Abstract
We investigated the mechanisms underlying the negative inotropic effect of the volatile anaesthetics halothane and isoflurane using twenty-two intact, right ventricular trabeculae of rat. [Ca2+]i was measured qualitatively using either fluo-3 or fura-2, loaded into the cytosol via the acetoxymethyl (AM) ester form. Diastolic sarcomere length was adjusted to 2.1-2.2 μm and experiments were performed at 21-23°C.
Halothane (02.5-3 %) and isoflurane (0.48-4 %) produced dose-dependent decreases in the amplitudes of the intracellular Ca2+ transients and twitch force. When the fluorescent Ca2+ indicator signals were corrected for changes in autofluorescence, neither volatile anaesthetic significantly changed diastolic [Ca2+]i.
The ability of halothane and isoflurane to induce Ca2+ release from the sarcoplasmic reticulum of quiescent trabeculae was examined. When the superfusate was Ca2+ and Na+ free (thereby preventing Na+- Ca2+ exchange and Ca2+ influx), 2 % halothane, but not 4 % isoflurane, evoked a transient increase in [Ca2+]i.
Halothane and isoflurane produced reversible, dose-dependent changes in cellular autofluorescence, the pattern of which was consistent with an increase in concentration of the reduced forms of nicotinamide adenine nucleotides and flavoproteins. This observation supports the putative inhibitory action of volatile anaesthetics at the site of Complex I of the mitochondrial electron transport chain.
Addition of the fatty acid hexanoate, a substrate that can be metabolized in the face of Complex I inhibition, did not appreciably attenuate the anaesthetic-induced negative inotropy; however, it greatly diminished autofluorescence changes.
To determine whether direct actions of the volatile anaesthetics on the contractile system contributed to the negative inotropy, external [Ca2+] was varied to modulate the amplitude of the Ca2+ transient. In the presence of 2 % halothane or 4 % isoflurane, restoration of the peak Ca2+ transient to control levels did not restore peak force. Moreover, halothane (1 %) and isoflurane (1.6 %) each reduced maximal Ca2+-activated force (attained using ryanodine tetani and a high external [Ca2+]) by around 15 %.
We conclude that the negative inotropic actions of halothane and isoflurane on intact cardiac muscle reflect both reduced availability of Ca2+ and decreased responsiveness of the contractile system to Ca2+. The inhibitory action of the volatile anaesthetics on mitochondrial function does not contribute significantly to the negative inotropy but may lead to changes in cellular autofluorescence and misinterpretation of fluorescent Ca2+ indicator signals.
Volatile anaesthetics, such as halothane and isoflurane, reduce the force of contraction of cardiac muscle at clinically relevant doses. Mechanisms of anaesthetic action at various steps in excitation-contraction coupling have been put forward to explain this observation (for a review, see Rusy & Komai, 1987). For example, halothane and isoflurane have been reported to decrease L-type Ca2+ channel current (Pancrazio, 1996), the magnitude and duration of which control the activation of sarcoplasmic reticular (SR) Ca2+ release (ryanodine receptor) units (Cannell, Cheng & Lederer, 1995). In addition to diminishing the trigger for Ca2+ release, volatile anaesthetics have also been shown to reduce the Ca2+ content of the SR (Katsuoka, Kobayashi & Ohnishi, 1989; Katsuoka & Ohnishi, 1989). This action is reputed to be brought about by both a non-specific increase in the permeability characteristics of the SR membrane (Frazer & Lynch, 1992) and increased Ca2+ efflux via the Ca2+ release channel (Herland, Julian & Stephenson, 1990). Whether isoflurane is capable of inducing Ca2+ release by the latter means is open to doubt since Connelly & Coronado (1994) found that this agent, unlike halothane, had no effect on the gating (or conductance) properties of isolated release channels incorporated into lipid bilayers.
Although no indices of contractility were reported, studies of isolated cardiac myocytes loaded with a fluorescent Ca2+ indicator have shown that one of the consequences of the actions of volatile anaesthetics on transmembrane Ca2+ movements is a decrease in the amplitude of electrically or caffeine-evoked Ca2+ transients (Wheeler, Rice, Hansford & Lakatta, 1988; Wilde, Knight, Sheth & Williams, 1991; Wilde, Davidson, Smith & Knight, 1993). At variance with the results of Wilde et al. (1993), however, Wheeler, Katz, Rice & Hansford (1994) reported that isoflurane did not affect the amplitude of caffeine-induced Ca2+ transients, a measurement which is often used to assay the Ca2+ content of the SR.
In addition to reducing the amount of Ca2+ released into the cytosol, the volatile anaesthetics have also been reported to alter the response of the contractile system to activator Ca2+. Various studies using chemically (Murat, Ventura-Clapier & Vassort, 1988) or mechanically (Su & Kerrick, 1978; Su & Bell, 1986) skinned cardiac muscle preparations have consistently shown that halothane and isoflurane decrease maximal Ca2+-activated force and the Ca2+ sensitivity of the contractile system. In contrast, in an elaborate study employing a range of chemical- and mechanical-skinning techniques, Herland, Julian & Stephenson (1993) found that the magnitude and, even, direction of the effect of volatile anaesthetics on maximal Ca2+-activated force and Ca2+ sensitivity was dependent on the particular technique employed.
The above situation is complicated by previously noted effects of volatile anaesthetics on cardiac energy metabolism. It has previously been deduced that volatile anaesthetics inhibit the re-oxidation of NADH by blocking the electron transport chain at the site of Complex I (Biebuyck, 1973; Berman, Kewley & Kench, 1974; Kissin, Aultman & Smith, 1983). Recently, Stephenson, Garzella, Wingrove, Claflin & Julian (1995) reported that halothane reversibly increased the autofluorescence of rat cardiac muscle excited with ultraviolet (UV) wavelengths of light, presumably secondary to an increase in [NADH] (in accord with the work of Kissin et al. 1983), and pointed out that this could lead to misinterpretation of fura-2 fluorescence signals if it were not realized.
The object of the present study was to shed light on the mechanisms by which volatile anaesthetics depress contractile function in an intact multicellular cardiac muscle preparation. As far as we are aware this is the first study to use a fluorescent Ca2+ indicator to examine the effects of halothane and isoflurane, two commonly used volatile anaesthetics, on intracellular Ca2+ handling in a multicellular cardiac muscle preparation. As alluded above, one of the potential problems with using fluorescent Ca2+ indicators to measure intracellular [Ca2+] is that various experimental conditions may change cellular autofluorescence and thereby corrupt the signals. In the light of this potential problem, we characterized the nature of the effects of volatile anaesthetics on autofluorescence elicited by exciting the muscle with ultraviolet and visible wavelengths of light. We found that the effects of volatile anaesthetics on autofluorescence were minimal when a fatty acid was supplied as a metabolic substrate and the fluorescent Ca2+ indicator fluo-3, which is excited by visible wavelengths of light, was used. In addition to shedding light on the effects of halothane and isoflurane on autofluorescence (and therefore mitochondrial function), we have studied the effects of these volatile anaesthetics on sarcoplasmic reticular function, Ca2+ availability and the responsiveness of the contractile system to Ca2+.
METHODS
Anaesthesia and isolation of the heart
Methods were approved by the Animal Ethics Committee of the University of Auckland. Male Wistar rats, weighing 200-300 g, were anaesthetized by intraperitoneal injection of 3.5-4.5 ml kg−1 of a warmed (37-38°C) mixture containing (mg ml−1): 0.079 fentanyl citrate, 2.5 fluanisone and 1.25 midazolam hydrochloride (a gift from Roche, New Zealand). Following induction of anaesthesia, venous access was established by inserting a 22-G Teflon cannula into a tail vein and an intravenous load of 2-3 ml of warmed plasma volume expander (Haemaccel; Behringwerke AG, Marburg, Germany) was slowly infused. A tracheal cannula was inserted via a tracheostomy and intermittent positive pressure ventilation with 100 % O2 provided. End-tidal CO2 was maintained in the range 4.5-5 %. After the thorax was opened, the heart was rapidly excised and briefly immersed in chilled (4°C) dissection solution to induce arrest. Within 30 s of excising the heart, coronary perfusion with dissection solution A (see below) was recommenced via a rotatable and horizontal aortic cannula which protruded into the dissection chamber.
