Abstract
We have recently shown that perception of intestinal stimuli increases by spatial summation phenomena. Our aim was to determine in humans whether intestinal perception depends on (a) the length of gut stimulated, and (b) the distance between stimuli.
In a first series of studies, we compared perception of isobaric intestinal distensions applied over a 3 cm segment and a 36 cm segment by means of two separate barostats (n = 8). In a second series of studies we compared perception of intestinal distensions applied simultaneously by two balloons sited 3, 12 or 48 cm apart (n = 6).
Distension of the 36 cm segment induced significantly greater perception than distension of the 3 cm intestinal segment (discomfort perceived at 20 ± 2 mmHg and 31 ± 2 mmHg, respectively; P < 0.05). Perception of intestinal balloon distension increased when a second stimulus was simultaneously applied, independently of the distance between the two balloons (the discomfort thresholds were 30 ± 11, 20 ± 6 and 28 ± 7% lower with simultaneous distensions 3, 12 and 48 cm apart, respectively).
We conclude that perception of intestinal distension is determined by the extension of the field of stimulation, and the summation effect is similar whether adjacent or distant fields are stimulated.
We have recently shown that visceral perception in humans is modulated by spatial summation phenomena. Specifically, we found that perception of intestinal balloon distension increased when a second balloon located 5 cm orally was inflated simultaneously, and distending volumes which were well tolerated at a single site became uncomfortable when applied at both sites simultaneously (Serra, Azpiroz & Malagelada, 1995). In the present study we wished to characterize further the phenomenon of spatial summation on gut perception, and we performed two series of studies, each with a specific aim. Our first aim was to determine whether spatial summation affects the entire stimulus- response curve; that is, whether normally unperceived stimuli become perceptible when a greater area of intestine is stimulated. This specific aim was addressed by comparing responses to isobaric distensions of a short and a long segment of intestine produced by two separate barostats. In our experimental design we also included experiments performed during inhibition of intestinal tone by glucagon administration (Diamant & Picazo, 1983; Rouillon, Azpiroz & Malagelada, 1991a), to circumvent putative interferences of gut accommodation on perception of distension. Our second aim was to determine whether the effects of spatial summation depend on the respective location, whether adjacent or distant, of the fields of stimulation in the intestine. This aim was addressed by a second series of studies comparing the responses to simultaneous balloon distensions applied 3, 12 and 48 cm apart.
METHODS
Participants
Fourteen healthy individuals (6 women and 8 men; age range, 21–36 years), without gastrointestinal symptoms and not on any medication, participated in the study after giving written informed consent. The protocol for the study had been previously approved by the Institutional Review Board of the Hospital General Vall d'Hebron.
The intestinal barostat
In series I studies (see ‘Specific procedures and experimental design’) we produced isobaric distensions of a short intestinal segment (3 cm) and of a long intestinal segment (36 cm) using two separate barostats (Azpiroz & Malagelada, 1990a; Rouillon, Azpiroz & Malagelada, 1991b). The barostat maintained a constant pressure within an intestinal bag by a feedback regulation of the volume of air inside the bag (Azpiroz & Malagelada, 1985, 1987). The bag was of cylindrical shape and fixed length, but radially oversized (18 cm perimeter). The feedback mechanism consisted of a strain gauge linked by an electronic relay to an air injection/aspiration system. Both the strain gauge and the air pump were independently connected by 160 cm long tube (0.78 and 2 mm i.d., respectively) to the intestinal bag. A dial in the electronic system allowed selection of the desired pressure level to be maintained in the bag. One barostat was connected to a 3 cm long bag and the other barostat to a 36 cm bag. The connecting lines of both barostats were tethered together forming a 5.6 mm o.d. tube assembly. The bags were mounted over the tube assembly with the ends air-tight sealed to the tube. The 36 cm bag was divided in two equal parts separated by 6 cm, to prevent rectification of the intestinal curvature during distension. Both parts shared pressure and volume lines with multiple side-holes, to ensure a common cavity along the entire double-compartment bag. The 3 cm bag was located midway between both compartments, 1.5 cm from each one, so that the short bag stimulated an area of intestine that corresponded to the mid-point of the long segment (Fig. 1).
Figure 1. Experimental model.
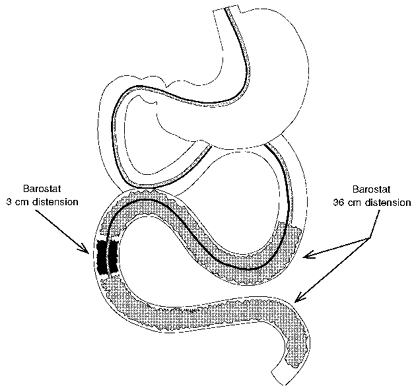
Isobaric intestinal distensions were applied over a short and a long segment by means of two separate barostats. The long bag was divided into two compartments sharing pressure and volume connections, and the short bag was located in-between.
