Abstract
The role of the cytokine leukemia inhibitory factor (LIF) in axotomy-induced sprouting of postganglionic sympathetic fibres into the dorsal root ganglia was examined in the adult rat.
Immunocytochemistry was used to study the distribution and density of tyrosine hydroxylase-immunoreactive (TH-IR) fibres within the lumbar dorsal root ganglia and lumbar spinal nerves 14 days following continuous intrathecal infusion of LIF (0.33 mg ml−1), or 14 days following unilateral peripheral nerve axotomy.
In LIF-treated animals, numerous pericellular TH-IR basket-like structures were observed surrounding sensory neurones, which were absent from controls.
The number of TH-IR fibres within the L3, L4 and L5 spinal nerves was significantly higher in LIF-treated animals than in control or saline-treated animals (P < 0.01, Student's t test).
Unilateral ligation of the L4 spinal nerve or unilateral sciatic nerve ligation was also associated with the formation of TH-IR baskets around sensory neurones and a significant increase in the number of TH-IR fibres within the lumbar spinal nerves (P < 0.01, Student's t test).
The percentage of neurones surrounded by TH-IR baskets within the L3 and L4 dorsal root ganglia following sciatic axotomy was significantly reduced in animals treated continuously for 2 weeks with a monoclonal antibody against the LIF receptor motif, gp130 (0.833 mg ml−1) (P < 0.05, Mann-Whitney U test). Antibody treatment did not reduce the axotomy-induced increase in TH-IR fibres within lumbar spinal nerves.
These results demonstrate that exogenous application of the axotomy-associated cytokine LIF is associated with sprouting of uninjured postganglionic sympathetic neurones around sensory neurones within the dorsal root ganglion. It is likely that increased LIF expression following peripheral axotomy plays an important role in the novel sympathetic sprouting observed within sensory ganglia following peripheral nerve injury.
Peripheral nerve injury has recently been shown to be associated with a novel form of sprouting of postganglionic sympathetic afferent fibres. Following either lumbar spinal nerve ligation (Chung, Kim, Na, Park & Chung, 1993), complete sciatic nerve axotomy (McLachlan, Janig, Devor & Michaelis, 1993) or chronic constriction injury of the sciatic nerve (Ramer & Bisby, 1997a), sympathetic fibres sprout into affected ganglia where some sensory neurone cell bodies become wrapped by elaborate pericellular arborizations. These arborizations display synapse-like structures and form functional connections with sensory neurones (McLachlan et al. 1993). In several current animal models of neuropathic pain, as well as in certain clinical circumstances, a complete repertoire of pain behaviour following nerve injury is dependent upon an intact sympathetic nervous system (Kim & Chung, 1991; Blumberg & Jänig, 1994; Desmules, Kayser, Weilfuggaza, Bertrand & Guilbauld, 1995). Under such circumstances an interaction between the sympathetic and sensory nervous system is likely. The sympathetic sprouting within the dorsal root ganglion (DRG) is an important observation, since it may represent an anatomical correlate of the close functional coupling between sympathetic and sensory activity which many clinical and experimental observations imply.
To date the mechanism by which this sprouting occurs is uncertain. In transgenic mice, overexpression of nerve growth factor (NGF) in the skin leads to the formation of tyrosine hydroxylase-immunoreactive (TH-IR) basket structures within the trigeminal ganglia (Davis, Albers, Seroogy & Katz, 1994). Recently, Zhou, Rush & McLachlan (1996) reported that the sympathetic sprouts observed within the DRG following sciatic nerve axotomy were associated with p75-immunoreactive glial cells. The authors suggested that the low-affinity neurotrophin receptor may function as a presenting molecule for neurotrophin-3 (NT3) or NGF to trigger sympathetic sprouting within the DRG. However, a recent study demonstrated significant levels of sympathetic sprouting within the DRG, after axotomy, in mice lacking the p75 low-affinity receptor (Ramer & Bisby, 1997b). This suggests that additional factors may be involved in the sympathetic sprouting observed following axotomy.
