Abstract
The presence of a hyperpolarization-activated pacemaker (If)-like current was tested in dedifferentiated adult rat ventricular myocytes up to 12 days in primary culture with the whole-cell patch clamp technique.
An If-like current was found and characterized on freshly isolated and cultured ventricular cells. Both activation and density of the current varied in relation to the stage of dedifferentiation. The current was activated from -92.0 ± 2.5 and -63.0 ± 1.0 mV at the beginning (4-day-cultured cells) and end of the dedifferentiation process (12 days), respectively. Its density measured at -170 mV progressively increased from -2.34 ± 0.36 to -6.12 ± 0.64 pA pF−1 between the two farthest stages of cellular remodelling. In freshly isolated cells the current was activated at -108.0 ± 1.5 mV and its current density measured at -170 mV was -1.97 ± 0.56 pA pF−1.
The current was blocked by extracellular CsCl (3 mM) in a voltage-dependent manner. Modification of reversal potentials obtained at various values of [K+]o (5.4 or 25 mM) and [Na+]o (140 or 30 mM) suggests that the current was carried by both K+ and Na+ ions.
It is concluded that the hyperpolarization-activated inward current, recorded in freshly isolated and in cultured ventricular cells has characteristics similar to those of If. In adult rat ventricular cells it is activated in a non-physiological potential range, but can be elicited in a more physiological range when the cells are remodelled through a dedifferentiated way. It is suggested that such a current could be implicated in ventricular arrhythmias developed in pathological events.
Pacemaker activity is a property of specialized myocytes that are able to generate spontaneous action potentials (Irisawa, 1978). For these cells, an inward current flowing during diastole (phase 4 depolarization) is required. One of the ionic components involved in diastolic depolarization is the pacemaker current If (DiFrancesco, 1985; Irisawa, Brown & Giles, 1993). It is a time-dependent inward current activated by hyperpolarization and is caesium (Cs+) sensitive. The If channel is permeable to monovalent cations, allowing both Na+ and K+ ions to pass through. It has been observed in the sino-atrial (SA) node (Yanagihara & Irisawa, 1980), frog sinus venosus (Bois & Lenfant, 1990), atrioventricular node (Noma, Irisawa, Kokobun, Kotake, Nishimura & Watanabe, 1980), atrium (Earm, Shimoni & Spindler, 1983; Zhou & Lipsius, 1992) and Purkinje fibres (DiFrancesco, 1981). If has also been observed in the chick embryonic ventricle where it begins to activate at around -50 to -60 mV, in the same voltage range of phase 4 depolarization (Sperelakis, 1982; Satoh & Sperelakis, 1991). However, it disappears during cardiac development and this disappearance parallels the reduction of the spontaneous pacemaker activity of these cells (Satoh & Sperelakis, 1991; Brochu, Clay & Shrier, 1992). In addition, If has recently been seen, in freshly isolated and cultured ventricular cells from newborn rats, to activate at around -70 mV (Robinson, Yu, Chang & Cohen, 1997). If has never been observed in adult mammalian ventricle in a physiological voltage range but it has been reported to exist in freshly isolated ventricular myocytes from adult guinea-pig, canine and rat hearts for potentials more negative than -120 mV (Yu, Chang & Cohen, 1993, 1995; Robinson et al. 1997). If has also been observed on ventricular cells isolated from hypertensive rats and from failing human hearts where it might favour the occurrence of spontaneous action potentials (Cerbai, Barbieri & Mugelli, 1994; Cerbai et al. 1997).
Adult cardiac ventricular myocytes have been shown to resume spontaneous contractility (Eppenberger, Hertig & Eppenberger-Eberhardt, 1994; Farès, Gomez & Potreau, 1996) when cultured in the ‘redifferentiated model’ (Jacobson & Piper, 1986). In these conditions, the cells maintained in serum-containing medium for periods up to 4 weeks undergo dedifferentiation and redifferentiation processes with reactivation of the early fetal program of expression for several genes (Eppenberger et al. 1994). Also, we have recently reported that T-type calcium current, which has been shown to be present in rat ventricular myocytes at the neonatal stage but absent in adult cells, can be re-expressed when adult cells were dedifferentiated in culture (Farès et al. 1996).
Here we report that a time-dependent inward current is activated on hyperpolarization in dedifferentiated adult rat ventricular myocytes in culture. We provide evidence that this current has characteristics similar to those of If and that its voltage range for activation depends on the dedifferentiation stage of the cells.
