Abstract
Neuronal retrograde tracing with the dye DiI (1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), in combination with immunohistochemical detection of choline acetyltransferase (ChAT) and vasoactive intestinal peptide (VIP), were used to identify the innervation of the mucosa of the guinea-pig proximal colon by submucosal neurones. Ussing chamber experiments were performed to measure changes in short circuit current (ΔIsc) evoked by electrical stimulation of the oral or anal end of the preparation.
The tracing studies revealed that the mucosa was primarily innervated by descending neurones (78%); the vast majority of these were VIP positive (85%). The numerically smaller ascending pathway (13%) was predominantly ChAT positive (69%). A small population (9%) of DiI-labelled neurones projected circumferentially.
Ussing chamber experiments revealed that oral electrical stimulation induced a significantly larger ΔIsc than anal stimulation. The VIP antagonist VIP(6–28) significantly reduced only orally induced ΔIsc. Anally induced ΔIsc were significantly more atropine sensitive that orally induced ΔIsc. Tissue incubation with carbachol or VIP significantly potentiated ΔIsc induced by VIP and carbachol, respectively, indicating cross-potentiation.
This study provides the first functional demonstration of polarized innervation patterns from submucosal neurones to enteric mucosa. The ascending ChAT and descending VIP pathways suggest the existence of reflexes resulting in preferential release of VIP or acetylcholine. The distinct pathways might favour the observed cross-potentiation of cholinergic and VIPergic mediated secretion.
Major gastrointestinal (GI) functions are under the control of the enteric nervous system (ENS). The ENS is a nervous system embedded within the wall of the gut and consists of two major plexuses: the myenteric plexus and the submucosal plexus. Both plexuses can regulate GI function independently of inputs from the central nervous system (for review see Wood, 1994). Myenteric neurones are primarily involved in the control of GI motility (for review see Wood, 1994). On the other hand, submucosal neurones are mainly responsible for mucosal functions ranging from mucosal fluid and electrolyte transport, mucosal blood flow, neuro-immune interaction and epithelial cell migration to regulators of lymphoid cell proliferation (Keast, 1987; Bustamante, Forshult & Lundgren, 1989; Ottaway, 1991; Cooke & Reddix, 1994; Vanner & Surprenant, 1996).
In order to regulate these GI functions, enteric neurones form neuronal networks. The most studied network today is the one controlling the peristaltic reflex and has been thoroughly described in the guinea-pig ileum (Bayliss & Starling, 1899; Bornstein, 1994; Wood, 1994; Costa, Brookes, Steele, Gibbins, Burcher & Kandiah, 1996). Different projections of myenteric motor neurones are believed to play a major role in adjusting motor activity of adjacent segments of the gut. The myenteric motor neurones involved are descending inhibitory neurones containing vasoactive intestinal peptide (VIP) and nitric oxide synthase (NOS), and ascending excitatory cholinergic neurones (Costa et al. 1996).
Recent studies have revealed the involvement of submucosal neurones in controlling blood flow and secretion reflexes in the colon (Frieling, Wood & Cooke, 1992; Hubel & Russ, 1993; Sidhu & Cooke, 1995; Vanner & Surprenant, 1996). Sidhu & Cooke (1995) showed that mucosal stroking induced a secretory response consisting of both a cholinergic and a non-cholinergic non-adrenergic component. Furthermore, submucosal cholinergic and non-cholinergic secretomotor neurones have been shown to project to the mucosa of the ileum (Song, Brookes, Steele & Costa, 1992). This suggests that mucosal secretion is modulated by at least two distinct mechanisms, one predominantly cholinergic and one probably VIPergic. In the guinea-pig colon, both carbachol and VIP have been shown to increase chloride secretion (Kuwahara & Radowicz-Cooke, 1988; Reddix, Kuwahara, Wallace & Cooke, 1994). Substance P localized in some cholinergic neurones has also been shown to have a similar effect on secretory processes (Kuwahara & Cooke, 1990).
Although the effects of various transmitters and modulatory substances on mucosal secretion have been documented, the neuronal circuitry involved in the control of mucosal functions in the guinea-pig colon has not yet been identified. One reason for this is the difficulty in characterizing a functional subclass of enteric neurones, since functionally distinct neurones are intermingled in one ganglion. Indeed, submucosal ganglia might contain secretomotor, vasomotor interneurones and/or sensory neurones (Cooke & Reddix, 1994). Therefore, a neuronal tracing method needs to be used to identify a specific functional subclass of neurones projecting to a defined target organ (Brookes & Costa, 1990).
In a previous study we demonstrated the presence of myenteric neurones projecting to the colonic mucosa (Neunlist & Schemann, 1997). Comparable data on the innervation of the colonic mucosa by submucosal neurones are not available. Therefore, the aim of this study was firstly to characterize the projection pattern and the neurochemical coding of submucosal neurones innervating the mucosa, and secondly to investigate the functional role of the innervation pattern in regulating secretory processes. Results have been previously published in abstract form (Neunlist, Reiche, Hoppe & Schemann, 1996; Frieling, Neunlist, Rupprecht, Becker, Häussinger & Schemann, 1997).
METHODS
Neuronal tracing experiments
The method used is similar to one described previously (Neunlist & Schemann, 1997). In brief, all dishes, glassware and surgical tools were sterile. Guinea-pigs of either sex (200–350 g) were killed by cervical dislocation followed by exsanguination. The abdomen was sprayed with 70% ethanol and opened. Specimens of proximal colon (5 cm distal from the caeco-colic junction) were removed and placed in aerated, sterile Krebs solution of the following composition (mm): NaCl, 117; KCl, 4.7; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 25; CaCl2, 2.5; glucose, 11.5; plus 1 μM nifedipine; pH 7.4.
A small incision was made at the oral side of the tissue for later identification of the neuronal projection pathways. The tissue (2–3 cm in length) was opened along the mesenteric border and the luminal content was flushed away. The tissue was agitated in aerated Krebs solution before being pinned out in a Sylgard-coated Petri dish. The mucosa was carefully removed except for a small window of about 1 cm × 1 cm. The tissue was washed with sterile aerated Krebs solution then pinned and maximally stretched, mucosa side up, in a large Sylgard-coated organ culture dish (9 cm diameter). Following the last wash, the retrograde tracer was applied to the mucosa.
The lipophyllic fluorescent retrograde tracer DiI (1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Molecular Probes) was used in all the experiments. The dye (1 mm in methanol) was evaporated onto small glass beads (diameter, 50–100 μm) that were lightly pressed onto the proximal colon mucosa at the anti-mesenteric border. Care was taken not to push the bead too deep into the mucosa.
