Abstract
In vivo rectal distension (RD) induces a neurally mediated colonic net water hypersecretion in rats. Interleukin-1β (IL-1β) also induces neural colonic water hypersecretion involving the release of prostaglandins (PGs) and a mast cell degranulation in rats. This study investigated in vivo the role of IL-1, PGs and mast cells in RD-induced colonic hypersecretion.
Proximal colonic net water flux was determined using [14C]polyethylene glycol (PEG) 4000 (mol. wt, 4000) in anaesthetized rats. On strips taken from the distal colon: (i) a histological analysis was performed to determine the number of mucosal mast cells (MMC); and (ii) histamine levels were measured by radioimmunoassay after stimulation with compound 48/80.
RD induced a net colonic water secretion that was blocked by i.c.v. administration of IL-1ra (an IL-1 receptor antagonist) and indomethacin, and by systemic treatment with doxantrazole and indomethacin. RD decreased the number of resident mast cells and the release of histamine from the distal colonic strips. Moreover, using SDS-PAGE immunoblotting the expression of IL-1β was detected in the brain.
These results suggest that, in rats, RD induces colonic net water hypersecretion by the activation of a neuro-immunological reflex pathway, involving brain IL-1β, PG release and peripheral mast cell degranulation.
Intestinal mechanical distension elicits active ion and water secretion (Swabb et al. 1982; Frieling, Wood & Cooke, 1992). In a previous study we have shown that rectal distension (RD) induced net water colonic hypersecretion in rats, in vivo, involving a neural long reflex pathway (Eutamene, Theodorou, Fioramonti & Bueno, 1997). This effect of RD involves an activation of splanchnic afferent fibres, and peripheral (NK1) and central (NK2 and NK3) tachykinin receptors (Eutamene, Theodorou, Fioramonti & Bueno, 1997). The role of intrinsic nervous system reflexes in the determining of secretory patterns induced by lumen distension, has been shown previously in vitro (Diener & Rummel, 1990; Frieling et al. 1992). The distension of rat colon descendens induced chloride secretion via a prostaglandin (PG) release, which can act directly on the epithelium or on the submucosal plexus (Diener & Rummel, 1990; Itasaka, Shiratori, Takahashi, Ishikawa, Kaneko & Suzuki, 1992). Moreover, the activation of arachidonic acid metabolism and subsequent formation of PGs in the intestine is generally accompanied by the active secretion of water and electrolytes (Bern, Sturbaum, Karayalcin, Berschneider, Wachsman & Powel, 1983).
Mast cells, as well as other immune cells, reveal an intimate association with nerve fibres in the central nervous system (CNS) and with sensory and autonomic fibres in the peripheral tissues (Dimitriadou, Lambracht-Hall, Reichler & Theoharides, 1990). Moreover, sensory neurotransmitters such as substance P (SP) can degranulate mast cells, leading to the release of inflammatory mediators such as prostaglandins, histamine and interleukin-1 (IL-1) (Dray & Bevan, 1993). Furthermore, it has been reported that SP, like other cationic amphilic neuropeptides, acts on mast cells by the direct activation of G proteins, inducing a histamine release known for its intestinal ion secretory effect (Castro, Harari & Russel, 1987). IL-1 also affects the intestinal ion transport, reducing Na+ and Cl− absorption in rabbit and chicken intestinal mucosa (Chiossone, Simon & Smith, 1990; Chang, Musch & Mayer, 1990). Therefore, in vivo, IL-1 induces a neurally mediated colonic water secretion in rats, involving mast cell degranulation, PGs and a tachykinin release (Theodorou, Eutamene, Fioramonti, Junien & Bueno, 1994; Eutamene, Theodorou, Fioramonti & Bueno, 1995). IL-1 is present in the CNS and particularly in hypothalamic nuclei or in the gastrointestinal tract, and can play a role in the control of gut functions at both levels. IL-1 centrally administered alters small intestine motility through the release of central prostaglandins (Fargeas, Fioramonti & Bueno, 1993). Moreover, a recent study has shown that the peripheral administration of IL-1 induces a plasma increase of SP and neurokinin A in the cerebrospinal fluid in rats (Bileviciute, Lundeberg, Ekblom & Theodorson, 1994).
This study was undertaken to determine: (1) the involvement of IL-1 and prostaglandins on RD-induced colonic net water hypersecretion; and (2) the role of mast cells in this effect.
METHODS
Sixteen groups of eight male Wistar rats weighing 250-300 g were used. The animals were housed individually and fed with laboratory pellets (Epinay, France).
Colonic net water flux movements
Fluid transport studies using the isolated loop technique were performed in vivo as previously described (Quito & Brown, 1987). Under urethane (2 g kg−1i.p.) anaesthesia, a mid-line laparotomy was performed to expose the large intestine. A 5 cm long segment of proximal colon, 1 cm from the caecocolonic junction, was ligated at its extremities and cannulated for intraluminal infusion. Cannulated segments were rinsed with warm saline, replaced in the abdominal cavity, and the laparotomy incision was closed. The rats were placed on heating pads in order to maintain their body temperature around 37°C throughout each experiment.