METHODS
Cell isolation and culture
Ventricular myocytes were dissociated and cultured as previously described (Farès et al. 1996). Briefly, adult (250-300 g) male Wistar rats were injected with 1000 i.u. heparin i.p. (Choay; Sanofi, Gentilly, France) and anaesthetized with ether; the heart was quickly removed via thoracotomy and transferred to an ice-cold Tyrode solution. The aorta was cannulated and the heart mounted on a Langendorff apparatus and successively perfused (at 37°C) with the following oxygenated solutions: 5 min with Tyrode solution to recover its spontaneous activity; 4 min with a nominally Ca2+-free Tyrode solution, and about 20 min with the same solution supplemented with 0.05 % collagenase (type II, Worthington), 0.06 mM CaCl2and 0.1 % bovine serum albumin (BSA). When the heart was flaccid, it was rinsed with Kraft-Brühe (KB) medium (Isenberg & Klöckner, 1982) for 2 min. The ventricles were cut off, chopped into small pieces and gently stirred in KB medium. The isolated cells were filtered, maintained for 1 h in the KB medium and gradually resuspended in normal Tyrode solution. Part of these freshly isolated cells were kept in Tyrode solution at room temperature (20 ± 2°C) for control experiments. For culture, other isolated cells were washed once in M199 medium (Gibco, Life Technologies) supplemented with 10−7 m insulin, 0.2 % BSA, 10 % fetal calf serum (Boehringer Mannheim) and 1 % antibiotics (penicillin, 100 i.u. ml−1; streptomycin, 50 i.u. ml−1; Sigma), seeded into 35 mm Petri dishes pretreated with laminin (20 μg ml−1; Sigma) and incubated for 30 min at 37°C for plating. The culture medium was changed and supplemented with 10 % fetal calf serum, and 10 μM cytosine 1-β-D arabinofuranoside (Sigma) to inhibit fibroblast proliferation. This medium was then renewed every 2 days.
Solutions
The Tyrode solution contained (mM): NaCl, 140; KCl, 5.4; CaCl2, 1.8; MgCl2, 1.8; Hepes, 10; glucose, 10; pH was adjusted to 7.35 with NaOH. To study the hyperpolarization-activated current, the extracellular control solution was modified to reduce the interference of components other than If, by adding to the Tyrode solution (mM): BaCl2, 8; MnCl2, 2; CdCl2, 0.2; 4-aminopyridine (Sigma), 0.5; plus tetrodotoxin (TTX; Sigma), 15 μM. For characterization of the current, the concentration of KCl was increased to 25 mM or that of NaCl was reduced to 30 mM and replaced by equimolar N-methyl-D-glucamine (NMDG). The internal solution in the patch pipettes (2-4 MΩ) contained (mM): potassium aspartate, 130; MgCl2, 2; Na2-ATP, 2; CaCl2, 2; EGTA, 5; Na-GTP, 0.1; disodium phosphocreatine, 5; Hepes, 10; adjusted to pH 7.20 with KOH.
Experimental protocols
Voltage clamp experiments were performed at room temperature in the whole-cell configuration of the patch clamp technique (Hamill, Marty, Neher, Sakmann & Sigworth, 1981). The electrical signal was recorded by a patch amplifier (RK 300, Biologic, Grenoble, France) and digitized through a conversion board (Labmaster TM40, Scientific Solutions, Solon, OH, USA). Programed voltage clamp sequences and data acquisition were performed by a PC compatible computer (Epson, PC AX20, Nagano, Japan) using the pCLAMP software (version 5.5.1, Axon Instruments).
Membrane capacitance (Cm) was estimated from the capacitance transient elicited by a 10 mV depolarizing step from a holding potential (Vh) value of -90 mV and calculated according to the equation:
where τc is the time constant of the membrane capacitance, I0 is the initial current value, I∞ is the amplitude of steady-state current and ΔVm is the amplitude of voltage steps. No capacitance correction was used. Series resistance (Rs) ranged from 3 to 8 MΩ and was compensated in all cells by about 80 %. If was elicited by hyperpolarization steps, varying from -60 to -170 mV from a holding potential of -40 mV.
Spontaneous activity was recorded in current clamp mode by means of the perforated patch method (Horn & Marty, 1988). For this, pipettes tips were filled with an antibiotic (Amphotericin B)-free solution containing (mM): KCl, 30; K2SO4, 50; MgCl2, 8; Hepes, 10; pH, 7.2 with KOH. It should be noted that chloride ions were partly substituted by sulphate to minimize junction potential (Horn & Marty, 1988). The rest of the pipette was backfilled with the same solution containing Amphotericin-B (150 μM; Sigma). Other specific protocols will be described in the text.
Data analysis
The amplitude of If was measured as the difference between the instantaneous current at the beginning of the hyperpolarizing step and the steady-state current recorded at the end of the step and normalized to Cm.
Specific conductance was determined as a function of membrane potential. At steady state the voltage dependence of If can be described by the equation:
where gf is the conductance calculated at membrane potential Vm, and Vrev the reversal potential of the fully activated current derived from the analysis of tail currents of Fig. 3. Conductance-voltage (gf-V) relations (Fig. 2F) were then obtained from the above equation as ratios between steady-state I-V curves If-V and Vm-Vrev. Conductance curves were fitted to the Boltzmann distribution:
where gfmaxV is the maximum conductance, V0.5 the half-maximal voltage of activation and S is the slope factor. This allowed estimation of the shifts of the voltage dependence of conductance measured as changes in V0.5.
Figure 3. Fully activated I-V relations of If.
