Abstract
Isolated longitudinal muscle-myenteric plexus preparations from guinea-pig ileum were used to investigate the activity of myenteric neurons when the tissue was stretched in the circumferential direction. Membrane potentials were recorded via flexibly mounted intracellular recording electrodes containing Neurobiotin in 1 M KCl. The preparations were stretched to constant widths (+20 % and +40 % beyond slack width).
Multipolar neurons (Dogiel type II morphology) discharged spontaneous action potentials and proximal process potentials during maintained stretching, three of twenty-one at +20 % stretch and seven of nine at +40 % stretch. At the maximum extent of stretch tried, +40 % beyond slack tissue width, action potentials in Dogiel type II neurons occurred at 10-33 Hz. Neurons with other morphologies were all uniaxonal. Some displayed spontaneous fast EPSPs or action potentials, three of forty-one at +20 % stretch and seven of nineteen at +40 % stretch.
In seven of eight Dogiel type II neurons, action potentials or proximal process potentials persisted when membrane hyperpolarization was imposed via the recording electrode. Action potential discharge was abolished by hyperpolarization in seven of nine uniaxonal neurons; the exceptions were two orally projecting neurons.
Dogiel type II and uniaxonal neurons were classified as rapidly accommodating if they discharged action potentials only at the beginning of a 500 ms intracellular depolarizing pulse and slowly accommodating if they discharged for more than 250 ms. For Dogiel type II neurons, three of thirteen were slowly accommodating at +20 % stretch and two of four at 40 % stretch. For uniaxonal neurons the corresponding data were twelve of twenty-six and fifteen of nineteen neurons. The slowly accommodating state was associated with increased cell input resistance in uniaxonal neurons.
The spontaneous action potential discharge in Dogiel type II and uniaxonal neurons ceased when the muscle was relaxed pharmacologically by nicardipine (3 μM) or isoprenaline (1 μM), although the applied stretch was maintained. At the same time, evoked spike discharge became rapidly accommodating
We conclude that many Dogiel type II neurons, and possibly some orally projecting uniaxonal neurons, are intrinsic, stretch-sensitive, primary afferent neurons that respond to muscle tension with sustained action potential discharge.
Motor reflexes in the intestine can be evoked by chemical or mechanical stimulation of the mucosa or by distension (see Furness & Costa, 1987). Identities of the motor neurons and interneurons of these reflexes have been postulated from electrophysiological responses to physiological stimuli and projections to muscle or other neurons (Furness, Bornstein, Pompolo, Young, Kunze & Kelly 1994; Costa, Brookes, Steele, Gibbins, Burcher & Kandiah 1996). Motor and interneurons belong to the class of neurons designated electrophysiologically as S, these neurons have only a single axon. Uniaxonal neurons fall into several shape categories, including Dogiel type I, filamentous and small simple cells (Furness, Bornstein & Trussell, 1988; Furness et al. 1994; Costa et al. 1996). The other electrophysiological class of neurons is termed AH, these possess two or more long axons and have Dogiel type II morphology (Bornstein, Furness & Kunze, 1994). Intracellular microelectrode studies suggest that chemosensory intrinsic primary afferent neurons are AH/Dogiel type II neurons (Kunze, Bornstein & Furness, 1995; Bertrand, Kunze, Bornstein, Furness & Smith, 1997). Further evidence that the AH/Dogiel type II neurons are primary afferent neurons is provided by a recent study using activity-dependent neuronal markers (Kirchgessner, Liu & Gershon, 1996), which demonstrated that Dogiel type II neurons in submucous ganglia are activated by physiological stimuli even when some of the fast EPSP transmission between enteric neurons is blocked.
Intrinsic sensory neurons that respond to stretch had not been directly identified prior to this study. Some are predicted to have cell bodies in the myenteric plexus because stretch-evoked motor reflexes can be elicited in the absence of the mucosa and submucosa (Magnus, 1904; Costa & Furness, 1976; Tsuji & Yokoyama, 1982; Grider, 1989). Furthermore, extracellular recording has demonstrated action potential discharge in myenteric neurons in response to local distortion (Wood, 1970; Wood 1973). The patterns of firing that were seen are consistent with the proposal that S neurons are interneurons and motor neurons and AH neurons are intrinsic primary afferent neurons with spatially restricted receptive fields close to the cell bodies (Furness et al. 1994).
In the experiments reported here, we have identified stretch-sensitive neurons electrophysiologically and morphologically using Neurobiotin-filled intracellular microelectrodes to record from myenteric nerve cells in the region where stretch was applied. We impaled myenteric neurons in isolated myenteric plexus-longitudinal muscle preparations in which there was spontaneous contractile activity while constant circumferential stretch was applied. Because activation of some sensory neurons excites interneurons and motor neurons via slow EPSPs, thereby decreasing action potential accommodation (Kunze, Bertrand, Furness & Bornstein, 1997), we have also examined the effects of tissue stretching on the action potential discharge characteristics of myenteric neurons.
METHODS
Guinea-pigs (160-280 g) were stunned and killed by exsanguination and severence of the spinal cord. All experiments were performed using 2-3 cm long segments of distal ileum excised from these animals. The segments were placed in a recording dish (volume, 4 ml), and opened along the line of mesenteric attachment. During dissection, the tissue was immersed in physiological saline solution (composition (mM): NaCl, 118; KCl, 4.8; NaHCO3, 25; NaH2PO4, 1.0; MgSO4, 1.2; glucose, 11.1; CaCl2, 2.5; equilibrated with 95 % O2 and 5 % CO2) and kept at room temperature (16-20°C). Opened segments were pinned mucosal side up, one edge was attached to a fixed silicone elastomer (Sylgard®; Dow Corning) base and the other edge was attached to a smaller base that could be moved laterally to stretch the tissue along its circumferential axis (Fig. 1). The mucosa, submucosa, and circular smooth muscle were removed to expose the myenteric plexus. Then pins were positioned so that the tissue was stretched flat in the longitudinal direction, but was loose in the circumferential direction. The smaller Sylgard® base was pulled away from the larger base by hand until the tissue was flat in the circumferential direction without applying tension; at this stage, the circumferential width of the slack tissue (slack width) was measured. The recording dish was then placed on the stage of an inverted microscope and continuously superfused (4 ml min−1) with physiological saline solution that had been preheated to yield a bath temperature of 35-37°C. The movable base was attached to a metal rod via a small tungsten wire hook which was embedded in the Sylgard®. During the experiment, the metal rod was mounted on a hydraulically driven micromanipulator that was operated manually.
Figure 1. Recording set-up.

Schematic diagram showing arrangement of myenteric plexus-longitudinal muscle preparation in the recording bath. One edge of the tissue was pinned to a large immobile block of Sylgard® and the other was pinned to a movable Sylgard® block. Myenteric neurons were impaled with a flexibly mounted recording electrode (R) and internodal strands were electrically stimulated by a tungsten wire stimulating electrode (S). The tissue was stretched circumferentially 20 % or 40 % beyond slack width. The bath was filled with oxygenated physiological saline at 37 °C and placed on the stage of an inverted microscope.
