Abstract
Tonic inhibition of sensory spinal neurones is well known to descend from the rostroventral medulla. It is not clear if this inhibition is dynamically activated by peripheral noxious stimuli.
Transection of the ipsilateral dorsolateral funiculus (DLF) removed a descending inhibition of multireceptive spinal neurones and disproportionally prolonged the after-discharge component of their response to a noxious cutaneous stimulus.
Microinjection of GABA or tetracaine into the medullary nucleus gigantocellularis pars alpha (GiA) similarly prolonged the after-discharge in response to noxious stimuli.
Recordings of GiA cells, initially using minimal surgery, revealed that many had low levels of spontaneous activity and responded vigorously to noxious stimuli applied to any part of the body surface. One hour after the surgery necessary to expose the spinal cord, GiA cells had a high firing rate but responded weakly to noxious stimuli.
The response of GiA cells to noxious stimuli was abolished by transection of only the DLF contralateral to the stimulus.
It is concluded that the inhibition of multireceptive dorsal horn neurones from GiA is dynamically activated by noxious cutaneous stimuli via a projection in the contralateral DLF. Surgical exposure of the spinal cord tonically activates this inhibition and masks the dynamic component.
It has been well established for many years that the rostroventral medulla (RVM) including the nucleus raphe magnus (NRM) and nucleus reticularis gigantocellularis pars alpha (GiA) is the major source of descending inhibition of spinal dorsal horn neurones via axons that run in the ipsilateral dorsolateral funiculus (DLF) (Basbaum & Fields, 1979; Ren & Dubner, 1996). It is also clear that the cells in this area of the brain respond to noxious stimuli (Wolstenscroft & West, 1982; Oliveras, Martin, Montagne & Vos, 1990). These data have clearly implied that the RVM is part of a dynamically activated supraspinal loop by which noxious stimuli activate the descending inhibition of multireceptive dorsal horn cells (Basbaum & Fields, 1984a). This has never been convincingly demonstrated, however (but see Cervero, Lumb & Tattershall, 1985), and the majority of studies have reported that the inhibition from the RVM is tonic in nature (Hall, Duggan, Moreton & Johnston, 1982; Foong & Duggan, 1986; Sandkuhler, Eblen-Zajjur, Fu & Forster, 1995). The phenomenon of diffuse noxious inhibitory controls (DNIC) (Le Bars, Dickenson & Besson, 1979) is a clear demonstration that supraspinal inhibition may be dynamically activated by noxious stimuli and early data implied that the NRM was a source of this inhibition (Dickenson, Le Bars & Besson, 1980). Recent work by Bouhassira, Bing & Le Bars (1993) and Bouhassira, Gall, Chitour & Le Bars (1995b) has denied this, however, and shown that the inhibition from the RVM is purely tonic and only cells in the subnucleus reticularis dorsalis in the posterior medulla contribute to the dynamically activated inhibition.
This paper demonstrates that cells in the GiA are dynamically activated by noxious stimuli and are a major source of descending inhibition. Surgical exposure of the spinal cord tonically activates this inhibition, however, and masks the dynamic component.
METHODS
All experiments were conducted on male Wistar rats weighing between 280 and 320 g. Experimental procedures were carried out in accordance with the Animals (Scientific Procedures) Act 1986.
Recording from dorsal horn neurones
Anaesthesia was induced with 4% Fluothane in O2 and urethane administered (1.2 g kg−1, i.p.). The Fluothane was gradually reduced to between 0 and 1% in O2 as required to ensure complete abolition of all withdrawal responses to noxious pinch. The animal was shaved as required, the trachea was exposed and a cannula was inserted. A cannula containing heparin (0.03%) saline was inserted into the right carotid artery to enable blood pressure to be monitored. The temperature of the animal was monitored by a rectal probe and maintained between 36.5 and 37.5°C by a thermostatically controlled heating blanket.
Cervical and lumbar laminectomies were performed as described in Rees & Roberts (1989). Briefly, the lumbar vertebrae L2 and L3 were rigidly clamped and the dorsal laminae removed back to S1. The dura mater was incised and pinned back laterally and the arachnoid removed. Cervical vertebrae C2-C4 were not clamped but otherwise subjected to the same procedure. Skin flaps were raised and the cord covered in warm paraffin oil.
Black platinum and gold plated tungsten electrodes sheathed in glass were used for recording from neurones in the lumbar spinal cord. Microelectrode tracks were made with a computer controlled stepper (Digitimer, Welwyn Garden City, UK) through the lumbar dorsal horn in the segment L5. Cells found at depths from 400 to 800 μm below the surface of the cord were tested with cutaneous brush/touch and strong pinch stimuli. These were studied further if shown to be multireceptive cells. Recordings were made via an AC preamplifier (Digitimer Neurolog) and a spike processor with a window discriminator (Digitimer D130) to distinguish action potentials from background noise. Data were analysed by a computer analysis programme (T. E. Salt, University College London, UK) that constructed peristimulus time histograms (PSTHs). The output from the spike processor was also fed to a polygraph (Grass Instruments, Quincy, MA, USA) that provided a continuous record of the firing rate and responses of cells during the entire experiment. Permanent records of the size and shape of the action potentials were made by means of an X-Y plotter (Rikadenki, Chessington, UK).