The systemic arterial blood pressure was monitored via a cannula placed in the right carotid artery and connected to a blood pressure transducer (P 1000 B, Narco Bio-Systems, Houston, TX, USA), and processed by a transducer coupler (type 7173, Narco Bio-Systems). The output was amplified and recorded (type 7070, Narco Bio-Systems). The heart rate was continuously measured, using the arterial pressure pulse to trigger a rate meter. Anaesthetized rats have a basal blood pressure of 100.3 ± 2.3 mmHg. During the RD application, we observed a decrease in blood pressure. This decrease was rapid at the onset (30 s), with an average fall of 10-20 mmHg. The maximum decrease occurred 30-40 s later and returned rapidly (2-3 min) to baseline during the remainder of the RD application.
The intestinal loops were infused with a Ringer solution containing (mM): Na+, 142.6; K+, 5.0; Cl−, 123.8; Mg2+, 1.2; Ca2+, 1.3; HCO3−, 25.0; HPO42−, 17.0; H2PO4−, 0.3; and glucose, 5.0. The solution also contained 1 μCi l−1 of [14C]polyethylene glycol (PEG) 4000 (mol. wt 4000) as a non-absorbed dilution marker of water flux and 5 g l−1 of unlabelled PEG 4000 as a carrier. The isolated colonic segment was infused at a constant rate of 6 ml h−1 and the effluent collected during consecutive 15 min periods over a total period of 360 min, with the first 2 h corresponding to the equilibration period.
14C activity in collected samples was determined by liquid scintillation. Water flux for each 15 min interval was calculated by the following formula:
where DPMs and DPMx are 14C concentration in buffer solution and effluent, respectively, expressed as disintegrations per minute (DPM), P is the rate of infusion (ml h−1), and L is the length (cm) of the isolated colonic segment. Water flux occurring over four consecutive 15 min intervals was averaged to obtain a mean net flux of water over 1 h periods. The net water flux was expressed in μl cm−1 h−1. Positive values represent net water absorption whereas negative values indicate a net secretion.
Rectal distension (RD) was performed with an arterial embolectomy catheter (Fogarty; Edwards Laboratoire, Inc., Santa Ana, CA, USA) introduced into the rectum (1 cm from the anus) and fixed at the base of the tail. Balloons were calibrated using an electronic caliper gauge, in order to define the mean diameters corresponding to the volumes applied. The balloon (2 mm diameter, 2 cm long) was inflated with a syringe filled with 2 ml of water corresponding to a pressure of 65-70 mmHg, and a diameter of 13.7 mm.
After a 120 min equilibration period, a rectal distension (RD) was applied for 3 h. Net water flux values were determined during the RD period and for one postdistension hour. In addition, the percentage of total recovery of [14C]PEG 4000 was calculated from the total volume of effluent collected over the experimental period, in order to test the eventual permeability of the marker across the intestinal wall. The recovery, which varied between 92 and 103 % of [14C]PEG 4000, did not indicate changes in the colonic permeability. Control experiments consisted of net water flux measurement with a non-inflated balloon inserted in the rectum (sham-RD).
In four distinct groups, the effect of indomethacin (a prostaglandin synthesis inhibitor) and doxantrazole (a mast cell stabilizing agent) were determined on RD-induced net water movement changes. Indomethacin was administered i.p. 15 min before RD at the dose of 5 mg kg−1, and i.c.v. at the dose of 0.5 mg kg−1. Similarly, doxantrazole was administered i.p. at 5 mg kg−1 and i.c.v. at 0.5 mg kg−1. Four other groups were used in order to determine the effect per se of i.p. and i.c.v. indomethacin and doxantrazole without inflation of the rectal balloon.
In a last series of experiments two groups of rats were used to evaluate the central and/or peripheral effect of IL-1ra, an IL-1 receptor antagonist, on RD-induced colonic net water secretion. IL-1ra was administered either by i.p. or i.c.v. route at a dose of 10 μg per rat, 15 min before RD. Furthermore, two sham-RD groups were used in order to determine the effect per se of IL-1ra at both central and peripheral level.
At the end of the experiments animals were killed by cervical dislocation.
Identification of mast cells and measurement of histamine release
Two groups of eight rats were used. They were anaesthetized under the same conditions as used for water flux measurement. A balloon was inserted into the rectum. In one group the balloon was inflated (65-70 mmHg, 13.7 mm) for 3 h, 2 h after the beginning of the anaesthesia. In a control group the balloon was maintained without inflation for 5 h. After the end of the distension period, the rats were exsanguinated as approved by our local ethical committee (agreement no. 96024 A). Blood and two segments (0.5 cm) of distal colon from each animal were taken. The first segment was fixed in Carnoy's solution, cleared in xylene, and embedded in paraffin blocks. Transverse sections (5 μm) were cut and stained with Alcian Blue-Safranin O according to Roberts, Jones & Stoddart (1990). Mast cell evaluation in mucosa and sub-mucosa was expressed in number per high-powered field (HPF, using 20 × objective). The number of intact mast cells per animal and per HPF is given as the mean value obtained for three sections.