Myenteric neurons located on the part of the tissue that was over the fixed Sylgard® base were impaled using two types of flexibly mounted glass pipettes. In the first case, glass pipettes with 2-3 cm long tapers were pulled using a Brown-Flaming P-87 pipette puller (Sutter Instruments), and impalements were maintained during spontaneous contractions because of the flexibility of the long taper. In the second case, pipettes were pulled with short (1 cm or less) tapers and, after filling the pipette with electrolyte, the stem was shortened by breaking the glass under water so that the overall length of the pipette was 1-2 cm. The shortened pipette was attached to a thin, flexible plastic strip using plastic putty (Blu-Tack, Bostik Ltd). In both cases, the signal was fed to the preamplifier via an unrestrained 40 μm thick silver wire. The plastic strip or the pipette with the long taper was attached to a Leitz micromanipulator. During active stretching, maintenance of the impalement was further aided by manually moving the pipettes using the Leitz micromanipulator while observing the tissue and micropipette through a dissecting microscope.
Glass micropipettes were filled with 4 % Neurobiotin (Vector Laboratories, Burlingame, CA, USA) in 1 M KCl. Electrode resistances were 80-200 MΩ. Recordings were made using an Axoclamp-2B amplifier (Axon Instruments). Signals were digitized at 1-10 kHz using a Digidata 1200A/D board (Axon Instruments) and stored using PC-based data acquisition software (AxoTape 2, Axon Instruments). Measurement of electrophysiological variables were done off-line using pCLAMP 6 (Axon Instruments) and Origin 4.1 (Microcal, Northampton, MA, USA). Extracellular stimuli were delivered to internodal nerve strands located circumferentially to the recording site via sharpened tungsten wires (10-50 μm diameter), insulated except at the tip. Stimulus pulses were of 0.1 ms duration and 0.1-0.5 mA intensity.
Resting membrane potential (Vm,rest) and spontaneous activity were measured without applying intracellular holding current after allowing the impalement to stabilize for at least 5 min. For spontaneous action potential discharge, spikes were detected using the ‘pick peaks’ module in Origin. Interspike interval histograms were plotted in Origin and the mean firing frequency taken as the reciprocal of the mean interspike interval. Input resistances and action potential accommodation were measured after injection of 500 ms duration depolarizing and hyperpolarizing current pulses (Kunze et al. 1997).
After recording, neurons were injected with Neurobiotin by passing depolarizing current pulses (1 nA, 500 ms duration) through the recording electrode. The tissue was then fixed overnight in 2 % formaldehyde plus 0.2 % picric acid in 0.1 M sodium phosphate buffer, pH 7.0, cleared in three changes of dimethylsulphoxide, and placed in phosphate-buffered saline (0.01 M sodium phosphate buffer in 0.9 % NaCl, pH 7). The tissue was then reacted with streptavidin coupled to Texas Red to reveal Neurobiotin fluorescence. The calcium binding protein calretinin was used as a marker for ascending interneurons and longitudinal muscle motor neurons (Costa et al. 1996). The calretinin was revealed using a polyclonal antibody (1 : 1000) (Code no. 7696; Swant Antibodies, Bellezona, Switzerland) and a secondary antibody (1 : 25) (Code no. 1034; Amersham) conjugated to fluorescein isothiocyanate.
Drugs used
For some experiments, isoprenaline (1 μM) or nicardipine (3 μM) were added to the superfusing solution to inhibit muscle contraction. Both drugs were purchased from Sigma-Aldrich.
Statistics
Unless otherwise stated, descriptive statistics of sample measurements are given as means ±s.d. with the number of samples (n) given in parentheses. Samples of mean frequency of spike discharge are given as the range and median of several mean values. When samples were compared, the probability associated with the null hypothesis of no difference is given as P. Statistical significance for the indicated two-tailed tests was set at P= 0.1.
RESULTS
Classification of impaled neurons
Intracellular recordings were made from nerve cells of preparations in two conditions of muscle cell activity, those in which muscle cells exhibited spontaneous contractile activity (actively contracting preparations) and those in which muscle cell contraction was inhibited by the L-type Ca2+ channel blocker nicardipine, or the β-receptor agonist, isoprenaline (non-contracting preparations). In preparations that were stretched with no relaxing agent present, spontaneous local areas of contractile activity were observed through the low power microscope that was used to position recording electrodes. This contractile activity was not observed when nicardipine (3 μM) or isoprenaline (1 μM) was present in the bathing solution. In non-contracting preparations, it was possible to place all neurons into either one of the two groups (AH and S neurons) that were originally described in the guinea-pig ileum (Hirst, Holman & Spence, 1974). Neurons had different electrophysiological states in actively contracting preparations, as described in detail below. In non-contracting tissue, AH neurons have Dogiel type II morphology and S neurons have single axons and cell bodies of several morphologies (Dogiel type I, filamentous and small simple) (Bornstein et al. 1994). Thus, morphological definitions have been used to identify the cell types examined in the present study. Only those neurons that were sufficiently filled with Neurobiotin to allow identification of the soma shape were included in the analysis.
Neural activity in stretched, actively contracting, tissue
Initial impalements were usually made in tissue that had been stretched circumferentially by 20 % beyond its slack width (+20 % stretch). Slack width is the width that the unrestrained tissue assumed in oxygenated physiological saline solution. After active and passive membrane properties of neurons were recorded, the tissue was slowly stretched further, at a rate of 10-15 μm s−1, until it was 40 % beyond slack width (+40 % stretch). If the impalement could not be maintained while the stretch was increased from +20 to +40 %, new impalements of neurons were made when the tissue was at +40 % stretch.
The soma morphology was identified for seventy-two of eighty-one neurons; twenty-four were Dogiel type II and forty-eight were uniaxonal. Each of the twenty-four Dogiel type II neurons had a hump on the descending phase of the action potential, but a single action potential was followed by a slow post-spike hyperpolarization lasting 2 s or more (a characteristic of these neurons in non-contracting tissue) in only eleven. None of the Dogiel type II neurons responded to focal electrical stimulation of internodal strands with a fast EPSP. None of the forty-eight uniaxonal neurons had a post-spike hyperpolarization lasting 2 s or more. Thirty-eight uniaxonal neurons responded with a fast EPSP to internodal strand stimulation, one had no synaptic response, and nine were not tested with internodal strand stimulation.