Dissection and mounting of trabeculae
The criteria for the selection of suitable trabeculae from the right ventricle was similar to that described by de Tombe & ter Keurs (1990). That is, only thin (112 ± 39 μm) and long (2.6 ± 0.5 mm) (means ±s.d., n = 22) trabeculae which were unbranched, uniform and freely ran from the free wall to the atrioventricular ring were selected (see Fig. 1A). Unfortunately, these criteria were met only about once in every three hearts. The isolated trabecula was transferred in a miniature glass spoon to the mechanical stage of an inverted microscope (Nikon Diaphot 300, Japan), which was located on a vibration isolation table (Newport, CA, USA). It was then mounted between a force transducer and fixed support, both of which were connected to 3-axis micromanipulators. The free wall end of the muscle was attached to the force transducer by means of a wire cradle. The other end was held by either a 100 μm-diameter stainless-steel hook (which was inserted into a preformed perforation in the remnant of valvular tissue; see Fig. 1A) or a nylon snare (loop of 30 μm-diameter nylon monofilament threaded through a miniature stainless-steel tube (length, 25 mm; i.d., 100 μm; wall thickness, 50 μm; Goodfellow, Cambridge, UK). Since care was taken to avoid abrading the trabecula during its dissection and mounting, it is assumed that the endothelium, a reputed modulator of cardiac contractility (McClellan, Weisberg & Winegrad, 1995), was not disrupted.
Figure 1. Isolation of trabecula from the right ventricle of a rat heart and typical fluo-3 fluorescence and force recordings.
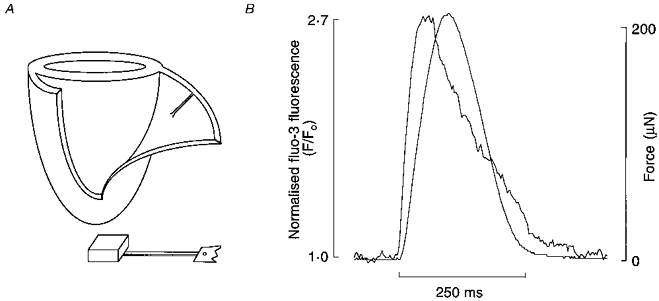
A, schematic diagram showing the typical location of a suitable trabecula in the right ventriclar free wall of a heart (atria and valves are not depicted) and its appearance after isolation. At one end of the isolated preparation, a small block of ventricular wall has been retained and, at the other, a small flap of tricuspid valve tissue remains attached to the remnant of the atrioventricular ring. Note the preformed perforation in the flap of valve. B, typical intracellular Ca2+ transient (measured using the fluorescent Ca2+ indicator fluo-3) and force transient simultaneously recorded without filtering or averaging.
After mounting, the muscle was superfused with dissection solution B (see below) for about 45 min and then with standard Krebs-Henseleit solution containing low [Ca2+] (0.25 mM) for a further 10 min or so before electrical field stimulation (provided by a Digitimer D100 via parallel platinum wire electrodes) was commenced. A stabilization period of at least 1 h was provided, during which time the muscle was stimulated at a rate of 0.5 Hz. A central portion of the muscle was imaged by a charge-coupled device (CCD) camera after being magnified × 400 (by use of a × 40 long working distance Nikon objective lens) and displayed on a video monitor. Muscle striations were clearly visible at this magnification and the sarcomere length was adjusted to 2.1-2.2 μm.
Muscle bath and solution delivery system
Solutions were supplied to the Perspex bath (volume, 450 μl) at a moderately high flow rate (8-9 ml min−1) using a 4-channel roller pump (Minipuls2, Gilson, France). The pulsatility of the solution caused by the roller pump was dampened before it entered the bath by feeding it first into an open, miniature, stainless-steel cylinder. At the bath outflow end, meniscus perturbations were abolished by placing the suction nozzle in a side-channel just distal to a thin and low barrier. Miniature 3-way solenoid valves (LFAA1201610H, The Lee Company, Westbrook, CT, USA) were used to switch solutions. Solution exchange, assessed by the rate of wash-out of fura-2 fluorescence, was 50 and 90 % complete in 1.5 and 7.5 s, respectively, when the flow rate was 8 ml min−1.
Solutions
The dissection and superfusing media were modified Krebs- Henseleit solutions which contained (mM): 118 NaCl, 3.82 KCl, 1.18 MgSO4, 1.18 KH2PO4, 24.8 NaHCO3 and 10 D-glucose. All solutions were equilibrated with 95 % O2-5 % CO2 and the pH was 7.4. One of the dissection solutions (dissection solution A) contained an additional 10 mM KCl (final [K+], 15 mM) and 20 mM 2,3-butanedione monoxime (BDM). The other one (dissection solution B) contained only an additional 20 mM BDM. BDM was added primarily to prevent contracture of the muscle in response to cutting. Both dissection solutions also contained a low [Ca2+] (0.25 mM).
In order to provide fatty acid as a metabolic substrate in selected experiments, the appropriate amount of the sodium salt of the short-chain fatty acid hexanoic acid was added directly to give a concentration of 1 mM. The advantages of using sodium hexanoate as a fatty acid substrate and at this concentration are outlined by Vuorinen, Ala-Rämi, Yan, Ingman & Hassinen (1995). CaCl2 was added from a 1 M stock solution; under control conditions, [Ca2+]o was 2 mM (unless stated otherwise). In experiments requiring external Ca2+ concentrations above 10 mM (ryanodine tetani series), MgSO4 and KH2PO4 were substituted for MgCl2 and KCl, respectively, to prevent the formation and precipitation of poorly soluble calcium salts.
Ca2+-free solutions
In the preparation of Ca2+- and Na+-free solutions, CaCl2 and KCl were omitted, 1 mM EGTA was added and the salts NaCl and NaHCO3 were replaced with LiCl and KHCO3, respectively. The main reason for using a Ca2+- and Na+-free solution was to inhibit the Na+-Ca2+ exchanger, so that it could not rapidly extrude Ca2+ and thereby mask the detection of Ca2+ release from the sarcoplasmic reticulum.
Addition of volatile anaesthetics
Halothane (a gift from ICI Pharmaceuticals, Auckland, New Zealand) and isoflurane (a gift from Abbott, Wellington, New Zealand) were added to solutions by passing the 95 % O2- 5 % CO2 gas mixture through agent-specific vaporizers (at a constant flow rate of 1.3 l min−1) and bubbling part of the outflow into the solution via a fine-porosity gas distribution tube. Solutions were allowed at least 10 min to equilibrate at each partial pressure of anaesthetic. An anaesthetic vapour analyser (Datex Ai/3, Helsinki, Finland) was used to calibrate the output of the vaporizers. In the present study volatile anaesthetic doses are expressed as volume per cent (vol. %). The partial pressure of the vaporized anaesthetic can be calculated by multiplying (vol. %/100) by ambient pressure. Concentrations of volatile anaesthetic are commonly expressed as multiples of the anaesthetic potency index MAC (minimal alveolar concentration), where 1 MAC is defined as the minimal alveolar (gas) concentration at one atmosphere ambient pressure required to prevent movement in response to a noxious stimulus in 50 % of animals (Eger, Saidman & Brandstater, 1965). The MAC values for the adult rat (at around 37°C body temperature) are 1.03 and 1.46 vol. % for halothane and isoflurane, respectively (Mazze, Rice & Baden, 1985).
Force measurement
Force was measured using a silicon strain gauge (model AE801, SensoNor, Horten, Norway). A short length of 250 μm-diameter stainless-steel wire was glued to the silicon beam of the transducer using a sparing amount of SupaGlue. This wire extended 10 mm beyond the tip of the beam before making a 90 deg turn and terminating in a configuration designed to hold the free wall end of the trabecula. The resonant frequency of the transducer in air was ∼0.6 kHz and the peak-to-peak noise was less than 0.1 mg (note, 0.1 mg is equivalent to ∼1 μN).
Force and fluo-3 fluorescence signals were each sampled at up to 1 kHz by a Macintosh computer via an analog-to-digital converter (NB-MIO-16, National Instruments Corporation, Austin, TX, USA) and software custom-written in LabVIEW® (Version 3.1, National Instruments Corporation). The force and fluorescence signals were also recorded on a chart recorder. All force and fluorescence signals depicted in the figures are unfiltered and are not averaged. At the end of experiments, trabeculae were fixed and embedded in resin. The cross-sectional areas of trabeculae could be readily measured after sectioning the trabecula-containing resin blocks and are, where appropriate, presented in figure legends.