Following bead application, the tissue was washed in Krebs solution. Sterile culture medium containing 1 μM nifedipine was then added to the Petri dish. The culture medium (Dulbecco's modified Eagle's medium/F12; Sigma Chemical Co.) was supplemented with 10% heat inactivated fetal calf serum (CC pro, Karlsruhe, Germany), 100 i.u. ml−1 penicillin, 100 mg ml−1 streptomycin, 5.25 μg ml−1 amphotericin B, 100 μg ml−1 gentamicin (Sigma Chemical Co.), and 2.1 g l−1 NaHCO3 and adjusted to pH 7.4. The tissue was maintained in a humidified incubator at 37°C and equilibrated with 5% CO2 and air for a period of 72 h. The dishes were placed on a rocking tray, shaking at a frequency of approximately 0.5–1 Hz. The culture medium was changed daily.
Immunohistochemistry
After the organotypic culture, the tissue was fixed for 12 h at 4°C in 2% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.44). The fixed tissue was then repeatedly washed with phosphate buffer. The longitudinal and circular muscle layers were carefully dissected to expose the submucosal plexus. The position of the DiI application site was marked by pushing a hole through the mucosa into the submucosa, using a fine needle. The mucosa was then removed.
Immunohistochemical detection of neuronal antigens was made using the procedure previously described (Neunlist & Schemann, 1997). Cells were permeabilized with successive washes of the tissue in phosphate-buffered saline (PBS)-NaN3 glycerol solution of various glycerol concentrations (30 min in 50% glycerol; 30 min in 80% glycerol; 120 min in 100% glycerol). The tissue was then thoroughly washed in PBS-NaN3 to remove the glycerol.
After the permeabilization procedure, the tissue was exposed at room temperature for 30–40 h in PBS-NaN3 and 4% goat or donkey serum with the following primary antibodies: rabbit anti-choline acetyltransferase (ChAT) (1:1000; Schemann, Schaaf & Mäder, 1995), goat anti-ChAT (1:100; Chemicon International, Temecula, CA, USA), rabbit anti-vasoactive intestinal polypeptide (VIP) (RAS-7161-N, 1:1000; Peninsula, Heidelberg, Germany) or rabbit anti-neurone specific enolase (NSE) (16625, 1:1000; Polysciences, Eppelheim, Germany). Specificity of antisera and antibodies used has been reported previously (Schemann et al. 1995). Tissues were then thoroughly rinsed with PBS-NaN3 and incubated for a period of 24 h with the following species-specific secondary antibodies: goat anti-mouse or anti-rabbit or donkey anti-goat IgG conjugated to dichlorotriazinyl aminofluorescin (DTAF; 1:200, Dianova, Hamburg, Germany). In some cases biotinylated secondary antibodies coupled to streptavidin AMCA (7-amino 4-methyl coumarin 3-acetic acid; Dianova) were used to enhance the fluorescent signal. The preparations were viewed under an epifluorescence microscope (Olympus IX 70) with the filter cubes UM41007 (beam splitter, 565 DCLP; Ex, HQ 545/30; Em, HQ610/75M) for DiI, U-MNIBA (beam splitter, DM 505; Ex, BP 470–490; Em, D520) for DTAF and U-MWU (beam splitter, DM 400; Ex, BP 330–385; Em, BP 460–490) for AMCA. There was no cross-detection between the three fluorophores-filter-cube combinations.
Pictures were taken with a black and white video camera (Model 4910, Cohu Inc., San Diego, CA, USA) connected to a Macintosh Computer via an IPlab card controlled by the IPLab Spectrum 3.0 software (Signal Analytics, Vienna, VA, USA). Frame integration and contrast enhancement were the only procedures used for image processing.
Cell counting
Only cells showing a granulated DiI filling and a clearly defined nucleus were counted as positive. The same criteria were used in previous studies (Song et al. 1992; Neunlist & Schemann, 1997). The position of each DiI-labelled neurone with respect to the application site was measured using a computerized stage mapping system (Märzhäuser and Kassen; Wetzlar, Germany) with a precision of 1 μm. Data were plotted and analysed with SigmaPlot and SigmaStat (SPSS Inc., Chicago, IL, USA) on a personal computer.
Nomenclature
In this paper the same notations are used as described previously (Neunlist & Schemann, 1997). In brief, each DiI-labelled neurone is located with respect to its cartesian X- and Y-co-ordinate from the DiI application site. The application site had the co-ordinate 0,0. Longitudinally projecting neurones were neurones whose co-ordinate verified: |X| >|Y|. Circumferentially projecting neurones verified: |X| < |Y|. Longitudinally projecting neurones were further classified into two categories: descending neurones i.e. neurones whose cell body was located oral from the DiI application site (X < 0) and ascending neurones i.e. neurones whose cell body was located anal from the DiI application site (X > 0). Distribution of DiI-labelled neurones are illustrated by their longitudinal projection along the X-axis and their circumferential projection along the Y-axis. The projection distance of the DiI-labelled soma from the tracer application site was calculated as d =√X2+Y2.
Ussing chamber experiments
Guinea-pigs of either sex (200–350 g) were killed by cervical dislocation followed by exsanguination. Segments of proximal colon (5–10 cm from the caeco-colic junction) were removed and a small incision was made at the oral end of the tissue for orientation. The piece of colon was then opened along the mesenteric border and pinned in a Sylgard-coated Petri dish. Preparations including mucosa and the submucosal plexus (0.78 cm2) were obtained after stripping the longitudinal and circular muscle layers.
The experimental methods for studying mucosal transport were similar to those described previously (Frieling et al. 1992). The chambers were equipped with a pair of Krebs-Ringer-agar bridges connected to calomel half-cells for measurement of transepithelial potential difference (PD) with respect to the luminal solution. Positive short-circuit currents (Isc) indicated a net anion current from serosa to lumen. A pair of Ag-AgCl disc electrodes were connected to a voltage clamp apparatus (VCC 600, Physiologic Instruments, Houston, TX, USA) to compensate for the solution resistance between the PD sensing bridges. Electrodes were juxtaposed between the Ussing chamber half-cells and the submucosal surface. Tissue conductance was calculated according to Ohm's law.
