Abstract
With a breakdown of the vascular-CNS barrier, serum enters the nervous system. Although this is a frequent pathophysiological event, knowledge of the effects of serum on the function of the nervous system is limited. In this study, we examined the effects of serum on the activity of ion channels in Müller cells: the principal glia of the retina.
Freshly dissociated Müller cells from the bovine and human retina were studied with the perforated-patch configuration of the patch clamp technique. In other experiments, electroretinograms (ERGs) were recorded from isolated rat retinas.
Perforated-patch recordings revealed that serum induced a calcium-permeable, non-specific cation (NSC) current. Approximately 40 s after induction of this current, an outwardly rectifying K+ current was also detected. Sensitivity to charybdotoxin and margatoxin indicated that this K+ current was due to the activation of KV1.3 channels. This increase in the KV1.3 current was dependent on extracellular calcium.
The NSC and KV1.3 currents were activated by serum in 100% and 95% of the sampled Müller cells, respectively. Also, in a minority (21%) of the cells, the inwardly rectifying K+ current was inhibited slightly. These changes in ion channel activity were associated with depolarization of the Müller cells.
We hypothesized that activation of NSC channels would reduce the siphoning of K+ via the Müller cells. Consistent with this idea, ERGs from isolated retinas showed serum-induced reductions in the slow PIII component, which is generated by Müller cells responding to light-evoked changes in the extracellular K+ concentration.
Lysophosphatidic acid (LPA), a component of serum, had effects on Müller cells that were qualitatively similar to those induced by serum.
Our observations demonstrate that exposure to serum alters the activity of multiple types of ion channels in Müller glial cells of the mammalian retina. When there is a breakdown of the blood-retina barrier, LPA may be one of the serum-derived molecules which regulates the physiology of Müller cells.
When there is a breakdown of the barrier between the circulatory and nervous systems, the function of the CNS is compromised. Whilst gross tissue swelling and distortion due to an influx of fluid from the vascular compartment can cause damage, knowledge of more subtle mechanisms by which a breakdown of this barrier alters function is limited. We hypothesize that serum-derived molecules enter the nervous system, induce receptor-mediated changes in cell function and thereby alter the activity of neural circuits.
In this study, we examined the effect of serum on the activity of ion channels in glial cells. One reason the glia are of interest is that serum leaking from the vascular system would almost certainly contact these cells, since they ensheath the blood vessels of the nervous system. We focused our study on the activity of ion channels, since they are involved in important glial functions such as the maintenance of K+ homeostasis (Newman & Reichenbach, 1996). Glial K+ channels are pathways for the redistribution of excess potassium. This redistribution via glial cells serves to limit wide swings in [K+]o which can alter neuronal excitability.
To identify and characterize the effects of serum on glial channels, we chose to study Müller cells, the predominant glia of the retina. A reason for selecting these glial cells is that their role in K+ redistribution has been particularly well studied (Newman, 1995). By a specialized mechanism of K+ spatial buffering, termed K+ siphoning (Newman, Frambach & Odette, 1994), K+ enters a Müller cell where [K+]o is high and exits where [K+]o is lower. The extensive information concerning K+ siphoning via Müller cells facilitates attempts to relate changes in ion channel activity to the function of these cells in regulating [K+]o. Another motivation for studying retinal cells is that a breakdown of the blood-retinal barrier is a frequently occurring, sight-threatening pathophysiological process (Gass, 1997).
Based on perforated-patch recordings from fresh bovine and human Müller cells, we now report that serum causes these glial cells to depolarize as a non-specific cation current and an outwardly rectifying K+ current are activated. In addition, the electroretinograms (ERGs) from isolated retinas exposed to serum showed changes consistent with the idea that the serum-induced changes in the activity of ion channels reduces the role of Müller cells in the redistribution of K+. We also found that lysophosphatidic acid (LPA), a component of serum, induces currents similar to those activated by whole serum. Thus, when there is a breakdown in the blood-retinal barrier, this glycerophospholipid may be one of the serum-derived molecules that regulates ion channel activity in Müller cells.
METHODS
Fresh Müller cells
Freshly dissociated human and bovine Müller cells were prepared as detailed previously (Kusaka, Dabin, Barnstable & Puro, 1996). In brief, approximately 0.5 cm × 0.5 cm pieces of retina were incubated in Earle's balanced salt solution supplemented with 0.5 mM EDTA, 1.5 mM CaCl2, 1 mM MgSO4, 20 mM glucose, 26 mM sodium bicarbonate, 15 u papain (Worthington Biochemicals Co.), 0.04% DNase, 2 mM cysteine and 12% chicken serum for 40 min at 30°C, whilst 95% oxygen-5% CO2 was bubbled through to maintain pH and oxygenation. The piece of retina was then washed with the appropriate bathing solution, drawn up into a glass pipette and gently ejected back into a microcentrifuge tube. A suspension of cells (∼0.1 ml) was placed in a recording chamber and allowed to settle ∼15 min prior to the addition of bathing solution to the chamber. Cells were examined at × 400 magnification with an inverted microscope equipped with phase-contrast optics. Müller cells were identified by their characteristic morphology (Puro, Yuan & Sucher, 1996). Experiments were performed within 3 h of cell dissociation.
Patch clamp recordings
Perforated-patch recordings from fresh Müller cells were made at room temperature (22–24°C). A gravity-fed system with multiple reservoirs was used to continuously perfuse (1–2 ml min−1) the recording chamber (0.5 ml volume) with various solutions. Whole-cell currents were monitored using the perforated-patch configuration of the patch clamp technique. Unless noted otherwise, the bathing solution consisted of (mM): 133 NaCl, 10 KCl, 1.8 CaCl2, 0.8 MgCl2, 10 Na-Hepes and 20 glucose, at pH 7.4 with the osmolarity adjusted to 305–310 mosmol l−1. Patch pipettes were pulled from Corning No. 7052 glass tubing (Gardner Glass Co., Claremont, CA, USA) using a multistage programmable puller (Sutter Instruments), coated with Sylgard No. 184 (Dow Corning) to within 100 μm of their tips and heat polished to tip diameters of 2–3 μm. A pipette tip was filled to approximately 400 μm from the tip by applying negative pressure to the back end of the pipette while briefly dipping the tip into the pipette solution, which, unless stated otherwise, consisted of (mM): 50 KCl, 65 K2SO4, 6 MgCl2 and 10 K-Hepes, at pH 7.4 with the osmolarity adjusted to ∼285 mosmol l−1. The remainder of the pipette was then backfilled with this solution supplemented with freshly mixed amphotericin B (240 μg ml−1) and nystatin (240 μg ml−1). The resistances of the pipettes used were 2–5 MΩ when tested in the bathing solution.
