Abstract
Using patch-clamp techniques, we have studied actions of dopamine and related compounds on granule neurones within the islands of Calleja in vitro, in slices of ∼200 μm thickness or as groups of varying cell number following enzymic digestion.
Prior to agonist application, island of Calleja granule cells displayed spontaneous stepwise shifts in whole-cell conductance ranging from 104 to 632 pS. The reversal potentials of these conductance changes ranged widely and matched the distribution of the cells' membrane potentials. Reversal potentials and membrane potentials shifted equally when cells were uniformly depolarized in 24 mm external K+.
Bath-applied dopamine elicited, after a delay of 4-9 min, an exaggerated form of the spontaneous behaviour that frequently gave way to a sudden large (up to thirtyfold) conductance change. At concentrations of 100-300 nm, a range of agonists with increasing affinity for the D3 receptor (apomorphine, quinpirole, 7-OH DPAT and PD 128907) triggered the response. The actions were neither mimicked by SKF-38393 nor antagonized by SCH-23390 (a selective D1 agonist and antagonist, respectively). Haloperidol reversibly blocked responses elicited by the D3/D2 agonist quinpirole. The action of effective agonists was maintained when transmitter release was abolished. Given the reported lack of D2 receptors in the islands of Calleja, these findings indicate a direct action of dopamine at the D3 receptor.
The dopaminergic effects were not affected by Gd3+ or substantial replacement of external Na+ with TEA, Tris or choline, eliminating stretch-activated channels but suggesting that if transmembrane channels were to be involved in this dopaminergic action they posseses a non-selective permeability to large cations. The reported presence of gap junctions in the islands of Calleja offers the explanation that these effects derive from enhanced activity of such channels or their hemi-constituents.
In testing the possible involvement of gap junctional coupling the following experimental observations were made: (i) alkalinization of slices mimicked the effect of D3 agonists; (ii) in cell groups, recording from pairs provided evidence of intercellular coupling, and mechanical separation of recorded neurones from neighbouring cells during the agonist-evoked response caused shutdown of the additional conductance; (iii) when applied to slices, the gap junctional blocker, 18α-glycyrrhetinic acid, whilst not preventing the full-blown dopamine response, significantly reduced both the variance of recorded granule cell input conductance and the cells’ apparent capacitance.
Taken together the results indicate a D3 action in granule cells, which is best explained by a dopaminergic promotion of intercellular coupling. The physiological relevance of such a mechanism is discussed.
Five subtypes of dopamine receptors (D1-5) are currently recognized (for review see Sokoloff & Schwartz, 1995). These fall into two broad classes, D1-like and D2-like, based on considerations of their pharmacology and effector coupling via GTP-binding protein and second messenger pathways. Currently, D1/D5 and D2-4, which have all been cloned and structurally identified, constitute these respective categories. Of the dopamine receptors, the function of the D3 receptor is least well characterized. While some success has been achieved in studying the pharmacology and effector linkage of the D3 receptor expressed in cultured cell lines (Pilon et al. 1994; Seabrook, Kemp, Freedman, Patel, Sinclair & McAllister, 1994), the role of this receptor in situ remains obscure. The distribution of D3 receptors within dopamine projection areas (Bjorkland & Lindvall, 1984) of mammalian brain is discretely localized, with a preferential expression in limbic regions (Sokoloff, Giros, Martres, Bouthenet & Schwartz, 1990). In particular, the areas with the highest concentration of both D3 mRNA and receptor expression are the islands of Calleja (IC) in the olfactory tubercle (OT) and nucleus accumbens (Landwehrmeyer, Mengod & Palacios, 1993a; Diaz et al. 1995). The islands are composed of granule cells which are reported to possess only D1 and D3 dopamine receptors. Therefore, whilst pharmacological characterization of dopamine receptor subtypes beyond the broad D1- or D2-like classification has been hampered by the lack, so far, of selective antagonists, the demonstration that in the IC the only D2-like receptors are a homogeneous population of D3 receptors offers an opportunity to probe the functional aspects of this latter subtype in these cells.
Although the functional role of the IC in vivo is uncertain, it is strategically positioned within the limbic forebrain, at an interface between this region and the thalamic nuclei which project to the frontal lobes; these are all areas of special relevance to the study of cognitive processing and the treatment of neuropsychiatric disorders (Alheid & Heimer, 1988). Furthermore, antagonist compounds active at the D2-like DA receptors are used to ameliorate schizophrenic conditions (Sokoloff & Schwartz, 1995), with scant knowledge of the cellular consequences of dopamine receptor activation within limbic areas, in particular, of the D3 subclass. Information about DA action in the IC sheds light on the physiological role for the D3 receptor in this structure and furthers the rationale for neuroleptic therapy. We have recently developed preparations to investigate the electrophysiological properties of IC granule cells in varying degrees of isolation in vitro (Halliwell & Horne, 1995). In the present study, therefore, using patch clamp techniques, we have studied the actions of dopamine and a range of congeners on whole-cell currents recorded from IC granule cells. We report that dopamine- and D3-selective agonists depolarize these granule cells by triggering a TTX- and Mn2+-resistant current which is often characterized by stepwise increases in cell conductance that reflect a minimum observable change of just over 100 pS. Consistent with morphological reports of gap junctions between granule cells (Ribak & Fallon, 1982), we also show that they can be electrically coupled and that, moreover, the conductance induced by exposure to D3 agonists is abolished by disrupting intercellular connections. We conclude that D3 receptor activation promotes electrical coupling in the islands of Calleja and that this is a mechanism whereby released dopamine can recruit a population of granule cells to a concerted action. Preliminary accounts of this work have appeared (Halliwell & Horne, 1996, 1997).
METHODS
Male Sprague-Dawley rats (10-15 days old) were decapitated without anaesthesia (according to licence) and the brain was rapidly removed and transferred to ice-cold saline medium (for composition see below). Using a Camden Vibroslice (Camden Instruments, Loughborough, UK) serial coronal slices, 200 μm thick, of basal forebrain were taken from the region containing the OT. Slices were then allowed a period of equilibration of at least 1 h in an artificial cerebrospinal fluid (ACSF) in an oxygenating chamber. The standard ACSF in which the slices were cut and maintained was composed of (mm): 120 NaCl, 3 KCl, 2.5 CaCl2, 1.25 MgCl2, 25 NaHCO3 and 11 glucose. All ACSF solutions were equilibrated with a 95 % O2-5 % CO2 gas mixture to maintain a pH of 7.4.
Experiments on aggregates of granule cells varying in number from five upwards - cell clumps (see Halliwell & Horne, 1995) - were also performed in order to record from two adjacent IC neurones simultaneously. To prepare for the isolation of cells, the region of the OT was extracted from the tissue slices, chopped into prisms not exceeding 0.5 mm along two dimensions and incubated in a high-Mg2+ (4.25 mm), low-Ca2+ (50 μm) solution which was otherwise identical to the standard ACSF. Trypsin was then added at a concentration of 0.375 mg ml−1 (approximately 1000-1275 benzoyl L-arginine ethyl ester units ml−1). The slice prisms were then incubated in this solution for 20-60 min at ∼32°C. At the end of this time, the trypsin solution was discarded and its vestiges inactivated by washing the prisms with ∼0.4 mg ml−1 bovine trypsin inhibitor. The inhibitor was then washed out by three changes of solution which also reduced the Mg2+ concentration and restored the Ca2+ concentration, by stages, to those of the standard ACSF. Cells were then isolated by passing the enzyme-treated slice prisms through the polished tips of Pasteur pipettes. Generally, two sizes of tip diameter were used, the smaller final size being approximately 0.5 mm internal diameter. The slices were not dissociated completely but rather were partly broken up in an attempt to isolate small regions of the IC relatively intact (see Figs 10 and 12). The partial dissociates were then plated onto glass coverslips that had previously been coated with, and rinsed free of the excess of, poly-D-lysine (5 μg ml−1) or Alcian Blue (1 %). Coverslips were then left in a moist, gassed environment for about 30 min to allow cells to adhere, after which the coverslips were submerged in standard ACSF until required later that day.
Figure 10. IC neurones can be electrically coupled.
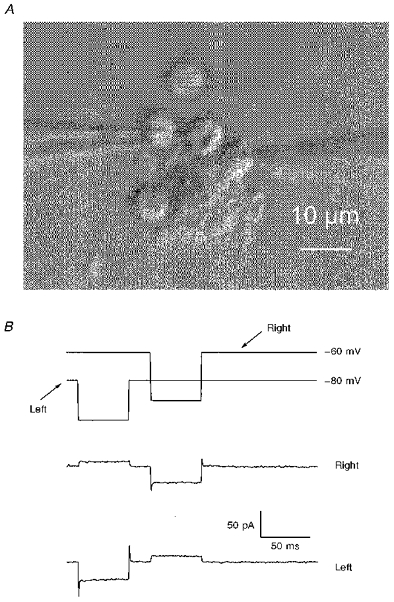
A, a grabbed video image of a clump of ∼6 granule cells in which 2 cells were subject to simultaneous voltage clamp by means of separate patch-clamp amplifiers. B, the upper panel is a record of the voltage commands to the patch electrodes: right electrode in A, steady holding potential, -60 mV; left electrode, -80 mV. The lower traces are averages (12) of the currents recorded simultaneously from each recording electrode ∼7 min after establishing whole-cell recording in both cells. The magnitude of the outward current drawn from one cell by hyperpolarizing the other indicates a coupling conductance of ∼300 pS which was present for the duration of the recording (∼12 min). The ACSF contained 0.5 μm TTX.
Figure 12. D3 agonist-induced conductance can be eliminated by breaking intercellular connections.
