Abstract
Dissociated rat superior cervical ganglion (SCG) neurons have been shown to possess a hyperpolarization-activated inwardly rectifying chloride current. The current was not altered by changes in external potassium concentration, replacing external cations with NMDG (N-methyl-d-glucamine) or by addition of 10 mM caesium or barium ions.
The reversal potential of the current was altered by changing external anions. The anion selectivity of the current was Cl− > Br− > I− > cyclamate. All substituted permeant anions also blocked the current.
The current was blocked by DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid), 9AC (anthracene-9-carboxylic acid) and NPPB (5-nitro-2-(3-phenylpropylamino)benzoic acid) but was unaffected by SITS (4-acetamido-4′-isothiocyanatostilbene-2,2′-disulphonic acid) and niflumic acid. The effective blockers were voltage dependent; DIDS and NPPB were more effective at depolarized potentials while 9AC was more effective at hyperpolarized potentials.
The current was enhanced by extracellular acidification and reduced by extracellular alkalinization. Reducing external osmolarity was without effect in conventional whole-cell recording but enhanced current amplitude in those perforated-patch recordings where little current was evident in control external solution.
The current in SCG neurons was blocked by external cadmium and zinc. ClC-2 chloride currents expressed in Xenopus oocytes were also sensitive to block by these divalent ions and by DIDS but the sensitivity of ClC-2 to block by cadmium ions was lower than that of the current in SCG neurons.
Reverse transcriptase-polymerase chain reaction (RT-PCR) experiments showed the presence of mRNA for ClC-2 in SCG neurons but not in rat cerebellar granule cells which do not possess a hyperpolarization-activated Cl− current.
The data suggest that ClC-2 may be functionally expressed in rat SCG neurons. This current may play a role in regulating the internal chloride concentration in these neurons and hence their response to activation of GABAA receptors.
Three main types of hyperpolarization-activated inwardly rectifying currents have been described. The two most commonly observed are currents carried by members of the KIR superfamily of inwardly rectifying potassium channels and a number of slowly activating, hyperpolarization-activated currents which are permeable to both sodium and potassium such as If, Ih and IQ (see Hille, 1992). The third group of hyperpolarization-activated inwardly rectifying currents are chloride (Cl−) currents.
Hyperpolarization-activated Cl− currents have now been found in a number of different non-neuronal cell types including rat osteoblastic cells (Chesnoy-Marchais & Fritsch, 1994), cortical astrocytes (Ferroni, Marchini, Schubert & Rapisarda, 1995), T84 epithelial cells (Fritsch & Edelman, 1996) and rat parotid acinar cells (Arreola, Park, Melvin & Begenisich, 1996). Fewer studies have been carried out on neurons, although such currents have been identified in Aplysia neurons (Chesnoy-Marchais, 1983) and hippocampal pyramidal neurons (Madison, Malenka & Nicoll, 1986; Staley, 1994). Two proteins have been cloned which when expressed in Xenopus oocytes resulted in hyperpolarization-activated inwardly rectifying chloride currents. They are ClC-2, which belongs to the ClC family of voltage-gated chloride channels (Thiemann, Gründer, Pusch & Jentsch, 1992), and phospholemman, which may form all or part of a chloride channel (Moorman, Palmer, John, Durieux & Jones, 1992).
Rat superior cervical ganglion (SCG) neurons were first shown to possess a hyperpolarization-activated current by Selyanko (1984). It was said to be a chloride current since it was augmented if recorded with a KCl or CsCl microelectrode solution rather than an acetate-based solution. The aim of this study is to characterize the properties of this current in more detail. In addition, we aim to compare and contrast the properties of this current with those of cloned hyperpolarization-activated chloride currents, in particular ClC-2. A preliminary report of some of these results has been given to The Physiological Society (Clark & Mathie, 1995).
METHODS
Neuron dissociation
Neurons were dissociated from superior cervical ganglia by a method described previously (see Wooltorton & Mathie, 1995). Briefly, Sprague-Dawley rats of either sex (age, 7-8 days) were killed by decapitation. The SCGs were removed and placed at 37°C in Hank's solution containing 20 i.u. ml−1 papain for 20 min. The ganglia were then incubated in a mixture of 400 i.u. ml−1 collagenase (Type I) and 16 mg ml−1 dispase (Grade II) for 45 mins and triturated every 15 min. Cells were then centrifuged and resuspended in standard Leibovitz L-15 medium. Isolated neurons were kept at 4°C and used for recording within 10 h. Cerebellar granule neurons were prepared as described previously (Watkins & Mathie, 1996). Granule cells were used for electrophysiological or reverse transcriptase-polymerase chain reaction (RT-PCR) experiments between 48 and 72 h after plating.
Current recording
Currents in neurons were recorded in the whole-cell configuration of the patch clamp technique at 20-23°C using an Axopatch-1D amplifier. For recordings, the external solution contained (mM): NaCl, 117.5 or 120; KCl, 2.5 or 0; MgCl2, 2.4; CaCl2, 0.1; glucose, 8; Hepes, 10; TEA, 10; TTX, 0.005; and the pipette solution contained (mM): CsCl, 125; Hepes, 5; MgCl2, 5; BAPTA, 0.1 (pH adjusted to 7.4 with CsOH). The osmolarity of the solutions was adjusted to 310-325 mosmol l−1 by the addition of sucrose. Hyposmotic external solutions had no added sucrose and had an osmolarity of 250-265 mosmol l−1. Perforated-patch recordings were made where indicated in the results. In those experiments the intracellular solution was supplemented with 240 μg ml−1 amphotericin B (see Watkins & Mathie, 1996). Drugs were applied by bath perfusion at a rate of 4-5 ml min−1. Complete exchange of bath solution occurred within 20-40 s. DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid), SITS (4-acetamido-4′-isothiocyanatostilbene-2,2′-disulphonic acid), 9AC (anthracene-9-carboxylic acid) and niflumic acid were obtained from Sigma, and NPPB (5-nitro-2-(3-phenylpropylamino)benzoic acid) was from RBI.
Currents were low-pass filtered at 5 kHz and data acquisition and analysis were done using pCLAMP software (Axon Instruments), an IBM compatible PC with a TL-1 interface, and Microsoft Excel and Origin (MicroCal). Data are presented as means ±s.e.m. with n as the number of neurons. Statistical analysis was performed using Student's t test with paired comparisons if relevant. Probabilities are given for two-tailed tests.
RT-PCR
Cell suspensions of SCG or cerebellar granule (CG) neurons were centrifuged at 1000 r.p.m. and the cell pellet used in the preparation of mRNA. Primers for ClC-2 were identical to those used by Smith, Clayton, Wilcox, Escudero & Staley (1995). They were 5′-GTA CCCCATGTAGCCCTCAGC-3′ (1948-1969) and 5′-CCG GAGCTCCTTTAGGGTGAC-3′ (2703-2683), PCR product 755 bp. Actin primers were 5′-TTGTAACCAACTGGGACGATATGG-3′ (1554-1577) and 5′-GATCTTGATCTTCAT GGTGCTAGG-3′ (2869-2846), PCR product 763 bp.