The pipettes were mounted in the holder of a Dagan 3900 patch clamp amplifier (Dagan Corp., Minneapolis, MN, USA) and sealed to the cell bodies of Müller cells. Seals generally formed over a period of 1 to 30 s and reached resistances of greater than 1 GΩ. As amphotericin-nystatin perforated the patch, the access resistance to the cell usually decreased to less than 20 MΩ within 20 min for the Müller cells analysed. Recordings were only used when the ratio of cell membrane to series resistance reached more than 10. This ratio was monitored periodically; if the ratio decreased to below 10, the analysis of the cell was terminated. We did not correct for series resistance, but the error due to the voltage drop across the patch pipette was always less than 10% of the applied voltage.
Currents were evoked by a voltage step protocol or by ramping membrane voltage (66 mV s−1) from negative to positive membrane potentials for the construction of continuous current-voltage plots. Currents were filtered at 1 kHz and digitally sampled (at 400 μs for steps and 1 ms for ramps) using a Lab Master DMA acquisition system (Axon Instruments), an IBM-compatible microcomputer (Gateway 2000, North Sioux City, SD, USA) and pCLAMP software (version 6, Axon Instruments), which also controlled voltage protocols and helped with data analysis. A scientific plotting program (ORIGIN version 4.1, MicroCal, Northhampton, MA, USA) was used for data exported from pCLAMP. After data collection, the recorded membrane potentials were corrected for liquid junction potentials, which were calculated using a computer program (Barry, 1993). Time courses for serum-induced currents (Figs 5 and 7) were determined by applying a voltage ramp protocol at 10 s intervals, plotting the current-voltage relationships and measuring the current amplitudes at the potentials of interest (see figure legends for details).
Figure 5. Time course for the activation of serum-induced currents in a bovine Müller cell.

A, plots of the percentage of the maximal inward or outward currents versus time. The inward current was measured at −74 mV, which is the equilibrium potential for potassium. The outward current was measured at the approximate reversal potential for the non-specific cation current, −15 mV. The inward current increased from a minimum of −6 pA to a maximum of −54 pA; the comparable values for the outward current were 190 pA and 591 pA, respectively. Currents were measured from I-V plots generated by voltage ramps initiated at 10 s intervals. B, I-V relationship prior to exposure to 10% serum (Control), 40 s after the onset of serum exposure (Initial effect) and 110 s later (Maximal effect). C, plot of the difference between the I-V curves during the control period and 40 s after the onset of the exposure to serum. D, plot of the difference between the I-V relationship during the control period and 150 s after the onset of exposure to serum. With exposure of a Müller cell to serum, a non-specific cation current is initially induced; subsequently, an outwardly rectifying current is activated.
Figure 7. Inward and outward currents induced in a bovine Müller cell during a 10 min exposure to serum.

A, plot of the change in inward current at −74 mV, which is the equilibrium potential of K+. B, plot of the change in outward current at −15 mV: the approximate reversal potential of the non-specific cation current. The current amplitudes were measured as in Fig. 5A. The induced inward and outward currents can remain activated during a 10 min exposure to serum.
Electroretinography
Albino rats were dark-adapted for 12 h and anaesthetized with i.p. injections of sodium pentobarbitone (30 mg kg−1). The animals were decapitated; both eyes were enucleated, and the retinas were removed under infrared light. The isolated retinas were placed in a bath of Ringer solution that had been oxygenated by bubbled 95% O2-5% CO2 and was at room temperature. The composition of the Ringer solution was (mM): 110 NaCl, 5 KCl, 0.8 Na2HPO4, 0.1 NaH2PO4, 30 NaHCO3, 1 MgSO4, 1.8 CaCl, 0.25 sodium glutamate and 22 glucose. A portion of the retina (approximately 0.3 cm × 0.35 cm) was placed photoreceptor-side up on a Millipore filter (type SC; Millipore) which had been perforated several times with ∼1 mm holes. The filter and retina were then positioned between two pieces of nylon mesh in a perfusion chamber. The retina was perfused at a flow rate of 3 ml min−1 with the oxygenated Ringer solution at 36.6 ± 0.5°C and pH 7.50 ± 0.05. When the perfusate was supplemented with 10% (v/v) bovine serum, the osmolarity of the perfusate changed by less than 1%. An antifoaming agent (∼100 p.p.m. Antifoam A) was added to the perfusion reservoirs containing the Ringer solution and the serum-containing perfusate.
Recordings were made using a micropipette positioned at the tips of the outer segments and referenced to a Ag-AgCl electrode (model E202; IVM, Healdsburg, CA, USA) located under the retina. The borosilicate (Corning No. 7740; Sutter Instruments) micropipettes were fabricated using a vertical puller (David Kopf Instruments, Tujunga, CA, USA) and filled with Ringer solution. The micropipettes had tip diameters of 5–15 μm and resistances of 5–25 MΩ. A micropipette entered the chamber from above and made an angle of approximately 70 deg with respect to the retina. Transretinal electroretinograms were evoked by electronic flashes (∼0.5 ms duration) of monochromatic light (500 nm). Potentials from the isolated retina were recorded with a high gain amplifier (CyberAmp, Axon Instruments), filtered at 400 Hz, digitized (A/D converter model NB-MIO-16X; National Instruments, Austin, TX, USA), then stored and subsequently analysed using a minicomputer (Apple) and LabView software (version 4.0, National Instruments).
Dialysis
Cellulose membrane tubings (Spectrum Medical Industries, Laguna Hills, CA, USA) with molecular weight cut-offs of 1000 or 6000–8000 were used for dialysis. The solution (4–8 ml) to be dialysed was placed in the dialysis tubing, which was tightly clipped at both ends and carefully checked for leaks. The tube was placed in a beaker containing ∼800 ml of Dulbecco's phosphate buffer solution (PBS), which was continuously stirred and totally replaced three times during the first 8 h. Dialysis was performed for ∼24 h at 4°C.
Chemicals
Charybdotoxin and pertussis toxin were purchased from Research Biochemicals; margatoxin and iberiotoxin were from Alomone Labs (Jerusalem, Israel). PBS and chicken serum were obtained from Gibco. Human serum was donated by one of the authors (D. G. P.). Bovine serum, 1-oleoyl-lysophosphatidic acid and other chemicals were from Sigma unless otherwise noted.