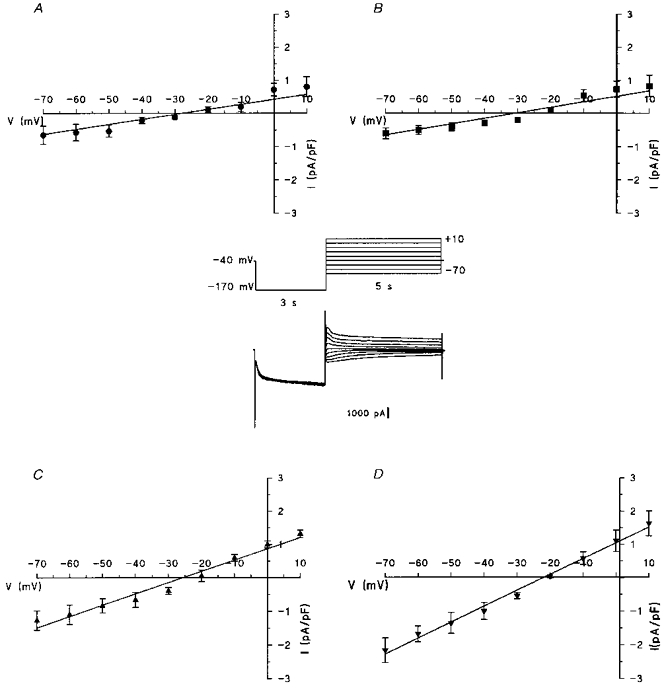
The tail current amplitude was measured after a hyperpolarizing pulse of -170 mV, normalized to membrane capacitance and plotted versus voltage values from +10 to -70 mV, for freshly isolated cells (A, n = 7), 4-day- (B, n = 6), 8-day- (C, n = 6) and 12-day-cultured cells (D, n = 10). Data points are means ±s.e.m. Inset, an example of current traces recorded from a 12-day-cultured cell.
Figure 2. If characterization in dedifferentiated cardiac cells.
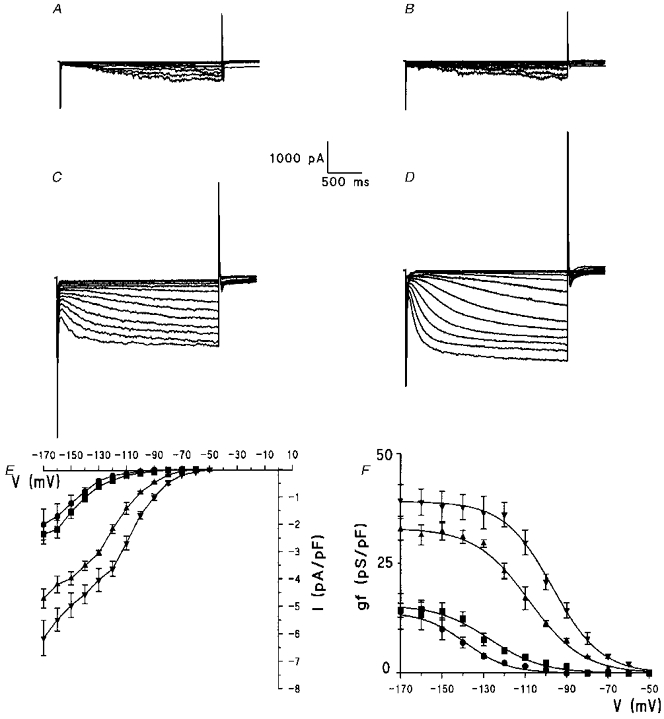
Example of current traces for freshly isolated cells (A), 4-day- (B), 8-day- (C), and 12-day-cultured cells (D) recorded during 2.4 s pulses in the range -60 to -170 mV from a holding potential of -40 mV. E, superimposed I-V curves of the hyperpolarizing-activated current recorded in various cells types. If was normalized with respect to membrane capacitance for freshly isolated cells (n = 10, •) and for 4-day- (n = 7, ▪), 8-day- (n = 4, ▴) and 12-day-cultured cells (n = 7, ▾). F, plot of mean conductance density (gf) versus step potential calculated from current densities reported in E, with the equation described in Methods. Curves were fitted to data by a Boltzmann distribution. Data points are means ±s.e.m.
Results from multiple experiments are expressed as means ±s.e.m. Statistical analysis was performed using analysis of variance, followed by the Newman-Keuls multiple range test for multiple experiments.
RESULTS
Correlation between cell dedifferentiation and the presence of a hyperpolarization-activated current
When cultured in the presence of M199 medium containing 10 % fetal calf serum, adult rat cardiomyocytes undergo drastic remodelling (Eppenberger et al. 1994; Farès et al. 1996). Figure 1 shows that the dedifferentiation process of the freshly isolated cells (Fig. 1A) is characterized by gradual morphological transitions including initial loss of most traits of the cardiac phenotype followed by the flattening and spreading into a polymorphic cell shape. Several types were defined (types I, II and III): type I corresponds to the cells cultured for about 4 days, when the freshly isolated rod-shaped myocytes were attached to the substrate (Fig. 1A) and progress to a rounded shape (Fig. 1B); after 8 days of culture, most cells were largely flattened and considerably bigger with apparent multinucleation as shown in Fig. 1C and correspond to type II; after 12 days in culture, fully spread cells, defined as type III, exhibited large triangular extensions (Fig. 1D) and showed spontaneous activity (Fig. 1E). Cm increased in relation to the dedifferentiation process with significant increases in type II and type III cells (see Table 1). Furthermore, it should be noted that there was a transient decrease of Cm during the first days of culture, which could be explained by initial loss of cardiac phenotype and particularly by reduced T-tubular connections (Lipp, Hüser, Pott & Niggli, 1996).