The second distal colonic segment was used for histamine release measurement. Briefly, the colonic segment (0.5 cm) was incubated for 1 h in a Ringer solution containing compound 48/80 (1.5 mg ml−1), a potent rat mast cell secretagogue (Riley & West, 1955). The histamine concentration in the supernatant and in the blood sample were measured with a radioimmunoassay (RIA) kit using polyclonal histamine antibodies (Immunotech, Marseille, France). In the tissues, the histamine level was expressed in nanomoles per microgram of total protein. Total tissue protein concentrations were determined according to the Bradford method (Bradford, 1976).
Central detection of IL-1β by SDS-PAGE and immunoblotting
IL-1β was detected in two groups of rats, the control group and the RD group, submitted to the same protocol as for mast cell identification and histamine measurement.
At the end of the RD period, animals were immediately infused intracardially with sterile Ringer solution. The brain was removed from the skull and immediately put into sterile vials containing 5 ml of RPMI 1640 plus 0.2 M AEBSF (4-(2-aminoethyl)-benzene sulphonyl fluoride; an irreversible serine protease inhibitor), 10 μM EDTA, 10 μM leupeptin and 1 μM pepstatin to inhibit protease activity. Tissues were then treated as described by Fontana (Fontana, Weber & Dayer, 1984). The concentrated brain fluids were fractionated by gel fitration according to Cannon & Dinarello (1985). Samples were applied to sterile columns (1 cm × 40 cm) packed with autoclaved sephadex G50 (fine, Pharmacia). The gel was equilibrated with RPMI 1640 containing 25 mM Hepes buffer and 5 × 10−5 M 2-mercaptoethanol at a flow rate of 1 ml min−1. The eluate was collected at one fraction per minute, or 1 ml per fraction. IL-1β, with a molecular mass of 17.5 kDa, was eluted from the G50 column in fractions 10-15 with the highest concentration in fraction 12, as described by Quan, Sunder & Weiss (1994).
Proteins from fractions 10-15 equilibrated in the sample buffer were separated by SDS-PAGE in 15 % polyacrylamide gels according to Laemmli (1970), and electrophoretically transferred into nitrocellulose membranes as described by Towbin, Staehelin & Gordon (1979), in 25 mM Tris-HCL, 192 mM glycine, 0.01 % (v/v) SDS, and 15 % methanol. Immunoblottings were performed using polyclonal rabbit anti-mouse IL-1β used at 1/100 (Genzyme, West Malling, Kent, UK), mouse IL-1β (Genzyme UK) and anti-rabbit IgG (peroxidase conjugated; Sigma) used at 1/10000. Immunolabelled bands were revealed by fluorography, using ECL reagents (Enhanced Chemiluminescence, Amersham, France) followed by 10 min exposure to hyperfilm MP (Amersham, France).
Drugs
Indomethacin and compound 48/80 were purchased from Sigma. Doxantrazole was a gift from Wellcome Research Laboratories (Beckenhan, England). IL-1ra was a gift from Synergen Inc., Boulder, CO, USA.
Statistics
Net water flux values obtained during the RD session were systemically compared hour per hour with their sham-RD corresponding values. The net water secretion was maximal during the second hour of distension. The values obtained during this period for the different treatments were used for statistical analysis. The values of net water flux, mast cell numbers and histamine concentrations were analysed using the Mann-Whitney U test for unpaired data and the Wilcoxon test for paired data. The criterion for statistical significance was P < 0.05. Data are expressed as means ±s.d. for each group of rats (n = 8).
RESULTS
Effect of rectal distension (RD) on colonic net water flux movements
Basal net water flux (NWF) in rats without inflation of the rectal balloon were 38.7 ± 39.6, 41.5 ± 40.9, 43.2 ± 35.7, 45.2 ± 39.1 and 39.6 ± 35.7 μl cm−1 h−1 (n = 8) over 5 h. Three-hour RD applications (69.8 ± 2 mmHg) reversed the net water absorption into a net secretion at the 2nd and 3rd hour of RD, with a maximal effect during the 2nd hour. The values obtained during the 2nd and 3rd hour of RD (-131.8 ± 62.6 vs. 43.2 ± 35.7; -50.0 ± 70.6 vs. 45.2 ± 39.1 μl cm−1 h−1, respectively) were significantly different (P < 0.05) from basal NWF values obtained in sham-RD rats. No significant change in net water flux values was observed during the 1st hour of RD vs. sham-RD rats.
The basal net water flux obtained during the first post-distension hour (20.6 ± 51.5 vs. 39.6 ± 35.7 μl cm−1 h−1) was not significantly different (P > 0.05) from basal values (Fig. 1).
Figure 1. Influence of rectal distension on proximal colonic net water flux movements in anaesthetized rats.