Loading of trabeculae with fluorescent Ca2+ indicators
Fura-2 or fluo-3 was loaded into trabeculae using its acetoxymethyl (AM) ester form. The respective AM ester form was initially solubilized in freshly prepared 10 % (w/v) Pluronic F127 in anhydrous dimethyl sulphoxide (DMSO; Aldrich). It was then added to Krebs-Henseleit solution which additionally contained 0.25 % bovine serum albumin, 1 mM CaCl2 and two or three drops of antifoam A (Sigma), added to suppress foaming during bubbling. The final incubation medium contained ∼10-12.5 μM fura-2 AM or ∼10 μM fluo-3 AM and less than 0.4 % DMSO. Trabeculae were superfused with fura-2 AM for 1-2 h so that, with an excitation wavelength of 360 nm, the fluorescence signal increased to 3-5 times the autofluorescence level. Trabeculae were readily loaded with fluo-3 after only 20-24 min exposure to the AM ester form, by which time the fluorescence signal had increased to ∼2-3 times the autofluorescence level. During loading trabeculae were stimulated at a rate of 0.1 Hz.
It should be noted that earlier attempts to load a fluorescein-derived Ca2+ indicator (fluo-3 or Calcium Green-1) were thwarted by its excessive rate of leakage from the muscle, initially deduced by noting a monotonic rise in background Calcium Green-1 fluorescence. To overcome this problem, the organic anion transport blocker probenecid (1 mM) was employed (for elaboration, see Di Virgilio, Steinberg & Silverstein, 1990) to reduce the rate of extrusion of the de-esterified (salt) form of fluo-3. Probenecid (0.35-2.63 mM) has previously been reported to produce a dose-dependent negative inotropic effect in rat atrial muscle (Erttmann, 1978). In contrast, when we studied the effect of 1 mM probenecid on peak twitch force in ventricular trabeculae we found that it was increased by 26 ± 4.9 % (n = 8). The mechanism underlying this positive inotropy is unclear.
Fluorescence measurement
Fura-2 fluorescence
A spectrophotometric system (Cairn Research, Faversham, Kent, UK) was attached to the inverted microscope to allow rapidly alternating dual-excitation and ratiometric measurements of fura-2 fluorescence. Excitation ultraviolet (UV) wavelengths of light were selected from the output of a 75 W xenon arc lamp by optical interference (bandpass) filters centred at 340, 360 and 380 nm (bandwidth, 12.5 nm). These filters were circumferentially arranged in a rotating wheel (operated at 40-120 Hz) which contained six filters: 3 × 340 nm, 2 × 380 nm and 1 × 360 nm. The UV excitation light was focused onto the trabecula by a Nikon CF Fluor × 20 objective lens (numerical aperture, 0.75) after transit via a liquid light guide and reflection by a dichroic mirror (400DPLC, Nikon). The fluorescent light collected by the objective lens was transmitted to the side-port of the microscope. A 600 nm dichroic mirror directed this light to the photomultiplier tube (PMT) via a 480 nm long-pass filter. Hence, the PMT was exposed to a wide window (480-600 nm) of the fura-2 emittance spectrum. In order to improve the signal-to-noise ratio of the ratiometric measurements, the signals of the 3 × 340 nm filters were averaged before division by the average signal of the 2 × 380 nm filters. The resultant fluorescence ratio (340/380) was used as a measure of intracellular Ca2+ concentration. In preliminary in vitro experiments we found that the relation between the fura-2 fluorescence ratio (340/380) and pCa was unaffected by either 2 % halothane or 4 % isoflurane.
Fluo-3 fluorescence
Fluo-3 was excited with visible light at a wavelength of 485 ± 11 nm and its fluoresence was measured at 530 ± 15 nm using an Omega Optical (Brattleboro, VT, USA) XF22 filter set. After subtraction of autofluorescence, the fluo-3 fluorescence signal (F) was normalized with respect to the resting fluorescence intensity (Fo) and expressed as F/Fo (see Fig. 1B). It is worth noting that, unlike fura-2, there is no significant spectral shift upon the binding of Ca2+ to fluo-3 and therefore this indicator is non-ratioable when used alone. Since motion artifacts may distort fluo-3 fluorescence signals, care was taken to mount the trabecula so that lateral movement was minimized.
Relation of fluorescence ratios to [Ca2+]i
In this study, indices of [Ca2+]i are presented as fluorescence ratios (340/380 or F/Fo) since the problems associated with chemical loading (using the AM ester forms), as discussed by Backx & ter Keurs (1993), may render calibration unreliable. Although the accurate quantification of [Ca2+]i would have been preferable, it is not a requisite for the interpretation of the present results.
Autofluoresence changes
The major contributors to mammalian cellular autofluorescence are the reduced nicotinamide adenine nucleotides (NAD(P)H; NADH > NADPH) and oxidized flavoproteins (Vuorinen et al. 1995). Like fura-2, NAD(P)H is excited by UV light (excitation maximum, ∼340 nm) and its broad emittance spectrum (emission maximum, ∼460 nm) considerably overlaps that of fura-2 (Stephenson et al. 1995). In the present study, we have made the reasonable assumption that an increase in the intensity of autofluorescence detected at 480-600 nm, while exciting the trabecula with 340 or 380 nm UV light, reflects an increase in mitochondrial [NAD(P)H] secondary to a rise of the NAD(P)H/NAD(P)+ ratio. Furthermore, since the spectral properties of the oxidized form of flavoproteins (excitation and emission maxima are 450 and 515 nm, respectively) are similar to those of the fluorescein-derivative fluo-3 (excitation and emission maxima are 506 and 526 nm, respectivley), we have assumed that the intensity of autofluorescence elicited by exciting the muscle with ∼485 nm while measuring emission at ∼530 nm reflects the redox state of flavoproteins.
Statistical analyses
Pooled data are expressed as means ±s.e.m. unless stated otherwise. In all results, n denotes the number of preparations from which the data were obtained. A total of twenty-two trabeculae were used for these experiments. An analysis-of-variance for repeated measures was applied where appropriate and statistical significance was determined at the 0.95 level of confidence.
RESULTS
Effects of halothane and isoflurane on intracellular Ca2+ transients and twitch force
The effects of halothane (0.25-3 %) and isoflurane (0.48-4 %) on normalized fluo-3 fluorescence signals (F/Fo) and peak twitch force are shown in Fig. 2. It can be clearly seen that halothane and isoflurane each produced a dose-dependent decrease in the amplitude of the intracellular Ca2+ transient (Fig. 2A) and twitch force (Fig. 2B), the former (halothane) exhibiting greater potency. The concentrations of halothane and isoflurane which produced half-maximal inhibition of force (IC50) were approximately 0.8 and 3 %, respectively. Note, for comparison, that the MAC (minimum alveolar (gas) concentration) values for halothane and isoflurane are 1.03 and 1.46 vol. %, respectively in the adult rat (Mazze et al. 1985). It should be noted that during this series of experiments there was a gradual decrease in the control twitch force over the 2 h or so during which a trabecula was alternately exposed to control solution and an anaesthetic dose, such that the control twitch force was reduced to 78 ± 9.3 % (n = 4) of the initial value by the end of the series. There was also a parallel decrease in the control normalized fluo-3 fluoresence signals during this series of experiments (indicated by the dotted lines in Fig. 2A). This may have been due partly to a gradual loss of fluo-3 from the cytosol, a likely possibility given that probenecid is generally reckoned to be a competitive inhibitor of the organic anion transport mechanism. Attenuation of fluo-3 fluorescence by photobleaching was not evident, even when a preparation was illuminated with excitation light continuously for up to 3 min.
Figure 2. Effects of halothane and isoflurane on intracellular Ca2+ transients and twitch force.
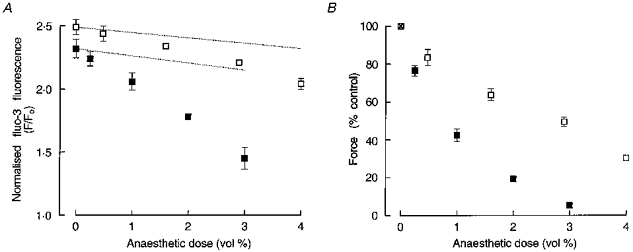
A, mean (±s.e.m., n = 4) effects of halothane (▪) and isoflurane (□) on the amplitude of normalized fluo-3 fluorescence (F/Fo) transients (a measure of intracellular [Ca2+]). In all four trabeculae, cumulative doses of isoflurane (0.48, 1.6, 2.9 and 4 %) were presented before those of halothane (0.25, 1, 2 and 3 %). The upper and lower dotted lines indicate the decrease in the amplitude of control F/Fo signals observed during the isoflurane and halothane dose-response series, respectively. B, effect of halothane (▪) and isoflurane (□) on peak twitch force (means ±s.e.m., n = 4). Values at each anaesthetic dose are expressed with respect to force produced in the immediately preceding control (anaesthetic-free) condition. Where no error bars are given, s.e.m. was smaller than the symbol.
