Abstract
Membrane currents were studied in single human blood eosinophils using the whole cell voltage clamp technique. The whole cell current-voltage relationship exhibited rectification about the membrane potential which followed the potassium equilibrium potential when [K+]o was raised. Elevation of [K+]o considerably potentiated inward current amplitude, and in some cells channel activity was discernible in the whole cell membrane current recordings. The single channel conductance was 24 ± 1 pS ([K+]o, 100 mm [K+]i, 140 mm), and eosinophils were found to have as few as three, and on average twenty, inward rectifier channels each.
The inward current was inhibited in a voltage-dependent manner by extracellular cations in order of potency Ba2+ > Cs+ > Na+. Intracellular acidification inhibited while alkalization augmented the inward current. Mg2+ contributed to rectification as dialysis with nominally Mg2+-free pipette solution was associated with an increase in the outward current during membrane polarization.
By reverse transcription-polymerase chain reaction (RT-PCR) using suitable primers on human eosinophil mRNA, an inward rectifier channel, Kir2.1, was identified, which is known from expression studies to have very similar properties to those found in this study.
Superoxide anion production or its stimulation by phorbol 12-myristate 13-acetate (PMA) was not significantly affected by depolarization with 140 mm [K+]o, or by 1 mm BaCl2.
It is concluded that the single channel currents and the whole cell current rectification observed in human blood eosinophils resulted from the presence of an inwardly rectifying potassium channel, probably Kir2.1.
The potential role of ion channels in regulating the behaviour of eosinophils was essentially unexplored until recently. Two independent studies have reported the existence and characterization of proton currents in freshly isolated human eosinophils (Gordienko, Tare, Parveen, Fenech, Robinson & Bolton, 1996; Schrenzel, Lew & Krause, 1996) and additionally, Ca2+-activated K+ channels have been identified in EoL-1, a cell line with properties claimed to resemble those of human eosinophils (Saito, Sato, Hisatome & Narahashi, 1996).
Alterations in the resting membrane potential produced as a result of channel activity may play a role in modulating cellular function in eosinophils. In a variety of cell types (see Hille, 1992) including cells of myeloid origin (for review see Gallin, 1991), anomalously or inwardly rectifying potassium channels have been identified and are presumed to have a role in the regulation of cell resting membrane potential. Inwardly rectifying potassium channels conduct ions more readily in the inward direction at membrane potentials negative to the equilibrium potential for potassium ions (EK). Activation of the inward rectifier is dependent on the electrochemical potential for potassium (Hagiwara & Yoshi, 1979) and is susceptible to inhibition by various extracellular cations such as Cs+, Ba2+ and Na+ (Gay & Stanfield, 1977; Standen & Stanfield, 1978, 1979) and intracellular cations including protons (Moody & Hagiwara, 1982). Cytoplasmic Mg2+ and/or polyamines (Matsuda, Saigusa & Irisawa, 1987; Lopatin, Makhina & Nichols, 1994) are responsible for rectification of the current by inhibiting the outward flow of K+ through inward rectifier channels during membrane polarization. This study reveals that the behaviour of the whole cell inward current upon membrane hyperpolarization of human blood eosinophils can be attributed to the presence of inward rectifier potassium channels. Some of the characteristics of this current are similar to those described in several other cells of myeloid origin (Gallin, 1991). It may serve to set the membrane potential of eosinophils close to the equilibrium potential for K+. Preliminary accounts of this work have previously been reported in abstract form (Tare, Gordienko, Parveen, Fenech, Robinson & Bolton, 1996; Tare, Gordienko, Parveen, Robinson & Bolton, 1997).
METHODS
Cell preparation
Blood was obtained by venipuncture from healthy, non-atopic human donors (male, 86%; female, 14%; age range, 25–54 years; who gave informed consent) and anticoagulated 9:1 (v/v) with 3.15% trisodium citrate. The suspension was centrifuged at 400 g for 20 min (20°C) after which the platelet-rich plasma was discarded and the remaining fraction mixed 4:1 (v/v) with 6% (w/v) dextran in Dulbecco's phosphate-buffered saline (PBS). After allowing the erythrocytes to sediment at 1 g (45 min, 20°C), 15 ml aliquots of the leucocyte-rich layer were carefully layered onto 25 ml aliquots of Ficoll-Hypaque® with a density of 1.077 g ml−1 and centrifuged at 400 g (20 min, 20°C). The granulocyte pellet was resuspended in Ca2+- and Mg2+-free Hanks’ balanced salt solution (HBSS) containing 2% (v/v) heat-inactivated fetal calf serum (HIFCS) and Phenol Red (10 μg ml−1), and washed by centrifugation at 339 g (7 min, 20°C). Residual erythrocytes were lysed by resuspending the cell pellet in 0.2% (w/v) sodium chloride, shaking for approximately 15 s, and then adding an equal volume of 1.6% (w/v) sodium chloride. After centrifugation at 339 g (7 min, 20°C), the granulocytes were washed twice in Ca2+- and Mg2+-free HBSS, containing 2% HIFCS (by centrifugation under the same conditions), and a sample was stained with 2% (w/v) Crystal Violet for counting in an Improved Neubauer Haemocytometer. The volume of cell suspension was adjusted to give a maximum of 1 × 107 cells ml−1 in ice-cooled HBSS, and monoclonal antihuman CD16 was added (2 μg per 107 cells). The mixed granulocytes were incubated for 15 min at 4°C on a roller mixer (Denley Spiramix 5) and then washed three times with HBSS (339 g, 7 min, 4°C). The granulocyte pellet was then resuspended in ice-cold HBSS and incubated for 45 min at 4°C with sheep antimouse IgG1 labelled with superparamagnetic polymer particles, prior to magnetic removal of the CD16+ cells. The cell suspension containing CD16− eosinophils was carefully aspirated and washed (339 g, 7 min, 20°C) with HBSS containing 2% HIFCS. The cell pellet was finally resuspended in HBSS containing 2% HIFCS at a cell number of approximately 1.0 × 106 cells ml−1. Viability of the cells was determined by Trypan Blue exclusion. Differential counts were performed after Haematoxylin and Eosin staining of fixed cytocentrifuge smears.
The cell suspension was stored at 4°C until use, generally within 16 h. An aliquot of cells was diluted in the physiological external solution supplemented with 10% HIFCS and allowed to settle onto a glass coverslip pre-coated with Sigmacote (Sigma). The preparation was mounted onto the stage of a Nikon Diaphot phase-contrast inverted microscope. The cells were allowed to settle for 15–20 min and were then thoroughly rinsed with control physiological external solution (BS I; see Table 1) before electrical recordings were commenced. All experiments were performed within about 1 h of plating the cells.
Table 1.
Composition of solutions (mM)
| Bath solutions(BS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BS | pH | NaCl | KCl | CaCl2 | MgCl2 | Hepes | Glucose | NMDG | Base/acid |
| I | 7·0 | 137·6 | 5 | 2 | 1 | 10 | 6 | — | 2.4 NaOH |
| II | 7·0 | 132·6 | 10 | 2 | 1 | 10 | 6 | — | 2.4 NaOH |
| III | 7·0 | 126·6 | 16 | 2 | 1 | 10 | 6 | — | 2.4 NaOH |
| IV | 7·0 | 117·6 | 25 | 2 | 1 | 10 | 6 | — | 2.4 NaOH |
| V | 7·0 | 78·6 | 64 | 2 | 1 | 10 | 6 | — | 2.4 NaOH |
| VI | 7·0 | 42·6 | 100 | 2 | 1 | 10 | 6 | — | 2.4 NaOH |
| VII | 7·0 | 2·6 | 140 | 2 | 1 | 10 | 6 | — | 2.4 NaOH |
| VIII | 7·0 | — | 5 | 2 | 1 | 10 | 6 | 140 | 137 HCl |
| Pipette solutions(PS) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS | pH | NaCl | KCl | CaCl2 | MgCl2 | BAPTA | EDTA | MgATP | Na2ATP | Buffer | HCl | KOH |
| I | 7·0 | 5 | 97·6 | 0.7 | — | 10 | — | 1 | — | 10 Hepes | — | 42·6 |
| II | 7·0 | 5 | 97·6 | 0.7 | — | 10 | — | 1 | — | 10 Hepes | — | 42·6 |
| III | 7·0 | 3 | 97·6 | 1.6 | — | — | 10 | — | 1 | 10 Hepes | 2 | 41·5 |
| IV | 7·0 | — | 97·6 | 1.6 | — | — | 10 | — | 1 | 10 Hepes | 5 | 45·7 |
| V | 7·0 | 3·88 | 97·6 | 0.7 | 0.74 | 10 | — | — | 0.56 | 10 Hepes | — | 41·8 |
| VI | 5·5 | — | 102 | 0.06 | 0.69 | 10 | — | — | 2.5 | 10 Mes | 3.6 | 38 |
| VII | 8·0 | 4 | 97.2 | 0.83 | 0.76 | 10 | — | — | 0.5 | 10 Taps | — | 42·6 |
In addition, all pipette solutions contained 0.02 mM GTP. All pipette solutions except pipette solutions II-IV also contained 5 mM creatine. Pipette solution IV also contained 200 μM low molecular weight heparin. The concentration of free calcium in pipette solutions I-IV was clamped to 0.01 μM using Ca2+-BAPTA or Ca2+-EDTA buffer. For the group of pipette solutions V-VII, the concentrations of Ca2+, Mg2+ and ATP were adjusted so that the free levels of these were maintained at the same concentration within this particular group, despite the different pH of these solutions. For pipette solutions V-VII, the free concentrations were 0.01 μM Ca2+, 0.28 mM Mg2+ and 0.17 mM ATP. The concentrations of Ca2+, Mg2+ and ATP required were calculated using EQCAL software (Biosoft, Cambridge, UK). NMDG, N-methyl-D-glucamine.
