Abstract
Climbing fibre-mediated excitatory postsynaptic potentials (CF-EPSPs) or currents (CF-EPSCs) were recorded from Purkinje cells in rat cerebellar slices using the whole-cell recording technique.
Climbing fibre responses displayed prominent paired-pulse depression (PPD). In the current-clamp recording mode, PPD resulted in a decreased number of spikelets in the second complex spike of the pair, and depression of the after-depolarization and after-hyperpolarization.
The mechanism of PPD was examined under voltage clamp. Manipulations that reduce transmitter release significantly affected PPD. These included lowering extracellular Ca2+ concentration and bath application of baclofen or adenosine.
Changing the number of stimulated climbing fibres, equivalent to changing the number of release sites, had no effect on PPD.
Selective manipulations of postsynaptic responsiveness had no effect on PPD. These included partial blockade of CF-EPSCs by a non-NMDA receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and changing the holding potential.
A rapidly dissociating AMPA receptor antagonist, 2,3-cis-piperidine dicarboxylic acid, inhibited the second CF-EPSC of the pair proportionately more than the first, suggesting that presynaptic release by the second pulse is decreased.
PPD at interstimulus intervals of 50 ms or longer (up to 3000 ms) was not significantly affected by manipulations that change postsynaptic glutamate receptor desensitization.
Blockade of metabotropic glutamate, GABAB and adenosine receptors had no effect on PPD, suggesting that presynaptic autoreceptors do not contribute to PPD.
These results indicate that decreased transmitter release is a major cause of PPD at cerebellar climbing fibre-Purkinje cell synapses.
Cerebellar Purkinje cells receive two distinct excitatory inputs from parallel fibres and climbing fibres. Parallel fibres consist of the bifurcated axons of granule cells, and form weak en passant synapses onto the dendritic spines of Purkinje cells (Palay & Chan-Palay, 1974; Ito, 1984). One Purkinje cell is estimated to receive inputs from as many as 150 000 parallel fibres. In contrast, climbing fibres originate from the inferior olive of the medulla and form strong excitatory synapses onto the proximal dendrites of Purkinje cells (Palay & Chan-Palay, 1974; Ito, 1984). Each Purkinje cell is innervated by only a single climbing fibre in the adult.
Purkinje cell firing of simple spikes normally ranges from 10 to 50 Hz depending on the strength of parallel fibre inputs and composes the major inhibitory cerebellar outputs (Ito, 1984). In contrast, climbing fibres have low firing rates around 1 Hz (Ito, 1984). The climbing fibre inputs cause a large depolarization of Purkinje cell dendrites and induce complex spikes that accompany significant Ca2+ influx (Knöpfel, Vrancesic, Staub & Gähwiler, 1991; Kano, Rexhausen, Dreessen & Konnerth, 1992; Konnerth, Dreessen & Augustine, 1992; Miyakawa, Lev-Ram, Lasser-Ross & Ross, 1992; Callaway, Lasser-Ross & Ross, 1995). The climbing fibre inputs can modulate simple spike discharge rates and thereby affect the output of the cerebellum (Ebner & Bloedel, 1981; McDevitt, Ebner & Bloedel, 1982; Montarolo, Palestini & Strata, 1982; Savio & Tempia, 1985; Sato, Miura, Fushiki & Kawasaki, 1992). Moreover, the climbing fibre-mediated Ca2+ transients are known to trigger long-lasting change in the strength of other synapses, such as long-term depression of parallel fibre synapses (Sakurai, 1990; Konnerth et al. 1992) and rebound potentiation of inhibitory synapses (Kano et al. 1992). It is thus important to characterize the nature of climbing fibre-mediated synaptic transmission to better understand the behaviour of cerebellar circuitry and its role in motor control.
Recent studies using whole-cell patch-clamp recording applied to cerebellar slices have revealed detailed features of climbing fibre synapses. For example, climbing fibre responses display significant depression to the second of a stimulus pair (paired-pulse depression, PPD) (Konnerth, Llano & Armstrong, 1990; Aiba et al. 1994; Kano et al. 1995). Mechanisms of this short-term synaptic plasticity are poorly understood. In view of the particularly long duration of PPD (3–4 s) compared with mean interspike intervals of complex spikes (around 1 s), PPD is presumed to have functional significance for controlling the size of climbing fibre-mediated excitatory postsynaptic potentials (CF-EPSPs).
In the present study, we demonstrate that, in current-clamp recording mode, PPD of CF-EPSPs resulted in a decreased number of spikelets in the complex spike and depression of the after-depolarization and after-hyperpolarization that followed the complex spike. Then, we examined the mechanisms of PPD in voltage-clamp recording mode. We selectively manipulated either presynaptic or postsynaptic factors of synaptic transmission, and examined the effects of these manipulations on PPD. Our results suggest that PPD is mainly due to decreased transmitter release from presynaptic terminals. Some of the results have been published in an abstract form (Hashimoto & Kano, 1996).
METHODS
Preparation
Parasagittal slices were prepared from Wistar rat cerebella as described previously (Edwards, Konnerth, Sakmann & Takahashi, 1989; Llano, Marty, Armstrong & Konnerth, 1991; Kano & Konnerth, 1992; Aiba et al. 1994). In brief, postnatal day 12–20 (P12-P20) rats were killed by cervical dislocation and the cerebellum was quickly removed. The cerebellum was kept in ice-cold standard saline (for composition see Solutions) bubbled with 95% O2 and 5% CO2. The block of the cerebellum was glued onto the stage of a chamber filled with ice-cold standard saline. Then, parasagittal cerebellar slices of 200–300 μm thickness were cut with a Vibroslicer (Campden Instruments, Loughborough, UK). The slices were kept at 32°C for at least 1 h in a chamber containing oxygenated standard saline. One slice was then transferred to a recording chamber where it was continuously perfused with oxygenated standard saline.
Recording
Recognition of layers within the cerebellar cortex and identification of Purkinje cells was easily achieved on slices when viewed using a × 40 water-immersion objective lens attached to an Olympus upright microscope (BH-2; Tokyo, Japan) (Edwards et al. 1989; Llano et al. 1991). All experiments were performed using the whole-cell configuration of the patch-clamp technique with borosilicate pipettes (Clark Electromedical Instruments, Pangbourne, UK) (resistance of 3–6 MΩ when filled with an intracellular solution). Ionic currents (voltage-clamp experiments) or membrane voltages (current-clamp experiments) were recorded with an Axopatch-1D patch-clamp amplifier (Axon Instruments) and stored on a DAT data recorder (Sony PC204) for later analysis. In voltage-clamp experiments, the holding potentials of Purkinje cells were set to −20-0 mV to inactivate voltage-dependent conductances. The pipette access resistance was compensated as explained by Llano et al. (1991b), when necessary. On-line data were acquired using the ITC-16 interface (Instrutech Co., Great Neck, NY, USA) and the PULSE program (HEKA, Lambrecht, Germany) on a Macintosh computer. The signals were filtered at 3 kHz and digitized at 20 kHz. For stimulation of climbing fibres, a glass pipette with 5–10 μm tip diameter filled with standard saline was used. Square pulses (duration, 0.1 ms; amplitude, 0–100 V) were applied for focal stimulation.