Dogiel type II neurons
Recordings were made from fifteen Dogiel type II neurons at +20 % stretch only, and from three at +40 % stretch only, and from six neurons under both conditions (Table 1). Three of twenty-one Dogiel type II neurons tested discharged spontaneous action potentials during +20 % stretch, whereas seven of nine were spontaneously active at +40 % stretch. These proportions are statistically different at P= 0.02 (χ2 test). Spontaneous events recorded from the impaled neurons included both soma action potentials and proximal process potentials (Fig. 2). The three neurons that fired spontaneously at +20 % stretch discharged action potentials irregularly at mean rates of 1.5-2.1 Hz. Two of the neurons with spontaneous activity at +40 % stretch fired in irregular bursts and the remaining five fired more regularly. During +40 % stretch, mean spike discharge rates ranged from 0.3 to 25 Hz (median, 4 Hz).
Table 1.
Proportions of neurons that were spontaneously active in tissue stretched to +20 or +40 %
| +20 % stretch | +40 % stretch | |
|---|---|---|
| Dogiel Type II neurons | ||
| Unpaired recordings | 2/15 | 3/3 |
| Paired recordings | 1/6 | 4/6 |
| All recordings | 3/21 | 7/9 |
| Uniaxonal neurons | ||
| Unpaired recordings | 2/29 | 2/7 |
| Paired recordings | 1/12 | 5/12 |
| All recordings | 3/41 | 7/19 |
Figure 2. Patterns of spontaneous spike discharge in Dogiel type II neurons.

Spontaneous spike discharge from three neurons during +40 % stretch of actively contracting tissue. The traces show firing patterns and shapes of individual spikes. A, neuron, spiking with full somatic action potentials (APs) which show a hump on the descending phase. B, a neuron with more regular action potential discharge than that shown in A. C, a neuron which discharged irregularly with only proximal process potentials during stretching. The electrotonic potential (proximal process potential, PPP) shown to the right lacks the hump characteristic of full somatic action potentials.
For the six neurons in which activity was recorded at both degrees of stretch, one discharged action potentials at both +20 % and +40 % stretch, four were without spontaneous activity in tissue extended by 20 % but spiked spontaneously at +40 % stretch, and the remaining one showed no activity in both conditions. The neuron that discharged during +20 % stretch increased its mean discharge rate from 2 to 25 Hz when the tissue was stretched to +40 % (Fig. 3).
Figure 3. Activity of a single Dogiel type II neuron at differing degrees of tissue stretch.

A, the neuron discharged action potentials at 1.8 Hz during +20 % stretch. B, the same neuron discharged at the higher rate of 33 Hz when the tissue was stretched to +40 %, this record was taken approximately 5 min after applying the stretch.
Eight spontaneously spiking Dogiel type II neurons, all included in the above samples, were hyperpolarized by ≥ 10 mV from Vm,rest by passing current through the recording electrode to determine whether the spontaneous spiking might be due to excitatory synaptic input to the soma or conduction from processes (Fig. 4). Two neurons at +20 % and six at +40 % stretch were tested. The discharge frequencies of spontaneous potentials were not altered by the hyperpolarization in seven neurons. The discharge of the remaining neuron (in tissue stretched +20 %) was abolished by hyperpolarization.
Figure 4. Effect of membrane hyperpolarization on spontaneous discharge in Dogiel type II neurons.

The records are from Dogiel type II neurons in tissues stretched to +40 %. A, for this neuron, hyperpolarizing current was manually applied starting at the time indicated by the vertical arrow. Hyperpolarization by more than 10 mV prevented full somatic action potential discharge but failed to abolish proximal process potential discharge. Partial removal of the hyperpolarizing current produced limited recovery of somatic spiking. Inset, trace showing single proximal process potential. B, injection of a prolonged hyperpolarizing current pulse caused spike discharge to cease in this neuron.
The state of excitability, measured by accommodation in action potential discharge during injection of suprathreshold depolarizing current pulses (500 ms) was tested in seventeen of the Dogiel type II neurons. Neurons in which action potential firing ceased within the first 250 ms were classified as rapidly accommodating (RA) whereas those that discharged action potentials for more than 250 ms were classified as slowly accommodating (SA) (Kunze et al. 1997).
During +20 % stretch, ten neurons were in the RA state and three were SA, and during +40 % stretch, two neurons were RA and two were SA. The proportion of Dogiel type II neurons that were in the SA state (5/17) was significantly larger than in our previous study (0/29; Kunze, Bornstein, Furness, Hendriks & Stephenson, 1994) in which the tissue, although under tension, was relaxed by addition of 3 μM nicardipine (P= 0.009, Fisher's exact test).
For RA neurons, Vm,rest was -58 ± 6 mV (n= 12) and input resistance (Rin) was 167 ± 46 MΩ (n= 7), and for SA neurons Vm,rest was -49 ± 9 mV (n= 5), and Rin was 227 ± 65 MΩ (n= 4). P, associated with no difference between RA and SA neurons, was 0.05 for Vm,rest and 0.09 for Rin (Mann-Whitney U test).
Paired comparisons were made for three of the seventeen Dogiel type II neurons, for which spike discharge accommodation was tested during both +20 and +40 % stretch. One neuron was RA for both degrees of stretch and fired only one action potential, and two were RA during +20 % stretch but became SA when the tissue was stretched to +40 % (Fig. 5). The change in the maximum number of action potentials discharged was from three to twenty-one for one neuron and from two to seven for the other.
Figure 5. Effect of tissue stretch on accommodation of spike discharge.

Firing patterns of impaled neurons during injection of 500 ms duration current pulses. A, records taken from a uniaxonal neuron during both +20 and +40 % stretch. Aa, during +20 % stretch, the neuron discharge accommodated rapidly, no matter what the intensity of the depolarizing current pulse. Ab, when the tissue was stretched to +40 % this neuron could fire throughout a 500 ms depolarizing pulse. B, records taken from a single Dogiel type II neuron. Ba, during +20 % stretch this neuron fired phasically during injection of a large 500 ms current pulse. Bb, when the tissue was stretched to +40 %, the same neuron fired throughout most of the duration of a smaller intensity depolarizing current pulse.
Uniaxonal neurons
Activity of forty-eight uniaxonal neurons was recorded, twenty-nine in tissue stretched to +20 %, seven in tissue stretched to +40 %, and twelve under both conditions (Table 1). Three of the forty-one neurons at +20 % stretch discharged action potentials spontaneously. The proportion of active neurons (7/19) was greater in tissue stretched by +40 % (P= 0.02; χ2 test). Mean firing rates were 0.2, 1.3, and 10 Hz for the three neurons at +20 % stretch; for neurons at +40 % stretch, mean firing rates ranged from 0.8 to 33 Hz (median, 2.0 Hz). Only two of the forty-eight uniaxonal neurons received ongoing spontaneous fast EPSPs; both were in tissue stretched to +20 % (Fig. 6). Five neurons showed spontaneous fast EPSPs separated by intervals of at least 5 s, four were at +20 % and one was at +40 % stretch.
Figure 6. Spontaneous fast EPSPs in a uniaxonal neuron.