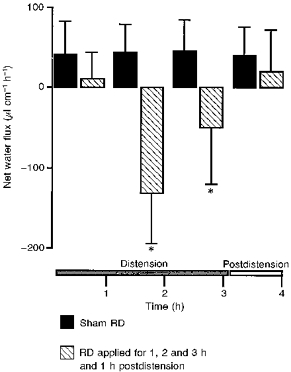
Note the net water hypersecretory effect induced by RD (P = 65-70 mmHg) during the 2nd and 3rd hour. Values are means ±s.d., n = 8. * Significantly different (P < 0.05) from corresponding control values.
Antagonism by doxantrazole and indomethacin
Peripheral administration of both doxantrazole (5 mg kg−1i.p.) and indomethacin (5 mg kg−1i.p.) suppressed the RD-induced colonic hypersecretory effect. NWF values obtained during the 2nd hour of RD (52.1 ± 39.5 and 132.0 ± 69.7 μl cm−1 h−1, respectively) were significantly different (P < 0.05) from the corresponding RD control values, and were not significantly different (P > 0.05) from the sham-RD values (Fig. 2).
Figure 2. Effect of peripheral and central administration of doxantrazole and indomethacin on RD-induced net water hypersecretion in anaesthetized rats.
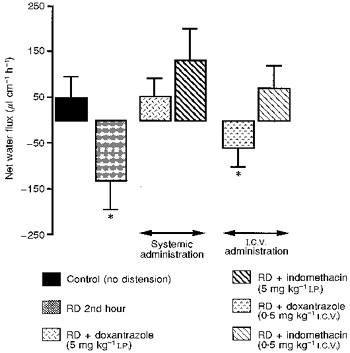
Note that at peripheral level both doxantrazole and indomethacin blocked the secretory effect induced by RD. At the central level indomethacin abolished the RD-induced net water hypersecretion, as opposed to doxantrazole, which had no effect. Values are means ±s.d., n = 8. * Significantly different (P < 0.05) from basal values.
The i.c.v. administration of doxantrazole (0.5 mg kg−1) had no effect on RD-induced colonic hypersecretion, and the values obtained (-60.3 ± 40.9 μl cm−1 h−1) were not significantly different (P > 0.05) from RD values. In contrast, indomethacin (0.5 mg kg−1 i.c.v.) blocked the RD hypersecretory effect, since the values obtained (69.9 ± 51.7 μl cm−1 h−1) were significantly different (P < 0.05) from the RD values, but were not significantly different (P > 0.05) from the control values (Fig. 2).
The indomethacin and doxantrazole administration had no effect per se on net water flux movements either at central or peripheral level.
Influence of central and peripheral IL-1ra pretreatment
Peripheral IL-1ra administration (10 μg per rat) did not completely block the RD hypersecretory effect; values obtained (-52.9 ± 48.7 μl cm−1 h−1) were not significantly different (P > 0.05) from RD values, but reduced it by 46 %. In contrast, the same dose of IL-1ra, i.e. 10 μg per rat, injected i.c.v., suppressed the RD-induced hypersecretion: the NWF value obtained (56.7 ± 49.8 μl cm−1 h−1) was significantly different (P < 0.05) from RD values, but not significantly different (P > 0.05) from sham-RD values (Fig. 3). Central and peripheral IL-1ra administration had no effect per se on net water flux movements.
Figure 3. Influence of central and peripheral IL-1ra administration on RD-induced colonic net water hypersecretory effect in anaesthetized rats.
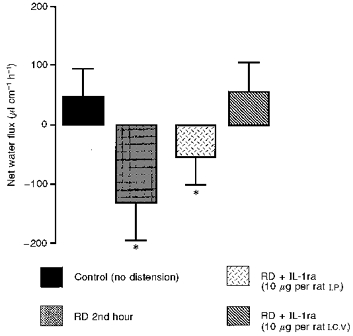
Note the suppression of RD-induced colonic net water hypersecretion by a central administration of IL-1ra, whereas IL-1ra administered i.p. had no effect on the RD-induced secretory effect. Values are means ±s.d., n = 8. * Significantly different (P < 0.05) from basal values.
Mast cell degranulation
Mast cells were visualized in tissues using the staining properties of their granule content. Consequently, only mast cells with intact granules were counted. The histological analysis showed that mast cells of control and RD-submitted animals were located in the colonic submucosa near blood vessels and nerves. The number of granulated mast cells stained by Alcian Blue-Safranin O in the distal control colonic segment was 8.4 ± 2.1 per HPF of mucosa. In contrast, the intact mucosal mast cell number of RD-treated rats was 4.9 ± 1.3, and was significantly different from the control values (P < 0.05) (Table 1). No significant difference was observed between submucosal mast cell numbers in control and RD-treated rats (2.1 ± 0.5 vs. 1.9 ± 0.8) (Table 1).
Table 1.
Mast cell number and histamine levels on distal colonic segment
| Mast cell number (per HPF; × 20) | Supernatant histamine (nmol (μg total proteins)−1) | Blood histamine (μmol) | ||
|---|---|---|---|---|
| Mucosa | Submucosa | |||
| Control | 8·4 ± 2·1 | 2·1 ± 0·5 | 0·24 ± 0·15 | 47·24 ± 9·32 |
| RD 2nd hour | 4·9 ± 1·3 * | 1·9 ± 0·8 | 0·16 ± 0·09 * | 103·28 ± 72·71 * |
Mast cell histological number analysis on distal colonic segment and histamine levels determination with RIA dosages on both blood sample and distal colonic segment were performed on distal colonic segment. Results are expressed as means ± S.D.; n = 8.
