknown but might be due to the different recording conditions. We recorded the block in high extracellular K+ and in the complete absence of extracellular Ca2+ (EGTA externally) whereas Sharma & Stevens (1996) analysed the block in high extracellular Na+ and in 0.1 mm Ca2+.
The adjacent asparagines mediate the mechanism controlling the strong voltage dependence
A key feature of the block by extracellular Mg2+ is its strong voltage dependence, manifested at the single channel level as increasingly longer dwell times with more negative potentials. The Woodhull model identifies the relative location of a blocking site (δ) in the transmembrane electric field based on the voltage dependence of the block. The voltage dependence of the block by Mg2+ in physiological solutions (e.g. Ascher & Nowak, 1988; Jahr & Stevens, 1990a) as well as in the present study places the apparent blocking site almost entirely across the transmembrane electric field. Our results demonstrate that the narrow constriction represents a critical blocking site for extracellular Mg2+, yet recent studies examining the block by impermeant organic cations suggest that it is positioned about 0.5-0.6 of the way across the electric field (Villarroel et al. 1995; Zarei & Dani, 1995). Since the adjacent NR2A-subunit asparagines form an apparent barrier for Mg2+ influx, it is unlikely that in terms of simple block Mg2+ penetrates any deeper into the channel than to the narrow constriction. Hence, following a simple Woodhull model, Mg2+ occupying its blocking site at the narrow constriction underlies a major part of the strong voltage dependence, perhaps about 0.5-0.6 of the 0.82 fractional electric distance.
Substitutions of the adjacent asparagines in the NR2A-subunit strongly attenuated the voltage dependence of the block. Over intermediate potentials (∼-40 to -10 mV), this effect is apparently not due to changes in permeation (Fig. 12). The strongest attenuation occurred when Q (0.57) was substituted at NR2A(N0) or when G (0.50), S (0.53) or Q (0.46) was substituted at NR2A(N + 1). Surprisingly, these values converge around 0.5-0.6, a value similar to the position of the narrow constriction. Assuming that the position of the narrow constriction in the transmembrane electric field is unchanged by the substitutions, this result suggests that much of the additional voltage dependence is an intrinsic property of the NR2A amino acid residues at the narrow constriction. This conclusion is consistent with the results of Zarei & Dani (1994) who found that the NMDA receptor channel has a single file pore suggesting that the strong voltage dependence does not arise by multi-ion occupancy. Apparently, although other sites may contribute to the entry and exit of extracellular Mg2+ to its blocking site at the narrow constriction, when Mg2+ occupies this deep site, no other sites in the channel are occupied. How is the additional voltage dependence generated at the narrow constriction? Two alternative explanations can be considered. First, the strong voltage dependence of the block has been suggested to arise by ion-ion interactions within the pore (Zarei & Dani, 1995), and either of the adjacent asparagines could represent the ‘permeant-ion’ site proposed by these authors. Alternatively, the entire M2 segment or parts of it could move in the electric field as a function of voltage, with the bound Mg2+ acting as the voltage sensor. In this view, changes in binding of Mg2+ to the adjacent NR2A-subunit asparagines would reduce the dwell time of Mg2+ making it less sensitive to the transmembrane electric field and correspondingly reducing the voltage dependence of the block.
Contribution of the N-site asparagine in the NR1-subunit to extracellular Mg2+ block
The NR1-subunit N-site asparagine, which is critical for Ca2+ permeation (Burnashev et al. 1992), appears to make only a weak contribution to the block by extracellular Mg2+. In all NR1(N0) mutant channels, the block by Mg2+ was strongly voltage dependent over intermediate potentials having δ values ranging from 0.80 (N0S) to 0.88 (N0Q), values that are very similar to those of wild-type (Table 2). However, the extent of block in these channels, compared with wild-type, was enhanced over intermediate potentials as seen by the higher voltage-independent affinity. Given that this effect occurred to an equivalent extent for the non-polar glycine and the polar serine and glutamine, all NR1(N0) substitutions may reflect to some extent a change in a general property of the channel. Indeed, when NR1(N0Q) is co-expressed with NR2A(N0Q), the resulting channels have multiple conductance levels, one of which has a higher affinity for Mg2+ than wild-type (Premkumar & Auerbach, 1996). This high-affinity site is weakly voltage dependent and presumably arises, at least in part, from the NR1(N0Q) substitution since NR1-NR2A(N0Q) channels do not display such behaviour (Sharma & Stevens, 1996). The net effect of multiple conductance levels with differential affinities for Mg2+ at the macroscopic level therefore appears to be an enhanced affinity with no change in the voltage dependence at least over intermediate potentials. At extreme potentials, more complex block patterns may arise.
Surprisingly, the substitution of a negative charge at NR1(N0) caused the strongest increase in the extent of Mg2+ block of all substitutions tested. Based on the available structural information, which places the NR1(N0) near the adjacent NR2A-subunit asparagines (Fig. 1B), this enhanced block could arise via an electrostatic mechanism and/or by offering Mg2+ an additional ligand. In summary, NR1(N0) substitutions do alter, albeit weakly, the block but the essential elements of the interaction between Mg2+ and the channel remain intact.
Conclusion
The amino acid residues forming the narrow constriction as contributed by the NR2-subunit in NMDA receptor channels represent a critical blocking site for extracellular Mg2+. The results do not define how the strong voltage dependence of the block arises but suggest that much of the voltage dependence of the block is an intrinsic, local property of NR2-subunits at the narrow constriction.