Records taken from a uniaxonal neuron in tissue stretched to +40 %. A, the neuron exhibited ongoing fast EPSPs which did not elicit action potentials even when the membrane was depolarized by current injection. B, record from same neuron as in A showing that hyperpolarizing current injection did not abolish the fast EPSPs.
Of the twelve neurons studied under both conditions, seven were quiescent and one discharged spontaneous action potentials at both +20 % and +40 % stretch. Four neurons that were quiescent during +20 % stretch discharged spontaneous spikes at +40 % stretch (Fig. 7). The neuron which spiked spontaneously during +20 % stretch increased its mean discharge rate from 1.3 to 2.3 Hz at +40 % stretch.
Figure 7. Patterns of spontaneous spike discharge in uniaxonal neurons.

Spontaneous spike discharge from two neurons during +40 % stretch in actively contracting tissue. Traces to the left show firing pattern, and to the right show shapes of spikes in expanded traces. A, neuron spiking with full somatic action potentials; there is no hump on the descending phase. This action potential was elicited by stimulation of an internodal strand and arises from a fast EPSP (• indicates position of stimulus). B, neuron with more regular action potential discharge than that shown in A. This neuron also lacked a hump on the descending phase of the action potential.
Nine uniaxonal neurons that fired spontaneous action potentials were hyperpolarized by current injection; three were in +20 % and six were in +40 % stretched tissue. Hyperpolarization abolished spiking in seven without revealing any underlying spontaneous fast EPSPs (Fig. 8). The spike discharge for two of the nine neurons, both of which were in preparations stretched by 20 %, was not notably altered by hyperpolarization and fast EPSPs were not evident (Fig. 8).
Figure 8. Effect of membrane hyperpolarization on spontaneous discharge in uniaxonal neurons.

A, hyperpolarization of this neuron extinguished spontaneous discharge. B, spontaneously discharging neuron. Manual injection of hyperpolarizing current commenced at the arrow but did not prevent spontaneous spike discharge.
Unpaired comparisons in firing accommodation were made between twenty-six uniaxonal neurons at +20 % and nineteen neurons at +40 % stretch. During +20 % stretch, fourteen neurons were RA and twelve were SA; during 40 % stretch four neurons were RA and fifteen were SA. Stretching to +40 % from +20 % was therefore associated with an increased proportion of uniaxonal neurons in the SA state (P= 0.03, χ2 test). At +20 % stretch, RA neurons fired between one and sixteen action potentials in response to 500 ms intracellular depolarizing current pulses (median, 2) for periods ranging from 10 to 230 ms (median, 38 ms); six neurons discharged with only one action potential at the beginning of the depolarizing pulse. SA neurons fired ten to thirty-three action potentials (median, 21) for periods ranging from 260 ms to the maximum possible 500 ms (median, 400 ms). In tissue at +40 % stretch, RA neurons fired one and sixteen action potentials (median, 2), two fired only once and the remaining two fired for 94 and 104 ms, respectively. SA neurons fired five to forty-eight action potentials (median, 22); for durations lasting from 256 ms up to the maximum 500 ms (median, 500 ms).
Overall, for RA neurons Vm,rest was -48 ± 5 mV (n= 18) and Rin was 198 ± 73 MΩ (n= 14) whereas for SA neurons Vm,rest was -48 ± 5 mV (n= 27) and Rin was 317 ± 136 MΩ. The input resistances of SA neurons exceeded those of RA neurons at P= 0.007 (Mann-Whitney U test).
Only one of the eighteen RA neurons but eight of the twenty-seven SA neurons exhibited spontaneous action potentials, indicating that for uniaxonal neurons there was a correlation between the nature of accommodation and the likelihood of spontaneous spike discharge occurring (P= 0.05, χ2 test).
Action potential accommodation was tested for twelve uniaxonal neurons under both degrees of stretch. One neuron was RA and seven were SA throughout, and four converted from RA to SA after the tissue was stretched from +20 % to +40 % (Fig. 5). The number of action potentials discharged increased from one to thirty-two (median, 6.5) during +20 % stretch to one to forty-eight (median, 23) during +40 % stretch (P= 0.003, Wilcoxon paired signed-rank test).
Effect of preventing contractile activity in stretched tissue
Contractile activity in the smooth muscle was inhibited by adding the L-type calcium channel antagonist nicardipine (3 μM) or of the β-adrenergic receptor agonist isoprenaline (1 μM) to the superfusate. At these concentrations, the drugs relax the smooth muscle of the guinea-pig ileum without affecting nerve transmission (isoprenaline: Kosterlitz, Lydon & Watt, 1970; nicardipine: Kaplita & Triggle, 1983)
Only one of seventeen Dogiel type II neurons and one of forty-five uniaxonal neurons spiked spontaneously in tissues exposed to nicardipine throughout the experiment. Thus, the proportions of spontaneously active Dogiel type II and uniaxonal neurons in non-contracting tissue were smaller than in contracting tissue (Table 2).
Table 2.
Proportions of spontaneously spiking neurons: nicadipine-paralysed (N) tissues compared with contracting (C) tissues
| +20 % stretch | +40 % stretch | |||
|---|---|---|---|---|
| C | N | C | N | |
| Uniaxonal neurons | 2/29 | 0/35 | 7/19 | 1/10 |
| Dogiel type II neurons | 2/15 | 0/15 | 7/9 | 1/2 |
Proportions of spontaneous myenteric neurons in nicardipine-paralysed tissues differ from those in contracting tissue at P= 0.08, Fisher's exact test.
Recordings were made from six neurons (1 Dogiel type II and 5 uniaxonal) which were initially superfused with normal physiological saline so that the muscle cells could develop tension. One uniaxonal neuron was in tissue that was stretched to +20 % and the others were in tissue stretched to +40 %; the degree of stretch was not altered during the experiment. All neurons fired spontaneously in actively contracting tissue, but became silent within 15 min after nicardipine was added to the superfusate (Fig. 9). Resting membrane potentials in normal solution were -52 ± 7 mV and with nicardipine they were -50 ± 6 mV. Cell input resistance fell from 334 ± 91 MΩ for neurons in actively contracting tissue to 257 ± 75 MΩ after the smooth muscle was relaxed (P= 0.04, Wilcoxon paired signed-rank test).
Figure 9.