P < 0·05 compared with control values.
The histamine level detected in the supernatant of the incubated distal colonic segment, after compound 48/80 stimulation, obtained from control animals, was 0.24 ± 0.15 nmol (μg total proteins)−1. Under the same stimulation conditions, the histamine released from the incubated distal colonic segment of RD-treated animals was 0.16 ± 0.09 nmol (μg total proteins)−1 and was significantly lower (P < 0.05) compared with the control values. In contrast, the blood histamine level obtained in RD-treated rats was 103.28 ± 72.71 μmol, a value significantly higher (P < 0.05) compared with control (47.24 ± 9.32 μmol) (Table 1).
Central IL-1 release
In contrast to the control samples, proteins from fractions 10-15 extracted from brain tissues of rats submitted to a RD of 2 h contained IL-1β (Fig. 4). IL-1β was detected in the brain lysates as uncleaved pro-IL-1β, migrating as a major band of 33 kDa, and IL-1β, in its mature form at 17 kDa. The 29 kDa band (Fig. 4) was recognized by anti-IL-1β in Western blots. It probably represents an intermediate proteolytic fragment. This last observation was also made by Rubartelli, Cozzolino, Talio & Sitia (1990) using IL-1β Western blot, with the fragment being released by human monocytes activated with lipopolysaccharides. Neither IL-1β nor pro-IL-1β signal bands were observed in brain from control rats (Fig. 4).
Figure 4. Immunoblotting analysis of brain IL-1β in rats submitted to RD for 2 h.
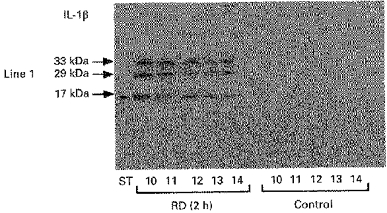
Note both signal bands at 33 and 17 kDa corresponding to a proIL-1β and IL-1β secretion, respectively. No signal band was observed in the brain of control rats. ST, standard marker.
DISCUSSION
This study confirms our previous results showing that rectal distension reverses the net water flux in the proximal colon, with a maximal effect during the second hour of RD application (Eutamene et al. 1997). In our study the inflation pressure used (65-70 mmHg), corresponding to 88.4-95.2 cmH2O, was high when compared with a pressure of 15 cmH2O used in other publications (Itasaka et al. 1992), a value thought to be generated by faecal pellets. However, previous experiments we have performed with 40 mmHg did not induce a reproducible colonic net water hypersecretion in rats (data not shown). As we previously discussed in a recent study (Eutamene et al. 1997), we suggest that the high pressure used, which is necessary to induce net water secretion, can mimick a pathophysiological situation such as bowel obstruction, which stimulates water secretion to facilitate intestinal transit by lubricating the luminal surface and by liquifying the intestinal contents.
Doxantrazole (a mast cell stabilizing agent) administered peripherally but not centrally blocks the RD-induced secretory effect, suggesting the involvement of peripheral mucosal mast cells (MMC) in this effect. The involvement of mast cells in this RD-induced effect is also supported by the histological examination that indicates a significant decrease in the number of intact mast cells in the distal colonic mucosa after RD application, and by the decrease of distal colonic MMC histamine release induced by compound 48/80 stimulation in vitro. Histamine measurement is frequently used as a mast cell degranulation criterion (Pearce, Befus, Gauldie & Bienenstock, 1982). Degranulatory polyamines such as compound 48/80 used in this method, promote mast cell histamine release, acting, like amphilic peptide, directly on a mast cell G protein, which induces mast cell degranulation (Mousli, Bronner, Bueb, Tschirhart, Gies & Landry, 1989). The stimulation of distal colonic segments taken from animals submitted to RD by compound 48/80, showed a decrease of the histamine level in the supernatant. Taken together, histological observations and in vitro histamine level measurement suggest a mast cell degranulation induced by RD, in this study.
We suggest that one or several mediators released by mast cell degranulation, such as histamine, serotonin, eicosanoids, interleukin-1 or substance P, are involved in the RD-induced colonic hypersecretion. Moreover, MMC and other immune cells reveal an intimate relationship with nerve fibres (Stead, Tomioka, Quinonez, Simon, Felten & Bienenstock, 1987) and may be one factor influencing the response of visceral afferents to physical, chemical and neurochemical stimuli (Minner, 1991).
Consequently we speculate that the hypersecretion induced by RD is mediated through a neuroimmune pathway, involving mast cell degranulation in the distal colon, which in turn can interact with sensitive fibres via the mediators released.