Cells were lysed and mRNA separated using magnetic dT beads (Dynal). Transcription and amplification were carried out using the enzyme rTth (Boehringer Mannheim). The PCR program was 2 min initial denaturation (95°C) then twenty-five cycles of heating to 95°C for 1 min then cooling to 62°C for 2 min. The reaction tubes were then incubated for a further 7 min at 62°C before placing on ice. PCR products on an agarose gel were visualized with ethidium bromide then photographed and digitized.
ClC-2 expression construct, cRNA synthesis and oocyte electrophysiology
Rat ClC-2 (rClC-2) cDNA (Thiemann et al. 1992) was inserted into the vector pTLN which contains Xenopusβ-globin sequences to boost expression (see Lorenz, Pusch & Jentsch, 1996). Capped cRNA was transcribed by SP6 RNA polymerase from 0.5 μg plasmid DNA after linearization with the restriction enzyme MluI using the mMessage mMachine cRNA synthesis kit (Ambion, Austin, TX, USA). Two to ten nanograms of cRNA (corresponding to 40-200 ng ml−1) were injected into manually defollicated Xenopus oocytes. Oocytes were kept at 16°C in modified Barth's solution (88 mM NaCl, 2.4 mM NaHCO3, 1.0 mM KCl, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 0.8 mM MgSO4, 10 mM Hepes, pH 7.6) for 3 days and analysed in ND96 saline (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes, pH 7.4). Standard two-electrode voltage clamp measurements were performed at room temperature (20-22°C) using a Turbotec amplifier (Npi Instruments) and pCLAMP 5.5 software (Axon Instruments).
RESULTS
Characteristics of the hyperpolarization-activated current in SCGs
Typical current traces generated in a single SCG neuron by applying hyperpolarizing voltage steps are shown in Fig. 1A. Currents, which activated slowly, consisted of an instantaneous current jump followed by a time-dependent relaxation and showed strong inward rectification. The time-dependent activation could be fitted by two exponential components with time constants (τ1 and τ2) of 0.33 ± 0.04 s (relative area, 24 ± 1 %) and 2.3 ± 0.2 s (area, 76 ± 1 %, n= 5), respectively, at -80 mV. Both of these time constants decreased with increasing hyperpolarization, so that at -110 mV τ1 decreased to 0.20 ± 0.02 s (area, 31 ± 4 %) while τ2 decreased to 1.3 ± 0.1 s (area, 69 ± 4 %). The relative areas of the two components did not change with voltage. The amplitude of the time-dependent current relaxation was quantified by subtracting the current measured 25 ms after the beginning of the test step from the current measured 2900 ms into the step. The currents do not appear to show any time-dependent inactivation and this was confirmed by applying hyperpolarizing voltage steps to -90 mV for 10 s. Even over this extended time course the current showed no inactivation.
Figure 1. A non-cationic hyperpolarization-activated current in SCG neurons.
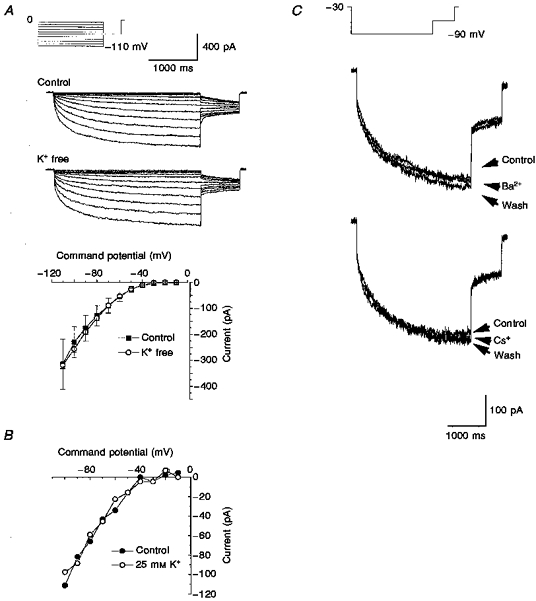
A, current traces in control and K+-free solutions were generated by applying hyperpolarizing voltage steps from between -10 and -110 mV for 3 s from a holding potential of 0 mV followed by a 0.8 s step to -60 mV once every 10 s. The graph shows mean current-voltage relationships for time-dependent current in control (▪) and K+-free (○) solutions. Data are means ±s.e.m.; n= 7 for control, n= 24 for K+-free solution. B, current-voltage relationships in control (•) and 25 mM K+ (○) solutions in a single cell. C, currents obtained in the presence and absence of 10 mM Ba2+ or Cs+ ions were evoked by 3 s steps to -90 mV from a holding potential of -30 mV followed by a 0.8 s step to -60 mV once every 10 s.
Hyperpolarization-activated K+ currents are blocked by caesium and/or barium and in addition their amplitude and activation are altered by changes in the external potassium concentration while hyperpolarization-activated non-selective cation currents are blocked by caesium or replacing external cations with N-methyl-D-glucamine (NMDG) (see Hille, 1992). Replacement of the external potassium with sodium had no effect on the current (Fig. 1A). Raising the external potassium concentration to 25 mM was also without effect at all potentials tested (Fig. 1B). At - 90 mV, the current amplitude was -171 ± 21 pA in control and -175 ± 77 pA (n= 6) in 25 mM potassium. Similarly, there was no significant difference (P > 0.07, Student's paired t test) in the current amplitude at -90 mV in control solutions (-133.2 ± 42.2 pA) and in NMDG-substituted solutions (-95.3 ± 25.9 pA, n= 10). Figure 1C illustrates the lack of effect of both barium (10 mM, n= 3) and caesium (10 mM, n= 3) ions. The lack of effect of the various changes in external potassium concentration, NMDG substitution and addition of either caesium or barium indicated that this current could not be regarded as a hyperpolarization-activated cation current. In all our experiments, no evidence was found for an Ih-like current as described recently by Lamas, Selyanko & Brown (1997) in cultured rat sympathetic neurons.
Anion selectivity of the current
The anion selectivity of the current was determined by replacing the NaCl in the external solution with the sodium salt of bromide, iodide or the organic anion cyclamate and measuring the reversal potential of tail currents following current activation using either a ramp or step pulse protocol (see Fig. 2A and B). After complete dialysis of the cells with normal pipette solution the chloride reversal potential should be close to 0 mV. The tail current amplitude on stepping to 0 mV was -2 ± 3 pA (n= 5; see also Fig. 2B), suggesting that the current is carried by Cl− ions. The mean changes in reversal potential were 2.2 ± 1.4 mV (n= 6), 8.8 ± 3.9 mV (n= 4) and 23.6 ± 2.1 mV (n= 5) for bromide, iodide and cyclamate solutions, respectively. These shifts in reversal potential gave relative permeability values of 0.91, 0.67 and 0.32 for bromide, iodide and cyclamate and a halide selectivity sequence for the current of Cl− > Br− > I−. Thus the inwardly rectifying current is a chloride current and will henceforth be denoted ICl,IR.