Figure 1. Changes in adult rat cardiomyocyte cytoarchitecture during long-term cell culture.
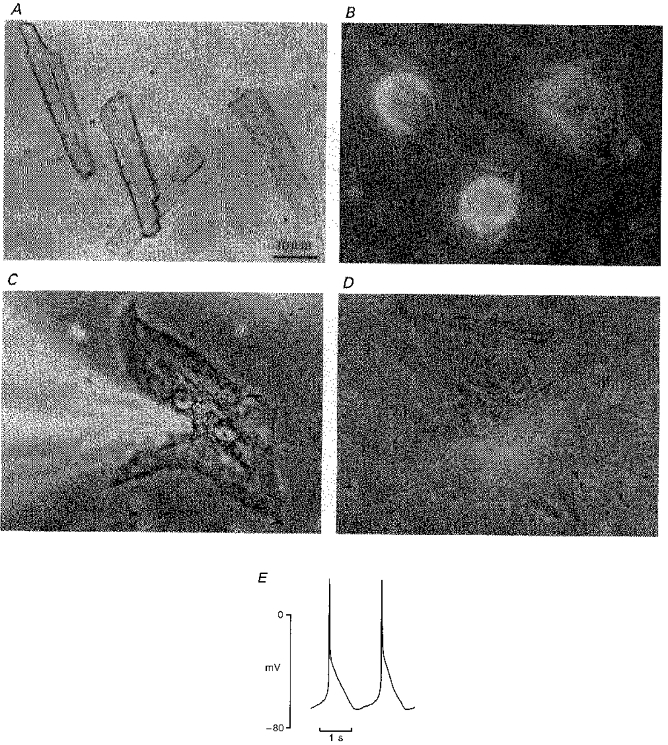
Phase-contrast micrograph (× 400) for freshly isolated cells (A) and for cells cultured for 4 (B), 8 (C) and 12 days (D) in M199 medium containing 10 % fetal calf serum. The originally rod-shaped cells (A) undergo a morphological transition, first rounding and attaching to the substrate (4-day-cultured cells, B), then spreading and flattening (8 day cells, C) and finally displaying a large polymorphic configuration (12 day cells, D). At this stage, myocytes develop spontaneous contractile activity (E). Note in C the shadow of the patch pipette used for voltage clamp.
Table 1.
Comparative data measured for If in freshly isolated cells and in the three types of cultured cells
| Freshly isolated cells | 4-day-cultured cells (Type I) | 8-day-cultured cells (Type II) | 12-day-cultured cells (Type III) | |
|---|---|---|---|---|
| Cm (pF) | 157 ± 8 (8) | 153 ± 5 (7) | 263 ± 12 *† (4) | 313 ± 12 *†‡ (7) |
| Ta (mV) | -108·0 ± 1·5 (10) | -92·0 ± 2·5 * (7) | -75·0 ± 2·0 *† (4) | -63·0 ± 1·0 *†‡ (7) |
| Vrev (mV) | -29·0 ± 0·1 (7) | -31·2 ± 0·1 (6) | -27·6 ± 0·1 (6) | -23·0 ± 0·1 (10) |
| gfmaxV (pS pF−1) | 13·90 ± 0·40 (10) | 15·45 ± 0·77 (6) | 33·02 ± 0·51 *† (4) | 39·15 ± 0·44 *†‡ (7) |
| gfmax (pS pF−1) | 15·00 ± 0·15 (7) | 16·58 ± 0·18 (6) | 33·57 ± 0·21 *† (6) | 47·36 ± 0·15 *†‡ (10) |
| V0·5 (mV) | -138·8 ± 0·9 (10) | -127·7 ± 2·0 * (7) | -108·1 ± 0·9 *† (4) | -96·9 ± 0·7 *†‡ (7) |
| S | 10·31 ± 0·80 | 12·60 ± 1·50 | 12·47 ± 0·71 | 11·66 ± 0·60 |
Cm, cell membrane capacitance; Ta, potential for activation threshold; Vrev, reversal potential; gfmaxV, maximum conductance obtained from Boltzmann fitting (Fig. 2); gfmax, slope of the fully activated current (Fig. 3); V0·5, half-maximum voltage; and S, slope factor. Values are means ± S.E.M.
P < 0·01 compared with freshly isolated cells
P < 0·01 compared with type I cells
P < 0·01 compared with type II cells.