Whole cell patch clamp recording technique
The conventional whole cell patch clamp technique (Hamill, Marty, Neher, Sakmann & Sigworth, 1981) was used to measure whole cell membrane currents and membrane potential. Electrophysiological recordings were performed essentially as described previously (Gordienko et al. 1996) under static bath conditions at room temperature (20–25°C). As determined from the actions of high [K+]o solutions on the holding current, external solution changes in the proximity of the cell under investigation were achieved within several seconds. The input amplifier used was an Axopatch 200A (Axon Instruments). Patch pipettes were made from borosilicate glass and were pulled in two stages using a Kopf vertical pipette puller (Model 720, David Kopf Instruments, Tujunga, CA, USA). The pipettes were fire polished and had tip resistances in the range 4–10 MΩ when filled with pipette solution and measured in standard external solution. The reference electrode was made from Ag-AgCl wire connected to the bath via an agar-salt bridge in order to minimize electrode junction potentials during solution changes. The passive membrane properties of eosinophils bathed in quasi-physiological solutions were determined from 128 randomly selected recordings. The cell membrane input resistance was 21.6 ± 1.9 GΩ. The cell membrane capacitance (Cm), calculated as a ratio of charge (estimated by integrating the area under the capacitive transients) to the pulse amplitude (10 mV), was found to be 2.6 ± 0.04 pF. Series resistance (Rs) was estimated by fitting a single exponential function to the decay of the capacitive current on the assumption that it discharges with a time constant τ=CmRs. The average series resistance was 16.7 ± 0.05 MΩ. No compensation of whole cell capacitance or series resistance was used, except where indicated (Fig. 6B). Whole cell current recordings were generally not corrected for leakage current, except where indicated (Figs 4B and 5A). In these cases, the leakage current determined from the linear portion of the current-voltage relationship between approximately 0 and −60 mV when [K+]o was 5 mM, was subtracted from all records, assuming that leakage was unchanged when [K+]o was elevated. The capacitive current (ic) arising during the ramp voltage protocol (ic=CmdV/dt, where dV/dt is the rate of ramp) was subtracted from the current-voltage relationships (Figs 4B and 5A). Unless stated otherwise, the holding potential used was −60 mV. The whole cell currents were filtered at 1 or 2 kHz by a 4-pole low-pass Bessel filter. Voltage protocols were generated and the resulting currents were acquired (at 3–5 kHz) using a CED 1401 interface connected to a 486 PC running CED software (Cambridge Electronic Design, Cambridge, UK). The current and voltage were also recorded on tape (Racal Store 4, Racal-Thermionic Ltd, Southampton, UK). Data were analysed using MicroCal Origin software (MicroCal Software, Inc., Northhampton, MA, USA). Statistical analyses of electrophysiological data were made using Student's t test for unpaired data with a probability value of < 0.05 considered statistically significant. Where appropriate, data are presented as the mean values ±s.e.m. of n cells studied. To allow for cell dialysis with pipette solution, recordings were commenced only after 2 min had elapsed following formation of whole cell configuration. In order to reduce variation arising from between cell comparisons when the effects of different pipette solutions were evaluated, all data within each specific experimental group were obtained at similar times following the establishment of the whole cell configuration, using the same sequence of voltage protocols. Testing of each pipette solution was strictly alternated in a sequential manner.
Figure 6. Time-dependent block of the inward current by barium.
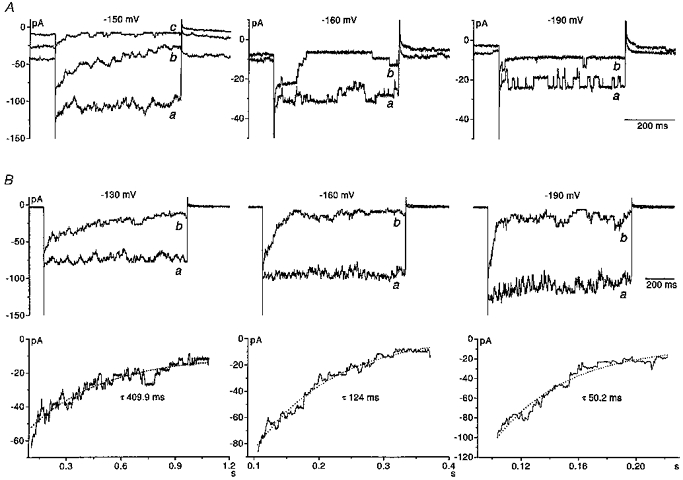
A, whole cell inward currents recorded in three different eosinophils in response to 500 ms-long hyperpolarizing voltage steps from the holding potential of −60 mV. All responses were recorded in the presence of 100 mM K+ solution (PS I, BS VI). A, left panel, a family of inward currents evoked by voltage steps to −150 mV in the absence (a) and presence of barium at 5 μM (b) and 30 μM (c). In the absence of barium, the inward current was very noisy although distinct channel openings could not be distinguished. In the presence of barium the opening and closing of channels was evident with more channels open at the beginning of the pulse and the number of channels open apparently decaying during the duration of the pulse. A, middle panel, inward currents evoked by voltage steps to −160 mV in the absence (a) and presence (b) of 5 μM barium. Distinct channel openings and closings were detectable in 100 mM K+ solution alone. In the presence of barium, the number of open channels rapidly declined following the onset of the pulse. A, right panel, inward currents evoked by voltage steps to −190 mV. The frequency of channel openings was high in 100 mM K+ solution. In comparison, in the presence of 30 μM barium, the frequency of channel opening was considerably reduced and rapid closure of channels at the onset of the pulse was not seen. Each panel is from a different cell. B, upper panels, inward currents recorded in an individual eosinophil in the presence of 100 mM K+ solution (PS I, BS VI). Currents were evoked by 1 s pulses by stepping membrane voltage from −10 mV to −130, −160 and −190 mV in the absence (a) and presence (b) of barium. Whole cell membrane capacitance and series resistance were compensated in these cells. B, lower panels, the decay of the current produced by barium block at each voltage was fitted with a single exponential function. The time constant for the decay of the current at each voltage is indicated in each of the corresponding traces.
Figure 4. Voltage-dependent block of the inward current by caesium.
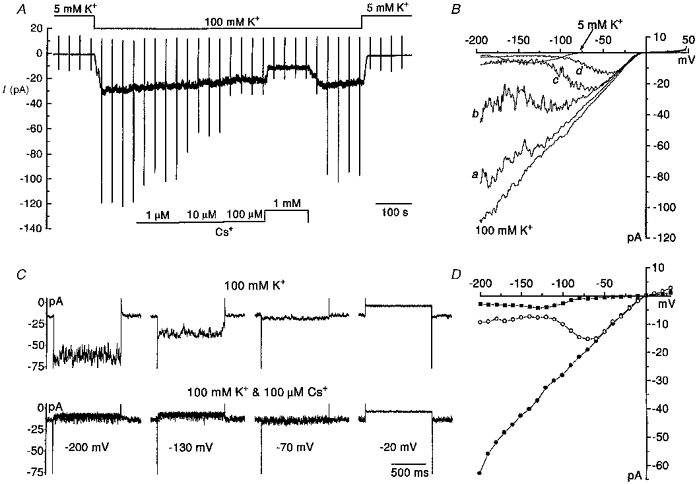
A, a continuous recording of whole cell membrane current in an individual eosinophil. The membrane potential was ramped at 30 s intervals. During this voltage protocol, the membrane potential was stepped from the holding potential of −60 mV to +50 mV where it was held for 20 ms and then this was followed by ramp repolarization of the membrane to −200 mV at a rate of 0.83 V s−1. On completion of the ramp, the membrane was returned to the holding potential. The eosinophil was exposed to a solution containing 100 mM K+ (PS I, BS VI) during which time four concentrations of caesium (1 μM to 1 mM) were applied. Following this, caesium was washed out and then [K+]o was subsequently returned to 5 mM. B, whole cell current-voltage relationships derived from the voltage ramps applied in A. Each relationship represents the average of 3–4 ramps in each solution. Each current-voltage relationship was corrected for leakage and capacitive current (ic). Current-voltage relationships were obtained in solutions when [K+]o was 5 and 100 mM, and also when caesium at 1 μM (a), 10 μM (b) 100 μM (c) and 1 mM (d) was added to the 100 mM K+ solution. C, effects of caesium on steady-state currents. A selection of inward currents recorded in a single eosinophil (different to the one in A and B) in response to 1 s long rectangular voltage pulses applied from the holding potential of −60 mV to voltages between −200 and −20 mV. Currents were recorded in 100 mM K+ solution in the absence (upper traces) and presence (lower traces) of 100 μM caesium. D, complete steady-state current-voltage relationships derived from currents evoked by rectangular voltage pulses applied at 7 s intervals from −200 to +30 mV in 10 mV increments. Shown are current-voltage relationships obtained when [K+]o was 5 mM (▪) and 100 mM (•), and when 100 μM caesium was included in the 100 mM K+ solution (○). C and D are from the same cell.
