Abstract
Peristalsis, which involves enteric nervous reflexes, is the co-ordinated movements of the longitudinal (LM) and circular (CM) muscle layers that propel intraluminal contents down the bowel. Although the movements of the CM during peristalsis are reasonably clear the relative movements of the LM are poorly understood.
We studied the oral and anal movements of the LM and CM during a peristaltic wave in isolated segments of guinea-pig distal colon. Dissection techniques were used to prevent mechanical interactions between the LM and CM; also, the colonic segment was passed through a partition to prevent mechanical disturbances created by a peristaltic wave in the bulk of the colon from influencing the end from which recordings were made.
Peristalsis was generated by slowly filling the lumen of the colon with fluid. At threshold, the LM and CM synchronously contracted oral (ascending excitation) to, and relaxed anal (descending inhibition) to, a peristaltic wave. The anal relaxation was followed by a contraction (descending excitation) of both muscle layers.
Atropine (1 μm) in the recording chamber reduced both the oral (LM by 40% and CM by 27%) and anal (LM by 36% and CM by 36%) contractile responses as well as the anal relaxation response in both muscle layers. Hexamethonium (300 μm) almost blocked the oral contractile responses of the LM and CM but had no affect on the anal responses of either muscle layer.
Nω-nitro-l-arginine (l-NA; 100 μm) reduced the oral contractile response of the LM and CM by 50%, the anal contractile response of the LM by 30%, and the anal relaxation response of the LM and CM by about 30%. The anal contractile response of the CM was unaffected by l-NA.
Apamin (0.5 μm) also reduced the evoked anal relaxation of both the LM and CM by about 50%. Further addition of l-NA nearly abolished the relaxation response in the LM, but did not cause any further reduction in the relaxation response of the CM observed in apamin alone.
It is concluded, that the LM and CM exhibit synchronous movements during peristalsis in the colon. Also, peristalsis consists of activation of ascending excitatory, and descending inhibitory and excitatory nervous pathways to the LM and CM, which are cholinergic and non-cholinergic, respectively. Nitric oxide is an important neuromodulator within the intrinsic nervous pathways.
At the turn of the century Bayliss & Starling (1899, 1900) demonstrated that distention of an intraluminal balloon in the extrinsically denervated canine small or large intestine evoked a contraction of the circular muscle oral, and a relaxation anal, to the stimulus. The free inflated balloon propagated down the intestine, was followed by a wave of constriction and preceded by a wave of relaxation of the circular muscle. As these responses were blocked by neural antagonists, they concluded that peristalsis was due to reflex activation of polarized intrinsic nervous pathways. In support of these conclusions, studies using tension recordings or intracellular microelectrodes have shown that distension or mucosal stimulation elicit polarized reflexes: contraction and excitatory junction potentials (EJPs), and relaxation and inhibitory junction potentials (IJPs), in the circular muscle of the small and large intestine, oral and anal to the stimulus, respectively (Hirst & McKirdy, 1974; Hirst, Holman & McKirdy, 1975; Costa & Furness, 1976; Smith, Bornstein & Furness, 1991; Smith, Bywater, Holman & Taylor 1992; reviewed in Smith & McCallum, 1995). Excitatory junction potentials have also been observed to follow evoked anal IJPs in the circular muscle of both the small and large intestine suggesting that contractions elicited by activation of descending excitatory nervous pathways may also play a major role in peristalsis (Hirst et al. 1975; Smith et al. 1992; Smith & McCallum, 1995).
The longitudinal and circular muscle layers of the intestine are innervated by different populations of motor neurons (Bornstein, Furness, Smith & Trussell, 1991; Smith, Bornstein & Furness, 1992; Smith & McCallum, 1995). These supply both an excitatory and inhibitory innervation to both muscle layers in the colon (Furness, 1969; Costa, Furness & Humphreys, 1986), suggesting that the two muscles can act independently of one another. Slow waves, however, occur synchronously in both muscle layers of the intestine (Bortoff, 1965; Smith, Reed & Sanders, 1987; Smith & Sanders, 1995). Slow waves in the longitudinal muscle elicit action potentials more often than those in the circular muscle which is probably why the longitudinal muscle exhibits rhythmic rocking or pendular movements, whereas contractions of the circular muscle occur less often (Bayliss & Starling, 1899, 1900; Mackennna & McKirdy, 1972). Although the movements of the circular muscle during peristalsis are relatively straight forward, the movements of the longitudinal muscle are unclear. Bayliss & Starling (1899) presented evidence that both muscle layers contract and relax together, behind and in front of a propagating balloon, respectively. Studies in isolated rabbit colon by Caprilli, Frieri & Vernia (see Figs 10–13 in Smith & Sanders, 1995) support these observations. They found that spontaneous action potentials (measured with serosal electrodes), attributed to electrical activity of the longitudinal muscle, were suppressed below (descending inhibition), and transiently increased above (ascending excitation) a propagating balloon. Yokohama & Ozaki (1990) measured the electrical activity of myenteric neurons and circular muscle with pressure electrodes together with contractions of the longitudinal muscle layers in guinea-pig ileum, and came to the conclusion that although the two muscles moved independently, at threshold both muscle layers contracted together.
Trendelenburg (1917) described the peristaltic reflex in isolated segments of small intestine. He concluded that in response to a continuous infusion of fluid into the lumen there was a graded contraction of the longitudinal muscle. This was followed by a wave-like contraction of the circular muscle which travelled down the intestine and expelled fluid from the lumen. The contraction of the longitudinal muscle is referred to as the preparatory phase or Type I contraction (Kosterlitz & Robinson, 1959; Kosterlitz & Lees, 1964). The all-or-none peristaltic contraction of the circular muscle is referred to as the Type II contraction. The Type I contraction is partly mediated by cholinergic nerves since it is reduced by atropine but resistant to hexamethonium, and to the passive mechanical interactions between the two muscle layers, since shortening of the gut can occur even after neural blockade (Kosterlitz & Robinson, 1959; Kosterlitz & Lees, 1964). The Type II contraction, on the other hand, is purely neural in origin since it is reduced or blocked by atropine and hexamethonium (Kosterlitz & Robinson, 1959; Kosterlitz & Lees, 1964; Smith & McCallum, 1995).
A modified Trendelenburg method has also been used to study peristalsis in the large intestine (Gary & Gillespie, 1955; Mackenna & McKirdy, 1972). Peristalsis in the large intestine is also dependent on enteric nervous reflexes since it is blocked by tetrodotoxin or hexamethonium (Crema, Frigo & Lecchini, 1970; Mackenna & McKirdy, 1972). In the large intestine peristalsis has a higher threshold and a slower conduction velocity than in the small intestine (Mackenna & McKirdy, 1972; Frigo & Lecchini, 1970). The movements of the muscle layers in the colon appear to be somewhat more complex than those in the small intestine. Although no clear preparatory phase was observed in the large intestine using the Trendelenburg technique (Mackenna & McKirdy, 1972), others have observed a phasic contraction followed by a more sustained contraction of the longitudinal muscle when a balloon was inflated in the isolated distal colon (Crema et al. 1970; Frigo & Lecchini, 1970). In preparations with a high degree of tone, however, distension initially produced relaxation of the longitudinal muscle (Frigo & Lecchini, 1970). The phasic contraction of the longitudinal muscle was followed after a short delay by contraction and relaxation of the circular muscle above and below the balloon respectively (Crema et al. 1970; Frigo & Lecchini, 1970). These muscle responses were inhibited by tetrodotoxin and hexamethonium (Crema et al. 1970).