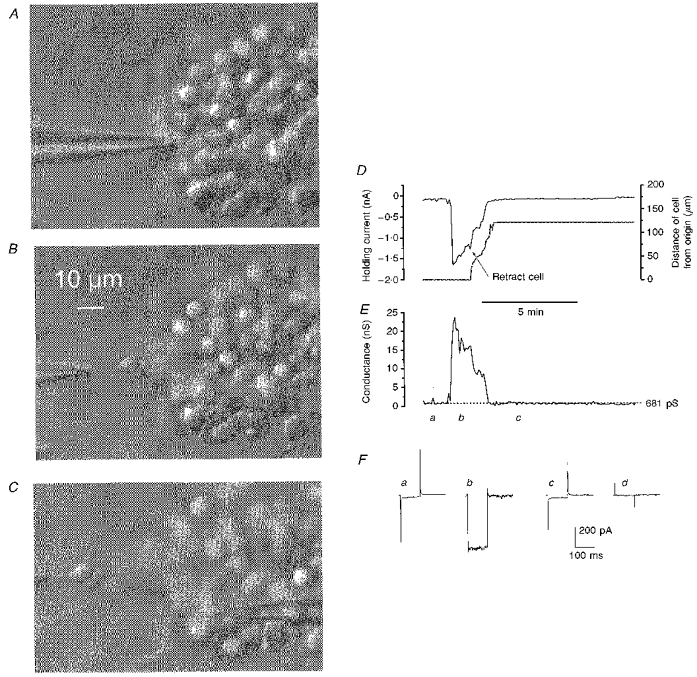
A-C, video grab images of an IC clump showing the removal of an intact granule cell from which whole-cell currents were continuously recorded. These images are of the preparation in which the responses shown in Fig. 11 were recorded, but captured after the cell recorded with the right-hand electrode (holding potential -60 mV and in focus in C) had deteriorated; the electrode on the left-hand side, which was moved away from the clump, thereby isolating the neurone, maintained it at a holding potential of -80 mV. D, simultaneous plots: left ordinate scale, the current required to hold the cell connected to the left-hand electrode at -80 mV; and right ordinate scale, the cell's distance from its original recording site during the separation procedure - both against time (abscissa). The time at which the retraction of the cell commenced is indicated on the graph. E, a plot to the same time scale as D of the retracted cell's conductance. The large conductance increase occurred suddenly 17.5 min after exposure of the preparation to 300 nm PD 128907 and was a continuation of a cycle of repeated inward currents some of which are depicted in Fig. 11, top trace. F, traces of averaged (n = 12) current driven by a 25 mV hyperpolarizing command from -80 mV recordedjust before the conductance increase in E (a), at the height of the increase (b), and following cell removal from the clump (c); d, the subtraction a - c. The ACSF contained 0.5 μm TTX.
The slices or plated coverslips were transferred singly to a continuously perfused recording bath that was fed with pre-gassed standard ACSF at a rate of about 1 ml min−1. Coverslips were pressed to the base of the bath where they adhered, cells uppermost, whereas the thin slices were restrained, fully submerged, by a small loop of silver wire. The recording bath had a transparent base formed by a 19 mm glass coverslip and was mounted on the fixed stage of an upright microscope (Nikon MM-11, Nikon Corporation, Tokyo) equipped with a ×40 water immersion lens and differential interference contrast (Nomarski) optics. Recording electrodes were placed under direct visual control by micromanipulators attached to the microscope. The recording electrodes were patch pipettes pulled on a horizontal Flaming/Brown type puller (P87, Sutter Instruments, Novato, CA, USA) from filamented thin-wall borosilicate glass (Clark Electromedical, Reading, UK; 1.2 mm o.d.) to a tip size of 1-2 μm and were used without further processing after pulling. Most recordings were made using patch pipettes containing the following solution (mm): 135 potassium gluconate, 8 NaCl, 10 Hepes, 0.2 EGTA, 2 Mg-ATP and 0.2 GTP (the nucleotides were freshly prepared and added to the other constituents in thawed aliquots of frozen stock for each experiment); pH adjusted to 7.2 with KOH. For a number of experiments alternative pipette solutions were used viz. (mm): 135 potassium methylsulphate or caesium or potassium methanesulfonate, 8 NaCl, 0.1 EGTA, 10 Hepes, 2 Mg-ATP and 0.2 GTP; pH adjusted to 7.2 with KOH, CsOH or HMeSO3 as appropriate. Pipettes had a resistance of about 2-8 MΩ, and were connected to an Axopatch-1D or Axopatch 200B for voltage-clamp experiments or an Axoclamp-2A for voltage recording with current clamp (amplifiers from Axon Instruments; high frequency cut-off filters set at 5 kHz).
Whole-cell intracellular recordings were obtained in the ruptured patch manner from granule cells in either slices or clumps. Whilst gently expelling a stream of solution with positive pressure, a pipette was brought close to a cell and a high resistance seal obtained by releasing the pressure and applying a little suction (a combination of the techniques of Edwards, Konnerth, Sakmann & Takahashi (1989) and Blanton, Lo Turco & Kriegstein (1989)). Intracellular access was obtained by applying further gentle suction to the pipette following the formation of a seal. Where measured, series resistances of up to 20 MΩ were apparent; however, capacitance transients proved difficult to compensate completely and thus series resistance measurements may not have been accurate. Whenever the measurable resistance was compensated (by up to 80 %) little difference was obtained either qualitatively or quantitatively and so this procedure was not routinely followed.
In cells where adequate recordings were obtained, control of the membrane potential and data acquisition was effected using the Clampex programme in the pCLAMP suite of IBM PC-compatible software (Axon Instruments). Such work was carried out mostly in voltage clamp; however, some experiments were performed in current clamp with the signal recorded digitally using Axotape software (Axon Instruments) and sample records acquired by pCLAMP. Records of current and voltage were also made on-line on a chart recorder (0-500 Hz bandwidth: Easygraph, Gould Electronics, Hainault, UK). Measurement of the recorded signals and curve fitting was performed using Clampfit with graphical representations and other curve fitting obtained using Origin software (Microcal Inc., Northampton, MA, USA). Data are expressed as means ±s.e.m. except where explicitly stated otherwise.
Drugs were administered in the perfusate; times of application indicated in figures include a dead time of about 2 min and, for slice experiments, a further diffusion time dependent on cell positioning within the slice. All bulk chemicals were purchased from BDH-Merck (Poole, UK); trimethylammonium chloride (TMA), choline chloride, Hepes, trizma, DMSO, 18α-glycyrrhetinic acid and poly-L-lysine were obtained from Sigma; tetraethylammonium chloride (TEA) was from Phase Separations (Deeside, Clwyd, UK). Trypsin (porcine or bovine pancreatic) and its inhibitor were purchased from Sigma or BDH-Merck. Poly-D-lysine was dissolved in 0.1 m H3BO3 at a concentration of 10 mg l−1 and the pH adjusted to 8.4 with NaOH. Dopamine agonists and antagonists employed in this study were all purchased from RBI. Most were kept as 10 mm frozen stock solution; exceptions were haloperidol which was kept as a 10 mm stock in DMSO and then diluted by 1000 for use and 18α-glycyrrhetinic acid which was kept as a 20 mm solution in DMSO and diluted by a factor of 2000 for application at 10 μm. All experimental procedures were carried out at room temperature (22-25°C).
RESULTS
Spontaneous behaviour of IC granule cells
Current clamp recordings were made from more than twenty-five IC granule cells visualized in 200 μm-thick slices of olfactory tubercle or in clumps of partly disaggregated islands. As previously reported for either experimental preparation (Halliwell & Horne, 1995) small holding currents (3-15 pA) were required to maintain IC neurones in an excitable state, owing to a rather positive resting potential in these cells, at which voltages sodium currents are inactivated. The input resistance of the neurones was characteristically high (ranging from 1.5 to 8.7 GΩ) and the membrane potential displayed wide fluctuations, which, if depolarizing, triggered action potential discharges, and much subthreshold voltage noise. In addition to this rapid irregular activity, more than 50 % of neurones displayed (within the constraints of their long membrane time constants with τ often > 50 ms) step changes (Fig. 1A) in membrane potential which were accompanied by marked changes in input resistance (Fig. 1A and B). In the example shown in Fig. 1B, the cell spent periods of up to 1 min at one of two membrane potential levels which were separated by 18 mV and characterized at the depolarized level by both increased noise and membrane conductance. Periods of increased membrane noise were not invariably concurrent with depolarization but were frequently accompanied by subthreshold ‘spikelets’ of a consistent amplitude (Fig. 1C).
Figure 1. Spontaneous voltage changes recorded from IC granule cells under conditions of current clamp.
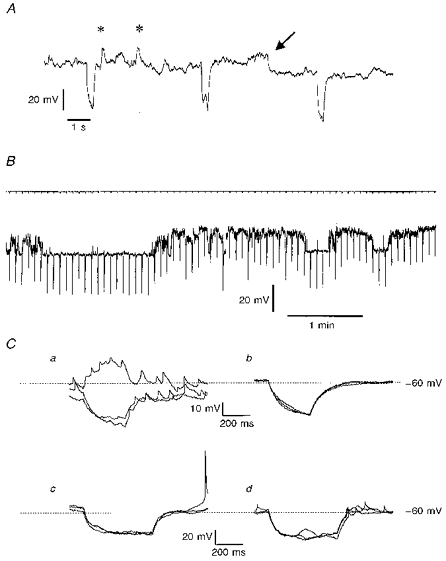
A, an extract of a membrane potential recording from an IC neurone in a slice, held hyperpolarized by a steady current of 10 pA of injected current. The periodic hyperpolarizations (every 5 s) are in response to additional superimposed 0.5 s current injections of 5 pA. On the trace are marked two putative synaptic events (*) and a ‘step’ change in membrane potential from -61 to -70 mV (arrow); see text for further details. B, from a different cell in a semi-isolated clump of neurones, a longer trace of membrane potential record with accompanying current record showing the level of steady clamping current (-7.5 pA) and at 0.2 Hz the imposition of 0.5 s, 5 pA hyperpolarizing current injections. The membrane potential wavered between preferred values: the more negative level was -79 mV. C, responses captured in two further neurones in response to 0.5 s current injections of 10 pA (Ca and b) or 15 pA (Cc and d). In Ca the record was noisy and small spikelets appeared on the recordings, whereas in Cb which was recorded 2 min after Ca, the voltage trace was relatively quiet and the spikelets were no longer observable; each panel shows 3 consecutive superimposed traces. Cc and d, 3 superimposed voltage traces are shown in each panel, showing responses to hyperpolarizing current in a cell which displayed both full-sized action potentials (Cc) and spikelets (Cd) which appeared 3 min later. Steady holding currents for the two cells, which were both in clumps of IC neurones, were - 5 pA (Ca and b) and -18 pA (Cc and d).