Ability of volatile anaesthetics to induce sarcoplasmic reticular Ca2+ release
When halothane, at doses above 1 %, was introduced to fluo-3-loaded trabeculae, a small and transient increase in the amplitude of both the intracellular Ca2+ transient and twitch force preceded the subsequently maintained decreases. The transient increase in intracellular Ca2+ signals was not readily detectable in fura-2-loaded trabeculae (n = 4), possibly due to the greater twitch-to-twitch variability in the peak amplitude of the signals. In contrast to halothane, when isoflurane (up to 4 %) was introduced to fluo-3-loaded trabeculae, the decrease in intracellular Ca2+ transients and twitch force which ensued was monophasic. The simplest explanation for this difference in response is that halothane readily augments Ca2+ release from the SR whereas isoflurane does not (see Introduction). This interpretation is supported by the observation that caffeine (1-2 mM), an agent well known to induce Ca2+ release from the sarcoplasmic reticulum, produces a similar biphasic response to that evoked by halothane (Katsuoka & Ohnishi, 1989; P. J. Hanley, unpublished observation).
The ability of halothane or isoflurane to induce Ca2+ release directly from intracellular stores in quiescent trabeculae was tested. As a first step, trans-sarcolemmal Ca2+ influx and Na+-Ca2+ exchange were prevented by superfusing the unstimulated preparation with a Na+- and Ca2+-free (1 mM EGTA) medium (see Methods). As can be seen in Fig. 3, the baseline of the normalized fluo-3 fluorescence signal declined between switching off electrical stimulation (indicated by the first arrow) and introducing the anaesthetic (second arrow). This decrease in resting [Ca2+]i may be attributable to an increased turnover rate of the sarcolemmal Ca2+ATPase under the Ca2+-free conditions that prevailed. When the trabecula was subsequently challenged with 2 % halothane in the absence of external Ca2+ and Na+, a small and transient increase in [Ca2+]i was observed which was unaccompanied by contracture. In order to determine whether isoflurane similarly released Ca2+ from the SR, this experimental protocol was repeated in the same preparation (data not shown). In contrast to 2 % halothane, the introduction of 4 % isoflurane did not increase [Ca2+]i. Similar results were obtained in three other trabeculae using comparable protocols. On average, the peak of the Ca2+ transient evoked by application of 2 % halothane under Na+- and Ca2+-free conditions was 20 ± 5 % (n = 4) of the amplitude of Ca2+ transients elicited by electrical stimulation under control conditions.
Figure 3. Intracellular Ca2+ transient evoked by the application of halothane under Ca2+- and Na+-free conditions.
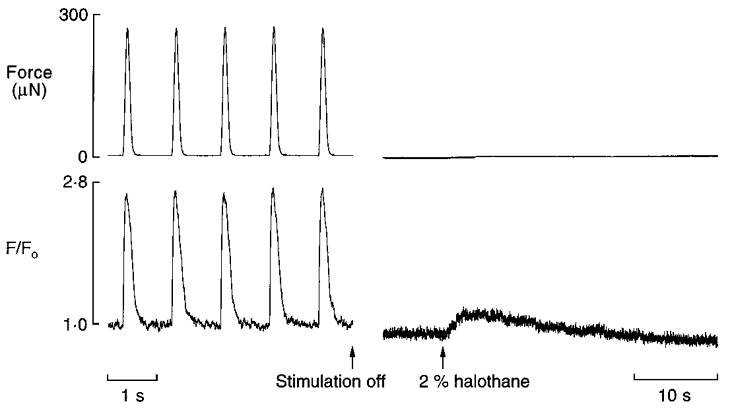
After electrical stimulation was switched off (indicated by the first arrow), the trabecula was superfused with Ca2+-free solution for 15 s followed by Ca2+- and Na+-free solution for a further 50 s. Subsequently, the preparation was exposed to 2 % halothane (indicated by the second arrow). Note that the baseline intracellular [Ca2+] had fallen between switching off electrical stimulation and introducing 2 % halothane. These recordings were taken more than 2.5 h after fluo-3 loading. Trabecula dimensions: length, 2.7 mm; cross-sectional area, 0.0078 mm2.
Effects of halothane and isoflurane on cellular autofluorescence
NAD(P)H autofluorescence
Figure 4 illustrates the importance of correcting for anaesthetic-induced changes in autofluorescence when using the Ca2+ indicator fura-2. In this particular example, the increase in diastolic 340/380 ratio evoked by 4 % isoflurane or 2 % halothane was inferred to be due to an increase in the NAD(P)H/NAD(P)+ ratio rather than an increase in the ratio of Ca2+-bound fura-2 to Ca2+-free fura-2. In four trabeculae we found that halothane and isoflurane produced dose-dependent and reversible increases in NAD(P)H autofluorescence. Moreover, when fura-2 fluorescence signals were corrected for volatile anaesthetic-induced changes in NAD(P)H autofluorescence (determined before dye loading) no increase in diastolic 340/380 ratio remained.
Figure 4. Effects of halothane and isoflurane on force and the fura-2 fluorescence ratio.
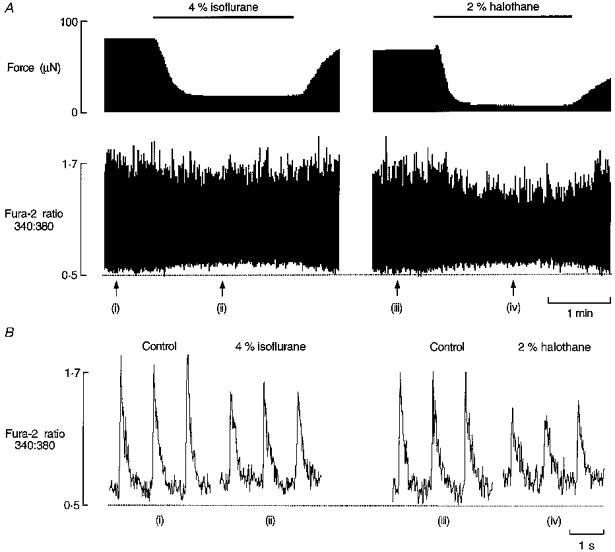
A, continuous records of force and the fura-2 fluorescence ratio (a measure of intracellular [Ca2+]), simultaneously measured in a trabecula electrically stimulated at a rate of 1 Hz. The bar indicates exposure to anaesthetic and the dotted line has been arbitrarily positioned to serve as a reference. Around 1.5 h elapsed between exposing the preparation to 4 % isoflurane and to 2 % halothane. B, consecutive fura-2 signals recorded at the points indicated by the arrows in panel A. Trabecula dimensions: length, 2.7 mm; cross-sectional area, 0.004 mm2.
Recently, Brandes & Bers (1996) reported that an increase in NAD(P)H autofluorescence was observed when the work of rat cardiac trabeculae was decreased. In the present study, two lines of experimental evidence suggest that the increase in NAD(P)H autofluorescence produced by volatile anaesthetics is not simply secondary to the concomitant decrease in twitch force (and presumably overall cellular ATPase activity): (i) in four trabeculae, we found that when twitch force was reduced to levels comparable to that produced by 2 % halothane or 4 % isoflurane by decreasing the external [Ca2+], there was no change in the diastolic 340/380 ratio and (ii) the introduction of 2 % halothane to a quiescent trabecula produced an increase in NAD(P)H autofluorescence. It should be borne in mind that, typical of cardiac muscle, the so-called quiescent trabecula has a high rate of energy expenditure, amounting to over 20 % of the rate observed in an active preparation (Daut & Elzinga, 1988).