Figure 2. Anion permeability of ICl,IR.
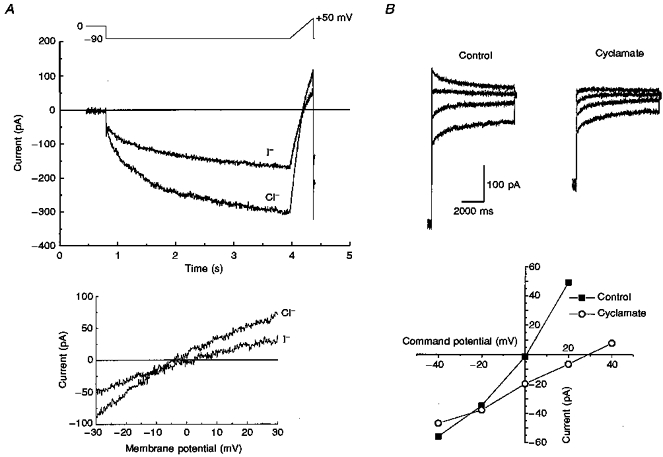
A, ramp tail currents generated in control and NaI external solutions. Currents were evoked by a hyperpolarizing step to -90 mV for 3.15 s to activate ICl,IR then ramped from -90 to +50 mV at 0.35 mV ms−1 once every 12 s. The lower graph shows the current-voltage relationships close to their reversal potentials. B, examples of tail currents obtained in control solutions and cyclamate external solutions. Tail currents (4.75 s in duration) are shown at -40, -20, 0 and +20 mV following a hyperpolarizing step to -90 mV for 4.058 s once every 12 s to activate ICl,IR. The lower graph shows the time-dependent tail current amplitudes plotted against tail current potential in control and cyclamate solutions.
The replacement of the external chloride with another anion not only caused a shift in the reversal potential but also resulted in reductions in the current amplitude at all potentials (see Fig. 2). Bromide caused a 29.6 ± 6.4 % (n= 6) reduction in control current at -90 mV, while iodide and cyclamate caused 45.3 ± 6.2 % (n= 4) and 40.9 ± 8.4 % (n= 5) inhibition of control current, respectively. A similar block has been seen for ClC-2 currents in Xenopus oocytes (Thiemann et al. 1992).
Block of ICl,IR by the inorganic cations, cadmium and zinc
The divalent cations cadmium and zinc have been shown to block hyperpolarization-activated chloride currents in a number of native cell types (Madison et al. 1986; Chesnoy-Marchais & Fritsch, 1994; Fritsch & Edelman, 1996). In the presence of 10 μM cadmium ICl,IR was reduced (Fig. 3A). A reduction in ICl,IR was also seen when currents were recorded in external solution containing 10 μM zinc. The effects of both cadmium and zinc were completely reversible upon wash (see Fig. 3A and B). Both ions were significantly more effective at depolarized test potentials than at hyperpolarized potentials. In the presence of cadmium ICl,IR at -50 mV was inhibited by 77.4 ± 6.7 % but at -110 mV ICl,IR was only reduced by 53.5 ± 3.5 % (P < 0.01, paired t test). Similarly for zinc, ICl,IR was inhibited by 77.0 ± 3.1 % at -60 mV while at -110 mV it was inhibited by only 27.3 ± 1.4 % (P < 0.02, paired t test). At -90 mV, 10 μM cadmium inhibited ICl,IR by 41.0 ± 2.8 % (n= 5) while 30 μM cadmium reduced ICl,IR current by 72.7 ± 3.4 % (n= 6), and at 100 μM, cadmium substantially reduced ICl,IR by 77.6 ± 4.4 % (n=13).
Figure 3. ICl,IR is blocked by Cd2+ and Zn2+.
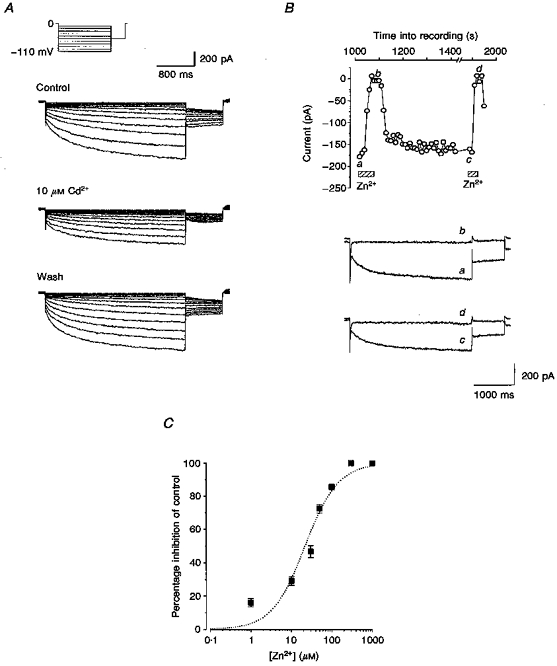
A, currents evoked by the same voltage protocol as Fig. 1A were obtained in control solution, in the presence of 10 μM Cd2+ and after wash. B, 300 μM Zn2+ completely and reversibly inhibits currents evoked by the same voltage protocol as Fig. 2B. Current traces a-d were recorded at the time points indicated. Internal solution contained 10 mM BAPTA and cell dialysis of 15 min had occurred before the data were recorded. C, concentration-response curve for inhibition by Zn2+ at -90 mV. Data for concentrations between 1 and 1000 μM (means ±s.e.m.; n=3 or 4 for each point) are fitted by the Hill equation with slope constrained to 1, giving an IC50 of 23 μM.
The concentration-dependent effects of zinc on ICl,IR at -90 mV are shown in Fig. 3C. The fitted curve indicated that the concentration of zinc that produced half the maximal inhibitory effect (IC50) was approximately 23 μM. At concentrations of 300 and 1000 μM, zinc completely abolished ICl,IR at -90 mV but even at these high concentrations the block was fully reversible (Fig. 3B).
Since cadmium is a well-known inhibitor of calcium currents, the block of ICl,IR by cadmium might suggest that ICl,IR is calcium dependent. However, niflumic acid (10 μM), which has been shown to block calcium-activated chloride currents in many different cell types had virtually no effect on ICl,IR (n=5, data not shown). Furthermore, the current was unaffected by Co2+ or calcium-free external solutions. When the internal solution contained 10 mM BAPTA (see Fig. 3B) to buffer intracellular calcium there was no change in ICl,IR or indeed its modulation by zinc. The results obtained therefore indicate that ICl,IR is not a calcium-activated or calcium-dependent chloride current.