Ocular tissue
Sprague-Dawley rats were bred in colonies at the University of Michigan and maintained under conditions of reduced lighting. Animal use conformed with the guidelines of the Society for Neuroscience and Association for Research in Vision and Ophthalmology. Bovine eyes were obtained soon after near-instantaneous death induced at a local licensed abattoir. Donor adult human eyes were supplied within 24 h post mortem by the Midwest Eye Bank and Transplantation Centre (Ann Arbor, MI, USA) or the National Disease Research Interchange (Philadelphia, PA, USA), both of which obtained proper consent from relatives. This study protocol was approved by the Institutional Review Board of the University of Michigan. All tissue was obtained and used in accordance with applicable laws and regulations.
Statistics
Unless otherwise stated, data are given as means ±s.e.m., and probability was evaluated using Fisher's exact test.
RESULTS
Serum-induced changes in the I-V relationship
We hypothesized that serum, which enters the retina with a breakdown of the blood-retinal barrier, may alter the physiology of the Müller cells. To test this idea, we obtained perforated-patch recordings from Müller cells freshly dissociated from the bovine retina. As illustrated in Fig. 1, exposure of Müller cells to 10% bovine serum was associated with an increase in an inward current at hyperpolarized potentials, an increase in an outwardly rectifying current at depolarized potentials and a depolarization of the resting membrane potential. These effects of serum were reversible.
Figure 1. Effect of serum on currents in a freshly dissociated bovine Müller cell.

Currents were monitored using the perforated-patch recording technique. A, currents evoked before (Control), during (Serum) and after (Recovery) exposure to 10% bovine serum. The clamp protocol is shown below (Stimuli). B, I-V relationship before, during and after exposure to serum. C, I-V relationship for the serum-induced current, which was calculated by subtracting currents recorded under control conditions from those recorded in the presence of serum. The inset shows the serum-induced currents obtained by digital subtraction. Current amplitudes used for the I-V plots were the means from 200–400 ms after the onset of a voltage step. Serum induces an outwardly rectifying current at depolarized potentials and an inward current at hyperpolarized voltages.
In a series of thirty-two Müller cells studied under similar recording conditions, serum induced an inward current in each of the sampled cells. In thirty of the thirty-two cells, this increase in inward current was associated with an increase of an outwardly rectifying current at depolarized membrane potentials. With exposure to serum, the membrane potential decreased by a mean of 7.5 ± 0.7 mV from −72 to −65 mV.
These serum-induced changes in the physiology of Müller cells are not limited to one species. A similar induction of an inward current at hyperpolarized potentials and an outward current at depolarized potentials also was observed in freshly dissociated human Müller cells (n = 3) that were exposed to human serum (Fig. 2). In addition, bovine serum activated an inward current and an outwardly rectifying current in sampled human Müller cells (n = 3). In other experiments, we found that exposure to chicken serum induced similar currents in bovine Müller cells (n = 2; data not shown).
Figure 2. Effect of human serum on Müller cells freshly dissociated from the human retina.

A, I-V relationship in the absence (Control) and presence of 10% serum. Currents were monitored by perforated-patch recording and evoked by ramping membrane voltage. B, plot of the difference between the I-V curves shown in A. Serum induces similar currents in human and bovine Müller cells.
The outwardly rectifying current induced by serum
To help characterize the currents induced by serum, we tested the effects of various peptidyl scorpion toxins known to block outwardly rectifying K+ channels. Figure 3A illustrates the effect of charybdotoxin (ChTX), which blocks large-conductance calcium-activated K+ channels (maxi-KCa) and various delayed K+ rectifiers (Hille, 1992). Comparison of the I-V relationship before and during exposure of bovine Müller cells to 10% bovine serum (Fig. 3A, upper panel) confirmed that serum induced an increase in outward current at depolarized membrane potentials and an increase in inward current at more hyperpolarized voltages; the membrane potential depolarized. After the addition of ChTX (100 nM) to the bathing solution (in the absence of serum), the basal outward current was reduced (Fig. 3A, upper panel) demonstrating that a component of the outward current in Müller cells is sensitive to ChTX. Of particular interest to this study, exposure to serum in the presence of ChTX did not induce an increase in outward current, but did induce an inward current (Fig. 3A, upper panel). The lower panel of Fig. 3A shows the I-V relationship of the ChTX-sensitive component of the serum-induced current. This ChTX-sensitive current had a threshold of activation of approximately −40 mV and was outwardly rectifying. Similar effects of ChTX were observed in four Müller cells. We conclude that ChTX blocks the outwardly rectifying current induced by serum.
Figure 3. Effects of charybdotoxin, iberiotoxin and margatoxin on Müller cell currents induced by serum.

A, top panel, I-V relationship under control conditions and in the presence of either 10% bovine serum, 100 nM charybdotoxin (ChTX) or serum plus ChTX. Bottom panel, plot of the difference between the I-V curves of the serum-induced currents in the absence and presence of ChTX. B, similar to A, with 50 nM iberiotoxin (IbTX) in place of ChTX. C, similar to A and B, with 1 nM margatoxin (MTX) in place of ChTX or IbTX. Freshly dissociated bovine Müller cells were monitored with perforated-patch recordings. The sensitivity of the serum-induced outwardly rectifying current to charybdotoxin and margatoxin, but not iberiotoxin, is consistent with the activation of KV1.3 channels.
In the next series of experiments, we examined the effect of toxins that selectively block a specific type of K+ channel. Iberiotoxin (IbTX, 50 nM), a specific blocker of maxi-KCa channels (Candia, Garcia & LaTorre, 1992), minimally affected the I-V relationships of Müller cells under control conditions or in the presence of serum (Fig. 3B). No effect on the serum-induced current was observed in any of the five Müller cells tested with IbTX.
In contrast, when Müller cells were exposed to margatoxin (MTX, 1 nM), which selectively blocks KV1.3 channels (Garcia-Calvo et al. 1993), the serum-induced outward current was markedly less than in the absence of this toxin (Fig. 3C). A similar effect was seen in three Müller cells. These observations suggest that the outwardly rectifying current induced by serum is mainly due to the activation of KV1.3 channels.
The inward current induced by serum
Although activation of KV1.3 channels plays a significant role in the serum-induced increase in the outwardly rectifying current, another type of channel must be responsible for the increase in inward current detected at hyperpolarized potentials (Figs 1C and 2B). To characterize this current, we examined the effect of serum on Müller cells which had their K+ channels blocked by Cs+ and Ba2+ in the bathing solution and Cs+ in the pipette solution (Fig. 4). Under these recording conditions, serum induced a current which had minimal rectification (Fig. 4B).
Figure 4. Effect of serum on the Müller cell currents recorded under K+ channel-blocking conditions.