Kottegoda (1969) found that contractions of the circular muscle appeared to alternate with those in the longitudinal muscle during peristalsis in the small intestine. Also that the two muscle layers responded differently to transmural nerve stimulation and various drugs. Kottegoda therefore put forward the hypothesis, which is now widely accepted, that the two muscle layers of the intestine are reciprocally innervated: when the longitudinal muscle contracts the circular muscle relaxes and vice versa.
Many investigators have assumed that by connecting a tension transducer to one end of a segment of intestine they are recording movements of the longitudinal muscle when the gut shortens or lengthens (Gary & Gillespie, 1955; Crema et al. 1970; Frigo & Lechinni, 1970; Mackenna & McKirdy, 1972). Gregory & Bentley (1968) drew attention to the possibility of passive mechanical interactions between the two muscle layers. They found that shortening of the gut was reduced when the circular muscle was prevented from contracting. Wood & Perkins (1970) systematically studied these mechanical interactions and clearly demonstrated that when the gut is radially stretched the length passively shortens and vice versa. These interactions, which result from the intestine acting like a constant volume cylinder, were unaffected by neural blockade and by removal of the longitudinal muscle.
The aims of this study were to re-investigate the relative movements of the longitudinal and circular muscle layers during peristalsis in the isolated guinea-pig distal colon. The distal colon generates robust responses of these muscles during propulsion (Frigo & Lecchini, 1970; Crema et al. 1970). In light of the above concerns, we have developed techniques and used a partitioned bath to allow us to mechanically isolate the oral or anal responses of the longitudinal and circular muscle layers from each other and from the mechanical disturbance caused by a peristaltic wave in an isolated segment of distal colon.
We present evidence that the longitudinal and circular muscle layers move synchronously both oral and anal to a peristaltic wave. A preliminary account of these findings has been published in abstract form (Robertson, Malarkey, Shuttleworth & Smith, 1997).
METHODS
Guinea-pigs weighing 250–350 g were killed in a specially constructed chamber with an overdose of CO2. The abdomen was then cut open, the distal colon removed and the pellets expelled by gently flushing the lumen with a modified Krebs solution (see below) applied via a syringe to the oral end of the segment. The extrinsic blood vessels and nerves along the mesenteric border of the colon were then carefully trimmed away.
Peristaltic bath
We modified the peristaltic apparatus described by Tonini, Frigo, Lecchini, D'Angelo & Crema (1981) by dividing the organ bath with a partition into a peristaltic chamber (length, 55 mm; width, 35 mm; volume, 60 ml) and recording chamber (length, 45 mm; width, 35 mm; volume 40 ml; Fig. 1). Both chambers contained continuously oxygenated Krebs solution at 37°C. After dissection (see below), the segment of colon was passed through a relatively tight greased hole (i.d., 1–1.5 mm) in a condom rubber membrane, which was sandwiched between two Perspex sheets that formed the partition. The colon was then mounted (mesenteric border below) at each end over rigidly held plastic tubes (i.d., 1.5 mm) as shown (Fig. 1). The dissected (see below) and intact ends of the segment were mounted in the recording chamber (see below) and peristaltic chamber, respectively. Each plastic tube formed the outlet or inlet for luminal fluid depending on whether anal or oral responses were recorded, respectively. The plastic tube in the recording chamber fitted snugly through the hole in the rubber membrane and protruded 2 mm into the peristaltic chamber; this insured that movement of the fluid with each peristaltic wave did not mechanically influence the end of the colon in the recording chamber from which recordings were made. It was essential to ensure that the plastic tube protruding through the hole in the membrane did not cause sufficient restriction to damage intrinsic nerve pathways running to the longitudinal and circular muscle layers in the recording chamber. The end of the segment in the peristaltic chamber was then cut to length (4 cm) and attached over the plastic tube in this chamber with an ‘O’ ring. Leaks between chambers were prevented by silicon grease around the hole in the membrane. Leaks were easily detected because the fluid in each chamber was maintained at a different level. Tetrodotoxin (1 μm) or atropine (1 μm) were routinely added to the recording chamber to ensure that there were no leaks between the two chambers; any leak of these drugs from the recording chamber into the peristaltic chamber reduced or blocked peristalsis.
Figure 1. Peristaltic apparatus.

A segment of distal colon was threaded through a hole in a rubber diaphragm of a partition and then anchored at each end over inflow and outflow tubes. The partition functionally divided the bath into a recording chamber and a peristaltic chamber. The dissected end of the colon, which consisted of only longitudinal muscle and myenteric plexus (LMMP), was pinned to a bead of silastic near the junction with the intact colon. The other end of the dissected strip was attached to an isometric tension transducer (TLM), which measured movements of the longitudinal muscle. Movements of the circular muscle were made with a frog heart clip attached to the intact segment near the junction with the dissected LMMP strip and to another isometric tension transducer (TCM). A peristaltic wave was generated in the segment of colon lying in the peristaltic chamber by infusing the lumen with Krebs solution via a peristaltic pump against a constant back pressure provided by the height of the outflow tube and the one-way valve (V). The strength of the peristaltic wave was measured with a pressure gauge connected to the outflow line. The expelled fluid was weighed in a bucket attached to a tension transducer.
Recording of mechanical activity of the longitudinal and circular muscle
Approximately 8 cm of distal colon was pinned lightly to the Sylgard (Dow Corning Corp., MI, USA) floor of a dissection dish. The oral or anal end of the segment was cut open for 15 mm along the mesenteric border. The cut end was pinned flat with the mucosa uppermost and then the mucosa and circular muscle removed by sharp dissection, leaving a full width strip of longitudinal muscle with attached myenteric plexus (LMMP preparation) from which tension recordings could be made. The integrity of the myenteric plexus in the dissected region was routinely checked after each experiment by NADPH-diaphorase staining (Fig. 2), which reveals nitrergic neurons and nerve fibres (see Sanders & Ward, 1992). The dissected end of the colon was threaded through the rubber membrane and over the plastic tube so that 7 mm of intact colon, from which recordings of circular muscle activity were made, was also in the recording chamber (Fig. 1).
Figure 2.

Dissected longitudinal muscle myenteric plexus preparation (LMMP) revealed by NADPH-diaphorase staining method. The mucosa, submucosa and circular muscle were removed to create a preparation with an intact myenteric plexus lying on the longitudinal muscle.
Longitudinal muscle activity
The full width LMMP strip was pinned onto a bead of silicon rubber (RTV sealant 732, Dow Corning Corp., MI, U.S.A) close (< 2 mm) to the junction with the intact segment that was mounted on the plastic tube in the recording chamber. The free end of the longitudinal muscle was tied by a silk thread to an isometric tension transducer (Grass FT 03 D).
