Abstract
Transcranial magnetic stimulation over motor areas of cerebral cortex in man can activate short latency bilateral cortical projections to the pharynx and oesophagus. In the present paper we investigate the interaction between pathways from each hemisphere and explore how activity in these pathways is modulated by afferent feedback from the face, pharynx and oesophagus.
Comparison of unilateral and bilateral stimulation (using interstimulus intervals (ISIs) of 1, 5 or 10 ms between shocks) showed spatial summation of responses from each hemisphere at an ISI of 1 ms, indicating that cortical efferents project onto a shared population of target neurones. Such summation was not evident at ISIs of 5 or 10 ms. There was little evidence for transcallosal inhibition of responses from each hemisphere, as described for limb muscles.
Single stimuli applied to the vagus nerve in the neck or the supraorbital nerve, which alone produce intermediate (onset 20-30 ms) and long (50-70 ms) latency reflex responses in the pharynx and oesophagus, were used to condition the cortical responses. Compared with rest, responses evoked by cortical stimulation were facilitated when they were timed to coincide with the late part of the reflex. The onset latency was reduced during both parts of the reflex response. No facilitation was observed with subthreshold reflex stimuli.
Single electrical stimuli applied to the pharynx or oesophagus had no effect on the response to cortical stimulation. However, trains of stimuli at frequencies varying from 0.2 to 10 Hz decreased the latency of the cortically evoked responses without consistently influencing their amplitudes. The effect was site specific: pharyngeal stimulation shortened both pharyngeal and oesophageal response latencies, whereas oesophageal stimulation shortened only the oesophageal response latencies.
Cortical swallowing motor pathways from each hemisphere interact and their excitability is modulated in a site-specific manner by sensory input. The latter may produce a mixture of excitation and inhibition at both brainstem and cortical levels.
The process of swallowing comprises an ordered sequence of sensory and motor events that transport food from mouth to stomach, whilst ensuring protection of the airway. It is functionally divided into three stages: oral, pharyngeal and oesophageal (Miller, 1982), reflecting the anatomical structures involved. The central neural control of swallowing is also divided, with cortical centres in conjunction with afferent feedback from the musculature acting to initiate and modulate the volitional swallow (Sumi, 1969; Car, 1970; Hockman, Beiger & Weerasuriya, 1979; Jean, 1990; Martin & Sessle, 1993), whilst the brainstem swallowing centre (Jean, 1990) generates the sequenced events of reflex swallowing via the V, IX, X and XII cranial nerve nuclei. The interaction between each of these elements is responsible for a normal swallow in healthy subjects, whilst their disruption will lead to swallowing dysfunction (Wiles, 1991).
The importance of suprabulbar influences in regulating swallowing has been established in animal studies, through disruption of cortical swallowing regions by lesioning, anaesthesia or cooling (Sumi, 1969, 1972b; Hockman et al. 1979; Martin & Sessle, 1993), and disturbing the normal swallowing pattern. Animal data have also demonstrated that repetitive stimulation of either hemisphere can evoke a swallow, that stimulation of both cerebral hemispheres, simultaneously, can enhance the number of swallows evoked compared with unilateral stimulation (Sumi, 1969), and that concurrent afferent excitation can facilitate swallowing in an intensity- and frequency-dependent manner (Sumi, 1969; Miller, 1972; Beiger & Hockman, 1976; Jean & Car, 1979).
We have shown that the cortical pathways to human swallowing musculature can be studied by applying transcranial magneto-electric stimuli to the motor cortex whilst recording the electromyographic (EMG) responses evoked from the oro-pharynx and upper oesophagus (Aziz et al. 1994; Hamdy et al. 1996). In addition, we have demonstrated that these pathways are present on both hemispheres, display marked inter-hemispheric asymmetry independent of handedness (Aziz, Rothwell, Hamdy, Barlow & Thompson, 1996; Hamdy et al. 1996), and can be facilitated by prior stimulation of cranial nerve afferents, which evoke reflex pharyngo-oesophageal EMG responses (Aziz, Rothwell, Barlow & Thompson, 1995; Hamdy, Aziz, Rothwell, Hobson, Barlow & Thompson, 1997). It remains uncertain, however, how the cortical centres on each hemisphere interact, or how the facilitation of the cortical swallowing pathways is modulated by afferent stimulation.
We therefore conducted the following series of studies, in healthy human volunteers, to explore (i) how bilateral stimulation of the cortical hemispheres would influence the cortically evoked swallow response, and (ii) whether the facilitation of the cortical swallowing pathways by afferent pathway stimulation displays intensity or frequency dependence.
METHODS
Subjects
All participants in the studies were healthy adult volunteers recruited from personnel affiliated with the research units involved in the project. None gave any history of swallowing problems. The project was formally approved by Salford & Trafford Ethics Committee. Subjects were given details of the experimental protocol and all gave full written informed consent before the studies were conducted.
Magnetic stimulation
Magnetic stimulation of the cerebral cortex and cranial nerves was performed using two commercially available magnetic stimulators (Magstim 200, MAGSTIM Company Limited, Whitland, Dyfield, UK).
Cortical stimulation
Focal magnetic stimulation of one hemisphere was performed using a 70 mm (outer diameter) figure of eight coil oriented at an angle of 45 deg to the parasagittal plane, tangential to the scalp surface and with the anterior edge of the bifurcation positioned over the site of interest.