Corresponding spontaneous events could be observed in IC cells under voltage clamp (Fig. 2A). Neurones in slices were routinely held at -80 mV; at this potential spontaneous step changes in holding current occurred, during which time it was possible to impose a ramp change in the membrane potential repeatedly from -105 to 0 mV and obtain averages of up to twelve responses (Fig. 2B). By assessing the slope of the linear part of the current-voltage (I-V) relation so generated (between -70 and -100 mV) and extrapolating these lines, the magnitude of the spontaneous conductance change and its reversal potential could be estimated (Fig. 2B). It was also commonplace to observe poorly resolved channel activity from the whole-cell current records, which could be subject to the same sort of analysis (Fig. 2C). Figure 2D summarizes results from twenty-two neurones which had a mean whole-cell conductance of 317 ± 21 pS and a mean zero current level (indicating resting potential) of -31 ± 3 mV. (A number of the cells were exposed to 0.5 μm TTX (12/22) and 1 mm Ba2+ (18/22), the latter to linearize the I-V curve. The effect of this concentration of Ba2+, tested directly in five neurones, was to shift the zero current level from - 55 ± 1.3 mV to -31.2 ± 2.6 mV.) Figure 2Da shows that the distribution of reversal potentials for spontaneous conductance changes in this population of cells was similar to the spread of resting potentials (zero current level: =Vm). The mean reversal potential was -25 ± 3 mV, zero current was -31 ± 3 mV (n = 22) and the minimum conductance increase ranged individually from 104-632 pS with a mean of 270 ± 27 pS (Fig. 2Db). However, there was no overall correlation between the reversal potential for the spontaneous conductance increase measured in a neurone and its resting potential (r = -0.035; P = 0.88, n = 22); approximately one-third (32 %) of the reversal potentials were negative to Vm and two-thirds were positive. The correspondence between the apparent reversal potential observed for spontaneous conductance changes and the population membrane potential was investigated in a further nineteen granule neurones in slices bathed in ACSF containing 24 mm K+ to reduce Vm uniformly, but with low Ca2+ (100 μm), elevated Mg2+ (6 mm) and 1 mm Mn2+ to prevent depolarization-evoked release of endogenous transmitters. As expected, this medium significantly depolarized the cells (to -14 ± 1.9 mV, P < 0.005, unpaired t test); the apparent reversal potential of spontaneous conductance changes (135-613 pS; mean, 367 ± 40 pS) which were observed in fifteen of the nineteen cells was shifted to -8 ± 2.1 mV (P < 0.005). The voltage protocol was modified for these experiments, the ramp running from -85 to +20 or +40 mV. Direct determination of the reversal potential was possible from the intersection of I-V relationships derived before and after the conductance changes, but profound inward rectification of the difference curves was encountered at potentials positive to the reversal potential in 11/15 cells; the remaining four cells displayed linear I-V relationships in this high-K+ medium. We were interested to ascertain, by comparing reversal potentials, whether Cl− permeability was a major component of the spontaneous conductance changes. In order, therefore, to determine the putative Cl− reversal potential, given the set transmembrane Cl− distribution of the present experiments, GABA (300 μm to 1 mm) was applied to four cells in the high-K+ medium; the measured GABA reversal potential was -61 ± 2.6 mV. Thus Cl− flux does not play a major role in these spontaneous events.
Figure 2. Analysis of the spontaneous current changes observed in voltage clamped IC neurones.
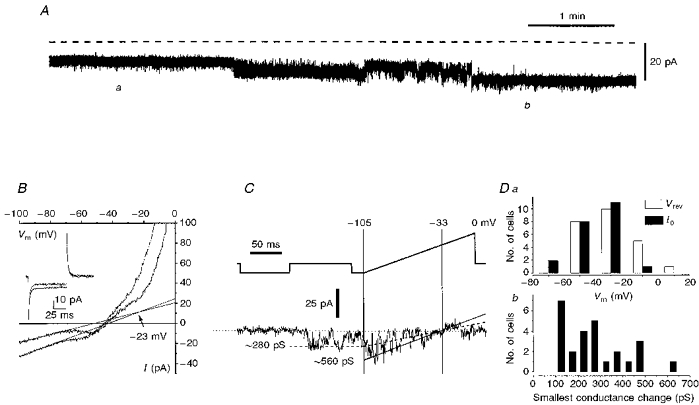
A, a ∼5 min extract of a chart recording of the whole-cell current in an IC granule cell voltage clamped at -80 mV (fluctuations caused by periodic clamp commands have been removed from the trace). The dashed line denotes the zero current level. B, current-voltage (I-V) relations for the cell depicted in A and recorded at the times marked a and b on the trace. The I-V plots were generated by plotting the currents driven by a ramp command from -105 mV to zero (at 0.6 V s−1), against the command potential (see upper trace in C for typical protocol). The linear region of each plot (between -100 and -70 mV) has been fitted with a straight line and the extrapolated intersection of these two lines has been taken to determine the apparent reversal potential of the observed conductance change; the lower conductance state was recorded at position a. Also in B are shown superimposed current traces driven at times denoted by positions a and b, by a 25 mV hyperpolarizing step command for 100 ms. C, faster current transitions in IC neurones. Upper trace (voltage) and lower trace (current) are shown. The current trace was generated by subtracting the average of 11 repeated responses of the granule cell to the indicated voltage protocol, when no fast transitions in current were observed, from a single trace in which a rapid current fluctuation was seen. Two predominant conductance states are indicated, based on the current reversal observed at the holding potential of -33 mV. Da, a plot of the distribution of current reversal potentials values for spontaneous conductance changes (□) and of the distribution of cell resting potentials (▪; as determined by the voltage at which clamp current is zero) in the same population of 22 IC neurones. Db, the frequency distribution of the smallest observed conductance change occurring spontaneously in the population of cells depicted in Da. All the data in this figure are from IC neurones studied in slice preparations; the data collected from the individual cells shown were from experiments in which 0.5 μm TTX and 1 mm Ba2+ were in the bathing medium.
The phenomena described above and observed in 3 mm K+ could still be seen when chemical synaptic transmission was blocked by omitting Ca2+ and adding 3 mm Mg2+ and 2 mm Mn2+ (n = 6) or in the presence of 50-100 μm GdCl3 (n = 4). While action potentials were blocked by 0.5 μm TTX the spontaneous shifts in membrane potential or current were resistant to this drug.
Dopamine evoked voltage responses from IC granule cells
When dopamine (DA) was applied to IC granule cells, contained within slices or cell clumps and artificially hyperpolarized to ∼-80 mV, the cells responded with a long latency (∼7 min) depolarization that was accompanied by a marked reduction of the cell input resistance (Fig. 3A). The depolarization was not dependent on intact chemical synaptic transmission since comparable effects were observed when the bathing solution contained no added Ca2+, 2 mm Mn2+ and additional (3 mm) Mg2+(Fig. 3B). In normal medium 0.5-3 μm DA produced a mean peak depolarization of 34 ± 5 mV (range, 10-45 mV; n = 9); in the ‘Ca-free’ ACSF the same concentrations applied produced a mean depolarization of 33 ± 6 mV (range, 10-49 mV; n = 6). The respective median percentage changes in input resistance were as follows: normal ACSF, 87 % - from 3.1 to 0.403 GΩ; ‘Ca-free’ ACSF, 67 % - from 2.31 to 0.762 GΩ. During the depolarization caused by DA, membrane voltage noise increased markedly and as indicated in Fig. 3A (*), the initial or recovery phase of the response could proceed in a stepwise manner, indicative of discrete changes of whole cell resistance and similar in form to the spontaneous changes described above. The DA-induced resistance decrease exceded that produced by an equivalent depolarization-evoked opening of K+-channels, predictable from the cells’ known current-voltage relationship (Halliwell & Horne, 1995) and was still present when 1 mm Ba2+ was included in the ACSF to eliminate outward rectification (cf. subsequent voltage-clamp data).
Figure 3. Dopamine-induced depolarizations in IC granule neurones.
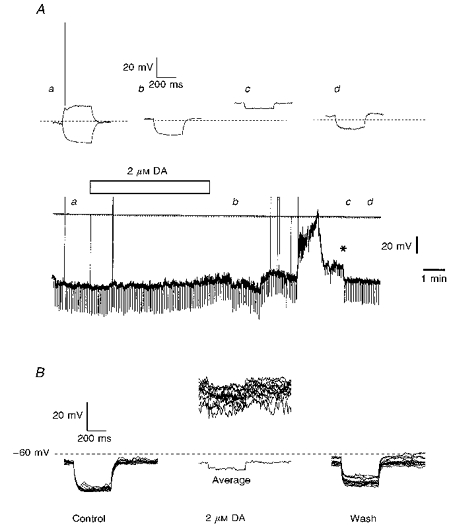
A, the lower part of this panel shows a chart record of the membrane potential of an IC neurone together with the representation of the clamping current to which it was exposed. The bar indicates the period for which 2 μm DA was applied to the slice preparation in the bathing medium. Faster voltage traces were caught at the timesindicated on the chart (a-d) and are displayed in the upper part of the panel. They are membrane responses to 10 pA current injections of 400 ms duration; the dotted line indicates a membrane potential of -70 mV. Note the stepwise recovery of the dopaminergic action (*). B, dopamine action is independent of the presence of conventional chemical transmission. Twelve superimposed traces of consecutive voltage responses triggered in an IC neurone by -15 pA current injections are shown before, at the height of dopaminergic action and following washout from the 2 μm DA exposure in ACSF containing no Ca2+ but with added Mg2+ (2 mm), Mn2+ (1 mm) and TTX (0.5 μm). The average of the cell's responses during the depolarization induced by DA is also shown but with a voltage displacement to allow comparison of resistance. Both A and B were slice experiments.