In the whole-cell configuration (Fig. 2A-D), an inward current was activated by hyperpolarization in freshly isolated cells and in the three types of cultured cells. Figure 2E illustrates the current density-voltage relations. At -170 mV, the mean current density of freshly isolated cells was -1.97 ± 0.56 pA pF−1 with a Cm of 157 ± 8 pF (n = 8). In type I cultured cells these values did not change significantly: -2.34 ± 0.36 pA pF−1 and 153 ± 5 pF (n = 7), respectively. At the same potential, a significant increase of current density was observed in type II (-4.68 ± 0.35 pA pF−1, n = 4) and in type III cells (-6.12 ± 0.64 pA pF−1, n = 7) in parallel with a rise of Cm (263 ± 12 and 313 ± 12 pF, respectively). Figure 2F illustrates conductance density-voltage relations obtained from current density curves of Fig. 2E (see Methods). The potential values for activation threshold (Ta) and for half-conductance density (V0.5) were largely negative in the freshly isolated cells and significantly decreased through the three types of cultured cells, leading to a shift of the conductance density curves to less negative voltages, without a change in the slope factor (S) values. Moreover, maximum values of conductance density (gfmaxV) were significantly increased for type II and III cells (see Table 1).
In Fig. 3 the fully activated current density for freshly isolated cells (A) and for the three types of cultured cells (B-D) was evaluated over a large range of potential values (+10 to -70 mV) by measuring the tail current amplitudes after a hyperpolarizing pulse to -170 mV. The analysis of the curves shows that there is no significant change in the reversal potential (Vrev) values estimated from the intersection between the curves and the voltage axis. The maximum values of conductance density that correspond to the slopes of the curves (gfmax), were similar to those obtained in Fig. 2, with the same increase in type II and III cultured cells (see Table 1).
Effect of extracellular caesium on the hyperpolarization-activated current
As reported in Purkinje fibres (DiFrancesco, 1982), in Purkinje cells (Callewaert, Carmeliet & Vereecke, 1984) and in SA node cells (DiFrancesco, Ferroni, Mazzanti & Tromba, 1986), Cs+ is known to exert a voltage-dependent block of f-channels at potentials negative to the reversal potential for the pacemaker current. In cells cultured for 12 days (type III cells), the effect of Cs+ was evaluated over a large range of potentials (+10 to -70 mV), by measuring the amplitude of tail currents following a hyperpolarizing step to -120 mV, which fully activates If (Fig. 4). Current traces recorded in control conditions (Fig. 4A) and after the addition of 3 mM CsCl show that the presence of Cs+ results in an almost complete block of the inward current elicited by the step to -120 mV (Fig. 4B). In panel C, tail current amplitude, normalized to membrane capacitance, was plotted against the step voltage. In the presence of Cs+ (filled circles), the negative region of the fully activated current density-voltage relation was characterized by a progressive reduction of the current showing a clear-cut voltage-dependent blockade. The reversal potential in the presence of Cs+ was identical to that measured in control conditions (24.7 ± 0.1 mV, n = 4). A similar effect of Cs+ was also observed on the current activated in the two other types of cultured cells and in freshly isolated cells (data not shown). The effect of Cs+ on the hyperpolarization-activated current in cultured ventricular cells was qualitatively similar to that reported from Purkinjes fibres (DiFrancesco, 1982), SA node cells (DiFrancesco et al. 1986), ventricular cells isolated from hearts of normotensive (Robinson et al. 1997) and hypertensive rats (Cerbai et al. 1994) and in ventricular myocytes isolated from failing human hearts (Cerbai et al. 1997). According to DiFrancesco (1982) the effect of Cs+ on If is voltage dependent and the current is completely blocked at voltages more negative than -120 mV. In these conditions the residual inward current observed in the presence of Cs+ between -30 and -70 mV (Fig. 4C) is probably a part of If not fully suppressed at this voltage range.
Figure 4. Effect of caesium on the hyperpolarizing-activated current.
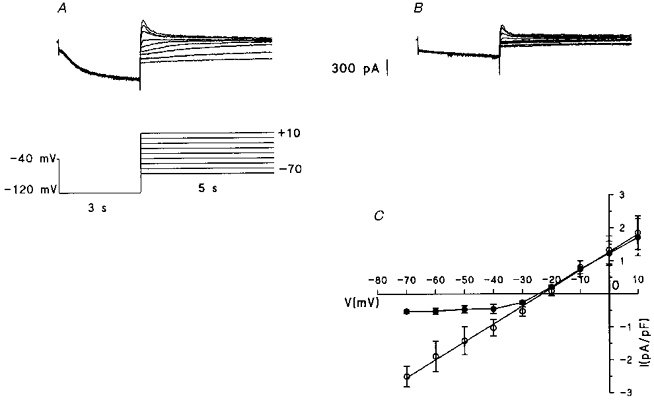
Current traces are recorded in a 12-day-cultured myocyte (Cm= 305 pF) in control conditions (A) and after the addition of 3 mM CsCl (B) with the voltage protocol reported in A. C, fully activated I-V curves for tail current amplitude, normalized to membrane capacitance and plotted against the step voltage in control (^) and in the presence of 3 mM CsCl (•). Data points are means ±s.e.m., n = 4.
Ionic selectivity of the hyperpolarization-activated current
If is dependent on external potassium and sodium in Purkinje fibres and SA node cells (Callewaert et al. 1984; DiFrancesco et al. 1986). In order to investigate whether potassium and sodium influence the hyperpolarization-activated current of cultured ventricular cells, their concentrations were modified in the perfusion solution.