A, I-V relationship in the absence and presence of 10% bovine serum. B, plot of the difference between the I-V curves shown in A. Recordings from fresh bovine Müller cells were made in a bathing solution containing (mM): 30 CsCl, 2 CaCl2, 0.8 MgCl2, 1.8 BaCl2, 10 Cs-Hepes, 20 glucose and 195 mannitol (pH 7.4; 309 mosmol l−1). The pipette solution consisted of (mM): 135 CsCl, 6 MgCl2 and 10 Cs-Hepes (pH 7.4; 284 mosmol l−1). With K+ channels blocked, serum induces a relatively linear current which has a reversal potential not far from 0 mV.
To begin to assess the ionic selectivity of this conductance, the change in reversal potential was determined with differing concentrations of external K+, Na+, Ca2+ and Cl− (Table 1). The mean reversal potential (Vrev) was −11 mV when the bathing solution contained (mM): 30 CsCl, 0 NaCl, 0 KCl, 2 CaCl2, 0.8 MgCl2, 1.8 BaCl2, 10 Cs-Hepes, 20 glucose and 195 mannitol, and the pipette (perforated-patch) solution consisted of (mM): 135 CsCl, 6 MgCl2 and 10 Cs-Hepes. Addition of 30 mM KCl to the bathing solution (with mannitol adjusted to maintain osmolarity) shifted the reversal potential to +1 mV. This 12 mV depolarization demonstrates a permeability to K+. Consistent with a permeability to other cations, substitution of 30 mM NaCl for 30 mM KCl resulted in a reversal potential of 0 mV. In addition to monovalent cations, the effect of external Ca2+ on the reversal potential of the serum-induced current was determined. Increasing [Ca2+]o from 2 to 20 mM caused a 14 mV hyperpolarization of the reversal potential (Table 1). To help exclude the possibility that this apparent shift in reversal potential was simply due to charge effects at the membrane surface, we also examined the effect of another divalent cation, Mg2+. The reversal potential was minimally affected when [Mg2+]o was increased to 20 mM (Table 1). This finding suggests that alteration of surface charge by a divalent cation was not a confounding issue. These experiments indicate that serum activates a conductance that is sensitive to cations, but minimally, if at all, to chloride.
Table 1.
Effect of changes in the ionic composition of the bathing solution on the reversal potential (Vrev) of the serum-induced current
| Series | [K+]o (mM) | [Na+]o (mM) | [Ca2+]o (mM) | [Mg2+]o (mM) | [Cl−]o (mM) | Vrev (mV ±s.e.m.) | n |
|---|---|---|---|---|---|---|---|
| Control | 0 | 0 | 2 | 0.8 | 39.2 | −11 ± 2 | 18 |
| KCl | 30 | 0 | 2 | 0.8 | 69.2 | +1 ± 5 | 3 |
| NaCl | 0 | 30 | 2 | 0.8 | 69.2 | 0. ± 1 | 4 |
| CaCl2 | 0 | 0 | 20 | 0.8 | 79.2 | +3 ± 4 | 9 |
| MgCl2 | 0 | 0 | 2 | 20.0 | 79.2 | −10 ± 2 | 3 |
Using the extended constant-field voltage equation with assumptions similar to those detailed by Mayer & Westbrook (1987), we calculated that PCa/PNa=PCa/PK= 9 (where P represents permeability) when the bathing solution contained a physiological concentration of calcium. Further studies are needed to more fully characterize the relative cation permeabilities over a wide range of concentrations. Taken together, these observations demonstrate a permeability to Ca2+ and support the idea that exposure of Müller cells to serum activates a Ca2+-permeable non-selective cation channel as well as a voltage-dependent K+ channel of the KV1.3 type.
Inward current activated before outward current
We observed that serum consistently activated the non-specific cation (NSC) current prior to the increase in the outward K+ current. The inward current generated by the flow of cations through the NSC channels was assayed at the equilibrium potential for K+ (−74 mV), where the net current passing through K+-selective channels would be zero. To detect the outward currents passing through KV1.3 channels, we monitored currents at −15 mV, the approximate reversal potential of the NSC current under the recording conditions used. In a series of twenty-five Müller cells, the interval between the onset of the inward current and the onset of the outward current was 40 ± 4 s. The inward current reached its half-maximal level 48 ± 5 s before the outward current increased to an equivalent amplitude.
This temporal sequence is illustrated in Fig. 5A. To help confirm that the non-specific cation current was activated prior to the outwardly rectifying current, we compared the I-V relationship before exposure to serum and soon after the initial serum-induced increase in the inward current (but before an increase in the outward K+ current). As shown in Fig. 5B and C, serum initially induced a current that had a relatively linear I-V relationship and reversed at approximately −15 mV. Later, after the KV1.3 channels were activated, the net induced current showed outward rectification and a reversal potential which was shifted towards the threshold for activation of these K+ channels (Fig. 5D).
The observation that Ca2+-permeable NSC channels are activated prior to the increase in the activity of the KV1.3 channels raised the possibility that this may be a cause-and-effect relationship. We hypothesized that an influx of Ca2+ via NSC channels is necessary for the activation of the outwardly rectifying K+ channels. An experiment examining this possibility is shown in Fig. 6. In a Ca2+-containing bathing solution, serum induced an increase in outward current at depolarized potentials and an increase in inward current at hyperpolarized potentials (Fig. 6A and B). However, in a bathing solution lacking Ca2+, the effect of serum on the outward current was minimal (Fig. 6A and B). The difference between the I-V curves for the serum-induced currents in the presence or absence of external Ca2+ (Fig. 6C) shows that nearly all of the outwardly rectifying current induced by serum was dependent on the presence of Ca2+ in the bathing solution. Similar findings were made in each of four Müller cells tested.
Figure 6. Dependence of the serum-induced outwardly rectifying current on extracellular calcium.

A, I-V relationship of a bovine Müller cell in a Ca2+-containing bathing solution in the absence (Control) or presence of serum, and in a Ca2+-free bathing solution in the absence or presence serum. B, I-V plots of the currents induced by serum in the presence or absence of Ca2+ in the bathing solution. C, plot of the difference between the I-V curves in B. The Ca2+-containing bathing solution consisted of (mM): 133 NaCl, 10 KCl, 1.8 CaCl2, 0.8 MgCl2, 10 Na-Hepes and 20 glucose. The Ca2+-free solution lacked CaCl2 and contained 3 mM EGTA. The outwardly rectifying current induced by serum is dependent on the presence of Ca2+ in the bathing solution.