A sheet of Sylgard with an opening of 4 mm × 3 mm was applied over the mucosa in order to record short circuit currents induced mainly by neurones projecting to the mucosal area under the Sylgard opening (see below). This window was centred in the middle of the preparation. Submucosal neurones were electrically stimulated in a randomized pattern (oral vs. anal) via pairs of aluminium foils located at the serosal side of the tissue. Pairs of these electrodes (2 mm wide) were situated between the two halves of the Ussing chamber, at the oral and anal side of the preparation at a distance of 4 mm from the centre of the Sylgard window. The distance from the stimulus site to the centre of the mucosal area under the Sylgard opening closely matches the average projection distance of submucosal neurones innervating the mucosa (see Results). Trains of constant voltage pulses were delivered by a Grass SD9 stimulator (Grass Instruments, Quincy, MA, USA). Preliminary experiments showed that electrically induced ΔIsc increased with increasing stimulus intensities ranging from 1 to 20 V (n = 8). However, the asymmetry between orally and anally induced ΔIsc was most significant at voltages between 5 and 10 V (n = 8). Although the asymmetry was observed with stimulus frequencies ranging from 3 to 30 Hz (n = 24), we decided to use 20 Hz in order to make a comparison with previous studies in our laboratory. For these reasons, we decided to use the following stimulus parameters: 8 V, 20 Hz given for 10 s with a pulse duration of 500 μs.
Preliminary experiments and comments on standard procedure
We carried out a number of experiments to validate our modified Ussing chamber configuration. Firstly, we estimated whether the Sylgard sheet with the centred opening restricted the stimulus-evoked currents, compared with the configuration without the sheet. In the presence of the Sylgard sheet the currents evoked by oral and anal stimulation decreased significantly by 66 and 72%, respectively.
We also determined the relative proportion of the preparation stimulated by the oral and anal electrodes using the configuration with the Sylgard sheet. Using an electrode configuration allowing us to stimulate the entire tissue, an average ΔIsc of 616 ± 38 μA cm−2 (n = 6) was measured. Compared with this value the responses to oral and anal stimulation (n = 31 for each) were reduced by 55 and 71%, respectively.
Experiments were carried out to verify that the electrical stimulation mainly occurs in close proximity to the electrodes and to determine the extent of current spread. We therefore interrupted the nerve pathways anal to the oral stimulation electrodes. This was achieved by coagulating a narrow band about 1 mm anal to the oral stimulation electrodes, with a heated cannula (500 μm diameter). Under these conditions, ΔIsc induced by oral stimulation was decreased by 94% compared with control (n = 3). At the same time, ΔIsc induced by the anal stimulation electrodes was not significantly changed (n = 3). We therefore concluded that our secretory responses were caused mainly by local stimulation.
Chemicals
All drugs used in this study were dissolved in deionized water. Carbachol, tetrodotoxin, atropine and hexamethonium were purchased from Sigma. Further substances used were: guinea-pig VIP and the VIP receptor antagonists VIP fragment 6–28 (8087, 10 μm; Peninsula, Belmont, CA, USA), a VIP antagonist (7124, 10 μm; Peninsula), the VIP receptor binding inhibitor (L-8-K; 7133, 10 μm; Peninsula), the VIP fragment 10–28 (1 μm; Sigma), [D-p-Cl-Phe6-Leu17]-VIP (V4380, 10 μm; Sigma) and VIP antiserum (1 : 400 to 1 : 2000; Peninsula). Drugs were added to the serosal side.
Statistics
Data are expressed as the mean ±s.e.m. Median values are given with their 25 and 75 percentiles. Distributions were tested for significant differences using the z test. Means were compared using Student's paired or unpaired t tests, and medians were compared using the Spearman Rank test. One-way or two-way analysis of variance (Student-Newman-Keuls test) was performed for multiple comparisons. Differences were considered as statistically significant at values of P < 0.05.
RESULTS
DiI labelling in the proximal colon
The results were obtained from twenty-one preparations (16 animals). The application of a DiI bead onto the mucosa resulted in the labelling of 77 ± 8 neurones (range, 31–178) in each preparation. The spatial distribution of all DiI-labelled neurones in the longitudinal and circumferential direction is represented in Fig. 1A-C. In the longitudinal direction, approximately 7% of the DiI-labelled neurones were located within 1 mm, 21% within 2 mm, 49% within 3 mm and 73% within 4 mm, either side of the bead application site. Along the circumferential axis, neurones were located closer to the application site with 60% of them located within 1 mm, 89% within 2 mm, 96% within 3 mm and 98% within 4 mm, either side of the application site.
Figure 1. Spatial distribution of DiI-labelled submucosal neurones around the DiI application site onto the colonic mucosa.
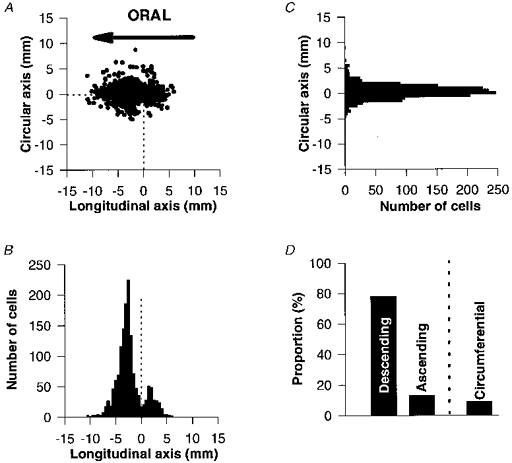
Labelled neurones were grouped into 0.5 mm bins. A, the DiI-labelled neurones are represented by their cartesian X- and Y-co-ordinates with respect to the DiI application site (0,0). All 1502 DiI-labelled cells from 21 preparations are illustrated. B, histogram of DiI-labelled neurones in the longitudinal axis. Descending neurones outnumbered ascending neurones. They also projected over significantly longer distances than those of ascending neurones. C, histogram of DiI-labelled neurones in the circumferential axis. Neurones are distributed uniformly along the Y-axis. D, histogram of the percentage of descending, ascending and circumferentially projecting neurones.
Most neurones had longitudinal projections (91%) and the remainder had circumferential projections (9%; P < 0.001). Longitudinally projecting neurones could be subdivided into descending (86%) (located orally from the DiI application site) and ascending (14%) neurones (located anally from the dye application site), these proportions being significantly different (P < 0.001) (Fig. 1D). Descending neurones projected over longer distances than ascending neurones (3.9 ± 0.2vs.2.8 ± 0.2 mm, respectively; P < 0.0005; n = 21). Circumferentially projecting neurones showed no preferential projection direction (50% negative direction; 50% positive direction). In addition, there was no significant difference between the projection distances of the two groups of circumferential neurones (2.9 ± 0.3vs.3.4 ± 0.4 mm; n = 21).