The barostat bags were finely folded for intubation. Once the bags were positioned, they were unfolded by slow inflation of air to a perceptible level. The bags were then completely deflated and connected to the respective barostat. The barostat volume recordings were filtered (0.5 Hz on-line filter) to reduce the magnitude of the rapid oscillations induced by respiratory motion (Rouillon et al. 1991a). The operating pressure in each barostat was individually adjusted at a level 1 mmHg above intra-abdominal pressure. Intra-abdominal pressure was established at 5.5 ± 0.3 mmHg in each barostat, by increasing intrabag pressure in 1 mmHg steps every minute, until the barostat recorded changes induced by respiratory motion (minimal distending pressure) (Azpiroz & Malagelada, 1987; Rouillon et al. 1991a). During the studies, the basal volume in each bag was verified periodically to exclude potential air leaks, by asking the subjects to perform a sustained Valsalva manoeuvre until the bag was completely collapsed. Pressurization of the barostat during the distensions introduces a magnification artifact in the volume recording due to air compression and deformation of the pump (Azpiroz, 1995). The distending volumes recorded during the studies were properly corrected by subtracting the corresponding compression factor which had been previously calculated.
Intestinal balloon distension
In series II studies (see ‘Specific procedures and experimental design’) we applied phasic distensions at different sites in the small intestine. We used a multilumen polyvinyl tube assembly (3.9 mm o.d.) that incorporated four latex balloons (3 cm fixed longitudinal axis each). The most distal balloon was located at the tip of the tube and the other balloons were located at a distance of 3, 12 and 48 cm, respectively. Intestinal distensions were produced by inflation of the most distal balloon, either alone or with simultaneous inflation of one of the other balloons with predetermined volumes of air. The balloons were inflated by the investigator using a syringe at a rate of 10 ml s−1. Since the resistance to airflow through the connecting tube (0.78 mm i.d.) was relatively high, manual inflation with the same syringe size provided reproducible flow rates (7–13 ml s−1 range). At the end of the distension the balloon was completely deflated. During the study, the balloons were connected to pressure transducers (model CDK 3, COBE, Lakewood, CO, USA), and the intraballoon pressures at each volume tested were recorded. Before each study we measured in vitro the intrinsic pressure achieved within each balloon during stepwise inflation at 8 ml increments up to 64 ml total volume. At each distending volume tested, the pressure recorded in vivo was corrected by subtracting the intrinsic pressure previously determined for the same inflation volume in vitro (Accarino, Azpiroz & Malagelada, 1995). We used high-compliance balloons made with condoms, that maintain intrinsic intraballoon pressures relatively low; during in vitro inflation with volumes up to 64 ml the pressure in none of the balloons exceeded 30 mmHg. Balloon distension was chosen for series II studies, because with this method distensions at multiple sites can be tested using relatively small connecting tubes. This simple and reliable method to produce graded intestinal distension has been extensively used in our laboratory (Azpiroz & Malagelada, 1990b; Rouillon et al. 1991a; Accarino, Azpiroz & Malagelada, 1992, 1995; Coffin, Azpiroz & Malagelada, 1994; Iovino, Azpiroz, Domingo & Malagelada, 1995; Serra et al. 1995).
Perception questionnaire
In both series I and II studies we used a graded questionnaire to measure the intensity and the type of sensations perceived, and an anatomical questionnaire to measure the location and extension of the sensations. The graded questionnaire included five scales specifically for scoring (a) abdominal pressure, (b) fullness, (c) colicky sensation, (d) stinging sensation and (e) other types of sensation (to be specified). Participants were asked to score any perceived sensation (one or more perceived simultaneously) on the respective scale(s). Each graphic rating scale combined verbal descriptors on a visual analog scale graded from 0 to 6 (Gracely, 1994; Azpiroz, 1995). All participants received standard instructions, specifying that score 0 represented no perception at all, score 5 represented discomfort, and score 6 represented a painful sensation, which was not intended, and was to be instantaneously reported for immediate discontinuation of the stimulus. Any sensation had to be scored on the scale based on its perceived intensity, and orientative descriptors were provided indicating that score 1 represented vague perception of mild sensation; score 2 represented definite perception of mild sensation; scores 3 and 4 represented vague and definite perception of moderate sensation, respectively. Participants were also told that, when appropriate, they could mark half-unit scores on the scale, so that twelve intensity grades were actually provided.
The anatomical questionnaire incorporated a diagram of the abdomen divided in nine regions: epigastrium, periumbilical area, hypogastrium, both hypochondria, flanks and ileal fossae. Participants were instructed to mark the location, i.e. abdominal region(s) or extra-abdominal, where the sensations were perceived. Before the study both questionnaires were fully explained to the participants. This type of questionnaire has been extensively used in our laboratory (Azpiroz & Malagelada, 1990a,b; Rouillon et al. 1991a,b; Accarino et al. 1992, 1995; Notivol, Coffin, Azpiroz, Mearin, Serra & Malagelada, 1995; Serra et al. 1995; Azpiroz, 1995).