Peripheral indomethacin administration blocks the RD-induced colonic secretory effect, and suggests the involvement of local prostaglandins in this effect, although biochemical PG measurement was not performed. PGs are known to be synthetized and released de novo by chemical or mechanical stimulation of the mucosa (Beubler & Juan, 1978). Moreover, the major source of PGs released in the intestine are fibroblasts, the vascular smooth muscle and nerves (Lawson & Powel, 1987). PGs in the gut originate in the lamina propia and submucosa, and their intestinal secretory action on the enterocytes is probably a direct one, via adenylate cyclase stimulation and via the elevation of 3′,5′-cyclic adenosine monophosphate concentration (Warhurst, Lees, Higgs & Turnberg, 1987), or by increasing intracellular Ca2+ concentration. Furthermore, we cannot exclude a possible intramural mechanism of action of endogenous PG release in the RD-induced colonic net water hypersecretion effect (Itasaka et al. 1992). Moreover, in vivo studies have demonstrated that in the anaesthetized rat ileum, lumen distension induced net fluid secretion, which was prevented by indomethacin, indicating the involvement of PGs in this effect (Mac Gregor & Lavigne, 1979). Furthermore, in the central and peripheral nervous systems, PGs can act on neural sites, causing an enhancement of acetylcholine release from myenteric ganglia and cholinergic nerve terminals and promoting intestinal fluid secretion (Yagasaki, Takai & Yanagiya, 1984). PGs can also directly activate nociceptive and non-nociceptive capsaicin-sensitive afferent fibres (Davies, Bailey & Goldenberg, 1984). Consequently, we suggest that the involvement of PGs in the RD-induced colonic hypersecretion is mediated through a neural pathway, since previous results showed that both systemic capsaicin treatment and atropine blocked the RD-induced colonic hypersecretory effect (Eutamene et al. 1997). However, in our model, we cannot exclude a direct PG efferent effect on the enterocyte. Furthermore, the time course effect of RD-induced colonic hypersecretion, with a maximal effect 2 h after RD application, could be explained by a delay corresponding to PG synthesis de novo after mast cell degranulation; these PGs are able to interact with nerve fibres in order to induce colonic hypersecretion.
Central indomethacin administration blocks the RD-induced colonic hypersecretion, suggesting PG central release induced by RD. This result is in agreement with a study showing that central administration of PGs can control jejunal water and ion transport in dog (Primi & Bueno, 1986). Furthermore, it is well established that central PG cardiovascular effects are mediated by vasopressin (AVP) release (Okuno, Lindheimar & Oparil 1982). Moreover, AVP has been shown to promote intestinal secretion in rat colon (Dennhardt, Lingelbach & Haberich, 1979). Consequently, we can speculate that AVP may be a possible hormonal factor released under PG influence in the RD-induced colonic hypersecretion.
IL-1ra administered i.c.v. blocked the RD-induced colonic hypersecretory effect. Moreover, SDS-PAGE and immunoblotting studies indicate central IL-1β secretion during the 2nd hour of RD suggesting central IL-1β release during the RD-induced colonic effect. Systemic IL-1 administration increases PG synthesis in the organum vasculosum of the lamina terminalis region (OVLT), and stimulates the production of PGs from hypothalamic tissue in vitro (Milton, Self & Hillhouse 1993). Taken together, these data support the hypothesis that brain IL-1 stimulates central PG production in the RD-induced colonic hypersecretion. In addition, we cannot exclude a possible peripheral IL-1 release, involved in the RD-induced colonic hypersecretion, although the i.c.v. dose of IL-1ra administered systemically did not completely block the RD hypersecretory effect, although it did reduce it by 46 %. Moreover, a cytokine-tachykinin interaction in the hypothalamus was recently demonstrated. IL-1β increased neurokinin A (NKA) secretion selectively in the median eminence and in the arcuate nucleus (Kalra, Dube & Kalra, 1994), where IL-1 co-exists with tachykinins and with preprotachykinin mRNAs and activates various members of the tachykinin family of peptides (Lindefor, Brodin, Theodorsson-Norheim & Ungerstedt, 1985). These observations highlight the idea that one of the sites of interaction between the cytokines and NKA is localized in the basal hypothalamus. In a previous study, we have shown an IL-1β colonic water hypersecretory effect in rats, via a neural pathway, involving spinal or central NK2 receptor activation, suggesting NKA release at these levels (Eutamene et al. 1995). Moreover, in a previous work we have also demonstrated a central NK2 receptor subtype activation in the RD-induced colonic hypersecretion in anaesthetized rats, supporting the hypothesis of release of NKA at the central level, which activate in turn an efferent vagal pathway to induce colonic net water hypersecretion through the release of secretagogues. We speculate that RD-induced colonic hypersecretion involves central IL-1 synthesis and release, which in turn can activate a neural pathway through NK2 receptor activation, suggesting NKA release and PG secretion, and finally causing colonic hypersecretion. The delay required for central synthesis and release of IL-1 can also explain the time course effect observed in the RD-induced secretory effect.
Finally, we conclude that RD induces net water colonic hypersecretion in anesthetized rats. This effect illustrates a complex neuroimmune pathway through both central IL-1 and PG release and peripheral mast cell degranulation.