Figure 5. Voltage-dependent block of the inward current by barium.
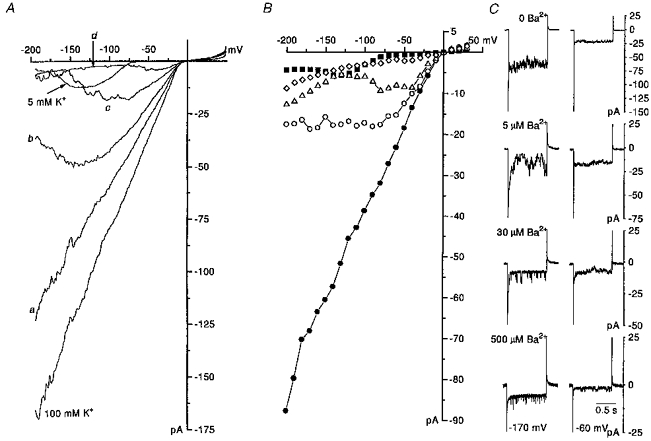
A, changes in whole cell current induced by ramp repolarization of the membrane. The same voltage protocol was used as described in Fig. 4. Average current-voltage relationships were recorded when [K+]o was 5 mM (PS I, BS I) and 100 mM (BS VI), and also when four concentrations of barium (1, 5, 30 and 500 μM; a, b, c and d, respectively) were added in ascending order to the 100 mM K+ solution. The relationships represent the average of 3–4 responses evoked in each solution. The current-voltage relationships were corrected for leakage and ic. The voltage ramps were applied at 30 s intervals. B, the effect of barium on the steady-state current-voltage relationship in a different cell to that in A. 1 s-long voltage pulses were applied every 7 s to voltages in the range −200 to +30 mV, in 10 mV increments. Holding potential was −60 and −10 mV when [K+]o was 5 and 100 mM, respectively. End pulse current amplitude was plotted against membrane voltage. Shown are current-voltage relationships when [K+]o was 5 mM (▪) and when [K+]o was 100 mM, either in the absence (•) or presence of barium at 5 μM (○), 30 μM (Δ) or 500 μM (⋄). C, a selection of inward currents evoked by 1 s-long hyperpolarizing voltage steps from the holding potential of −10 mV to −170 and −60 mV. All responses were recorded in an individual eosinophil (same cell as in B) when [K+]o was 100 mM (PS I, BS VI) either in the absence or presence of barium (5, 30, 500 μM).
Molecular biology
Cloning of the human eosinophil Kir2.1
Three separate samples of total RNA were prepared from 106 normal human eosinophils (isolated as described above) according to the method of Chomczynski & Sacchi (1987). For reverse transcription-polymerase chain reaction (RT-PCR) 1 μg of total RNA was reverse transcribed using oligo(dT) primers (Promega, Southampton, UK) and Superscript II (Gibco) reverse transcriptase. Forward and reverse primers were designed based on a conserved portion of previously cloned Kir2.0 channels. The forward primer corresponded to bases 550–573 and the reverse primer corresponded to bases 1120–1142 of Kir2.1 from human hippocampus (GenBank accession number, GBAcc.No., U16861). PCR amplification was carried out using Klentaq (Clontech, Cambridge Bioscience, Cambridge, UK) under the following conditions: 94°C, 2 min, followed by 35 cycles of 94°C, 30 s; 60°C, 4 min; 68°C, 4 min; and a final extension step of 68°C, 10 min. Samples were run on a 1% agarose gel; a band corresponding to a 592 bp product was subcloned into pGEM-T vector (Promega) and isolated clones sequenced using a Pharmacia T7 sequencing kit and Perkin Elmer ABI automatic sequencer. A search, using the Fasta program (GCG Wisconsin Package, WI, USA) running on the Seqnet facility (Daresbury Laboratory, Warrington, UK), revealed > 96% similarity with several Kir2.1 clones in GenBank. 5′- and 3′-primers for the beginning and end of the coding sequence of these Kir2.1 clones were then used to amplify, by PCR, a clone spanning the whole of the coding region of a Kir from the human eosinophil cDNA using Klentaq and the following PCR parameters: 94°C, 1 min; followed by 35 cycles of 94°C, 30 s and 68°C, 6 min; with a final extension step of 68°C, 6 min.
Other Kir channels were also investigated by RT-PCR using the methods and conditions described. The forward and reverse primers used were bases 411–441 and 1065–1093 of Kir1.1 (GBAcc.No. U12543), bases 5–27 and 443–464 of Kir3.4 (GBAcc.No. X83584), bases 510–533 and 1363–1387 of Kir6.1 (GBAcc.No. D42145), bases 444–470 and 1254–1280 of Kir6.2 (GBAcc.No. D50581), respectively.
Superoxide anion production
The production of superoxide anion was measured spectrophotometrically at 550 nm as the superoxide dismutase-inhibitable reduction of cytochrome c. Eosinophils (2 × 105) were equilibrated for 3 min at 37°C in 1 ml prewarmed BS I buffered with 10 mM Hepes or 1 ml prewarmed test solution (composition, mM: 5 NaCl, 140 KCl, 2 CaCl2, 1 MgCl2, 6 glucose, 10 Hepes), each containing 80 μM cytochrome c. In some experiments the incubation solutions also contained 1 mM BaCl2. Cells were stimulated by the addition of phorbol 12-myristate 13-acetate (PMA) at final concentrations of 1 or 10 nM and quickly mixed. Rates of superoxide production were measured during the linear phase of production using an extinction coefficient of 21.0 mM−1 cm−1. The reduction of cytochrome c was entirely due to superoxide formation in these assays because the reduction was ablated by addition of 20 μg ml−1 superoxide dismutase. Statistical comparisons of superoxide anion production were made by analysis of variance. Probability differences were estimated by the least significant difference test with values < 0.05 being considered statistically significant.
Solutions
The composition of external bath and pipette solutions used in this study is listed in Table 1. The standard control bath solution used was one containing 5 mM [K+]o (BS I). In a majority of experiments the pH of internal and external solutions was 7.0. This choice was a compromise between the need to reduce the influence of the outward proton current over most of the voltage range tested whilst at the same time maintaining the whole cell configuration for sufficient periods of time to enable the recording of data.
Chemicals
Adenosine 5′-triphosphate (disodium or magnesium salt), BAPTA, barium chloride, caesium chloride, creatine, cytochrome c (from horse heart), dextran from Leuconostoc mesenteroides (510 kDa), EDTA, guanosine 5′-triphosphate (GTP; sodium salt), heparin (low molecular weight, MW ≈ 3000, sodium salt), Hepes, Histopaque-1077, Mes, phorbol 12-myristate 13-acetate, superoxide dismutase (E.C.1.15.11, from bovine erythrocytes), Taps and all other chemicals for the molecular biology were obtained from Sigma. HBSS with and without Phenol Red and Superscript II were obtained from Gibco BRL, Paisley, UK. The remaining chemicals and reagents were obtained as follows: [35S]dATP and Dulbecco's PBS from ICN Flow, Thame, Oxfordshire, UK; Dynabeads® M-450 sheep antimouse IgG from Dynal (UK) Ltd, New Ferry, Wirral, UK; heat inactivated fetal calf serum from Labtech International Ltd, Uckfield, East Sussex, UK; Klentaq from Clontech; murine monoclonal IgG1 antihuman CD16 from Coulter Electronics Ltd, Luton, Bedfordshire, UK; pGEM-T ligation kit from Promega; T7 polymerase Sequencing kit from Pharmacia Biotech, St Albans, UK; and tinctorial stains and all other reagents from BDH, Poole, UK.
RESULTS
Whole cell steady-state inward current
The cells were bathed in, and dialysed with, quasi- physiological solutions (BS I, PS I) and the membrane potential of the eosinophils was clamped to −60 mV (Gordienko et al. 1996). Hyperpolarizing voltage steps to levels more negative than approximately −80 mV evoked rapidly activating inward currents (Fig. 1A) which were increasingly ‘noisy’ in appearance with membrane hyperpolarization to levels more negative than −120 mV, although the average current amplitude was sustained throughout the duration of the 0.5 s pulse. The steady-state current-voltage relationship constructed for currents evoked by test voltage pulses over the range −200 to +50 mV is shown in Fig. 1B. Eosinophils exhibited strong inward rectification, with little outward current recorded even at the most positive range of the voltages tested. The current-voltage relationship was U-shaped in the range −80 to −200 mV. The maximum inward current usually peaked within the voltage range −130 to −140 mV and was on average 6.9 ± 0.7 pA (n = 13) in amplitude. The characteristics of the current-voltage relationship were similar regardless of whether recordings were made from cells that had adhered to the base of the recording chamber or were in suspension.
Figure 1. Whole cell steady-state inward current and the influence of [K+]o.