Stimulation parameters for spinal cells: cutaneous stimuli
All neurones had cutaneous receptive fields on the ipsilateral hindlimb. The search stimulus for these cells was gentle mechanical stimulation with a camel hair brush or glass probe. The centre of the receptive field was localized by a fine glass probe. Noxious cutaneous stimulation was applied to the centre of the receptive field by means of a calibrated heat lamp controlled for time of application and temperature by an adjustable control unit. A thermocouple touching the skin in the centre of the receptive field recorded the temperature achieved onto a polygraph chart record. Consistent responses were evoked by the lamp and were repeated at 10 min intervals to avoid peripheral or central sensitization.
Stimulation parameters for spinal cells: stimulation of cut dorsal roots at C fibre intensity
The responses of dorsal horn neurones to electrical stimulation of cut dorsal roots were also studied. The roots were laid across bipolar silver wire electrodes in the paraffin pool. C fibre stimulation at up to 22 V (100 × A fibre threshold), 0.2 ms, was applied 3–15 times at a rate of 1 Hz. This electrical stimulation caused ‘wind up’ of the response of the dorsal horn neurone (i.e. a progressive increase in excitation with each stimulation).
Electromyograph (EMG) studies
These studies were performed to determine the flexor reflex response to noxious stimuli in animals not subjected to extensive surgery. Rats were anaesthetized by the methods described previously, but no surgery was performed on these animals (i.e. no tracheal or carotid artery cannulae and no ear bars were inserted). Bipolar electromyography electrodes were made from 4 μm insulated silver wires that were threaded through 24-gauge hypodermic needles and bent into barbs. These were inserted into the biceps femoris of the hindlimb of the rat to record multiunit activity. Flexor reflex responses were elicited every 10 min by means of a calibrated heat lamp applied to the plantar surface of the hindpaw. Once three constant responses had been evoked the dorsal skin was incised and lumbar and cervical laminectomies performed to determine the effects of this surgical procedure.
Dorsolateral funiculus (DLF) lesions
In the course of some studies, lesions of the DLF of the spinal cord were made at the cervical level under close microscopic control. A pair of Dumont forceps (coated in Pontamine Sky Blue (PSB) to aid later histological analysis) were used to transect the funiculus. In some experiments a reversible cord block was achieved using topical application of a local anaesthetic (tetracaine 2%) to the DLF.
Microinjections into the brain
These studies involved injection of inhibitory drugs (0.5–1.0 μl 200 mM GABA, or 0.5–1.0 μl 2% tetracaine) into the brain in order to determine the effects of localized disruption of function in selected areas on the responses of spinal neurones. On completion of the laminectomy the skull was exposed and trepanned. A 10 μl Hamilton syringe was sterotaxically positioned over the hole with a micromanipulator. It contained the drug solution dissolved in 4% saline coloured with PSB to enable histological verification of the injection site. The stereotaxic co-ordinates were taken from the atlas of Paxinos & Watson (1986).
Following three control responses from a spinal neurone, the syringe was lowered to the target depth and the drug slowly expelled over a 3 min period. The syringe was withdrawn and further responses evoked from the spinal neurone at 10 min intervals for up to 1 h. At the end of the study, the ipsilateral DLF was transected and further responses evoked from the spinal neurone. This was to enable comparison between the effects of the microinjection and ipsilateral DLF transection.
Electrophysiological recordings in the medullary gigantocellularis pars alpha (GiA)
The microinjection experiments revealed that a potentially dynamic component of descending inhibition was disrupted by GABA injections into the GiA. Recordings were therefore taken from cells in this area. ‘Low surgery’ procedures were used. Animals were anaesthetized with urethane (1.2 g kg−1, i.p.) with additional Fluothane (1–2%) given via a face mask. Lignocaine gel (2%) was applied to the ears before the rat was put in the stereotaxic frame. The electrocardiogram (ECG) was monitored as an indicator of the state of the animal. A hole was drilled in the skull above the GiA and the dura mater removed. The co-ordinates used for the GiA recordings were −2.0 mm AP, −1.0 mm lateral, −0.25 mm vertical from the ear bar zero.
The spontaneous activity and the nociceptive responses of GiA cells were recorded with tungsten microelectrodes. Noxious stimuli were applied by a metal block (3 cm × 3 cm) heated to 52°C and applied for 10 s to the skin of the anaesthetized animal. A grid of 3 cm × 3 cm squares was drawn on the back and flanks of the rat so that the receptive field of the GiA cell could be mapped. The effects of cervical and lumbar laminectomies were studied on the firing rate and nociceptive responses of the GiA cell. The position of the recorded cells could be calculated from the tracks left by the recording electrode when the brain was sectioned. The ascending pathway mediating the nociceptive responses of GiA cells was studied by progressive transection of spinal tracts at cervical levels. This was done using Dumont forceps under microscopic control.
Analysis of data
The frequency of action potential discharge was measured from the calibrated chart record or the PSTH as appropriate. Data are expressed as means ±s.e.m. Calculation of significance of differences used Student's paired t test. P < 0.05 was considered significant.
Histological analysis
At the end of the experiment the animal was deeply anaesthetized and perfused with formal saline, and the brain and spinal cord removed for histological determination of the electrode track, tetracaine/GABA injection site or location of spinal lesions. The CNS was stored in 25% formalin in isotonic saline for at least 48 h prior to sectioning on a microtome. Sections (50 μm) were cut and the relevant sections mounted on gelatinized slides before staining with Neutral Red.