Solutions
The composition of standard saline was (mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3 and 20 glucose, which was bubbled continuously with a mixture of 95% O2 and 5% CO2. In most of the experiments, bicuculline methochloride (10 μm) was present in the saline to block spontaneous inhibitory postsynaptic currents (Konnerth et al. 1990; Kano et al. 1992). In experiments using low Ca2+ Ringer solutions (Fig. 1), Ca2+ was replaced with equimolar concentration of Mg2+ to keep the total concentration of divalent cations constant. The ratio of Ca2+ to Mg2+ concentration (in millimoles) is expressed throughout as [Ca2+]/[Mg2+]. The standard pipette solution for voltage-clamp experiments contained (mM): 60 CsCl, 30 caesium D-gluconate, 20 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 20 TEA-Cl, 4 MgCl2, 4 ATP and 30 Hepes (pH 7.3, adjusted with CsOH). That for current-clamp experiments contained (mM): 5 KCl, 115 potassium D-gluconate, 4 MgCl2, 4 ATP and 50 Hepes (pH 7.3, adjusted with KOH). All experiments were carried out at a bath temperature of 32°C.
Figure 1. Complex spikes and accompanying after-depolarization and after-hyperpolarization in current-clamp recording mode.

Responses were elicited by paired stimuli to the climbing fibre at two interpulse intervals. A, first response to a stimulus pair (single sweep record) shown with low gain and high time resolution (a), and with high gain and with slow time resolution (b). The dashed line in b shows the baseline membrane potential before the stimulus. Downward and upward arrows in b indicate the after-depolarization and after-hyperpolarization, respectively. B, second response to a stimulus pair (single sweep record) at interstimulus interval (ISI) of 500 ms; details as for A. C, second response to a stimulus pair (single sweep record) at interstimulus interval of 1000 ms; details as for A. Note the decreased number of spikelets in the complex spikes (oblique arrows in Ba and Ca) and depression of after-depolarization (downward arrows) in the second responses.
Bicuculline methochloride, baclofen, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), 2-amino-5-phosphonopentanoic acid (AP-5), (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), (S)-α-methyl-4-carboxyphenylglycine (MCPG), (S)-2-amino-2-methyl-4-phosphonobutanoic acid (MAP4), 2-hydroxysaclofen, L-trans-pyrrolidine-2,4-dicarboxylate (PDC) and (+)-cis-2,3-piperidinedicarboxylic acid (PDA) were purchased from Tocris Cookson (Bristol, UK). Adenosine, diazoxide and theophylline were from Sigma.
RESULTS
In parasagittal cerebellar slices prepared from P12-P20 rats, climbing fibres were stimulated in the granule cell layer, and Purkinje cells were recorded in the whole-cell configuration (Konnerth et al. 1990; Llano et al. 1991; Kano & Konnerth, 1992; Kano et al. 1992, 1995). Climbing fibre-mediated excitatory postsynaptic currents (CF-EPSCs) are known to display significant depression to the second of a stimulus pair (PPD) (Konnerth et al. 1990; Aiba et al. 1994; Kano et al. 1995). The duration of PPD measured in slice preparation (3–4 s) is much longer than mean interspike intervals of complex spikes (around 1 s) recorded in vivo (Ito, 1984). This strongly suggests that CF-EPSP amplitudes and the shapes of complex spikes are dynamically controlled in vivo depending on the firing rate of climbing fibre afferents. To test this possibility in the slice preparation, we first examined CF-EPSPs and complex spikes under physiological recording conditions using the current-clamp technique. For this purpose, potassium based pipette solution was used and bicuculline was omitted from the external solution.
PPD affects the shape of complex spikes and reduces after-potentials
Climbing fibre stimulation induced typical complex spikes which consisted of large EPSPs, one clear Na+ spike and several spikelets with lower amplitudes (Fig. 1Aa). In addition, each complex spike was accompanied by after-depolarization and -hyperpolarization (Fig. 1Ab). When paired climbing fibre stimuli were applied, the number of spikelets decreased in the second complex response with little change in the initial Na+ spikes (Fig. 1Ba and Ca). It should be noted that this effect was evident even with an interstimulus interval of 500 ms which is much longer than the duration of the after-hyperpolarization induced by the first complex spike (Fig. 1Bb and Cb). This suggests that PPD of the EPSP is the main cause of the decrease of spikelet numbers in the second complex response. Moreover, after-depolarization and -hyperpolarization that followed the second complex spike were also significantly reduced (Table 1). These results suggest that PPD of climbing fibre-mediated EPSPs is functionally significant in controlling Purkinje cell excitability.
Table 1.
Effects of paired climbing fibre stimulation on EPSP and after-potentials
| After-depolarization | After-hyperpolarization | |||
|---|---|---|---|---|
| Amplitudea (mV) | Duration (ms) | Amplitudeb (mV) | Durationc (ms) | |
| 1st response | 7.0 ± 2.4 (10) | 110.5 ± 27.9 (10) | 3.7 ± 0.7 (10) | 149.1 ± 32.6 (9) |
| 2nd response | ||||
| ISI, 400 ms | 5.6 ± 2.6* (10) | 128.1 ± 63.9 (10) | 2.9 ± 1.5* (10) | 129.1 ± 33.3 (9) |
| ISI, 800 ms | 5.8 ± 2.9 (10) | 129.5 ± 52 (10) | 2.8 ± 1.1* (10) | 125.2 ± 32* (9) |
All data are expressed as means ±s.d. with the sample size given in parentheses.
P < 0.05 compared with the value of the first response (Wilcoxon signed-rank test).
Amplitudes of after-depolarization were measured 50 ms after the stimulus artefacts.
Amplitudes of after-hyperpolarization were measured at the peak.
Durations of after-hyperpolarization were measured as the times between the data points crossing the half-maximum amplitudes.
The rest of the experiments were undertaken to identify the locus responsible for PPD. Purkinje cells were whole-cell voltage clamped to holding potentials of −20 to 0 mV and CF-EPSCs were recorded in the presence of bicuculline methochloride (10 μm) to block GABAA receptor-mediated inhibitory postsynaptic currents. We adopted various experimental manipulations which affect transmitter release from presynaptic terminals or the responsiveness of the postsynaptic membrane.