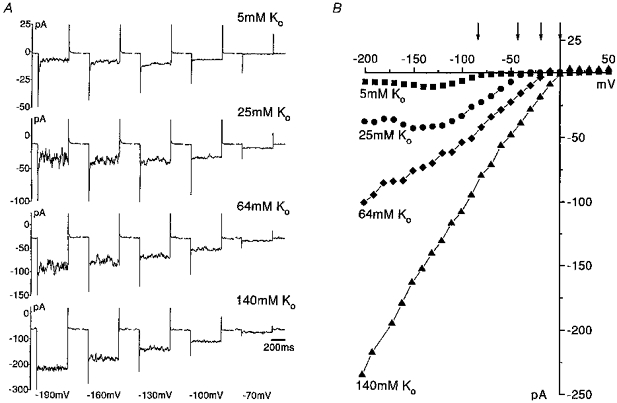
A, a selection of inward currents recorded in an individual eosinophil in response to 500 ms duration hyperpolarizing voltage pulses applied from a holding potential of −60 mV to voltage levels between −190 and −70 mV. The eosinophil was dialysed with physiological pipette solution (PS I) and bathed in external solutions of different [K+]: 5, 25, 64 and 140 mM (BS I, IV, V and VII, respectively). B, effect of raising [K+]o on the complete steady-state whole cell current-voltage relationship over the voltage range −200 to +50 mV (voltage pulses were applied every 10 s in 10 mV increments), in the same cell as in A. The current measured was the steady-state current during the pulse. Presented are current-voltage relationships obtained when [K+]o was 5 mM (▪), 25 mM (•), 64 mM (♦) and 140 mM (▴). Holding potential was −60 mV throughout. The slope conductance measured in the linear section (voltage range used is indicated in parentheses after each conductance) of the current-voltage relationship in the potential range negative to EK was 0.24 nS (−110 to −80 mV), 0.53 nS (−110 to −40 mV), 0.67 nS (−110 to −20 mV) and 1.16 nS (−150 to −10 mV) when [K+]o was 5, 25, 64 and 140 mM, respectively. The arrows indicate the theoretical equilibrium potential for K+ calculated for each different [K+]o/[K+]i gradient.
Effects of raising extracellular potassium on the inward current
The characteristics of the inward currents described above are suggestive of the involvement of an anomalously rectifying/inwardly rectifying potassium conductance. This type of potassium conductance has been described in a variety of different cells (see Hille, 1992) including several inflammatory cell types (Gallin, 1991). To characterize further the properties of the inward current in human eosinophils, the effects of raising [K+]o were investigated in voltage clamp mode.
Exposure of eosinophils to solutions containing elevated [K+] (BS IV, V and VII) increased the holding current and significantly potentiated the magnitude of the evoked inward currents (Fig. 1A). The voltage range over which inward rectification occurred was shifted positively and the zero-current potential under voltage clamp closely followed the equilibrium potential for potassium ions when external potassium was raised (Fig. 1B). The slope conductance of the inward current determined from the linear section of the current-voltage relationships increased as [K+]o was raised and the values in solutions containing 5, 16, 25, 64 and 100 mM K+ (BS I, III-VI, respectively) were 0.15 ± 0.02 nS (n = 13), 0.27 ± 0.05 nS (n = 5), 0.30 ± 0.06 nS (n = 6), 0.51 ± 0.15 nS (n = 3) and 0.49 ± 0.1 nS (n = 5), respectively. Linear regression of a double logarithmic plot of these values of slope conductance versus[K+]o gave a slope of 0.42 (r = 0.98); thus slope conductance is roughly proportional to the square root of [K+]o.
The U-shaped appearance of the current-voltage relationship seen when [K+]o was 5 mM became pronounced with modest increases in [K+]o (e.g. Fig. 1B, [K+]o= 25 mM). However, as the concentration of potassium was further raised, the relationship became roughly linear (Fig. 1B). The amplitude of the current fluctuations observed during hyperpolarizing voltage steps in control solution was considerably magnified when [K+]o was raised. In cells where the U-shaped current-voltage relationship was prominent in control solution, the random current fluctuations during the hyperpolarizing voltage pulses further increased in amplitude on elevation of [K+]o (Fig. 1A). In contrast, in those cells where conductance was low over the U-shaped portion of the current-voltage relationship in control solution, it was often possible to distinguish the opening and closing of single channels in the whole cell current recording when [K+]o was raised to high levels (Fig. 2A). Both the frequency of channel opening and the number of channels open increased as the membrane was increasingly hyperpolarized to levels beyond EK. Single channel events could not be distinguished from the noise of the inward current at voltages around EK, and no single channel events were detectable during outward current. There was also an intermediate group where it was possible to detect some single channel events that were superimposed on larger fluctuations in current. In the group of cells where channel openings were distinct (Fig. 2A), unitary current-voltage plots were constructed for single channel currents (Fig. 2B). Slope conductance was determined from the straight line fitted to the data. In four cells, the conductance of single inward rectifier channels was found on average to be 24 ± 1 pS ([K+]o= 100 mM, PS I, BS VI). Given that the average slope conductance in the steepest region of the steady-state current-voltage relationship in the voltage range negative to EK was 0.49 ± 0.1 nS (n = 5; when [K+]o was 100 mM), then it is estimated that eosinophils have, on average, about twenty active inwardly rectifying channels under these conditions.
Figure 2. Effects of [K+]o on whole cell current and the membrane potential.
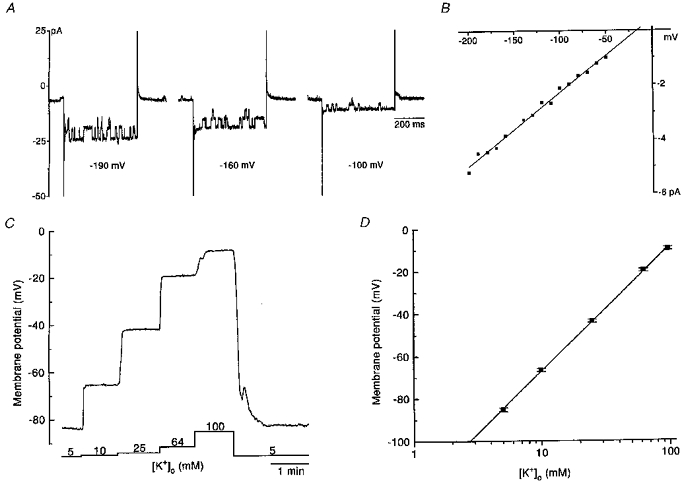
A, a sample of inward currents evoked by 500 ms duration hyperpolarizing pulses applied from the holding potential of −60 mV to levels in the range −190 to −100 mV in the presence of 100 mM K+ (PS I, BS VI). The opening and closing of single channels was also discernible at the holding potential. The frequency and amplitude of this channel activity increased during the hyperpolarizing pulses. B, the complete single channel current-voltage relationship determined from the cell in A. A straight line was fitted through the points. In this cell the slope conductance was 27 pS. C, membrane potential (zero current holding potential) recording from an eosinophil made in current clamp mode. The eosinophil was sequentially superfused with solutions of increasing [K+] (10, 25, 64 and 100 mM, PS I, BS II, BS IV-VI). Raising [K+]o evoked depolarization of the membrane. The membrane potential rapidly returned to the resting level on washout of the high potassium solution. D, relationship between membrane potential and [K+]o. Average results ±s.e.m. obtained in 11 cells for each different [K+]o tested. A linear regression line was fitted to the points and the slope of this line was 58.2 mV decade−1 (r = 0.999). A and C are from different cells.
Effects of [K+]o on the membrane potential
The membrane potential of eosinophils, determined as the zero current holding potential, was measured in current clamp mode following formation of the whole cell configuration. Membrane potential was recorded when cells were bathed in solutions where [K+]o was varied from 5 to 100 mM. Elevation of [K+]o evoked graded, concentration dependent, depolarization of the membrane (Fig. 2C). The average results obtained in eleven cells is shown in Fig. 2D. The slope of the linear regression line fitted through these points revealed that for a 10-fold increase in [K+]o, the membrane is depolarized by 58.2 mV. This is the same value as the slope of 58.2 mV decade−1 at 25°C predicted by the Nernst equation for a pure potassium conductance. Thus the membrane potential of the eosinophil behaves like a K+ electrode.
Influence of external Na+
Although in voltage clamp mode the current-voltage relationship appeared U-shaped when [K+]o was 5 or 25 mM, it became approximately linear as [K+]o was raised further (Fig. 1B). In these experiments, the concentration of KCl in the solutions was increased by equimolar replacement of the NaCl (Table 1). Given that Na+ is a known weak blocker of inward rectifier channels in several types of cells, the effects of Na+ removal on the inward current were investigated by equimolar replacement of extracellular Na+ with N-methyl-D-glucamine (NMDG+) (Fig. 3). The voltage protocol was applied initially when the cells were bathed in control solution ([K+]o, 5 mM; [Na+]o, 140 mM; PS I, BS I; Fig. 3A) and then repeated following 2 min superfusion with the Na+-free/NMDG+ solution (BS VIII; Fig. 3B). The inward currents evoked by hyperpolarizing pulses in the most negative range of the voltages tested were larger in amplitude in Na+-free solution (Fig. 3B) and the current-voltage relationship became nearly linear in the absence of extracellular sodium (Fig. 3C). Similar results were observed in a further five cells. The amplitude of the inward current evoked in Na+-free solution at −200, −180 and −160 mV was on average 2-fold, 1.8-fold and 1.4-fold larger, respectively (n = 6). The amplitudes of current fluctuations observed during hyperpolarizing voltage pulses were smaller in Na+-free solution. Generally, current fluctuations seen in control solution were larger over the voltage range in which the effect of Na+ block was greatest (e.g. Fig. 3A, between −140 and −200 mV).