RESULTS
The nociceptive responses of dorsal horn cells
The cells recorded were between 400 and 800 μm from the surface of the lumbar spinal cord. They all had receptive fields on the ipsilateral hindpaw and were multireceptive cells excited by gentle brushing or touch and vigorously excited by the thermal noxious stimulus. The mean baseline firing rate of the cells was 3.3 ± 0.7 spikes s−1. The mean amplitude of the response to a 10 s noxious stimulus that reached 50°C was 42.1 ± 7.6 spikes s−1 and the mean duration was 11.6 ± 2.9 s (n = 18).
Effect of ipsilateral DLF transection on the dorsal horn cell response to noxious heat
Transection of the ipsilateral DLF at cervical levels very markedly increased the size of responses to the standardized noxious thermal stimulus applied to the centre of the receptive field (Fig. 1). The baseline firing rate of the cells increased to 151.5% of control (5.0 ± 0.6 spikes s−1, n = 40, not significant). The peak firing rate increased to 151% of control (63.5 ± 10.1 spikes s−1, not significant), and the after-discharge increased to 598% of control (69.3 ± 7.8 spikes s−1, P < 0.001). Similar lesions to adjacent areas in dorsal columns or lateral funiculi did not reliably increase any of these measures. Complete spinal transection did not enhance the effects of ipsilateral DLF transection.
Figure 1. Effects of ipsilateral DLF section on the response properties of multireceptive dorsal horn cells.
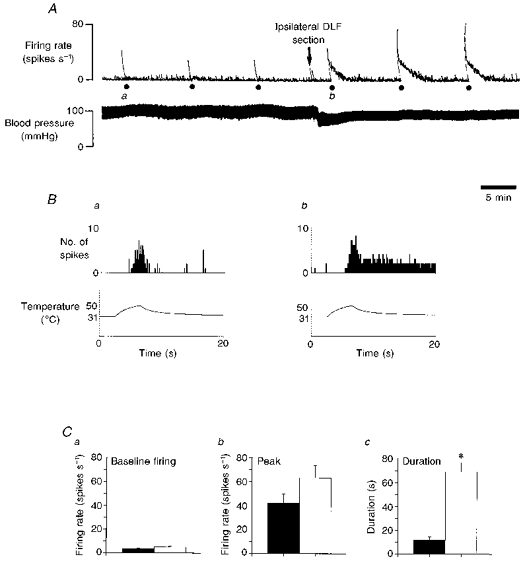
A, polygraph chart record of the firing rate of a multireceptive dorsal horn cell recorded 622 μm below the surface of the cord. The lower trace shows the blood pressure of the animal. Noxious stimulation (50 °C) was given at 10 min intervals (indicated by •). It can be seen that the three control responses had a similar peak amplitude and very little after-discharge. At the point indicated by the arrow, the ipsilateral DLF was transected. The reduction in blood pressure did not alter the recorded amplitude of the action potential, which was carefully monitored throughout the study. The response to noxious stimulation was greatly increased, however. B, these graphs are peristimulus time histograms (PSTHs) of the responses of the same cell shown in A. Below the PSTHs are shown the skin temperature of the receptive field with time. PSTHs a and b are the responses labelled a and b, respectively, in A. The bin size of the PSTHs was 100 ms. The ipsilateral DLF transection increased the peak firing frequency from about 5 to about 6 spikes (100 ms)−1. By far the greatest effect was on the duration of the after-discharge. This was prolonged beyond the analysis period of the PSTH but can be clearly seen in A. C, histograms of the effects of ipsilateral DLF section on the responses of 40 multireceptive dorsal horn cells to noxious stimulation. Control values before (▪) and the values after (□) ipsilateral DLF transection are shown. a, change in baseline firing rate; b, change in peak firing rate (both not significant); and c, change in duration of the after-discharge; *P < 0.001.
Tetracaine mimics the effects of physical transection of the ipsilateral DLF
Tetracaine was used to confirm that the effects of mechanical transection of the ipsilateral DLF were due to the cutting, and not due to the mechanical stimulation of axons. In four studies a tissue pad soaked in 2% tetracaine in 4% saline was applied to the ipsilateral DLF at the cervical level. After about 5–10 min the response of multireceptive cells to noxious heat was prolonged. The increase in the after-discharge was to 381% of control (9.6 ± 1.7 to 36.6 ± 4.2 spikes s−1, n = 4, P < 0.003) and the increase in the peak amplitude of the response was to 133.7% of control (36.4 ± 3.4 to 48.8 ± 2.4 spikes s−1, n = 4, not significant). This effect was reversible on wash of the tetracaine with saline (n = 2).
The effects of ipsilateral DLF transection on the response of multireceptive spinal cells to C fibre stimulation of the cut dorsal root
The repetitive electrical stimulation of high threshold fibres in a cut dorsal root (100 times A fibre threshold) was also used to activate multireceptive dorsal horn neurones. This was done to avoid sensitization of peripheral nociceptors, to provide a more intense activation of a larger group of dorsal horn cells and to study the effects of ipsilateral DLF transection on wind up.
Between three and fifteen stimuli at 1 Hz were applied depending upon the response of the cell. Typically, the first stimulus evoked 1–2 spikes and subsequent stimuli evoked increasingly larger responses (wind up). The discharge was sustained for 3–6 s after the end of the stimulus train. Cutting the ipsilateral DLF (Fig. 2B) caused wind up to appear earlier in the train, caused the peak discharge to be increased to 143% of control (from 33.8 ± 3.0 to 48.4 ± 3.2 spikes s−1, P < 0.001) and principally increased the duration to 1183% of control (from 9.3 ± 1.8 to 110 ± 14.1 s, n = 16, P < 0.001, Fig. 3). As in previous experiments, ipsilateral DLF section also increased the baseline firing rate to 145% of control. These results strongly indicate that the inhibition descending in the ipsilateral DLF is very largely tonically active in these animals. Although the disproportionate prolongation of responses may be suggestive of an additional dynamically activated inhibition, this could equally result from disruption of tonic inhibition.