Effect of increasing the external potassium concentration
The measurement of tail current amplitude was used again to evaluate the reversal potential of If at two extracellular potassium concentrations. Current traces in Fig. 5 were obtained in type III cells superfused with Tyrode solution containing either 5.4 mM (Fig. 5A) or 25 mM [K+]o (Fig. 5B). The most obvious effect caused by the augmentation in [K+]o was a marked increase in If amplitude. Tail current amplitudes, obtained in four different cells superfused with 5.4 mM [K+]o and normalized to membrane capacitance, are plotted as a function of tail step potential in the I-V curve of Fig. 5C (open squares). The best fit line through data points gave a linear relation that intersected the X-axis at -22.8 ± 0.1 mV with a gfmax of 44.50 ± 3.00 pS pF−1. In the presence of 25 mM [K+]o, the reversal potential was -13.8 ± 0.3 mV and gfmax was 90.20 ± 3.50 pS pF−1. Such a shift of the reversal potential is comparable to that reported for If in Purkinje fibres (DiFrancesco, 1982) and SA node cells (DiFrancesco et al. 1986) exposed to a similar increase in extracellular K+. The rise in conductance as a consequence of increasing [K+]o is also typical for If in primary cardiac pacemaker cells (DiFrancesco et al. 1986; Frace, Maruoka & Noma, 1992).
Figure 5. Effects of changes in external potassium and sodium concentrations on the fully activated current.
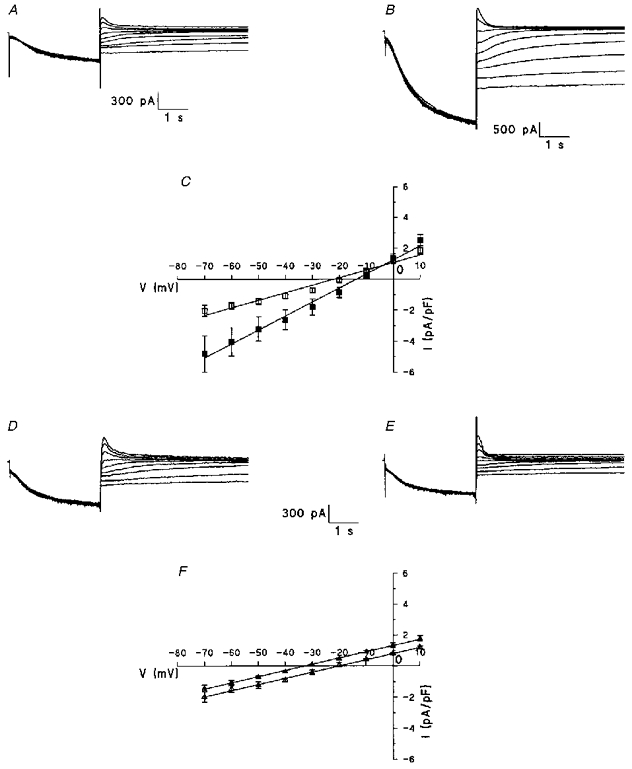
A-C, effect of increasing K+ concentration from 5.4 (A and C: □) to 25 mM (B and C: ▪). Note the positive shift of the reversal potential and the increase of the curve slope in C (n = 4). D-E, effect of decreasing Na+ concentration from 140 (D and F: ▵) to 30 mM (E and F: ▴). Note the negative shift of the reversal potential without changing the slope in F (n = 6). In panels C and F, data points are means ±s.e.m.
On the other hand, freshly isolated cells exhibit a similar shift of Vrev to less negative values (shift of Vrev of 11 ± 5 mV, n = 3) when increasing [K+]o from 5.4 to 25 mM. These data are compatible with those obtained by Yu et al. (1995) on adult cells isolated from guinea-pig hearts.
Effect of reducing the external sodium concentration
Figure 5D-F reports the effect of reducing the external sodium concentration from 140 mM (control conditions) to 30 mM on the hyperpolarization-activated current in type III cultured cells with the above protocol. The fully activated current in control (Fig. 5D) was decreased after reducing the external sodium concentration to 30 mM (Fig. 5E). The fully activated current density curves show that data points obtained from six cells in control and 30 mM [Na+]o, respectively, were fitted by linear relations (Fig. 5F). In both conditions, gfmax was comparable (39.40 ± 0.90 and 39.70 ± 0.70 pS pF−1 in 140 and 30 mM [Na+]o, respectively) suggesting that the conductance of the channel is not affected by reducing extracellular sodium. However, the reversal potential obtained in control (-20.5 ± 0.2 mV) is significantly shifted toward hyperpolarization (-33.6 ± 0.4 mV) in reduced sodium conditions. The shift is comparable to that reported for If in SA node cells (DiFrancesco et al. 1986) or frog sinus venosus cells (Bois & Lenfant, 1990) exposed to a similar reduction in extracellular sodium. Figure 5F also shows faster current kinetics in low sodium solution. This observation needs further experiments to explain this.