Effects of lowering extracellular Ca2+ concentration
To change the presynaptic factors, we first examined the effect of lowering extracellular Ca2+ concentration on PPD. Ca2+ was replaced with equimolar Mg2+ to keep the concentration of divalent cations constant. This manipulation decreases release of transmitter from presynaptic terminals without affecting the postsynaptic responsiveness (Katz & Miledi, 1968; Manabe, Wyllie, Perkel & Nicoll, 1993; Andreasen & Hablitz, 1994).
Figure 2 shows an example in which CF-EPSCs in response to paired stimuli (interstimulus interval of 40 ms) were recorded in standard saline ([Ca2+]/[Mg2+], 2.0/1.0) and three different low Ca2+, high Mg2+ extracellular solutions. The [Ca2+]/[Mg2+] (in millimoles) of these solutions were: 0.25/2.75, 0.5/2.5, and 1.0/2.0. In different low Ca2+, high Mg2+ solutions, the amplitudes of both the first and second CF-EPSCs were smaller than for controls, but the decrease was greater for the first CF-EPSCs (Fig. 2A and B). Hence, the ratio of the second to the first CF-EPSC amplitude increased (Fig. 2A, superimposed traces and Fig. 2C).
Figure 2. Suppression of synaptic transmission by decreasing extracellular Ca2+ concentration and its effect on PPD.
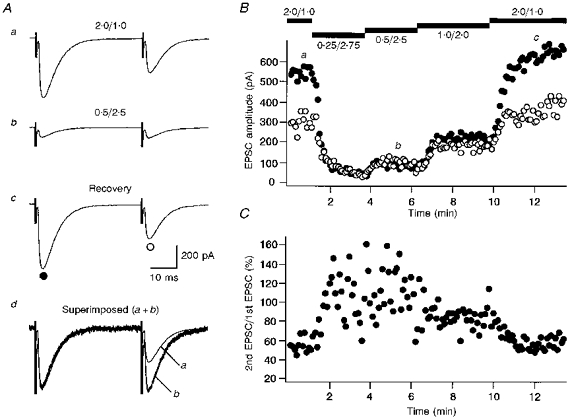
A, specimen records of CF-EPSCs (average of five consecutive responses) in response to paired stimuli (40 ms interval). Records a-c were taken sequentially in extracellular solutions with the following [Ca2+]/[Mg2+] in millimoles: a, 2.0/1.0; b, 0.5/2.5; c, 2.0/1.0 (recovery). In d, the first CF-EPSC from Ab is scaled to the amplitude of the first CF-EPSC from Aa and superimposed (a+b). The points at which these records were taken are indicated in B. B and C, amplitudes of the first (○) and the second (•) CF-EPSCs (B) and the amount of PPD calculated as the ratio of the second to the first CF-EPSC amplitude (C) plotted against time.
Excitatory synaptic inputs projecting onto rodent Purkinje cells are mediated exclusively by non-NMDA receptors (Llano et al. 1991; Perkel, Hestrin, Sah & Nicoll, 1991; Aiba et al. 1994; Kano et al. 1995). To check whether lowering extracellular Ca2+ concentration had any side effects on postsynaptic receptors, the responsiveness of Purkinje cells to ionophoretically applied AMPA, a non-NMDA receptor agonist, was examined. A pipette with a tip diameter of 1–2 μm was filled with Ca2+-free saline containing 1 mM AMPA, and positioned near the proximal dendrites. AMPA was ejected with negative current pulses (duration, 100 ms; amplitude, −150 to −250 nA) from retention currents of +50 nA. The amplitudes of AMPA-induced currents in low Ca2+ solutions were 101.8 ± 10.5% (n = 6) for 0.25/2.75, 104.1 ± 13.7% (n = 6) for 0.5/2.5, and 95.5 ± 9.5% (n = 7) for 1.0/2.0 (means ±s.d., relative to the AMPA-induced currents in standard saline). These observations indicated that lowering extracellular Ca2+ concentration did not change AMPA responsiveness of the Purkinje cells.
Effects of reducing transmitter release using baclofen or adenosine
Next, we took another approach to changing transmitter release from presynaptic terminals. Baclofen and adenosine are known to act on presynaptic GABAB and A1 receptors, respectively, inhibiting transmitter release (Dunwiddie, 1985; Lupica, Proctor & Dunwiddie, 1992; Prince & Stevens, 1992; Scanziani, Capogna, Gähwiler & Thompson, 1992; Isaacson, Solis & Nicoll, 1993; Pfrieger, Gottmann & Lux, 1994). Figure 3 shows an example in which baclofen (50 μm) was bath applied while recording CF-EPSCs in response to paired stimuli. Baclofen readily reduced the amplitudes of both the first and second CF-EPSCs (Fig. 3A and B). The reduction was greater for the first EPSCs than for the second EPSCs (Fig. 3A and B), which resulted in the increase of the CF-EPSC ratio (Fig. 3Ad and C).
Figure 3. Suppression of synaptic transmission by baclofen and its effect on PPD.
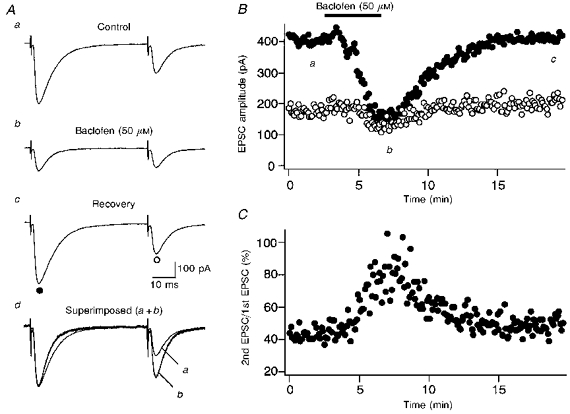
A, records of CF-EPSCs (average of five consecutive responses) in response to paired stimuli (50 ms interval). Records a-c were taken sequentially before (a), during (b), and after (c) bath application of baclofen (50 μm). The times at which these records were taken are indicated in B. In d, the first CF-EPSC from Ab is scaled to the amplitude of the first CF-EPSC from Aa and superimposed (a+b). B and C, amplitudes of the first (○) and the second (•) CF-EPSCs (B) and the amount of PPD (C) plotted against time.
Adenosine (100 μm) similarly induced a reduction of both the first and second EPSCs and increased the CF-EPSC ratio (data not shown).
Effects of a presynaptic manipulation equivalent to changing the number of release sites
Both lowering external Ca2+ and application of baclofen or adenosine are presumed to act, at least in part, by changing the Ca2+ concentrations at the presynaptic terminals. Hence, both manipulations are likely to affect the probability of transmitter release from presynaptic terminals. To manipulate another presynaptic factor, namely the number of release sites, we selected Purkinje cells that were innervated by multiple climbing fibres. Our recent results indicate that about 40% of mouse Purkinje cells have such multiple climbing fibre innervation at the developmental stage which is comparable to rats used in the present study (Kano et al. 1995).