Focal bilateral stimulation was performed using two identical magnetic stimulators and two figure of eight coils, one positioned over each hemisphere. Both stimulators were attached to a timing device (Bistim Module, MAGSTIM Company Limited), the output of which was programmed to discharge the stimulators at intervals varying from 1-10 ms.
Diffuse stimulation of both hemispheres was performed using a single magnetic stimulator connected to a 90 mm (outer diameter) circular coil, which, when discharged over the vertex of the cranium, provided diffuse stimulation of the cortex beneath the coil.
Cranial nerve stimulation
This was performed using a magnetic stimulator connected to a small (50 mm outer diameter) figure of eight coil, which provided focal stimulation of an area of tissue approximately one square centimetre beneath its bifurcation.
Stimulation of the vagus nerve was performed by discharging the coil over the right side of neck, 2 cm below the angle of the jaw, at the anterior border of the sternocleidomastoid muscle (Aziz et al. 1994, 1995).
Stimulation of the trigeminal nerve was performed by positioning the centre of the figure of eight coil over the right supra-orbital branch of the trigeminal nerve (Hamdy et al. 1997). This was chosen because it is a purely afferent branch, and because its excitation evokes a bilateral blink reflex (Kimura, Powers & Van Allen, 1969), thereby confirming that effective stimulation had occurred.
Combined cranial nerve and cortical stimulation
This was performed using the two magnetic stimulators connected to the timing device, programmed to discharge both stimulators at intervals varying from 10-100 ms.
Electrical stimulation
Electrical stimulation of the pharynx and oesophagus was performed using two pairs of bipolar platinum ring electrodes built into a 3 mm diameter intraluminal catheter (Gaeltec, Dunvegan, Isle of Skye, UK). The electrode pairs were sited 5 and 12 cm from the distal tip of the catheter, the proximal electrode pair being used to stimulate the mucosa of the pharynx and the distal pair to stimulate the mucosa of the upper oesophagus. Each electrode pair had an inter-electrode distance of 1 cm and each was connected to an electrical stimulator device (Stimulator Model DS7, Digitimer, Welwyn Garden City, Hertfordshire, UK) via a trigger generator (Neurolog System, Digitimer), which delivered trains of stimuli (pulse duration, 0.1 ms; voltage, 280 V) repetitively, at frequencies of 0.2-10 Hz.
Combined sensory and cortical stimulation
This was performed by connecting both the magnetic stimulator and the electrical stimulator to a timing device (Neurolog System, Digitimer), programmed to discharge the magnetic stimulator 100 ms after the last pulse of a train of twenty-five electrical stimuli for each frequency studied.
Electromyographic recording
The swallow muscle groups chosen for study were the pharyngeal muscles and the striated muscle of the upper oesophagus.
EMG responses were detected using a second intraluminal catheter identical to that used for electrical stimulation. The proximal electrode pair was used to detect pharyngeal EMG responses and the distal electrode pair to detect oesophageal EMG responses.
Each electrode pair was connected to a pre-amplifier (CED 1902, Cambridge Electronic Design, Cambridge, UK) with filter settings of 5-2000 Hz. Response signals were then collected through a laboratory interface (CED 1401 plus, Cambridge Electronic Design) at a sampling rate of 4-8 kHz and fed into a 486 Sx desktop computer (Mitac Europe Ltd, Shropshire, UK) for immediate display, data collection and averaging. During each study, electrode contact was monitored at 10 min intervals by observing the real time EMG responses to a wet swallow.
Manometric recording
Two solid-state strain gauge transducers (Gaeltec) were incorporated into the recording catheter, one between each electrode pair. The output of each transducer was amplified and connected to a solid-state data logger (TDS 9090 Forth Computer, Triangle Digital Services Ltd, London, UK), and then down loaded onto a 486 Sx desktop computer for display and storage. Manometric measurements were made from the pharynx and oesophagus at the start of, and during, each study. This enabled the upper oesophageal sphincter to be identified, and the distal electrode pair to be maintained in position, 3 cm below its lower margin.
Experimental protocols
All the protocols described below were presented to, and approved by, the Salford Health Authority Ethics Committee. Throughout each study, the volunteer sat comfortably in a chair, and the vertex of the cranium was identified according to the international 10-20 system (Jasper, 1958). The pharyngo-oesophageal catheter for recording EMG responses was then inserted either trans-orally or trans-nasally, depending on subject preference.
Study 1: effect of single and bilateral hemispheric stimulation on cortically evoked EMG responses
Eight volunteers (five male, three female; mean age, 29 years; age range, 22-35 years) were studied. First, to determine the optimal sites for cortical stimulation for each subject, a preliminary mapping study was performed by discharging the figure of eight coil over multiple grid points, 1 cm apart, in an 8 cm × 8 cm area anterior and lateral to the vertex, using stimulation intensities of 2.2 tesla (T) (100 % stimulator output). The sites evoking the greatest EMG responses for each muscle group on each hemisphere were identified and marked on the scalp.
Next, a series of cortical stimulations were performed over these marked positions, starting at a subthreshold intensity and increasing in 5 % steps until the threshold intensity for evoking EMG responses of greater than 30 μV in five out of ten consecutive trials was found. The stimulation coil was then repositioned over one or other hemisphere, the order of study being randomized between subjects, and at each site the coil was discharged at the following three intensities: (i) 10 % below threshold intensity, (ii) threshold intensity, and (iii) 10 % above threshold intensity. Three stimuli were delivered at each intensity, with an interval of 15 s between each stimulation, and the EMG responses to each stimulus recorded.