DISCUSSION
The present results indicate that the time-dependent inward current exists in dedifferentiated adult rat ventricular myocytes in primary culture. In type III cultured cells, this current has the electrophysiological characteristics of If present in pacemaker cardiac cells. It is activated upon hyperpolarization, is caesium sensitive and is selective for Na+ and K+. A similar inward current activated by hyperpolarizations can be observed in less dedifferentiated cells in culture and even in freshly isolated cells. However, this current is activated at more negative voltages and its density is smaller than this of If recorded in type III cells. Such a current previously reported in freshly isolated adult rat ventricular cells (Robinson et al. 1997), but not observed in all cells (Cerbai, Barbieri & Mugelli, 1996), will need further characterization to be clearly considered as If.
Analysis of the conductance density curves shows that the electrophysiological characteristics change with the time of culture: a shift of potential activation threshold towards less negative values (-92 mV for type I to -63 mV for type III cells) and an increase of the conductance density from type I to type III cells (by about 150 %). The present shift may be due to the increasing activities of cAMP which directly acts on f-channels (DiFrancesco & Tortora, 1991) and/or the increasing activities of protein kinases and decreasing activities of phosphatases, enhancing the phosphorylation of channels (Yu et al. 1995). It is interesting to note that a shift of the voltage dependence of If towards large negative values inversely occurs from newborn to adult rat ventricle myocytes (Robinson et al. 1997). This negative shift may be due to a gene switch, a developmentally regulated translational or post-translational alteration in the channel, or the appearance (or disappearance) of an associated protein.
The rise of conductance should be explained by an overexpression of the f-channel gene leading to an augmentation in the number of functional channels. Such an upregulation during culture of adult ventricular cardiomyocytes was already reported for several genes: β-myosin heavy chain (Eppenberger-Eberhardt, Flamme, Kurer & Eppenberger, 1990; Dubus, Rappaport, Barrieux, Lompré, Schwartz & Samuel, 1993), atrial natriuretic factor (Clark, Rudnick, LaPres, Andersen & LaPointe, 1993; Swynghedauw, 1993), T-type calcium channels (Farès et al. 1996). This process is generally correlated to an increase in cell size and to cytoskeletal changes (Eppenberger et al. 1994).
Finally, the present results show that type III cultured cells exhibit pacemaker activity with a maximum diastolic potential around -60 mV and a marked depolarization phase (Fig. 1E). As reported in a previous study (Farès et al. 1996), the resting membrane potential measured in control Tyrode solution significantly decreases throughout the culture in serum-containing medium from -77 ± 3 mV (n = 20) for freshly isolated cells to -65 ± 4 mV (n = 21) in 8-day-old cultured cells and -63 ± 2 mV (n = 12) for the maximum diastolic potential measured in spontaneously beating cells after 13 days in culture (type III). Such values can be compared to those recorded in primary pacemaker cells (Irisawa, 1978).
A recent report demonstrated that cardiomyocytes in biopsies from patients with chronic myocardial ischaemia and marked anterior wall motion abnormalities showed signs of dedifferentiation rather than necrotic properties (Ausma et al. 1995). Moreover, it has been suggested that If contributes to the increased propensity for ventricular arrhythmias of hypertrophied rat heart and of human failing heart (Cerbai et al. 1994, 1997). In these conditions, the present model of dedifferentiated adult ventricular myocytes in primary culture seems to offer an opportunity to investigate the potential role of If in the genesis of ventricular arrhythmias.
Acknowledgments
This work was supported by grants from CNRS, University of Poitiers and Fondation Langlois.
References
- Ausma J, Schaart G, Thone F, Shivalkar B, Flameng W, Depre C, Vanoverschelde J-L, Ramaekers F, Borgers M. Chronic ischemic viable myocardium in man: aspects of dedifferentiation. Cardiovascular Pathology. 1995;4:29–37. doi: 10.1016/1054-8807(94)00028-p. 10.1016/1054-8807(94)00028-P. [DOI] [PubMed] [Google Scholar]
- Bois P, Lenfant J. Isolated cells of the frog sinus venosus: properties of the inward current activated during hyperpolarization. Pflügers Archiv. 1990;416:339–346. doi: 10.1007/BF00392071. [DOI] [PubMed] [Google Scholar]
- Brochu RM, Clay JR, Shrier A. Pacemaker current in single cells and aggregates of cells dissociated from the embryonic chick heart. Journal of Physiology. 1992;454:503–515. doi: 10.1113/jphysiol.1992.sp019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G, Carmeliet E, Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. Journal of Physiology. 1984;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E, Barbieri M, Mugelli A. Characterization of the hyperpolarization-activated current, If, in ventricular myocytes isolated from hypertensive rats. Journal of Physiology. 1994;481:585–591. doi: 10.1113/jphysiol.1994.sp020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E, Barbieri M, Mugelli A. Occurrence and properties of hyperpolarization-activated current If in ventricular myocytes from normotensive and hypertensive rats during aging. Circulation. 1996;94:1674–1681. doi: 10.1161/01.cir.94.7.1674. [DOI] [PubMed] [Google Scholar]