Flavoprotein autofluorescence
We observed that halothane and isoflurane consistently caused dose-dependent and reversible decreases in flavoprotein autofluorescence, elicited by illuminating the trabecula with light at ∼485 nm while detecting fluorescence emission at ∼530 nm. Figure 5 illustrates the importance of accounting for autofluorescence changes when interpreting the effects of volatile anaesthetics on intracellular [Ca2+] measured using the Ca2+ indicator fluo-3. In Fig. 5 the effects of 4 % isoflurane on twitch force and flavoprotein autofluorescence were recorded in an electrically stimulated trabecula before loading with fluo-3. Note that a small increase in fluorescence is associated with internal shortening during muscle contraction. After loading, the isoflurane challenge was repeated and, as can be seen in this example, the apparent anaesthetic-induced reduction in diastolic [Ca2+]i could be almost completely accounted for by the decrease in flavoprotein autofluorescence. Furthermore, as in the case of NAD(P)H autofluorescence, we were able to show that this reduction in autofluorescence was peculiar to an action of the anaesthetic rather than a consequence of the concomitant decrease in force. Figure 6B shows that reduction of twitch force by lowering the external [Ca2+] had virtually no effect on flavoprotein autofluorescence whereas 4 % isoflurane decreased autofluorescence (Fig. 6A). Similar results were obtained in two other trabeculae.
Figure 5. Effect of isoflurane on flavoprotein autofluorescence.
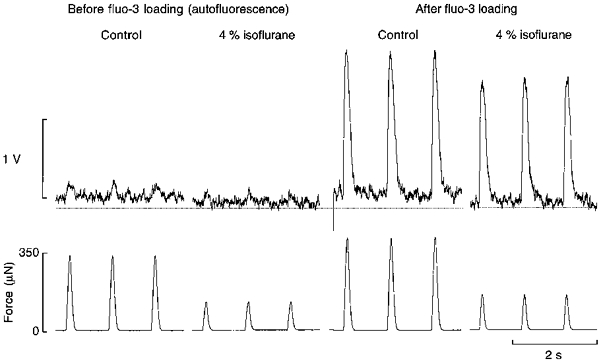
Flavoprotein autofluorescence and force simultaneously measured in a trabecula of length 3 mm and cross-sectional area 0.012 mm2. After the trabecula had been loaded with fluo-3, isoflurane (4 %) was reintroduced. The dotted line has been arbitrarily located as a reference. Note the comparable decrement of diastolic fluorescence in the presence of isoflurane before and after loading with the fluorescent Ca2+ indicator fluo-3.
Figure 6. Comparison of the effects of reduced [Ca2+]o and isoflurane on flavoprotein autofluorescence.
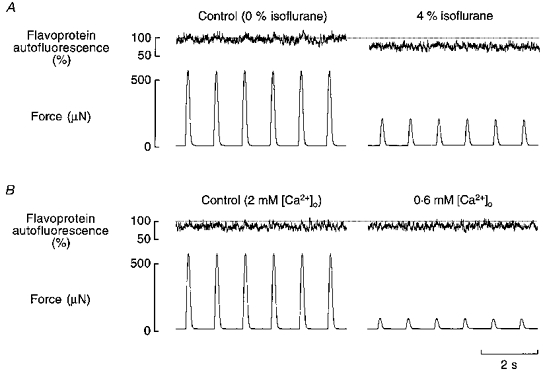
The effects of 4 % isoflurane (A) and of reduced [Ca2+]o (B) on flavoprotein autofluorescence and force in the same trabecula. Note the reduction of control autofluorescence between exposing the preparation to 4 % isoflurane and lowering the [Ca2+] to 0.6 mM. In B, an external [Ca2+] of 0.6 mM was chosen so that the reduction in force would be at least as great as that produced by 4 % isoflurane. Trabecula dimensions: length, 2.8 mm; cross-sectional area, 0.016 mm2.
Dependence of anaesthetic-induced changes in autofluorescence on metabolic substrate
Taken together, the reciprocal effects of volatile anaesthetics on the intensity of autofluorescence attributable to NAD(P)H and flavoproteins support the notion that volatile anaesthetics inhibit the electron transport chain at the site of Complex I. In order to bypass this block in the respiratory chain and to restore oxidative phosphorylation, we supplied a fatty acid as a metabolic substrate. Figure 7A and B shows representative records of the effects of volatile anaesthetics, in this case 2 % halothane, on flavoprotein autofluorescence in the absence (Fig. 7A) and presence (Fig. 7B) of the fatty acid hexanoate (1 mM). Figure 7A shows that when glucose was the sole metabolic substrate, the introduction of 2 % halothane caused a decrease in autofluorescence intensity. It can also be seen in Fig. 7A that when hexanoate was provided as a metabolic substrate there was a decrease of both steady-state twitch force (as has been observed in the isolated, perfused rat heart; Hassinen, Ito, Nioka & Chance, 1990) and flavoprotein autofluorescence. It is worth noting that fatty acids are preferentially utilized as metabolic substrate by the heart in vivo. Interestingly, the reintroduction of 2 % halothane had little further effect on the redox state of flavoproteins while 1 mM hexanoate was present. In the same preparation, we also examined the effects of 2 % halothane on NAD(P)H autofluorescence and, additionally, the effects of 4 % isoflurane on both flavoprotein and NAD(P)H autofluorescence. In each case, the anaesthetic-induced changes in autofluorescence were greatly reduced when hexanoate was present as a metabolic substrate. Similar results were obtained in three other trabeculae.
Figure 7. The effect of halothane on flavoprotein autofluorescence in the absence and presence of the fatty acid hexanoate.
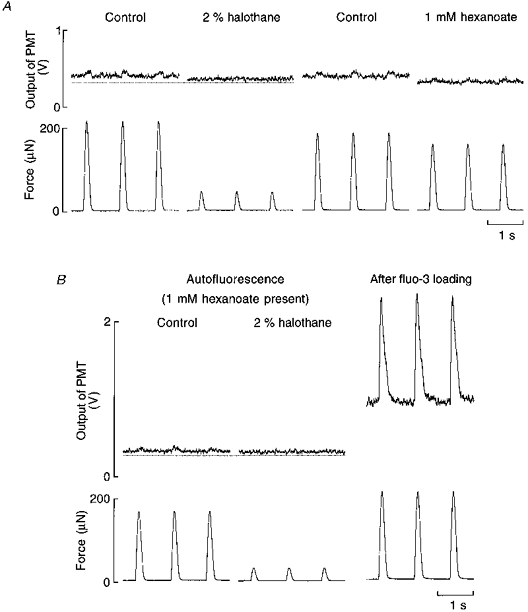
A, typical records showing the effects of 2 % halothane and 1 mM hexanoate on flavoprotein autofluorescence, scaled as voltage output of the photomultiplier tube (PMT), and force. The dotted line has been arbitrarily located for reference. B, effect of 2 % halothane on flavoprotein autofluorescence and force in the presence of hexanoate. Note that the halothane-induced decrease in autofluorescence is greatly diminished when hexanoate is present. The records on the right show the typical effects of fluo-3 loading on baseline fluorescence intensity. Trabecula dimensions: length, 2.4 mm; cross-sectional area, 0.006 mm2.
Direct effects of volatile anaesthetics on the contractile system
As a first step in investigating the effects of halothane and isoflurane on the responsiveness of the contractile system to Ca2+, we tested whether force would recover concomitantly with restoration of the amplitude of the Ca2+ transient by elevation of the external [Ca2+] during exposure to the anaesthetic. Hexanoate was supplied as a metabolic substrate in order to minimize anaesthetic-induced autofluorescence changes. As can be seen in Fig. 8, elevation of the external [Ca2+] from 2 to 4 mM while the trabecula was exposed to 4 % isoflurane (Fig. 8A) or 2 % halothane (Fig. 8B) restored the peak of the fluo-3 fluorescence transient (relative to control conditions) but did not fully restore force. At these anaesthetic doses, we were unable to restore force completely by further raising the [Ca2+]o and attempts to do so typically induced excitation-contraction coupling failure. Similar results were obtained in two further experiments. The inability of restored Ca2+ transients to restore force in the presence of 4 % isoflurane or 2 % halothane is consistent with the interpretation that these anaesthetics reduce the responsiveness of the contractile system to Ca2+ in intact cardiac muscle.
Figure 8. Effect of restoring the amplitude of the intracellular Ca2+ transient on force in the presence of halothane or isoflurane.