A strong candidate molecule is leukemia inhibitory factor (LIF). LIF is a multifunctional cytokine and a member of the haemopoietin cytokine family defined by their interaction with the common receptor motif, gp130. Many of the gp130 cytokines, including LIF, play a role in phenotypic maturation during development of the peripheral nervous system (PNS) and promote neurite extension and morphologic maturation in cultured embryonic neuroblasts (Mehler & Kessler, 1995). In the adult nervous system LIF is normally undetectable (Yamamori, 1991) but is induced at the site of peripheral nerve axotomy (Banner & Patterson, 1994) and is retrogradely transported and accumulates within the DRG in a specific population of nociceptive-specific neurones (Thompson, Vernallis, Heath & Priestley, 1997). Following injury to the mature nervous system LIF also acts as an important phenotypic specifying factor (see Zigmond et al. 1996). Evidence exists therefore that LIF regulates phenotypic responses and may have actions in the mature nervous system in addition to its developmental role. The results from the present study extend the known roles of LIF in the adult PNS demonstrating that LIF is associated with sprouting of sympathetic fibres within the DRG following peripheral nerve axotomy.
Some of these results have been presented earlier in abstract form (Thompson & Majithia, 1997).
METHODS
Animal surgery
Experiments were performed in adult Wistar rats of either sex weighing 200-250 g. Three animals served as a control group for determining the degree of tyrosine hydroxylase immunoreactivity in normal uninjured, untreated dorsal root ganglia. In a second group of animals a laminectomy was performed between the L5 and L6 vertebrae using sterile precautions. The dura was cut and a silastic tube (external diameter, 0.6 mm) passed intrathecally so that its tip lay approximately between the L2 and L3 dorsal root ganglia. The silastic tube was connected to a mini-osmotic pump (Alzet, Alza Corp., Palo Alto, CA, USA; type 2002, mean fill volume, 242 μl; mean pumping rate, 0.55 μl h−1). The pumps were filled with rat serum albumin (RSA; 5 mg ml−1) in phosphate-buffered saline (PBS; n= 2) or this buffer plus recombinant human LIF (rhLIF; 0.33 mg ml−1, a gift from J. Heath, Birmingham; n= 6). Pumps were left in place for 14 days. In a third group (n= 3), the L4 spinal nerve was located and tightly ligated with 5/0 Mersilk and a 5 mm distal portion of the nerve was resected to prevent regeneration. In a final group of animals (n= 8), the common sciatic nerve was exposed at mid-thigh level by blunt dissection and either tightly ligated (5/0 Mersilk) and a 10 mm distal portion resected to prevent regeneration (n= 4) or the sciatic nerve was sectioned and the proximal stump placed into a pliable Gortex cuff (n= 4). The sectioned nerve was secured by a 10/0 prolene suture passed through the epineurium and into the base of the cuff. The cuff was connected to a mini-osmotic pump (Alzet, type 2002) via a silicon cannula (Vygon, Nutricath, Ecouen, France; external diameter, 1.3 mm) and filled with monoclonal anti-human gp130 antibody (0.833 mg ml−1, R&d Systems, Abingdon, UK, in PBS, n= 4). Surgery in all groups was carried out under sodium pentobarbitone anaesthesia (40 mg kg−1) under aseptic conditions. All wounds were closed in layers and skin incisions dusted with antibiotic powder (Cicatrin). All animals recovered without incident. Post surgery, animals were inspected daily and showed normal weight gain and grooming activity. Autotomy was not observed in any experimental group. All experiments were carried out with UK Home Office approval and in accordance with IASP (International Association for the Study of Pain) guidelines on the use of animals in the study of pain (Zimmermann, 1983).
After 14 days animals were deeply anaesthetized (sodium pentobarbitone, 40 mg kg−1, i.p.) and exsanguinated by transcardial perfusion with cooled heparinized PBS (200 ml) followed by a 4 % solution of paraformaldehyde (500 ml). Following perfusion, lumbar ganglia L3-L5 were dissected and postfixed in 4 % paraformaldehyde for 2 h prior to cryoprotection overnight in 20 % sucrose solution.
Immunostaining procedures
Tissue was frozen sectioned (15 μm thick) on a cryostat, thaw mounted on microscope slides and air dried. Sections were then processed with antibodies to the enzyme tyrosine hydroxylase (TH), a rate-limiting enzyme for the synthesis of noradrenaline, using single labelling immunofluoresence. Incubations consisted of 1 h in 10 % normal goat serum followed by 24 h in the primary reagent (rabbit antiserum to TH, 1 : 1000 dilution; Affinity, UK). Sections were incubated for 4 h in the secondary reagent which consisted of tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit antibody (Jackson, 1 : 400). All incubations were carried out at room temperature (20-22°C); antisera were diluted in PBS containing 0.2 % Triton X-100 and 0.1 % sodium azide. Immunofluorescent sections were washed in PBS and mounted in PBS-glycol (1 : 3) containing 2.5 % diazobicycl-(2,2,2)-octane (antifading agent, Sigma). Sections were viewed using a Leica epifluorescence microscope using N2 (TRITC) filter blocks. Images of TH-IR fibres and baskets were photographed using Ilford T max negative film and digitized at 900 pixels inch−1 using a Microtek 35T Plus film scanner and Adobe Photoshop software (Adobe Systems Inc., CA, USA) and printed using a Sony UP-D8800 digital printer.