Figure 4 shows a typical record from Purkinje cells innervated by two climbing fibres. During the first 10 min of recording (Fig. 4Aa and Ba), two climbing fibres were stimulated. Then the stimulus intensity was reduced to activate one of the two climbing fibres while recording continued (Fig. 4Ab and Bb). There was no change in the CF-EPSC ratio (Fig. 4Ac and C) in spite of the sudden reduction of the CF-EPSC amplitude (Fig. 4A and B).
Figure 4. Changing the number of stimulated climbing fibres does not change the amount of PPD.
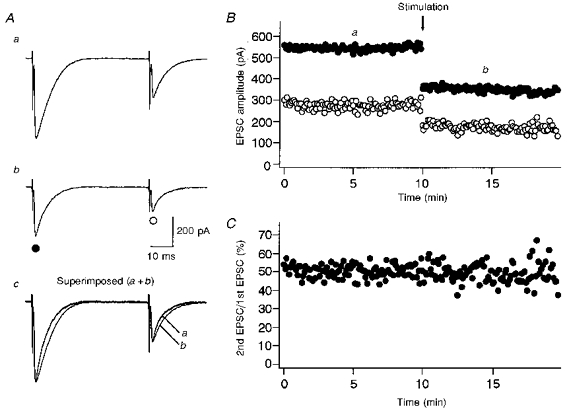
A, specimen records of CF-EPSCs (average of five consecutive responses) in response to paired stimuli (50 ms interval) in a Purkinje cell innervated by two climbing fibres. Records in response to stimulating two climbing fibres (a), and one climbing fibre (b) are shown. In c, the first CF-EPSC from Ab is scaled to the amplitude of the first CF-EPSC from Aa and superimposed (a+b). The times at which these records were taken are indicated in B. B, amplitudes of the first (○) and the second (•) CF-EPSCs plotted against time. The arrow indicates the time at which the stimulus strength was decreased to change the number of stimulated climbing fibres. C, amount of PPD plotted against time. Note that no change in the amount of PPD was induced by changing the number of stimulated climbing fibres.
Effects of changing the postsynaptic responsiveness
The manipulations of the synaptic strength adopted so far have all focused on changing the presynaptic factors. We then examined whether manipulations selectively affecting the postsynaptic responsiveness might have any effect on PPD.
The first selective postsynaptic manipulation was to reduce the amplitudes of CF-EPSCs by partially blocking the postsynaptic non-NMDA receptors using CNQX (Fig. 5). Bath application of CNQX (1 μm) markedly reduced both the first and second CF-EPSCs to about 15% of control (Fig. 5A and B), whereas the CF-EPSC ratio was not significantly affected by CNQX application (Fig. 5Ac and C).
Figure 5. Partial blockade of the postsynaptic non-NMDA receptors by CNQX does not change the amount of PPD.

A, specimen records of CF-EPSCs (average of five consecutive responses) in response to paired stimuli (50 ms interval) taken before (a) and during (b) bath application of CNQX (1 μm). In c, the first CF-EPSC from Ab is scaled to the amplitude of the first CF-EPSC from Aa and superimposed (a+b). The times at which specimen records were taken are indicated in B. B and C, amplitudes of the first (○) and second (•) CF-EPSCs (B) and the amount of PPD (C) plotted against time.
The second selective postsynaptic manipulation involved changing the holding potential and thereby the driving force for the synaptic currents. The holding potential was shifted from −20 to −10 mV, or from −10 to 0 mV, while continuously recording EPSCs to paired climbing fibre stimuli. Changing the holding potential caused a stepwise reduction of CF-EPSC amplitudes, but the CF-EPSC ratio remained constant throughout the recording period (data not shown).
Summary of the effects of experimental manipulations on PPD
All the data from experiments with various pre- and post-synaptic manipulations at an interstimulus interval of 40 or 50 ms are summarized in Fig. 6A. The ratio of PPD (CF-EPSC ratio during an experimental manipulation/CF-EPSC ratio in control) was plotted against the ratio of the first CF-EPSC amplitudes (first CF-EPSC amplitude during an experimental manipulation/first CF-EPSC amplitude in control).
Figure 6. Summary graphs for the effects of all experimental manipulations on PPD.

A, summary graph for experiments performed with interstimulus intervals of 40 or 50 ms. Ordinate: ratio of PPD (the ratio of the second to the first CF-EPSC obtained during an experimental manipulation, divided by the ratio of the second to the first CF-EPSC obtained during control period before the manipulation). Abscissa: ratio of the first CF-EPSC amplitude (the amplitude recorded during an experimental manipulation, divided by the value obtained during control period before the manipulation). All of the individual experiments are plotted. B, similar to A, but for experiments with interstimulus intervals of 1000 ms. Vh, holding potential.
Lowering extracellular Ca2+ concentration resulted in a change in the CF-EPSC ratio which was reciprocal with respect to the first EPSC amplitude (Fig. 6A, open circles). Baclofen and adenosine caused similar change in the CF-EPSC ratio (Fig. 6A, open squares and open triangles, respectively). In contrast, changing the number of stimulated climbing fibres caused no change in the ratio of PPD over the wide range of change in the first EPSC ratio (Fig. 6A, filled triangles). Two manipulations to change postsynaptic responsiveness with CNQX and by changing the holding potentials caused no change in the ratio of PPD over the wide range of change in the first EPSC ratio (Fig. 6A, filled squares for CNQX and filled circles for holding potential). These data are consistent with those presented in Figs 2–5.
Effects of lowering extracellular Ca2+ concentration and changing the holding potentials were examined also on PPD at an interstimulus interval of 1000 ms (Fig. 6B). Although the ratio of PPD was smaller, the results of these two manipulations were essentially similar to those with shorter interstimulus intervals (Fig. 6B). Lowering extracellular Ca2+ concentration resulted in a change in the CF-EPSC ratio which was reciprocal with respect to the first EPSC amplitude (Fig. 6B, open circles), whereas changing the holding potentials caused no consistent change in the ratio of PPD (Fig. 6B, filled circles).
These results strongly suggest that PPD is mainly due to a decrease in transmitter release from presynaptic terminals.