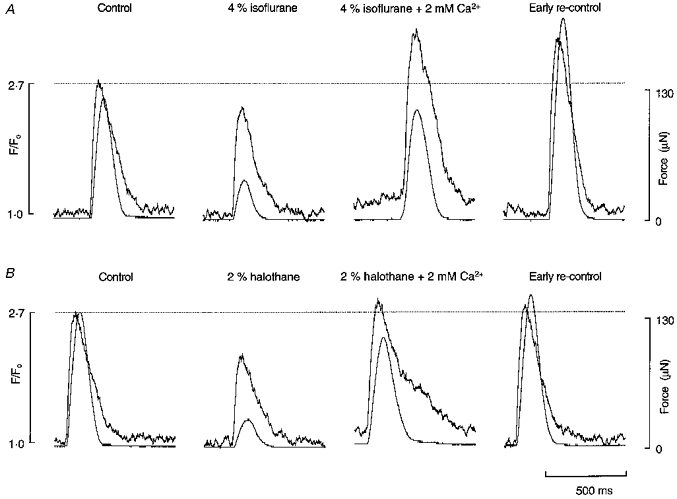
While the fluo-3-loaded trabecula was exposed to 4 % isoflurane (A) or 2 % halothane (B) the external [Ca2+] was increased from 2 to 4 mM in order to restore the amplitude of the intracellular Ca2+ transient to above the level observed under control conditions (indicated by the dotted line). Same preparation as in Fig. 7.
A number of mechanisms can be invoked to explain the reduced responsiveness of the contractile proteins to Ca2+ in the presence of a volatile anaesthetic. We tested whether a reduction in maximal Ca2+-activated force contributed to the reduced response of the contractile system to Ca2+. Maximal activation of the contractile system was achieved by tetanizing trabeculae (elicited by brief bursts of 12 Hz stimulation) in the presence of 5 μM ryanodine and high external Ca2+ concentrations (> 10 mM). We found that equivalent elevations of fluo-3 fluorescence (i.e. activator Ca2+) were difficult to attain in the presence of 2 % halothane or 4 % isoflurane, even when tetani were elicited with an external [Ca2+] of 30 mM. Thenceforward, we used lower doses of these anaesthetics which would be expected to impose less resistance to trans-sarcolemmal Ca2+ influx. Attainment of maximal Ca2+-activated force was assumed when force reached a plateau while the [Ca2+]i continued to rise. In order to reduce the likelihood of elevations in the concentrations of intracellular H+ and Pi (inorganic phosphate), which have inhibitory actions on Ca2+-regulated force development, the duration of tetani was kept to less than 6 s. Figure 9A shows the temporal relation between Ca2+ and force during a typical tetanus; note that the fine ripples on the force plateau occur at the same frequency as the rate of stimulation, i.e. 12 Hz. On average, when 1 % halothane or 1.6 % isoflurane were present, maximal Ca2+-activated force was reduced by 15 ± 4.6 % and 15 ± 3.3 %, respectively (Fig. 9B; n = 3).
Figure 9. Effects of halothane and isoflurane on maximal Ca2+-activated force.
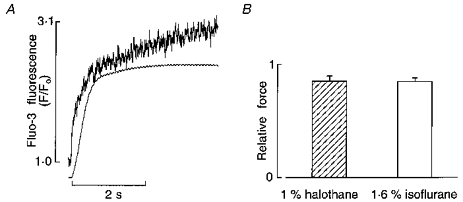
A, temporal relation between intracellular [Ca2+] and force during a typical tetanus elicited by stimulating at a rate of 12 Hz in the presence of 5 μM ryanodine and 20 mM external [Ca2+]. B, summary of effects of 1 % halothane and 1.6 % isoflurane on maximal Ca2+-activated force.
DISCUSSION
We have shed light on the mechanisms by which halothane and isoflurane decrease force in intact cardiac muscle using rat ventricular trabeculae loaded with a fluorescent Ca2+ indicator. Care was taken to characterize the effects of halothane and isoflurane on cellular autofluorescence intensity (and, indirectly, mitochondrial function) in order to avoid misinterpretation of the fluorescent Ca2+ indicator signals.
Autofluorescence changes and mitochondrial function
Halothane has previously been deduced to inhibit the electron transport chain at the level of Complex I (see Introduction). Consistent with this putative action, Kissin et al. (1983) showed that halothane and other volatile anaesthetics (e.g. isoflurane and diethyl ether) reversibly increased NAD(P)H autofluorescence in isolated, perfused rat hearts. Inhibition of the electron transport chain at the site of Complex I would be expected to produce a reciprocal change in flavoprotein autofluorescence. For example, in the isolated perfused rat liver, rotenone (an archetypical inhibitor of Complex I) has been shown to evoke an increase in NAD(P)H autofluorescence while simultaneously decreasing flavoprotein autofluorescence (Scholz, Thurman, Williamson, Chance & Bücher, 1969). Accordingly, our observation that halothane and isoflurane decrease flavoprotein autofluorescence (Figs 5–7), while concomitantly increasing NAD(P)H autofluorescence, is consistent with the interpretation that volatile anaesthetics effect inhibition of the electron transport chain at the site of Complex I.
The ability of volatile anaesthetics to inhibit the electron transport chain and possibly to reduce the rate of ATP synthesis has been invoked as a mechanism to explain, at least in part, the negative inotropic effect of these agents (Berman et al. 1974). However, halothane has been shown not to produce a change in steady-state concentrations of high-energy phosphates in 31P NMR studies employing either Langendorff-perfused rat (Dedrick & Allen, 1983) or rabbit (Murray, Blanck, Rogers & Jacobus, 1987) hearts. In the latter study, the authors reported that intracellular pH was unchanged by 1.5 % halothane, suggesting that the negative inotropic action of this agent is not mediated by intracellular lactic acidosis secondary to its inhibitory effects on oxidative phosphorylation. In the present study, we provide indirect evidence that the negative inotropic effect of volatile anaesthetics is not due to a reduction in energy supply. When we supplied a fatty acid (hexanoate) in order to restore oxidative phosphorylation in the face of inhibition of Complex I by halothane or isoflurane, the negative inotropic effect of these agents was unchanged. However, the anaesthetic-induced changes in NAD(P)H and flavoprotein autofluorescence were greatly reduced (Fig. 7). The most likely explanation for this observation is that, whereas Complex I has a major role in the oxidation of carbohydrates, such as pyruvate, its function is much less important in the oxidation of fatty acids.
Intracellular Ca2+ availability
Our results clearly show that the inhibition of force produced by halothane or isoflurane in rat ventricular trabeculae is associated with a dose-dependent decrease in the amplitude of the intracellular Ca2+ transient (Fig. 2). It is worth noting that the reduction in the amplitude of the fluo-3 fluorescence transient produced by the anaesthetics may be exaggerated due to a concomitant reduction of internal movement. However, equivalent results were obtained in four fura-2-loaded trabeculae which were excited with rapidly alternating 340 and 380 nm wavelengths of excitation light.
Volatile anaesthetics have similarly been shown to decrease peak twitch [Ca2+]i in guinea-pig papillary muscles which have been microinjected with aequorin (Bosnjak, Aggarwal, Turner, Kampine & Kampine, 1992). One of the limitations of aequorin, however, is that it is not very sensitive to low Ca2+ concentrations (Lee, Westerblad & Allen, 1991) and, accordingly, Bosnjak et al. (1992) were unable to determine the effects of volatile anaesthetics on diastolic [Ca2+]i. Another potential problem with using aequorin as a tool for studying the effects of anaesthetics on Ca2+ handling is that the light-emitting properties of aequorin may be altered by direct interaction of the anaesthetic with this photoprotein (Baker & Schapira, 1980). The use of fura-2 or fluo-3 to measure Ca2+ has a number of advantages over aequorin such as the option of chemical loading (using the AM ester form of the indicator) and the ability to measure the relatively low concentrations of Ca2+ associated with the resting myocyte. Our study of trabeculae loaded with either fura-2 or fluo-3 shows that, provided due account is taken of anaesthetic-induced changes in autofluorescence, neither halothane nor isoflurane produces significant changes of diastolic [Ca2+]i.
One of the principal mechanisms by which volatile anaesthetics reduce the intracellular Ca2+ transient is probably via inhibition of L-type Ca2+ channel current (Pancrazio, 1996). Our observation that halothane and isoflurane, particularly at high doses, decreased the magnitude of the rise in [Ca2+]i during tetani, when 5 μM ryanodine was present to suppress sarcoplasmic reticular function, is consistent with the interpretation that these agents are inhibitors of Ca2+ influx via voltage-activated Ca2+ channels.