Circular muscle activity
The mechanical activity of the circular muscle was measured with a frog heart clip attached via the serosal surface to the underlying circular muscle of the intact colon in the recording chamber approximately 1–2 mm before the junction with the LMMP strip. The clip was attached to the side of the colon (angle of 60 deg from the antimesenteric border), which minimized interference with neural pathways running to the LMMP strip, and to another isometric tension transducer (Grass FT 03 D) via a silk thread. Care was taken to ensure that the thread was orthogonal to the long axis of the gut so that only movements of the circular muscle layer were recorded. The tension of the longitudinal and circular muscle was then stretched against the rigid (inlet or outlet) tube in the recording chamber to give an initial tension of 0.7 g.
Recording of oral and anal responses
When recording the anal responses of the longitudinal and circular muscle, the peristaltic pump, which provided luminal fluid for generating peristalsis, was connected to the tube in the peristaltic chamber which became the inflow tube and the tube in the recording chamber became the outflow tube (see Fig. 1). When recording oral responses the connections were reversed. When the anal responses of the longitudinal and circular muscle were measured the peristaltic wave was generated near the inflow tube in the peristaltic chamber and gradually moved towards the partition, the fluid being expelled through the outflow tube running under the recording sites. When the oral responses of the two muscles were measured the peristaltic wave was initiated just below the partition and moved down the segment away from the recording site, the fluid being expelled through the luminal tube in the peristaltic chamber.
Equilibration
The segment was equilibrated for 1.5–2 h. During this time the peristaltic bath slowly heated up to a stable working temperature of 37°C. The oxygenated Krebs solution in each chamber was replaced every 30 min throughout an experiment.
Peristalsis
After equilibration, the lumen was perfused with oxygenated Krebs solution at 37°C at a rate of 0.2 ml min−1 via a peristaltic pump (Masterflex 7523–30 with cartridge 3519–85, Cole-Palmer, USA) which initiated peristaltic waves in the peristaltic chamber of constant amplitude and duration. To prevent run-down of peristalsis the infusion pump was stopped and the lumen emptied by gently sucking out most of the fluid with a syringe connected to the outflow line and the preparation allowed to rest for 10–15 min before the pump was restarted. In this way peristaltic waves of similar strength could be recorded for up to 6 h. During the rest period there were occasional weaker spontaneous peristaltic waves which originated at various sites along the colon. Only those preparations were considered for analysis in which peristaltic waves were initiated at the oral end of the colon and travelled the full length of the segment spanning the peristaltic chamber.
Peristaltic waves were measured with a pressure gauge (Gould PT 23 10) connected to the outflow tube, which monitored the pressure in the lumen of that portion of the segment of colon in the peristaltic chamber (Fig. 1). The outflow tube was connected to a one-way valve so as to restrict backflow of fluid during a peristaltic wave; the end of the outflow tube was situated 3 cm above the inflow tube. The volume of Krebs solution ejected with each peristaltic wave was determined by expelling the fluid into a bucket which was weighed with an isometric tension transducer (Grass FT 03 D).
The luminal pressure, tension of the longitudinal and circular muscle layers and ejected fluid volume were recorded on a four-channel Gilson Medical Electronics 5/6H Recorder (WI, USA).
Drugs and solutions
In order to determine the pharmacology of the responses to a peristaltic wave, drugs were only added to the recording chamber. If a drug persistently altered the tone of the longitudinal or circular muscle the tension on the muscle was re-adjusted back to its original 0.7 g of tension. The drugs used in these studies were apamin, atropine sulphate, hexamethonium bromide, Nω-nitro-L-arginine (l-NA), L-arginine, D-arginine and tetrodotoxin (TTX; Sigma, USA). Stock solutions (10 mm) were prepared in saline (0.9% NaCl) or distilled H2O. Drugs were added to the bath in volumes less than 1% of bath volume. Corresponding volumes of solvents had no effect.
The composition of the modified Krebs solution was (mM): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; and glucose 11.5. The solution was gassed continuously with carbogen, a mixture containing 3% CO2-97% O2 (v/v) to give a solution with a pH of 7.3–7.4.
Analysis of data and statistics
Some of the measured parameters associated with a peristaltic wave are shown schematically in Fig. 3. The effect of drugs on the area under responses of the longitudinal and circular muscle layers to a peristaltic wave were assessed by comparing the mean responses to at least six peristaltic waves measured before and after the equilibration of the responses to a drug applied to the recording chamber. The area under a particular response and other parameters were measured using a Jandel Opaque Digitizing Tablet and Sigma Scan software (Scientific Measuring System, Jandel Scientific, California, USA) and results stored on computer. All data are presented as means ±s.e.m. taken from n guinea-pigs. Statistical analysis of results was performed with Student's t tests for either paired or unpaired data. A significance level of 0.05 was used for all statistical tests.
Figure 3. Parameters associated with responses to a peristaltic wave.
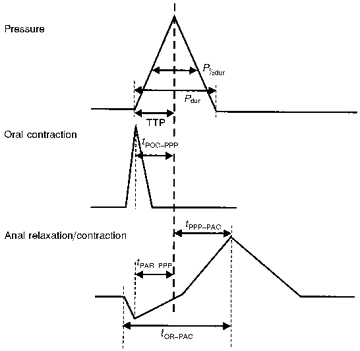
Pdur, duration of pressure pulse; P½dur, duration of pressure pulse at half-amplitude; TTP, time to peak; tPOC-PPP, time from peak of oral contraction to peak of pressure pulse; tPPP-PAC, time from peak of pressure pulse to peak of anal contraction; tPAR-PPP, time from peak of relaxation to peak of pressure pulse; tOR-PAC, time from onset of relaxation to peak of anal contraction.
RESULTS
Regular peristaltic waves of frequency 0.6 ± 0.1 min−1 were generated in the segment of distal colon lying in the peristaltic chamber by constant infusion of Krebs solution into the oral end of the lumen at a rate of 0.2 ml min−1. The threshold volume for generating a peristaltic wave was 0.30 ± 0.01 ml. Visual inspection showed that each peristaltic wave was a moving complex consisting of an oral constriction that completely occluded the lumen and a prominent anal dilatation of the gut that propagated distally over the full length of the colon. This complex was similar to that reported in the rabbit distal colon by Mackenna & McKirdy (1972) who also used fluid as a stimulus. Each peristaltic wave was associated with a transient rise in intraluminal pressure of amplitude 6.0 ± 0.4 mmHg, time-to-peak (TTP) of 16.3 ± 2.4 s (n = 20), duration of 25.35 ± 0.80 s (n = 20), and a small volume (0.24 ± 0.02 ml) of ejected fluid. These waves were of similar amplitude and duration to those recorded in the absence of the partition suggesting that the rubber membrane did not significantly alter peristalsis. By dividing the length (4 cm) of the intestine in the peristaltic chamber by the duration of the pressure pulse we obtained a conduction velocity of 1.57 ± 0.04 mm s−1, which was similar to the value of 1.5 ± 0.06 mm s−1 obtained by Frigo & Lecchini (1970), who used freely moving, inflated, intraluminal balloons, and to 0.5–1.5 mm s−1 by Costa & Furness (1976), who used epoxy resin coated pellets, as a stimulus for peristalsis in the guinea-pig distal colon.