Figure 3. Inhibition of the inward current by external Na+.
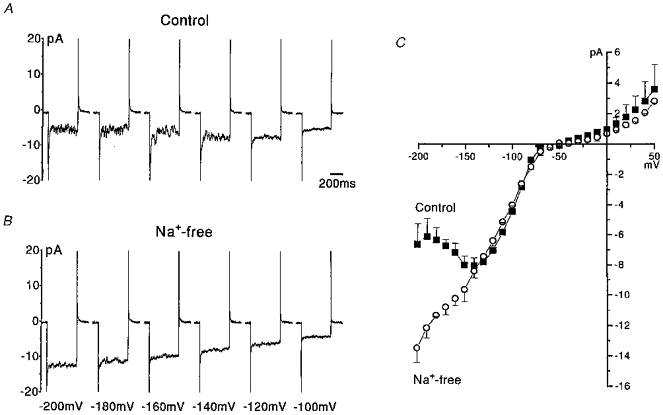
A, a selection of whole cell inward currents activated in a single eosinophil by 500 ms duration voltage steps applied from a holding potential of −60 mV to voltages in the range −200 to −100 mV. The cell was bathed in control solution where [K+]o was 5 mM and [Na+]o was 140 mM (PS I, BS I). B, augmentation of the inward current following 2 min superfusion with Na+-free/NMDG+-containing solution ([K+]o = 5 mM, [NMDG+]o = 140 mM, BS VIII). C, current-voltage relationships for the entire voltage range tested (−200 mV to +50 mV, in 10 mV increments). Shown are the average current-voltage relationships recorded in the same cell arising from data obtained during two exposures to control (▪) and to Na+-free (○) solutions.
Blockade of the inward current by extracellular caesium and barium
Susceptibility to blockade by the extracellular cations caesium and barium is a characteristic of inward rectifier potassium conductance reported in a wide variety of cell types. The actions of these cations were studied in human eosinophils when [K+]o was 100 mM (BS VI), under which conditions the inward current is amplified, and when extracellular Na+ was reduced from 140 to 45 mM. Given the difficulty in maintaining whole cell recordings in eosinophils for long periods of time, the effects of several concentrations of blockers were examined on current-voltage relationships generally determined using ramp voltage protocols (refer to Fig. 4 legend for details) rather than rectangular voltage protocols.
Caesium
Addition of caesium to the 100 mM K+ (BS VI) solution produced concentration-dependent inhibition of the inward holding current and the ramp-evoked inward currents (Fig. 4A and B). Caesium produced voltage-dependent block of the inward current at concentrations ≥ 1 μM. As the concentration of caesium was increased, the voltage dependence of the block was shifted to less negative values. The extent of the block was gradually relieved with membrane polarization such that caesium (1 mM) was without effect on inward currents evoked by voltages more positive than about −30 mV. The reversal potential of the current in 100 mM K+ solution was unchanged in the presence of caesium. The extent of the block produced by caesium was similar, regardless of whether the membrane voltage was ramped from negative to positive (data not shown) or positive to negative. The characteristics of the voltage-dependent block were also comparatively similar in steady-state conditions (Fig. 4D). The block produced by caesium was characteristically noisy or ‘flickery’ in appearance (Fig. 4C) and this noise became even more intense with higher concentrations (Fig. 4). This probably reflects the open channel block produced by caesium ions. The blocking effects of caesium were reversible (Fig. 4A).
Barium
Barium is characteristically a potent inhibitor of the inward rectifier current. Exposure to barium in the continued presence of 100 mM K+ solution (BS VI) caused a reduction of the inward holding current and the inward current evoked by voltage ramps (Fig. 5A) and steps (Fig. 5B and C). Barium was a more potent blocker than caesium, as substantial inhibition was already apparent over the entire negative voltage range at 1 μM (Fig. 5Aa) and block was almost complete at 500 μM (Fig. 5Ad and B). Barium was without effect on the reversal potential of the current.
When step changes in voltage were applied, the time dependence of the barium block was revealed and detectable at both the macroscopic current level and the single channel level (Fig. 6A and B). In cells exhibiting large inward currents in the presence of 100 mM K+ solution where discrete single channel events could not be resolved, the time dependence of the barium block was manifested as current decay during a constant voltage pulse (Fig. 6A, left panel). In those cells where single channels events were distinguishable in the presence of 100 mM K+ solution (Fig. 6A, middle panel, a), it was observed that the number of channels open at the onset of the pulse was often greater than that at the end of the pulse in the presence of barium (Fig. 6A, middle panel, b). However, this was not always apparent, especially when the frequency of channel opening was considerably reduced in the presence of barium (Fig. 6A, right panel, b).
The rate of decay of the macroscopic current in the presence of barium increased as the membrane voltage was made more negative. In two cells, the rate of the barium-induced current decay was investigated under conditions where cell capacitance and series resistance had been compensated following formation of the whole cell configuration. This allowed a more precise characterization of the decay of the current at the onset of the voltage step. Barium at a concentration of 5 μM was used so that inhibition was seen as decay of the macroscopic current rather than closure of a few single channels. Examples of currents evoked by stepping from the holding potential of −10 mV to three different voltages in a single eosinophil, in the absence (a) and presence (b) of barium, are shown in Fig. 6B (upper panels). A single exponential function was fitted to the decay of the current in the presence of barium at each voltage tested (lower panels). In two cells the average time constant for onset of barium block was 350, 158.2 and 51.9 ms for currents evoked at −130, −160 and −190 mV, respectively. Thus, the rate of barium block increased with membrane negativity.
The extent of the voltage dependence of the barium block was revealed when current-voltage relationships were determined from currents elicited by 500–1000 ms-long rectangular voltage pulses (Fig. 5B and C), which on occasions may not have been long enough to reach steady-state level at certain voltages (Fig. 5C). Block was greater for current-voltage relationships derived from steps than from ramps. Barium at 30 μM produced a severe reduction in inward current (Fig. 5B). Cell loss precluded proper evaluation of the effects of barium using hyperpolarizing voltage pulses of much longer duration. The blocking actions of barium were reversible, although this took longer than for caesium.
Voltage ramps are a convenient way of obtaining complete current-voltage relationships rapidly by the use of a single voltage command. This method is particularly useful in cells such as the eosinophil where it is difficult to maintain whole cell recordings for sufficiently long periods in order to allow the testing of several concentrations of blockers. When channel block is relatively rapid, as with caesium, ramps are a suitable and rapid method of establishing the degree of block at different potentials. Although ramps have also been used in barium studies on cells of myeloid origin (Gallin & Sheehy, 1985; Piguet & North, 1992; DeCoursey, Kim, Silver & Quandt, 1996) the rate of ramping needs to be extremely slow in order that the steady-state block by barium can be accurately evaluated at each potential. This is particularly so if membrane voltage is ramped from negative to positive voltages and, unlike current-voltage relationships derived from positive to negative ramps (which resembled those obtained by rectangular pulses). The current-voltage relationships derived from ramps applied in the negative to positive direction were poorly voltage dependent (not shown). Slowing the rate of ramping to 0.42 and 0.28 V s−1 did not alter the appearance of these current-voltage relationships. Whole cell recordings were not maintained if the rate of ramping was considerably reduced.
In addition to Na+, Cs+ and Ba2+, the inward current was also slightly sensitive to the presence of external Mg2+ (data not shown). Inward currents were potentiated by removal of Mg2+ over a similar voltage range as in the case of Na+, although to a lesser extent.
Superoxide anion production
Upon activation, eosinophils generate an oxidative burst which results in the production of the superoxide anion. We investigated whether the presence of the inward rectifier had any influence on this function of the eosinophils by examining the effects of 1 mM barium on superoxide anion production. Under resting condition, basal rate of superoxide anion production was unchanged by the presence of barium (control, 335 ± 99, versus 1 mM Ba2+, 225 ± 93 pmol (106 cells)−1 min−1, n = 7 experiments, 3 donors). Net production of superoxide anion was significantly augmented by stimulation with the phorbol ester PMA (1 and 10 nM, 3235 ± 633 and 22062 ± 720 pmol (106 cells)−1 min−1, respectively, n = 7) and this was not significantly altered by the presence of barium (2715 ± 577 and 21621 ± 863 pmol (106 cells)−1 min−1, 1 and 10 nM PMA, respectively, n = 7). Depolarization of the membrane by elevation of [K+]o was also without effect on production of superoxide anion (1 nM PMA and 140 mM , 3987 ± 543 pmol (106 cells)−1 min−1; 10 nM PMA and 140 mM , 23099 ± 1257 pmol (106 cells)−1 min−1, n = 7; 1 nM PMA, 140 mM and 1 mM Ba2+, 3219 ± 392 pmol (106 cells)−1 min−1; 10 nM PMA, 140 mM and 1 mM Ba2+, 22499 ± 1417 pmol (106 cells)−1 min−1, n = 7). Thus block of the inward rectifier does not compromise the ability of the cells to undertake a respiratory burst.