One pathway for an influx of Ca2+ into Müller cells is via the serum-activated Ca2+-permeable NSC channels. Although a role for the voltage-gated calcium channels expressed by Müller cells (Puro, Hwang, Kwon & Chin, 1996) is not completely excluded, this seems unlikely since the serum-induced depolarization (to a mean resting membrane potential of −65 mV, n = 32) did not reach the threshold (approximately −25 mV) for activation of the L-type calcium channels (Puro & Mano, 1991). Rather, we suggest that the NSC channels activated by serum allow an influx of Ca2+ which initiates as yet unidentified events leading to the activation of KV1.3 channels.
The serum-induced currents over a longer time course
In the experiments illustrated in Figs 1–5, Müller cells were exposed to serum for 1–2 min. Since a persistent change in the physiology of the Müller cells is likely to be of functional significance, we asked whether the NSC and Kv1.3 channels remain activated during a longer exposure to serum.
We studied a series of six bovine Müller cells in which stable perforated-patch recordings were maintained for ∼20 min. In each of the six cells studied, the induced inward current persisted during a 10 min exposure to 10% serum. Figure 7A illustrates this observation for one of the cells studied. We conclude that NSC channels remain activated throughout a 10 min exposure to serum.
Similarly, in three of the six Müller cells exposed to serum for 10 min, the induced outwardly rectifying current was also sustained (Fig. 7B). However, in the three other Müller cells, the induced increase in the outwardly rectifying current was transient and returned to the basal level within 5 min of the onset of perfusion with the serum-containing solution. Although the regulation of the outwardly rectifying K+ channels is complex, our findings suggest that at least some Müller cells continue to have activated KV1.3 channels during a longer-term exposure to serum.
Serum and KIR channels
As shown in Figs 1C, 2B, 5D and 6B, the characteristic I-V relationship of the serum-induced current in bovine and human Müller cells consists of an inward current at hyperpolarized potentials and, in 95% (36/38) of the cells, an outwardly rectifying current at depolarized potentials. However, in a minority (21%; 8/38) of the responsive Müller cells, there was also a lessening of the net induced current at voltages more hyperpolarized than the equilibrium potential of K+ (Fig. 8).
Figure 8. Example of a bovine Müller cell in which the net inward current induced by serum lessens at potentials hyperpolarized to the equilibrium potential of K+ (EK).

A, I-V relationship before (Control) and during exposure to serum. B, plot of the difference between the I-V curves shown in A. In a minority of the sampled Müller cells, the net current induced by serum decreases at voltages negative to EK, −74 mV.
We hypothesized that the portion of the I-V curve with a negative slope reflects an inhibition of the inwardly rectifying K+ (KIR) channels, the predominant channels active in Müller cells at hyperpolarized potentials (Brew, Gray, Mobbs & Attwell, 1986; Newman, 1993; Puro & Stuenkel, 1995; Kusaka & Puro, 1997). To help test this hypothesis, we assessed the effects of serum on Müller cells assayed under conditions in which K+-selective channels were blocked by Cs+ and Ba2+ in the bathing solution and Cs+ in the pipette solution. Consistent with our hypothesis, we did not detect a negative slope in any of the I-V plots of the serum-induced current in the sample of thirty-one Müller cells assayed under K+ channel-blocking conditions (e.g. Fig. 4). This difference in incidence (8/38 versus 0/31) was significant (P = 0.007). These observations suggest that, in addition to activating Ca2+-permeable non-specific cation channels and KV1.3 channels, serum may also partially inhibit the KIR channels in at least some Müller cells.
Effect of serum on the electroretinogram (ERG)
Our experiments demonstrate that serum induces changes in the current-voltage relationship of Müller cells freshly dissociated from the retina. The question arose as to whether serum affects Müller cells in the intact retina. To begin to address this issue, we examined the effect of serum on the ERG of the isolated rat retina.
We postulated that the serum-induced activation of non-specific cation channels would reduce K+ siphoning by Müller cells and thereby alter components of the ERG which are generated as these glia siphon K+. It seems likely that the activation of non-specific cation channels diminishes the transretinal K+ current since Müller cells would lose their K+ selectivity. As a result, the movement of Na+ and Ca2+, in addition to K+, would balance the transmembrane K+ fluxes induced by changes in [K+]o. Also, the serum-induced depolarization of Müller cells would reduce the electrochemical gradient for the influx of K+ and thereby may diminish the siphoning of K+. Based on these premises, we assessed the effect of serum on the slow PIII component of the ERG. This component was selected since there is general agreement (Ripps & Witkovsky, 1985; Karwoski, Xu & Yu, 1996) that slow PIII is mainly due to the response of Müller cells to the light-evoked decrease in [K+]o that occurs adjacent to the photoreceptors (Oakley & Green, 1976).
Figure 9 illustrates one of our experiments using an isolated retina. Figure 9A confirms that the retina generated a satisfactory ERG in response to a brief (∼0.5 ms) flash of light. Exposure to the glutamate analogue 2-amino-4-phosphonobutyric acid (APB, 50 μM) blocked the b-wave of the ERG and revealed a long-lasting negative component, i.e. slow PIII (Fig. 9B). After establishing that the peak amplitude of slow PIII was stable (2% maximal change in 6 min), the retina was perfused with a bathing solution containing APB plus 10% bovine serum (Fig. 9C). With exposure to serum, the amplitude of slow PIII decreased by 26%. The amplitude remained reduced throughout a 5 min exposure to serum. With wash-out of the serum, the amplitude returned towards the control level. In all of the four retinas tested, exposure to serum reversibly reduced slow PIII. The mean reduction was 21 ± 2%, a significant (P = 0.003, Student's t test) decrease. This decrease in the amplitude, but not the time course, of the slow PIII component of the ERG is consistent with serum causing a reduction in the siphoning of K+ by Müller cells in the retina.
Figure 9. Effect of serum on the ERG of an isolated rat retina.

A, ERG evoked by a brief (≈0.5 ms) flash of light at time zero. B, ERG of the same retina as in A after the addition of 50 μM 2-amino-4-phosphonobutyric acid (APB) to the perfusate. C, ERGs displayed on a slower time base before (Control), during (Serum) and 5 min after (Recovery) exposure of the retina to 10% bovine serum. APB was in the perfusate at all times. For all panels, the intensity of the flash was 3.6 log quanta rod−1 flash−1. Serum reduces the slow PIII component of the ERG.