Effects of organic chloride channel blockers on ICl,IR
In addition to niflumic acid, four other organic chloride channel blockers have been investigated. SITS at 1 mM was without effect at any potential tested. At -110 mV the control current was -477.9 ± 39.6 pA (n=3) while the current in 1 mM SITS was -494.9 ± 56.7 pA; the difference was not significant (P > 0.5, paired t test).
In contrast to the lack of effect observed with SITS and niflumic acid, DIDS, 9AC and NPPB were all able to reversibly reduce the current amplitude. The mean inhibition of the current by DIDS (1 mM) was 35.3 ± 6.1 % (n=3) at -90 mV. NPPB produced a substantial inhibition at a concentration of only 200 μM, with the mean inhibition at -90 mV being 58.6 ± 4.9 % (n=7), while 1 mM 9AC reduced the current by 33.5 ± 6.8 % (n=5) at -90 mV. Block by 9AC was slow to occur, taking almost three times as long to reach maximal effect as the block produced by either DIDS or NPPB.
Figure 4 shows plots of the mean current-voltage relationships obtained in DIDS, 9AC and NPPB together with their respective controls. All three blockers were shown to reduce the current over the entire voltage range studied. DIDS and NPPB both appeared to be more effective at blocking the current at the more depolarized command potentials. For DIDS there was a significant difference between the block at -60 and that at -110 mV. The mean inhibition was 60.5 ± 1.7 % (n=3) at a potential of -60 mV, and at -110 mV it was 21.9 ± 4.8 % (n=3) (P < 0.01, paired t test). Similarly, NPPB caused an inhibition of 83.5 ± 10.4 % at -70 mV but only of 52.5 ± 9.8 % (n=4) at -110 mV. In contrast the effect of 9AC significantly increased with increasing hyperpolarization so that at -60 mV the current was inhibited by only 29.4 ± 3.9 % (n=3) while at -110 mV the current was inhibited by 58.6 ± 2.5 % (n=3, P < 0.05, paired t test).
Figure 4. Effects of DIDS, 9AC and NPPB on ICl,IR.
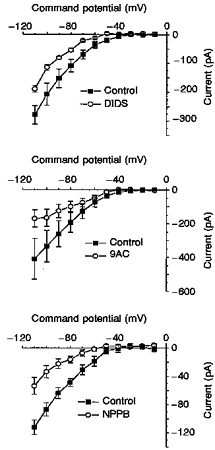
A, current-voltage relationships recorded in control and 1 mM DIDS using the protocol described in Fig. 1A. B, effect of 1 mM 9AC on the control current-voltage relationship. C, current-voltage relationships generated in control and then in the presence of 200 μM NPPB. All data are the means ±s.e.m. (n=3, 3 and 4 for DIDS, 9AC and NPPB, respectively).
Effects of changes in the pH of the extracellular solution on ICl,IR
The extracellular pH was reduced by 0.5 or increased by 0.6 of a pH unit, from pH 7.4 to either pH 6.9 or pH 8.0. A typical example of the effect that a reduction in the extracellular pH had on ICl,IR is shown in Fig. 5. Current traces generated at pH 8.0 were smaller in magnitude than those recorded in control (pH 7.4). Similar reductions were observed in a further three cells where the pH was increased. In contrast, a decrease in pH to 6.9 resulted in an increase in current amplitude (see Fig. 5) and similar increases in current were seen for four further cells.
Figure 5. Effect of changes in extracellular pH on ICl,IR.
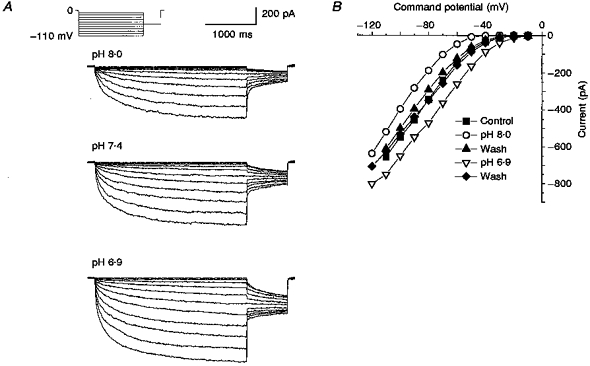
A, current traces generated using the protocol described for Fig. 1A in pH 8.0, 7.4 and 6.9 external solutions. B, plot of current-voltage relationships in control (pH 7.4), pH 6.9 and pH 8.0 external solutions together with two washes in control external solution.
The current traces shown in Fig. 5A were obtained from the same neuron and the corresponding current-voltage relationships are plotted in Fig. 5B. Figure 5B shows that the current amplitudes recorded in control (▪) and after two washes (▴ and ♦, washes for pH 8.0 and pH 6.9, respectively) were not different. It also shows that increasing the extracellular pH caused a reduction in current amplitude at all potentials, while reducing the extracellular pH resulted in an increase in current at all potentials. The decrease in current at pH 8.0 appeared to be more pronounced at the more depolarized command potentials. At -60 mV the current was inhibited by 68.3 ± 4.3 % while at -110 mV it was inhibited by only 27.3 ± 4.2 % (n=4 for the data at -60 mV; n=3 for the data at -110 mV). This reduction was found to be significant (P < 0.003). The effects of pH 6.9 were also found to be more potent at the more depolarized potentials. At -40 mV the current amplitude was increased by 257.2 ± 15.2 % but at -110 mV it was only increased by 38.1 ± 10.7 % (n=5 at -40 mV; n=3 at -110 mV). Raising the external pH from 7.4 to 9.4 resulted in a complete inhibition of the current amplitude (n=3, data not shown).
Effect of hypotonic external solution on ICl,IR
Figure 6A shows typical current traces obtained in control (osmolarity of between 310 and 325 mosmol l−1) and in hypotonic (osmolarity of between 250 and 265 mosmol l−1) external solutions using conventional whole-cell recording methods. There was no difference in the current amplitudes measured in the two solutions at any potential tested. At -110 mV the current in control was - 636.7 pA while that in hypotonic external was -645.5 pA. The same lack of effect was observed in a further four cells. The hypotonic solution was applied for between 3.5 and 7 min.
Figure 6. Effect of a hypotonic external solution on ICl,IR recorded using conventional whole-cell and amphotericin B perforated patches.