Internal magnesium contributes to rectification
In various cell types, the gating properties of inward rectifier potassium channels are also known to be modulated by intracellular cations (Matsuda et al. 1987; Lopatin et al. 1994). In the present whole cell study, the influence of internal magnesium was investigated. Eosinophils were dialysed with one of two pipette solutions. The control pipette solution contained 1 mM Mg2+ in the form of MgATP (PS II). The nominally Mg2+-free pipette solution (PS III) contained no added Mg2+ (1 mM Na2ATP was used) and 10 mM of the calcium and magnesium chelator EDTA was included. The eosinophils were bathed in standard external physiological solution where [K+]o was 5 mM (BS I). All recordings commenced 2 min after formation of the whole cell configuration to allow time for equilibration of the pipette solution. Each of the pipette solutions was tested on eosinophils from the same batches.
Dialysis of eosinophils with magnesium-free pipette solution (PS III) was generally without significant effect on the inward current (Fig. 7A). In contrast, the magnitude of the outward currents recorded at voltages more positive than −60 mV was significantly augmented in cells dialysed with magnesium-free solution (Fig. 7A). In whole cell studies it is not possible to remove by dialysis the influence of the other possible cations implicated in rectification; namely intracellular polyamines. However, in an attempt to neutralize some of the cationic charges, the sulphated polyanionic oligosaccharide heparin (low molecular weight, 200 μM) was included in the magnesium-free pipette solution (PS IV). The augmentation of the outward current observed with the magnesium-free pipette solution was virtually absent when heparin was present (Fig. 7B). Only small augmentation was observed when compared with the magnesium-containing solution (PS II) at voltages from +30 to +50 mV. However, within this voltage range augmentation could be possibly occurring at the level of either the proton or the potassium current. It was also observed that the magnitude of the inward current was significantly attenuated in the magnesium-free heparin-containing group (PS IV) compared with the magnesium-free group (PS III) (Fig. 7B).
Figure 7. Augmentation of the outward current in the absence of internal Mg2+.
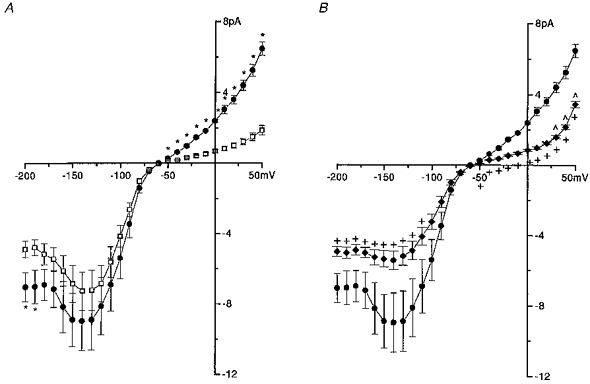
A, cells from the same batches were dialysed with pipette solution which either contained Mg2+ (□, n = 8, PS II) or was nominally free of Mg2+ (•, n = 7, PS III). All cells were bathed in the same external physiological solution (BS I). The membrane potential of the cells was clamped at −60 mV. Voltage steps, 500 ms in duration, were applied at 10 s intervals to voltages from −200 to +50 mV in 10 mV increments. Steady-state current amplitude was plotted against corresponding test membrane potential. Data are shown as mean values ± s.e.m. *Significant difference (P < 0.05) between □ and •. B, the average current-voltage relationship for the group of cells dialysed with a pipette solution containing 200 μM heparin in a nominally magnesium-free solution (♦, n = 12, PS IV) was included with the data for the nominally Mg2+-free group as already seen in A (•). +, significant difference (P < 0.05) between ♦ and •; ω, significant difference between ♦ and □ (not shown in this graph but in A).
Modulation of inward current by intracellular pH
When the eosinophil respiratory burst oxidase is engaged to produce superoxide anions there is as a result of this reaction intracellular generation of protons. This may result in the cytoplasmic aspect of the channels being exposed to a range of micro-environment pHs. We therefore investigated the effects of altering internal pH on the inward whole cell current.
The pH of the pipette solutions was clamped at 7.0, 5.5 or 8.0 (PS V-VII, respectively) using 10 mM Hepes, Mes or Taps, respectively. The pH of the external solution was 7.0 in all cases (BS I). The levels of free Ca2+ and Mg2+ in each pipette solution were adjusted to take into account the different pH conditions so that the final free concentration in each pipette solution was 0.01 and 280 μM, respectively. Voltage steps (1 s duration, −200 to +30 mV in 10 mV increments) were applied 2 min following formation of whole cell configuration to allow sufficient time for dialysis of the eosinophils. Cells from the same batches were tested.
Cytoplasmic acidification reduced the magnitude of the inward current and this reached significance within the voltage range −130 to −100 mV (Fig. 8). The current- voltage relationship for the inward current was almost linear compared with that when pHi was 7.0 (Fig. 8). Alkalization of the cytoplasm was observed to have the opposite effect. The magnitude of the inward current was significantly augmented over the voltage range −200 to −80 mV on alkalization of the cytoplasm. The U-shaped appearance of the inward current-voltage relationship when pHi was 7.0 was accentuated when pHi was 8.0 (Fig. 8).
Figure 8. Influence of pHi on the inward current.
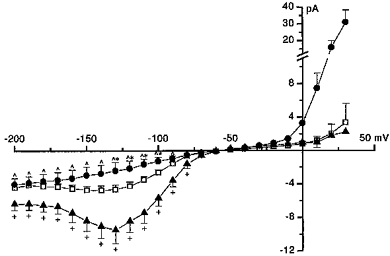
End pulse current amplitude was plotted against test membrane potential for eosinophils where pH of the internal solution was clamped at 7.0 (□, n = 12, PS V), 5.5 (•, n = 8, PS VI) and 8.0 (▴, n = 8, PS VII) (means ± s.e.m). The pH of the external solution was clamped at 7.0 (BS I). Relationships were constructed from currents evoked by 1 s duration pulses applied at 7 s intervals from the holding potential of −60 mV to voltages from −200 to +30 mV in 10 mV increments. Significance is only indicated for the inward current. *Significant difference ( P < 0.05) between □ and •; +, significant difference (P < 0.05) between □ and ▴; ω, significant difference (P < 0.05) between • and ▴. There was no significant difference in the amplitude of the outward current when cells were dialysed with internal solution in which the pH was either clamped at 7.0 or 8.0. The outward current was significantly (P < 0.05) larger in cells that were dialysed with internal solution where the pHi was 5.5 compared with those where the internal pHi was clamped to 7.0 (at voltages of −40 mV and more positive) or to 8.0.
The outward current was unaltered by cytoplasmic alkalization but was significantly enhanced in the voltage range −40 to +30 mV with acidification. When pHi is 5.5, the proton conductance starts to contribute to the whole cell outward current within this voltage range as the proton motive force is significantly augmented (equilibrium potential for protons was shifted to −87 mV when pHi was 5.5, compared with 0 and +58 mV when pHi was 7.0 and 8.0, respectively; Gordienko et al. 1996).
Reduction of extracellular pH to 5.5 also inhibited the inward current in eosinophils (not shown) but its effects were only slowly and incompletely reversed on washout with pHo 7.0 or 8.0.
Identification of Kir2.1 from human eosinophil cDNA
Three batches of total RNA were prepared using eosinophils from three different donors. Using primers based on the coding regions of several Kir channel groups (Kir1.1, Kir2.1, Kir3.4, Kir6.1, Kir6.2), RT-PCR of total RNA from the three samples was performed separately. A strong band around 1650 bp was observed in all three samples. This band was cut from one of the three samples, ligated and sequenced several times and the nucleotide sequence compared to the whole of the GenBank and EMBL databases using the GCG program module Fasta. The coding sequence was 1653 bp long from start to stop codons. The amino acid sequence was found to be 99.5% homologous to HH-IRK1, the Kir2.1 from human heart (Raab-Graham, Radeke & Vandenberg, 1994; GBAcc.No. U12507) and 97.9% homologous to mouse macrophage Kir2.1 (Kubo, Baldwin, Jan & Jan, 1993; GBAcc.No. X73052). We have called our sequence HEos-IRK (GBAcc.No. AF011904). Other bands for Kir3.4 and Kir6.1 were observed but, compared with the band for Kir2.1, these bands were exceptionally faint and only barely detectable. No bands for Kir1.1 or Kir6.2 were seen.
DISCUSSION
The strong rectification shown by the whole cell current, especially in raised external potassium concentration, the effects of varying [K+]o, the actions of Ba2+, Cs+ and Na+ ions and the single channel conductance leaves little doubt that human blood eosinophils have inward rectifier channels resembling closely those seen in a variety of other cell types. The inwardly rectifying behaviour of the current in human eosinophils is strikingly reminiscent of that in oocytes injected with cRNA encoding Kir2.1, an inward rectifier channel from mouse macrophages (Kubo et al. 1993). Properties of Kir2.1 resemble the channel described in our experiments in conductance (23 versus 24 pS in this study), block by cations, and rectification. A channel 97.0% and 99.5% similar in predicted amino acid sequence to that of Kir2.1 from murine macrophage and human heart (HH-IRK1), repectively, was detected by RT-PCR of human eosinophil RNA with specific primers amplifying the entire coding region of Kir2.1. The presence of an inwardly rectifying channel in human eosinophils (which we named HEos-IRK) closely resembling Kir2.1 is sufficient to explain their inwardly rectifying current-voltage behaviour. We exclude other known inward rectifiers as accounting for our findings on the basis of differences in their properties. Although weak bands on electrophoresis of PCR amplification products consistent with Kir6.1 and Kir3.4 were also detected in eosinophils, the former is weakly rectifying (unlike HEos-IRK) and the latter is receptor coupled and dependent upon formation of heteromultimers with Kir3.0 family members (Velimirovic, Gordon, Lim, Navarro & Clapham, 1996), and thus distinguishable from HEos-IRK by these characteristics.