References
- Bern MJ, Sturbaum CW, Karayalcin SS, Berschneider HM, Wachsman JT, Powel DW. Immune system control of rat and rabbit colonic electrolyte transport: Role of prostaglandins and enteric nervous system. Journal of Clinical Investigation. 1983;83:1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beubler E, Juan H. PGE-release, blood flow, and transmucosal water movement after mechanical stimulation of the rat jejunal mucosa. Naunyn-Schmiedeberg's Archives of Pharmacology. 1978;305:91–95. doi: 10.1007/BF00497010. [DOI] [PubMed] [Google Scholar]
- Bileviciute I, Lundeberg T, Ekblom A, Theodorson E. The effect of a single intraperitoneal dose of rhIL-1a on substance P neurokinin A, calcitonin gene-related peptide and neuropeptide Y like immunoreactivity in cerebrospinal fluid, plasma and knee joint synovial fluid in the rat. Regulatory Peptides. 1994;53:71–76. doi: 10.1016/0167-0115(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Bradford MA. Rapid and sensitive method for the quantification of microgram of protein utilizing the principle of protein-dye binding. Annals of Biochemistry. 1976;72:248–245. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Dinarello CA. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985;227:1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- Castro GA, Harari Y, Russel D. Mediators of anaphylaxis-induced ion transport changes in small intestine. American Journal of Physiology. 1987;253:540–548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- Chang EB, Musch MW, Mayer L. Interleukin 1 and 3 stimulate anion secretion in chicken intestine. Gastroenterology. 1990;98:1518–1524. doi: 10.1016/0016-5085(90)91084-j. [DOI] [PubMed] [Google Scholar]
- Chiossone DC, Simon PL, Smith PL. Interleukin-1 effect on rabbit ileal mucosa ion transport in vitro. European Journal of Pharmacology. 1990;180:217–228. doi: 10.1016/0014-2999(90)90305-p. [DOI] [PubMed] [Google Scholar]
- Davies P, Bailey PJ, Goldenberg MM. The role of arachidonic acid oxygenation products in pain and inflammation. Annual Review of Immunology. 1984;2:335–357. doi: 10.1146/annurev.iy.02.040184.002003. 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- Dennhardt R, Lingelbach B, Haberich FJ. Intestinal absorption under the influence of vasopressin: studies in unanasthetised rats. Gut. 1979;20:107–113. doi: 10.1136/gut.20.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener M, Rummel W. Distension-induced secretion in the rat colon: mediation by prostaglandins and submucosal neurons. European Journal of Pharmacology. 1990;178:47–57. doi: 10.1016/0014-2999(90)94792-v. 10.1016/0014-2999(90)94792-V. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39:209–224. doi: 10.1016/0306-4522(90)90234-u. 10.1016/0306-4522(90)90234-U. [DOI] [PubMed] [Google Scholar]
- Dray A, Bevan S. Inflammation and hyperalgesia: highlighting the team effort. Trends in Pharmacological Sciences. 1993;14:287–290. doi: 10.1016/0165-6147(93)90041-H. 10.1016/0165-6147(93)90041-H. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Theodorou V, Fioramonti J, Bueno L. Implication of NK1 and NK2 receptors in rat colonic hypersecretion induced by interleukin-1β: role of nitric oxide. Gastroenterology. 1995;109:483–489. doi: 10.1016/0016-5085(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Theodorou V, Fioramonti J, Bueno L. Rectal distension-induced colonic net water secretion in rats involves tachykinins, capsaicin sensory and vagus nerves. Gastroenterology. 1997;112:1595–1602. doi: 10.1016/s0016-5085(97)70041-6. [DOI] [PubMed] [Google Scholar]
- Fargeas MJ, Fioramonti J, Bueno L. Central action of Interleukin-1β on intestinal motility in rats: mediation by two mechanisms. Gastroenterology. 1993;104:377–383. doi: 10.1016/0016-5085(93)90404-z. [DOI] [PubMed] [Google Scholar]
- Fontana A, Weber E, Dayer JM. Synthesis of interleukin 1 endogenous pyrogen in the brain of endotoxin treated mice: a step in fever induction. Journal of Immunology. 1984;133:1696–1698. [PubMed] [Google Scholar]
- Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: distension evoked ion transport in the guinea pig distal colon. American Journal of Physiology. 1992;263:91–96. doi: 10.1152/ajpgi.1992.263.1.G91. [DOI] [PubMed] [Google Scholar]
- Itasaka S, Shiratori K, Takahashi T, Ishikawa M, Kaneko K, Suzuki Y. Stimulation of intraluminal secretory reflex by luminal distension pressure in rat distal colon. American Journal of Physiology. 1992;263:108–114. doi: 10.1152/ajpgi.1992.263.1.G108. [DOI] [PubMed] [Google Scholar]