Ca2+ release from the sarcoplasmic reticulum
Inhibition of L-type Ca2+ channel current by halothane and isoflurane would be expected to lead to a progressive twitch-to-twitch reduction of the calcium content of the sarcoplasmic reticulum. However, volatile anaesthetics have also been reported to promote Ca2+ loss from the sarcoplasmic reticulum via direct actions on this organelle. In fact, an abundance of evidence exists to show that halothane, in clinically relevant doses, is capable of inducing Ca2+ release from the cardiac sarcoplasmic reticulum (Herland et al. 1990; Connelly & Coronado, 1994). On the other hand, controversy exists as to whether isoflurane is likewise a potent stimulator of sarcoplasmic reticular Ca2+ release (Connelly & Coronado, 1994; Wheeler et al. 1994). At least at high doses, isoflurane has been reported to evoke a prominent Ca2+ transient, followed by a sustained increase in diastolic [Ca2+]i, when applied to a suspension of resting rat cardiac myocytes (Katsuoka et al. 1989).
In the present study we provide two lines of evidence to suggest that isoflurane, unlike halothane, does not readily induce Ca2+ release from the SR. First, the application of halothane, but not isoflurane, to electrically stimulated trabeculae caused a transient increase in Ca2+ transients and force before the negative inotropy was manifest. Note that the transient augmention of Ca2+ transients and force is presumed to be secondary to facilitated Ca2+ release from the sarcoplasmic reticulum by halothane (Robinson, Harrison, Winlow, Hopkins & Boyett, 1993; Wheeler, Rice, duBell & Spurgeon, 1997). Second, when halothane, but not isoflurane, was introduced to quiescent trabeculae in which trans-sarcolemmal Ca2+ influx and Na+- Ca2+ exchange were inhibited, a transient increase in [Ca2+]i was observed (Fig. 3). We infer that the source of Ca2+ for the rise in [Ca2+]i under these circumstances is the sarcoplasmic reticulum. Hence, we conclude that halothane, but not isoflurane, is capable of readily inducing Ca2+ release from the sarcoplasmic reticulum. We cannot, of course, rule out the possibility that isoflurane may induce a slow, and therefore not readily detectable, release of Ca2+ from the sarcoplasmic reticulum.
Response of the contractile system to Ca2+
In addition to reducing cytosolic Ca2+ delivery, volatile anaesthetics may also alter the response of the contractile system to a given amount of Ca2+. One approach to examine the effects of volatile anaesthetics on the responsiveness of the contractile system to Ca2+ is to use skinned ventricular muscle preparations so that the composition of the intracellular environment can be controlled. However, studies using skinned preparations have yielded conflicting results regarding the effects of halothane and isoflurane on the force-[Ca2+]i relation. Although Herland et al. (1993) used high doses of anaesthetic, these authors were able partially to reconcile these conflicting reports by accounting for the different effects of various skinning techniques on the subsequent behaviour of the contractile system. For example, whereas halothane and isoflurane shifted the force-[Ca2+]i relation to lower Ca2+ concentrations in mechanically skinned rat ventricular muscle, further disruption of the membrane by treatment with a detergent (saponin or Triton X-100) produced the opposite shift (decreased Ca2+ sensitization). Hence, the effects of volatile anaesthetics on the force-[Ca2+]i relation depend on the extent to which the native regulatory system of the contractile machinery has been disturbed by the particular skinning procedure employed.
There have been few studies of the effects of volatile anaesthetics on the responsiveness of the contractile system to Ca2+ in intact cardiac muscle. In work with aequorin-loaded guinea-pig papillary muscle Bosnjak et al. (1992) reported that 1.6 % isoflurane shifted the relation between peak Ca2+ and force to higher Ca2+ concentrations whereas 1.2 % halothane had no effect. In contrast, we found that when the external [Ca2+] was elevated in the presence of 2 % halothane or 4 % isoflurane so that the peak of the Ca2+ transient was restored to control levels (or a little above) force was not restored (see Fig. 8). These results indicate that part of the negative inotropic effect of both isoflurane and halothane in intact rat ventricular muscle reflects reduced responsiveness of the contractile system to Ca2+.
We examined the effects of a single dose of halothane and isoflurane on maximal Ca2+-activated force using ryanodine tetani in the presence of high external Ca2+ concentrations (> 10 mM). Halothane (1 %) and isoflurane (1.6 %) each reduced maximal Ca2+-activated force by about 15 %. These findings accord with previous results obtained using skinned rat ventricular muscle preparations (Murat et al. 1988). In further work with chemically skinned rat cardiac muscle, Murat, Lechene & Ventura-Clapier (1990) examined the effects of volatile anaesthetics on force and stiffness during rapid length perturbations at controlled levels of contractile activation. Halothane, enflurane and isoflurane each: (i) decreased active stiffness, (ii) increased the stiffness/force ratio and (iii) increased the time constant of force recovery. These findings indicate that these volatile anaesthetics decrease: (i) the number of force-generating cross-bridges, (ii) the force generated per cross-bridge and (iii) the rate of actomyosin ATPase activity. The mechanisms by which volatile anaesthetics alter the number and kinetics of myosin cross-bridge attachments to actin may involve a direct interaction with the contractile proteins. This notion is supported by the observations that volatile anaesthetics have been demonstrated to interact directly with and to change the properties of various proteins (Baker & Schapira, 1980).
Conclusions
Halothane and isoflurane reversibly increase NAD(P)H autofluorescence while simultaneously decreasing flavoprotein autofluorescence, consistent with an inhibitory effect of these volatile anaesthetics on the electron transport chain at the site of Complex I. When a fatty acid is supplied as metabolic substrate in order to bypass this block in the electron transport chain, the effects of volatile anaesthetics on autofluorescence are greatly reduced whereas the negative inotropy persists. The combined use of hexanoate and a fluorescent Ca2+ indicator excited by visible wavelengths of light (fluo-3) minimizes anaesthetic-induced changes of autofluorescence. Halothane and isoflurane inhibit force development of intact cardiac muscle by reducing intracellular Ca2+ availability and concomitantly reducing the responsiveness of the contractile proteins to Ca2+. Furthermore, halothane, but not isoflurane, readily promotes the release of Ca2+ from the sarcoplasmic reticulum, an action which would lead to a reduction of its Ca2+ content.
Acknowledgments
This work was supported by grants from the Health Research Council of New Zealand, the New Zealand Lotteries Grant Board (Health Research) and the Auckland Medical Research Foundation. The authors are grateful to Stuart Glasson for writing software and to Phil Lacey for electronic workshop support. We thank Professors Britton Chance and Henk E. D. J. ter Keurs for helpful advice. P. J. Hanley was the recipient of a Health Research Council of New Zealand Post-graduate Scholarship.