Finally, bilateral hemispheric stimulation of the chosen sites was performed at the subthreshold, threshold and suprathreshold intensities, using interstimulus intervals (ISIs) of 1, 5 and 10 ms, three paired stimuli being delivered at 15 s intervals for each stimulation intensity at each ISI, and the responses recorded.
Study 2: effect of cranial nerve stimulation intensity on cortically evoked EMG responses
Six volunteers (four male, two female; mean age, 32 years; age range, 22-45 years) were studied. First, the figure of eight coil was discharged over the right supra-orbital nerve or the right vagus nerve at an intensity of 30 % stimulator output and increased in 5 % steps until EMG responses were evoked in both muscle groups. The lowest intensity which induced reflex responses in 50 % of trials for both muscle groups was defined as the threshold stimulus intensity for that nerve.
Next, the cerebral cortex of each subject was diffusely stimulated, initially at a discharge intensity of 30 %, increasing in 5 % steps until EMG responses of greater than 30 μV were obtained in both muscle groups on at least five out of ten consecutive trials, this being defined as the threshold intensity. Three stimuli, 15 s apart, were then delivered at 20 % above the threshold stimulus intensity and the EMG responses to each recorded.
Finally, a series of stimuli were delivered to the trigeminal and vagus nerves, in random order, at the following intensities: (i) 5 % stimulator output (‘sham’ excitation), (ii) 10 % below threshold, (iii) threshold and (iv) 10 % above threshold. Each stimulus was followed by diffuse stimulation of the cortex, at 20 % above threshold intensity, at either 10, 20, 30, 50, or 100 ms intervals. Three stimulations, each 15 s apart, were delivered for each interval, and the EMG responses from each muscle group were recorded.
Study 3: effect of repetitive pharyngeal and oesophageal stimulation on cortically evoked EMG responses
Seven volunteers were studied (four male, three female; mean age, 33 years; age range, 22-44 years) were studied. First, the electrical stimulation catheter was inserted alongside the EMG recording catheter, with the distal electrode pair positioned in the upper oesophagus and the proximal pair in the pharynx. Next, the cerebral cortex was diffusely stimulated, at an intensity of 30 %, and then intensity was increased in 5 % steps until responses of greater than 30 μV were obtained in 50 % of trials in both the pharynx and oesophagus; this intensity being defined as the threshold. Three stimuli, 15 s apart, were then delivered at 20 % above threshold and the EMG responses recorded.
Finally, repeated electrical stimulation of the pharynx or oesophagus was performed, at a constant stimulus intensity which was just perceived by the subject, and at varying frequencies (0.2, 0.5, 1, 5 and 10 Hz). One hundred milliseconds after the delivery of the last of a series of twenty-five stimuli, the cerebral cortex was stimulated at 20 % above threshold intensity. The procedure was repeated three times at each stimulation frequency, and the EMG responses from each muscle group recorded.
Definition of terms
Response latency: the interval between the onset of the stimulus and the onset of the EMG response, expressed in milliseconds (ms).
Response amplitude: the maximum peak-to-peak voltage of the EMG response, expressed in microvolts (μV).
Response facilitation: the enhancement of the EMG response, ascertained as either a reduction in the response latency or an increase in the response amplitude.
Data analysis
For each muscle group, the mean values of the EMG responses evoked in studies 1-3 were calculated and used for data analysis. The normality of the data was then assessed using the Shapiro-Wilk W test (Altman, 1991). In study 1, responses evoked following bilateral hemispheric stimulation were compared both with those obtained following unilateral hemispheric stimulation of either side, and with the calculated sum of the responses of the two individual hemispheres. Student's paired, two-tailed, t test was used for the normally distributed latencies and the Wilcoxon signed-rank sum test for the non-normally distributed response amplitudes. In study 2, the cortically evoked EMG responses following cranial nerve stimulation at each intensity were compared with the responses following sham stimulation, using repeated measures ANOVA, with post hoc Student's t tests to determine where the differences lay. In study 3, the cortically evoked responses obtained during pharyngeal and oesophageal stimulation for each frequency were compared with those evoked by cortical stimulation alone, again using repeated measures ANOVA and post hoc Student's t tests, where appropriate. Results are expressed in the text as means ± standard error of the mean (s.e.m.) unless stated otherwise. A P value of 0.05 or less was taken to indicate that any observed differences were unlikely to have occurred by chance.
RESULTS
As reported in previous studies (Aziz et al. 1994; Hamdy et al. 1996), cortical stimulation evoked early and late EMG responses in both the pharynx and oesophagus. The early responses were biphasic or triphasic and were reproducible within individuals. The late responses were, in contrast, polyphasic and inconsistently evoked. All results described below relate to the early responses. Because the experiments were conducted in relaxed subjects without overt voluntary facilitation, response amplitudes were small but reproducible (Aziz et al. 1994).
Comparison of unilateral and bilateral hemispheric stimulation on cortically evoked EMG responses
The mean stimulus intensities used for subthreshold, threshold and suprathreshold stimulus pairings were 64 ± 5, 74 ± 5 and 84 ± 5 %, respectively, for the right hemisphere, and 68 ± 5, 78 ± 5 and 88 ± 5 %, respectively, for the left hemisphere.