Control for the specificity of the DiI labelling
In order to verify the specificity of the neuronal labelling method, different controls were undertaken. First, the segment of mucosa was removed from the submucosa and pinned back to its original position before a DiI bead was applied. No submucosal neurones were labelled after 3 days of culture (n = 4 from 3 animals). Only a diffusely stained area was seen directly under the application site. We evaluated further whether submucosal neurones might also project to the circular muscle. Therefore, in three preparations (2 animals), the longitudinal and myenteric plexus were removed and a DiI bead was applied onto the serosal side of the circular muscle. No submucosal neurones were labelled following 3 days of organ culture in these preparations. We therefore concluded that the submucosal neurones labelled by mucosal application of the dye projected exclusively to the mucosa.
Neurochemical coding of the submucosal plexus of the guinea-pig proximal colon
In order to characterize the neurochemical coding of the DiI-labelled submucosal neurones, we first determined the general coding of the submucosal plexus in the proximal colon. Previous studies on the guinea-pig ileum have revealed the existence of two independent submucosal populations based on their neurochemical coding: one positive for ChAT and the other for VIP (Furness, Costa & Keast, 1987). Therefore, we determined the cholinergic and VIPergic phenotype of the submucosal plexus. NSE immunoreactivity was used as a general neuronal marker to determine the total number of neurones per ganglion.
Submucous ganglia in the proximal colon contained on average 28 ± 1 NSE-immunoreactive neurones (median, 24 (10;40 (25 and 75 percentiles)); 767 ganglia). Every ganglion contained on average 13 ± 1 ChAT-immunoreactive neurones (median, 11 (5;18); 1125 ganglia) and 15 ± 1 VIP-positive neurones (median, 13 (8;19); 358 ganglia). Three preparations double-labelled for ChAT and VIP, revealed a total of 27 ± 17 ChAT- and VIP-positive neurones per ganglion (median, 24 (14;35); 358 ganglia). These data suggest further that submucosal neurones in the proximal colon are either in their vast majority VIP or ChAT positive. Therefore, VIP-positive neurones represented 53 ± 1% and ChAT-positive neurones 47 ± 1%. Only 1.4 ± 0.3% of the neurones were VIP and ChAT positive.
Neurochemical coding of DiI-labelled neurones
The general results on neurochemical coding of DiI-labelled neurones are illustrated using a representative preparation (Fig. 2) stained for ChAT and VIP immunoreactivity. This preparation revealed that the majority of DiI-labelled neurones were VIP immunoreactive and not ChAT immunoreactive. A marked polarity in the chemical coding between ascending and descending labelled neurones was also revealed (Fig. 2). The vast majority of descending neurones were VIP immunoreactive while the majority of ascending neurones were ChAT immunoreactive. The global analysis of twelve preparations revealed that 73 ± 3% of the DiI-labelled neurones were VIP positive compared with 23 ± 4% that were ChAT positive. For the descending neurones 85 ± 3% were VIP immunoreactive (Fig. 3) while only 11 ± 3% were ChAT immunoreactive. In contrast, 69 ± 8% of the ascending neurones were ChAT immunoreactive (Fig. 4) while only 24 ± 8% were VIP positive.
Figure 2. Spatial distribution of DiI-labelled submucosal neurones projecting to the mucosa and stained for ChAT (•) and VIP (○) immunoreactivity.
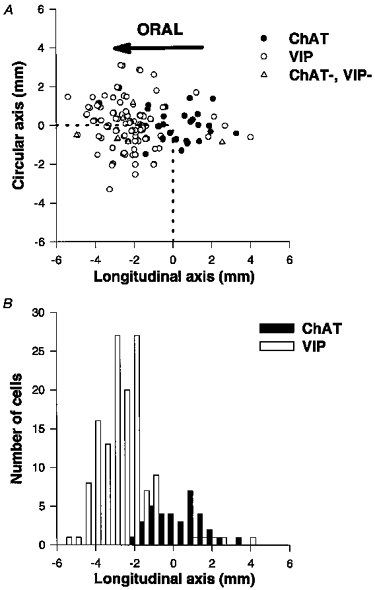
DiI-labelled neurones were grouped into 0.5 mm bins. Data are from one representative preparation. A, the DiI-labelled neurones are represented by their cartesian X and Y co-ordinates with respect to the DiI application site (0,0). Neurones not immunoreactive for either ChAT or VIP are represented by ▵. B, histogram of DiI-labelled neurones in the longitudinal axis. The majority of the descending neurones are VIP positive while there is no preferential projection for the ChAT-immunoreactive neurones.
Figure 3. Neurochemical coding of a descending DiI-labelled neurone.
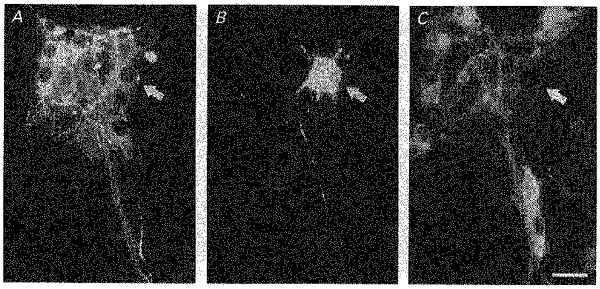
Immunohistochemical demonstration of choline acetyltransferase (ChAT) and vasoactive intestinal peptide (VIP) in one descending DiI-labelled submucosal neurone projecting to the mucosa (arrow in B). The neurone is VIP positive (arrow in A) but ChAT negative (arrow in C). Scale bar, 25 μm.
Figure 4. Neurochemical coding of ascending DiI-labelled neurones.
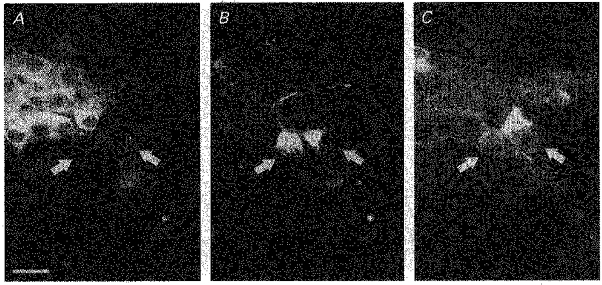
Immunohistochemical demonstration of ChAT and VIP in two ascending DiI-labelled submucosal neurones projecting to the mucosa (arrows in B). These neurones were VIP negative (arrows in A) but ChAT positive (arrows in C). Scale bar, 25 μm.