Voltage-clamp analysis of the action of dopamine and its congeners
Actions of agonists with affinity for Dopamine D3 receptors
IC granule cells in slices were routinely voltage clamped to -80 mV in ACSF that contained 0.5 μm TTX and sometimes 1 mm Ba2+ to linearize the I-V relation. Dopamine (10-300 nm, n = 3), apomorphine (10-300 nm, n = 8), quinpirole (100 nm, n = 7), 7-OH DPAT (100-300 nm, n = 3), and PD 128907 (300 nm, n = 5) were bath-applied with very similar results regardless of whether Ba2+ was present (Table 1). These are agonists ranging from the non-selective (dopamine and apomorphine) through D2-like preferring (quinpirole) to increasingly D3 selective (7-OH DPAT and PD 12807); see Sokoloff & Schwartz, 1995. Two main features of the response to effective dopaminergic agonists were noted. First, an increase in the occurrence of membrane current fluctuations occurred in most neurones, but without a marked overall change in the holding current. Second, after a long and variable latency, an abrupt inward current occurred, which could last for many minutes and draw large currents (often in excess of 3 nA) from the circuitry clamping the granule cell under study (Figs 4 and 6); we have termed this the ‘cataclysmic’ phase of the dopaminergic response and it was accompanied by up to a thirtyfold conductance increase. Around 65 % of the neurones made a full or near full recovery upon washout of the agonist. Upon recovery from the ‘cataclysmic’ phase, the other feature of dopaminergic action frequently resumed. During this latter response phase, the minimal response, the neurones displayed transitions in whole-cell holding current; these were either fast channel-like steps or of a slow ramp-like form taking several milliseconds to stabilize (Figs 4 and 5). Steps in holding current were caused by observable conductance changes as measured by changes in the current driven by rectangular or ramp voltage commands. The smallest conductance change measured during the minimal response phases flanking the large (‘cataclysmic’) conductance change was ∼120 pS. Ramp commands from -110 mV to 0 mV were imposed before, at different phases of the response and following drug washout, to determine the reversal potential for the conductance change induced by the DA agonists (Fig. 5). Overall, the minimal conductance changes during the flanking phases reversed at -34.4 ± 2.5 mV (range -8 to -57 mV, n = 22); the reversal potential of the maximal (‘cataclysmic’) conductance change was -4.2 ± 1.2 mV (range +12 to -19 mV, n = 21). Where measured (n = 16), 50 % of the reversal potentials for the minimal effects of dopamine agonists and all the reversal potentials for the ‘cataclysmic’ response were more positive than Vm values of the neurones in which they were observed. Table 1 summarizes the data for each of the agonists in terms of the response features described above. Dopaminergic responsiveness of IC granule cells was still observed in the ‘Ca2+-free’ ACSF under voltage clamp conditions (see, e.g. Figure 8).
Table 1.
Effectiveness of various dopaminergic agonists in eliciting conductance changes in island of Calleja granule cells
| Agonist | Latency (min) | Vrev,min (mV) | Vrev,max (mV) | Initial conductance (pS) | Maximal conductance (nS) | Increase (%) | n |
|---|---|---|---|---|---|---|---|
| Dopamine (300 nm) | 7·9 ± 2 * | -32·7 ± 4·5 | -6·3 ± 0·9 | 241 ± 47 | 6·48 ± 3·5 | 2589 | 3 |
| Apomorphine (10-300 nm) | 6·1 ± 0·9 | -36·3 ± 4·3 | -5·8 ± 4·0 † | 533 ± 101 | 5·66 ± 1·8 | 1271 | 8 |
| Quinpirole (100 nm)§ | 6·6 ± 1·1 | -32·0 ± 4·1 | -6·2 ± 2·3‡ | 592 ± 141 | 11·81 ± 3·37 | 3202 | 5 |
| 7-OH DPAT (100-300 nm)§ | 7·7 ± 0·73 | -42 ± 13 | 2·7 ± 4·8 | 280 ± 27 | 5·5 ± 1·6 | 1693 | 3 |
| PD 128907 (300 nm)§ | 6·0 ± 0·8 | -26·0 ± 4·3 | -4·6 ± 1·4¶ | 305 ± 43 | 8·25 ± 1·93 | 3083 | 5 |
The latency was the time between switching to agonist containing ACSF and the onset of the ‘cataclysmic’ component - the large inward current and conductance increase observed at -80 mV.
The value for dopamine was derived from current-clamp observations (n = 7); the remainder from voltage-clamp experiments. Reversal potentials at the start of responding (the minimal component; Vrev,min) and at the peak of inward current (Vrev,max) were determined from intersections of extrapolated I-V curves as indicated in Fig. 5. Neuronal input conductance prior to agonist application (initial conductance) and at the peak inward current (maximal conductance) were determined by the slopes of I-V relations between -70 and -100 mV also as indicated in Fig. 5.
Experimental series undertaken in ACSF that contained 1 mm Ba2+ Paired t tests were employed to test for differences in Vrev,min and Vrev,max and yielded exact probabilities of P = 0·013
for apomorphine, P = 0·016
for quinpirole and P = 0·021
for PD 128907.
Figure 4. Apomorphine induces a delayed inward current characterized by a stepwise increase and decrease.
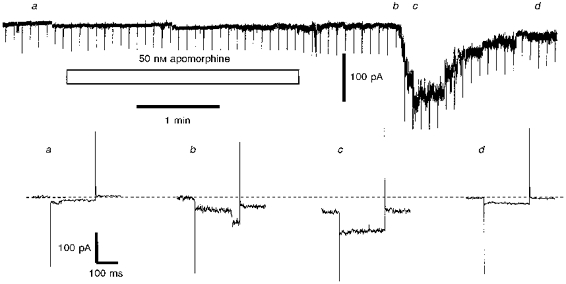
The upper part of the figure shows a chart record of the current required to hold an IC neurone at -80 mV; every 5 s the cell was stepped to -100 mV to assess whole-cell conductance and the traces so generated at the timesindicated (a-d) are displayed in the lower half of the figure. Apomorphine (50 nm) was applied as indicated by the bar. Note that prior to apomorphine exposure the neurone displayed small current fluctuations (cf. Fig. 2) and a clear transition is resolved in trace b: the magnitude of the latter conductance change was 606 pS. The ACSF contained 0.5 μm TTX and the preparation was a slice preparation.
Figure 6. D3- but not D1-like dopamine agonists evoke activity in IC neurones.
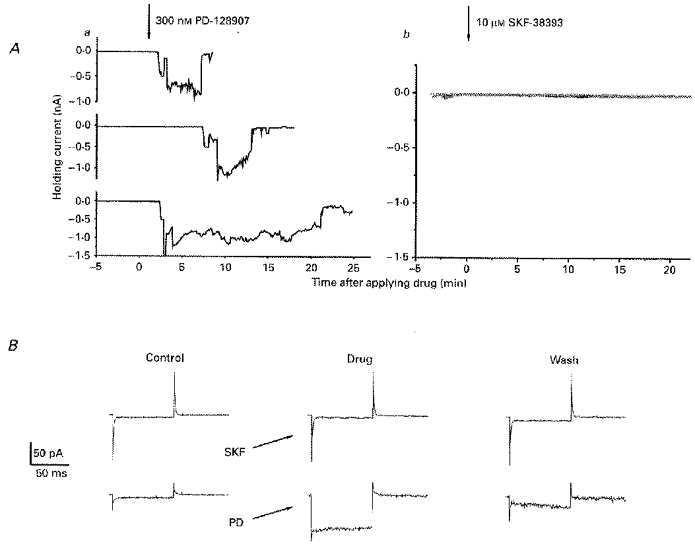
A, each panel shows plots of holding current (ordinate) required to hold IC neurones at -80 mV against the time (abscissae) after drug exposure (> 5 min) for 3 neurones exposed tothe D3-selective compound PD 128907 (300 nm; a) and3 neurones (different to those in a; superimposed on a single graph) exposed to 10 μm SKF-38393, a D1 agonist (b). B, a direct comparison of the effects of 10 μm SKF-38393 and 300 nm PD 128907 in one neurone (not included in the experiments of A); the traces are membrane currents driven by 25 mV hyperpolarizing steps from -80 mV before, during (marked SKF and PD) and after exposure to the drug. SKF-38393 induced no systematic response but PD 128907 caused a 2 nA inward current which is not shown. All the data were from slices bathed in ACSF containing 0.5 μm TTX and 1 mm Ba2+.
Figure 5. The reversal potential of dopaminergic effects observed in IC neurones.