Figure 2. The effects of microinjection of GABA and transection of the ipsilateral DLF on the response of multireceptive dorsal horn cells to C fibre stimulation of a cut dorsal root.
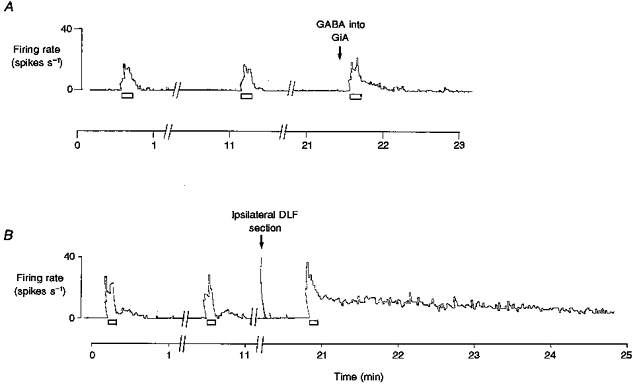
A and B are polygraph chart traces of the firing frequency of two different multireceptive dorsal horn cells. Stimulation of the dorsal roots at C fibre intensity was applied for the periods indicated by the open bars. The records are broken as indicated (//) because responses were evoked at 10 min intervals. The arrow in A indicates where 0.1 μmol GABA was microinjected into the GiA. The arrow in B indicates where the ipsilateral DLF was transected. Both procedures slightly increased the peak amplitude and markedly prolonged the after-discharge. In every cell studied, the effects of DLF transection were greater than the effects of GABA microinjected unilaterally into the GiA.
Figure 3. The effects of section of the ipsilateral DLF and microinjections of GABA and tetracaine on the duration of the after-discharge of multireceptive dorsal horn cells.
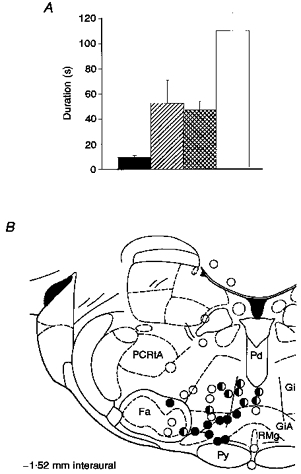
A, histogram of the effects of microinjections of GABA, tetracaine and section of the ipsilateral DLF on the mean duration of the after-discharge of 16 multireceptive dorsal horn cells. The microinjection of either tetracaine ( ) or GABA (
) or GABA ( ) into the GiA significantly prolonged the after-discharge as compared with control (▪) (P < 0.001). The effects of the two drugs did not differ however (n.s.). Ipsilateral DLF section (□) had significantly greater effects than the microinjections (P < 0.03). B, coronal section illustrating the location of microinjections into the brain (−1.0 to −3.0 mm AP). As the effects of GABA and tetracaine did not differ (see A) they have not been differentiated in this figure. •, microinjections which increased the duration of the after-discharge to more than 200% of control;
) into the GiA significantly prolonged the after-discharge as compared with control (▪) (P < 0.001). The effects of the two drugs did not differ however (n.s.). Ipsilateral DLF section (□) had significantly greater effects than the microinjections (P < 0.03). B, coronal section illustrating the location of microinjections into the brain (−1.0 to −3.0 mm AP). As the effects of GABA and tetracaine did not differ (see A) they have not been differentiated in this figure. •, microinjections which increased the duration of the after-discharge to more than 200% of control; 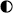 , areas that caused 120–200% of control; ○, areas where microinjections had little or no effect. Abbreviations: Fa, facial nucleus; PCRtA, parvocellular reticular nucleus alpha; Pd, predorsal bundle; Py, pyramidal tract; RMg, raphe magnus; Gi, nucleus gigantocellularis reticularis; and GiA, nucleus gigantocellularis reticularis pars alpha.
, areas that caused 120–200% of control; ○, areas where microinjections had little or no effect. Abbreviations: Fa, facial nucleus; PCRtA, parvocellular reticular nucleus alpha; Pd, predorsal bundle; Py, pyramidal tract; RMg, raphe magnus; Gi, nucleus gigantocellularis reticularis; and GiA, nucleus gigantocellularis reticularis pars alpha.
The electromyographic response to noxious stimuli
The predominance of tonic inhibition may be due to the extensive surgery necessary for the recording from spinal dorsal horn cells. To determine the effects of cervical and lumbar laminectomy on nociceptive responses, EMG responses were recorded from animals subjected initially to no surgery (Fig. 4). Following three baseline control responses (29.6 ± 2.1 units), the laminectomies were performed.
Figure 4. The effects of surgery on the EMG response to noxious pinch.
A, EMG studies were performed in animals that initially had no surgery. The flexor reflex responses to calibrated noxious pinch were evoked at 5 min intervals. The surgery performed was identical to the cervical and lumbar laminectomies described elsewhere (indicated by the arrow). It took 70 min to complete. It can be seen that in this study the responses were facilitated for more than 10 min post surgery. After this time the responses were smaller than controls. B, this histogram shows the mean amplitude of EMG responses (as a percentage of control) in 12 studies. Responses were potentiated by the surgery for 30 min and subsequently depressed.