VIP-positive DiI-labelled neurones were usually located in the middle of the ganglion or at the edges, relatively far from the connection with interganglionic fibre tracts (Fig. 3). VIPergic neurones projected preferentially in the longitudinal direction (91%), rather than the circumferential direction (9%; P < 0.0001). In addition, the proportion of VIPergic descending neurones (95%) was significantly greater than VIPergic ascending neurones (5%; P < 0.0001). Also, VIPergic descending neurones projected over longer distances than VIPergic ascending neurones (4.2 ± 0.3vs.2.5 ± 0.3 mm; P < 0.05; n = 6).
Cholinergic neurones were usually located at the edge of the ganglia, close to the junction between interganglionic fibre tracts and ganglia (Fig. 4). A significant proportion of the ChAT-immunoreactive cells projected in the longitudinal (73%) compared with the circumferential direction (27%; P < 0.0001). There was no preferential projection in the longitudinally projecting neurones (50% ascending vs. 50% descending; P = 0.92) (Fig. 2). ChAT-positive descending neurones projected over longer distances than ChAT-positive ascending neurones (2.8 ± 0.3vs.1.9 ± 0.2 mm; P < 0.05; n = 7). In addition, descending cholinergic neurones projected over significantly shorter distances than descending VIPergic neurones (P < 0.05; n = 6).
Ussing chamber experiments
Since our retrograde tracing studies suggested a polarized innervation pattern of the mucosa, we performed Ussing chamber experiments to investigate the possible functional role of such an innervation.
The guinea-pig proximal colon exhibited baseline short circuit current (Isc) and conductance similar to that reported previously by other investigators (Kuwahara & Radowicz-Cooke, 1988). Basal Isc averaged 18 ± 2 μA cm−2 and total tissue conductance was 10.0 ± 0.2 mS cm−2 (n = 31 preparations from 12 animals). Neurally induced changes in Isc (ΔIsc) were measured as a function of oral or anal electrical stimulation using a modified Ussing chamber (see Methods) (Fig. 5A). Oral stimulation was used to preferentially stimulate descending neurones and anal stimulation to preferentially stimulate ascending neurones.
Figure 5. Diagram of the configuration for electrical stimulation in the Ussing chamber and neurally evoked changes in short circuit current (ΔIsc) as a function of the location of the stimulating electrodes.
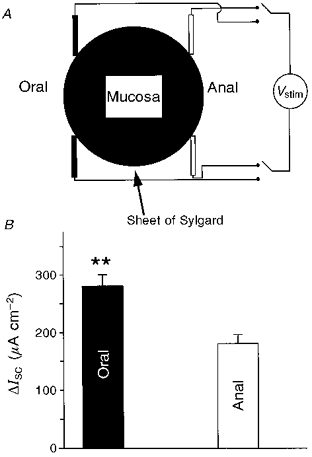
A, illustration of the electrical stimulation protocol of the mucosa/submucosa preparation. The mucosa was covered with a sheet of Sylgard with a 4 mm × 3 mm hole in the middle. This configuration allowed us to record mainly ΔIsc induced under this area. Two pairs of aluminium foil electrodes are placed on serosal side at the oral and anal ends of the preparation, in order to preferentially stimulate descending and ascending neurones, respectively. Vstim, stimulation potential. B, neurally evoked ΔIsc with oral stimulation was always significantly larger than that evoked by anal stimulation (**P < 0.005).
Both oral and anal stimulation induced reproducible increases in Isc. The amplitude of ΔIsc resulting from oral stimulation was significantly greater than that resulting from anal stimulation (278 ± 19 vs. 179 ± 14 μA cm−2; P < 0.001; n = 31) (Fig. 5B). The augmented response to oral electrical stimulation was always obtained, irrespective of whether the electrical stimulation was first initiated at the oral or anal side of the tissue. Blockade of nicotinic receptors with hexamethonium (100 μm) had no effect on basal Isc; however, the amplitudes of orally and anally induced ΔIsc were significantly reduced by 29 ± 6 and 22 ± 5%, respectively, compared with control (P < 0.005; n = 9). Nevertheless, even in the presence of hexamethonium, orally induced ΔIsc remained significantly higher than anally induced ΔIsc (P < 0.005).
Pharmacology of neurally evoked secretion
The VIP antagonist VIP(6–28) (10 μm) did not modify baseline Isc when added to the serosal side of the Ussing chamber. VIP(6–28) significantly reduced ΔIsc evoked by oral stimulation by 43 ± 7% (P < 0.001; n = 9) but only slightly and insignificantly reduced ΔIsc induced by anal stimulation (6 ± 13%; P = 0.31; n = 9) (Fig. 6A). In addition, among the various VIP antagonists tested (see Methods), only VIP(6–28) could inhibit VIP-induced secretion. VIP(6–28) at a concentration of 10 μm significantly reduced ΔIsc induced by 1 and 10 nM VIP by 67 and 32%, respectively. At higher VIP concentrations, the reduction was no longer significant, reaching only 8% at 1 μm VIP. VIP(6–28) did not affect ΔIsc induced by carbachol concentrations of 10 nM to 10 μm (n = 4 for each concentration).
Figure 6. Effect of the VIP antagonist VIP(6–28) and atropine on neurally evoked ΔIsc as a function of the electrode position.
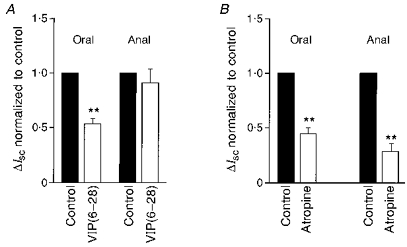
Neurally mediated ΔIsc values are normalized to control values obtained in the absence of antagonists. A, VIP(6–28) (10 μm) only significantly reduced neurally induced ΔIsc during oral stimulation of the preparation (**P < 0.005). B, atropine (1 μm) significantly reduced neurally induced ΔIsc for both oral and anal stimulation. The reduction was significantly larger for anal than oral stimulation (**P < 0.005).
Addition of atropine (1 μm) to the serosal side of the preparation did not modify the basal Isc. In contrast to VIP(6–28), atropine significantly reduced ΔIsc induced by oral and anal stimulation by 53 ± 3 and 73 ± 4%, respectively (Fig. 6B). However, this inhibitory effect of atropine was significantly more pronounced for anal than for oral stimulation (P < 0.005; n = 10).