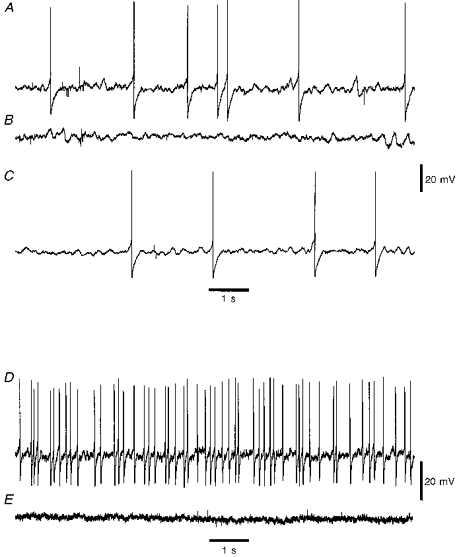
Effect of muscle relaxants on activity of Dogiel type II neuronsA, spontaneous discharge in a neuron in actively contracting tissue stretched to +40 %. B, spontaneous discharge in the same neuron was extinguished after exposure to 1 μM isoprenaline. C, spontaneous discharge was partially restored after 15 min washout with normal physiological saline. D, spontaneous discharge in a second Dogiel type II neuron in tissue stretched to +40 %. E, exposure of tissue to 3 mM nicardipine extinguished spontaneous discharge in the second neuron.
Isoprenaline-containing solution was substituted for the normal saline for six Dogiel type II neurons, each of which was initially firing spontaneously in +40 % stretched tissue. All neurons fell silent after 10 min exposure to isoprenaline. For three neurons, return to normal physiological saline partly restored spontaneous spiking after a delay of 20-30 min (Fig. 9). The initial resting membrane potential was -45 ± 7 mV and this changed to -50 ± 6 mV after addition of isoprenaline (P= 0.04, Wilcoxon paired signed-rank test). Input resistances were measured for only two neurons in this group; addition of isoprenaline decreased Rin from 296 to 260 MΩ for one and from 340 to 294 MΩ for the other neuron.
The effect of muscle relaxants on firing accommodation was tested by injecting 500 ms duration depolarizing current pulses (Fig. 10). Five uniaxonal and two Dogiel type II neurons were in the SA state at +40 % stretch. Nicardipine (3 μM) was used to relax the tissues containing the five uniaxonal neurons and one Dogiel type II neuron; isoprenaline (1 μM) was used for the remaining Dogiel type II neuron. The muscle relaxants converted all seven neurons to the RA state. For the uniaxonal neurons, the maximum number of action potentials during a 500 ms depolarization in contracting tissue was five to forty-eight (median, 22), after adding the muscle relaxant this decreased to one to two (median, 1) (P= 0.02, Wilcoxon paired signed rank test). Resting membrane potentials before and after muscle relaxation were -50 ± 2 and -54 ± 5 mV, respectively. Input resistances decreased from 329 ± 94 to 258 ± 76 MΩ (P= 0.04, Wilcoxon paired signed rank test). The Dogiel type II neurons had Vm,rest values of -39 and -61 mV in contracting tissue and hyperpolarized to -47 and -65 mV, respectively. Input resistances of the Dogiel type II neurons were not measured.
Figure 10.

Effect of pharmacological relaxation of the tissue on accommodation of spike dischargeFiring of neurons during injection of 500 ms duration current pulses. A, recording from a uniaxonal neuron in +40 % stretched tissue. In actively contracting tissue, this neuron discharged throughout most of the period of current injection. When the smooth muscle was relaxed by adding nicardipine, the neuron discharged only a single action potential at the beginning of the injection of an identical current pulse. B, Dogiel type II neuron spiked throughout a depolarizing current pulse when recorded from in actively contracting, +40 % stretched tissue. The same neuron fired only at the beginning of the depolarization, caused by injecting an identical current pulse, after the tissue was relaxed with isoprenaline.
Neural activity during rapid stretch
Dogiel type II neurons
Recordings were made from thirteen Dogiel type II neurons during a rapid, 0.7- 1.0 mm s−1, movement which stretched the tissue from +20 to +40 %. For ten of these neurons, neither spikes nor fast EPSPs were observed during the active stretch which lasted about 1 s and at the end of which the impalement was lost. Three neurons spiked during active stretching. One spiked transiently, another started to fire during active stretching but continued to spike after the tissue had reached +40 % stretch, the remaining neuron spiked twice during the active stretch after which the impalement was lost (Fig. 11).
Figure 11.

Neuron activity during rapid circumferential stretching of tissueIntracellular recordings from neurons during rapid (0.7-1 mm s−1) stretching of the tissue. The broad horizontal arrows indicate the approximate time of onset of the stretch. A, transient volley of small proximal process potentials evoked in a Dogiel type II neuron, one of which is shown on an expanded time base. B, in another Dogiel type II neuron, stretching was associated with the onset of action potential discharge which did not adapt. C, rapid stretching elicited two fast EPSPs in a uniaxonal neuron. One of these is shown on an expanded time base. D, another uniaxonal neuron responded during rapid stretch with sustained membrane depolarization and steady action potential discharge.
Uniaxonal neurons
Recordings were taken from fourteen uniaxonal neurons while the tissue was stretched rapidly. Eight neurons showed no action potentials or fast EPSPs during the movement and impalements were lost near the end of active stretching for four of these. Two neurons responded with a slow onset, sustained depolarization, which was accompanied by the discharge of full action potentials which did not accommodate. The remaining four neurons responded with a transient (median, 0.6 s; range, 0.1-1.5 s) volley of fast EPSPs which ceased within 1 s or less of their onset (Fig. 11).
Projection patterns of impaled neurons
Dogiel type II neurons
Dogiel type II neurons are smooth, round or oval, with two or more long, branching processes. Dogiel type II neurons never projected orally for more than 1 mm and all projected circumferentially. A subset of Dogiel type II neurons had an additional anal projection as previously described by Brookes et al. (1995), who also reported that the anally projecting neurons were almost exclusively of the dendritic subtype. Three of the nine spontaneously spiking Dogiel type II neurons had a single anally projecting collateral process that could be traced for four, five and nine rows of ganglia (about 2-6 mm) before becoming undetectable. All three neurons remained active during hyperpolarization and two neurons possessed filamentous dendrites. The two neurons that did not discharge at +40 % stretch projected circumferentially, but not anally.
For the seventeen Dogiel type II neurons whose spike accommodation was tested, all but one projected only circumferentially. The remaining neuron possessed a dye-filled collateral that projected anally for five rows of ganglia; this neuron was in the SA state.
Three Dogiel type II neurons responded with spikes on rapid stretching of the tissue. Of the two that only discharged transiently, one projected anally as well as circumferentially, and the other projected only circumferentially. The neuron whose spike discharge did not adapt projected only circumferentially, and this was also the case for the neurons that failed to respond during the rapid stretch.
Uniaxonal neurons
All forty-eight uniaxonal neurons had round or oval somata and either lamellar (Dogiel type I neurons) or filamentous (filamentous neurons) dendrites, or they lacked prominent dendrites (‘simple’ neurons) (Furness et al. 1988). Of the nine neurons that spiked spontaneously at +20 or +40 % stretch, five were Dogiel type I, two were filamentous, and two were simple neurons.