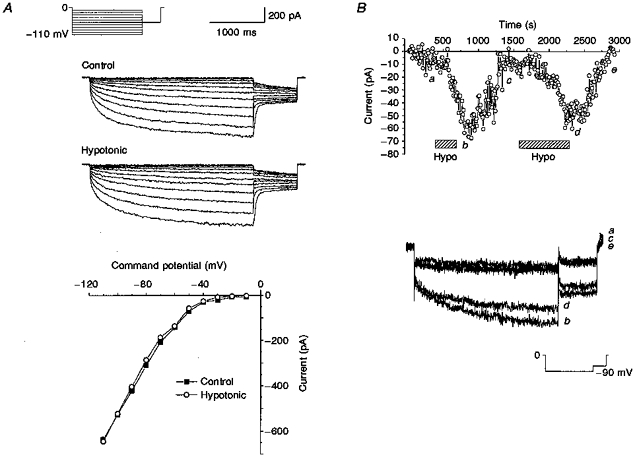
A, ICl,IR current-voltage relationships in conventional whole-cell recording in control and hypotonic external solution. Voltage protocol as described for Fig. 1A.B, amplitude of ICl,IR at -90 mV plotted against recording time in a perforated-patch recording. Hypotonic external solution was applied at the points indicated by the hatched bars. Current traces recorded as follows: a, in control; b, during the first application of hypotonic solution; c, after washout of the effects of hypotonic external solution; d, during the second application of hypotonic solution; e, after washout of the effects of the second application of hypotonic external solution. Protocol as described for Fig. 1C except that the holding potential was 0 mV.
Using the amphotericin B perforated-patch clamp method to record currents it was found that while some cells had little or no hyperpolarization-activated current present in control solution (-6.0 ± 1.0 pA, n=3) others did have currents (-44.0 ± 5.7 pA, n=4 at -90 mV). The current traces recorded using the amphotericin B perforated-patch technique activated slowly at hyperpolarized potentials with time constants of activation which were close to those determined for currents recorded using the conventional whole-cell method. Current-voltage relationships showed that the threshold for activation was approximately -40 mV and the currents showed strong inward rectification, both features of currents recorded using the conventional whole-cell method. Interestingly, it appeared that the cells that had little or no hyperpolarization-activated current present under control conditions responded with an increase in current amplitude upon the application of hypotonic external solution. Figure 6B shows the amplitude of a current recorded at -90 mV using amphotericin B perforated patches. In control there was little or no current present, the mean amplitude of the current recorded between 10 and 500 s into the recording was -4.9 ± 0.8 pA (n=50 sample points). When the osmolarity of the external solution was reduced, however, the current amplitude increased substantially. This increase in current amplitude was reversible upon returning to control external. For the cells which responded to hypotonic solution, the current amplitude increased to -55.7 ± 8.1 pA (n=3) upon hypotonic challenge.
Relationship of ICl,IR to ClC-2 current
These functional properties suggest that ICl,IR shares a number of similarities with the current carried by the cloned chloride channel, ClC-2. RT-PCR experiments (Fig. 7A) show that ClC-2 mRNA is indeed expressed in rat SCG neurons. It has previously been shown, by in situ hybridization, that cerebellar granule (CG) neurons do not express ClC-2 (Smith et al. 1995). The RT-PCR of mRNA from these cells using primers for ClC-2 was therefore carried out as a negative control. In Fig. 7A mRNA from SCG (lane 1) and CG (lane 3) neurons has been amplified by RT-PCR using ClC-2 primers. A DNA band is visible in lane 1 but not in lane 3, indicating that, as expected, CG neurons do not have ClC-2 mRNA while SCG neurons, on the other hand, do. In lanes 2 and 4 mRNA from SCG and CG neurons was amplified using primers for actin. Actin is a ubiquitously expressed protein and therefore acts as a positive control. Electrophysiological experiments (Fig. 7B) show the presence of ICl,IR in SCG neurons and the absence of such a current in a CG neuron using identical recording conditions.
Figure 7. ClC-2 mRNA is present in SCG neurons.
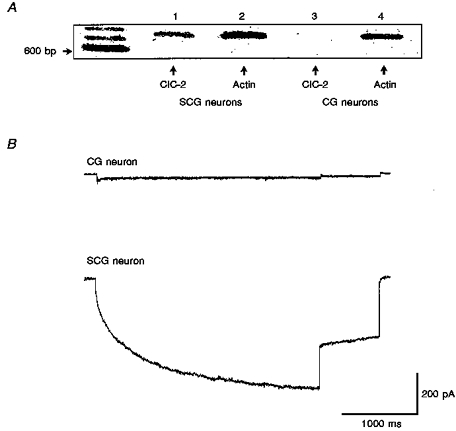
A, image of a gel showing DNA bands obtained from SCG (lanes 1 and 2) and cerebellar granule (CG) neurons (lanes 3 and 4) by RT-PCR using primers for ClC-2 and actin. Size markers on the left are 600, 700 and 800 bp. Actin mRNA is present in both neurons (lanes 2 and 4) but ClC-2 mRNA is only present in SCGs (compare lanes 1 and 3). B, typical current traces recorded in a CG neuron (top) and SCG neuron (bottom) in response to the same voltage protocol as Fig. 1C except that the holding potential was 0 mV.
There is a lack of detailed information concerning the effects of a number of the compounds which block ICl,IR on expressed ClC-2 currents. Experiments were carried out to determine whether cadmium and zinc were also effective blockers of ClC-2 channels expressed in Xenopus oocytes (Fig. 8). The time-dependent activation of ClC-2 currents could also be fitted by two exponential components with time constants (τ1 and τ2) of 0.73 ± 0.09 s (area, 21 ± 2 %) and 6.4 ± 0.3 s (area, 79 ± 2 %, n=5) at -100 mV (compared with time constants of 0.23 ± 0.02 s (area, 28 ± 4 %) and 1.6 ± 0.1 s (area, 72 ± 4 %, n=5) at the same potential for ICl,IR). As with ICl,IR both of these time constants decreased with increasing hyperpolarization, so that at -140 mV τ1 decreased to 0.42 ± 0.08 s (area, 24 ± 6 %) while τ2 decreased to 5.5 ± 0.2 s (area, 76 ± 6 %). Cadmium blocked the ClC-2 channel expressed in oocytes with an IC50 value of 280 μM (Fig. 8A and B) while zinc (30 μM) produced around 45 % inhibition of ClC-2 current (Fig. 8C). In these oocyte experiments more than 10 min were required to reverse block by high concentrations of zinc and cadmium. As for ICl,IR, DIDS (1 mM) was found to be an effective blocker of ClC-2 channels (Fig. 8D and E) with a block of 41 ± 7 % at -140 mV (n=4).
Figure 8. ClC-2 is blocked by Cd2+, Zn2+and DIDS.