General procedure
The studies were conducted in a quiet, isolated room, and the testing period did not exceed 3 h. Participants were intubated after an 8 h fast. The intraluminal probes were introduced through the mouth into the intestine. The probes were positioned under fluoroscopic control in the desired location. Specifically, in series I studies the orad end of the most proximal bag was located 5 cm distal to the duodeno-jejunal junction, and in series II studies the most orad balloon was located in the distal duodenum. During the study the subjects rested supine in bed at an angle of 30 deg to the horizontal and were asked to relax quietly. In series I studies an intravenous line was established and saline, either alone as control or with glucagon, was continuously perfused at 25 ml h−1 using an infusion pump (model Perfusor®; B. Braun, Melsungen, Germany). Pressure and volume within the barostat bags in series I studies, and pressure within the latex balloons in series II studies, were recorded on a paper polygraph (model 6006; Letica, Barcelona, Spain).
After allowing 10 min for stabilization, we performed intestinal distensions (by means of the barostats in series I studies and by means of the latex balloons in series II studies). Phasic distensions of 1 min duration were performed at fixed increments up to the threshold for discomfort, that is until the subject perceived any kind of sensation at the level of discomfort (score 5 or greater).
Individual stimuli were randomly applied at 5 min intervals without the participants knowing the type or the intensity of the stimulus applied. In series II studies, 2 ml of residual air were maintained within each balloon between the distensions, to record phasic intestinal activity. No stimuli were performed during or 10 min after a period of activity of the interdigestive motor complex (phase III) recorded either by the barostats in series I studies (Rouillon et al. 1991a) or by the balloons in series II studies. Stimuli performed within the 10 min period before a period of activity were repeated afterwards.
Specific procedures and experimental design
Series I studies
In eight subjects (3 women, 5 men) we compared by stimulus-response trials perception of intestinal distensions of a 3 cm and a 36 cm segment. Intestinal distensions at each site were randomly applied in 4 mmHg increments up to the respective threshold for discomfort. Distensions were first performed during i.v. saline infusion and then repeated 15 min after the onset of i.v. infusion of glucagon (Glucagon Novo; Laboratorios Novo, Madrid, Spain). Glucagon was infused as a 4.8 μg kg−1 bolus followed by 9.6 μg kg−1 h−1 continuous infusion. To prevent the total duration of the study from exceeding the 3 h limit period, during glucagon administration the stimulus-response trials at each site started at a pressure level 12 mmHg below the pressure that had previously induced discomfort during saline administration in the same subject. At least two distensions were performed during glucagon infusion with each barostat. All stimuli were performed blindly, without the participants knowing the moment when the stimuli were applied, the type of stimuli or the mechanisms explored in the study. Each subject participated only in one study.
Series II studies
In six subjects (3 women, 3 men) we compared perception of simultaneous distensions of two balloons located 3, 12 and 48 cm apart. We first determined by short stimulus-response trials the distending volume in the three proximal balloons that was first perceived. Phasic distensions of 10 s duration were tested in 8 ml increments at 10 s intervals up to the level of perception. After a 5 min period the same stimuli were applied for 1 min at 5 min intervals, and the volumes were readjusted when required to induce clear perception without discomfort (score 2–4). We then performed probing distensions with the distal balloon either alone or with simultaneous inflation of one of the other balloons to the pre-established volume that elicited perception. Probing distensions of the caudad balloon were performed in 8 ml stepwise increments up to the respective threshold for discomfort (perception score 5 or greater).
Data analysis
In each individual stimulus-response trial we defined the threshold for perception as the lowest distending level (distending pressure in series I studies, and distending volume in series II studies) that induced any type of perception (perception score 1 or greater) and the threshold for discomfort as the lowest distending level that produced any sensation at the level of discomfort (perception score 5 or greater). Stimulus-response curves were then calculated by plotting the perception scores at different distending pressures in series I studies and at different distending volumes in series II studies. In series I studies, all distending levels were expressed as pressures above intra-abdominal levels.
In each subject we counted the number of times each sensation was perceived, and under the different conditions tested, we calculated the frequency of each sensation in relation to the total number of stimuli perceived; the frequency was expressed as percentage. Furthermore, to determine whether the type of perception depended on the stimulus intensity, we compared the sensations perceived at different stimulus magnitude as we have done before (Accarino et al. 1992, 1995). For each subject, we divided the number of perceived distensions into a lower half (i.e. weak stimuli) and a higher half (i.e. strong stimuli), and we then compared the type of sensations elicited by strong and weak stimuli. In the anatomical questionnaire we also measured, for each experimental condition, the percentage of stimuli perceived in more than one abdominal region.