Responses of the longitudinal and circular muscle layers
Figure 4 shows the characteristic oral (A) and anal (B) responses of both the LM and CM to a peristaltic wave.
Figure 4. Responses to a peristaltic wave.

A and B, oral and anal responses, respectively, to a peristaltic wave taken from two different tissues. Top trace, mechanical activity of longitudinal muscle (LM); 2nd trace, mechanical activity of circular muscle (CM); 3rd trace, intraluminal pressure (P); 4th trace, ejected fluid volume (EFV). Oral and anal responses aligned on peaks of their associated pressure pulses. Arrows indicate onset of relaxation and inflection in pressure wave. Prep, preparatory phase. Note that the peaks of the oral contraction coincide with those of the anal relaxation of the LM and CM.
Oral responses
At a threshold level of luminal distention, both the longitudinal muscle (LM) and circular muscle (CM) layers oral to the point of generation of the peristaltic wave (above the partition) exhibited a transient contraction which occurred simultaneously in both muscle layers. The peak of the oral contraction (POC) in the LM and CM occurred 5.0 ± 1.0 s and 4.7 ± 0.8 s (P > 0.05, n = 20), respectively, before the peak of the pressure pulse (PPP) (tPOC-PPP), suggesting that these contractions initiated peristalsis. Although the amplitude (LM, 35.50 ± 3.87 mN; CM, 43.41 ± 3.82 mN; P > 0.05; n = 20) and TTP (LM, 4.8 ± 0.4 s; CM, 6.5 ± 1.2 s; P > 0.2, n = 20) of these contractions were similar for both muscle layers, the duration (LM, 10.0 ± 0.8 s; CM, 14.2 ± 1.3 s; P < 0.01, n = 20) and the duration at half-amplitude (LM, 4.8 ± 0.6 s; CM, 6.4 ± 0.6 s; P < 0.05, n = 20; amplitude duration) of the contraction was significantly shorter in the LM than in the CM.
Anal responses
The anal responses during a peristaltic wave were complex and biphasic and consisted of a transient relaxation (descending inhibition) followed by a sustained contraction of both the longitudinal and circular muscle layers (Fig. 4). The peak of the anal relaxation (PAR) in the LM and CM occurred 4.2 ± 0.4 s and 5.4 ± 1.1 s (tPAR-PPP; P > 0.05, n = 20) before the peak of the pressure wave, and at about the same time as the peak of the oral contraction. The peak of the anal contraction (PAC), which also usually occurred at about the same time in both the LM and CM, followed the peak of the pressure wave (PPP) by 4.9 ± 0.8 s and 5.2 ± 0.8 s (tPAC-PPP; P > 0.05, n = 20), respectively (Fig. 4).
Anal relaxation
The amplitude (LM, 7.55 ± 0.43 mN; CM, 6.75 ± 0.49 mN; P > 0.3; n = 20), TTP (LM, 3.1 ± 0.4 s; CM, 3.7 ± 0.5 s; P > 0.2) and duration (LM, 8.1 ± 0.9 s; CM, 9.2 ± 1.8 s; P > 0.4) of the relaxation were similar in both muscle layers.
Anal contraction
The amplitude (LM, 44.75 ± 4.38 mN; CM, 54.00 ± 6.13 mN; P < 0.04), duration (LM, 13.0 ± 0.9 s; CM, 18.5 ± 3.2 s; P < 0.05), duration at half-amplitude (LM, 6.5 ± 0.7 s; CM, 9.0 ± 1.0 s; P < 0.03) and TTP (LM, 4.7 ± 0.4 s; CM, 8.9 ± 1.5 s; P < 0.02) of the anal contraction (PAC) were less for the LM than for the CM. The peaks of the contractions occurred 10.3 ± 1.3 s in the LM and 12.5 ± 1.5 s in the CM (P < 0.03, n = 20; tOR-PAC) after the onset of relaxation (OR), respectively.
Peristaltic complex
The relationship between the oral contractile responses and the anal relaxation responses of the LM and CM during a peristaltic wave can be observed by aligning the peaks of their associated pressure pulses (Fig. 4). The peak of the oral contraction occurs at the same time as the peak of the anal relaxation, suggesting that as the distention threshold is reached, the gut contracts orally and relaxes anally at the same time. The duration of the complete contractile complex measured from the onset of the oral contraction to the end of the anal contraction was 20.8 ± 1.2 s for the LM and 24.7 ± 2.2 s for the CM which were similar to the duration of the pressure pulse (24.35 ± 0.8 s) suggesting that both the oral and anal contractions of the LM and CM contribute to the peristaltic wave. The often observed inflection on the declining phase of the pressure pulse may result from activation of the anal contraction (Fig. 4).
Preparatory phase of peristalsis
Oral response during filling
The contractile activity of both muscle layers situated orally to a peristaltic wave usually gradually increased toward threshold for the initiation of peristalsis (Fig. 4), suggesting a stretch activation of ascending excitatory nervous pathways.
Anal response during filling
The contractile activity of both muscle layers situated anally to a peristaltic wave would gradually increase following a peristaltic wave and then decreased before threshold for peristalsis was reached (Fig. 4). This increasing inhibition of contractile activity immediately preceding a peristalsis wave was most probably caused by activation of descending inhibitory nervous pathways. These phenomena were more obvious in the CM than in the LM. Following a peristaltic wave there was usually a decrease in tone in both the longitudinal and circular muscle layers on both the oral and anal sides of the wave which was presumably caused by the removal of distention due to the emptying of the lumen.
Effect of neural blockade
All the oral and anal responses to the peristaltic wave were neural in origin since they were completely blocked by tetrodotoxin (TTX; 0.5–1 μm) in the recording chamber (see Fig. 5).
Figure 5. Effect of tetrodotoxin.

Effect of tetrodotoxin (TTX; 1 μm) in the recording chamber on oral and anal responses of LM and CM during a peristaltic wave. A, control oral mechanical responses of LM and CM (top two traces). B, oral mechanical responses of LM and CM after TTX (top two traces). C, control anal mechanical responses of LM and CM. D, anal mechanical responses after TTX.
If the myenteric plexus was lesioned (4 experiments) or damaged during sharp dissection then the strip of longitudinal muscle with myenteric plexus attached did not respond to a peristaltic wave, suggesting that propagated activity through the myenteric plexus was essential for the response of the LM.
Pharmacology of responses in the longitudinal and circular muscle layers
The effects of drugs on the responses of the LM and CM described below all occurred when the drugs were added to the recording chamber only.
Effect of cholinergic antagonists
In the guinea-pig small and large intestine, both the longitudinal and circular muscle layers are innervated by cholinergic motor neurons which have nicotinic receptors (see McConologue & Furness, 1994).