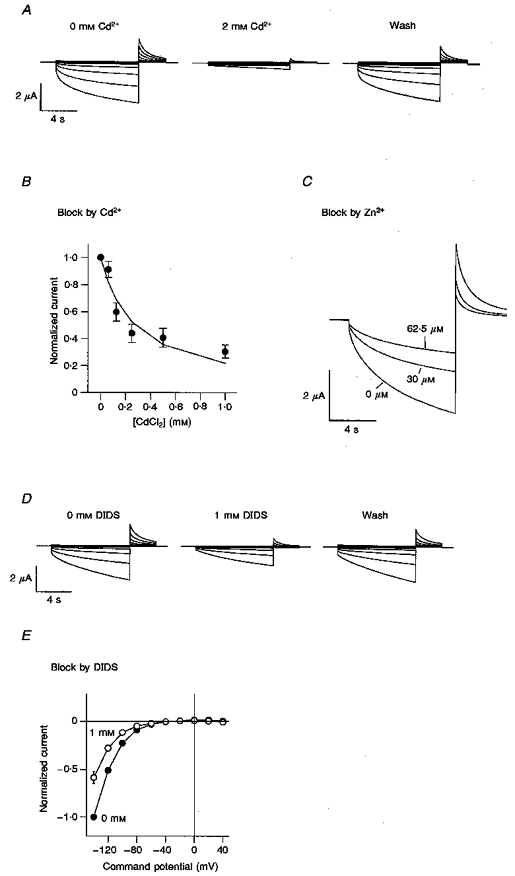
A, voltage clamp traces from an oocyte expressing rClC-2 before (left) and after a 1 min perfusion with 2 mM Cd2+ (middle) and after 3 min wash (right). Voltage was clamped for 9 s at values between +40 and -140 mV in steps of 20 mV from a holding voltage of -30 mV, followed by a 3 s step to +40 mV. B, block of ClC-2 by increasing concentrations of Cd2+ using the same voltage protocol as Fig. 8A except with a constant test step to -120 mV. Normalized data from 5 oocytes are shown. The IC50 is 280 μM when fitted with a Hill equation with slope constrained to 1. C, block of ClC-2 by Zn2+ using the same voltage protocol as Fig. 8B. Traces are shown in control and in the presence of 30 and 62.5 μM Zn2+. D, voltage clamp traces from an oocyte expressing rClC-2 before (left) and after a 1 min perfusion with 1 mM DIDS (middle) and after 3 min wash (right) using the same voltage protocol as Fig. 8A. E, current-voltage relationships obtained from 4 cells in the absence or presence of 1 mM DIDS. Currents have been normalized to the currents at -140 mV in the absence of DIDS.
DISCUSSION
Rat SCG neurons possess a hyperpolarization-activated chloride current (ICl,IR) which is activated at potentials more negative than -30 mV. This current is not carried by cations since it was unaffected by agents which block hyperpolarization-activated cation currents such as IQ (Halliwell & Adams, 1982; Lamas et al. 1997) or inwardly rectifying potassium currents (see Hille, 1992). Many of the properties of ICl,IR are, however, similar to other hyperpolarization-activated chloride currents such as those that have been recorded in rat osteoblastic cells (Chesnoy-Marchais & Fritsch, 1994), T84 epithelial cells (Fritsch & Edelman, 1996) and Aplysia neurons (Chesnoy-Marchais, 1983). The threshold for activation of ICl,IR is within the range observed for the other currents and all of these currents exhibit inward rectification. In addition, the halide selectivity sequence found for ICl,IR, Cl− > Br− > I−, is the same as that determined for the current in T84 epithelial cells (Fritsch & Edelman, 1996). It is of interest that the human isoform of ClC-2 has been cloned from a cDNA library from T84 cells (Thiemann et al. 1992; Cid, Montrose-Rafizadeh, Smith, Guggino & Cutting 1995) and expression confirmed in this cell line by Northern blotting (Thiemann et al. 1992).
DIDS, NPPB and 9AC were all able to inhibit ICl,IR while SITS and niflumic acid were ineffective. All three effective compounds reduced the amplitude of ICl,IR in a voltage-dependent manner but DIDS and NPPB exhibited a different voltage dependence to that of 9AC. Both DIDS and NPPB were more effective at depolarized potentials while 9AC produced a greater effect at hyperpolarized potentials. An additional difference is that 9AC appeared to have a much slower onset of action than the other two compounds. A similar voltage-dependent effect of DIDS was observed for the hyperpolarization-activated chloride current in rat osteoblastic cells (Chesnoy-Marchais & Fritsch, 1994). We have found that DIDS is also an effective blocker of ClC-2 channels expressed in Xenopus oocytes.
9AC is a potent blocker of skeletal muscle chloride channels both in the intact tissue (Bryant & Morales-Aguilera, 1971) and when ClC-1, the major skeletal muscle chloride channel, is expressed in Xenopus oocytes (Steinmeyer, Ortland & Jentsch, 1991). However, although the block by 9AC of ClC-1 is very potent (> 80 % block at 0.1 mM), more than 10 min is required to reach maximal effect (Jentsch, 1994). In addition, vesicles from skeletal muscle transverse tubules, incorporated into planar lipid bilayers, were only inhibited by 9AC when it was applied to the cytoplasmic side of the membrane (Ide, Hidaka & Kasai, 1995). An intracellular site of action would explain the slow onset of the 9AC-induced inhibition of ICl,IR. 9AC is an effective inhibitor of ClC-2 (Thiemann et al. 1992).
ICl,IR was found to be sensitive to both acidification and alkalization of the external recording solution. A half-unit decrease in pH resulted in an increase in current amplitude while a 0.6 unit increase in pH decreased the amplitude of ICl,IR. Arreola, Melvin & Begenisich (1995) have suggested that there may be negatively charged groups which, under control conditions, normally restrict the flow of chloride ions, but which are neutralized by a decrease in pH. The hyperpolarization-activated chloride current in Aplysia neurons was reported to exhibit the same sensitivity to external pH as was observed in this study and the effects were attributed to shifts in the activation curves (Chesnoy-Marchais, 1983). ClC-2 is similarly sensitive to both decreases and increases in external pH (Pusch & Jentsch, 1994; Jordt & Jentsch, 1997) and studies on this channel support the idea that changes in pH alter channel activation since a constitutively active mutant of ClC-2 is virtually pH insensitive (Jordt & Jentsch, 1997).
A reduction in the external osmolarity caused an increase in current amplitude that was fully reversible. This only occurred, however, when ICl,IR was recorded using amphotericin B perforated patches and then only in neurons which did not display an appreciable current under control conditions. The characteristics of ICl,IR are completely different to typical volume-activated chloride currents. Such currents are activated rapidly by depolarization, display outward rectification, have a halide selectivity sequence of I− > Br− > Cl− and require ATP (Anderson, Sheppard, Berger & Welsh, 1992; Ackerman, Wickman & Clapham, 1994; Nilius et al. 1994; Arreola et al. 1995, 1996). A number of currents which share common features with ICl,IR, such as the hyperpolarization-activated chloride currents of rat osteoblastic cells (Chesnoy-Marchais & Fritsch, 1994), mandibular duct cells (Komwatana, Dinudom, Young & Cook, 1995), T84 cells (Fritsch & Edelman, 1997) and the ClC-2-induced chloride current (Thiemann et al. 1992; Gründer, Thiemann, Pusch & Jentsch, 1992) do exhibit sensitivity to changes in the external osmolarity. However, in rat osteoblastic cells and mandibular duct cells, the effects of a hyposmotic solution are opposite to those demonstrated for ICl,IR in that the currents in these cells are reduced under hyposmotic conditions. In contrast, ClC-2 current (Gründer et al. 1992) is increased in magnitude by a reduction in the external osmolarity.