In series I studies, the intestinal pressure-volume relationship was established by plotting the distending pressures against the corrected intrabag volumes at the different levels of distension. We also measured the intestinal reflex response to the distension applied with one barostat by geometrically averaging the volume recorded by the other barostat during the 1 min period preceding the distension, and during the last 30 s of the distension. The reflex response in intestinal tone was measured as the volume change, e.g. relaxation as isobaric expansion, and expressed as percentage change.
Statistical analysis
In each group of subjects (series I and II studies) we calculated the mean values (±s.e.m.) of the parameters measured in the stimulus-response trials under the different experimental conditions tested. In series I studies, the responses to short and long distensions with and without glucagon administration were compared by paired analysis using the Wilcoxon test. Reflex responses in intestinal tone were analysed by paired comparisons of barostat volumes before and during the different distension levels applied with the other barostat (see above) using the Wilcoxon test. In series II studies, analysis of variance for repeated measures was performed by the Friedman test; Dunn's test was used for post hoc comparisons.
RESULTS
Series I studies
Perception of intestinal distension
Distension of the short (3 cm) intestinal segment induced stimulus-related perception: low pressures were unperceived and perception increased from the perception threshold (18 ± 2 mmHg) up to the threshold for discomfort (31 ± 2 mmHg). Distension of the long (36 cm) intestinal segment was also stimulus related, but the entire stimulus-response curve was shifted to the left: both the pressure thresholds for perception (12 ± 1 mmHg) and for discomfort (20 ± 2 mmHg) were significantly smaller (Fig. 2). Hence, distension at the pressure threshold for perception in the long segment was completely unperceived when applied in the short segment, and only distension at the pressure threshold for discomfort in the long segment induced minimal perception in the short segment (1.1 ± 0.4 perception score, P < 0.05versus long distension).
Figure 2. Perception of fixed-pressure intestinal distensions applied over a 3 cm segment and a 36 cm segment.
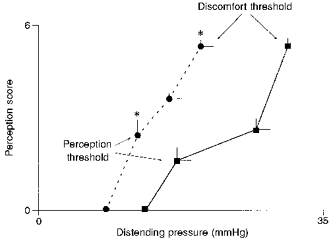
Values are means ±s.e.m. at the threshold and subthreshold for perception and discomfort. Note that distension of the 36 cm segment (•) induced significantly higher perception scores than isobaric distensions of the 3 cm segment (▪) (*P < 0.05).
Regardless of the length of intestine stimulated, configuration of the stimulus-response curves maintained a relation of proportionality. Thus, in the short segment, the pressure at the discomfort threshold was 1.9 ± 0.2 times higher than that at the perception threshold, and a similar relation was observed in the long segment where the pressure at the discomfort threshold was 1.7 ± 0.2 times higher than that at the perception threshold (Fig. 2).
Symptoms induced by intestinal distension
Intestinal distensions were perceived as abdominal pressure, fullness, a colicky sensation and a stinging sensation (Fig. 3). Subjects invariably reported one single sensation in response to intestinal distension. However, the distensions were not always perceived alike, but repeated distensions could elicit different sensations in the same individual (average, 1.9 ± 0.3). The symptom pattern was unrelated to the length of intestine stimulated; the same type of sensation was elicited by short and long distensions (Fig. 3). Most sensations (80 ± 12% of the responses to short distension and 82 ± 12% of those to long distension) were referred to the epigastrium and periumbilical regions. The area of referral was somewhat larger, but not statistically significant, when the long segment of intestine was distended (24 ± 8 and 42 ± 13% of the sensations induced by short and long distensions, respectively, were perceived over more than one abdominal region). Neither the symptoms nor the referral pattern were related to the stimulus intensity: the type of sensations, the percentage of sensations referred to the epigastrium and periumbilical regions and the percentage of sensations perceived over more than one abdominal region in response to weak and strong stimuli were not significantly different.
Figure 3. Abdominal sensations induced by intestinal distension.
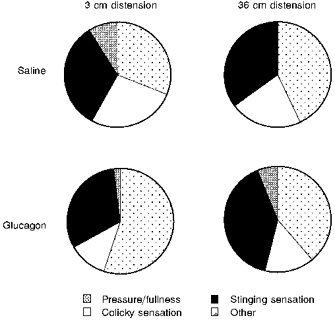
In each subject we calculated the frequency of each sensation in relation to the total number of stimuli perceived; mean values in 8 subjects are shown. Note that neither the length of distension nor the inhibition of intestinal motility by glucagon affected the symptom pattern.
Intestinal response to distension
In both the short and the long intestinal segments, intraluminal volumes were related to the pressure levels set by the barostats (Fig. 4). The local response to distension was similar regardless of the length of the segment distended. Distensions of the long segment were only performed up to 20 mmHg, due to lesser tolerance, but within this interval the shape of the pressure-volume curve paralleled that obtained in the short intestinal segment (Fig. 4).
Figure 4. Compliance of the 3 cm and the 36 cm intestinal segments.