Possible cross-potentiation between VIP and carbachol was also investigated using the Ussing chamber technique. For this purpose, suprathreshold concentrations of VIP (0.1 μm) or carbachol (1 μm) were added to the bath 5 min before performing concentration-response experiments for carbachol or VIP, respectively. To eliminate neuronally mediated effects of VIP and carbachol, these experiments were carried out in the presence of 1 μm TTX (n = 4). In the presence of TTX both VIP and carbachol increased ΔIsc dose dependently (Fig. 7A and B). The effects of carbachol were dramatically increased by the background application of VIP. Under these conditions, ΔIsc induced by 0.1, 1 and 10 μm carbachol were 4.5-, 7.7- and 5.4-fold higher, respectively, than the expected additive responses (Fig. 7A). Similarly, the effects of VIP were substantially increased by the background application of carbachol. ΔIsc induced by 0.01, 0.1 and 1 μm VIP were 4.0-, 5.7- and 5.2-fold higher, respectively, than the expected additive responses (Fig. 7B). Cross-potentiation was only observed when the substance administered as background stimulus, could alone evoke a ΔIsc. Subthreshold application of either VIP or carbachol did not lead to any potentiation.
Figure 7. Cross-potentiation studies for VIP- and carbachol-evoked ΔIsc.
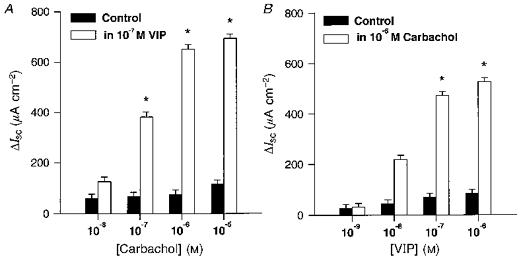
The data were obtained in the continuous presence of 1 μm TTX. A, concentration-response curve of carbachol-induced ΔIsc in the presence (□) or absence (▪) of 0.1 μm VIP. ΔIsc induced by carbachol plus ΔIsc induced by the background application of VIP are shown. Carbachol effects are significantly potentiated in the presence of 0.1 μm VIP (indicated by *). B, concentration-response curve of VIP-induced ΔIsc in the presence (□) or absence (▪) of 1 μm carbachol. ΔIsc induced by VIP plus ΔIsc induced by the background application of carbachol-VIP effects are shown. Values are significantly potentiated in the presence of 1 μm carbachol (indicated by *).
DISCUSSION
This study has demonstrated for the first time, polarized projection patterns in the innervation of the guinea-pig proximal colon mucosa by submucosal neurones. It has also revealed the functional involvement of these circuits on neurally mediated ion secretion by selective electrical stimulation of neural pathways with different projections. The mucosa is primarily innervated by descending neurones and to a lesser extent by ascending neurones. The vast majority of these descending neurones are VIP immunoreactive whereas ascending neurones are primarily ChAT immunoreactive.
It has previously been suggested that submucosal neurones containing VIP or neuropeptide Y, the latter being a marker for a subset of cholinergic neurones in the submucosal plexus of the guinea-pig, are secretomotor neurones (Furness, Bornstein, Murphy & Pompolo, 1992). The results of the present study support this by demonstrating that both populations of VIP- and ChAT-positive cells project to the mucosa. It is unlikely that our retrograde tracing reflected innervation of the muscularis mucosae. Although Messenger (1993) also found a prominent VIPergic innervation of the muscularis mucosa in the proximal colon, myectomy experiments revealed that the majority of these fibres had their origin in the myenteric and not in the submucosal plexus. If we had labelled predominantly submucosal motor neurones innervating the muscularis mucosa, they should have been primarily substance P (SP)-encoded cells (Messenger, 1993), which are also ChAT positive (authors’ unpublished observation). Since the ChAT population in our tracing study is much smaller than the VIP population, we might assume that very few motor neurones to the muscularis mucosa were labelled.
Results obtained from the neurochemical coding of the entire submucosal population revealed that VIP and ChAT antibodies label separate populations of almost equal size. It is therefore striking that 73% of the DiI-labelled submucosal neurones projecting to the mucosa were VIPergic while only 23% were cholinergic. A predominant VIPergic innervation of the mucosa was also observed in the small intestine using similar tracing techniques (Song et al. 1992). The lower percentage of ChAT-positive DiI-labelled cells compared with their overall proportion in the submucosal plexus, might be explained by the fact that we labelled the specific functional class of neurones that innervate the mucosa. Interneurones whose axons project within the plexus would not be labelled. Previous studies indicated the existence of cholinergic interneurones in the guinea-pig submucosal plexus (Furness et al. 1992). This was confirmed further by results from the Ussing chamber experiments. Hexamethonium, which blocks synaptic transmission from cholinergic interneurones, decreased the secretion evoked by oral and anal stimulation, without affecting the asymmetry in secretion between the two stimulation sites.
In Ussing chamber experiments, we demonstrated the involvement of the polarized innervation by the submucosal plexus on mucosal secretory processes. Thus oral electrical stimulation of the preparation resulted in a greater secretory response than stimulation at the anal side. This asymmetry might be explained in view of our tracing results. They revealed that a given mucosal area is predominantly innervated by descending neurones. Therefore, oral stimulation might activate a higher number of neurones than anal stimulation.
In addition, this study revealed that only orally induced secretion had a significant VIPergic component. This is consistent with results from the tracing studies that indicated that 95% of the VIPergic neurones were descending. There may be several reasons for the inability of the VIP antagonist to totally abolish the orally evoked secretion. Firstly, it might reflect the presence of an additional non-VIPergic component in the secretory response, induced by oral stimulation. This is likely because at least the VIPergic submucosal neurones in the small intestine contain a number of additional pro-secretory substances (Keast, 1987). Secondly, we found a cholinergic component in the orally evoked responses, which strongly supported the existence of descending cholinergic pathways demonstrated by our tracing studies. Finally, the VIP antagonist used may not have been potent or specific enough to block all VIP receptors on epithelial cells, although VIP(6–28) has been reported to be a potent inhibitor of VIP binding to VIP receptors on guinea-pig pancreatic acini (Fishbein et al. 1994). Insufficient blockade of VIP-induced secretion by various VIP antagonists has been reported previously (Burleigh & Kirkham, 1993; Reddix et al. 1994).