The muscarinic antagonist atropine (1 μm) reduced the tone of the LM by 26.7 ± 5.3% and to a lesser extent that of the CM by 8.0 ± 1.6% (P < 0.02; n = 6). Atropine also reduced the rhythmic contractions of both muscle layers. After the tone had been restored by increasing the tension of the muscle back to control levels it was found that atropine had also reduced the excitatory and inhibitory peristaltic responses of both muscle layers by similar amounts (Fig. 6). Atropine reduced the area under the oral contractile response of the LM and CM by 39.63 ± 4.4 and 27.14 ± 7.48% of control (P > 0.05; n = 6), respectively, (Fig. 6A and B), and the area under the anal contractile response of the LM and CM by 36.32 ± 6.04 and 35.51 ± 7.34% of control, respectively, (P > 0.05; n = 6). Atropine also reduced the area under the anal relaxation response in both the LM and CM layers to 63.26 ± 8.85 and 63.03 ± 10.52% of control, respectively, (P > 0.05; n = 6; Fig. 6C and D), which was a similar reduction in both muscle layers.
Figure 6. Effect of atropine.

Effect of atropine (1 μM) in the recording chamber on oral and anal responses of LM and CM during a peristaltic wave. A, control oral mechanical responses of LM and CM (top two traces). B, oral mechanical responses of LM and CM after atropine (top two traces). C, control anal mechanical responses of LM and CM. D, anal mechanical responses after atropine. Note that atropine reduces oral and anal contractile response of LM and CM as well as relaxation of both muscle layers. Note that P and EFV are unaffected by atropine.
Hexamethonium (300 μm), an antagonist of nicotinic receptors, also greatly reduced the area under the oral excitatory responses of the LM and CM to 13.70 ± 4.48 and 26.98 ± 8.65% (n = 6) of control respectively, which was a similar reduction in both muscle layers (P > 0.05; Fig. 7A and B). In contrast, hexamethonium had no significant effect (P > 0.05) on either the anal relaxations (LM, 111.8 ± 41.3%; CM, 102.7 ± 22.7%; n = 8) or contractions (LM, 78.3 ± 28.4%; CM, 98.4 ± 16.4%; n = 8) of either muscle layer associated with a peristaltic wave (Fig. 7C and D).
Figure 7. Effect of hexamethonium on oral and anal responses of LM and CM during a peristaltic wave.

Effect of hexamethonium (300 μM) on oral and anal responses of LM and CM during a peristaltic wave. A, control oral mechanical responses of LM and CM. B, oral responses after hexamethonium. C, control anal mechanical responses of LM and CM. D, anal mechanical responses after hexamethonium. Note that P and EFV are unaffected by hexamethonium.
These results suggest that there is an asymmetry in neurotransmission between ascending and descending nervous pathways. Cholinergic ascending interneurons appear to activate cholinergic excitatory motor neurons to both the longitudinal and circular muscle layers, whereas non-cholinergic descending interneurons activate inhibitory and excitatory cholinergic motor neurons anal to the stimulus.
Effect of blocking nitric oxide synthesis
A variety of substances have been implicated in inhibitory neurotransmission to the muscle in the gastrointestinal tract. These include adenosine triphoshate (ATP), nitric oxide (NO) and vasoactive inhibitory polypeptide (VIP) (Grider & Makhlouf, 1987; Sanders & Ward, 1992; He & Goyal, 1993). NO can function as an inhibitory neurotransmitter to both the LM and CM (Sanders & Ward, 1992; He & Goyal, 1993; Bartho & Lefebvre, 1995); as a neuromodulator which depresses excitability and increases the threshold for peristalsis (Waterman & Costa, 1994) or as a retrograde messenger in descending inhibitory pathways (Yuan, Bornstein & Furness, 1995). NO can also produce contraction of the LM by releasing ACh (Bartho & Lefebvre, 1995).
To test whether NO was involved in generating the oral and anal responses of the LM and CM to a peristaltic wave we added Nω-nitro-L-arginine (l-NA, 100 μm), which is a specific competitive inhibitor of nitric oxide synthase (Keef, Murray, Sanders & Smith, 1997), to the recording chamber.
Oral excitatory responses
L-NA (100 μm) had no effect on the tone of either the LM or CM. It did however, reduce the area under the oral excitatory responses of both muscle layers to 41.11 ± 6.72% in the LM and 52.29 ± 10.55% in the CM of control (n = 7), which was similar for both muscles (P > 0.05; Fig. 8A and B).
Figure 8. Effect of Nω-nitro-l-arginine.
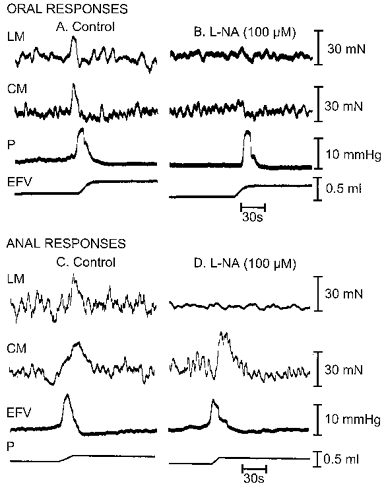
Effect of Nω-nitro-l-arginine(L-NA; 100 μM) on oral and anal responses of LM and CM during a peristaltic wave. A, control oral mechanical responses of LM and CM. B, oral responses of LM and CM after L-NA. C, control anal responses of LM and CM. D, anal responses of LM and CM after L-NA. Note that in this example L-NA almost completely abolished the anal responses of the LM (see D).
Anal excitatory responses
L-NA (100 μm) also reduced the area under the anal contractile response of the LM to 62.60 ± 5.89% of control. L-NA, however, either enhanced or had no affect on the anal contractile response of the CM which averaged out as 144.00 ± 21.20% of control (P > 0.05; n = 7).
Anal inhibitory responses
L-NA (100 μm) also reduced the anal relaxation response of the LM to 58.1 ± 5.97% (P < 0.0001; n = 5) and CM to 69.7 ± 10.12% (P > 0.05; n = 5) of control (Fig. 8C and D). L-NA (100 μm) had no effect on either the oral or anal responses if L-arginine (3 mM) was added to the recording chamber 20 min before the subsequent addition of L-NA (3 oral and 3 anal experiments), a protocol previously used by Yuan et al. (1995). In contrast, prior incubation with d-arginine (3 mM) for 20 min did not affect the reduction in the responses caused by the further addition of L-NA (100 mM; 3 oral and 3 anal experiments).
Effect of apamin on inhibitory neurotransmission
The bee venom apamin, which blocks potassium channels on the muscle, has been used to distinguish two phases of inhibitory neurotransmission to the longitudinal and circular muscle layers of the small and large intestine (Costa et al. 1986). It abolishes the fast component of the inhibitory junction potential in the circular muscle of the guinea-pig ileum and colon but leaves the slow component intact (Smith et al. 1992; He & Goyal, 1993). The fast component appears to be due to adenosine triphosphate (ATP) and the slow component to nitric oxide (He & Goyal, 1993; review McConologue & Furness, 1994).
The bee venom apamin (0.5 μm) increased the tone of the LM by 44.0 ± 2.7% (n = 7) and the CM by 18.7 ± 2.7% (n = 7), which was significantly less than that of the LM (P < 0.01). It also increased the amplitude of phasic contractions in both the LM and CM. Apamin reduced the relaxation response of both the LM and CM to 49.18 ± 9.87 and 47.27 ± 11.59% (P < 0.02; n = 7) of control, respectively (Fig. 9A and B). It also reduced the anal contractile response of the LM by 33.2 ± 1.8% and CM by 7.2 ± 2.2% (P < 0.05; n = 7) of control, respectively.