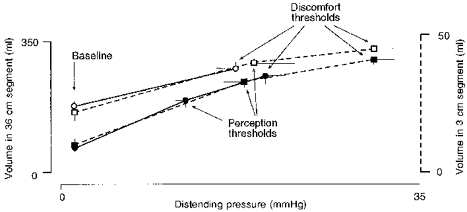
Values are means ±s.e.m. Note that compliance curves of the 3 cm and the 36 cm distensions were superimposable, both during i.v. saline (▪ and •, respectively) and during i.v. glucagon (□ and ○, respectively). Glucagon relaxed the intestine and reduced the extension ratio similarly in both segments. The perception threshold for 36 cm distension during i.v. glucagon was not determined (see ‘Specific procedures and experimental design’).
Distension of the short intestinal segment induced a reflex relaxation that was recorded by the long segment barostat. Such reflex relaxation was statistically significant at the threshold for discomfort (the intestine relaxed by 55 ± 12%; P < 0.05) and below (at 27 ± 2 mmHg, perception scored 2.3 ± 0.5 and the intestine relaxed by 37 ± 12%; P < 0.05), but no significant relaxation occurred at the perception threshold (5 ± 9% reflex volume change). The bag of the barostat located in the short segment collapsed during 65 ± 15% of the distensions applied with the long barostat. This effect seemed artifactual, because the volume changes in the short bag were abrupt and coincided with the modification of the pressure in the long bag. Hence, no reflex responses could be measured during distension of the long segment.
Effect of glucagon
Glucagon produced marked intestinal relaxation that was of similar magnitude in the short and in the long segments of intestine: the volume measured by the barostat at baseline pressure increased during glucagon infusion by 142 ± 31 and by 190 ± 34%, respectively (Fig. 4). This relaxatory effect was maintained throughout the infusion period: baseline volume measured by the long barostat 10 min after starting glucagon infusion was 181 ± 17 ml and remained at 176 ± 18 ml at the end of the infusion period.
Glucagon modified the intestinal response to distension. At each distending pressure intestinal volumes were larger during glucagon infusion, but the differences were more prominent at lower pressures (Fig. 4). Hence, glucagon lowered basal intestinal tone and reduced the extension ratio of the intestine. For instance, by distending the long segment from baseline to the highest pressure level tested, the volume expanded by 64 ± 15% during glucagon administration and by 338 ± 67% during saline infusion (P < 0.05). Corresponding values in the short segment were larger (197 ± 91% volume expansion during glucagon versus 535 ± 196% during saline; P < 0.05), due to the higher pressures tolerated. During glucagon administration, no further relaxation could be induced by intestinal reflexes on account of the profound relaxation induced by the drug. Thus, distension of the short segment induced no reflex response in the long segment, even at the highest pressure tested (−3 ± 2% volume change).
Despite the profound inhibitory effect of glucagon on intestinal activity, clear-cut differences in perception were still observed depending on the length of the intestine stimulated. The discomfort threshold was significantly lower in the long segment of intestine than in the short segment (17 ± 2 mmHg and 31 ± 2 mmHg, respectively; P < 0.05), and at the highest pressure level applied at both sites (17 ± 2 mmHg) perception was markedly higher with long than with short distensions (perception scores were 5.3 ± 0.2 and 0.5 ± 0.3, respectively; P < 0.05).
Isobaric distensions of the long intestinal segment induced somewhat higher perception during glucagon administration than during saline, but the differences did not reach statistical significance. For instance, at 17 ± 2 mmHg (highest pressure tested) perception scored 3.9 ± 0.5 during saline (n.s. versus glucagon). Glucagon administration did not affect perception of the short distensions, and modified neither the type of symptoms (Fig. 3) nor the referral pattern in response to short and long distensions.
Series II studies
Control distensions
Probing distensions with the most caudad balloon alone, induced volume-related perception. Small distending volumes were unperceived, but volumes from the perception threshold and above induced progressively more intense perception up to the threshold for discomfort (Fig. 5). Intestinal balloon distensions induced sensations similar to the distensions performed in series I studies with the barostat: abdominal pressure/fullness (53 ± 13%), and colicky (25 ± 17%) and stinging sensations (13 ± 8%). Perception was predominantly localized in the epigastrium and periumbilical area (80 ± 8% of the perceived sensations) and 47 ± 15% of the sensations were referred over more than one abdominal region.
Figure 5. Stimulus-related perception of phasic intestinal balloon distensions.
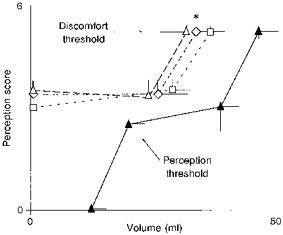
Distensions were tested alone (▴, control) and with simultaneous conditioning distensions applied either at 3 cm (▵), 12 cm (□) or 48 cm (⋄) orally. Values are means ±s.e.m. at the threshold and subthreshold for discomfort and perception. Note that discomfort was perceived at significantly smaller volume when a second stimulus was simultaneously applied, independently of the distance between the two balloons (*P < 0.05 for all vs. control).