- Kalra PS, Dube MG, Kalra SP. The effects of interleukin-1β on the hypothalamic tachykinin, neurokinin A. Brain Research. 1994;662:178–184. doi: 10.1016/0006-8993(94)90810-9. 10.1016/0006-8993(94)90810-9. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly to the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson LD, Powell DW. Bradykinin-stimulated eicosanoid synthesis and secretion by rabbit ileal components. American Journal of Physiology. 1987;252:783–790. doi: 10.1152/ajpgi.1987.252.6.G783. [DOI] [PubMed] [Google Scholar]
- Lindefor N, Brodin E, Theodorsson-Norheim E, Ungerstedt U. Regional distribution and in vivo release of tachykinin-like immunoreactivities in rat brain: evidence for regional difference in relative proportions of tachykinins. Regulatory Peptides. 1985;10:217–230. doi: 10.1016/0167-0115(85)90016-3. 10.1016/0167-0115(85)90016-3. [DOI] [PubMed] [Google Scholar]
- MacGregor IL, Lavigne ME. Inhibition by indomethacin of intestinal distension induced secretion in the rat. Journal of Surgical Research. 1979;26:167–170. doi: 10.1016/0022-4804(79)90095-7. 10.1016/0022-4804(79)90095-7. [DOI] [PubMed] [Google Scholar]
- Milton NGN, Self CH, Hillhouse EW. Effects of pyrogenic immunomodulators on the release of corticotrophin-releasing factor-41 and prostaglandin E2 from the intact rat hypothalamus in vitro. British Journal of Pharmacology. 1993;109:88–93. doi: 10.1111/j.1476-5381.1993.tb13535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minner PB. Systemic mastocytosis and regional gastrointestinal mast cell disease. In: Read NW, editor. Irritable Bowel Syndrome. Oxford: Blackwell Scientific Publications; 1991. pp. 174–189. [Google Scholar]
- Mousli M, Bronner C, Bueb JL, Tschirhart E, Gies JP, Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. Journal of Pharmacology and Experimental Therapeutics. 1989;250:329–335. [PubMed] [Google Scholar]
- Okuno T, Lindheimar MD, Oparil S. Central effects of prostaglandin E2 on blood pressure and plasma renin activity in rats. Hypertension. 1982;4:809–816. doi: 10.1161/01.hyp.4.6.809. [DOI] [PubMed] [Google Scholar]
- Pearce FL, Befus A, Gauldie J, Bienenstock J. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. Journal of Immunology. 1982;128:2481–2486. [PubMed] [Google Scholar]
- Primi MP, Bueno L. Central nervous system influence of prostaglandin E2 on jejunal water and electrolyte transport in conscious dogs. Gastroenterology. 1986;91:1427–1432. doi: 10.1016/0016-5085(86)90196-4. [DOI] [PubMed] [Google Scholar]
- Quan N, Sunder SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. Journal of Neuroimmunology. 1994;49:125–134. doi: 10.1016/0165-5728(94)90188-0. 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Quito FL, Brown DL. (D-ala2, met5)-enkephalinamide: CNS-mediated inhibition of prostaglandin-stimulated intestinal fluid and ion transport in the rat. Peptides. 1987;8:1029–1033. doi: 10.1016/0196-9781(87)90132-x. 10.1016/0196-9781(87)90132-X. [DOI] [PubMed] [Google Scholar]
- Riley JF, West GB. Tissue mast cells: studies with a histamine liberator of low toxicity (compound 48/80) Journal of Pathology and Bacteriology. 1955;69:269–282. doi: 10.1002/path.1700690135. [DOI] [PubMed] [Google Scholar]
- Roberts ISD, Jones CJP, Stoddart RW. Lectin histochemistry of the mast cell: heterogeneity of rodent and human mast cell populations. Histochemical Journal. 1990;22:73–80. doi: 10.1007/BF01885784. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO Journal. 1990;5:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestine are in intimate contact with peptidergic nerves. Proceedings of the National Academy of Sciences of the USA. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swabb EA, Hyness RA, Marnane WG, McNeil JS, Decker RA, Tai YH, Donowitz M. Intestinal filtration-secretion due to increased intraluminal pressure in rabbits. American Journal of Physiology. 1982;242:65–75. doi: 10.1152/ajpgi.1982.242.1.G65. [DOI] [PubMed] [Google Scholar]
- Theodorou V, Eutamene H, Fioramonti J, Junien JL, Bueno L. Interleukin-1 induces a neurally mediated colonic secretion in rats: involvement of mast cells and prostaglandins. Gastroenterology. 1994;106:1493–1500. doi: 10.1016/0016-5085(94)90402-2. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst G, Lees M, Higgs NB, Turnberg LA. Site and mechanisms of action of kinins in rat ileal mucosa. American Journal of Physiology. 1987;252:G293–301. doi: 10.1152/ajpgi.1987.252.3.G293. [DOI] [PubMed] [Google Scholar]
- Yagasaki O, Takai M, Yanagiya I. Acetylcholine release from the myenteric plexus of guinea-pig ileum by prostaglandin E1. Journal of Pharmacy and Pharmacology. 1984;33:521–525. doi: 10.1111/j.2042-7158.1981.tb13851.x. [DOI] [PubMed] [Google Scholar]