Figure 9. Effect of apamin.
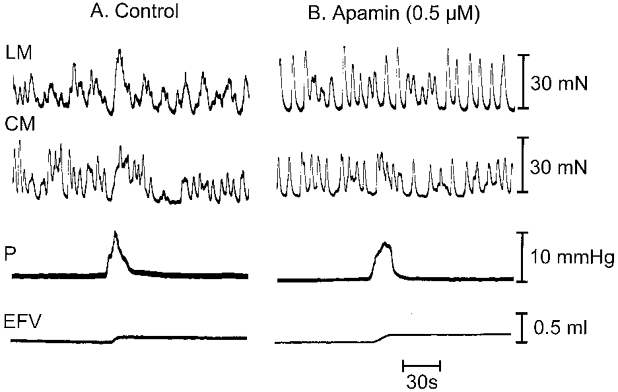
Effect of apamin (0.5 μM) in recording chamber on anal responses of LM and CM during a peristaltic wave. A, control anal mechanical responses of LM and CM (top two traces). B, anal responses of LM and CM after apamin (top two traces).
After apamin, the addition of L-NA (100 μm) to the recording chamber caused a further reduction in the relaxation response of the LM to 30.00 ± 9.09% (P < 0.03). We could not, however, detect a further reduction in the relaxation response of the CM (45.77 ± 12.6%; n = 7) (P > 0.05) with the subsequent addition of L-NA.
Preparatory phase
The oral increase in contractile activity of the LM and CM during the preparatory phase was reduced by either atropine, hexamethonium or L-NA, whereas, the anal inhibition of spontaneous activity in these muscles was not observed in apamin or after L-NA.
DISCUSSION
We designed a preparation that allowed us to mechanically isolate and compare the oral and anal responses of the longitudinal (LM) and circular muscle (CM) layers of the guinea-pig distal colon during a peristaltic wave. The partitioned bath also enabled us to isolate that part of the intestine from which recordings were made from mechanical disturbances caused by a peristaltic wave in the bulk of the colon. There was little temporal delay between the responses of the LM and CM to a peristaltic wave as they were recorded within 2 mm of each other. These responses were dependent on the integrity of intrinsic neural pathways since they were blocked by TTX in the recording chamber. The responses of the LM were also blocked by lesions or damage to the myenteric plexus suggesting that the integrity of neural pathways within the dissected region were essential for activating motor neurons innervating the LM layer. As recordings were made within 7 mm of the partition, the neurally evoked responses of the LM and CM to a peristaltic wave resulted from propagated activity in short ascending and descending nervous pathways. The partition enabled us to investigate some aspects of the pharmacology of the responses of the LM and CM since drugs could be added to the recording chamber without interfering with propulsion in the peristaltic chamber.
Synchronous movements of the longitudinal and circular muscle
Both the oral and anal responses of the LM and circular CM in an isolated segment of distal colon were found to be similar, with both muscle layers contracting at the same time, oral (ascending excitation) to the wave and relaxing (descending inhibition), and then contracting (descending excitation) together below the wave. This implies that the intrinsic ascending and descending nervous pathways activating the LM and CM during peristalsis are similarly organized to ensure that the muscles contract and relax synchronously as originally observed by Bayliss & Starling (1899). Garry & Gillespie (1955) also found that the longitudinal and circular muscles contracted and relaxed together in response to stimulation of pelvic and sympathetic nerves, respectively. The responses of the two muscle layers during a peristaltic wave are, however, more complex than those envisioned by Bayliss and Starling as they include descending excitation, which has been observed previously in response to distention or mucosal stimulation in both the small and large intestine (Hirst et al. 1975; Smith & Furness, 1988; Smith et al. 1992). Descending excitation probably manifests itself as the wave of descending contraction that has been observed to start at the oral end, and advance slowly down the rabbit and guinea-pig distal colon (Mackenna & McKirdy; 1972; Costa & Furness, 1975).
Preparatory phase
During the filling of the lumen there was often a gradual increase and decrease in the oral and anal spontaneous contractile activity of both the LM and CM, respectively. This suggests that low threshold polarized ascending excitatory and descending inhibitory pathways are activated by increasing luminal distension, before the more ‘all or none’ threshold for peristalsis is reached.
Commonality of ascending and descending pathways
The transient oral contraction of both the LM and CM occurred just before the peak of the pressure pulse generated by a peristaltic wave suggesting these contractions, which are activated by intraluminal stretch, drive peristalsis. As the oral contractions of the LM and CM occurred at the same time, were of similar duration, equally sensitive to hexamethonium, and had a similar threshold for activation, this suggests that the same sensory neurons and ascending cholinergic interneurons activated excitatory motor neurons to each muscle layer. Furthermore, as these oral contractions peaked at the same time as the anal relaxations this implies also that, at threshold both the ascending excitatory and descending inhibitory neural pathways are reflexly activated together. This is supported by electrophysiological studies of reflex responses in CM to distention and mucosal stimulation which have shown that oral EJPs and anal IJPs occur after similar latencies (Smith & Furness, 1988; Smith et al, 1992).
The late descending contractile response of the LM and CM may be due to either activation of descending excitatory pathways by distention or as a result of a migrating anally propagated contractile event that is initiated by a build up of activity in descending nervous excitatory pathways. Anally propagated contractions of the CM, which arise spontaneously or are initiated by distention, have previously been observed in the guinea-pig distal colon (Costa & Furness, 1976). These anally migrating waves of contraction can expel uninflated balloons from the colon (Frigo & Lecchini, 1970).
The oral and anal mechanical responses of the LM and CM to a peristaltic wave correlate with the oral and anal electrical responses to distention obtained previously with suction electrodes that were mounted on the serosa of the guinea-pig distal colon (Smith et al. 1992). In the latter experiments, distention evoked an oral excitatory junction potential (EJP; ascending excitation) and an anal inhibitory junction potential (IJP; descending inhibition) which was sometimes followed by a prolonged burst of suprathreshold EJPs (descending excitation). The serosal electrodes may have picked up the co-ordinated electrical activity from both muscle layers.
Pharmacology of reflex pathways
Effect of cholinergic antagonists
Both the oral and late anal contraction of the LM and CM were inhibited by atropine, suggesting that the cholinergic motor neurons supplying each muscle layer (McConalogue & Furness, 1994) are activated by both ascending and descending interneurons. Previous electrophysiological studies support this conclusion since atropine sensitive EJPs are evoked by distension both oral (ascending excitation) and anal (descending excitation) to the stimulation site in the guinea-pig distal colon (Smith et al. 1992). Also, fast excitatory postsynaptic potentials (FEPSPs) are activated in most longitudinal muscle motor neurons and in some circular muscle motor neurons by both oral and anal reflex stimulation of the mucosa or by distention (Smith et al. 1992; Smith & Sanders, 1995). Immunohistochemical studies in the guinea-pig ileum also suggest that ascending cholinergic (Chat positive) and descending non-cholinergic (bombesin, vasoactive intestinal polypeptide (VIP), nitric oxide synthase (NOS)) interneurons, as well as local sensory neurons, synapse with excitatory cholinergic motor neurons supplying the LM (Pompolo & Furness, 1995).