Data analysis
Pericellular TH-IR basket structures
Following both peripheral nerve injury and intrathecal LIF infusion, many neurones were either totally or partially encircled by fine TH-IR fibres. The number of neuronal profiles surrounded by these structures, referred to as ‘TH-IR baskets’, was counted on immunostained sections. Those profiles which were encircled for at least 50 % of their circumference by TH-IR fibres were regarded as possessing baskets. The number of profiles with TH-IR baskets was expressed as a percentage of the total number of neuronal profiles counted under dark-field illumination in the same section. The total area of each DRG section was examined. An average of six sections per ganglion was counted for each animal (range, 3-12). In order that counts were not biased towards particular regions of the ganglion, sections were analysed at a minimum interval of 120 μm.
TH-IR fibres in the spinal nerve
An estimation of the numerical density of fibres in the peripheral nerve was obtained by counting TH-IR fibres within the spinal nerve approximately 0.5-1.00 mm distal to the DRG. A calibrated bar (250 μm length) was used to count TH-IR fibres under epifluorescence (× 400 magnification) in 15 μm thick longitudinal sections of the DRG with attached spinal nerve. The bar was aligned perpendicularly to the long axis of the spinal nerve and all fibres along its length were counted. Because of the small number of sections obtained with spinal nerve and ganglia in continuum, three sections were selected at 15 μm intervals close to the widest diameter of the nerve. Fibre density per spinal nerve was presented as the mean number of fibres per millimetre. Because sympathetic axons often travel in groups in Remak bundles within the peripheral nerve, it is often difficult to detect single axons at the level of the light microscope. The measure of fibre density is likely therefore to be an underestimate in all groups. The percentage of neuronal profiles with TH-IR baskets was compared between groups using Mann-Whitney rank sum tests. The density of TH-IR fibres in the spinal nerve was compared between different groups using Student's t test. A P value of less than 0.05 was considered significant.
RESULTS
TH-IR fibres in the DRG following intrathecal LIF and peripheral nerve injury
In sections of DRG taken from untreated animals (n= 3), or those with pumps delivering buffer intrathecally alone for 14 days (n= 2), TH-IR fibres were sparse. When present they appeared as fine varicose fibres confined to regions of the ganglia containing axon bundles passing through the ganglion or were associated with blood vessels. These fibres had a spiral ‘railroad track’ appearance, characteristically following the circumference of the vascular wall. TH-IR fibres did not penetrate areas of the ganglion occupied by the cell somata and were never seen to form perivascular baskets around neurones. Because of their similar appearance, data from untreated and saline-treated animals were grouped together.
Fourteen days following intrathecal administration of LIF (0.33 mg ml−1) a large increase in the number of TH-IR fibres within the lumbar DRG was observed. Fine TH-IR sprouts were seen to penetrate into the DRG parenchyma from both the spinal nerve and from blood vessels within the DRG. TH-IR basket-like structures surrounding DRG neurones were observed in all lumbar dorsal root ganglia examined (segments L3-L5; Fig. 1). These structures were absent from control animals.
Figure 1. Immunofluorescence photomicrographs showing TH-IR basket structures surrounding neuronal profiles within the L4 DRG following intrathecal LIF, L4 spinal nerve ligation or sciatic nerve ligation.

A, DRG section from a control animal showing a single small diameter TH-IR neuronal profile (arrow). These cells were found very rarely and are likely to be dopaminergic sensory neurones. TH-IR baskets were not present in control tissue. B and D, examples of TH-IR basket structures observed within the L4 DRG 14 days following sciatic nerve ligation (B) or L4 spinal nerve ligation (D). TH-IR baskets formed around both large and small diameter profiles (arrows). C, E and F, TH-IR baskets within the L4 DRG 14 days following continuous intrathecal infusion of rhLIF. Baskets formed around large (C), small (E) and intermediate (F) diameter profiles (arrows). Numerous varicose TH-IR fibres were also seen coursing between neurones (arrowheads). Scale bars, 20 μm.