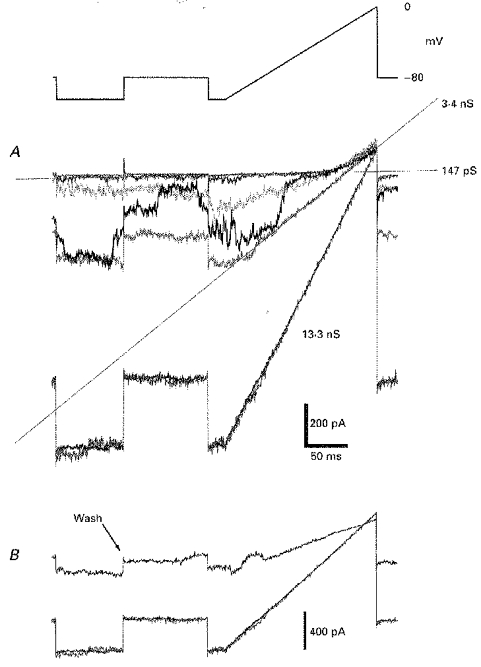
The upper panel of this figure depicts the voltage protocol imposed on a cell to measure the induced conductance change and its reversal potential. A, these superimposed traces were recorded from a cell before and as it commenced its response to 300 nm DA and are membrane currents driven by the voltage command above, collected at 5 s intervals. The individual traces are bracketed by averages of 12 repeated commands before DA exposure (fitted with the 147 pS conductance line) and after maximal DA-induced conductance was established, 30 s after the onset of the DA response (the 13.3 nS conductance line). The slope of the fourth trace was fitted by a line indicating an intermediate conductance of 3.4 nS. Note the marked transitional conductance states exhibited during the third trace and the progressive migration to less negative potentials of the apparent reversal potential of the response with increasing conductance. B, in this panel are superimposed currents showing the partial recovery from this dopamine application; the trace labelled ‘wash’ was captured 4.5 min after the maximal effect had developed. All these effects were recorded in the presence of 10 μm SCH-23390 in addition to 0.5 μm TTX. Slice preparation.
Figure 8. The reversal potential of the maximal D3 agonist effect is unaffected in low external [Na+].
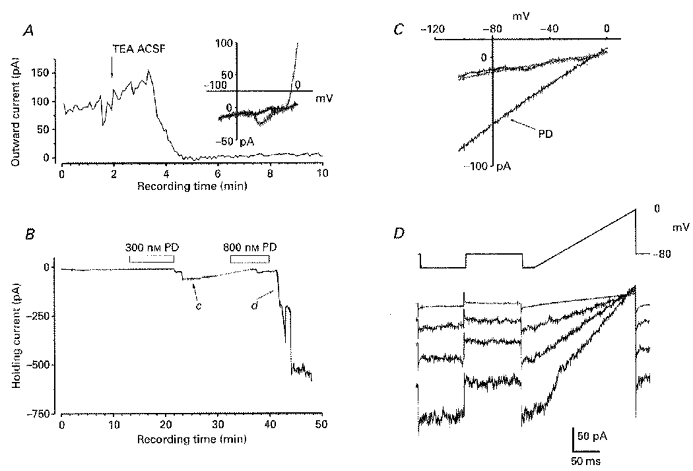
The effects of PD 128907 were tested in ‘TEA ACSF’ which contained no Ca2+, in which 120 mm NaCl was replaced by 120 mm TEA Cl and to which supplementary Mg2+ (2 mm) and Mn2+ (1 mm) was added. A, the main graph shows a plot of the outward current (ordinate) elicited by a ramp depolarization to 0 mV (as depicted in the upper part of D) against experimental recording time (abscissa) for the first 10 min of recording; at the time marked by the arrow the perfusing solution was switched from the normal ACSF to TEA ACSF. The inset graph shows this cell's I-V relation which was acquired before (grey), and 2 min after exposure to the TEA ACSF (black), and was generated by a ramp command from -105 mV to 0 mV. B, the graph shows a plot of holding current (ordinate) required to hold the IC neurone at -80 mV against the experimental recording time (abscissa) and bars indicate when PD 128907 was added to the perfusing medium. The dotted line denotes a break in recording. C, I-V plots for the cell, generated as in A (inset), before, during the inward current that followed exposure to 300 nm PD 128907 (marked PD and from recordings taken at time c on graph shown in B) and after recovery (∼40 min of recording: grey line). D, currents driven by the indicated protocol after the recovery from the first application of PD 128907 (top current trace - an average of 12 responses) and individual traces recorded consecutively at 15 s intervals around the time of the onset of inward current following the second application of PD 128907 (marked d on graph B). The responses shown here were generated in a slice preparation and were notably similar to those obtained in normal ACSF.
Actions of agonists with affinity for dopamine D1-like receptors and of dopamine D1- and D2-like antagonists
Dopaminergic agonist responses were not blocked by the D1-like receptor antagonist SCH-23390 (10-50 μm, n = 5: see Fig. 5), nor were they elicited by application of up to 10 μm SKF-38393 (n = 3), which is an agonist at D1-like DA receptors (Fig. 6). Consistent with the above agonist effects being mediated by actions at D2-like DA receptors, the action of the prototypical D2/3 agonist quinpirole (at 100 nm) which was observed in 7/7 neurones was completely prevented by prior inclusion of 10 μm haloperidol in the ACSF bathing the slices for at least 30 min (n = 4); in the cell shown in Fig. 7, 100 nm quinpirole was effective following washout of haloperidol, which previously antagonized the actions of 500 nm of this agonist.
Figure 7. Reversible antagonism of the dopaminergic effects by haloperidol.
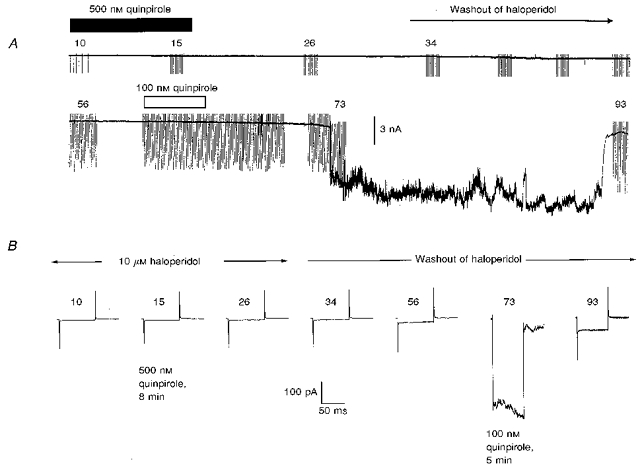
A, a chart record of the IC granule cell membrane current at a maintained holding potential of -80 mV, with periodic superimposed test voltage-clamp commands imposed at the indicated recording times (in minutes) after achieving the whole-cell recording configuration. The experiment commenced with 10 μm haloperidol in the ACSF bathing the slice and the antagonist was withdrawn after 30 min of recording; 500 nm quinpirole was applied in the presence of haloperidol (dark bar) and, following washout of the haloperidol, was reapplied at 100 nm (open bar). B, currents driven by 25 mV hyperpolarizing steps (averages of 12) acquired at times during the experiment as indicated in A. The traces labelled quinpirole indicate responses recorded following exposure to 8 min or 5 min of one of the two concentrations of quinpirole. The ACSF also contained 0.5 μm TTX and 1 mm Ba2+.
Ionic mechanisms underlying dopaminergic action
Despite the wide range of reversal potentials for the initial, minimal phase of the dopamine agonist-induced currents giving no clear indication of the nature of the ionic permeation, the more homogeneous reversal potential of the ‘cataclysmic’ phase is consistent with the activation of a non-specific cation conductance. One candidate current would be the gadolinium-sensitive stretch-activated current (Yang & Sachs, 1989) that could be secondary to induced cell volume changes. However, application of agonist in the presence of 50 or 100 μm Gd3+ (n = 5) continued to evoke undiminished responses (data not shown). To address the possibility that a Ca2+- or agonist-activated non-specific conductance might underlie the response, we attempted to shift the reversal potential of the large induced conductance by substituting more than 80 % of the extracellular Na+ with TEA (Vrev, -6.3; n = 3), Tris (Vrev, -6.0; n = 3) or choline (Vrev, +10; n = 3). The absence of a marked change (overall Vrev, 0 ± 3; n = 9; see Fig. 8) suggests that if a truly transmembrane conductance is activated during the large inward current, it is a non-specific conductance with an uncharacteristically large pore size to allow passage of these larger ions with the same apparent ease as Na+. Inspection of the reversal potentials of minimal activity induced by dopamine agonists in low-Na+ medium revealed similar values to those obtained in control ACSF (reversal potential with TEA replacement, -28 ± 6 mV; n = 3) and spontaneous events also appeared unaffected.
Tests for electrical coupling between IC neurones
Cell alkalinization
The IC contain high levels of mRNA for the gap junction protein connexin 32, which persist into adulthood (Micevych & Abelson, 1991) and are consistent with ultrastructural identification of gap junctions in the IC of the adult rat (Ribak & Fallon, 1982). We addressed the possibility that such junctions might be involved in the present dopaminergic responsiveness by observing the effects of cell alkalinization on IC granule cells in slices, a procedure known to open gap junctions (Spray, Harris & Bennett, 1981). Application to four slices of ACSF containing 10 mm trimethyl ammonium (TMA), which should shift pHi to ∼pH 8 (cf. Pocock & Richards, 1992), mimicked the action of the dopaminergic agonists. Both minimal conductance changes and the large (‘cataclysmic’) conductance change were reversibly induced (Fig. 9). The latency to the onset of the precipitate inward current observed at -80 mV was 7.7 ± 1.1 min and it reversed close to zero potential (2.3 ± 4.9 mV).
Figure 9. A cell alkalinizing procedure mimics the D3 agonist effects.