Atropine also reduced the tone and phasic contractions in both the LM and CM suggesting that cholinergic motor neurons to both muscle layers were active, perhaps because of the local stretch applied to the tissue or because of their inherent spontaneous activity (Smith & Shuttleworth, 1996). Atropine, as well as reducing the contractions of the LM and CM, also reduced the anal relaxation in both the LM and CM. This suggests that inhibition of acetycholine release, perhaps by inhibiting activity in cholinergic motor neurons, may also contribute towards descending relaxation. Others have also observed that atropine reduces descending inhibition in the colon (Crema et al. 1970).
The nicotinic antagonist hexamethonium substantially reduced the oral contractile responses of both the LM and CM by a similar amount, but had little effect on the anal relaxation or anal contraction. This suggests that ascending cholinergic interneurons activate cholinergic motor neurons with short projections to both the LM and CM, whereas, descending non-cholinergic interneurons activate both inhibitory and cholinergic excitatory motor neurons to the LM and CM. These differences in neurotransmission between ascending and descending nervous pathways are supported by the immunohistochemical studies described above by Pompolo & Furness (1995), and by the effects of cholinergic antagonists on the electrical and mechanical studies of reflex responses to distension or mucosal stimulation in the CM of the small and large intestine (Costa & Furness, 1976; Smith & Furness, 1988; Smith et al. 1991). Evoked oral contractions and EJPs are substantially reduced or abolished by muscarinic and nicotinic antagonists, whereas, the anal relaxations and IJPs are largely unaffected by these drugs
Effect of blocking nitric oxide synthesis
The NO synthesis antagonist L-NA reduced both the oral contractile and anal relaxation response of the LM and CM. Nitric oxide synthase is contained in anally projecting interneurons (Pompolo & Furness, 1995). In the colon, nitric oxide may also act as a retrograde messenger to influence neurotransmission in both ascending excitatory and descending inhibitory neural pathways to the LM and CM, similar to that postulated for guinea-pig ileum (Yuan et al. 1995). Studies in the guinea-pig ileum and rat colon suggest that NO can elicit a TTX sensitive contraction of the LM that is reduced by atropine and substance P antagonists suggesting that NO can excite cholinergic and/or tachykinin containing motor neurons innervating the LM (see Bartho & Lefebvre, 1995). Since both L-NA and apamin reduced the anal relaxation response of the LM and CM, both NO and ATP may also be released from inhibitory motor neurons innervating both muscle layers (see Sanders & Ward, 1992; He & Goyal, 1993; McConologue & Furness, 1994; Bartho & Lefebvre, 1995). The enhancement of the anal contractile response of the CM by L-NA being due to removal of an underlying relaxation of the CM. As the anal relaxation response was not completely blocked by L-NA and apamin, this suggests other transmitters such as VIP may also play a role in the inhibition of the LM and CM (Costa et al. 1986; Grider & Makhlouf, 1987; He & Goyal, 1993). The reduction in the anal contractile response of the LM by L-NA may have been due to a direct effect of NO on the longitudinal muscle (Bartho & Lefebvre, 1995). We are currently investigating the possible roles of NO in the regulation of ascending and descending nervous pathways in the distal colon.
Conclusions
We have shown that although the responses of the longitudinal and circular muscle layers during peristalsis are complex, the enteric nerve pathways are arranged so that both muscle layers contract and relax at the same time. A tentative neural circuit describing the reflex pathways underlying peristalsis in the distal colon is shown in Fig. 10.
Figure 10. Tentative neural circuit describing peristalsis.
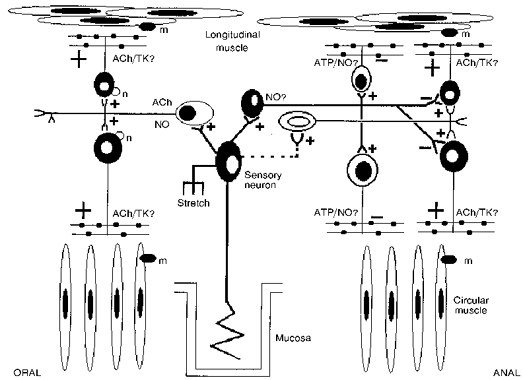
Sensory neurons sensitive to stretch or mucosal stimulation activate ascending cholinergic (and nitrergic?) interneurons which activate excitatory cholinergic motor neurons to both the LM and CM. The sensory neurons also activate descending non-cholinergic (and nitrergic?) interneurons which activate inhibitory motor neurons to both the LM and CM, and presumably, suppress activity in excitatory cholinergic motor neurons. Descending non-cholinergic interneurons also activate, after a delay, cholinergic excitatory motor neurons. ACh, acetylcholine; NO, nitric oxide; TK, tachykinins; n, nicotinic receptor; m, muscarinic receptor.
The complex responses of the LM and CM must be well co-ordinated to produce the smooth propulsion of a pellet down the colon. The pellet is preceded by relaxation and followed by contraction of the CM (see Costa & Furness, 1976). The oral contraction of the longitudinal muscle would tend to pull the intestine over the rear of a pellet; the simultaneous contraction of both muscle layers ensuring that contraction of the LM also contributes to propulsion by providing a vector of force along the intestine. Presumably, as the pellet is propelled into the relaxed or accommodating segment by the oral contraction of both the LM and CM, the descending excitatory responses in both muscles provide extra force to propel the bolus and re-establish the tone of the muscle layers. A schematic model showing activation of the CM during peristalsis is shown in Fig. 11. Thus, peristalsis in the colon consists of activation of ascending excitatory and descending inhibitory nervous reflexes, and an anally propagating contractile motor complex or a propagated ‘off’ type contraction as seen in the oesophagus (Dodds, Christensen, Dent, Wood & Arndorfer, 1978).
Figure 11. Activation of the circular muscle during peristalsis in the distal colon.

During filling there is an activation of low threshold ascending excitatory and descending inhibitory nervous pathways. At threshold there is a transient oral contraction and anal relaxation of the circular muscle, the oral contraction forcing the bolus into the accommodating segment. The anal relaxation gives way to contraction resulting from activation of descending excitatory nervous pathways which aids in driving the bolus down the intestine.
Acknowledgments
William Robertson was a visiting research scholar from the University of Ulster (Coleraine, UK). He was supported by a grant from the National Institutes of Health (USA): first award (R29) DK 45713.