The lack of effect of hypotonic solutions on ICl,IR in conventional whole-cell recordings may indicate that ICl,IR is already fully activated in those neurons. This suggests, in turn, that some intracellular constituent may tonically regulate ICl,IR and this may be lost through dialysis in such recordings. A related dependence on recording configuration has been observed for a swelling-activated chloride current in chick heart cells (Hall, Zhang & Lieberman, 1995). Activation of this current was seen in conventional but not perforated-patch recordings and current activation was shown to require dephosphorylation of a cAMP-dependent phosphorylation site on the channel (Hall et al. 1995). It is of interest that the hyperpolarization-activated chloride current in hippocampal pyramidal neurons is enhanced by activation of protein kinase A (Staley, 1994) and that Fritsch & Edelman (1997) have recently suggested that the sensitivity of the hyperpolarization-activated chloride current in T84 cells to changes in extracellular osmolarity depends on the phosphorylation state of the channels.
The lack of inhibition of ICl,IR by barium ions coupled with the inhibition by cadmium shows that this current is unlikely to be the same as that recorded upon expression of phospholemman in Xenopus oocytes since the phospholemman-induced current is blocked by barium with an IC50 of 0.36 mM (Moorman et al. 1992) but is unaffected by cadmium (Kowdley, Ackerman, John, Jones & Moorman, 1994). Another important difference is that phospholemman-induced currents in Xenopus oocytes have an anion selectivity of I− > Br− > Cl−, the reverse of ICl,IR (Kowdley et al. 1994).
ClC-2, when expressed in Xenopus oocytes, carries a hyperpolarization-activated chloride current (see Results and Thiemann et al. 1992) with kinetic and activation properties similar to that found for ICl,IR in SCG neurons. The anion permeability sequence of Cl− > Br− > I− > cyclamate, block by these permeant anions, regulation by changes in extracellular pH and block by DIDS, 9-AC, cadmium and zinc are all properties that are shared with ICl,IR (see Results and Thiemann et al. 1992; Jordt & Jentsch, 1997). Furthermore, we have shown that the mRNA for ClC-2 is present in SCG neurons but not rat CG neurons. Taken together, these results suggest that ClC-2 is present in rat SCG neurons and may be functionally expressed. However, when examined in greater detail, some of these properties do, in fact, show some differences between ClC-2 and ICl,IR. For example, the time constants of activation of ClC-2 currents in oocytes are slower than those for ICl,IR at a given potential. Similarly ClC-2 currents in oocytes are much less sensitive to block by Cd2+ than ICl,IR, with an IC50 value of 280 μM compared with around 30 μM for ICl,IR. The Cd2+ sensitivity of ICl,IR is similar to that seen for the hyperpolarization-activated chloride current in hippocampal pyramidal neurons (Madison et al. 1986; Staley, 1994), a current previously attributed to expression of ClC-2 (Smith et al. 1995).
At present it is not clear whether these differences reflect differences between expression systems and thus differences in post-translational modifications of the channels between cell types, whether it may indicate the possible contribution of associated subunits in some cells which are as yet unidentified, or even whether the current in SCG neurons is not, in fact, ClC-2.
These issues will be best addressed by successful functional expression of ClC-2 in a mammalian expression system together with attempts at more direct molecular experimental approaches to block functional expression of ClC-2 in SCG neurons. Such experiments would also help to determine whether the current activated by hypotonic solutions in perforated-patch recordings is due to functional ClC-2 expression.
Unlike cadmium, zinc seems to be equally effective in both cell types; 30 μM zinc produced around 45 % inhibition of ClC-2 currents compared with an IC50 of 23 μM (at -90 mV) for zinc on ICl,IR. Zinc has also been found to be effective at blocking the hyperpolarization-activated chloride current in hippocampal pyramidal neurons (Staley, 1994). In the CNS, zinc has been shown to have a neuromodulatory role. It can be localized to synaptic vesicles of neurons in the central nervous system where the concentration of zinc is estimated to be as much as 300 μM (Frederickson, 1989). This may be released from synapses in a calcium-dependent manner and would be enough to completely block ICl,IR.
In non-neuronal cells, an increase in chloride efflux is known to be an important part of regulatory volume decrease and such a role has been suggested previously for ClC-2 (Gründer et al. 1992). In hippocampal pyramidal neurons, the hyperpolarization-activated chloride current has been proposed to maintain the relationship between the chloride equilibrium potential and the resting membrane potential and therefore determines the direction of chloride flux upon activation of GABAA receptors (Staley, 1994). Similarly, expression of ClC-2 in DRG neurons causes a negative shift in the Cl− equilibrium potential and attenuates GABAA receptor-mediated depolarizations in these cells (Staley, Smith, Schaak, Wilcox & Jentsch, 1996). There are functional GABAA receptors on rat SCG neurons (e.g. Adams & Brown, 1975) and some evidence indicates that GABA may be a neurotransmitter within the SCG. For example, fibres that are positive for GABA have been shown to form basket-like structures around and presumably innervate some of the principal neurons of the SCG (Dobó, Kása, Wenthold & Wolff, 1989; Párducz, Dobó, Joo & Wolff, 1992). In addition, Eugène (1987) has shown that GABA can mediate fast depolarizing postsynaptic potentials in rat SCG neurons while Adams & Brown (1975) have shown that GABA may either depolarize or hyperpolarize SCG neurons, depending on the resting potential of the cell. Therefore if ICl,IR has a role in the regulation of the internal chloride concentration in SCG neurons it may affect either the magnitude or the direction of the response of these neurons to GABAA receptor activation (see also Staley et al. 1996).
Acknowledgments
The MRC and The Wellcome Trust are thanked for support. S.C. was an MRC student. Thanks to G. Jones for providing cerebellar granule cells and M. Docherty for expert advice on the RT-PCR experiments.