PPD in the presence of a low affinity AMPA receptor antagonist PDA
The time course of free glutamate in the cleft of AMPA receptor-mediated synapses can be estimated by the displacement of rapidly dissociating antagonists of AMPA receptors, such as PDA (Tong & Jahr, 1994). This approach can detect changes in glutamate clearance from the synaptic cleft, because the longer glutamate is present, the more AMPA receptors will become unbound by PDA. This method has been used to detect increases in effective concentration of free glutamate during paired-pulse facilitation in cultured hippocampal neurones (Tong & Jahr, 1994). To estimate changes in effective glutamate concentration at climbing fibre synaptic clefts during PPD, paired CF-EPSCs were evoked at 1000 ms intervals with and without preincubation of 1 mM PDA (Fig. 7A and B). Because PDA is a weak NMDA receptor agonist, AP-5 (100 μm) was included to eliminate the contribution of NMDA receptor activation. PDA inhibited the second CF-EPSC of the pair proportionately more (56.5 ± 3.6%) than the first (64.7 ± 3.6%, mean ±s.e.m., n = 8, P < 0.0001, Student's paired t test) (Fig. 7B). This indicates that glutamate concentration in the cleft during the second CF-EPSC is significantly lower than that during the first, and further supports the suggestion that PPD is due to the decrease in transmitter release.
Figure 7. A low affinity AMPA receptor antagonist, PDA, affects the amount of PPD.
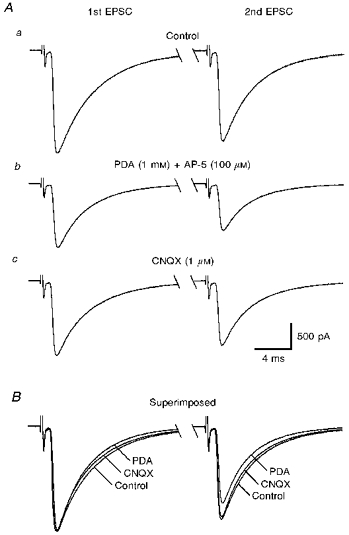
A, specimen records of paired CF-EPSCs (average of five consecutive responses) at interstimulus intervals of 1000 ms, taken sequentially before (a), during bath application of PDA (1 mM) together with AP-5 (100 μm) (b), and during bath application of CNQX (1 μm) (c). B, the first CF-EPSC from Ab and Ac are scaled to the amplitude of the first CF-EPSC from Aa and superimposed. Note that the second CF-EPSC in PDA is smaller than that of control or in CNQX.
Contribution of postsynaptic glutamate receptor desensitization on PPD
Takahashi, Kovalchuk & Attwell (1995) reported that application of diazoxide (500 μm), which preferentially blocks desensitization of AMPA receptors (Yamada & Rothmann, 1992), significantly affected PPD at relatively short interstimulus intervals (30 ms). To examine whether this is also true for PPD at longer interstimulus intervals, we measured PPD in the presence of diazoxide (500 μm) at interstimulus intervals of 50–3000 ms.
As reported previously (Barbour, Keller, Llano & Marty, 1994; Takahashi et al. 1995), diazoxide significantly prolonged the duration of CF-EPSCs (compare Fig. 8Aa with Ba). However, diazoxide had no significant effects on PPD at interstimulus intervals of 50 ms, as the CF-EPSC ratio was little affected by diazoxide (compare Fig. 8Aa with Ba, Fig. 8C). Diazoxide also had no significant effect on PPD at longer interstimulus intervals up to 3000 ms (not shown), indicating that diazoxide does not change the time course of PPD. Lowering extracellular Ca2+ concentration greatly influenced the amount of PPD in the presence of diazoxide (Fig. 8Bb and c). The CF-EPSC ratios in various extracellular Ca2+ concentrations were similar in the standard and diazoxide containing solutions (Fig. 8C). These results suggest that desensitization of postsynaptic glutamate receptors does not contribute significantly, if at all, to PPD of CF-EPSCs at interstimulus intervals of 50 ms or longer (up to 3000 ms).
Figure 8. Removal of glutamate receptor desensitization by diazoxide does not significantly affect PPD.
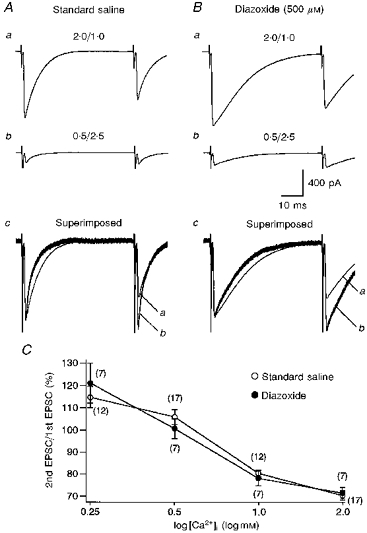
A, specimen records of CF-EPSCs (average of five consecutive responses) at interstimulus interval of 50 ms obtained in standard saline. Records in a and b were taken in extracellular solutions with [Ca2+]/[Mg2+] of 2.0/1.0 and 0.5/2.5, respectively. In c, the first CF-EPSC from b is scaled to the amplitude of the first CF-EPSC from a and superimposed. B, similar to A, but for records after switching to external solution containing diazoxide (500 μm). C, PPD of CF-EPSCs at four different extracellular [Ca2+]/[Mg2+] (0.25/2.75; 0.5/2.5; 1.0/2.0; 2.0/1.0) in the standard (○) and 500 μm diazoxide (•)-containing saline. Data are expressed as means ±s.e.m. and the sample numbers are given in parentheses.
Because diazoxide might enhance transmitter release as reported previously in cyclothiazide (Diamond & Jahr, 1995; Barnes-Davies & Forsythe, 1995), we took another non-pharmacological approach to assess the possible contribution of glutamate receptor desensitization. We used a triple-pulse protocol under recording conditions in which the transmitter release probability was low and postsynaptic desensitization was minimal (Barnes-Davies & Forsythe, 1995). Lowering extracellular Ca2+ concentration to 0.5 mM ([Ca2+]/[Mg2+], 0.5/2.5) resulted in significant reduction of the first CF-EPSC amplitude to 24.6 ± 4.1% (mean ±s.e.m.) of that in the normal Ca2+ concentration of 2 mM ([Ca2+]/[Mg2+], 2.0/1.0) (Fig. 9A). The ratio of the second to the first CF-EPSC (P2/P1) was 100.0 ± 2.7%, whereas that of the third to the second CF-EPSC (P3/P2) was 96.8 ± 4.1% (n = 10) (Fig. 9B). When the extracellular Ca2+ concentration was further reduced to 0.25 mM ([Ca2+]/[Mg2+], 0.25/2.75), the amplitude of the first EPSC was only 6.7 ± 2.4% of that in the normal Ca2+ concentration (Fig. 9A). The second CF-EPSC was potentiated over the first CF-EPSC (P2/P1=125.8 ± 11.1%) (Fig. 9B), which was, however, not significantly different from the P2/P1 value in the 0.5 mM Ca2+ solution (P = 0.09). A mixture of depression and facilitation appears to be present at this synapse. However, even in 0.25 mM Ca2+ solution, there appeared to be no significant facilitation of P3 over P2. The P3/P2 value in 0.25 mM Ca2+ solution (102.1 ± 2.6%) was not significantly different from that in 0.5 mM Ca2+ solution (96.8 ± 4.1%, P = 0.30) (Fig. 9B). The effect of depression seems to be significant even when the transmitter release probability is very low and postsynaptic desensitization was minimal.