For a variable period of time after the surgery the EMG responses were greatly facilitated to 148% of control, (43.9 ± 1.9 units, n = 11). Subsequently, in all animals, EMG responses were reduced to 32% of control, (9.5 ± 3.0 units, n = 11, P < 0.001). The period of facilitation ranged from 20 to 45 min (28.7 ± 8.2 min). These data indicate a complex effect of surgery on nociceptive responses resulting in a marked and sustained inhibition.
Microinjection of tetracaine or GABA into the RVM
The effects of microinjection of tetracaine or GABA into the RVM are summarized in Fig. 3. During all these tests, the dorsal roots were stimulated at C fibre intensity and it is clear that microinjections in, or very close to, GiA gave the greatest prolongation of the after-discharge on multireceptive spinal neurones. Following tetracaine microinjection, ten of the sixteen cells studied showed an increase in the response to C fibre stimulation. The mean increase in amplitude was to 129.5% of control, from 32.6 ± 3.1 to 42.2 ± 8.5 spikes s−1 (P < 0.002, n = 10) and the mean increase in duration was to 565% of control, from 9.3 ± 1.8 to 52.5 ± 18.4 s (P < 0.005, n = 10). In two cells there was a decrease in amplitude, two were inhibited completely and in two there was no effect. GABA (200 mM) was injected into the GiA while recording from six multireceptive neurones. The mean increase in amplitude was to 124.2% of control, from 33.9 ± 5.4 to 42.2 ± 4.8 spikes s−1 (P < 0.003, n = 6). The duration of the response was increased to 485.5% of control, from 9.6 ± 1.1 to 47.1 ± 7.0 s (P < 0.002, n = 6). Figure 2A illustrates an example of this effect of GABA on the wind up of a multireceptive neurone. The effect of microinjection of either GABA and tetracaine was 46% of the effects of transection of the ipsilateral DLF.
The response of GiA cells to noxious stimuli
GiA cells were recorded in twenty-four animals subjected to minimal surgery. No lumbar or cervical laminectomy and no cannulations were performed. A small scalp incision was made and a 1 mm burr used to trepan the skull. The dura mater was reflected. Under these conditions thirty-nine of the forty-three GiA cells (91%) recorded were spontaneously active. The mean firing rate was 9.3 ± 1.2 spikes s−1. Thirty-six of the forty-three GiA cells studied were excited and seven were inhibited by noxious stimuli (a block heated to 50°C applied to the depilated skin for 10 s). None of the GiA cells responded to brush/touch cutaneous stimuli. All the cells had a diffuse bilateral receptive field, responding to a noxious stimulus applied in a random sequence to any limb or any area of the back or flank. Responses from all areas studied were not significantly different from each other. The mean excitation was 38.4 ± 2.5 spikes s−1 (Fig. 5).
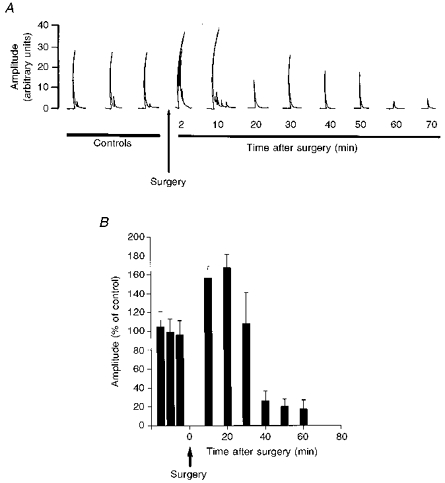
Figure 5. GiA cells have diffuse receptive fields.
All cells in the GiA were unresponsive to brush/touch stimulation but most were excited by noxious stimulation (50 °C), applied to any part of the body. A, four PSTHs (bin size, 100 ms) of the response of a cell in the left GiA. Each PSTH shows the response of the cell to noxious stimulation applied to the skin area indicated by the arrows for the period shown by the open bar. B, coronal section illustrating the position of extracellular recordings in the RVM (−1.5 to −2.5 mm AP). •, cells that were excited by noxious stimulation; ▪, cells that were inhibited; ○, the location of cells that were unresponsive to noxious stimulation. Abbreviations are as listed in the legend to Fig. 3, except LPGi, lateral paragigantocellular nucleus.
When a GiA cell was isolated that gave constant excitatory responses, cervical and lumbar laminectomy was performed in each animal (Fig. 6). GiA cells were very strongly but variably excited during the surgery. This was followed within 1–2 min by a reduction of spike activity (from 9.3 ± 1.2 to 3.8 ± 1.5 spikes s−1, to 35%, P < 0.001). After 15–60 min (34.2 ± 9.1 min), the cells resumed activity and the firing frequency progressively rose to a sustained frequency of 29.6 ± 5.1 spikes s−1 (318% of presurgery control, P < 0.01, see Fig. 6C).