Figure 2A shows the percentage of neuronal profiles with TH-IR baskets in the ipsilateral L3, L4 and L5 dorsal root ganglia 14 days following L4 spinal nerve or sciatic nerve ligation compared with the effects of intrathecal LIF. A significantly higher percentage of neuronal profiles had TH-IR baskets following spinal nerve ligation than following intrathecal LIF, both within the ligated segment (L4) and neighbouring, intact segments (L3 and L5). The percentage of profiles with TH-IR baskets in the L3, L4 and L5 dorsal root ganglia following sciatic nerve ligation was not significantly different to that observed following intrathecal LIF but was significantly different from the effect of spinal nerve ligation in two segments (L3 and L4). The mean number of TH-IR baskets per section and the percentage observed in each lumber DRG following L4 spinal nerve ligation and sciatic nerve axotomy are shown in Table 1.
Figure 2. Effects of peripheral axotomy and intrathecal LIF upon the percentage of TH-IR baskets within lumbar DRG (A), the number of TH-IR fibres within lumbar spinal nerves (B) and the effect of anti-gp130 antibody upon sciatic nerve axotomy.
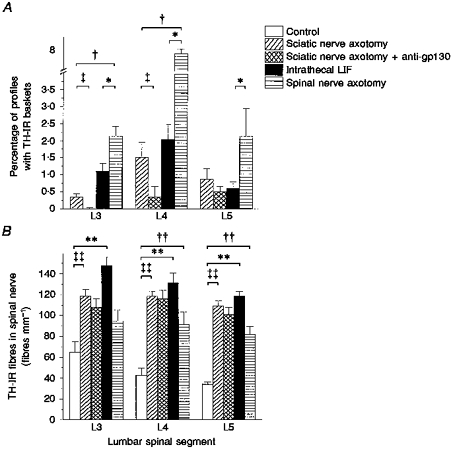
A, TH-IR baskets were present 14 days following sciatic nerve axotomy, intrathecal LIF and L4 spinal nerve axotomy. Anti-gp130 antibody significantly reduced the percentage of baskets in L3 and L4 DRG following sciatic nerve axotomy. No baskets were observed in control tissue. *P < 0.05, spinal nerve axotomy vs. intrathecal LIF; †P < 0.05, spinal nerve axotomy vs. sciatic nerve axotomy; ²P < 0.05, sciatic nerve axotomy vs. sciatic nerve axotomy + anti-gp130 (Mann-Whitney U test). B, TH-IR fibres were present within the spinal nerve in all experimental groups. **P < 0.01, intrathecal LIF vs. control; ²²P < 0.01, sciatic nerve axotomy vs. control; ††P < 0.01, spinal nerve axotomy vs. control (Student's t test). There was no significant effect of anti-gp130 upon TH-IR fibres following sciatic nerve axotomy.
Table 1.
The percentage of profiles surrounded by TH-IR baskets per ganglion and number of TH-IR baskets per section within the DRG (A) and the number of TH-IR fibres within the spinal nerve (B)
| A. TH-IR baskets in the DRG | ||||||
|---|---|---|---|---|---|---|
| Experimental group | L3 | L4 | L5 | |||
| (baskets per section) | (%) | (baskets per section) | (%) | (baskets per section) | ||
| LIF | 2.8 ± 0.6 | 1.15 ± 0.22 (6)* | 5.6 ± 1.0 | 2.07 ± 0.46 (6)* | 1.6 ± 0.5 | 0.65 ± 0.18 (6)* |
| Spinal nerve ligation | 4.7 ± 1.3 | 2.18 ± 0.28 (3) | 23.0 ± 4.8 | 7.82 ± 0.20 (3) | 5.9 ± 1.6 | 2.18 ± 0.82 (3) |
| Sciatic nerve ligation | 0.8 ± 0.1 | 0.39 ± 0.08 (4);† | 2.7 ± 0.4 | 1.55 ± 0.45 (4)† | 2.6 ± 0.8 | 0.91 ± 0.32 (4) |
| Sciatic nerve ligation + anti-gp130 | 0.1 ± 0.0 | 0.04 ± 0.02 (4)‡ | 0.8 ± 0.6 | 0.38 ± 0.32 (4)‡ | 1.6 ± 0.6 | 0.54 ± 0.16 (4) |
| B. TH-IR fibres in the spinal nerve | ||||
|---|---|---|---|---|
| Experimental group | L3 (fibres mm−1) | L4 (fibres mm−1) | L5 (fibres mm−1) | |
| Control | 64.3 ± 9.9 (5) | 42.8 ± 6.4 (5) | 34.0 ± 2.0 (5) | |
| LIF | 147.6 ± 8.5 (6)** | 130.9 ± 9.9 (6)** | 118.4 ± 4.8 (6)** | |
| Spinal nerve ligation | 81.9 ± 7.1 (3) | 91.2 ± 12.2 (3)** | 94.8 ± 10.0 (3)** | |
| Sciatic nerve ligation | 119.0 ± 5.9 (4)** | 118.4 ± 4.9 (4)** | 108.7 ± 5.1 (4);** | |
| Sciatic nerve ligation + anti-gp130 | 107.7 ± 8.3 (4) | 116.2 ± 8.0 (4) | 101.3 ± 6.6 (4) |
No baskets were observed in control animals.