Figure 9. CF-EPSCs in response to triple-pulse stimulation.

A, specimen records of CF-EPSCs (average of ten consecutive responses) in response to triple-pulse stimuli (interstimulus interval of 50 ms) in extracellular solutions with [Ca2+]/[Mg2+] of 2.0/1.0 (standard saline) (a), 0.5/2.5 (b), 0.25/2.75 (c). All data are from the same neurone. The ratios of the second to the first CF-EPSC (P2/P1) and that of the third to the second CF-EPSC (P3/P2) are indicated below each trace. B, summary bar graphs of the P2/P1 and P3/P2 values obtained in the 0.5/2.5 (left, n = 10) and 0.25/2.75 (right, n = 5) solutions. Data are expressed as means +s.e.m.
To examine further the possible contribution of postsynaptic desensitization to PPD, paired CF-EPSCs were evoked with and without preincubation of a glutamate uptake inhibitor, PDC (100 μm). Under these recording conditions, glutamate concentration in the synaptic cleft will be higher than control, and desensitization of glutamate receptors, if any, will be enhanced. As reported by Takahashi et al. (1995), PDC prolonged the CF-EPSC time course. The decay time constant (fitted by a single exponential; Llano et al. 1991) was 6.7 ± 0.5 ms before, and 9.9 ± 0.7 ms during PDC application (mean ±s.e.m., n = 5, P < 0.05). However, the extent of PPD in the presence of PDC (56.5 ± 4.2%) was not significantly different from that of the control (55.4 ± 3.4%, mean ±s.e.m., n = 5, P = 0.46), suggesting that postsynaptic desensitization does not play a major role in PPD.
Taken together, these three lines of experimental evidence strongly suggest that desensitization of postsynaptic glutamate receptors does not contribute significantly, if at all, to PPD of CF-EPSCs at interstimulus intervals of 50 ms or longer.
Effects of metabotropic glutamate, GABAB or adenosine receptor antagonists on PPD
One possible mechanism of the reduction of transmitter release by the second climbing fibre impulse would be that glutamate released from climbing fibre terminals during the first pulse binds to presynaptic autoreceptors, and reduces Ca2+ influx to terminals during the second pulse either by inhibiting Ca2+ channels or by opening K+ channels. We demonstrate that application of a metabotropic glutamate receptor (mGluR) agonist, ACPD (5 μm), reversibly depressed CF-EPSCs (Fig. 10A) presumably through activating mGluRs at climbing fibre presynaptic terminals. ACPD at 5 μm has been shown preferentially to activate group II mGluR (mGluR2/3) (Pin & Duvoisin, 1995). In contrast, a selective group III mGluR (mGluR4/6/7/8) agonist, L-(±)-2-amino-4-phosphonobutyric acid (L-AP4, 10 μm), was much less effective in depressing CF-EPSCs (data not shown). The mean magnitude of depression was 74.5 ± 4.6% for ACPD (5 μm, n = 10) and 96.1 ± 3.9% for L-AP4 (10 μm, n = 5) (mean ±s.e.m.). The ACPD-induced depression of CF-EPSCs was effectively antagonized by MCPG (500 μm), a non-selective group I/group II mGluR antagonist (Sekiyama et al. 1996) (Fig. 10B). The magnitude of ACPD-induced depression was only 90.2 ± 1.6% in the presence of MCPG (500 μm, n = 10). These results suggest that autoreceptors at climbing fibre terminals are mainly type II mGluRs and they are blocked by MCPG (500 μm). We then examined whether blockade of mGluRs has any effect on PPD of CF-EPSCs. We found that MCPG (500 μm) did not significantly affect the extent of PPD (Fig. 10C). Moreover, MAP4 (500 μm), a selective group III mGluR antagonist (Sekiyama et al. 1996), also had no effect on PPD. Magnitudes of PPD were 66.1 ± 2.1% before, and 65.7 ± 2.3% during MCPG application (mean ±s.e.m., n = 12, interstimulus interval of 40 ms), and 52.9 ± 1.9% before, and 52.8 ± 2.5% during MAP4 application (n = 5). These results indicate that activation of presynaptic mGluRs by glutamate released by the first pulse plays no significant role, if any, in PPD of CF-EPSCs.
Figure 10. Blockade of metabotropic glutamate receptors has no effect on PPD.
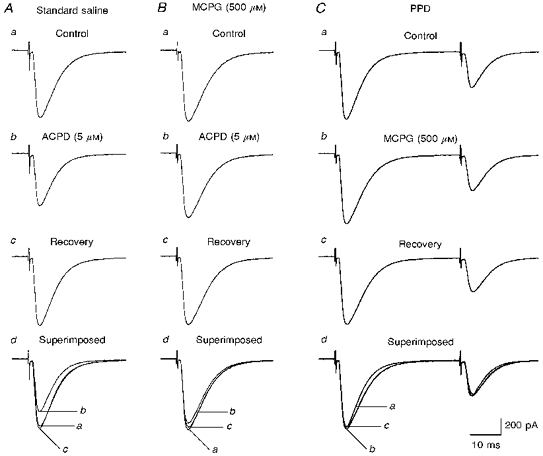
A, specimen records of CF-EPSCs (average of five consecutive responses) taken sequentially before (a), during bath application of ACPD (5 μm) (b), and after washing out ACPD (recovery) (c). In d, the CF-EPSC traces from a, b and c are superimposed. B, similar to A, but in the presence of MCPG (500 μm). Note that MCPG significantly antagonized the ACPD-induced depression of CF-EPSC. C, specimen records of paired CF-EPSCs (average of five consecutive responses) at interstimulus intervals of 40 ms. Records were taken sequentially before (a), during bath application of MCPG (500 μm) (b), and after washing out MCPG (recovery) (c). In d, the CF-EPSC traces in a, b, and c are superimposed. All records in A, B and C were obtained from the same Purkinje cell.