Responses to bilateral stimulation at an ISI of 1 ms were often larger than the sum of the responses to each (unilateral) stimulus given alone (Figs 1 and 2). This was significant for responses in the pharynx when subthreshold intensities were used, and approached significance in both pharynx and oesophagus at higher intensities (P < 0.06; Wilcoxon signed-rank sum test). Responses were no longer facilitated when the ISI was lengthened to 5 or 10 ms. Response latency decreased by 0.8-1 ms with bilateral stimulation at an ISI of 5 ms, but not at ISIs of 1 or 10 ms.
Figure 1. Effects of increasing intensities of bilateral hemispheric stimuli on the cortically evoked swallow muscle responses.
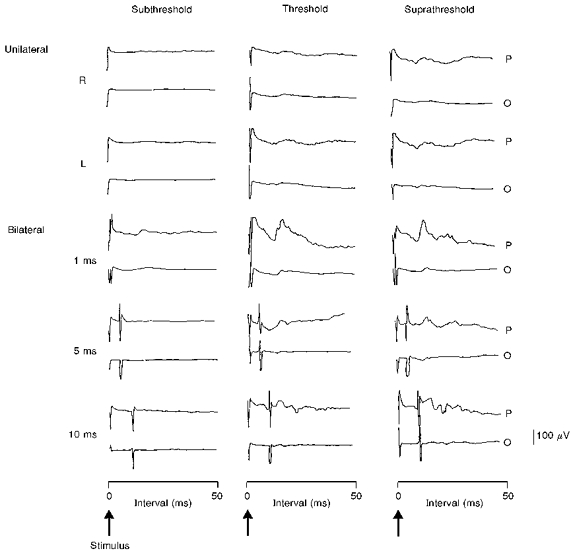
The mean (of 3 trials) cortically evoked pharyngeal (P) and oesophageal (O) EMG responses in one individual are shown for each stimulation intensity following unilateral right (R) and left (L) hemisphere stimulation, and then bilateral hemispheric stimulation at ISIs of 1, 5 and 10 ms. It can be seen that subthreshold bilateral hemispheric stimulation only evokes pharyngeal responses at an ISI of 1 ms. In comparison, bilateral stimulation at threshold and suprathreshold intensities enhances both the pharyngeal and oesophageal response amplitudes at an ISI of 1 ms, which reduces at ISIs of 5 and 10 ms.
Figure 2. Comparison of bilateral and unilateral hemisphere stimulation on the cortically evoked swallow muscle responses at increasing intensities.
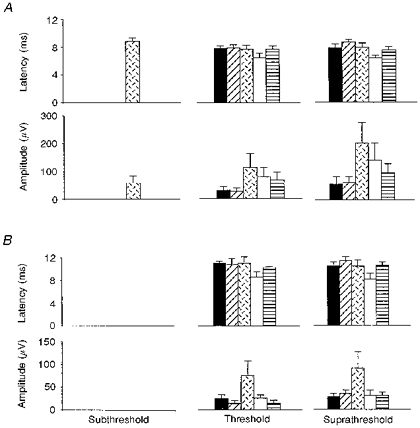
Histogram plots of the group (n= 8) mean (±s.e.m.) response latencies and amplitudes, for the pharynx (A) and oesophagus (B), are shown for each stimulation intensity following unilateral right (▪) and left ( ) hemisphere stimulation, and bilateral hemisphere stimulation with ISIs of 1 ms (
) hemisphere stimulation, and bilateral hemisphere stimulation with ISIs of 1 ms ( ), 5 ms (□) and 10 ms (
), 5 ms (□) and 10 ms ( ). In both the pharynx and oesophagus, bilateral stimulation, at threshold and suprathreshold intensities, always increased the response amplitudes at an ISI of 1 ms, and shortened the response latencies at an ISI of 5 ms compared with single hemisphere stimulation. In contrast, at subthreshold intensities, bilateral stimulation enhanced only the pharyngeal responses, and only at an ISI of 1 ms.
). In both the pharynx and oesophagus, bilateral stimulation, at threshold and suprathreshold intensities, always increased the response amplitudes at an ISI of 1 ms, and shortened the response latencies at an ISI of 5 ms compared with single hemisphere stimulation. In contrast, at subthreshold intensities, bilateral stimulation enhanced only the pharyngeal responses, and only at an ISI of 1 ms.
Effect of cranial nerve stimulation intensity on cortically evoked EMG responses
We have reported previously (Hamdy et al. 1997) that single suprathreshold stimuli applied alone to either the trigeminal or vagus nerve produce an intermediate and a late reflex response in the pharynx and oesophagus, with onset latencies of about 20-30 and 50-70 ms, respectively. If such stimuli are given 50 or 100 ms prior to a cortical shock, then the cortical potential occurs within the later part of the reflex response: its amplitude is facilitated, and the onset latency is reduced. Surprisingly, with such stimuli the onset latency of the cortical response is reduced at an ISI of 30 ms, even though its amplitude is unaffected.
In the present experiments, we compared the effects of giving three different intensities of peripheral stimulation. The subthreshold, threshold, and suprathreshold intensities used for the trigeminal nerve were 36 ± 5, 46 ± 5 and 56 ± 5 %, respectively, whereas for the vagus nerve they were 32 ± 5, 42 ± 5 and 52 ± 5 %, respectively.