Lysophosphatidic acid mimics the effects of serum
Having found that exposure to serum changes the current- voltage relationship in Müller cells, we wished to identify a specific molecule in serum that could mediate these effects. To help narrow the range of molecular weights for candidate molecules, we dialysed the bovine serum using a membrane that allowed diffusion of molecules with molecular weights of less than 6000–8000. Dialysing the serum significantly (P < 0.001) reduced the incidence of induced currents. Only one out of the twelve bovine Müller cells sampled showed an induction of current during exposure to the dialysed serum. In contrast, all of the thirty-two bovine Müller cells were responsive to the non-dialysed serum. These observations suggested that a molecule(s) with a molecular weight of less than ∼6000-8000 was likely to be of interest in our search.
We then tested the effect of serum dialysed with a membrane that allowed diffusion of molecules with molecular weights of less than ∼1000. This dialysed serum also had a low probability (2/13) of activating currents in the sampled bovine Müller cells. This incidence was significantly (P < 0.001) less than with the non-dialysed serum. These experiments led us to focus our search on molecules with molecular weights of less than 1000. One candidate molecule was lysophosphatidic acid (LPA). This glycerophospholipid has a molecular weight of 436, is present in serum at concentrations of up to 28 μM (Tokumura, Iimori, Nishioka, Kitahara, Sakashita & Tanaka, 1994) and activates a specific G-protein-coupled receptor present on many types of cells (Moolenaar, 1995; Hecht, Weiner, Post & Chun, 1996; An, Dickens, Bleu, Hallmark & Goetzl, 1997). We found that eleven of the eighteen Müller cells sampled were responsive to LPA.
Figure 10 shows the I-V relationship for bovine Müller cells before and during exposure to 25 μM LPA. The I-V plot of the LPA-induced current in Fig. 10A is qualitatively similar to the current typically induced by non-dialysed serum (Figs 1C, 2B, 5D and 6B). In a minority of the responsive cells (2/11), the net inward current induced by LPA diminished at voltages hyperpolarized to the equilibrium potential of K+ (Fig. 10B): a phenomenon also observed in 21% of the Müller cells exposed to serum (Fig. 8).
Figure 10. Effects of lysophosphatidic acid (LPA) on currents in a freshly dissociated bovine Müller cell.
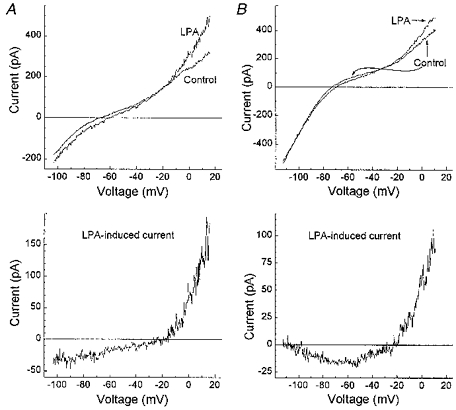
Currents were monitored using the perforated-patch recording technique. A and B: top panels, I-V relationship before (Control) and during exposure to 25 μM LPA; bottom panels, plots of the differences between the Control and LPA I-V curves in the top panels. As observed with serum, the effects of LPA varied at hyperpolarized potentials: most of the responsive cells had LPA-induced currents with an I-V relationship as illustrated in A; however, in a minority of cells, the net inward current induced by LPA diminished at hyperpolarized voltages (B). LPA induces currents in Müller cells that are qualitatively similar to those induced by serum.
Since LPA in serum is bound to albumin (MW, 68 000), we wished to establish that our method of dialysis removed LPA in the presence of albumin. To address this issue, we performed electrophysiological experiments using a 40-fold dilution of either the standard stock solution of 1 mM LPA and 0.1% bovine albumin or the stock solution after dialysis at a molecular weight cut-off of 1000. As noted, using our standard stock solution (final concentrations: LPA, 25 μM; BSA, 0.0025% (0.4 μM)), eleven out of eighteen bovine Müller cells showed an induction of an inward current at hyperpolarized potentials and an outward current at depolarized potentials. In contrast, only one of the nine sampled Müller cells had changes in its I-V relationship when exposed to the diluted stock solution which had been dialysed. This incidence of responses was significantly (P = 0.019) lower than when the non-dialysed stock solution was used. We conclude that our method of dialysis removed LPA from the albumin-containing solution.
Studies with various types of cells have shown that LPA activates multiple types of G-protein-coupled pathways (Moolenaar, 1995). Both pertussis toxin (PTX)-sensitive and -insensitive mechanisms can mediate the effects of LPA. To learn more about the mechanism of action of LPA in Müller cells, we tested the effect of PTX on the ability of LPA to induce currents in these cells. Retinas were removed from bovine eyes and exposed for ∼18 h at 4°C to 400 ng ml−1 of PTX in Earle's balanced salt solution supplemented with 0.5 mM EGTA, 1.5 mM CaCl2, 1 mM MgCl2, 20 mM glucose and 26 mM NaHCO3, with the pH adjusted with CO2 to 7.4. The Müller cells were then dissociated from the retinas and studied as usual, except for the continued presence of 400 ng ml−1 of PTX. In four out of the six Müller cells sampled, LPA induced increases in inward and outward currents. PTX did not significantly (P = 1.0) block the effects of LPA on ion channels in Müller cells. These results suggest that LPA regulates Müller cell channels by a PTX-insensitive mechanism.
We suspect that, in addition to LPA, there are other molecules in serum which regulate the activity of ion channels in Müller cells. This is based on our finding that LPA appears to be less effective than serum. For example, significantly (P < 0.001) fewer Müller cells were responsive to 25 μM LPA (11/18) than to 10% serum (32/32), which, based on measurements of Tokumura et al. (1994), contains < 2.8 μM LPA. Also, the depolarization of the resting membrane potential in responsive cells was significantly (P = 0.025, Student's t test) less during exposure to LPA (4.5 ± 0.8 mV) than with exposure to serum (7.5 ± 0.7 mV). In addition, serum, but not LPA (25–200 μM), induced a detectable decrease in the slow PIII component of the isolated retinal ERG.
Taken together, these findings are consistent with LPA being one of a number of serum-derived molecules which modulate the physiology of Müller cells. However, the identities of other molecules which have effects similar to those of LPA are unknown. Although thrombin (MW, 36 000) inhibits the KIR channels of cultured Müller cells, it does not appear to activate non-specific cation channels (Puro & Stuenkel, 1995). Albumin affects the physiology of certain glia (Nadal, Fuentes, Pastor & McNaughton, 1997), but is too large to have been one of the active molecules removed from serum by our dialysis. Further studies are needed to identify other low molecular weight molecules which are present in the serum and affect the electrophysiological properties of Müller cells.