Another possibility would be that other neurotransmitters such as GABA or adenosine bind to their receptors at climbing fibre presynaptic terminals and inhibit transmitter release. GABA could be released from inhibitory interneurones that might be activated polysynaptically by the first climbing fibre stimulus. Adenosine might be co-released with glutamate from climbing fibre terminals. We first verified that 2-hydroxysaclofen (500 μm), a selective GABAB receptor antagonist, and theophylline (100 μm), an adenosine receptor antagonist, effectively antagonized the depressions of CF-EPSCs induced by baclofen (50 μm) and adenosine (100 μm), respectively. Magnitudes of baclofen-induced depression were 79.3 ± 3.6% before, and 92.4 ± 0.6% during 2-hydroxysaclofen application (mean ±s.e.m., n = 4). Magnitudes of adenosine-induced depression were 85.4 ± 4.4% before, and 94.4 ± 2.3% during theophylline application (n = 3). We then examined effects of 2-hydroxysaclofen and theophylline on PPD. We found neither 2-hydroxysaclofen (500 μm) nor theophylline (100 μm) significantly affected the extent of PPD. Magnitudes of PPD were 72.5 ± 2.1% for before, and 73.7 ± 2.1% for during 2-hydroxysaclofen application (n = 9, interstimulus interval of 50 ms), and 71.3 ± 2.0% for before, and 71.9 ± 2.3% for during theophylline application (n = 8). These results suggest that neither GABAB nor adenosine receptors are involved in PPD.
DISCUSSION
PPD affects the complex spikes and the following after-potentials
The present results clearly indicate that the shape of a complex spike is significantly influenced by the preceding complex spike depending on the interspike interval. Using the paired-pulse protocol under current-clamp recording conditions with physiological extracellular and pipette solutions, we have shown that the number of spikelets of the second complex spike decreased at interstimulus intervals up to 500 ms (Fig. 1). This depressant effect is not likely to result from the after-hyperpolarization that follows the complex spike. First, under our recording conditions, the duration of after-hyperpolarization was much shorter than the duration of the depressant effect on the second complex spikes. Second, the shape of the complex spike was not affected by artificial hyperpolarization of 5–10 mV by direct current injection which was larger than the after-hyperpolarization (authors’ unpublished observation). Therefore, PPD of the CF-EPSP appears to be a major cause of the depressant effect on the complex spike.
Besides this depressant effect, we have found that the amplitude and duration of the after-depolarization and after-hyperpolarization are significantly reduced following the second complex spike (Table 1). A Ca2+-activated K+ conductance is reported to be one factor that produces after-hyperpolarization (Llinás & Sugimori, 1980a, b). Therefore, the depression can be explained by the reduction of the Ca2+ influx during the second complex spike.
An alternative mechanism for the depression of the second complex spike and after-hyperpolarization would be that climbing fibre stimulation caused a concomitant activation of cortical inhibitory interneurones that form synaptic contacts on Purkinje cells. Callaway et al. (1995) reported that Ca2+ influx induced by climbing fibre stimulation is significantly depressed by simultaneous activation of inhibitory interneurones in the molecular layer. The shape of complex spikes, however, is not affected by cortical inhibition when recorded from Purkinje cell somata (Callaway et al. 1995). In contrast, a clear decrease in the number of spikelets in the second complex spikes was consistently observed by the paired-pulse protocol (Fig. 1). This indicates that the depressant effect of paired-pulse stimulation is stronger than that produced by cortical inhibition. In the presence of bicuculline to suppress cortical inhibition, we observed that the paired-pulse protocol produced a clear reduction of the number of spikelets in the second complex spike. Furthermore, each complex spike is followed by clear after-depolarization and after-hyperpolarization the duration and amplitude of which are equivalent to those recorded in normal external solution (authors’ unpublished observation). These results suggest that climbing fibre stimulation used in the present study did not produce significant concomitant activation of inhibition on Purkinje cells. Thus, the results of the present study suggest that PPD of climbing fibre mediated EPSP is a major cause for the depressant effect on the second complex spike and the after-potentials.
Decrease in transmitter release is the major cause for PPD
Several lines of evidence presented in the present study are consistent with the notion that PPD of CF-EPSC at interstimulus intervals of 50 ms or longer is due exclusively to presynaptic change. First, lowering extracellular Ca2+ concentration, which is known to decrease Ca2+ influx to presynaptic terminals and reduces transmitter release, caused reduction of the amplitudes of CF-EPSCs and significantly changed the extent of PPD measured as the ratio of the second to the first CF-EPSC amplitude (Fig. 2). Second, baclofen and adenosine, both of which are known to inhibit transmitter release from presynaptic terminals (Dunwiddie, 1985; Lupica et al. 1992; Prince & Stevens, 1992; Scanziani et al. 1992; Isaacson et al. 1993; Pfrieger et al. 1994), caused reduction of CF-EPSC amplitudes that is accompanied by a reciprocal change of the CF-EPSC ratio (Fig. 3). On the other hand, changing the number of presynaptic release sites had no effect on the CF-EPSC ratio, when tested in multiply innervated Purkinje cells by stimulating a different number of climbing fibres (Fig. 4). Two manipulations that selectively affect postsynaptic responsiveness had no effect on PPD. These included partial blockade of postsynaptic non-NMDA receptors by CNQX (Fig. 5), and changing the holding potentials. These results suggest that PPD at interstimulus intervals of 50 ms or longer is purely of presynaptic origin. The cause of PPD appears to be reduction of the amount of transmitter release rather than decrease in the number of release sites.
As previously reported by Takahashi et al. (1995) blocking the desensitization of postsynaptic glutamate receptors using diazoxide (500 μm) significantly prolonged the duration of CF-EPSC and reduced the CF-EPSC ratio (Fig. 8). This clearly indicates that the transmitter released at each climbing fibre impulse leaves significant desensitization of postsynaptic glutamate receptors, which leads to reduction of the amplitude of subsequent CF-EPSCs. This effect, however, did not appear to contribute to PPD at interstimulus intervals of 50 ms or longer. Three lines of experimental evidence strongly support this notion. First, diazoxide had almost no effect on the amount of PPD (Fig. 8A and B). The presence of diazoxide did not influence the modulating effect of lowering external Ca2+ (Fig. 8C). Second, the triple-pulse protocol revealed that the effect of depression was still significant even when the transmitter release probability is very low and postsynaptic desensitization was minimal (Fig. 9). Third, PPD was not affected by a glutamate uptake inhibitor, PDC. Under these recording conditions, glutamate concentration in the synaptic cleft were elevated and postsynaptic desensitization was facilitated. These results support the suggestion that PPD is due to presynaptic change and that desensitization of postsynaptic glutamate receptors does not significantly contribute to PPD.
Precise mechanisms of the reduction of transmitter release by the second climbing fibre impulse remain to be elucidated. The present results that mGluR, GABAB or adenosine receptor antagonists had no effect on PPD suggest that negative feedback of transmitter release through presynaptic autoreceptors is unlikely to operate at climbing fibre synapses. We cannot exclude, however, the possibility that some unidentified substance released from climbing fibre terminals contributes to this negative feedback. Another possibility would be that the first climbing fibre impulse causes partial depletion of a readily releasable transmitter pool and it takes 1–3 s to refill completely.