It is a common characteristic of inward rectifier channels that they are blocked by extracellular cations such as Na+ (Standen & Stanfield, 1979; Fukushima, 1982; Harvey & Ten Eick, 1989), Ba2+ and Cs+ (Hagiwara, Miyazaki & Rosenthal, 1976; Gay & Stanfield, 1977; Hagiwara, Miyazaki, Moody & Patlak, 1978; Standen & Stanfield, 1978; Fukushima, 1982; Harvey & Ten Eick, 1989). The inward rectifiers of cells of myeloid origin are also blocked by these cations (Gallin & Sheehy, 1985; Lindau & Fernandez, 1986; Randriamampita & Trautmann, 1987; Gallin & McKinney, 1988; McKinney & Gallin, 1988; Kawa, 1989; Wieland, Chou & Gong, 1990; Piguet & North, 1992; Wischmeyer, Lentes & Karschin, 1995; DeCoursey et al. 1996). The order of potency of the blocking action of these cations in eosinophils was Ba2+ > Cs+ > Na+.
In sodium-containing solution ([Na+]o= 140 mM, [K+]o= 5 mM; BS I, PS I), the inward current was instantly activated upon membrane hyperpolarization and it was relatively sustained throughout the voltage pulse, even at very negative test voltages. In contrast, time-dependent inactivation of the inward rectifier current at voltages more negative than approximately −100 mV is a feature reported in other cells of myeloid origin such as human macrophages (Gallin & McKinney, 1988), PMA-differentiated human THP-1 macrophages (DeCoursey et al. 1996), human leukaemic (HL-60) PMA-differentiated macrophages (Wieland et al. 1990), the murine macrophage J774.1 cell line (Gallin & Sheehy, 1985; Randriamampita & Trautmann, 1987; McKinney & Gallin, 1988), rat basophilic leukaemia cells (RBL-2H3) (Lindau & Fernandez, 1986; Piguet & North, 1992; Wischmeyer et al. 1995), and newt neutrophils (Kawa, 1989). In the human eosinophil the current-voltage relationship has a U-shaped form, similar in appearance to the end-pulse or steady-state current-voltage relationships reported in these other cells of myeloid origin. The current inactivation in these other cell types has been mainly attributed to time- and voltage-dependent block of the inward rectifier by extracellular Na+ ions, although a Na+-independent process may also participate. Indeed, on removal of extracellular Na+, the steady-state current in cells of myeloid origin became larger in amplitude (Lindau & Fernandez, 1986; McKinney & Gallin, 1988; DeCoursey et al. 1996) and the steady-state current-voltage relationships became more nearly linear (Gallin & McKinney, 1988; Kawa, 1989; Wishmeyer et al. 1995). The inward current in human eosinophils did not appear to exhibit substantial time-dependent inactivation, presumably because extracellular Na+ produces a virtually instantaneous block at the onset of the voltage pulse, the time course of which could not be satisfactorily resolved from the decay of the capacitive transient. Removal of external sodium potentiated the current at potentials negative to about −130 mV, consistent with this explanation.
The Cs+ block was strikingly flickery in nature (Fig. 4C, lower panel). The flickery block is not seen in other cells of myeloid origin, but has been observed in single channel recordings of the inward rectifier in tunicate egg cells (Fukushima, 1982) and ventricular heart cells (Matsuda, Matsuura & Noma, 1989). The flickering is produced by apparent rapid transitions between open and blocked states. Whole cell recordings from cell types of myeloid origin which express larger numbers of inward rectifier channels results in a failure to resolve the nature of the block at this level. Barium block was voltage dependent but slower to develop and could be followed in some cells either as single channel events during a hyperpolarizing pulse (presumably largely blocking events) or in other cells as a decay of the whole cell current with a time constant in the 50–500 ms range.
External Mg2+ was also found to inhibit the inward current over the same voltage range as Na+. In cardiac ventricular myocytes, both extracellular Mg2+ and Ca2+ produce fast open channel block of the inward rectifier (even faster than Cs+) which is manifested as an apparent decrease in unit current amplitude (Shioya, Matsuda & Noma, 1993).
The amplitude of the inward rectifier current was smaller than that seen in other cells of myeloid origin. This fact probably makes single channel events more apparent in whole cell recordings when [K+]o was elevated in some eosinophils. Those eosinophils having very small inward currents in control solution generally exhibited resolvable single channel activity when [K+]o was elevated. In contrast, in eosinophils where the current was large, distinct openings were not discernible when [K+]o was raised, and the current was very noisy due presumably to the summation of numerous channel events. From records where single openings could be resolved, as few as three channels were active in 100 mM K+ solution at −200 mV in some cells. In cells exhibiting larger currents it was estimated that up to 20 channels per cell or 7 channels pF−1 were active. This probably explains why the currents are small in eosinophils compared with other cells of myeloid origin. In the murine macrophage J774.1 cell line, inward rectifier channel density was estimated to be approximately 1100 channels per cell or 47 channels pF−1 (McKinney & Gallin, 1988). The single channel conductance of the inward rectifier in eosinophils was 24 pS (100 mM [K+]o, 140 mM [K+]i), which fits well within the 20–30 pS range which has been reported for the inward rectifier in other cells of myeloid origin (human macrophage, 28 pS, 150 mM [K+]o, Gallin & McKinney, 1988; PMA-differentiated human THP-1 macrophages, 30 pS, symmetrical high K+, DeCoursey et al. 1996; RBL-2H3 cells, 26 pS, 130 mM [K+]o, Lindau & Fernandez 1986; 25 pS, 140 mM [K+]o, Wischmeyer et al. 1995; J774.1 cells, 20 pS, 75 mM [K+]o, McKinney & Gallin, 1988) and for other cell types, e.g. cardiac ventricular cells (22 pS, 150 mM [K+]o, Matsuda et al. 1987). On occasions subconductance levels were sometimes observed. Whether observed in the absence or presence of barium, the single channel activity was only detected at potentials well negative to EK. No outward single channel openings were ever observed. This observation suggests that rectification occurs at the single channel level (McKinney & Gallin, 1988).
The gating properties of inward rectifier potassium channels are known to be modulated by intracellular cations. In some cells such as ventricular heart cells, rectification of the K+ current is abolished on removal of intracellular Mg2+ (Matsuda et al. 1987), whereas in other cells Mg2+ is only partially responsible for rectification or not involved at all (Silver & DeCoursey, 1990). More recently, studies using inside-out patches have revealed that intracellular polyvalent organic cations such as the polyamines, are capable of producing voltage-dependent block of the inward rectifier (Lopatin et al. 1994). The human eosinophil inward rectifier is a case where internal Mg2+ is partially responsible for rectification because internal Mg2+ produced a voltage-dependent block when the membrane was polarized to levels beyond EK. In our whole cell experiments we attempted to neutralize intracellular cations by addition of heparin to the intracellular milieu but this manoeuvre paradoxically restored complete rectification of the current.
The outward current through the inward rectifier was potentiated at all voltages in the absence of intracellular Mg2+. It is likely that there was some contamination of the outward current with the voltage-activated outward proton current in the very positive region of the voltage range tested. The transmembrane pH gradient was deliberately set at zero so that the proton current would only be activated significantly at potentials more positive than 0 mV. Additionally, since the [Ca2+]i was clamped to 0.01 μM, the proton current would not emerge until voltages in excess of approximately +20 mV (Gordienko et al. 1996). Thus, significant potentiation of the outward current in the absence of internal Mg2+ was seen at voltages well before those sufficient for activation of the proton current.
The cytoplasmic domain of the inward rectifier channels may well be subjected to varying pH conditions depending on the activation state of the eosinophil. Cytoplasmic acidification inhibited the inward current in eosinophils, as has also been previously observed in several cell types such as starfish oocytes (Moody & Hagiwara, 1982), ventricular myocytes (Ito, Vereecke, Carmeliet, 1992), skeletal muscle (Blatz, 1984), and also the cloned IRK1 expressed in Xenopus oocytes (Sabirov, Okada & Oiki, 1997). In contrast to the observations in guinea-pig ventricular myocytes (Ito et al. 1992), cytoplasmic alkalization potentiated the inward whole cell current. Internal protons are thought to inhibit some channels by screening the surface charges around the cytoplasmic aspect of the channel. H+ may bind within the channel pore thus affecting K+ permeation (Moody & Hagiwara, 1982). Cytoplasmic acidification was observed to increase close times of inward rectifier K+ channels in guinea-pig ventricular myocytes (Ito et al. 1992) and abolish single channel activity of IRK1 expressed in Xenopus oocytes (Sabirov et al. 1997). By binding to the intracellular site of the channel, protons may cause the channels to stay in the closed state for longer periods.