- Cerbai E, Pino R, Porciatti F, Sani G, Toscano M, Maccherini M, Giunti G, Mugelli A. Characterization of the hyperpolarization-activated current, If, in ventricular myocytes from human failing heart. Circulation. 1997;95:568–571. doi: 10.1161/01.cir.95.3.568. [DOI] [PubMed] [Google Scholar]
- Clark WA, Rudnick SJ, LaPres JJ, Andersen LC, LaPointe MC. Regulation of hypertrophy and atrophy in cultured adult heart cells. Circulation Research. 1993;73:1163–1176. doi: 10.1161/01.res.73.6.1163. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pacemaker current in calf Purkinje fibres. Journal of Physiology. 1981;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pacemaker channel in calf Purkinje fibres: effects of potassium, caesium, and rubidium. Journal of Physiology. 1982;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and development. Progress in Biophysics and Molecular Biology. 1985;46:163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ferroni A, Mazzanti M, Tromba C. Properties of the hyperpolarization-activated current (if) in cells isolated from the rabbit sino-atrial node. Journal of Physiology. 1986;377:61–68. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac ‘pacemaker’ (if) channels by intracellular cyclic-AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Dubus I, Rappaport L, Barrieux A, Lompré AM, Schwartz A, Samuel JL. Contractile protein gene expression in serum-free cultured adult rat cardiac myocytes. Pflügers Archiv. 1993;423:455–461. doi: 10.1007/BF00374941. [DOI] [PubMed] [Google Scholar]
- Earm YE, Shimoni Y, Spindler A. A pacemaker-like current in the sheep atrium and its modulation by catecholamines. Journal of Physiology. 1983;342:569–590. doi: 10.1113/jphysiol.1983.sp014869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppenberger-Eberhardt M, Flamme I, Kurer V, Eppenberger HM. Reexpression of α-smooth muscle actin isoform in cultured adult rat cardiomyocytes. Developmental Biology. 1990;139:269–278. doi: 10.1016/0012-1606(90)90296-u. [DOI] [PubMed] [Google Scholar]
- Eppenberger HM, Hertig C, Eppenberger-Eberhardt M. Adult rat cardiomyocytes in culture. A model system to study the plasticity of the differentiated cardiac phenotype at the molecular and cellular levels. Trends in Cardiovascular Medicine. 1994;4:187–193. doi: 10.1016/1050-1738(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Farès N, Gomez J-P, Potreau D. T-type calcium current is expressed in dedifferentiated adult rat ventricular cells in primary culture. C. R. Académie des Sciences, Paris. 1996;319:569–576. [PubMed] [Google Scholar]
- Frace AM, Maruoka F, Noma A. External K+ increases Na+ conductance of the hyperpolarization-activated current in rabbit cardiac pacemaker cells. Pflügers Archiv. 1992;421:97–99. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membranes patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiological Reviews. 1978;58:461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiological Reviews. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Klöckner U. Calcium tolerant ventricular myocytes prepared by preincubation in a ‘KB medium’. Pflügers Archiv. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Jacobson SL, Piper HM. Cell cultures of adult cardiomyocytes as models of the myocardium. Journal of Molecular and Cellular Cardiology. 1986;18:661–678. doi: 10.1016/s0022-2828(86)80939-7. [DOI] [PubMed] [Google Scholar]
- Lipp P, Hüser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. Journal of Physiology. 1996;497:589–597. doi: 10.1113/jphysiol.1996.sp021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A, Irisawa H, Kokobun S, Kotake H, Nishimura M, Watanabe Y. Slow current systems in the A-V node of the rabbit heart. Nature. 1980;285:228–229. doi: 10.1038/285228a0. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Yu H, Chang F, Cohen IS. Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. Pflügers Archiv. 1997;433:533–535. doi: 10.1007/s004240050309. [DOI] [PubMed] [Google Scholar]
- Satoh H, Sperelakis N. Identification of the hyperpolarization-activated inward current in young embryonic chick heart myocytes. Journal of Developmental Physiology. 1991;15:247–252. [PubMed] [Google Scholar]
- Sperelakis N. Pacemaker mechanisms in myocardial cells during development of embryonic chick hearts. In: Bouman LN, Jongsma HJ, editors. Cardiac Rate and Rhythm. The Hague: Martinus Xijboff Publishers; 1982. pp. 129–165. [Google Scholar]
- Swynghedauw B. Remodeling of the heart in chronic pressure overload. Basic Research in Cardiology. 1993;86:I99–105. [PubMed] [Google Scholar]
- Yanagihara K, Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflügers Archiv. 1980;385:11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yu H, Chang F, Cohen IS. Pacemaker current exists in ventricular myocytes. Circulation Research. 1993;72:232–236. doi: 10.1161/01.res.72.1.232. [DOI] [PubMed] [Google Scholar]
- Yu H, Chang F, Cohen IS. Pacemaker current if in adult canine cardiac ventricular myocytes. Journal of Physiology. 1995;485:469–483. doi: 10.1113/jphysiol.1995.sp020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZF, Lipsius SL. Properties of the pacemaker current (If) in latent pacemaker cells isolated from cat right atrium. Journal of Physiology. 1992;453:503–523. doi: 10.1113/jphysiol.1992.sp019242. [DOI] [PMC free article] [PubMed] [Google Scholar]