P < 0.05, LIF vs. spinal nerve ligation
P < 0.05, sciatic nerve ligation vs. spinal nerve ligation
P < 0.05, sciatic nerve ligation vs. sciatic nerve ligation
anti-gp130 (Mann-Whitney U test).
P < 0.01, experimental groups vs. control (Student's t test). Values are means ±s.e.m The number of animals is given in parentheses.
TH-IR fibres in the spinal nerve following intrathecal LIF administration and peripheral nerve injury
Following intrathecal LIF infusion, the number of TH-IR fibres in the spinal nerve was significantly greater than in control or saline-treated animals in all lumbar segments investigated (Figs 2B and 3). Ligation of the L4 spinal nerve was also associated with a significant increase in the number of TH-IR fibres within the ligated nerve (L4) and one of the neighbouring intact segments (L5). Sciatic nerve ligation also induced a significant increase in the number of TH-IR fibres within all ipsilateral spinal segments investigated. The mean number of TH-IR fibres within the spinal nerve for each group is shown in Table 1.
Figure 3. Immunofluorescence photomicrographs showing TH-IR fibres within the L4 lumbar spinal nerve following intrathecal LIF, L4 spinal nerve axotomy or sciatic nerve axotomy.

A, TH-IR fibres within the L4 spinal nerve in a control animal. The density of TH-IR fibres within the L4 spinal nerve was significantly increased 14 days following sciatic nerve axotomy (B), 14 days following intrathecal LIF infusion (C) or 14 days following L4 spinal nerve axotomy (D). Scale bar, 50 μm.
The effect of anti-gp130 antibody on TH-IR baskets in the DRG and fibres in the spinal nerve following sciatic ligation
Figure 2A shows the effect of 14 days continuous delivery of anti-gp130 antibody (0.833 mg ml−1) to the proximal stump of the sciatic nerve on the percentage of neuronal profiles with TH-IR baskets in the L3, L4 and L5 dorsal root ganglia. In two of the three ganglia examined, a significant difference was observed between those animals treated with the antibody and those with sciatic ligation alone (L3 and L4). Continuous delivery of the anti-gp130 antibody did not completely eliminate basket formation in any of these three dorsal root ganglia. In contrast, the number of TH-IR fibres observed within lumbar spinal nerves following delivery of anti-gp130 antibody to the sciatic nerve was unaltered when compared with sciatic ligation alone (Fig. 2B). The mean percentage and number of TH-IR baskets per section and TH-IR fibres per millimetre within the spinal nerves following anti-gp130 treatment are presented in Table 1.
DISCUSSION
This study demonstrates that intrathecal infusion of LIF induces noradrenergic fibre sprouting and basket formation in intact dorsal root ganglia in the adult rat. Intrathecal delivery does not damage sensory or sympathetic neurones. An important observation therefore is that nerve injury itself is not a priori for sympathetic sprouting into the DRG. There is a large body of evidence on the other hand which indicates that LIF will directly interact with sympathetic neurones. LIF plays an important role in the modulation of neurotransmitter phenotype in both the intact adult sympathetic nervous system (Bamber, Masters, Hoyle, Brinster & Palmiter, 1994) and following sympathetic axotomy (Rao et al. 1993). Although there is evidence that LIF promotes neuronal growth in vitro this is the first observation that LIF is associated with sprouting of the intact adult sympathetic nervous system in vivo.