Five of these neurons projected anally, two projected orally, and two had axons that ended in expansion bulbs within the ganglion containing the soma. One anally projecting neurons had an axon ending in an expansion bulb. None of the spiking neurons had processes with extensive collateral branching or preterminal boutons in neighbouring ganglia. The axons of the two orally projecting and the remaining four anally projecting neurons could not be traced to their targets. Only the two orally projecting neurons continued spiking spontaneously when hyperpolarized. None of the spontaneously spiking neurons was immunoreactive for calretinin.
Of the twelve neurons that did not spike at +40 % stretch, seven projected anally, one projected orally, one projected to the tertiary plexus and two had expansion bulbs in their own ganglia but did not project beyond it. The labelling of the remaining neuron was insufficient to determine its projection. Only the neuron projecting to the tertiary plexus and the one projecting orally were positive for calretinin immunoreactivity. Both neurons that received continuous fast EPSP input but did not spike projected orally and were positive for calretinin immunoreactivity.
For uniaxonal neurons that were RA during injection of the depolarizing current pulses, eleven had projections that could be traced anally or locally, six projected orally, and one was not sufficiently filled to determine the projection; all were negative for calretinin immunoreactivity. Twenty-four of the SA neurons projected anally or locally, one projected orally; two projected to the tertiary plexus and were positive for calretinin.
Fourteen uniaxonal neurons were recorded from during rapid, active stretch. Five of eight unresponsive neurons projected anally, two projected orally (these were calretinin negative), and two were insufficiently filled to determine the direction of axon projection. Of the six neurons that responded during rapid stretch, four had axons that projected anally and the remaining two could not be traced beyond their ganglia due to poor Neurobiotin filling.
DISCUSSION
Possible identification of Dogiel type II neurons as primary afferent neurons responsive to stretch
The present study indicates that many myenteric neurons with Dogiel type II morphology respond to mechanical forces in the external musculature. These neurons sometimes fired action potentials and/or proximal process potentials spontaneously under conditions of moderate sustained stretch and either increased their firing or began firing when the stretch was increased. Proximal process potentials occur when action potentials, which reach the cell body via its processes, fail to initiate a regenerative action potential in the soma. Their presence indicates that action potentials were generated in the processes of Dogiel type II neurons, not in the nerve cell bodies. This conclusion is reinforced by the finding that when the membrane was artificially hyperpolarized via the recording electrode, the spontaneous discharge was maintained. Thus it is unlikely that the spontaneous firing was secondary to a synaptic activation of the cell bodies of Dogiel type II neurons.
The responses of Dogiel type II neurons to stretch were similar to those reported with extracellular recording by Wood (1970, 1973), who identified units that discharged when the ganglion from which records were taken was distorted, or when tension was applied to the adjacent muscle, in cat and guinea-pig small intestine. The units fired repetitively during the mechanical stimulus at mean frequencies of 40 Hz, but abruptly ceased firing when the stimulus was removed. In the present study, some Dogiel type II neurons fired at high rates throughout periods of stretch. Wood (1975) used the term ‘slowly adapting mechanoreceptor’ to describe units with similar discharge characteristics, which fired action potentials when local distortion was applied with a glass probe. Some slowly adapting mechanoreceptors fired action potentials when the gut wall moved spontaneously, as was observed in the present work. This suggests that the slowly adapting mechanoreceptors identified by Wood may be Dogiel type II primary afferent neurons. Wood (1989) pointed out that the receptive fields of the slowly adapting mechanoreceptors are very small, not much larger than the ganglia that contain them. This is consistent with the predominantly local projections of the Dogiel type II neurons (Bornstein, Hendriks, Furness & Trussell, 1991b). It is thus not surprising that responses in myenteric mechanoreceptive neurons have not been previously recorded with intracellular microelectrodes. Intracellular recording of responses to distension have previously been made at 20 mm or greater longitudinal distances from the stimulus, for the obvious reason that the stimulus is likely to dislodge the electrode at sites closer than this. The events that were recorded intracellularly at a distance were synaptic potentials, in interneurons and motor neurons (Hirst, Holman & McKirdy, 1975; Bornstein, Furness, Smith & Trussell, 1991a; Smith, Bornstein & Furness, 1992).
The degree of stretch, 20-40 %, that was applied in the present experiments is in the range of diameter increase (40-70 %) that is observed when the guinea-pig small intestine is distended with fluid and is free to generate spontaneous emptying reflexes (Schulze-Delrieu, Brown & Custer-Hagen, 1991).
Only three of thirteen Dogiel type II neurons responded with spikes during the active phase of a rapid stretch applied to the tissue. These neurons could be the rapidly adapting mechanosensory neurons identified in the extracellular recording studies of Wood (see Wood, 1975). It is not yet clear whether these neurons are a subset of those that are sensitive to sustained stretch.
The present work suggests that a proportion of Dogiel type II neurons are intrinsic primary afferent neurons that are responsive to stretch. In other studies, some myenteric Dogiel type II neurons have been shown to be chemosensitive primary afferent neurons that respond to changes in the lumenal chemical environment (Kunze et al. 1995; Bertrand et al. 1997). However, it has not yet been determined whether all Dogiel type II neurons are likely to be primary afferent neurons, or whether some of these neurons respond to several modes of sensory stimulation.
Mechanism of detection of stretch
The action potential discharge in the Dogiel type II neurons that occurred in stretched tissue disappeared if contractile activity in the muscle was substantially reduced or prevented either with an L-type Ca2+ channel blocker or with a β-receptor agonist. This indicates that active tension in the muscle cells caused excitation of the primary afferent neurons in the present experiments. When intestinal muscle is stretched, the muscle cells react by depolarization and action potential generation which develops tension in the tissue (Bülbring, 1955). Local tensions within the tissue are therefore greater for the same degree of stretch if the muscle cells contract, compared with when they do not. The tension in the muscle could be communicated to the neurons electrically, mechanically, or chemically. An electrical communication to the neurons from the muscle seems unlikely. There is no evidence of electrical connection between these elements, which would be presumably via gap junctions. If such connections existed, they would have been likely to be revealed in experiments in which intracellular injection of dye has been used to explore cell-to-cell connections (e.g. Hanani, Zamir & Baluk, 1989). It is feasible that mechanical stresses in the muscle are communicated to the ganglia. Ultrastructural analysis of intestinal smooth muscle during tension development indicates that the muscle cells exert force on the connective tissue (collagen and elastin) in which they are embedded (Gabella, 1984) and it has been demonstrated that the ganglia are distorted by muscle movement (Gabella & Trigg, 1984). Moreover, processes of muscle cells form peg and socket-like relationships with glial cells at ganglion surfaces and may pull directly on the ganglia (Komuro, 1988). Forces communicated to the afferent neurons could open deformation-sensitive channels, resulting in action potential generation in processes and perhaps in the cell body. It is also feasible that the active smooth muscle cells could release substances such as adenosine, potassium, lactic acid or inorganic phosphate that may excite the afferent neurons.