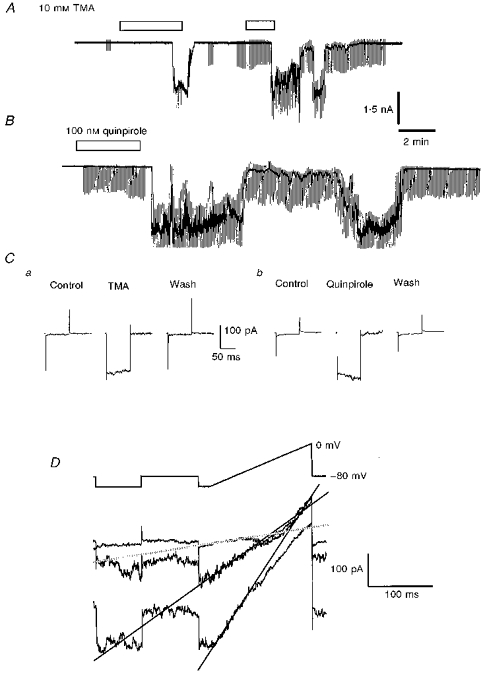
A, a chart record of IC granule cell membrane current at a maintained holding potential of -80 mV with periodic superimposed test voltage-clamp commands, showing currents induced by repeat applications of 10 mm trimethylammonium Cl (TMA) (bars). B, a similar record to that shown in A but depicting the response of another IC neurone to a single application of 100 nm quinpirole. C, conductance measurements for the cells depicted in A (a) and B (b) made by imposing 25 mV hyperpolarizing steps before, at the peak of drug action and following recovery; each trace displayed is the average of 12 current responses driven by the potential commands. D, three superimposed current traces driven by the indicated protocol and acquired from a cell different from that in A, at the start of the inward current elicited by exposure of the slice to 10 mm TMA; lines are fitted to the currents over the range -100 to -70 mV and their intersections give estimates of the reversal potentials of conductance changes (cf. Fig. 5). All examples in this figure were from slice experiments with 0.5 μm TTX present; in the quinpirole experiment the ACSF also included 1 mm Ba2+.
Paired recordings from granule cells
To investigate the possibility of electrical coupling more directly, we prepared clumps of IC neurones from lightly trypsinized and mechanically disaggregated olfactory tubercle slices, which could be morphologically identified according to our previously established criteria (Halliwell & Horne, 1995). Owing to easier visualization of the IC neurone surface membrane, these preparations allowed paired recordings from granule cells, which were individually voltage-clamped to test for electrical coupling. One pair of cells from twenty-seven recorded pairs was demonstrably coupled upon establishment of whole-cell recording, with a junctional conductance of ∼290 pS (Fig. 10) and one of these cells (in a clump of ∼6) responded further to 300 nm PD 128907 by the opening of an additional 20 pA channel at -60 mV holding potential (not shown). Seventeen other neurone pairs were tested for responsiveness to DA (300 nm, n = 7), PD 128907 (300-600 nm, n = 5), quinpirole (100 nm, n = 1), or TMA (10 mm, n = 4) and 79 % of the thirty-four cells responded with a current response typical of the IC neurones recorded within intact slices. In eleven of the neurone pairs, both cells displayed a dopaminergic response but this was devoid of any apparent coincident activity; illustrated in Fig. 11 is the independent response to 300 nm PD 128907 of two such IC neurones. One of these cells developed a typical long lasting high conductance state, during which time the recording pipette was withdrawn, pulling the neurone away from the clump and breaking intercellular contacts (Fig. 12A-C). Disruption of the intercellular contacts was effected in a stepwise manner with an corresponding reduction in holding current and measured cell conductance (Fig. 12D and E). Finally the conductance of the cell was restored to the same value that it displayed prior to drug exposure, with no attendant effect on cell capacitance (Fig. 12E and F), demonstrating that the conductance induced by the dopaminergic agonist resided in the contacts between the recorded cell and its neighbours. Similar results were obtained in two other IC neurones in which the large conductance state was elicited by 300 nm DA or 100 nm quinpirole and in one cell in which it developed spontaneously.
Figure 11. IC neurones which are close neighbours can respond similarly but independently to a D3 agonist.
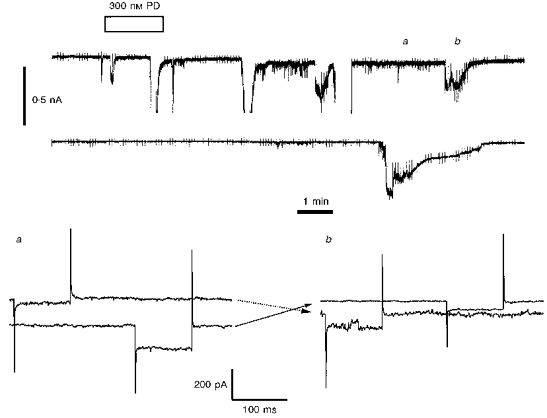
Upper panel, simultaneous chart records of whole cell currents from 2 neurones in a large isolated clump of IC granules (see Fig. 12). The upper record was from a cell at a holding potential of -80 mV and the lower record from a cell at a holding potential of -60 mV. The application of 300 nm PD 128907 (for the second time) is indicated. Missing sections in the upper trace denote the occurrence of off-scale currents. Lower panel, simultaneous currents recorded in the two neurones at the times indicated a and b including responses to hyperpolarizing commands of -25 mV. In each pair of traces the neurone held initially at -80 mV is the first to be hyperpolarized. The cross-over arrows indicate that as one neurone is subject to a standing inward current induced by the agonist, the other is quiescent, whilst during the recovery phase of the formerly active neurone the other cell starts to develop the agonist-induced response. The ACSF contained 0.5 μm TTX.
Effects of a gap junction blocker
The effects of pre-incubation with (> 3 h) and the maintained presence of the gap junction blocker 18α-glycyrrhetinic acid (GA; Davidson & Baumgarten, 1988; Goldberg et al. 1996) were tested on twelve granule neurones in slices. Cells were recorded with pipettes containing a Cs+-based intracellular solution to minimize cell K+ conductance and any effects of GA thereon. Ten micromolar GA failed to prevent either spontaneous or D3 agonist-evoked responses. In view of reports that GA only reduces coupling rather than abolishing it altogether (Goldberg et al. 1996), a more statistical approach was adopted and a comparison was made with a sample of a further ten neurones recorded with the same intracellular solution but in the absence of GA. Given the possibility of sustained electrical coupling between IC neurones (see previous Results section), the effects of GA on observed whole cell conductance and capacitance were assessed in these two samples to ascertain whether any GA-sensitive coupling could bestow additional components on the cell being patch clamped. The whole cell conductance measured from currents driven by a hyperpolarizing 25 mV step from -80 mV holding potential was 407 ± 80 pS for the sample of cells in the absence of GA and 300 ± 33 pS for cells exposed to GA (P = 0.22, unpaired t test); the variance ratio, F, for these two samples was 5.41 (P < 0.05), suggesting that GA reduces the variability in cell conductance. Apparent cell capacitance measured from the integral of the capacity transient generated by the 25 mV step command was significantly lower (3.16 ± 0.48 pF) with GA present than under control conditions (5.09 ± 0.70 pF; P < 0.05, unpaired t test) indicating that GA could reduce the effective membrane area controlled by the clamp circuitry by attenuating intercellular coupling.
DISCUSSION
Spontaneous activity in granule cells of the islands of Calleja
In this study we have extended observations which were initially reported in the context of describing the membrane properties of IC neurones (Halliwell & Horne, 1995). A feature which we report now is the appearance of particular modes of behaviour that the granule cells display. These are characterized by the sudden steps in membrane potential or holding current that accompany discrete steady conductance changes. Membrane noise increases during the depolarizing events that are marked by a conductance increase. Increased noise can occur independently of potential changes and small spikelets are frequently seen at this time. The latter occur in neurones that are quite capable of generating full-blown action potentials and are reminiscent of fast prepotentials, now believed to represent the electrotonic coupling through gap junctions at various sites within the mammalian CNS (see, e.g. Logan, Pickering, Gibson, Nolan & Spanswick, 1996). There is convincing ultrastructural evidence of gap junctions in the IC and this persists into adulthood (Ribak & Fallon, 1982); moreover, the IC are areas which have high levels of mRNA for connexin 32 (Micevych & Abelson, 1991), one of a family of proteins that form gap junction structures (e.g. Bennett et al. 1991; Dermietzel & Spray, 1993). Direct evidence for electrical coupling of about 300 pS between IC neurones has been obtained in the present study, albeit in only one pair so far. Taken together, these observations show that electrical coupling can occur and support the notion that changes in the efficiency of coupling between IC neurones account for their spontaneous activity and labile membrane potentials. It follows from this hypothesis that the electrical driving force underlying the observed responses would be the difference in voltage between that of the recorded cell and the average membrane potential of the neurone (or neurones) to which it couples; in these circumstances the distribution of apparent reversal potentials for the conductance changes should be predictable from the distribution of population membrane potentials. In support, this was borne out experimentally. The hypothesis also predicts that, given a random spread of membrane potential values, coupling of neurones should lead to both hyperpolarizing and depolarizing effects being observed, which was found to be the case. The variability of IC membrane potential probably reflects this situation. Moreover, when the granule cells were depolarized by application of a high K+-containing ACSF, a positive shift in both the apparent reversal potential of the spontaneous conductance changes and the population membrane potential was observed to occur in parallel.
It is necessary, however, to consider alternative explanations. The magnitude and ionic nature of the spontaneous conductance changes deserve some comment. The smallest observed change was ∼100 pS, which could correspond to a large single channel opening between the intracellular compartment and the extracellular space. Volume-activated or other non-specific ion channels would be likely candidates but for (i) reversal potentials significantly different from zero and with a very wide range of values, and (ii) a failure to be blocked in the presence of up to 100 μm Gd3+(Yang & Sachs, 1989). The reversal potentials also argue against the participation of large conductance K+ or Cl− channels given the present set of reversal potentials of -85 and -61 mV, respectively. Long open and closed times often with little evidence of intervening transitions do not accord with the activity of these candidate channels either. Much larger discrete conductance changes, up to ∼600 pS in magnitude, are even more difficult to account for in terms of a single channel model. By the process of elimination, therefore, the gap junction hypothesis remains the most attractive.