Our functional study revealed that atropine reduced the orally evoked secretion and more significantly the anally evoked secretion. This is in agreement with our tracing studies showing that cholinergic neurones were symmetrically distributed around the application site but that the majority of ascending neurones were cholinergic. The atropine-resistant component may be due to release of additional neurotransmitters, like neuropeptide Y, cholecystokinin, calcitonin gene-related peptide, somatostatin, dynorphin or SP, which are co-localized in cholinergic secretomotor neurones (Bornstein & Furness, 1992; Song et al. 1992; Kirchgessner, Tamir & Gershon, 1992). In addition, it has to be considered that electrical stimulation will also result in transmitter release from nerve fibres that originate from myenteric neurones (Neunlist & Schemann, 1997). These have been shown to be cholinergic and may also contain SP (Song, Brookes & Costa, 1991; Neunlist & Schemann, 1997).
The potentiation studies indicated at least one functional role of an innervation by two pro-secretory substances. We have shown a significant cross-potentiation of carbachol- and VIP-induced secretion. The potentiation of the carbachol-induced chloride and mucin secretion by VIP was also observed in colonic epithelial cell lines (Dharmsathaphorn & Pandol, 1986; Laburthe et al. 1989). Since the secretagogue mechanism of VIP and ACh are different (Cooke & Reddix, 1994), the double innervation of the mucosa could have a protective function to insure secretion if one pathway fails. Another possibility could be that cholinergic and VIPergic neurones are part of two networks, both promoting secretion but activated by different mechanisms. For instance, the secretory response induced by cholera toxin has been shown to be mediated primarily by submucosal VIPergic neurones (Jiang, Kirchgessner, Gershon & Surprenant, 1993; Cooke & Reddix, 1994). On the other hand, distension or stroking-induced secretion was shown to involve mainly cholinergic and/or serotoninergic pathways within the submucosal plexus (Frieling et al. 1992; Sidhu & Cooke, 1995).
The functional significance of a polarized innervation of the colonic mucosa is less obvious than that of the myenteric neurones innervating the muscle since both ACh and VIP induce mucosal secretion. A simple explanation may be that a polarized projection is linked to the respective neurochemical coding of the cells. It may be a general principle in the gut that cholinergic motor neurones project orally and VIPergic motor neurones project anally. At least for the innervation pattern of the lower oesophageal sphincter, and of the circular muscle in the stomach, ileum and colon, it appears that this polarity is conserved (Schemann & Schaaf, 1995; Costa et al. 1996; Brookes, Bao, Hodgson & Costa, 1996; Reiche, Pfannkuche & Schemann, 1997; Sang, Williamson & Young, 1997). Various pieces of evidence suggest spatial and temporal co-ordination of both secretory and contractile activity in the intestine (Greenwood & Davidson, 1987; Cooke, Wang & Rodgers, 1993). Therefore it is tempting to speculate that such a mucosal reflex would be timely activated together with the peristaltic reflex. Further studies are required to assess the possible functional role of this polarized projection pattern in the modulation of mucous secretion, T-cell proliferation, proliferation of enterocytes and mucosal blood flow (Bustamante et al. 1988; Laburthe et al. 1989; Ottaway, 1991; Vanner & Surprenant, 1996). A recent study has revealed the existence of distinct secretion pathways for colonic mucus secretion, one involving a muscarinic pathway and another a cAMP-dependent pathway (Epple, Kreusel, Hanski, Schulzke, Riecken & Fromm, 1997).
In conclusion, our study has revealed for the first time the existence of a polarized enteric innervation pattern in the colonic mucosa and has demonstrated the role of such an innervation in neurally evoked secretory processes. Activation of ascending submucosal pathways primarily led to cholinergically mediated secretion whereas activation of descending pathways induced secretion mediated by VIP and acetylcholine. Although both substances act individually as secretagogues, activation of both pathways would potentiate secretion.
Acknowledgments
The authors wish to thank Susanne Hoppe for her excellent technical support. We thank Lisa Baldwin for carefully reading the manuscript. This work was supported by SFB 280 to M. S., by grants from the Institut National de la Santé et de la Recherche Médicale (Bourse de Formation à l'Etranger) and a Marie Curie Fellowship from the European Community to M. N. and by grants Fr 733/3-3 to T. F.
References
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. Journal of Physiology. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC. Local neural control of intestinal motility: nerve circuits deduced for the guinea-pig small intestine. Clinical and Experimental Pharmacology and Physiology. 1994;21:441–452. doi: 10.1111/j.1440-1681.1994.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB. Enteric neurons and their chemical coding. In: Holle GE, Wood JD, editors. Advances in the Innervation of the Gastrointestinal tract. Amsterdam: Elsevier Science Publisher; 1992. pp. 101–114. [Google Scholar]
- Brookes SJH, Bao NC, Hodgson WM, Costa M. Characterization of excitatory and inhibitory motor neurons to the guinea pig lower oesophageal sphincter. Gastroenterology. 1996;111:108–117. doi: 10.1053/gast.1996.v111.pm8698189. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Costa M. Identification of enteric motor neurones which innervate the circular muscle of the guinea pig small intestine. Neuroscience Letters. 1990;118:227–230. doi: 10.1016/0304-3940(90)90633-k. [DOI] [PubMed] [Google Scholar]
- Burleigh DE, Kirkham SE. Lack of effect of three putative vasoactive intestinal peptide receptor antagonists on vasoactive peptide-induced secretory responses in rat colon. European Journal of Pharmacology. 1993;249:239–242. doi: 10.1016/0014-2999(93)90439-o. [DOI] [PubMed] [Google Scholar]
- Bustamante SA, Forshult M, Lundgren O. Evidence for an intramural nervous control of epithelial cell migration in the small intestine of the rat. Acta Physiologica Scandinavica. 1989;135:469–475. doi: 10.1111/j.1748-1716.1989.tb08605.x. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Reddix R. Neural regulation of intestinal electrolyte transport. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 2083–2132. [Google Scholar]