References
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. Journal of General Physiology. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PR, Brown DA. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. The Journal of Physiology. 1975;250:85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. American Journal of Physiology. 1992;263:L1–14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Volume-activated chloride channels in rat parotid acinar cells. The Journal of Physiology. 1995;484:677–687. doi: 10.1113/jphysiol.1995.sp020695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola J, Park K, Melvin JE, Begenisich T. Three distinct chloride channels control anion movements in rat parotid acinar cells. The Journal of Physiology. 1996;490:351–362. doi: 10.1113/jphysiol.1996.sp021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant SH, Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. The Journal of Physiology. 1971;219:367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Characterization of a chloride conductance activated by hyperpolarization in Aplysia neurones. The Journal of Physiology. 1983;342:277–308. doi: 10.1113/jphysiol.1983.sp014851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D, Fritsch J. Activation by hyperpolarisation and atypical osmosensitivity of a Cl− current in rat osteoblastic cells. Journal of Membrane Biology. 1994;140:173–188. doi: 10.1007/BF00233706. [DOI] [PubMed] [Google Scholar]
- Cid LP, Montrose-Rafizadeh C, Smith DI, Guggino WB, Cutting GR. Cloning of a putative human voltage-gated chloride channel (ClC-2) cDNA widely expressed in human tissues. Human Molecular Genetics. 1995;4:407–413. doi: 10.1093/hmg/4.3.407. [DOI] [PubMed] [Google Scholar]
- Clark S, Mathie A. A hyperpolarization-activated chloride current in acutely isolated rat superior cervical ganglion (SCG) neurons. The Journal of Physiology. 1995;485.P:48–49 P. [Google Scholar]
- Dobó E, Kása P, Wenthold RJ, Wolff JR. Evidence for GABAergic fibers entering the superior cervical ganglion of rat from the preganglionic nerve trunk. Histochemistry. 1989;92:133–136. doi: 10.1007/BF00490232. [DOI] [PubMed] [Google Scholar]
- Eugéne D. Fast non-cholinergic depolarizing postsynaptic potentials in neurons of rat superior cervical ganglia. Neuroscience Letters. 1987;78:51–56. doi: 10.1016/0304-3940(87)90560-x. 10.1016/0304-3940(87)90560-X. [DOI] [PubMed] [Google Scholar]
- Ferroni S, Marchini C, Schubert P, Rapisarda C. Two distinct inwardly rectifying conductances are expressed in long term dibutyryl-cyclic-AMP treated rat cultured cortical astrocytes. FEBS Letters. 1995;367:319–325. doi: 10.1016/0014-5793(95)00588-z. 10.1016/0014-5793(95)00588-Z. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. International Review of Neurobiology. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Fritsch J, Edelman A. Modulation of the hyperpolarization-activated Cl− current in human intestinal T84 epithelial cells by phosphorylation. The Journal of Physiology. 1996;490:115–128. doi: 10.1113/jphysiol.1996.sp021130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch J, Edelman A. Osmosensitivity of the hyperpolarisation-activated chloride current in human intestinal T84 cells. American Journal of Physiology. 1997;272:C778–786. doi: 10.1152/ajpcell.1997.272.3.C778. [DOI] [PubMed] [Google Scholar]
- Gründer S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Hall SK, Zhang J, Lieberman M. Cyclic AMP prevents activation of a swelling-induced chloride-sensitive conductance in chick heart cells. The Journal of Physiology. 1995;488:359–369. doi: 10.1113/jphysiol.1995.sp020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Research. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels in Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates Inc; 1992. [Google Scholar]
- Ide T, Hidaka J, Kasai M. An anion channel from transverse tubular membranes incorporated into planar bilayers. Biochimica et Biophysica Acta. 1995;1237:115–120. doi: 10.1016/0005-2736(95)00091-g. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Molecular biology of voltage-gated chloride channels. Current Topics in Membrane Transport. 1994;42:35–57. [Google Scholar]
- Jordt S-E, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO Journal. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komwatana P, Dinudom A, Young JA, Cook DI. Osmotic sensitivity ofthe hyperpolarisation-activated Cl− current in mouse mandibular duct cells. Cell Physiology and Biochemistry. 1995;5:243–251. doi: 10.1152/ajpgi.1995.268.5.G806. [DOI] [PubMed] [Google Scholar]
- Kowdley GC, Ackerman SJ, John JE, Jones LR, Moorman JR. Hyperpolarisation-activated chloride currents in Xenopus oocytes. Journal of General Physiology. 1994;103:217–230. doi: 10.1085/jgp.103.2.217. 10.1085/jgp.103.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer linopirdine (DuP 996) on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. European Journal of Neuroscience. 1997;9:605–616. doi: 10.1111/j.1460-9568.1997.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Lorenz C, Pusch M, Jentsch TJ. Heteromultimeric ClC chloride channels with novel properties. Proceedings of the National Academy of Sciences of the USA. 1996;93:13362–13366. doi: 10.1073/pnas.93.23.13362. 10.1073/pnas.93.23.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Malenka RC, Nicoll RA. Phorbol esters block a voltage-sensitive chloride current in hippocampal pyramidal cells. Nature. 1986;321:695–697. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- Moorman JR, Palmer CJ, John JE, Durieux ME, Jones LR. Phospholemman expression induces a hyperpolarisation activated chloride current in Xenopus oocytes. Journal of Biological Chemistry. 1992;267:14551–14554. [PubMed] [Google Scholar]
- Nilius B, Sehrer J, Viana F, De Greef C, Raeymaekers L, Eggermont J, Droogmans G. Volume activated Cl− currents in different mammalian non-excitable cell types. Pflügers Archiv. 1994;428:364–371. doi: 10.1007/BF00724520. [DOI] [PubMed] [Google Scholar]
- Párducz A, Dobó E, Joo F, Wolff JR. Termination pattern and fine structural characteristics of GABA- and [Met]enkephalin-containing nerve fibers and synapses in the superior cervical ganglion of adult rat. Neuroscience. 1992;49:963–971. doi: 10.1016/0306-4522(92)90372-9. [DOI] [PubMed] [Google Scholar]
- Pusch M, Jentsch TJ. Molecular physiology of voltage-gated chloride channels. Physiological Reviews. 1994;74:813–827. doi: 10.1152/physrev.1994.74.4.813. [DOI] [PubMed] [Google Scholar]
- Selyanko AA. Cd2+ suppresses a time-dependant Cl− current in rat sympathetic neurone. The Journal of Physiology. 1984;350:49 P. [Google Scholar]
- Smith RL, Clayton GH, Wilcox CL, Escudero KW, Staley KJ. Differential expression of an inwardly rectifying chloride conductance in rat brain neurons: A possible mechanism for cell-specific modulation of postsynaptic inhibition. Journal of Neuroscience. 1995;15:4057–4067. doi: 10.1523/JNEUROSCI.15-05-04057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K. The role of the inwardly rectifying chloride conductance in postsynaptic inhibition. Journal of Neurophysiology. 1994;72:273–284. doi: 10.1152/jn.1994.72.1.273. [DOI] [PubMed] [Google Scholar]
- Staley K, Smith R, Schaak J, Wilcox C, Jentsch TJ. Alteration of GABAA receptor function following gene transfer of the ClC-2 chloride channel. Neuron. 1996;17:543–551. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Thiemann A, Gründer S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Watkins CS, Mathie A. A non-inactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. The Journal of Physiology. 1996;491:401–412. doi: 10.1113/jphysiol.1996.sp021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JRA, Mathie A. Potent block of potassium currents in rat isolated sympathetic neurones by the uncharged form of amitriptyline and related tricyclic compounds. British Journal of Pharmacology. 1995;116:2191–2200. doi: 10.1111/j.1476-5381.1995.tb15053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