We previously (Halliwell & Horne, 1995) discounted the possibility of such coupling on the basis of comparing our measurements of resting input resistance with those of other investigators working with comparably sized neurones - cerebellar or olfactory bulb granule cells, for which there was no a priori presumption of electrical coupling. We now revise our position in the light of present evidence and in view of recent reports of much higher input resistance for cerebellar granule cells (up to 10 GΩ) encountered in thin slice preparations (Brickley, Cull-Candy & Farrant, 1996) and the possibility that olfactory bulb granule cells could also be electrically coupled (Reyher, Lubke, Larsen, Hendrix, Shipley & Baumgarten, 1991). Assuming the true input resistance of an IC granule cell to be around 10 GΩ and that coupling to others of a similar resistance was via a gap junction conductance of 100 pS, we estimate that only one additional coupled cell is required to reduce the observed input resistance to 7 GΩ, and if the cell to be coupled is itself coupled to three others, then the observed input resistance falls to around 3 GΩ. It is not difficult to envisage how, in practice, a variably coupled collection of just a few IC neurones could give rise to input resistances of the order of 3 GΩ and, as coupling efficiency fluctuated, produce observed conductance changes of between 100 and 600 pS. Results of the experiments with the gap junction blocker GA are also consistent with the hypothesis, there being a reduction in the resting conductance of cells in the presence of 10 μm GA. Whilst this result was not statistically significant, the reason for this was the inhomogeneity of variance between the two sets of experimental data. If permeant gap junctions were contributing to the observed overall variation in the magnitude of cell conductance, then a reduction in variance would be the predicted outcome of gap junction blockade. A previous report has suggested that GA reduces gap junctional conductance by about 75 % (Goldberg et al. 1996). It is noteworthy in this context that GA significantly reduced the capacitative load observed during our recordings, again suggesting the possibility of multiples rather than single cells. In our previous discussion of possible electrical synapses between IC neurones we left the proviso that some factor or factors might be required for the promulgation of coupling (Halliwell & Horne, 1995). In the next section, which discusses the action of dopamine and its congeners on IC activity, the possibility is raised that dopamine itself could be one such factor.
The mechanism of dopaminergic action in island of Calleja granule cells
Administration of dopamine and its congeners active at the D3 subclass of DA receptors induced a complex but consistent sequence of events when applied at maximal concentrations of ∼2 μm. From a quiescent state a latency of several minutes ensued before first a minimal response was encountered. The minimal response was initially indistinguishable from the spontaneous events except that the smallest induced conductance changes tended to be larger than those occurring spontaneously. In most cells, particularly after repeated applications of effective agonists, the minimal phase of response gave way to a precipitate depolarization or large inward current which we have termed the ‘cataclysmic’ response. During the ‘cataclysmic’ phase the cells sampled by the recording electrode became very leaky; a thirtyfold increase in conductance was not uncommon, for periods of up to tens of minutes. Cell death or electrode-cell seal breakdown would be an obvious explanation, were it not for an impressive degree of recovery; indeed, it was possible to extract cells from the experimental preparation even before recovery (see Fig. 12) to restore former conductance levels, or, alternatively pull off excised or nucleated patches (J. V. Halliwell, unpublished observations). Evidence for cyclical activity was also obtained, in particular following repeated agonist application. Substitution of more than 80 % of extracellular Na+ by ions having larger ionic radii failed to modify the form of either the minimal or ‘cataclysmic’ activity, the reversal potential for the latter remaining close to zero. This would suggest either the conductance induced by DA was between compartments unaffected by changes in extracellular [Na+] or involved an additional transmembrane pathway characterized by a large pore size and lack of ionic selectivity. Bearing in mind also a resistance to Gd3+ block and reversal potentials far from those for Cl− and K+, few channel types fit this picture and, therefore, the involvement of gap junction channels is the most likely explanation. In further support of this, intracellular alkalinization (cf. Spray, Harris & Bennett, 1981) generated a very similar pattern of activity to that evoked by dopaminergic agonists. As discussed above, dramatic conductance changes could be brought about by coupling relatively few cells. A substantial interconnectivity, extending to neurones on the periphery of the preparation that might be depolarized and/or leaky, could account for the present experimental observations. A further possibility, also involving gap junctions, or at least their hemiconstituents (e.g. De Vries & Schwartz, 1992) is that such pathways, present in plasmsa membrane, but unpaired as a result of cell-cell separation in vitro could be opened as a consequence of dopamine receptor activation. Gap junction hemichannels have even larger unitary conductance and low ionic selectivity and their permeating currents share features in common with the present response. When unpaired hemichannels have been studied in retinal horizontal cells grown at low density (De Vries & Schwartz, 1992) or expressed experimentally in oocytes, their currents have been variously induced: e.g. by reducing extracellular [Ca2+] or by voltage change (Ebihara, Berthoud & Beyer, 1995) and they are as demonstrably modulable by neurotransmitters and second messengers (De Vries & Schwartz, 1992) as is functional electrical coupling (e.g. Pereda, Nairn, Wolszon & Faber, 1994).
It is clear, therefore, that intercellular gap junctional conductance can be affected by a variety of conditions and an additional way it has been studied in the nervous system has been to investigate dye coupling of cells - the intercellular diffusion of permeant marker substances with molecular weights of up to ∼1000. Dopamine via a D1-like receptor reduces both electrical and dye coupling in retinal horizontal cells (e.g. De Vries & Schwartz, 1989) and through a similar receptor decreases dye transfer in neonatal cortex (Rörig, Klausa & Sutor, 1995) and the neostriatum (Onn & Grace, 1994) although D1-receptor activation enhances coupling at the electrical synapse onto the goldfish Mauthner cell (Perada et al. 1994). A D1-like receptor is also implicated in reducing transfer of Lucifer Yellow between neurones of the core region of the nucleus accumbens and also in the surrounding shell of that structure (O'Donnell & Grace, 1993). Interestingly O'Donnell & Grace (1993) found that D2-like agonists potentiated dye coupling in the shell region, which contains a single large Calleja island (Meyer, Gonzalez-Hernandez, Carrillo-Padilla & Ferres-Torres, 1989) and shares many other similarities (Alheid & Heimer, 1988) with the olfactory tubercle, including a marked presence of D3 receptors (Landwehrmeyer et al. 1993a; Diaz et al. 1995). The contribution of the particular subclass of D2-like receptor responsible for potentiated dye coupling could not have been distinguished pharmacologically by the compounds that O'Donnell & Grace (1993) had available and it is possible that these effects were due to activation of D3 receptors in the accumbens.
The pharmacology of dopaminergic responses in the islands of Calleja
There is consensus (see Diaz et al. 1995) that, of the D2-like dopamine receptors, the islands of Calleja contain only the D3 subclass; in addition they also posses D1 (and possibly D5; Sunahara et al. 1991) receptors. Demonstration of message for these receptors within the somata of these intrinsic cells firmly establishes the granule neurones as targets for the effects of synaptically released dopamine (Landwehrmeyer et al. 1993a; Diaz et al. 1995). The well established pharmacological separation of dopamine action as being via D1-like or D2-like receptor populations can therefore be employed in IC in vitro preparations to classify any observed dopaminergic effects (Sokoloff & Schwartz, 1995). The present dopaminergic actions were initiated directly at DA receptors since they were still observed under conditions where transmitter release was blocked. The progressive effects of dopaminergic stimulation were refractory to the D1 antagonist SCH-23390 and were not reproduced by the D1 agonist SKF-38393. Reversible antagonism of the D3/D2 agonist quinpirole by haloperidol indicates a D2-like pharmacology.
Furthermore, submicromolar concentrations of compounds with known selectivity for the D3 receptor - 7-OH DPAT and PD 128907 (see Sokoloff & Schwartz, 1995) - evoke responses similar to those produced by dopamine. Given the receptor population reported to be present in the IC, these results strongly suggest that DA-mediated enhancement of coupling is initiated via the D3 receptor.
The functional consequences of postsynaptic or somatic D3 receptor activation in situ have not previously been defined, there being a doubt about the specificity of available compounds used to analyse dopaminergic effects on D3 receptors in the ventral tegmental area of the midbrain (Bowery et al. 1996). Mitogenesis, c-Fos production, reduction of adenylyl cyclase activity and inhibition of calcium currents have all been variously described as a consequence of agonist activation in a number of cell lines forced to express the D3 receptors (see Sokoloff & Schwartz, 1995). Clearly the effects of activating any G-protein-coupled receptors depends on the effector system to which they are linked, whereby divergent cell-specific signal cascades could be triggered. The effector system(s) underlying the present experimental observations are entirely unknown and could only be speculated upon at the present time. The extended latency before observing effects of dopaminergic agonists suggests that a biochemical cascade of some complexity might be involved in mediating the response; the final path for affecting gap junctional conductance could include phosphorylation/dephosphorylation of channel constituents or a shift in the intracellular pH. There is no shortage of possible ways that different intracellular chemical mechanisms could interact to bring this about. Moreover, putative gap junctional coupling of IC neurones permits the intercellular transfer of chemical messengers with possible recruitment of additional cells that may not even possess DA receptors. The oscillatory responses that follow cellular alkalinization accord with this notion.