Although the mechanism by which LIF induces sympathetic sprouting is unclear, it is worthwhile considering likely locations of action of LIF in the present study. Regarding exogenous intrathecal administration, it is possible that LIF was distributed systemically at a sufficient concentration for a direct effect upon sympathetic terminals within peripheral effector organs or within the postganglionic sympathetic chain itself. The most probable site of interaction, however, is likely to be either within the spinal nerve or DRG. Intrathecally delivered substances, such as neurotrophins, penetrate into the spinal cord and dorsal roots and have biological effects within the DRG (Bennett, French, Priestley & McMahon, 1996; Verge, Gratto, Karchewski & Richardson, 1996). The conditions under which endogenous LIF is expressed have been well documented. Axotomy is a potent stimulus for LIF induction. Sciatic nerve axotomy leads to LIF expression within Schwann cells in both proximal and distal nerve stumps (Banner & Patterson, 1994; Sun & Zigmond, 1996) but not within the DRG (Curtis et al. 1994; Sun & Zigmond, 1996). LIF is, however, retrogradely transported to the DRG by sensory neurones (Curtis et al. 1994), in particular by small diameter nociceptive-specific neurones (Thompson et al. 1997). It is unlikely that the presence of LIF within these sensory neurone cell bodies may act directly as a trophic factor for sympathetic sprouting since many TH-IR baskets in this and other studies formed around large diameter cell profiles. Retrogradely transported LIF, or a secondary mediator, however, may be re-released into the DRG and act in a paracrine fashion, but this is yet to be demonstrated. Alternatively, LIF may act directly upon sympathetic neurones to induce sprouting. Transection of postganglionic sympathetic nerve trunks induces a substantial induction of LIF within relevant ganglia (Sun, Rao, Zigmond & Landis, 1994). 125I-labelled LIF may also be retrogradely transported by sympathetic neurones (Ure & Campenot, 1994). Accumulation or expression of LIF within postganglionic sympathetic neurones may therefore be an important step in sympathetic sprouting following nerve injury. The process by which such expression or accumulation triggers sprouting within the DRG is unknown.
There is considerable variation in the extent and time course of the sympathetic sprouting in the DRG between the various models of neuropathic pain currently employed. Lumbar spinal nerve transection produces a rapid (2 days) sprouting of adrenergic fibres into the DRG and peripheral nerve (Chung, Lee, Yoon & Chung, 1996) whilst the time course of basket formation following sciatic axotomy is much slower (Ramer & Bisby, 1997a). Comparison of the extent of basket formation between experimental groups in the present study would suggest that the time course of LIF-induced TH-IR basket formation most closely follows that observed following sciatic axotomy. Interestingly, the number of TH-IR fibres observed within the spinal nerve in the present study was not significantly different between experimental groups. It is likely that the increase in TH-IR fibre density within the spinal nerve in all three groups progresses more rapidly than the formation of TH-IR baskets within the DRG.
Bathing of the proximal end of the sciatic nerve with an antibody against the LIF receptor component gp130 significantly reduced the number of TH-IR baskets within the lumbar ganglia. This demonstrates that a significant portion of the sympathetic sprouting response to axotomy may be initiated by peripherally released factors which bind the gp130 motif. gp130 is the defining component of a number of closely related cytokines which include interleukins 6 and 11 (IL-6, IL-11), oncostatin-M (OSM), cardiotrophin-1 (CT-1) and ciliary derived neurotrophic factor (CNTF). Whilst the role of some of these factors such as OSM and CT-1 within the nervous system is undetermined, it is possible that others such as IL-6 or CNTF may play a significant part in the response to injury of either sympathetic or sensory nervous systems. Although CNTF may mimic the effect of LIF on peptide induction in sympathetic neurones under certain circumstances in vitro (Sun et al. 1994), there is good evidence that many of the biological actions of the gp130 family of cytokines are not strictly interchangeable (Piquet-Pellorce, Grey, Mereau & Heath, 1994). There is no evidence therefore that CNTF may play a role in sympathetic ganglia following axotomy in vivo (Zigmond et al. 1996). Indeed it is well known that, following peripheral nerve axotomy, expression of CNTF is significantly decreased (Sendtner, Stocki & Thoenen, 1992). A definitive answer to this point, however, awaits the development of gp130 ligand-specific antagonists.
In contrast to its effect upon the DRG, anti-gp130 was without effect upon the number of fibres present within the spinal nerve. Assuming significant inactivation of this receptor component by the antibody, it is likely that further factors may act as a stimulus to sympathetic sprouting. Neurotrophins are obvious candidates. Sympathetic axons in the central nervous system sprout in response to NGF (Isaacson, Saffran & Crutcher, 1992); however, data regarding the role of NGF following peripheral nerve axotomy are equivocal. Although there is a modest rise in NGF mRNA within the DRG following sciatic axotomy (Sebert & Shooter, 1993), and at the site of injury (Heumann, Korsching, Badtlow & Thoenen, 1987), retrograde transport of NGF to the DRG declines (Heumann et al. 1987) together with a reduced trkA synthesis (Verge, Riopelle & Richardson, 1989). Indeed many axotomy-induced responses within the DRG may be reversed by exogenous application of NGF. It is likely that multiple factors are involved in the sympathetic sprouting observed following peripheral axotomy and that the contributions of each factor may differ depending upon the type (transection vs. constriction) and location (proximal vs. distal) of the lesion. It has been demonstrated recently that the extent of sympathetic sprouting within the DRG is inversely related to the distance between the DRG and the injury site (Kim, Na, Nam, Park, Hong & Kang, 1996).