It could be postulated that axon reflexes in the enteric processes of spinal primary afferent neurons were responsible for initiating the activity observed in the Dogiel type II neurons. However, it has been shown that enteric reflexes in response to muscle stretch are not diminished after the axons of spinal primary afferent neurons have been cut and allowed to degenerate (Furness, Johnson, Pompolo & Bornstein, 1995). Moreover, this interpretation would require transmission from the spinal primary afferent neurons to the processes of the Dogiel type II neurons. Pharmacological studies indicate that transmission from spinal primary afferent neurons, within the gut wall, is via tachykinins, which generate slow EPSPS in enteric neurons (Holzer & Holzer-Petsche, 1997). Slow EPSPs generate trains of action potentials, but trains arising from the processes of Dogiel type II neurons are not observed when nerve fibre tracts that innervate myenteric ganglia are stimulated.
Interaction between Dogiel type II neurons
Electrophysiological and structural evidence indicates that Dogiel type II neurons are synaptically connected to each other, and that transmission at these synapses is via slow EPSPs (Pompolo & Furness, 1988; Kunze, Furness & Bornstein, 1993; Bertrand et al. 1997). In unstretched ileum, and in tissue in which active contraction is blocked, Dogiel type II neurons all exhibit prolonged post-spike hyperpolarizations following a single action potential (Bornstein et al. 1994). These hyperpolarizations are due to opening of calcium-dependent potassium channels; transmitters of slow EPSPs inhibit opening of these channels, depolarize the neurons and increase their input resistance. In the present study, only eleven of twenty-four Dogiel type II neurons exhibited a prolonged post-spike hyperpolarization in stretched tissue, suggesting that most Dogiel type II neurons received transmission via slow EPSPs during stretch. This conclusion is supported by the greater proportion of slowly accommodating Dogiel type II neurons observed in stretched, actively contracting, intestine. In tissue without contractile activity, AH/Dogiel type II neurons fire only at the beginning of depolarizing current pulses, that is, they are rapidly accommodating (Kunze et al. 1994). In contrast, five of seventeen Dogiel type II neurons in stretched, actively contracting, tissue in the present work were slowly accommodating. It is probable that this change in state was associated with synaptic input from excitatory neurons. Mutual excitation of Dogiel type II neurons that are mucosal chemosensors has been described previously, and it was concluded that primary afferent neurons form a self-reinforcing network (Wood, 1994; Bertrand et al. 1997).
Activity of uniaxonal/S neurons
S neurons discharged spontaneous action potentials in stretched, actively contracting tissue and more S neurons spiked spontaneously in highly stretched than in moderately stretched tissue. Increasing the degree of stretch induced activity in formerly non-spiking neurons or increased the frequency of ongoing spike discharge.
An explanation of the activity observed in S neurons in stretched tissue is that the neurons received excitatory synaptic input from the Dogiel type II neurons. It was shown by studies in which pairs of neurons were simultaneously impaled that AH/Dogiel type II neurons transmit to S neurons via slow EPSPs (Kunze et al. 1993). These slow EPSPs increase the input resistances of S neurons and in many S neurons they block an early onset rectifier, thus converting the neurons from rapidly accommodating to slowly accommodating (Kunze et al. 1997). In non-contracting, unstretched preparations, Kunze et al. (1997) found that three of forty-five S neurons were slowly accommodating and data collected by Bornstein et al. (1994) indicated that ten of 115 were SA (13/160 or 8 % overall). A much higher proportion of slowly accommodating neurons was found in stretched, actively contracting tissue in the present work 45 % (12/26) at +20 % stretch and 78 % (15/19) at +40 % stretch. Moreover, SA neurons in stretched tissue had greater input resistances than RA neurons (320 compared with 200 MΩ) and blocking contractile activity decreased the input resistance. These results are consistent with the hypothesis that S neurons receive slow excitatory input from the primary afferent neurons during stretch and generation of active tension. This parallels conclusions of another study which showed that chemosensitive enteric primary afferent neurons excite S neurons via slow EPSPs (Kunze et al. 1997). This conclusion is also consistent with the proposal by Wood (1975) that intrinsic primary mechanoreceptive sensory neurons communicate with second-order neurons via slow EPSPs, although Wood's suggestion was based on extracellular recording from myenteric neurons and so he was unable to identify the second-order neurons as S neurons. It is possible, but unlikely, that stretch directly causes membrane changes (block of rectifier channels and closure of K+ channels) that mimic the effects of slow EPSPs.
Some S neurons responded to stretch with fast EPSPs. These synaptic potentials could be from excitatory inputs from primary sensory neurons (Stebbing & Bornstein, 1996), or from interneurons (Bornstein et al. 1991a; Portbury et al. 1995).
Two S neurons were spontaneously active in stretched tissue, remained spontaneously active when they were hyperpolarized and showed no evidence of receiving spontaneous fast EPSPs. Both these neurons projected orally. It is feasible that these two S neurons were directly responsive to stretch. Such neurons may have dual roles as primary afferent neurons and inter- or motor neurons.
Conclusion: circuits for reflexes induced by muscle stretch
The present experiments can be related to previous work in which the enteric nerve circuits have been analysed morphologically, histochemically and by recording from muscle cells, interneurons and motor neurons (Furness et al. 1994; Furness Bornstein, Kunze, Bertrand, Kelly & Thomas, 1996). It is concluded that Dogiel type II neurons with cell bodies in the myenteric ganglia are the primary afferent neurons for the reflexes and that these excite interneurons of polysynaptic reflex paths and motor neurons of monosynaptic reflex paths via slow EPSPs to cause contraction of circular muscle oral to and relaxation anal to the region of stretch. It is further suggested that the stretch-induced contraction of smooth muscle is important for the excitation of the primary afferent neurons.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (grant no. 963213) and by the National Institutes of Health (USA; grant DDK 09162 to P.P.B.).