Simultaneous distensions at different sites
Probing distensions with the caudad balloon were also performed with simultaneous conditioning distensions applied either 3, 12 or 48 cm orally. The volumes used for the conditioning distensions were similar in the three balloons (28 ± 5 ml at 3 cm, 30 ± 4 ml at 12 cm, and 28 ± 4 ml at 48 cm). Conditioning distensions alone (with sham distensions of the caudad balloon) induced clear perception without discomfort (Fig. 5).
Probing distensions with the caudad balloon induced significantly higher perception when a conditioning distension was simultaneously applied (Fig. 5). Distending volumes in the caudad balloon that were well tolerated alone induced discomfort with simultaneous conditioning distensions (perception scores were significantly higher with simultaneous distension of balloons sited 3, 12 or 48 cm apart than with a single distension; P < 0.05 for all). These effects of spatial summation were similar regardless of the distance between the distending balloons (Fig. 5).
The higher perception induced by simultaneous conditioning cannot be explained on the basis of increased intestinal compliance, because the pressures in the balloons were similar whether distensions were applied alone or simultaneously. The symptom pattern elicited by intestinal distension was not modified by simultaneous conditioning. Reported sensations with conditioning were: pressure/fullness (60 ± 13%), a colicky sensation (22 ± 16%) and a stinging sensation (14 ± 6%); 76 ± 9% of the sensations were localized in the epigastrium and periumbilical area (pooled data for conditioning distensions at 3, 12 and 48 cm).
DISCUSSION
We have shown in humans that phenomena involving spatial summation may substantially modify gut perception. Thus, the surface area of intestine exposed to a distending stimulus determines the intensity of perception. Summation effects are similar whether adjacent or distant fields are stimulated, at least over the proximal half of the small bowel.
Intestinal distension activates mechanosensitive afferents that follow sympathetic pathways, splanchnic nerves and ascending spinal tracts, to reach the brain cortex and induce symptoms such as abdominal pressure, fullness, and colicky and stinging sensations (Jones, 1938; Ray & Neill, 1947; Sengupta & Gebhart, 1994; Azpiroz, 1995). The sensitivity of the proximal small intestine is very uniform (Rouillon et al. 1991a,b), with perception predominantly referred to the abdominal midline (Jones, 1938; Bentley & Smithwick, 1940; Ray & Neill, 1947). Perception of intestinal distension is related to stimulus and increases from the perception threshold up to the threshold for discomfort (Rouillon et al. 1991a,b; Accarino et al. 1992, 1995; Azpiroz, 1995). In the present study we found that perception also depends on the extension of the intestine stimulated, conceivably on the number of afferents activated. Spatial summation affected perception rather uniformly at all levels of stimulation. Hence, in series I studies the whole stimulus-response curve was shifted to the left by increasing the field of stimulation: pressure levels that were unperceived when applied over a short intestinal segment induced perception when applied over a longer segment, and likewise well-tolerated pressures became uncomfortable. The type and the location of the sensations are not related to the stimulus intensity (Accarino et al. 1992, 1995) and seemed neither affected by spatial summation phenomena.
We further showed that spatial summation in the small intestine occurs independently of the spatial distribution of the fields of stimulation. Thus, in series II perception in response to stimulation of a discrete field in the distal jejunum (by balloon distension) increased by additional stimulation of a similar field located at a certain distance. The magnitude of the effect was equivalent when both fields were either contiguous, separated but within the same intestinal region, or even in different anatomical segments, such as duodenum and jejunum.
Animal data suggest that primary viscerosensory neurons are scarce and have relatively large reception fields (McMahon & Morrison, 1982; Blumberg, Haupt, Jänig & Kohler, 1983). The response in the reception field is not homogeneous, the discharge threshold being higher in the periphery (Jänig & Koltzenburg, 1991). The fact that summation does not fade by separating the fields of stimulation would suggest that it results from central processing of sensory input. However, the detailed organization of sensory innervation of the gut in humans is still unknown. Neither the extension and overlap of reception fields, nor the convergence of afferent input have been characterized. Hence, we cannot establish on the basis of our data at which level of the gut-to-brain sensory network summation takes place. It is also unknown whether the same phenomenon occurs in the ileum or colon, when the stimuli are applied at more distant sites of the gut, or even on other abdominal viscera. In sharp contrast with the distinct summative effect of visceral stimuli, somatic stimulation reduces visceral perception (Coffin et al. 1994), a phenomenon, like counter-irritation or stimulation analgesia, presumably mediated by a supraspinal circuitry via descending spinal pathways (De Broucker, Cesaro, Willer & Le Bars, 1990; Ness & Gebhart, 1991; Woolf & Thompson, 1994).