Previous studies have shown a correlation between sympathetic sprouting in the DRG and the development of behavioural signs of neuropathic pain (Chung et al. 1996; Ramer & Bisby, 1997a). Exogenous LIF will also induce mechanical allodynia in nerve-intact rats (Thompson, Dray & Urban, 1996). Although the effect of LIF in these latter experiments was acute, measured in terms of hours, it is tempting to speculate upon a link between LIF expression and the sympathetic nervous system in the development of neuropathic pain behaviours following peripheral nerve injury. It remains to be demonstrated whether a functional connection occurs between LIF-induced sympathetic sprouts and sensory neurones within the DRG. Interventions, however, which reduce the expression of LIF at the site of nerve injury or within sympathetic ganglia, may reduce sympathetic sprouting and may have a concomitant effect upon the degree of neuropathic pain experienced following peripheral nerve trauma.
Acknowledgments
This research was supported by the Medical Research Council (UK). I should like to thank Professor J. Heath and Dr A. Vernallis for the gift of rhLIF used in this study, Professor J. Munson for the gift of Gortex tubing, Professor J. Priestley for use of the digital Sony printer and Professor S. McMahon for use of facilities and useful discussion.
References
- Bamber BA, Masters BA, Hoyle GW, Brinster RL, Palmiter RD. Leukemia inhibitory factor induces neurotransmiter switching in transgenic mice. Proceedings of the National Academy of Sciences of the USA. 1994;91:7839–7843. doi: 10.1073/pnas.91.17.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proceedings of the National Academy of Sciences of the USA. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DLH, French J, Priestley JV, McMahon SB. NGF but not NT-3 or BDNF prevents A-fibre sprouting into lamina II of the spinal cord that occurs following axotomy. Molecular and Cellular Neuroscience. 1996;8:211–220. doi: 10.1006/mcne.1996.0059. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Jänig W. Clinical manifestations of reflex sympathetic dystrophy and sympathetically maintained pain. In: Wall PD, Melzack R, editors. Textbook of Pain. 3. Edinburgh: Churchill Livingston; 1994. pp. 685–697. [Google Scholar]
- Chung K, Kim HJ, Na HS, Park MJ, Chung JM. Abnormalities of sympathetic innervation in the area of an injured peripheral nerve in a rat model of neuropathic pain. Neuroscience Letters. 1993;162:85–88. doi: 10.1016/0304-3940(93)90566-4. [DOI] [PubMed] [Google Scholar]
- Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglion of the injured nerve in a rat neuropathic pain model. Journal of Comparative Neurology. 1996;376:241–252. doi: 10.1002/(SICI)1096-9861(19961209)376:2<241::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS. Retrograde axonal transport of LIF is increased by peripheral nerve injury: Correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Davis BM, Albers KM, Seroogy KB, Katz DM. Overexpression of nerve growth factor in transgenic mice induces novel sympathetic projections to primary sensory neurones. Journal of Comparative Neurology. 1994;349:464–474. doi: 10.1002/cne.903490310. [DOI] [PubMed] [Google Scholar]
- Desmules JA, Kayser V, Weilfuggaza J, Bertrand A, Guilbauld G. Influence of the sympathetic nervous system in the development of abnormal pain-related behaviours in a rat model of neuropathic pain. Neuroscience. 1995;67:941–951. doi: 10.1016/0306-4522(95)00098-4. 10.1016/0306-4522(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Badtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. Journal of Cell Biology. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson LG, Saffran BN, Crutcher KA. Nerve growth factor-induced sprouting of mature, uninjured sympathetic axons. Journal of Comparative Neurology. 1992;326:327–336. doi: 10.1002/cne.903260302. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Na HS, Nam HJ, Park KA, Hong SK, Kang BS. Sprouting of sympathetic fibres into the dorsal root ganglion following peripheral nerve injury depends upon the injury site. Neuroscience Letters. 1996;212:191–194. doi: 10.1016/0304-3940(96)12811-1. 10.1016/0304-3940(96)12811-1. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. Sympathectomy alleviates mechanical allodynia in an experimental model for neuropathy in the rat. Neuroscience Letters. 1991;134:131–134. doi: 10.1016/0304-3940(91)90524-w. 10.1016/0304-3940(91)90524-W. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Jänig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Cytokines and neuronal differentiation. Critical Reviews in Neurobiology. 1995;9:419–416. [PubMed] [Google Scholar]