References
- Bartho L, Lefebvre RA. Nitric oxide-mediated contraction of enteric smooth muscle. Archives Internationales de Pharmacodynamie et de Therapie. 1995;329:53–66. [PubMed] [Google Scholar]
- Bayliss W, Starling EH. The movements and innervation of the small intestine. Journal of Physiology. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss W, Starling EH. The movements and innervation of the large intestine. Journal of Physiology. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. Journal of Neuroscience. 1991;11:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff A. Electrical transmission of slow waves from longitudinal to circular muscle. American Journal of Physiology. 1965;209:1254–1260. doi: 10.1152/ajplegacy.1965.209.6.1254. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of nerve pathways and their pharmacology. Naunyn-Schmiederberg's Archives of Pharmacology. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB, Humphreys CMS. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn-Schmiedeberg's Archives of Pharmacology. 1986;332:79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- Crema A, Frigo GM, Lecchini S. Pharmacological analysis of the peristaltic reflex in the isolated colon of the guinea-pig or cat. British Journal of Pharmacology. 1970;39:334–345. doi: 10.1111/j.1476-5381.1970.tb12897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds WJ, Christensen J, Dent J, Wood JD, Arndorfer RC. Esophageal contractions induced by vagal stimulation in the opossum. American Journal of Physiology. 1978;235:E392–401. doi: 10.1152/ajpendo.1978.235.4.E392. [DOI] [PubMed] [Google Scholar]
- Frigo GM, Lecchini S. An improved method for studying the peristaltic reflex in the isolated colon. British Journal of Pharmacology. 1970;39:346–356. doi: 10.1111/j.1476-5381.1970.tb12898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. An electrophysiological study of the innervation of the smooth muscle of the colon. Journal of Physiology. 1969;205:549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary RC, Gillespie JS. The responses of the musculature of the colon of the rabbit to stimulation, in vitro, of the parasympathetic and sympathetic outflows. Journal of Physiology. 1955;128:557–576. doi: 10.1113/jphysiol.1955.sp005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Bentley GA. The peristaltic reflex in the isolated guinea-pig ileum during drug-induced spasm of the longitudinal muscle. Australian Journal of Experimental Biology and Medical Science. 1968;46:1–16. doi: 10.1038/icb.1968.1. [DOI] [PubMed] [Google Scholar]
- Grider JR, Makhlouf GM. Colonic peristalsis: identification of vasoactive intestinal peptide as a mediator of descending relaxation. American Journal of Physiology. 1987;251:G40–45. doi: 10.1152/ajpgi.1986.251.1.G40. [DOI] [PubMed] [Google Scholar]
- He XD, Goyal RK. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. Journal of Physiology. 1993;461:485–490. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, McKirdy HC. Two descending nerve pathways activated by distention of guinea-pig small intestine. Journal of Physiology. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, McKirdy HC. A nervous mechanism for descending inhibition in guinea-pig small intestine. Journal of Physiology. 1974;238:129–143. doi: 10.1113/jphysiol.1974.sp010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef KD, Murray DC, Sanders KM, Smith TK. Modulation of canine colonic electrical and contractile activity by basal nitric oxide release. Journal of Physiology. 1997;499:773–786. doi: 10.1113/jphysiol.1997.sp021968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz HW, Lees GM. Pharmacological analysis of intrinsic intestinal reflexes. Pharmacological Reviews. 1964;16:301–339. [PubMed] [Google Scholar]
- Kosterlitz HW, Robinson JA. Reflex contractions of the longitudinal muscle coat of the isolated guinea-pig ileum. Journal of Physiology. 1959;146:369–379. doi: 10.1113/jphysiol.1959.sp006198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottegoda SR. An analysis of the possible nervous mechanisms involved in the peristaltic reflex. Journal of Physiology. 1969;200:687–712. doi: 10.1113/jphysiol.1969.sp008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConalogue K, Furness JB. Gastrointestinal neurotransmitters. In: Fuller P, Shulkes A, editors. The Gut as an Endocrine Organ. Vol. 8. London: Bailliere Tindall; 1994. pp. 51–76. chap. 3. [DOI] [PubMed] [Google Scholar]
- Mackenna BR, McKirdy HC. Peristalsis in the rabbit distal colon. Journal of Physiology. 1972;220:34–54. doi: 10.1113/jphysiol.1972.sp009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompolo S, Furness JB. Sources of inputs to longitudinal muscle motor neurons and ascending interneurons in the guinea-pig small intestine. Cell and Tissue Research. 1995;280:549–560. doi: 10.1007/BF00318359. 10.1007/s004410050384. [DOI] [PubMed] [Google Scholar]
- Robertson WJ, Malarkey DW, Shuttleworth CW, Smith TK. Relative movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig large intestine. Gastroenterology. 1997;112:A815. [Google Scholar]
- Sanders KM, Ward SM. Nitric oxide as a mediator of non-adrenergic noncholinergic neurotransmission. American Journal of Physiology. 1992;262:G379–392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interaction between reflexes evoked by distention and by stimulation of the mucosa of the guinea-pig ileum. Journal of the Autonomic Nervous System. 1991;34:69–76. doi: 10.1016/0165-1838(91)90009-r. 10.1016/0165-1838(91)90009-R. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distention and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea-pig small intestine. Journal of Neuroscience. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Bywater RAR, Holman ME, Taylor GS. Electrical responses of the muscularis externa to distension of the isolated guinea-pig distal colon. Journal of Gastrointestinal Motility. 1992;4:145–156. [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological study in the isolated guinea-pig ileum. Journal of the Autonomic Nervous System. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, McCallum RW. Serotonin mediation of intestinal peristalsis. In: Garginella T, editor. Regulatory Mechanisms in GI Function. New York: CRC Press; 1995. pp. 219–240. chap. 6. [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. American Journal of Physiology. 1987;252:C290–299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Smith TK, Sanders KM. Motility of the large intestine. In: Yamada T, Alpers DH, Owyang C, Powell DW, editors. Textbook of Gastroenterology. I. Philadelphia: J. B. Lippincott Co, Silverstein; 1995. pp. 234–260. chap. 10. [Google Scholar]
- Smith TK, Shuttleworth CW. Electrophysiological and morphological investigation of longitudinal muscle motor neurons in the guinea-pig ileum. Society for Neuroscience. 1996;22:417–426. [Google Scholar]
- Tonini M, Frigo G, Lecchini S, D'Angelo L, Crema A. Hyoscine-resistant peristalsis in guinea-pig ileum. European Journal of Pharmacology. 1981;71:375–381. doi: 10.1016/0014-2999(81)90181-3. 10.1016/0014-2999(81)90181-3. [DOI] [PubMed] [Google Scholar]
- Trendelenburg P. Pysiologische und pharmakologische Versuche uber die Dunndarm Peristaltick. Naunyn-Schmeideberg's Archives of Pharmacology. 1917;81:55–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- Waterman SA, Costa M. The role of enteric inhibitory motorneurons in peristalsis in the isolated guinea-pig ileum. Journal of Physiology. 1994;477:459–468. doi: 10.1113/jphysiol.1994.sp020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD, Perkins WE. Mechanical interactions between longitudinal and circular axes of small intestine. Journal of Neurophysiology. 1970;42:582–593. doi: 10.1152/ajplegacy.1970.218.3.762. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Osaki T. Frontiers in Smooth Muscle Research. Alan R. Liss, Inc; 1990. Contractions of the longitudinal and circular muscle of the small intestine; pp. 483–492. [PubMed] [Google Scholar]
- Yuan SY, Bornstein JC, Furness JF. Pharmacological evidence that nitric oxide may be a retrograde messenger in the enteric nervous system. British Journal of Pharmacology. 1995;114:428–432. doi: 10.1111/j.1476-5381.1995.tb13244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