Subthreshold conditioning stimulation of either the trigeminal or the vagus nerve had no effect on cortically evoked EMG responses (P= 0.4, ANOVA; Fig. 3). However, stimulation at threshold intensities again produced a differential effect on amplitude and latency. The only effect of threshold stimulation on response amplitude was facilitation of pharyngeal responses following trigeminal nerve stimulation (P < 0.02, ANOVA), vagus nerve stimulation failing to induce any effect on the pharyngeal and oesophageal responses (P= 0.2, ANOVA). The increase in pharyngeal response amplitude occurred only at an ISI of 100 ms after stimulation of the trigeminal nerve (P < 0.05, Student's t test). In contrast, onset latencies were reduced after stimulation of either site, (P < 0.01, ANOVA), occurring specifically at ISIs of 30, 50 or 100 ms (P < 0.03, Student's t test; Fig. 3).
Figure 3. Comparisons of the time course effects of increasing intensities of trigeminal and vagal stimulation on the cortically evoked swallow muscle response.
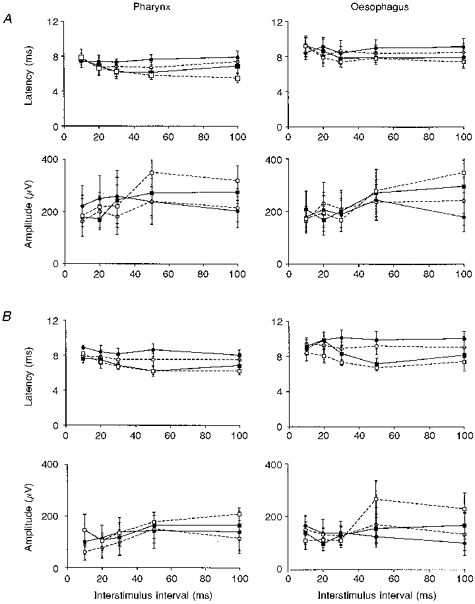
A series of graphs are shown, comparing the effects of increasing intensities of prior trigeminal (A) and vagal (B) nerve stimulation on the group (n= 6) mean (±s.e.m.) cortically evoked response latencies and amplitudes in the pharynx and oesophagus at increasing interstimulus intervals. It can be seen that suprathreshold (□, dashed line) and threshold (▪, continuous line) cranial nerve stimulation facilitate both the pharyngeal and oesophageal responses in comparison with subthreshold (○, dashed line) or sham (•, continuous line) stimulation. The time course of the reduction in response latencies, occurring at ISIs of 30-100 ms appears uninfluenced by increasing cranial nerve stimulus intensities. In contrast, the time course for the increases in response amplitudes, occurring at ISIs of 50-100 ms, are seen to shorten in the pharynx following stronger trigeminal nerve stimulation and in the oesophagus following stronger vagal stimulation.
Effects of pharyngo-oesophageal stimulation on cortically evoked EMG responses
Sensory threshold was lower in the pharynx (12.9 ± 1.9 mA) than in the oesophagus (16.3 ± 2.2 mA) (P < 0.05). Single stimuli at threshold or 10 % suprathreshold intensity had no consistent effect on cortically evoked responses. In view of this, we used trains of stimuli at each site, given at a range of frequencies, to condition cortically evoked responses.
Neither pharyngeal nor oesophageal stimulation had any consistent effect on the amplitude of cortically evoked responses (P= 0.3, ANOVA). In contrast, latencies were often shortened following stimulation of either site (P < 0.01 and P < 0.02, ANOVA, for pharyngeal and oesophageal responses, respectively). Pharyngeal stimulation at high frequencies (5 and 10 Hz) shortened the onset latency of responses in both pharynx and oesophagus (P < 0.03, Student's t test; Fig. 4). At low frequencies (0.2 and 0.5 Hz), however, only the oesophageal response latencies were shortened (P < 0.03, Student's t test). Oesophageal stimulation had a less consistent effect, shortening the latency of oesophageal responses at frequencies of 0.5 and 5 Hz (P < 0.02, Student's t test), with little effect on the latency of pharyngeal responses (Fig. 4).
Figure 4. Comparison of increasing frequencies of pharyngeal and oesophageal stimulation on the cortically evoked swallow muscle responses.
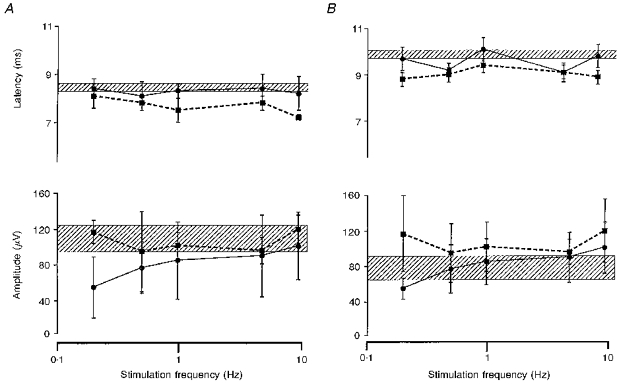
Histographic plots of the group (n= 7) mean (±s.e.m.) cortically evoked pharyngeal (A) and oesophageal (B) EMG responses following repetitive pharyngeal (▪, dashed line) and oesophageal (•, continuous line) stimulation at increasing frequencies. The hatched area for each plot indicates the mean (±s.e.m.) range of response values to cortical stimulation alone. The stimulation frequency axes are plotted on a logarithmic scale. Pharyngeal stimulation affects both the cortically evoked pharyngeal and oesophageal response latencies, the former in a frequency-dependent manner. Oesophageal stimulation, however, only affects the cortically evoked oesophageal response latencies, showing some degree of frequency specificity. In addition, it can be seen that whilst response latencies are clearly influenced both by pharyngeal and oesophageal stimulation, response amplitudes are not significantly affected.