- Piquet-Pellorce C, Grey L, Mereau A, Heath JK. Are LIF and related cytokines functionally equivalent? Experimental Cell Research. 1994;213:340–347. doi: 10.1006/excr.1994.1208. 10.1006/excr.1994.1208. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bisby MA. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain. 1997a;70:237–244. doi: 10.1016/s0304-3959(97)03331-9. 10.1016/S0304-3959(97)03331-9. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bisby MA. Reduced sympathetic sprouting occurs in dorsal root ganglia after axotomy in mice lacking low-affinity neurotrophin receptor. Neuroscience Letters. 1997b;228:9–12. doi: 10.1016/s0304-3940(97)00356-x. 10.1016/S0304-3940(97)00356-X. [DOI] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser J, Patterson PH, Zigmond RE, Brulet P, Landis SC. Leukemia inhibitory factor mediates injury response but not a target directed transmitter switch in sympathetic neurons. Neuron. 1993;11:1175–1185. doi: 10.1016/0896-6273(93)90229-k. 10.1016/0896-6273(93)90229-K. [DOI] [PubMed] [Google Scholar]
- Sebert ME, Shooter EM. Expression of messenger RNA for neurotrophic factors and their receptors in the rat dorsal root ganglion and sciatic nerve following injury. Journal of Neuroscience Research. 1993;36:357–367. doi: 10.1002/jnr.490360402. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Stocki KA, Thoenen H. Synthesis and localisation of CNTF in the sciatic nerve of the adult rat after lesion and during regeneration. Journal of Cell Biology. 1992;118:139–204. doi: 10.1083/jcb.118.1.139. 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Rao MS, Zigmond RE, Landis SC. Regulation of vasoactive intestinal polypeptide expression in sympathetic neurons in culture and after axotomy: The role of cholinergic differentiation factor/LIF. Journal of Neurobiology. 1994;25:415–430. doi: 10.1002/neu.480250407. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Leukemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. European Journal of Neuroscience. 1996;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Thompson SWN, Dray A, Urban L. Leukemia inhibitory factor (LIF) induces mechanical allodynia but not thermal hyperalgesia in the juvenile rat. Neuroscience. 1996;71:1091–1094. doi: 10.1016/0306-4522(95)00537-4. 10.1016/0306-4522(95)00537-4. [DOI] [PubMed] [Google Scholar]
- Thompson SWN, Majithia A. Leukemia inhibitory factor (LIF) and IL-6 induce sympathetic sprouting and basket formation in intact adult rat sensory ganglia. Society for Neuroscience Abstracts. 1997;23:565.17. [Google Scholar]
- Thompson SWN, Vernallis AE, Heath JK, Priestley JV. LIF is retrogradely transported by a distinct population of adult rat sensory neurons: co-localization with trkA and other neurochemical markers. European Journal of Neuroscience. 1997;9:1244–1251. doi: 10.1111/j.1460-9568.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- Ure DR, Campenot RB. Leukemia inhibitory factor and nerve growth factor are retrogradely transported and processed by cultured rat sympathetic neurons. Developmental Biology. 1994;162:339–347. doi: 10.1006/dbio.1994.1091. 10.1006/dbio.1994.1091. [DOI] [PubMed] [Google Scholar]
- Verge VM, Gratto KA, Karchewski LA, Richardson PM. Neurotrophins and nerve injury in the adult. Philosophical Transactions of the Royal Society. 1996;B 351:423–430. doi: 10.1098/rstb.1996.0038. [DOI] [PubMed] [Google Scholar]
- Verge VM, Riopelle RJ, Richardson PM. Nerve growth factor receptors on normal and injured sensory neurons. Journal of Neuroscience. 1989;10:926–934. doi: 10.1523/JNEUROSCI.09-03-00914.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T. Localization of cholinergic differentiation factor/leukemia inhibitory factor mRNA in the rat brain and peripheral tissues. Proceedings of the National Academy of Sciences of the USA. 1991;88:7298–7302. doi: 10.1073/pnas.88.16.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Rush RA, McLachlan EM. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the rat dorsal root ganglion after peripheral nerve transection. Journal of Neuroscience. 1996;16:2901–2911. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Hyatt-Sachs H, Mohney RP, Schreiber RC, Shadiack AM, Sun Y, Vaccariello SA. Changes in neuropeptide phenotype after axotomy of adult peripheral neurons and the role of leukemia inhibitory factor. Perspectives on Developmental Neurobiology. 1996;4:75–90. [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