Physiological consequences of dopaminergic action in the islands of Calleja
The physiological role of the olfactory tubercle in general and the islands of Calleja in particular remains obscure (see discussion in Halliwell & Horne, 1995), although recently a link to cardiovascular control has been suggested for the IC (McKitrick, Krukoff & Calaresu, 1992; Calaresu, Zhang, Chitravanshi & McKitrick, 1994). In spite of this, descriptions of the anatomy of the IC and their counterparts in vertebrate species are detailed (see Meyer et al. 1989 for references); granule cell clusters with IC-like morphology are seen in the brain of man (in the anterior perforated substance, the human vestige of the olfactory tubercle- ventral striatum) where they, too, contain D3 receptors (Landwehrmeyer, Mengod & Palacios 1993b; Murray, Ryoo, Gurevich & Joyce, 1994). In all species, the granule cells are associated with large (> 20 μm diameter) neurones residing in a cell-sparse (hilar) region that forms a dorsal concavity in the overall shape of islands; the large cells possess dendrites which extend only as far as the outer edges of the granule cell clusters and intimate contacts have been described between IC granules and these dendrites at the light microscope level (Fallon, Riley, Sipe & Moore, 1978; Millhouse & Heimer, 1984; Meyer & Wahle, 1986; Millhouse, 1987; Meyer et al. 1989). As well as gap junctions, conventional chemical synapses are apparent within the IC complexes (Ribak & Fallon, 1982). The granule cells are probably GABAergic, being positive for the GABA synthetic enzyme glutamic acid decarboxylase (Mugnaini & Oertel, 1985; Smith, Parent, Seguela & Descarries, 1987) and consistent with this we have preliminary evidence that IPSPs are triggered in IC neurones following stimulation of other granules (J. V. Halliwell, unpublished observations). The consensus is that IC granules are entirely intrinsic and their main targets are the large (hilar) cells with which the islands associate, and which provide the efferents from this area (Fallon, Loughlin & Ribak, 1983). Dopaminergic innervation of the IC is sparse, being limited to the outer borders; this has led to the suggestion that synaptically released DA may have to diffuse considerable distances to gain access to D3 receptors (Diaz et al. 1995). Even in the face of avid uptake processes, this is reasonable in view of the fact that, of the DA receptor subtypes studied so far, the D3 receptor has the highest affinity for agonists (Sokoloff & Schwartz, 1995). Our bath applications of DA and other D3 agonists may mimic this putative transmission process quite well. The coupling of granule cells under the influence of D3 receptor activation, then, would convert neuronal microcircuits normally responsible for point to point inhibition into a wholesale inhibitory syncytium preventing efferent activity from the IC complex. Moreover, electrical excitatory coupling would simultaneously override the possible effects of disinhibition between individual granule cells in order to maximize their inhibitory influence.
Acknowledgments
This work was supported by The Wellcome Trust.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Sáez JC. Gap junctions: new tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bjorkland A, Lindvall O. Dopamine-containing systems in the CNS. In: Bjorkland A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. part 1. Vol. 2. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- Blanton MG, Turco JJ, Kriegstein AR. Whole-cell recording from neurones in slices of reptilian and mammalian cerebral cortex. Journal of Neuroscience Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bowery BJ, Razzaque Z, Emms F, Patel S, Freedman S, Bristow J, Kulagowski J, Seabrook GR. Antagonism of the effects of (+)-PD 128907 on midbrain dopamine neurones in rat brain slices by a selective D2 receptor antagonist L-741,626. British Journal of Pharmacology. 1996;119:1491–1497. doi: 10.1111/j.1476-5381.1996.tb16063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. Journal of Physiology. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaresu FR, Zhang J, Chitravanshi VC, McKitrick DJ. Cardiovascular and single unit responses elicited by stimulation of the islands of Calleja and by changes in arterial pressure. Brain Research. 1994;655:45–50. doi: 10.1016/0006-8993(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellullar communication. Structure-activity relationships. Journal of Pharmacology and Experimental Therapeutics. 1988;246:1104–1107. [PubMed] [Google Scholar]
- Dermietzel R, Spray DC. Gap junctions in the brain: where, what type, how many and why? Trends in Neurosciences. 1993;16:186–192. doi: 10.1016/0166-2236(93)90151-b. 10.1016/0166-2236(93)90151-B. [DOI] [PubMed] [Google Scholar]
- Vries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. Journal of Physiology. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries SH, Schwartz EA. Hemi gap-junction channels in solitary horizontal cells of the catfish retina. Journal of Physiology. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Lévesque D, Lammers CH, Griffon N, Martres M-P, Schwartz J-C, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-c. 10.1016/0306-4522(94)00527-C. [DOI] [PubMed] [Google Scholar]
- Ebihara L, Berthoud VM, Beyer EC. Distinct behaviour of connexin56 and connexin46 gap junctional channels can be predicted from the behaviour of their hemi-gap-junctional channels. Biophysical Journal. 1995;68:1796–1803. doi: 10.1016/S0006-3495(95)80356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch-clamp recordings from synaptically connected neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE, Ribak CE. The islands of Calleja complex of rat basal forebrain. III. Histochemical evidence for a striatopallidal system. Journal of Comparative Neurology. 1983;218:91–120. doi: 10.1002/cne.902180106. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Riley JN, Sipe JC, Moore RY. The islands of Calleja: organization and connections. Journal of Comparative Neurology. 1978;181:375–395. doi: 10.1002/cne.901810209. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Bechberger JF, Hearn SS, Shivers RR, Macphee DJ, Zhang Y-C, Naus CCG. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Experimental Cell Research. 1996;222:48–53. doi: 10.1006/excr.1996.0006. [DOI] [PubMed] [Google Scholar]
- Halliwell JV, Horne AL. Membrane properties of the granule cells of the islands of Calleja of the rat studied in vitro. Journal of Physiology. 1995;487:421–440. doi: 10.1113/jphysiol.1995.sp020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell JV, Horne AL. Dopamine action in the rat islands of Calleja in vitro. Journal of Physiology. 1996;494P:82–83P. [Google Scholar]
- Halliwell JV, Horne AL. The spontaneous behaviour of rat island of Calleja granule cells in vitro: participation of gap junctions? Journal of Physiology. 1997;501P:26–27P. [Google Scholar]
- Landwehrmeyer B, Mengod G, Palacios JM. Differential visualization of dopamine D2 and D3 receptor sites in rat brain. A comparative study using in situ hybridization histochemistry and ligand binding autoradiography. European Journal of Neuroscience. 1993a;5:145–153. doi: 10.1111/j.1460-9568.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer B, Mengod G, Palacios JM. Dopamine D3 receptor mRNA and binding sites in human brain. Molecular Brain Research. 1993b;18:187–192. doi: 10.1016/0169-328x(93)90188-u. [DOI] [PubMed] [Google Scholar]
- Logan SD, Pickering AE, Gibson IC, Nolan MF, Spanswick D. Electrotonic coupling between rat sympathetic preganglionic neurones in vitro. Journal of Physiology. 1996;495:491–502. doi: 10.1113/jphysiol.1996.sp021609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKitrick DJ, Krukoff TL, Calaresu FR. Expression of c-fos protein in rat brain after electrical stimulation of the aortic depressor nerve. Brain Research. 1992;599:215–222. doi: 10.1016/0006-8993(92)90394-o. [DOI] [PubMed] [Google Scholar]
- Meyer G, Gonzalez-Hernandez T, Carrillo-Padilla F, Ferres-Torres R. Aggregations of granule cells in the basal forebrain (islands of Calleja): Golgi and cytoarchitectonic study in different mammals, including man. Journal of Comparative Neurology. 1989;284:405–428. doi: 10.1002/cne.902840308. [DOI] [PubMed] [Google Scholar]
- Meyer G, Wahle P. The olfactory tubercle of the cat I. Morphological components. Experimental Brain Research. 1986;62:515–527. doi: 10.1007/BF00236030. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Abelson L. Distribution of mRNAs coding for liver and heart gap junction proteins in the rat central nervous system. Journal of Comparative Neurology. 1991;305:96–118. doi: 10.1002/cne.903050110. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. Granule cells of the olfactory tubercle and the question of the Islands of Calleja. Journal of Comparative Neurology. 1987;265:1–24. doi: 10.1002/cne.902650102. [DOI] [PubMed] [Google Scholar]
- Millhouse OE, Heimer L. Cell configurations in the olfactory tubercle of the rat. Journal of Comparative Neurology. 1984;228:571–597. doi: 10.1002/cne.902280409. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals of the rat CNS as revealed by GAD immunohistochemistry. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. part 1. Vol. 4. Amsterdam: Elsevier; 1985. pp. 436–608. [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proceedings of the National Academy of Sciences of the USA. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. Journal of Neuroscience. 1993;13:3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Dye coupling between rat striatal neurons recorded in vivo: compartmental organization and modulation by dopamine. Journal of Neurophysiology. 1994;71:1917–1934. doi: 10.1152/jn.1994.71.5.1917. [DOI] [PubMed] [Google Scholar]
- Pereda AE, Nairn AC, Wolszon LR, Faber DS. Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell. Journal of Neuroscience. 1994;14:3704–3712. doi: 10.1523/JNEUROSCI.14-06-03704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon C, Lévesque D, Dimitriadou V, Griffon N, Martres M-P, Schwartz J-C, Sokoloff P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108–15 neuroblastoma-glioma hybrid cell line. European Journal of Pharmacology. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Pocock G, Richards CD. Hydrogen ion regulation in rat cerebellar granule cells studied by single-cell fluorescence microscopy. European Journal of Neuroscience. 1992;4:136–143. doi: 10.1111/j.1460-9568.1992.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Reyher CK, Lubke J, Larsen WJ, Hendrix GM, Shipley MT, Baumgarten HG. Olfactory bulb granule cell aggregates: morphological evidence for interperikaryal electrotonic coupling via gap junctions. Journal of Neuroscience. 1991;11:1485–1495. doi: 10.1523/JNEUROSCI.11-06-01485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Fallon JH. The island of Calleja complex of rat basal forebrain. I. Light and electron microscopic observations. Journal of Comparative Neurology. 1982;205:207–218. doi: 10.1002/cne.902050302. [DOI] [PubMed] [Google Scholar]
- Rörig B, Klausa K, Sutor B. Dye coupling between pyramidal neurons in developing rat prefrontal and frontal cortex is reduced by protein kinase A activation and dopamine. Journal of Neuroscience. 1995;15:7386–7400. doi: 10.1523/JNEUROSCI.15-11-07386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Kemp JA, Freedman SB, Patel S, Sinclair HA, McAllister G. Functional expression of human D3 dopamine receptors in differentiated neuroblastoma × glioma NG 108–15 cells. British Journal of Pharmacology. 1994;111:391–393. doi: 10.1111/j.1476-5381.1994.tb14746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Parent A, Seguela P, Descarries L. Distribution of GABA-immunoreactive neurons in the basal ganglia of the squirrel monkey (Saimiri sciureus) Journal of Comparative Neurology. 1987;259:50–64. doi: 10.1002/cne.902590105. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Schwartz J-C. Novel dopamine receptors half a decade later. Trends in Pharmacological Sciences. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MVL. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Guan H-C, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Tol HHM, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Yang X-C, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]