Functional significance of PPD
After-hyperpolarization and -depolarization are important factors to determine the firing pattern of CNS neurones. Indeed, recordings from Purkinje cells in vivo in awake decerebrate cat indicate that firing rate of simple spikes is significantly enhanced after each complex spike in the majority of Purkinje cells (Ebner & Bloedel, 1981; McDevitt et al. 1982; Sato et al. 1992). We assume that after-depolarization following the complex spike is a major determinant of this facilitation of simple spike firing, because its duration corresponds to the duration of after-depolarization recorded in the present study (Table 1). Thus, the interspike interval of complex spikes can greatly influence the Purkinje cell excitability and firing pattern by modulating the duration of after-depolarization. The present results suggest that PPD of climbing fibre mediated EPSP contributes to this modulation.
Besides short-term modulation of simple spike firing, climbing fibre-induced Ca2+ transients are known to trigger long-term depression of parallel fibre synapses (Sakurai, 1990; Konnerth et al. 1992) and rebound potentiation of inhibitory synapses (Kano et al. 1992). The present results suggest that PPD reduces the number of spikelets during the second complex spike (Fig. 1). If this change accompanies reduction of the climbing fibre induced Ca2+ influx, it is possible that not only the number but also the pattern of climbing fibre inputs may determine the efficacy for inducing these two forms of long-term synaptic plasticity.
Acknowledgments
The authors thank Dr N. Kawai for continual encouragement throughout the course of this study, K. Matsumoto and Y. Hirano for excellent technical assistance, and Drs R. Kado and F. Tempia for critically reading the manuscript. This work was supported by grants to M. K. from the Japanese Ministry of Education, Science, Sports and Culture, the Uehara Foundation, the Ciba-Geigy Foundation for the Promotion of Science, the Kato Memorial Bioscience Foundation and the Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation (JST).
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ. Paired-pulse facilitation in the dentate gyrus: a patch-clamp study in rat hippocampus in vitro. Journal of Neurophysiology. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. Journal of Physiology. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. Journal of Neuroscience. 1995;15:2777–2787. doi: 10.1523/JNEUROSCI.15-04-02777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV. The physiological role of adenosine in the central nervous system. International Review of Neurobiology. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Bloedel JR. Temporal patterning in single spike discharge of Purkinje cells and its relationship to climbing fiber activity. Journal of Neurophysiology. 1981;45:933–947. doi: 10.1152/jn.1981.45.5.933. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch-clamp recordings from neurons of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Pre- and post-synaptic mechanisms of paired-pulse depression of climbing fiber to Purkinje cell synapses in the cerebellum. Neuroscience Research Supplement. 1996;20:S30. [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S. Impaired synapse elimination during cerebellar development in PKCγ mutant mice. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Kano M, Konnerth A. Cerebellar slices for patch clamp recording. In: Kettenmann H, Grantyn R, editors. Practical Electrophysiological Methods. New York, USA: Wiley-Liss; 1992. pp. 54–57. [Google Scholar]
- Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic currents in cerebellar Purkinje cells. Nature. 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. Journal of Physiology. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöpfel T, Vrancesic L, Staub C, Gähwiler BH. Climbing fibre responses in olivo-cerebellar slice cultures. II. dynamics of cytosolic calcium in Purkinje cells. European Journal of Neuroscience. 1991;3:343–348. doi: 10.1111/j.1460-9568.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Dreessen J, Augustine JG. Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proceedings of the National Academy of Sciences of the USA. 1992;89:7051–7055. doi: 10.1073/pnas.89.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proceedings of the National Academy of Sciences of the USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Marty A, Armstrong CM, Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slice. Journal of Physiology. 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. Journal of Physiology. 1980a;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. Journal of Physiology. 1980b;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Proctor WR, Dunwiddie TV. Presynaptic inhibition of excitatory synaptic transmission by adenosine in rat hippocampus: analysis of unitary EPSP variance measured by whole-cell recording. Journal of Neuroscience. 1992;12:3753–3764. doi: 10.1523/JNEUROSCI.12-10-03753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt CJ, Ebner TJ, Bloedel JR. The changes in Purkinje cell simple spike activity following spontaneous climbing fiber inputs. Brain Research. 1982;237:484–491. doi: 10.1016/0006-8993(82)90460-7. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJA, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. Journal of Neurophysiology. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Lev-Ram V, Lasser-Ross N, Ross WN. Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. Journal of Neurophysiology. 1992;68:1178–1189. doi: 10.1152/jn.1992.68.4.1178. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Palestini M, Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. Journal of Physiology. 1982;332:187–202. doi: 10.1113/jphysiol.1982.sp014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. New York, Heidelberg, Berlin: Springer-Verlag; 1974. [Google Scholar]
- Perkel DJ, Hestrin S, Sah P, Nicoll RA. Excitatory synaptic currents in Purkinje cells. Proceedings of the Royal Society. 1990;B 241:116–121. doi: 10.1098/rspb.1990.0074. [DOI] [PubMed] [Google Scholar]
- Pfrieger DJ, Gottmann K, Lux HD. Kinetics of GABAB receptor-mediated inhibition of calcium currents and excitatory synaptic transmission in hippocampal neurons in vitro. Neuron. 1994;12:97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Prince DA, Stevens CF. Adenosine decreases neurotransmitter release at central synapses. Proceedings of the National Academy of Sciences of the USA. 1992;89:8586–8590. doi: 10.1073/pnas.89.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M. Calcium in an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proceedings of the National Academy of Sciences of the USA. 1990;87:8383–8385. doi: 10.1073/pnas.87.9.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Miura A, Fushiki H, Kawasaki T. Short-term modulation of cerebellar Purkinje cell activity after spontaneous climbing fiber input. Journal of Neurophysiology. 1992;68:2051–2062. doi: 10.1152/jn.1992.68.6.2051. [DOI] [PubMed] [Google Scholar]
- Savio T, Tempia F. On the Purkinje cell activity increase induced by suppression of inferior olive activity. Experimental Brain Research. 1985;57:456–463. doi: 10.1007/BF00237832. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gähwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Sekiyama N, Hayashi Y, Nakanishi S, Jane DE, Tse HW, Birse EF, Watkins JC. Structure-activity relationships of new agonists and antagonists of different metabotropic glutamate receptor subtypes. British Journal of Pharmacology. 1996;117:1493–1503. doi: 10.1111/j.1476-5381.1996.tb15312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Kovalchuk Y, Attwel D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. Journal of Neuroscience. 1995;15:5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rothman SM. Diazoxide blocks glutamate desensitization and prolongs excitatory postsynaptic currents in rat hippocampal neurons. Journal of Physiology. 1992;458:409–423. doi: 10.1113/jphysiol.1992.sp019424. [DOI] [PMC free article] [PubMed] [Google Scholar]