DISCUSSION
In previous studies we have shown that is it possible to investigate some of the cortical pathways involved in control of the pharynx and upper oesophagus using transcranial magnetic brain stimulation in conscious human subjects (Aziz et al. 1994, 1995; Hamdy et al. 1996, 1997). In the present paper we extend these findings by showing, first, how the pathways from each hemisphere interact to control these mid-line structures, and second, how the excitability of the pathways is influenced by afferent input from the face, pharynx and oesophagus.
Interaction between pathways from each hemisphere
The present data confirm that single stimuli applied over either hemisphere can elicit EMG responses both in the pharynx and oesophagus. In addition, the experiments with pairs of stimuli suggest that spatial facilitation can occur between the responses from each hemisphere. Thus, single stimuli applied to either hemisphere, which on their own were too small to evoke any EMG response, could evoke clear activity when given together. At suprathreshold intensities, the size of responses to pairs of stimuli was larger than the sum of the responses to each stimulus given alone, and the latency was shorter. These results are compatible with a shared population of brainstem interneurones or motoneurones receiving combined input from both hemispheres. Thus, as described in animal experiments, fibres from the motor and pre-motor cortex of both cerebral hemispheres probably converge, via interneurones of the central pattern generator (CPG), onto motor nuclei of the V, IX, X and XII cranial nerves (Car, 1970; Hockman et al. 1979; Miller, 1986). This overlap of inputs from each hemisphere may well explain why swallowing can be maintained in a large percentage of patients after unilateral cerebral stroke (Barer, 1989). It also suggests that both cerebral hemispheres are involved in initiating volitional swallows, possibly following pre-initiation processing (intention to initiate motor activity) in other cortical swallowing regions such as the insula (Mesulam & Mufson, 1982), or in supplementary motor areas (Penfield & Rasmussen, 1950), or via ascending afferent feedback (Miller, 1972).
There were subtle differences in hemispheric summation for the pharynx and oesophagus. Spatial facilitation for the pharyngeal responses was clear even with subthreshold stimuli, whereas this was not the case for the oesophagus. In addition, although facilitation was maximal at an interstimulus interval of 1 ms in both muscles, some degree of facilitation was seen at all three intervals in the pharynx, but not in the oesophagus. It is unclear why these differences occurred, although they may be due to the fact that cortical input to the pharynx is probably larger than to the oesophagus (Hamdy et al. 1996) and that input to the pharynx can induce distal inhibition of motor activity within the oesophagus (Jean, 1990), which could have influenced the oesophageal response to the second cortical stimulus. If facilitation were caused by the arrival of descending volleys at shared interneurones or motoneurones, then a 1 ms ISI would produce larger amplitude facilitation than a 5 or 10 ms ISI, because the excitatory post synaptic potentials (EPSPs) would sum on their rising phases. Facilitation at 5 and 10 ms would be correspondingly smaller because summation would occur on the falling phase of the initial EPSP. It is interesting to note that it was only when an interval of 5 ms was used that the latency of the response to combined stimulation was shorter than the response to single stimuli given alone. The reason for this is probably that at 5 ms, the first EPSP raises the excitability of shared brainstem neurones nearer to threshold so that they can respond more quickly to the arrival of the second input. This is compatible with the fact that the latency decrement is similar to that seen when responses are elicited in pre-active rather than relaxed muscle. No latency decrease was evident with an ISI of 1 ms, but this is probably because the maximum possible reduction (i.e. 1 ms) was within the noise of our measurements of onset latency in these muscles.
It is of interest that when the two hemispheres were stimulated sequentially (at ISIs of 5-10 ms), no convincing inhibition was seen. This is in contrast to the effects seen in hand muscles, where sequential magnetic stimuli of each hemisphere, at ISIs of 5-30 ms, inhibited the motor response, possibly via transcallosal interactions that may exist, at least for limb muscles, to ensure strictly unilateral movement (Ferbert, Priori, Rothwell, Day, Colebatch & Marsden, 1992). Our data indicate that the inter-hemispheric interactions for mid-line structures such as the pharynx and oesophagus, which are represented on both hemispheres and have bilateral projections to their motor nuclei, differ from those which have predominantly unilateral representation, although in the absence of direct recordings from the cortico-bulbar tracts, some transcallosal inhibition cannot be completely excluded, given that increased excitability of downstream neurons (at ISIs of 1-5 ms) could have masked any reduction in motor cortex output.
Interaction between afferent input and responses to cortical stimulation
In a previous paper we reported the effect of suprathreshold vagal or trigeminal stimuli on the response to cortical stimulation (Hamdy et al. 1997). The present paper extends these findings by using a range of different intensities of conditioning shock. Single suprathreshold stimuli applied to the vagus or trigeminal nerves elicit both an intermediate and a late reflex response in the pharynx and oesophagus by exciting afferent pathways from the face and neck, which then converge on interneurones of the nucleus of the tractus solitarius in the brainstem (Aziz et al. 1995; Hamdy et al. 1997). The former reflex response is small and has a latency of 20-30 ms; the latter is larger and longer lasting, with a latency to onset of about 50-70 ms. When the cortical stimulation is timed so that the evoked responses occur at the time of the late reflex (ISI, 50-100 ms), then facilitation is clear, and the onset latency of the cortical component is reduced. Presumably, neuronal pools in the brainstem are facilitated by the reflex input, and become more responsive to descending input from cortex. The situation is more difficult to understand when the interstimulus interval is short (20-30 ms), and cortical responses occur during the initial part of the reflex. At these times, the latency is reduced but there is no facilitation of the size of the cortically evoked responses. In order to account for this discrepancy between the effect on latency and amplitude, we proposed that reflex input could have had two effects: excitation at the brainstem and suppression at the cortex. The result would be that a given stimulus evoked a smaller descending volley which impinged on more excitable downstream structures.