DISCUSSION
The results show that exposure to serum alters the activity of various types of ion channels in the Müller glial cells of the mammalian retina. Our perforated-patch recordings from freshly dissociated human and bovine Müller cells revealed that serum activates a calcium-permeable, non-specific cation channel. Also, serum increases an outwardly rectifying K+ current which, based on its sensitivity to charybdotoxin and margatoxin, is due to the activation of KV1.3 channels. In addition, inwardly rectifying K+ (KIR) channels can be partially inhibited when Müller cells are exposed to serum. These effects of serum on ion channel activity are mimicked by lysophosphatidic acid, suggesting that this may be one of the serum-derived molecules regulating the physiology of Müller cells when there is a breakdown of the blood-retinal barrier.
It seems likely that exposure to serum causes a reduction in K+ siphoning via Müller cells. Normally, a Müller cell is almost exclusively permeable to K+ (Newman, 1985); thus, K+ current entering a Müller cell is balanced by K+ exiting from other regions of the cell. However, when non-specific cation channels are activated, K+ can be balanced, at least in part, by the movement of Na+ and Ca2+. As a result, the transretinal K+ current and K+ siphoning would be reduced. In addition, the siphoning of K+ may be reduced due to the depolarization caused by the activation of the non-specific cation channels and, to a lesser extent, by the modest inhibition of the KIR channels. This possibility is based on the observation (Newman, 1985; Kusaka & Puro, 1997) that normally the membrane potential (Vm) of a Müller cell is close to the equilibrium potential for K+ (EK). Under these conditions, a slight change in [K+]o creates an electrochemical gradient for a flux of K+ across the cell membrane. However, when the Vm is depolarized relative to EK, a greater change in [K+]o is needed in order to induce an influx of K+. Since an influx of K+ is essential for glia to spatially buffer K+ (Orkand, Nichols & Kuffler, 1966), a larger change in [K+]o would be required to initiate K+ siphoning via Müller cells. Thus, we predict that exposure to serum reduces the siphoning of K+ by Müller cells in the mammalian retina.
Consistent with these theoretical considerations, we observed that serum reduced the slow PIII component of the ERG of the isolated retina. This is of interest since slow PIII is generated by Müller cells (Ripps & Witkovsky, 1985; Karwoski et al. 1996) responding to the decrease in [K+]o adjacent to photoreceptors hyperpolarized by light; evidence supports the idea that K+ is siphoned via Müller cells from the inner retina, where [K+]o is higher, to the outer (photoreceptor) portion of the retina where [K+]o is lower (Frishman, Yamamoto, Bogucka & Steinberg, 1992). Thus, although a direct effect of serum on other elements of the retina, such as the photoreceptors, is not excluded by ERG recordings, the observed reduction in the amplitude of the slow PIII component is in agreement with the prediction that the siphoning of K+ by Müller cells is reduced when serum enters the retina.
Whilst the activation of the non-specific cation channels and the partial inhibition of the KIR channels accounts for the depolarization of Müller cells exposed to serum, the functional significance of the serum-induced increase in the activity of the KV1.3 channels is uncertain. With a threshold of activation of approximately −40 mV, it appears that these outwardly rectifying K+ channels would be active only under pathophysiological conditions, such as ischaemia and cell injury, which are associated with profound depolarization. Under these conditions, the KV1.3 channels may serve as pathways for the redistribution of K+ by the Müller cells. In addition, the membrane potential of depolarized Müller cells would tend to stabilize at the threshold of activation for these K+ channels. A stabilization of the membrane potential would maintain an electrochemical gradient for an influx of Ca2+ via the Ca2+-permeable, non-specific cation channels: a role postulated for the KV1.3 channels expressed by lymphocytes (Lin et al. 1993). However, the consequences, beneficial and/or harmful, of a sustained influx of Ca2+ on the function of Müller cells are not known.
We found that the effects of LPA on Müller cell currents are qualitatively similar to those induced by serum. LPA, the simplest physiologically occurring glycerophospholipid, has been identified as an intercellular messenger with a broad range of biological effects on a variety of cell types (Moolenar, 1995). This platelet-derived component of serum binds to a specific G-protein-coupled receptor (Hecht et al. 1996; An et al. 1997) with both PTX-sensitive (Gi-type) and PTX-insensitive (Gq-type) G-proteins being activated (Moolenaar, 1995). In our study, the effects of LPA on ion channels in Müller cells were PTX insensitive, consistent with the involvement of the Gq pathway. Based on our physiological evidence that Müller cells express LPA receptors, we propose that, with a breakdown of the blood-retinal barrier, LPA-containing serum enters the retina, activates LPA receptors on Müller cells and induces changes in the activity of multiple types of ion channels.
A breakdown of the blood-retinal barrier and a subsequent decrease in visual function is a common occurrence in numerous retinal disorders, such as diabetic retinopathy, retinal venous occlusions and trauma (Gass, 1997). Despite the frequency and significance of a breakdown of the blood-retinal barrier, the mechanisms by which this pathophysiological process decreases retinal function are poorly understood. Whilst it seems reasonable to assume that physical changes secondary to tissue swelling and distortion are important factors causing a disruption of function, our observations suggest an additional mechanism: namely, that serum-derived molecules activate receptors on retinal cells and thereby change the physiology of the cells. In the case of LPA and Müller cells, the depolarization induced by the activation of the non-specific cation channels is likely to reduce K+ siphoning by these glia. With less K+ siphoning, we predict that K+ homeostasis will be altered and neuronal function disturbed.
A role for LPA is not limited to the retina, since LPA receptors are found in many tissues including the brain (An et al. 1997). Interestingly, the brain has the greatest abundance of LPA receptors of any of the fifteen tissues examined by An et al. (1997). Thus, a breakdown in the blood-brain barrier is likely to be associated with LPA-induced effects. Whether LPA regulates the activity of ion channels in glial cells located at sites in the CNS other than the retina is not known. However, a recent report suggests that cerebral astrocytes in vitro express functional LPA receptors (Keller, Steiner, Mattson & Steiner, 1996). The abundance of LPA receptors and the relatively high concentration of LPA (Das & Hajra, 1989) within the CNS also raises the possibility that this glycerophospholipid may be synthesized within the nervous system and play a functional role under physiological, as well as pathophysiological, conditions.
Exposure to serum/LPA is not the only condition in which a non-specific cation current is activated in Müller cells. The nitric oxide-cGMP pathway also activates calcium-permeable, non-specific cation channels (Kusaka et al. 1996), which, however, differ from those induced by serum/LPA since extracellular calcium partially blocks the cyclic nucleotide-gated, but not the serum-activated channels. Taken together, these observations demonstrate that multiple intercellular signals regulate the activity of various types of non-specific cation channels expressed by mammalian Müller cells.