References
- Bertrand PP, Kunze WAA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of the myenteric neurons in the small intestine to chemical stimulation of the mucosa. American Journal of Physiology. 1997;273:G1–14. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Kunze WAA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? Journal of the Autonomic Nervous System. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. Journal of Neuroscience. 1991a;11:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Hendriks R, Furness JB, Trussell DC. Ramifications of the axons of AH-neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. Journal of Comparative Neurology. 1991b;314:437–451. doi: 10.1002/cne.903140303. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Song Z-M, Ramsay GA, Costa M. Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. Journal of Neuroscience. 1995;15:4013–4022. doi: 10.1523/JNEUROSCI.15-05-04013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E. Correlation between membrane potential, spike discharge and tension in smooth muscle. The Journal of Physiology. 1955;128:200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Brookes SJH, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of nerve pathways and their pharmacology. Naunyn-Schmiedeberg's Archives of Pharmacology. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Furness JB, Bornstein JC, Kunze WAA, Bertrand PP, Kelly H, Thomas EA. Experimental basis for realistic large scale computer simulation of the enteric nervous system. Clininical and Experimental Pharmacology and Physiology. 1996;23:786–792. doi: 10.1111/j.1440-1681.1996.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Bornstein JC, Pompolo S, Young HM, Kunze WAA, Kelly H. The circuitry of the enteric nervous system. Neurogastroenterology and Motility. 1994;6:241–253. [Google Scholar]
- Furness JB, Bornstein JC, Trussell DC. Shapes of nerve cells in the myenteric plexus of the guinea-pig small intestine revealed by the intracellular injection of dye. Cell and Tissue Research. 1988;254:561–571. doi: 10.1007/BF00226506. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The Enteric Nervous System. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterology and Motility. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Gabella G. Structural apparatus for force transmission in smooth muscles. Physiological Reviews. 1984;64:455–477. doi: 10.1152/physrev.1984.64.2.455. [DOI] [PubMed] [Google Scholar]
- Gabella G, Trigg P. Size of neurons and glial cells in the enteric ganglia of mice, guinea-pigs, rabbits and sheep. Journal of Neurocytology. 1984;13:49–71. doi: 10.1007/BF01148318. [DOI] [PubMed] [Google Scholar]
- Grider JR. Identification of neurotransmitters regulating intestinal peristaltic reflex in humans. Gastroenterology. 1989;97:1414–1419. doi: 10.1016/0016-5085(89)90384-3. [DOI] [PubMed] [Google Scholar]
- Hanani M, Zamir O, Baluk P. Glial cells in the guinea pig myenteric plexus are dye coupled. Brain Research. 1989;497:245–249. doi: 10.1016/0006-8993(89)90269-2. 10.1016/0006-8993(89)90269-2. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, McKirdy HC. Two descending nerve pathways activated by distension of guinea-pig small intestine. The Journal of Physiology. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. The Journal of Physiology. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part 1. Expression, release and motor function. Pharmacology and Therapeutics. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. 10.1016/S0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Kaplita PV, Triggle DJ. Actions of Ca2+ antagonists on the guinea-pig ileal myenteric plexus preparation. Biochemical Pharmacology. 1983;32:65–68. doi: 10.1016/0006-2952(83)90653-6. 10.1016/0006-2952(83)90653-6. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu M-T, Gershon MD. In situ identification and visualization of neurons that mediate enteric and enteropancreatic reflexes. Journal of Comparative Neurology. 1996;371:270–286. doi: 10.1002/(SICI)1096-9861(19960722)371:2<270::AID-CNE7>3.0.CO;2-#. 10.1002/(SICI)1096-9861(19960722)371:2<270::AID-CNE7>3.3.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Komuro T. Direct contacts between Auerbach's ganglia and smooth muscle cells in the small intestine of the rat. Neuroscience Letters. 1988;92:27–29. doi: 10.1016/0304-3940(88)90736-7. 10.1016/0304-3940(88)90736-7. [DOI] [PubMed] [Google Scholar]
- Kosterlitz HW, Lydon RJ, Watt AJ. The effects of adrenaline, noradrenaline and isoprenaline on inhibitory α- and β-adrenoceptors in the longitudinal muscle of the guinea-pig ileum. British Journal of Pharmacology. 1970;39:398–413. doi: 10.1111/j.1476-5381.1970.tb12903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Bertrand PP, Furness JB, Bornstein JC. Influence of the mucosa on the excitability of myenteric neurons. Neuroscience. 1997;76:619–634. doi: 10.1016/s0306-4522(96)00408-3. 10.1016/S0306-4522(96)00408-3. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. 10.1016/0306-4522(95)00067-S. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB, Hendriks R, Stephenson DSH. Charybdotoxin and iberiotoxin but not apamin abolish the slow after-hyperpolarization in myenteric plexus neurons. Pflügers Archiv. 1994;428:300–306. doi: 10.1007/BF00724511. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bornstein JC. Simultaneous intracellular recordings from enteric neurons reveal that myenteric AH neurons transmit via slow excitatory postsynaptic potentials. Neuroscience. 1993;55:685–694. doi: 10.1016/0306-4522(93)90434-h. [DOI] [PubMed] [Google Scholar]
- Magnus R. Versuche am überlebenden Dünndarm von Säugetieren. II. Mitteilung: Die Beziehungen des Darmnervensystems zur automatischen Darmbewegung. Archiv für die gesampte Physiologie. 1904;102:349–363. [Google Scholar]
- Pompolo S, Furness JB. Ultrastructure and synaptic relationships of calbindin-reactive, Dogiel type II neurons, in myenteric ganglia of guinea-pig small intestine. Journal of Neurocytology. 1988;17:771–782. doi: 10.1007/BF01216705. [DOI] [PubMed] [Google Scholar]
- Portbury AL, Pompolo S, Furness JB, Stebbing MJ, Kunze WAA, Bornstein JC, Hughes S. Cholinergic, somatostatin-immunoreactive interneurons in the guinea pig intestine: morphology, ultrastructure, connections and projections. Journal of Anatomy. 1995;187:303–321. [PMC free article] [PubMed] [Google Scholar]
- Schulze-Delrieu K, Brown BP, Custer-Hagen T. Contraction and accommodation of guinea pig duodenum in vitro. American Journal of Physiology. 1991;261:364–372. doi: 10.1152/ajpgi.1991.261.2.G364. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. Journal of Neuroscience. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing MJ, Bornstein JC. Electrophysiological mapping of fast excitatory synaptic inputs to morphologically and chemically characterised myenteric neurons of guinea-pig small intestine. Neuroscience. 1996;73:1017–1028. doi: 10.1016/0306-4522(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Yokoyama S. Peristaltic reflex in the small intestine deprived of mucosa. Japanese Journal of Smooth Muscle Research. 1982;18:218–220. [Google Scholar]
- Wood JD. Electrical activity from single neurons in Auerbach's plexus. American Journal of Physiology. 1970;219:159–169. doi: 10.1152/ajplegacy.1970.219.1.159. [DOI] [PubMed] [Google Scholar]
- Wood JD. Electrical discharge of single enteric neurons of guinea-pig small intestine. American Journal of Physiology. 1973;225:1107–1113. doi: 10.1152/ajplegacy.1973.225.5.1107. [DOI] [PubMed] [Google Scholar]
- Wood JD. Neurophysiology of Auerbach's plexus and control of intestinal motility. Physiological Reviews. 1975;55:307–324. doi: 10.1152/physrev.1975.55.2.307. [DOI] [PubMed] [Google Scholar]
- Wood JD. Electrical and synaptic behavior of enteric neurons. In: Schultz SG, Wood JD, Ranner BB, editors. Handbook of Physiology. Bethesda, MD, USA: American Physiological Society; 1989. pp. 465–516. [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 423–482. [Google Scholar]