The present data show that, as might be expected, threshold stimuli have effects similar to, but smaller than, those seen with suprathreshold intensities. In general, the time course of effects is similar, except that facilitation begins earlier at higher intensities. There were, however, apparent differences between the cranial nerves in effecting facilitation: for example, an increase in the response amplitude was observed following threshold conditioning of the trigeminal nerve but not the vagus nerve. This may indicate that whilst afferent inputs from both sites have comparable effects in exciting brainstem (swallowing) circuitry, ascending vagal input to the cortex is largely inhibitory (Rutecki, 1990) unless more vigorous stimuli are applied. Furthermore, the data also show that facilitation is not evident at subthreshold intensities. The implication is that afferent input evoked by such stimuli does not reach the population of neurones excited by descending cortical input. This may be because there are several synapses in the afferent pathway before convergence with cortical inputs.
Single stimuli applied, in pilot studies, to either the pharynx or oesophagus had no effect on the responses evoked by cortical stimulation. Because of this we used a train of repetitive stimuli, and gave the cortical test shock 100 ms after the end of the train, a time at which there had been maximum effects in the experiments using cranial nerve conditioning shocks. Under such conditions, stimulation of the pharynx or oesophagus demonstrated both frequency- and site-specific properties. At the highest frequencies, pharyngeal stimulation facilitated cortically evoked responses in both pharynx and oesophagus, whereas oesophageal stimulation had only limited effects on responses from the oesophagus alone. This indicates that pharyngeal sensation has a more powerful facilitatory effect on the cortical swallowing pathways than oesophageal sensation. These differences probably reflect the differing properties of the fibres innervating these regions. Sensation from the pharynx is carried largely by the glossopharyngeal nerve and the superior laryngeal nerve (SLN), stimulation of which is the most potent trigger of swallowing (Jean, 1990), whereas that from the upper oesophagus is conveyed via the recurrent laryngeal nerve, which does not trigger reflex swallowing (Miller, 1982; Jean, 1990). In addition, it is recognized that convergence of afferent fibres from the pharynx, in the nucleus of the tractus solitarius of the brainstem swallowing centre, is much more extensive than that from the upper oesophagus (Sessle, 1973).
Our observation that the highest stimulation frequencies of the pharynx also produced the greatest facilitation suggests that there may exist, at least for the pharynx, a frequency-dependent pattern for afferent feedback. In support of this, animal studies have indicated that the facilitation of reflex swallowing by SLN and glossopharyngeal nerve stimulation is also frequency dependent, with an optimal frequency of 30-50 Hz (Sinclair, 1971; Miller, 1972; Jean & Car, 1979; Weerasuriya, Bieger & Hockman, 1980).
Although there was a clear shortening of response latency, repetitive stimulation of the pharynx or oesophagus had no consistent effects on the amplitude of cortically evoked responses. This is similar to the results seen with cranial nerve conditioning at short intervals. As in the latter case, it might be that conditioning with pharyngeal or oesophageal stimuli excited brainstem motoneurones, whilst the motor cortex was inhibited. Direct excitation of brainstem vagal neurones seems likely in view of the strong projections from the pharynx and oesophagus to the CPG of the brainstem (Jean & Car, 1979). The mechanism of a possible cortical inhibition is less obvious. It is plausible that the same stimuli could produce direct inhibition of motor cortex. Alternatively, since the duration of the sensory stimulation was at least 2.5 s, it could be that subjects volitionally attempted to suppress (at a cortical level) the reflex swallowing which such stimuli can induce (Miller, 1982). In support of the first explanation, animal data have demonstrated that repetitive stimulation of pontine swallowing regions, receiving afferent input from the pharynx, inhibits cortical swallowing neurones, a finding that was not observed with similarly applied single stimuli (Sumi, 1972a). In addition, it is also known that repetitive stimulation of the human vagus nerve can suppress the frequency of epileptiform seizures, inferring a reduction in cortical excitability (Rutecki, 1990). While future animal studies may elucidate further the exact relationship between the short latency responses explored in our study and those activated during swallowing, it is possible that cortical inhibition may ensure that once brainstem CPG is activated, cortical discharge is suppressed, so that reflex swallowing can occur without interruption by other volitional commands to swallowing musculature.
Acknowledgments
The authors wish to thank Ms Josephine Barlow of the Gastrointestinal Physiology Laboratory at Hope Hospital for her assistance and Ms Fiona Campbell in the Research and Development Support Unit at Hope Hospital for her statistical advice. This work was conducted with the aid of a project grant from the Stroke Association. Dr S. Hamdy is a Clinical Training Fellow of the Medical Research Council, United Kingdom.