In series I studies we took advantage of the barostat to produce isobaric distensions regardless of the intestinal length and circumference. Although no direct measurement could be performed, several lines of reasoning suggest that the long barostat produced the same degree of radial distension as the short barostat. Intestinal distension induces intestinal relaxatory reflexes that could have different effects in the long and short distending segments: a more pronounced relaxatory effect would result in proportionally larger intraluminal volumes, and hence larger radial distension. However, the pressure-volume curve had a similar configuration in both intestinal segments, indicating that the local response to distension was similar at both sites. To further validate this point we performed experiments with glucagon administration. Glucagon has been shown to suppress intestinal motor activity with similar effects in the proximal and distal jejunum in humans, and hence it would eliminate the possible effects of accommodation reflexes (Diamant & Picazo, 1983; Rouillon et al. 1991a,b). In the present study, glucagon markedly reduced both basal tone and intestinal extension ratio, and furthermore completely abolished the intestinal reflexes induced by short distensions. However, glucagon did not alter the spatial summation phenomena, and still the differences in perception remained patent.
Using the barostat we also observed that distension of the short segment below the level of discomfort induced relaxation of the long intestinal segment. This reflex response is probably related to the intestino-intestinal inhibitory reflexes, which are driven by sympathetic pathways, and may relay either at the level of the prevertebral ganglia or at the spinal cord (Peterson & Youmans, 1945; Szurszewski, 1981). Some data suggest that intestino-intestinal inhibitory reflexes may also be mediated by intrinsic pathways in the enteric nervous system (Frantzides, Sarna, Matsumoto, Lang & Condon, 1987). We have previously shown that intestino-intestinal reflexes in humans are dissociable from perception (Rouillon et al. 1991a), suggesting that both responses are independently induced by specific mechanisms.
It has been shown that reflex intestinal responses may be modulated by different mechanisms (Johansson, Jonsson & Ljung, 1968) including spatial summation phenomena (Peterson & Youmans, 1945). However, we could not detect any consistent changes in response to long distensions, because in most cases the bag in the short segment collapsed during the distension. These effects did not appear to be related to a true intestinal contraction, but rather seemed a mechanical artifact, conceivably related to intestinal kinking of loops stretched by the long barostat.
Extending our observations to the pathophysiological arena, it would appear that the intestine may tolerate circumscribed activation of sensory terminals by a nociceptive condition without perception, but recruitment of additional areas of the gut, even at distant sites, may mount a symptomatic alert.
Acknowledgments
This work was supported in part by the Spanish Ministry of Education and Science (DGICYT). Dr Serra was supported by a scholarship from the Spanish Ministry of Education and Science. The authors thank Maite Casaus and Anna Aparici for technical support, and Gloria Santaliestra and Montse Domenech for secretarial assistance. This work was presented in part at the annual meeting (1995) of the American Gastroenterological Association, San Diego, CA, USA.
References
- Accarino AM, Azpiroz F, Malagelada J-R. Symptomatic responses to stimulation of sensory pathways in the jejunum. American Journal of Physiology. 1992;263:G673–677. doi: 10.1152/ajpgi.1992.263.5.G673. [DOI] [PubMed] [Google Scholar]
- Accarino AM, Azpiroz F, Malagelada J-R. Selective dysfunction of mechanosensitive intestinal afferents in the irritable bowel syndrome. Gastroenterology. 1995;108:636–643. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- Azpiroz F. Sensitivity of the stomach and the small bowel: human research and clinical relevance. In: Gebhart GF, editor. Progress in Pain Research and Management, vol. 5, Visceral Pain. Seattle: IASP Press; 1995. pp. 391–428. [Google Scholar]
- Azpiroz F, Malagelada J-R. Physiologic variations in canine gastric tone measured by an electronic barostat. American Journal of Physiology. 1985;248:G229–237. doi: 10.1152/ajpgi.1985.248.2.G229. [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Malagelada J-R. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology. 1987;92:934–943. doi: 10.1016/0016-5085(87)90967-x. [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Malagelada J-R. Isobaric intestinal distension in humans: sensorial relay and reflex gastric relaxation. American Journal of Physiology. 1990a;258:G202–207. doi: 10.1152/ajpgi.1990.258.2.G202. [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Malagelada J-R. Perception and reflex relaxation of the stomach in response to gut distension. Gastroenterology. 1990b;98:1193–1198. doi: 10.1016/0016-5085(90)90333-v. [DOI] [PubMed] [Google Scholar]