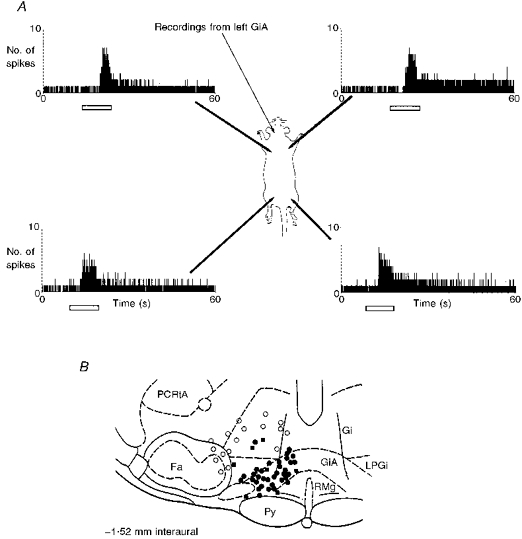
Figure 6. The effects of surgery on the baseline firing rate of GiA cells and their response to noxious stimuli.
A, continuous polygraph record of the activity of a GiA cell before, during and after cervical laminectomy. The first 15 min of the trace shows the firing frequency of the cell after the surgery necessary to record its activity but before the major surgery necessary to expose the spinal cord. The laminectomy was done during the time indicated by the open bar, causing a massive and intermittent excitation of the cell. After the surgery the cell became virtually silent for 15 min, although occasional action potentials confirmed that the recording location had not been lost. Subsequently, the cell recommenced spontaneous activity, eventually settling to a steady firing level almost double the original frequency. B, two polygraph records of another cell recorded before and 40 min after completion of the cervical laminectomy. The records show the increased baseline firing rate caused by major surgery and the change in response to noxious cutaneous stimuli (50 °C). The peak firing frequency evoked by the stimulus has changed very little but the response was largely occluded by the increased baseline firing rate. C, histogram of the mean baseline firing rate of a population of GiA cells and the effects of surgery with time (n = 8). The first three columns show the mean firing rate (+s.e.m.) during 5 min periods before surgery began. The next column shows the mean firing rate during the surgery. This took between 25 and 35 min and was completed at time 0 on the X-axis. For 20 min after the surgery GiA cell firing was reduced but subsequently increased to above control.
During the depressed period after surgery, GiA cells did not respond to noxious stimuli. During the subsequent period, when baseline firing was elevated, responses to noxious stimuli were of a similar amplitude to controls, (35.2 ± 5.1 spikes s−1; 91% of controls, not significant, see Fig. 6B). The percentage change from baseline, however, was reduced compared with presurgery controls (presurgery, 413%; postsurgery, 120%, P < 0.01).
The spinal tract mediating the responses of GiA cells
Although the responses of GiA cells were smaller following cervical laminectomy, it was possible to study the effects of tractotomy on these responses. Lesions of the ipsilateral DLF did not significantly alter GiA responses to noxious heat. Lesions of the contralateral DLF abolished responses of GiA cells. Following exposure of the spinal cord, noxious stimuli produced a peak firing rate of 120% of the baseline firing rate (35.2 ± 5.1 spikes s−1). Following DLF lesion, contralateral to the site of noxious stimulation, this response was reduced to 102% of the baseline firing rate (30.1 ± 3.6 spikes s−1, not significantly different from baseline) (Fig. 7). Lesions of other tracts including the ipsilateral and contralateral anterolateral funiculus or dorsal columns did not significantly alter responses of GiA cells. After DLF transection, hemisection of the cord ipsilaterally or contralaterally did not further alter responses of GiA cells.
Figure 7. The contralateral DLF is the ascending limb of the dynamically activated, supraspinal inhibition.
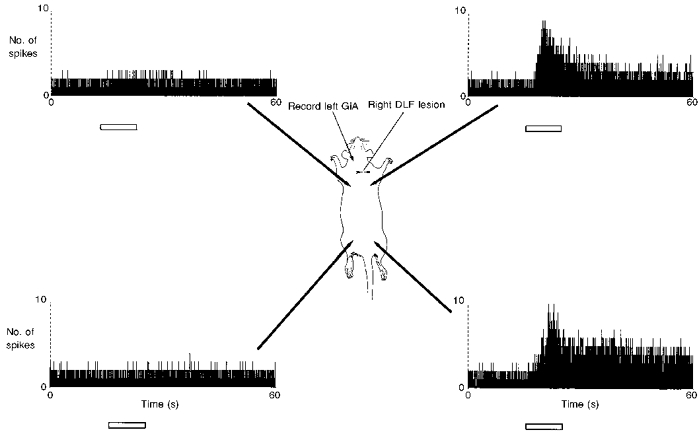
The details of this figure are very similar to those for Fig. 5 except that in this rat a cervical laminectomy had been performed. One hour had elapsed to stabilize the background firing rate of the left side GiA cell, and a right side transection of the DLF had been made. The laminectomy enhanced the background firing of the cell (compare with Fig. 5). Responses to stimulation of left and right sides of the body were of similar amplitude. The right DLF transection at cervical level abolished all responses to noxious stimulation (50 °C) on the left side but did not alter responses to right side stimulation. This suggests that the axons mediating the nociceptive responses of GiA cells run in the DLF, contralateral to the cutaneous stimulation.
DISCUSSION
The first experiments studied the time course of the responses of multireceptive dorsal horn cells to noxious stimuli and to afferent C fibre stimulation. Transection of the ipsilateral DLF increased the baseline firing rate of spinal cells to 150%, enhanced the peak response to stimulation to 142% and disproportionately prolonged the after-discharge of the cells to 648% of control values. Acute transection of the ipsilateral DLF is known to remove much of the tonic inhibition of dorsal horn neurones (Basbaum & Fields, 1984b) and would be expected also to remove any dynamic modulation of this inhibition by noxious stimuli (Villaneuva, Chitour & Le Bars, 1986). The dynamically activated component of the inhibition would not affect baseline firing rates and could only influence the later parts of the nociceptive response of the spinal cell. It is possible therefore that the disproportionate prolongation of the after-discharge reflects the removal of inhibition that is initiated by the noxious stimulus itself. Most reports of the effects of DLF transection on the responses of dorsal horn cells were discussed in terms of removal of tonic inhibition (Hall et al. 1982; Foong & Duggan, 1986; Sandkuhler et al. 1995) but the disproportionate prolongation of responses has been illustrated by others (for example Cervero et al. 1985).