The non-specific cation channels of Müller cells are likely to be of functional importance. We propose that exposure of Müller cells to serum decreases K+ siphoning by mechanisms involving the activation of these channels. Specifically, activation of non-specific cation channels reduces the selectivity of the cell membrane to K+ and causes a depolarization of the cell, resulting in a diminished electrochemical driving force for the influx of K+ and a smaller transretinal K+ current. By these mechanisms, the molecular composition of the microenvironment may regulate the role of Müller cells in maintaining K+ homeostasis in the retina.
Acknowledgments
This work was supported by grants from the NIH: grant nos EY06931 (D. G. P.), EY00379 (D. G. G.) and EY00785 (Core). D. G. P. is a Research to Prevent Blindness Senior Scientific Investigator. We thank Dr D. Thompson and Dr B. Hughes for helpful discussions.
References
- An S, Dickens MA, Bleu T, Hallmark OG, Goetzl EJ. Molecular cloning of the human edg2 protein and its identification as a functional cellular receptor for lysophosphatidic acid. Biochemical and Biophysical Research Communications. 1997;231:619–622. doi: 10.1006/bbrc.1997.6150. 10.1006/bbrc.1997.6150. [DOI] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. Journal of Neuroscience Methods. 1993;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Brew H, Gray PTA, Mobbs P, Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediated K+ buffering. Nature. 1986;324:466–468. doi: 10.1038/324466a0. [DOI] [PubMed] [Google Scholar]
- Candia S, Garcia ML, LaTorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophysical Journal. 1992;63:583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AK, Hajra AK. Quantification, characterization and fatty acid composition of lysophosphatidic acid in different rat tissues. Lipids. 1989;24:329–333. doi: 10.1007/BF02535172. [DOI] [PubMed] [Google Scholar]
- Frishman LJ, Yamamoto F, Bogucks J, Steinberg RH. Light-evoked changes in [K+]o in proximal portion of light-adapted cat retina. Journal of Neurophysiology. 1992;67:1201–1212. doi: 10.1152/jn.1992.67.5.1201. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ, Garcia ML. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. Journal of Biological Chemistry. 1993;268:18866–18874. [PubMed] [Google Scholar]
- Gass JDM. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. St Louis, MO, USA: Mosby; 1997. [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. Journal of Cell Biology. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer; 1992. [Google Scholar]
- Karwoski CJ, Xu X, Yu H. Current-source density analysis of the electroretinogram of the frog: methodological issues and origin of components. Journal of the Optical Society of America. 1996;A 13:549–556. doi: 10.1364/josaa.13.000549. [DOI] [PubMed] [Google Scholar]
- Keller JN, Steiner MR, Mattson MP, Steiner SM. Lysophosphatidic acid decreases glutamate and glucose uptake by astrocytes. Journal of Neurochemistry. 1996;67:2300–2305. doi: 10.1046/j.1471-4159.1996.67062300.x. [DOI] [PubMed] [Google Scholar]
- Kusaka S, Dabin I, Barnstable CJ, Puro DG. cGMP-mediated effects on the physiology of bovine and human retinal Müller (glial) cells. Journal of Physiology. 1996;497:813–824. doi: 10.1113/jphysiol.1996.sp021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka S, Puro DG. Intracellular ATP activates inwardly rectifying K+ channels in human and monkey retinal Müller (glial) cells. Journal of Physiology. 1997;500:593–604. doi: 10.1113/jphysiol.1997.sp022045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Boltz RC, Blake JT, Nguyen M, Talento A, Fischer PA, Springer MS, Sigal NH, Slaughter RS, Garcia ML, Kaczorowski GJ, Koo GC. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. Journal of Experimental Medicine. 1993;177:637–645. doi: 10.1084/jem.177.3.637. 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. Journal of Physiology. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. Journal of Biological Chemistry. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin induces calcium waves in rat cortical astrocytes. Glia. 1997;19:343–351. doi: 10.1002/(sici)1098-1136(199704)19:4<343::aid-glia7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Newman E A. Membrane physiology of retinal glial (Müller) cells. Journal of Neuroscience. 1985;5:2225–2239. doi: 10.1523/JNEUROSCI.05-08-02225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Inward-rectifying potassium channels in retinal glial (Müller) cells. Journal of Neuroscience. 1993;13:3333–3345. doi: 10.1523/JNEUROSCI.13-08-03333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell regulation of extracellular potassium. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995. pp. 717–731. [Google Scholar]
- Newman EA, Frambach DA, Odette LL. Control of extracellular potassium levels by retinal glial K+ siphoning. Science. 1984;225:1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Reichenbach A. The Müller cell: a functional element of the retina. Trends in Neurosciences. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Oakley B, II, Green D G. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. Journal of Neurophysiology. 1976;39:1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Oakley B, II, Katz BJ, Xu Z, Zheng J. Spatial buffering of extracellular potassium by Müller (glial) cells in the toad retina. Experimental Eye Research. 1992;55:539–550. doi: 10.1016/s0014-4835(05)80166-6. [DOI] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. Journal of Neurophysiology. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Puro DG, Hwang J-J, Kwon O-J, Chin H. Characterization of an L-type calcium channel expressed by human retinal Müller (glial) cells. Molecular Brain Research. 1996;37:41–48. doi: 10.1016/0169-328x(96)80478-5. 10.1016/0169-328X(96)80478-5. [DOI] [PubMed] [Google Scholar]
- Puro DG, Mano T. Modulation of calcium channels in human retinal glial cells by basic fibroblast growth factor: a possible role in retinal pathobiology. Journal of Neuroscience. 1991;11:1873–1880. doi: 10.1523/JNEUROSCI.11-06-01873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG, Stuenkel EL. Thrombin-induced inhibition of potassium currents in human retinal glial (Müller) cells. Journal of Physiology. 1995;485:337–348. doi: 10.1113/jphysiol.1995.sp020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG, Yuan JP, Sucher NJ. Activation of NMDA receptor-channels in human retinal Müller glial cells inhibits inward-rectifying potassium currents. Visual Neuroscience. 1996;13:319–326. doi: 10.1017/s0952523800007562. [DOI] [PubMed] [Google Scholar]
- Ripps H, Witkovsky P. Neuron-glia interaction in the brain and retina. Progress in Retinal Research. 1985;5:181–220. 10.1016/0278-4327(85)90009-4. [Google Scholar]
- Tokumura A, Iimori M, Nishioka Y, Kiahara M, Sakashita M, Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. American Journal of Physiology. 1994;36:C204–210. doi: 10.1152/ajpcell.1994.267.1.C204. [DOI] [PubMed] [Google Scholar]