- Cooke HJ, Wang Y-Z, Rogers R. Coordination of Cl− secretion and contraction by a histamine H2 receptor agonist in guinea pig distal colon. American Journal of Physiology. 1993;265:G973–978. doi: 10.1152/ajpgi.1993.265.5.G973. [DOI] [PubMed] [Google Scholar]
- Costa M, Brookes SJH, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. Journal of Clinical Investigation. 1986;77:348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple HJ, Kreusel KM, Hanski C, Schulzke JD, Riecken EO, Fromm M. Differential stimulation of intestinal mucin secretion by cholera toxin and carbachol. Pflügers Archiv. 1997;433:638–647. doi: 10.1007/s004240050325. [DOI] [PubMed] [Google Scholar]
- Fishbein VA, Coy DH, Hocart SJ, Jiang NY, Mrozinski JE, Mantey SA, Jensen RT. A chimeric VIP-PACAP analogue but not VIP pseudopeptide function as VIP receptor antagonists. Peptides. 1994;15:95–100. doi: 10.1016/0196-9781(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Frieling T, Neunlist M, Rupprecht C, Becker K, Häussinger D, Schemann M. Identification and functional implication of differential projection of submucosal neurons in guinea pig proximal colon. Gastroenterology. 1997;112:A734. [Google Scholar]
- Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: distension evoked ion transport in the guinea pig colon. American Journal of Physiology. 1992;26:G91–96. doi: 10.1152/ajpgi.1992.263.1.G91. [DOI] [PubMed] [Google Scholar]
- Furness JB, Bornstein JC, Murphy R, Pompolo S. Roles of peptides in transmission in enteric nervous system. Trends in Neurosciences. 1992;15:66–71. doi: 10.1016/0166-2236(92)90029-8. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M, Keast JR. Choline acetyltransferase and peptide immunoreactivity of submucous neurons in the small intestine of the guinea pig. Cell and Tissue Research. 1987;237:329–336. doi: 10.1007/BF00217152. [DOI] [PubMed] [Google Scholar]
- Furness JB, Young HM, Pompolo S, Bornstein JC, Kunze WAA, McConalogue K. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology. 1995;108:554–563. doi: 10.1016/0016-5085(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Greenwood B, Davison JS. The relationship between gastrointestinal motility and secretion. American Journal of Physiology. 1987;252:G1–7. doi: 10.1152/ajpgi.1987.252.1.G1. [DOI] [PubMed] [Google Scholar]
- Hubel KA, Russ L. Mechanisms of the secretory response to luminal propionate in rat descending colon in vitro. Journal of the Autonomic Nervous System. 1993;43:219–229. doi: 10.1016/0165-1838(93)90328-r. [DOI] [PubMed] [Google Scholar]
- Jiang MM, Kirchgessner A, Gershon MD, Surprenant A. Cholera toxin-sensitive neurons in guinea pig submucosal plexus. American Journal of Physiology. 1993;27:G86–94. doi: 10.1152/ajpgi.1993.264.1.G86. [DOI] [PubMed] [Google Scholar]
- Keast JR. Mucosal innervation and control of water and ion transport in the intestine. Reviews of Physiology Biochemistry and Pharmacology. 1987;109:3–59. doi: 10.1007/BFb0031024. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. Journal of Neuroscience. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara A, Cooke HJ. Tachykinin-induced anion secretion in the guinea pig distal colon: role of neural and inflammatory mediators. Journal of Pharmacology and Experimental Therapy. 1990;252:1–7. [PubMed] [Google Scholar]
- Kuwahara A, Radowicz-Cooke HJ. Epithelial transport in guinea-pig proximal colon: influence of enteric neurones. Journal of Physiology. 1988;395:271–284. doi: 10.1113/jphysiol.1988.sp016918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Augeron C, Rouyer-Fessard C, Roumagnac I, Maoret JJ, Grasset E, Laboisse C. Functional VIP receptors in the human mucus-secreting colonic epithelial cell line CL.16E. American Journal of Physiology. 1989;256:G443–450. doi: 10.1152/ajpgi.1989.256.3.G443. [DOI] [PubMed] [Google Scholar]
- Messenger JP. Immunohistochemical analysis of neurons and their projections in the proximal colon of the guinea pig. Archives of Histology and Cytology. 1993;56:459–473. doi: 10.1679/aohc.56.459. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Reiche D, Hoppe S, Schemann M. Characterization of enteric circuits innervating the mucosa of the guinea pig proximal colon. Neurogastroenterology and Motility. 1996;8:185. [Google Scholar]
- Neunlist M, Schemann M. Projections and neurochemical coding of myenteric neurons innervating the mucosa of the guinea pig proximal colon. Cell and Tissue Research. 1997;287:119–125. doi: 10.1007/s004410050737. [DOI] [PubMed] [Google Scholar]
- Ottaway CA. Neuroimmunomodulation in the intestinal mucosa. Gastroenterology Clinics of North America. 1991;20:511–529. [PubMed] [Google Scholar]
- Reddix R, Kuwahara A, Wallace L, Cooke HJ. Vasoactive intestinal polypeptide: a transmitter in submucous neurons mediating secretion in guinea pig distal colon. Journal of Pharmacology and Experimental Therapy. 1994;269:1124–1129. [PubMed] [Google Scholar]
- Reiche D, Pfannkuche H, Schemann M. Identification of myenteric neurones innervating the mucosa of the guinea pig stomach. Clinical and Autonomic Research. 1997;7:47. [Google Scholar]
- Sang Q, Williamson S, Young HM. Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. Journal of Anatomy. 1997;190:209–222. doi: 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Schaaf C. Differential projection of cholinergic and nitroxidergic neurons in the myenteric plexus of guinea pig stomach. American Journal of Physiology. 1995;269:G186–195. doi: 10.1152/ajpgi.1995.269.2.G186. [DOI] [PubMed] [Google Scholar]
- Schemann M, Schaaf C, Mäder M. Neurochemical coding of enteric neurons in the guinea pig stomach. Journal of Comparative Neurology. 1995;353:161–178. doi: 10.1002/cne.903530202. [DOI] [PubMed] [Google Scholar]
- Sidhu M, Cooke HJ. Role for 5-HT and ACh in submucosal reflexes mediating colonic secretion. American Journal of Physiology. 1995;269:G349–351. doi: 10.1152/ajpgi.1995.269.3.G346. [DOI] [PubMed] [Google Scholar]
- Song ZM, Brookes SJ, Costa M. Identification of myenteric neurons which project to the mucosa of the guinea-pig small intestine. Neuroscience Letters. 1991;129:294–298. doi: 10.1016/0304-3940(91)90484-b. [DOI] [PubMed] [Google Scholar]
- Song ZM, Brookes SJ, Steele PA, Costa M. Projections and pathways of submucous neurons to the mucosa of the guinea-pig small intestine. Cell and Tissue Research. 1992;269:87–98. doi: 10.1007/BF00384729. [DOI] [PubMed] [Google Scholar]
- Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. American Journal of Physiology. 1996;271:G223–230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 423–482. [Google Scholar]