In the second series of experiments, a principal source of the DLF-mediated inhibition was shown to be in the RVM, principally in the nucleus reticularis gigantocellularis pars alpha (GiA). The microinjection of tetracaine or GABA had qualitatively similar effects on the response of multireceptive spinal neurones to those of DLF section. The after-discharge component of the response to activation of nociceptive afferents was again disproportionately prolonged by disruption of GiA cell function. Quantitatively, the effects of microinjection were smaller than those of DLF section, presumably reflecting the smaller number of cells disrupted by the microinjection.
With few exceptions (Herrero & Headley, 1995), all observations of the responses of multireceptive dorsal horn neurones must be preceded by extensive surgical exposure of the spinal cord and the massively noxious stimulation that this involves may well alter the balance between tonic and dynamically activated inhibition. The next experiments were therefore conducted on anaesthetized rats initially subjected to minimal surgery. EMG recording of the flexor reflex to noxious heating of the footpad gave constant nociceptive responses. Surgery (cervical and lumbar laminectomy) almost doubled the amplitude of EMG responses for 29 min and subsequently more than halved the response amplitudes for the remaining hours of the experiment. These effects demonstrate very clearly that the surgery necessary for the recording of spinal neurones, or the selective lesioning of funiculi at cervical levels, has profound and rather complex effects on nociceptive responses. This has been reported previously by Clarke & Matthews (1990) who observed that the preparation of an animal for stereotaxic recording can cause a severe and long lasting depression in the excitability of neurones in the trigeminal sensory nuclei and an increase in the threshold of the jaw-opening reflex. Surgical procedures are, of course, a source of strong diffuse noxious stimuli and would be expected therefore to initiate the same descending inhibitions reported for DNIC (Le Bars et al. 1979). The initial period of enhanced responsiveness was not expected, however, and no explanation can presently be offered.
In the third experiment, recordings of RVM cells were made in animals that again were subjected to the minimum surgery necessary. The baseline firing rate and the response of these cells to a noxious heat stimulus were recorded and, subsequently, the same surgical procedures instituted as in the previous experiment. Noxious stimuli and surgical incisions excited the majority of RVM cells but, following the surgery, these cells were markedly depressed for 34 min and did not respond to noxious stimuli. After this period the cells recovered their spontaneous activity and discharged at a much enhanced rate for the rest of the experiment. This complex effect of surgery on RVM cells correlates well with the effects of surgery on nociceptive EMG responses. When RVM cells are silent, nociceptive EMG responses were enhanced and when RVM cells displayed increased tonic firing, the EMG responses were much reduced.
Bouhassira et al. (1993) and Bouhassira, Chitour, Villaneuva & LeBars (1995a) report that they studied the nociceptive responses of spinal neurones more than 1 h after surgery was completed. At this time, therefore, it may be expected that cells in the RVM have a much enhanced discharge rate and spinal dorsal horn neurones are subjected to tonic inhibition from the RVM. Under these circumstances, and as the present data demonstrate, the response of RVM cells to noxious stimuli is significantly occluded by their high and sustained firing rate and dynamically activated inhibition would be much less apparent than in animals not subjected to surgery. Thus the conclusion by Bouhassira et al. (1993, 1995a) that inhibition from the RVM is tonic may be true only for animals subjected to considerable preparatory surgery.
Although the dynamic response of RVM cells to noxious stimuli is largely occluded by the laminectomy necessary for access to the spinal cord, the majority of RVM cells continued to show a smaller but repeatable response to noxious stimuli. We investigated the ascending fibres responsible for this activation by systematic transections of the cervical cord. Only transection of the contralateral DLF reliably effected significant reductions in the nociceptive response of RVM cells. We therefore conclude that this tract contains the majority of the fibres that activate RVM cells.
McMahon & Wall (1988) demonstrated that many nociceptive spinal lamina I cells project to the brain by the contralateral DLF and concluded from their experiment that lamina I cells might activate brainstem circuits which in turn influence deep dorsal horn cells. Rees & Roberts (1993) drew the same conclusion and produced a schematic diagram involving the anterior pretectal nucleus in which lamina I cells, projecting via the contralateral DLF, activate descending inhibition of deep multireceptive spinal cells via the ipsilateral DLF. The present data fully support these proposals although the involvement of supraspinal structures other than the RVM has not been studied.
It may be concluded from these observations that some cells in the RVM are excited by a nociceptive input from the contralateral DLF. These cells have diffuse receptive fields and a baseline firing rate that is increased by the amount of surgery done to the animal. It is not possible to determine from these or other reported experiments if the RVM cells are silent in the absence of surgery or noxious stimulation but the possibility should be considered that the major source of the frequently reported tonic inhibition descending in the ipsilateral DLF (Hall et al. 1982; Foong & Duggan, 1986; Sandkuhler et al. 1995) is an artefactual consequence of the surgery necessary to record or disrupt the inhibition. In the absence of surgery, it is probable that the early phase of the nociceptive response of a multireceptive spinal neurone would be more vigorous than that seen after surgery but would be rapidly truncated by the activation of RVM cells and the onset of supraspinal inhibition.