References
- Backx PH, Ter Keurs HEDJ. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. American Journal of Physiology. 1993;264:H1098–1110. doi: 10.1152/ajpheart.1993.264.4.H1098. [DOI] [PubMed] [Google Scholar]
- Baker PF, Schapira AHV. Anaesthetics increase light emission from aequorin at constant ionised calcium. Nature. 1980;284:168–169. doi: 10.1038/284168a0. [DOI] [PubMed] [Google Scholar]
- Berman MC, Kewley CF, Kench JE. Contribution of inhibition of NADH-dehydrogenase to the cardiotoxic effects of halothane. Journal of Molecular and Cellular Cardiology. 1974;6:39–47. doi: 10.1016/0022-2828(74)90005-4. [DOI] [PubMed] [Google Scholar]
- Biebuyck JF. Effects of anaesthetic agents on metabolic pathways: fuel utilization and supply during anaesthesia. British Journal of Anaesthesia. 1973;45:263–268. doi: 10.1093/bja/45.3.263. [DOI] [PubMed] [Google Scholar]
- Bosnjak ZJ, Aggarwal A, Turner LA, Kampine JM, Kampine JP. Differential effects of halothane, enflurane, and isoflurane on Ca2+ transients and papillary muscle tension in guinea pigs. Anesthesiology. 1992;76:123–131. doi: 10.1097/00000542-199201000-00018. [DOI] [PubMed] [Google Scholar]
- Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophysical Journal. 1996;71:1024–1035. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Connelly TJ, Coronado R. Activation of the Ca2+ release channel of cardiac sarcoplasmic reticulum by volatile anesthetics. Anesthesiology. 1994;81:459–469. doi: 10.1097/00000542-199408000-00025. [DOI] [PubMed] [Google Scholar]
- Daut J, Elzinga G. Heat production of quiescent ventricular trabeculae isolated from guinea-pig heart. Journal of Physiology. 1988;398:259–275. doi: 10.1113/jphysiol.1988.sp017041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedrick D, Allen PD. NMR studies of myocardial high energy phosphates and sodium as mediators of negative inotropic effects of volatile anesthetics. Anesthesiology. 1983;59:A23. [Google Scholar]
- de Tombe PP, Ter Keurs HEDJ. Force and velocity of sarcomere shortening in trabeculae from rat heart. Effects of temperature. Circulation Research. 1990;66:1239–1254. doi: 10.1161/01.res.66.5.1239. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Steinberg TH, Silverstein SC. Inhibition of fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- Eger EI, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology. 1965;26:756–763. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- Erttmann RR. Kinetics and inotropic action of probenecid in guinea-pig heart in vitro. Experientia. 1978;34:1620–1621. doi: 10.1007/BF02034712. [DOI] [PubMed] [Google Scholar]
- Frazer MJ, Lynch C., III Halothane and isoflurane effects on Ca2+ fluxes of isolated myocardial sarcoplasmic reticulum. Anesthesiology. 1992;77:316–323. doi: 10.1097/00000542-199208000-00015. [DOI] [PubMed] [Google Scholar]
- Hassinen I, Ito K, Nioka S, Chance B. Mechanism of fatty acid effect on myocardial oxygen consumption. A phosphorus NMR study. Biochimica et Biophysica Acta. 1990;1019:73–80. doi: 10.1016/0005-2728(90)90126-o. [DOI] [PubMed] [Google Scholar]
- Herland JS, Julian FJ, Stephenson DG. Halothane increases Ca2+ efflux via Ca2+ channels of sarcoplasmic reticulum in chemically skinned rat myocardium. Journal of Physiology. 1990;426:1–18. doi: 10.1113/jphysiol.1990.sp018124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herland JS, Julian FJ, Stephenson DG. Effects of halothane, enflurane, and isoflurane on skinned rat myocardium activated by Ca2+ American Journal of Physiology. 1993;264:H224–232. doi: 10.1152/ajpheart.1993.264.1.H224. [DOI] [PubMed] [Google Scholar]
- Katsuoka M, Kobayashi K, Ohnishi ST. Volatile anesthetics decrease calcium content of isolated myocytes. Anesthesiology. 1989;70:954–960. doi: 10.1097/00000542-198906000-00012. [DOI] [PubMed] [Google Scholar]
- Katsuoka M, Ohnishi ST. Inhalation anaesthetics decrease calcium content of cardiac sarcoplasmic reticulum. British Journal of Anaesthesia. 1989;62:669–673. doi: 10.1093/bja/62.6.669. [DOI] [PubMed] [Google Scholar]
- Kissin I, Aultman DF, Smith LR. Effects of volatile anesthetics on myocardial oxidation-reduction status assessed by NADH fluorometry. Anesthesiology. 1983;59:447–452. doi: 10.1097/00000542-198311000-00016. [DOI] [PubMed] [Google Scholar]
- Lee JA, Westerblad H, Allen DG. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. Journal of Physiology. 1991;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan G, Weisberg A, Winegrad S. Endothelin regulation of cardiac contractility in absence of added endothelin. American Journal of Physiology. 1995;268:H1621–1627. doi: 10.1152/ajpheart.1995.268.4.H1621. [DOI] [PubMed] [Google Scholar]
- Mazze RI, Rice SA, Baden JM. Halothane, isoflurane, and enflurane MAC in pregnant and nonpregnant female and male mice and rats. Anesthesiology. 1985;62:339–341. doi: 10.1097/00000542-198503000-00021. [DOI] [PubMed] [Google Scholar]
- Murat I, Lechene P, Ventura-Clapier R. Effects of volatile anesthetics on mechanical properties of rat cardiac skinned fibers. Anesthesiology. 1990;73:73–81. doi: 10.1097/00000542-199007000-00012. [DOI] [PubMed] [Google Scholar]
- Murat I, Ventura-Clapier R, Vassort G. Halothane, enflurane, and isoflurane decrease calcium sensitivity and maximal force in detergent-treated rat cardiac fibers. Anesthesiology. 1988;69:892–899. doi: 10.1097/00000542-198812000-00015. [DOI] [PubMed] [Google Scholar]
- Murray PA, Blanck TJJ, Rogers MC, Jacobus WE. Effects of halothane on myocardial high-energy phosphate metabolism and intracellular pH utilizing 31P NMR spectroscopy. Anesthesiology. 1987;67:649–653. doi: 10.1097/00000542-198711000-00006. [DOI] [PubMed] [Google Scholar]
- Pancrazio JJ. Halothane and isoflurane preferentially depress a slowly inactivating component of Ca2+ channel current in guinea-pig myocytes. Journal of Physiology. 1996;494:91–103. doi: 10.1113/jphysiol.1996.sp021478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Harrison SM, Winlow W, Hopkins PM, Boyett MR. The effect of halothane on intracellular calcium and contraction in ventricular cells isolated from rat hearts. Journal of Physiology. 1993;473:110P. [Google Scholar]
- Rusy BF, Komai H. Anesthetic depression of myocardial contractility: a review of possible mechanisms. Anesthesiology. 1987;67:745–766. doi: 10.1097/00000542-198711000-00020. [DOI] [PubMed] [Google Scholar]
- Scholz R, Thurman RG, Williamson JR, Chance B, Bücher T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. Journal of Biological Chemistry. 1969;244:2317–2324. [PubMed] [Google Scholar]
- Stephenson DG, Garzella P, Wingrove DE, Claflin DR, Julian FJ. Autofluorescence changes caused by halothane can lead to erroneous interpretation of fura-2 signals in cardiac muscle. Biophysical Journal. 1995;68:A418. [Google Scholar]
- Su JY, Bell JG. Intracellular mechanism of action of isoflurane and halothane on striated muscle of the rabbit. Anesthesia and Analgesia. 1986;65:457–462. [PubMed] [Google Scholar]
- Su JY, Kerrick WGL. Effects of halothane on Ca2+-activated tension development in mechanically disrupted rabbit myocardial fibers. Pflügers Archiv. 1978;375:111–117. doi: 10.1007/BF00584232. [DOI] [PubMed] [Google Scholar]
- Vuorinen KH, Ala-Rämi A, Yan Y, Ingman P, Hassinen IE. Respiratory control in heart muscle during fatty acid oxidation. Energy state or substrate-level regulation by Ca2+? Journal of Molecular and Cellular Cardiology. 1995;27:1581–1591. doi: 10.1016/s0022-2828(95)90458-1. [DOI] [PubMed] [Google Scholar]
- Wheeler DM, Katz A, Rice RT, Hansford RG. Volatile anesthetic effects on sarcoplasmic reticulum Ca content and sarcolemmal Ca flux in isolated rat cardiac cell suspensions. Anesthesiology. 1994;80:372–382. doi: 10.1097/00000542-199402000-00017. [DOI] [PubMed] [Google Scholar]
- Wheeler DM, Rice RT, duBell WH, Spurgeon HA. Initial contractile response of isolated rat heart cells to halothane, enflurane, and isoflurane. Anesthesiology. 1997;86:137–146. doi: 10.1097/00000542-199701000-00018. [DOI] [PubMed] [Google Scholar]
- Wheeler DM, Rice RT, Hansford RG, Lakatta EG. The effect of halothane on the free intracellular calcium concentration of isolated rat heart cells. Anesthesiology. 1988;69:578–583. doi: 10.1097/00000542-198810000-00019. [DOI] [PubMed] [Google Scholar]
- Wilde DW, Davidson BA, Smith MD, Knight PR. Effects of isoflurane and enflurane on intracellular Ca2+ mobilization in isolated cardiac myocytes. Anesthesiology. 1993;79:73–82. doi: 10.1097/00000542-199307000-00012. [DOI] [PubMed] [Google Scholar]
- Wilde DW, Knight PR, Sheth N, Williams BA. Halothane alters control of intracellular Ca2+ mobilization in single rat ventricular myocytes. Anesthesiology. 1991;75:1075–1086. doi: 10.1097/00000542-199112000-00020. [DOI] [PubMed] [Google Scholar]