Acknowledgments
We thank Peter H. Seeburg for his generous support, Drs C. Beck, N. Burnashev, D. Feldmeyer, J. Mosbacher and A. Villarroel for their comments on the manuscript, H. Spiegel for expert secretarial assistance, and M. Kaiser and S. Grünewald for technical assistance. This work was supported in part by a long-term Human Frontier Science Fellowship (L. P. W.).
References
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. Journal of Physiology. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Günther W, Seeburg PH, Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pacemaker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. Journal of Physiology. 1982;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke JJ, Drake SK, Hazard AL, Peersen OB. Molecular tuning of ion binding to calcium signaling proteins. Quarterly Reviews of Biophysics. 1994;27:219–290. doi: 10.1017/s0033583500003012. [DOI] [PubMed] [Google Scholar]
- Frausto da Silva JJR, Williams RJP. The Biological Chemistry of the Elements. 1. Oxford: Clarendon Press; 1991. [Google Scholar]
- French RJ, Shoukimas JJ. An ion's view of the potassium channel. Journal of General Physiology. 1985;85:669–698. doi: 10.1085/jgp.85.5.669. 10.1085/jgp.85.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates, Inc.; 1992. [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual Review of Neuroscience. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Iino M, Ozawa S, Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. Journal of Physiology. 1990;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. A quantitative description of NMDA receptor-channel kinetic behavior. Journal of Neuroscience. 1990a;10:1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. Journal of Neuroscience. 1990b;10:3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate channels. Biophysical Journal. 1990;57:1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Wollmuth LP, Karlin A, Seeburg PH, Sakmann B. Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron. 1996;17:343–352. doi: 10.1016/s0896-6273(00)80165-8. 10.1016/S0896-6273(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Li-Smerin Y, Johnson JW. Kinetics of the block by intracellular Mg2+ of the NMDA-activated channel in cultured rat neurons. Journal of Physiology. 1996;491:121–135. doi: 10.1113/jphysiol.1996.sp021201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:261–263. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Miller RJ. Excitatory amino acid receptors, second messengers and regulation of intracellular Ca2+ in mammalian neurons. Trends in Pharmacological Sciences. 1988;11:254–260. doi: 10.1016/0165-6147(90)90254-6. 10.1016/0165-6147(90)90254-6. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. Journal of Physiology. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MS, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. Journal of Physiology. 1978;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Masaki H, Yamakura T, Mishina M. Identification by mutagenesis of a Mg2+-block site of the NMDA receptor channel. Nature. 1992;358:673–675. doi: 10.1038/358673a0. 10.1038/358673a0. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovsky P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Nowak LM, Wright JM. Slow voltage-dependent changes in channel open-state probability underlie hysteresis of NMDA responses in Mg2+-free solutions. Neuron. 1992;8:181–187. doi: 10.1016/0896-6273(92)90119-x. 10.1016/0896-6273(92)90119-X. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Auerbach A. Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. Neuron. 1996;16:869–880. doi: 10.1016/s0896-6273(00)80107-5. 10.1016/S0896-6273(00)80107-5. [DOI] [PubMed] [Google Scholar]
- Ruppersberg JP, Kitzing von E, Schoepfer R. The mechanism of magnesium block of NMDA receptors. Seminars in the Neurosciences. 1994;6:87–96. 10.1006/smns.1994.1012. [Google Scholar]
- Sakurada K, Masu M, Nakanishi S. Alteration of Ca2+ permeability and sensitivity to Mg2+ and channel blockers by a single amino acid substitution in the N-methyl-D-aspartate receptor. Journal of Biological Chemistry. 1993;268:410–415. [PubMed] [Google Scholar]
- Schneggenburger R, Ascher P. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. 10.1016/S0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- Sharma G, Stevens CF. A mutation that alters magnesium block of N-methyl-D-aspartate receptor channels. Proceedings of the National Academy of Sciences of the USA. 1996;93:9259–9263. doi: 10.1073/pnas.93.17.9259. 10.1073/pnas.93.17.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Thieleczek R. Activation of the contractile apparatus of skinned fibres of frog by the divalent cations barium, cadmium and nickel. Journal of Physiology. 1986;380:75–92. doi: 10.1113/jphysiol.1986.sp016273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AK, Li-Smerin Y, Johnson JW, Reynolds IJ. Mechanisms of glutamate-stimulated Mg2+ influx and subsequent Mg2+ efflux in rat forebrain neurones in culture. Journal of Physiology. 1996;492:641–657. doi: 10.1113/jphysiol.1996.sp021334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A, Burnashev N, Sakmann B. Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophysical Journal. 1995;68:866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Sakmann B. Intracellular Mg2+ interacts with structural determinants of the narrow constriction contributed by the NR1-subunit in the NMDA receptor channel. Journal of Physiology. 1998;506:33–52. doi: 10.1111/j.1469-7793.1998.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Seeburg PH, Sakmann B. Differential contribution of the NR1- and NR2A-subunits to the selectivity filter of recombinant NMDA receptor channels. Journal of Physiology. 1996;491:779–797. doi: 10.1113/jphysiol.1996.sp021257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. Journal of General Physiology. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM, Dani JA. Ionic permeability characteristics of the N-methyl-D-aspartate receptor channel. Journal of General Physiology. 1994;103:231–248. doi: 10.1085/jgp.103.2.231. 10.1085/jgp.103.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM, Dani JA. Structural basis for explaining open-channel blockade of the NMDA receptor. Journal of Neuroscience. 1995;15:1446–1454. doi: 10.1523/JNEUROSCI.15-02-01446.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