Acknowledgments
The support of The Wellcome Trust and the British Brain and Spine Foundation is gratefully acknowledged by the authors. We also thank Mr T. Gould, Mr C. J. Stent, Mr G. Pitt and Mr R. M. Jones for expert technical help during the course of this work.
References
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and the rat: further studies on the anatomy of pain modulation. Journal of Comparative Neurology. 1979;187:513–532. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms. In: Wall PD, Melzack R, editors. Textbook of Pain. Edinburgh: Churchill Livingstone; 1984a. pp. 142–152. [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control system: brainstem spinal pathways and endorphin circuitry. Annual Review of Neuroscience. 1984b;7:309–398. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Bing Z, Le Bars D. Studies of brain structures involved in diffuse noxious inhibitory controls in the rat: the rostral ventromedial medulla. Journal of Physiology. 1993;463:667–687. doi: 10.1113/jphysiol.1993.sp019616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhassira D, Chitour D, Villaneuva L, Le Bars D. The spinal transmission of nociceptive information: modulation by the caudal medulla. Neuroscience. 1995a;69:931–938. doi: 10.1016/0306-4522(95)00269-o. [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Gall O, Chitour D, Le Bars D. Dorsal horn convergent neurones: negative feedback triggered by spatial summation of nociceptive afferents. Pain. 1995b;62:195–200. doi: 10.1016/0304-3959(94)00270-O. [DOI] [PubMed] [Google Scholar]
- Cervero F, Lumb BM, Tattershall JEH. Supraspinal loops that mediate visceral inputs to thoracic spinal cord neurones in the cat: involvement of descending pathways from raphe and reticular formation. Neuroscience Letters. 1985;56:189–194. doi: 10.1016/0304-3940(85)90127-2. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Matthews B. The thresholds of the jaw-opening reflex and trigeminal brainstem neurones to tooth-pulp stimulation in acutely and chronically prepared cats. Neuroscience. 1990;36:105–114. doi: 10.1016/0306-4522(90)90354-7. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Le Bars D, Besson J-M. An involvement of nucleus raphe magnus in diffuse noxious inhibitory controls in the rat. Neuroscience Letters. 1980;(suppl. 5):S375. [Google Scholar]
- Foong FW, Duggan AW. Brain-stem areas tonically inhibiting dorsal horn neurones: studies with microinjection of the GABA analogue piperidine-4-sulphonic acid. Pain. 1986;27:361–371. doi: 10.1016/0304-3959(86)90160-0. 10.1016/0304-3959(86)90160-0. [DOI] [PubMed] [Google Scholar]
- Hall JG, Duggan AW, Moreton CR, Johnson SM. The location of brainstem neurones tonically inhibiting dorsal horn neurones of the cat. Brain Research. 1982;244:215–222. doi: 10.1016/0006-8993(82)90080-4. 10.1016/0006-8993(82)90080-4. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Headley PM. The dominant class of somatosensory neurones recorded in the spinal dorsal horn of awake sheep has wide dynamic range properties. Pain. 1995;61:133–138. doi: 10.1016/0304-3959(94)00152-5. 10.1016/0304-3959(94)00152-5. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, Besson J-M. Diffuse noxious inhibitory controls (DNIC): lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6:305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wall PD. Descending excitation and inhibition of spinal cord lamina I projection neurons. Journal of Neurophysiology. 1988;59:1204–1219. doi: 10.1152/jn.1988.59.4.1204. [DOI] [PubMed] [Google Scholar]
- Oliveras J-L, Martin G, Montagne J, Vos B. Single unit activity at ventromedial medulla level in the awake, freely moving rat: effects of noxious heat and light tactile stimuli onto convergent neurons. Brain Research. 1990;506:19–30. doi: 10.1016/0006-8993(90)91194-l. 10.1016/0006-8993(90)91194-L. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. London: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Rees H, Roberts MHT. Antinociceptive effects of dorsal column stimulation in the rat: involvement of the anterior pretectal nucleus. Journal of Physiology. 1989;417:375–388. doi: 10.1113/jphysiol.1989.sp017807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H, Roberts MHT. The anterior pretectal nucleus: a proposed role in sensory processing. Pain. 1993;53:121–135. doi: 10.1016/0304-3959(93)90072-W. 10.1016/0304-3959(93)90072-W. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. Journal of Neurophysiology. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Eblen-Zajjur A, Fu Q-G, Forster C. Differential effects of spinalization on discharge patterns and discharge rates of simultaneously recorded nociceptive and non-nociceptive spinal dorsal horn neurones. Pain. 1995;60:55–65. doi: 10.1016/0304-3959(94)00088-V. 10.1016/0304-3959(94)00088-V. [DOI] [PubMed] [Google Scholar]
- Villaneuva L, Chitour D, Le Bars D. Involvement of the dorsolateral funiculus in the descending spinal projections responsible for diffuse noxious inhibitory controls in the rat. Journal of Neurophysiology. 1986;56:1185–1195. doi: 10.1152/jn.1986.56.4.1185. [DOI] [PubMed] [Google Scholar]
- Wolstencroft JH, West DC. Functional characterisation of raphe-spinal systems. In: Sjolund BH, Bjorklund A, editors. Brain Stem Control of Spinal Mechanisms. Amsterdam: Elsevier; 1982. pp. 359–380. [Google Scholar]