- Bentley FH, Smithwick RH. Visceral pain produced by balloon distension of the jejunum. Lancet. 1940;2:389–391. [Google Scholar]
- Blumberg H, Haupt P, Jänig W, Kohler W. Encoding of visceral noxious stimuli in the discharge patterns of visceral afferent fibres from the colon. Pflügers Archiv. 1983;398:33–40. doi: 10.1007/BF00584710. [DOI] [PubMed] [Google Scholar]
- Coffin B, Azpiroz F, Malagelada J-R. Somatic stimulation reduces perception of gut distension. Gastroenterology. 1994;107:1636–1642. doi: 10.1016/0016-5085(94)90802-8. [DOI] [PubMed] [Google Scholar]
- De Broucker T, Cesaro P, Willer J C, Le Bars D. Diffuse noxious inhibitory controls in man. Involvement of the spino reticular tract. Brain. 1990;113:1223–1234. doi: 10.1093/brain/113.4.1223. [DOI] [PubMed] [Google Scholar]
- Diamant B, Picazo J. Spasmolytic action and clinical use of glucagon. In: Lefebvre J, editor. Handbook of Experimental Pharmacology. 66/II. Berlin: Springer-Verlag; 1983. pp. 611–643. [Google Scholar]
- Frantzides CT, Sarna SK, Matsumoto T, Lang IM, Condon RE. An intrinsic neural pathway for long intestino-intestinal inhibitory reflexes. Gastroenterology. 1987;92:594–603. doi: 10.1016/0016-5085(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Gracely RH. Studies of pain in normal man. In: Wall PD, Melzac R, editors. Textbook of Pain. 3. Edinburgh: Churchill Livingstone; 1994. pp. 315–336. [Google Scholar]
- Iovino P, Azpiroz F, Domingo E, Malagelada J-R. The sympathetic nervous system modulates perception and reflex responses to gut distension in humans. Gastroenterology. 1995;108:680–686. doi: 10.1016/0016-5085(95)90439-5. [DOI] [PubMed] [Google Scholar]
- Jänig W, Koltzenburg M. Receptive properties of sacral primary afferent neurons supplying the colon. Journal of Neurophysiology. 1991;65:1067–1077. doi: 10.1152/jn.1991.65.5.1067. [DOI] [PubMed] [Google Scholar]
- Johansson B, Jonsson O, Ljung B. Tonic supraspinal mechanisms influencing the intestinointestinal inhibitory reflexes. Acta Physiologica Scandinavica. 1968;72:200–204. doi: 10.1111/j.1748-1716.1968.tb03842.x. [DOI] [PubMed] [Google Scholar]
- Jones CM. Digestive Tract Pain: Diagnosis and Treatment; Experimental Observations. New York: McMillan; 1938. [Google Scholar]
- McMahon SB, Morrison JFB. Spinal neurones with long projections activated from the abdominal viscera of the cat. Journal of Physiology. 1982;322:1–20. doi: 10.1113/jphysiol.1982.sp014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. Journal of Neurophysiology. 1991;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- Notivol R, Coffin B, Azpiroz F, Mearin F, Serra J, Malagelada J-R. Gastric tone determines the sensitivity of the stomach to distension. Gastroenterology. 1995;108:330–336. doi: 10.1016/0016-5085(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Peterson C, Youmans WB. The intestino-intestinal inhibitory reflex: threshold variations, sensitization and summation. American Journal of Physiology. 1945;143:407–412. [Google Scholar]
- Ray BS, Neill CL. Abdominal visceral sensation in man. Annals of Surgery. 1947;126:709–724. [PubMed] [Google Scholar]
- Rouillon J-M, Azpiroz F, Malagelada J-R. Reflex changes in intestinal tone: relationship to perception. American Journal of Physiology. 1991a;261:G280–286. doi: 10.1152/ajpgi.1991.261.2.G280. [DOI] [PubMed] [Google Scholar]
- Rouillon J-M, Azpiroz F, Malagelada J-R. Sensorial and intestino-intestinal reflex pathways in the human jejunum. Gastroenterology. 1991a;101:1606–1612. doi: 10.1016/0016-5085(91)90398-5. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Gastrointestinal afferents and sensation. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3. Vol. 1. New York: Raven; 1994. pp. 483–519. [Google Scholar]
- Serra J, Azpiroz F, Malagelada J-R. Perception and reflex responses to intestinal distension are modified by simultaneous or previous stimulation. Gastroenterology. 1995;109:1742–1749. doi: 10.1016/0016-5085(95)90739-4. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Physiology of mammalian prevertebral ganglia. Annual Review of Physiology. 1981;43:53–68. doi: 10.1146/annurev.ph.43.030181.000413. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson JW. Stimulation fibre-induced analgesia: transcutaneous electrical nerve stimulation (TENS) and vibration. In: Wall PD, Melzack R, editors. Textbook of Pain. 3. Edinburgh: Churchill Livingstone; 1994. pp. 1191–1208. [Google Scholar]
- Youmans WB. Innervation of the gastrointestinal tract. In: Code CF, editor. Handbook of Physiology, section 6, The Gastrointestinal System, vol. 4, Motility. Washington DC: American Physiological Society; 1968. pp. 1655–1663. [Google Scholar]