Extracellular acidification also inhibited the inward current but its effects were incompletely reversed on washout. Similar effects of external acidification are also observed in starfish oocytes (Moody & Hagiwara, 1982) and skeletal muscle (Blatz, 1984). Thus the inward rectifier in eosinophils may also be modulated by protonation of its extracellular domain as well. Recently it has been shown that extracellular protons fully and reversibly inhibit the IRK1 expressed in Xenopus oocytes and cause a reduction of single channel conductance (Sabirov et al. 1997).
The fact that neither varying [K+]o nor BaCl2 had any effect on superoxide anion production whether stimulated or not by PMA argues for a minimal effect of membrane potential in controlling this type of human blood eosinophil activity. Although the membrane potential is ‘quiescent’ in some eosinophils, in others ‘spontaneous’ depolarizations of variable amplitude are observed (M. Tare & D. V. Gordienko, unpublished observations). The significance of these fluctuations in membrane potential is unknown, although depolarization may be associated with some aspect of cellular activation. In human neutrophils superoxide production is associated with depolarization and is thought to be caused by electron transfer through the NADPH oxidase (Henderson, Chappell & Jones, 1987). The spontaneous depolarizations seen in human eosinophils may, in part, reflect changes in basal superoxide production.
The function of the eosinophil inward rectifier, which is the dominant whole cell current within the ‘normal’ membrane potential range and when [K+]o is raised, is unclear and requires further study. It may be that the inward rectifier contributes to the stabilization of the resting membrane potential close to EK and, indeed, the membrane potential closely follows EK when [K+]o is raised. Eosinophils may conceivably be exposed to high microenvironmental [K+]o in the vicinity of cellular damage but despite this, the small outward current through the inward rectifier still allows the cell to respond to increases in conductance which depolarize and thus may activate the proton conductance (Gordienko et al. 1996). In the murine macrophage J774.1 cell line, the inward rectifier current is larger and the resting membrane potential is more negative in long term adherent cells than in non-adherent cells (Gallin & Sheehy, 1985). However, in eosinophils this current was similar regardless of whether they were adherent (≤ 1.5 h) or in suspension, but further experiments will be required to investigate the effects of longer periods of adherence or locomotion.
In conclusion, the results of this study strongly suggest that human eosinophils have inward rectifier channels of the Kir2.1 subtype. The importance of this channel type, and indeed of the membrane potential, in the regulation of eosinophil behaviour awaits elucidation.
Acknowledgments
This work was supported by the Medical Research Council (M.T., S.P. and J.E.C.) and by Pfizer Central Research (D.V.G.). The authors thank Dr A. V. Zholos for helpful discussions. This work has benefited from the use of the SEQNET facility.
References
- Blatz AL. Asymmetric proton block of inward rectifier K channels in skeletal muscle. Pflügers Archiv. 1984;401:402–407. doi: 10.1007/BF00584343. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Kim SY, Silver MR, Quandt FN. Ion channel expression in PMA-differentiated Human THP-1 macrophages. Journal of Membrane Biology. 1996;152:141–157. doi: 10.1007/s002329900093. [DOI] [PubMed] [Google Scholar]
- Fukushima Y. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording. Journal of Physiology. 1982;331:311–331. doi: 10.1113/jphysiol.1982.sp014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin EK. Ion channels in leukocytes. Physiological Reviews. 1991;71:775–811. doi: 10.1152/physrev.1991.71.3.775. [DOI] [PubMed] [Google Scholar]
- Gallin EK, McKinney LC. Patch-clamp studies in human macrophages: single-channel and whole-cell characterization of two K+ conductances. Journal of Membrane Biology. 1988;103:55–66. doi: 10.1007/BF01871932. [DOI] [PubMed] [Google Scholar]
- Gallin EK, Sheehy PA. Differential expression of inward and outward potassium currents in the macrophage-like cell line J774.1. Journal of Physiology. 1985;369:475–499. doi: 10.1113/jphysiol.1985.sp015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay LA, Stanfield PR. Cs+ causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977;267:169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Tare M, Parveen S, Fenech CJ, Robinson C, Bolton TB. Voltage-activated proton current in eosinophils from human blood. Journal of Physiology. 1996;496:299–316. doi: 10.1113/jphysiol.1996.sp021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. Journal of Physiology. 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Rosenthal NP. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. Journal of General Physiology. 1976;67:621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. Journal of Physiology. 1979;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Ten Eick RE. Voltage-dependent block of cardiac inward-rectifying potassium current by monovalent cations. Journal of General Physiology. 1989;94:349–361. doi: 10.1085/jgp.94.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LM, Chappell JB, Jones OTG. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with a H+ channel. Biochemical Journal. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Ito H, Vereecke J, Carmeliet E. Intracellular protons inhibit inward rectifier K+ channel of guinea-pig ventricular cell membrane. Pflügers Archiv. 1992;422:280–286. doi: 10.1007/BF00376214. [DOI] [PubMed] [Google Scholar]
- Kawa K. Electrophysiological properties of three types of granulocytes in circulating blood of the newt. Journal of Physiology. 1989;415:211–231. doi: 10.1113/jphysiol.1989.sp017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan L Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lindau M, Fernandez JM. A patch-clamp study of histamine-secreting cells. Journal of General Physiology. 1986;88:349–368. doi: 10.1085/jgp.88.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- McKinney LC, Gallin EK. Inwardly rectifying whole-cell and single-channel K currents in the murine macrophage cell line J774.1. Journal of Membrane Biology. 1988;103:41–53. doi: 10.1007/BF01871931. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Matsuura H, Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. Journal of Physiology. 1989;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Moody WJ, Hagiwara S. Block of inward rectification by intracellular H+ in immature oocytes of the starfish Mediaster aequalis. Journal of General Physiology. 1982;79:115–130. doi: 10.1085/jgp.79.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P, North RA. The inward rectifier potassium conductance in rat basophilic leukemia cells. Journal of Cellular Physiology. 1992;151:269–275. doi: 10.1002/jcp.1041510208. [DOI] [PubMed] [Google Scholar]
- Raab-Graham KF, Radeke CM, Vandenberg CA. Molecular cloning and expression of a human heart inward rectifier potassium channel. NeuroReport. 1994;5:2501–2505. doi: 10.1097/00001756-199412000-00024. [DOI] [PubMed] [Google Scholar]
- Randriamampita C, Trautmann A. Ionic channels in murine macrophages. Journal of Cell Biology. 1987;105:761–769. doi: 10.1083/jcb.105.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Okada Y, Oiki S. Two-sided action of protons on an inward rectifier K+ channel (IRK1) Pflügers Archiv. 1997;433:428–434. doi: 10.1007/s004240050296. [DOI] [PubMed] [Google Scholar]
- Saito M, Sato R, Hisatome I, Narahashi T. RANTES and platelet-activating factor open Ca2+-activated K+ channels in eosinophils. FASEB Journal. 1996;10:792–798. doi: 10.1096/fasebj.10.7.8635697. [DOI] [PubMed] [Google Scholar]
- Schrenzel J, Lew DP, Krause K-H. Proton currents in human eosinophils. American Journal of Physiology. 1996;271:C1861–1871. doi: 10.1152/ajpcell.1996.271.6.C1861. [DOI] [PubMed] [Google Scholar]
- Shioya T, Matsuda H, Noma A. Fast and slow blockades of the inward-rectifier K+ channel by external divalent cations in guinea-pig cardiac myocytes. Pflügers Archiv. 1993;422:427–435. doi: 10.1007/BF00375067. [DOI] [PubMed] [Google Scholar]
- Silver MR, DeCoursey TE. Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence and absence of internal Mg2+ Journal of General Physiology. 1990;96:109–133. doi: 10.1085/jgp.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. Journal of Physiology. 1978;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle. Journal of Physiology. 1979;294:497–520. doi: 10.1113/jphysiol.1979.sp012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare M, Gordienko DV, Parveen S, Fenech C, Robinson C, Bolton TB. Electrophysiological properties of human eosinophils. Journal of Physiology. 1996;491.P:90–91P. doi: 10.1113/jphysiol.1996.sp021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare M, Gordienko DV, Parveen S, Robinson C, Bolton TB. Identification of an inward rectifier K+ current in eosinophils from human blood. British Journal of Pharmacology. 1997;120:15P. [Google Scholar]
- Velimirovic BM, Gordon EA, Lim NF, Navarro B, Clapham DE. The K+ channel inward rectifier subunits form a channel similar to neuronal G protein-gated K+ channel. FEBS Letters. 1996;379:31–37. doi: 10.1016/0014-5793(95)01465-9. [DOI] [PubMed] [Google Scholar]
- Wieland SJ, Chou RH, Gong Q-H. Macrophage-colony-stimulating factor (CSF-1) modulates a differentiation-specific inward-rectifying potassium current in human leukemic (HL-60) cells. Journal of Cellular Physiology. 1990;142:643–651. doi: 10.1002/jcp.1041420326. [DOI] [PubMed] [Google Scholar]
- Wischmeyer E, Lentes K-U, Karschin A. Physiological and molecular characterization of an IRK-type inward rectifier K+ channel in a tumour mast cell line. Pflügers Archiv. 1995;429:809–819. doi: 10.1007/BF00374805. [DOI] [PubMed] [Google Scholar]