References
- Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1991. Comparing groups: continuous data; pp. 179–228. [Google Scholar]
- Aziz Q, Rothwell JC, Barlow J, Hobson A, Alani S, Bancewicz J, Thompson DG. Esophageal myoelectric responses to magnetic stimulation of the human cortex and the extracranial vagus nerve. American Journal of Physiology. 1994;267:G827–835. doi: 10.1152/ajpgi.1994.267.5.G827. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Rothwell JC, Barlow J, Thompson DG. Modulation of esophageal responses to stimulation of the human brain by swallowing and by vagal stimulation. Gastroenterology. 1995;109:1437–1445. doi: 10.1016/0016-5085(95)90628-2. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Rothwell JC, Hamdy S, Barlow J, Thompson DG. The topographic representation of esophageal motor function on the human cerebral cortex. Gastroenterology. 1996;111:855–862. doi: 10.1016/s0016-5085(96)70053-7. [DOI] [PubMed] [Google Scholar]
- Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. Journal of Neurology, Neurosurgery and Psychiatry. 1989;52:236–241. doi: 10.1136/jnnp.52.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieger D, Hockman CH. Suprabulbar modulation of reflex swallowing. Experimental Neurology. 1976;52:311–324. doi: 10.1016/0014-4886(76)90174-6. [DOI] [PubMed] [Google Scholar]
- Car A. La commande corticale du centre deglutiteur bulbaire. The Journal of Physiology (Paris) 1970;62:361–386. [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. The Journal of Physiology. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Hobson A, Barlow J, Thompson DG. Cranial nerve modulation of human cortical swallowing motor pathways. American Journal of Physiology. 1997;272:G802–808. doi: 10.1152/ajpgi.1997.272.4.G802. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, Tallis RC, Thompson DG. The cortical topography of human swallowing musculature in health and disease. Nature Medicine. 1996;2:1217–1224. doi: 10.1038/nm1196-1217. 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- Hockman CH, Beiger D, Weerasuriya A. Supranuclear pathways of swallowing. Progress in Neurobiology. 1979;12:15–32. doi: 10.1016/0301-0082(79)90009-1. 10.1016/0301-0082(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The 10–20 electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jean A. Brainstem control of swallowing: localisation and organisation of the central pattern generator for swallowing. In: Taylor A, editor. Neurophysiology of the Jaws and Teeth. London: Macmillan Press; 1990. pp. 294–321. [Google Scholar]
- Jean A, Car A. Inputs to the swallowing medullary neurones from the peripheral fibres and the swallowing cortical area. Brain Research. 1979;178:567–572. doi: 10.1016/0006-8993(79)90715-7. 10.1016/0006-8993(79)90715-7. [DOI] [PubMed] [Google Scholar]
- Kimura J, Powers JM, Van Allen MW. Reflex response of the orbicularis oculi muscle to supraorbital stimulation: study in normal subjects and in peripheral facial paresis. Archives of Neurology. 1969;21:193. doi: 10.1001/archneur.1969.00480140093009. [DOI] [PubMed] [Google Scholar]
- Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the Old World Monkey. III: Efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Characteristics of the swallowing reflex induced by peripheral nerve and brainstem stimulation. Experimental Neurology. 1972;34:210–222. doi: 10.1016/0014-4886(72)90168-9. 10.1016/0014-4886(72)90168-9. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Deglutition. Physiological Reviews. 1982;62:129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Neurophysiological basis of swallowing. Dysphagia. 1986;1:91–100. [Google Scholar]
- Penfield WP, Rusmussen T. The Cerebral Cortex of Man. New York: Macmillan; 1950. [Google Scholar]
- Rutecki P. Anatomical, physiological and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31:S1–S6. doi: 10.1111/j.1528-1157.1990.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Excitatory and inhibitory inputs to single neurones in the solitary tract nucleus and adjacent reticular formation. Brain Research. 1973;53:319–331. doi: 10.1016/0006-8993(73)90217-5. 10.1016/0006-8993(73)90217-5. [DOI] [PubMed] [Google Scholar]
- Sinclair WJ. Role of the pharyngeal plexus in initiation of swallowing. American Journal of Physiology. 1971;221:G1260–1263. doi: 10.1152/ajplegacy.1971.221.5.1260. [DOI] [PubMed] [Google Scholar]
- Sumi T. Some properties of cortically evoked swallowing in rabbits. Brain Research. 1969;15:107–120. doi: 10.1016/0006-8993(69)90313-8. 10.1016/0006-8993(69)90313-8. [DOI] [PubMed] [Google Scholar]
- Sumi T. Reticular ascending activation of frontal cortical neurones in rabbits, with special reference to the regulation of deglutition. Brain Research. 1972a;46:43–54. doi: 10.1016/0006-8993(72)90004-2. 10.1016/0006-8993(72)90004-2. [DOI] [PubMed] [Google Scholar]
- Sumi T. Role of the pontine reticular formation in the neural organisation of deglutition. Japanese The Journal of Physiology. 1972b;22:295–314. doi: 10.2170/jjphysiol.22.295. [DOI] [PubMed] [Google Scholar]
- Weesasuriya A, Bieger D, Hockman CH. Interaction between primary afferent nerves in the elicitation of swallowing. American Journal of Physiology. 1980;239:R407–414. doi: 10.1152/ajpregu.1980.239.5.R407. [DOI] [PubMed] [Google Scholar]
- Wiles CM. Neurogenic dysphagia. Journal of Neurology, Neurosurgery and Psychiatry. 1991;54:1037–1039. doi: 10.1136/jnnp.54.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]


