Abstract
We have used highly purified right-side-out luminal and basolateral membrane vesicles (LMVs and BLMVs) isolated from rat medullary thick ascending limb (MTAL) to study directly the possible roles of the LMV and BLMV Na+-H+ exchangers in the transport of NH4+.
Extravesicular NH4+ ((NH4+)o) inhibited outward H+ gradient-stimulated 22Na+ uptake in both types of vesicles. This inhibition could not be accounted for by alteration of intravesicular pH (pHi).
Conversely, in both plasma membrane preparations, the imposition of outward NH4+ gradients stimulated 22Na+ uptake at the acidic pHi (6.60) of MTAL cells, under conditions in which possible alterations in pHi were prevented. All NH4+ gradient-stimulated Na+ uptake was sensitive to 0.5 mM 5-(N,N-dimethyl)-amiloride.
The BLMV and LMV Na+-H+ exchangers had a similar apparent affinity for internal H+ (H+i), with pK (-log of dissociation constant) values of 6.58 and 6.52, respectively.
These findings indicate that NH4+ interacts with the external and internal transport sites of the LMV and BLMV Na+-H+ antiporters, and that both of these exchangers can mediate the exchange of internal NH4+ ((NH4+)i) for external Na+ (Na+o) at the prevailing pHi of MTAL cells.
We conclude that operation of the BLMV Na+-H+ exchanger on the NH4+-Na+ mode may represent an important pathway for mediating the final step of NH4+ absorption, whereas transport of NH4+ on the apical antiporter may provide negative feedback regulation of NH4+ absorption.
Reabsorption of ammonia by the mammalian renal medullary thick ascending limb of Henle (MTAL) plays a critical role in maintaining acid-base balance (for review, see Good, 1994). Active absorption of NH4+ from MTAL causes accumulation of ammonia in the interstitium, thereby driving its secretion into the collecting duct (Good, Knepper & Burg, 1984). Since the MTAL is not accessible on the kidney surface, the majority of the studies to reveal the mechanism of vectorial NH4+ transport in MTAL cells have been performed using the technique of isolated tubule perfusion (Burg, 1982). These studies have provided evidence that the majority of the ammonium absorption occurs by the active transport of NH4+ (Good et al. 1984; Watts & Good, 1993; Good, 1994). Cation substitution and the use of inhibitors have established that the first step of active NH4+ absorption is essentially mediated by substitution of NH4+ for K+ on the apical Na+-K+-2Cl− cotransporter (Good, 1994; Watts & Good, 1994a). On the other hand, the basolateral transport pathway(s) that could mediate NH4+ exit has not been identified.
Recent physiological studies have detected Na+-H+ exchange activity in both luminal and basolateral membranes of the mouse (Sun, Kikeri & Hebert, 1992) and rat (Good, George & Watts, 1995) MTAL perfused in vitro. More recently, we have demonstrated the presence of two isoforms of the Na+-H+ exchanger (NHE) in the rat MTAL; NHE-3 is expressed on the apical domain while NHE-1 is exclusively present on the basolateral membrane (Attmane-Elakeb et al. 1996). Several studies have established that NH4+ can serve as a substrate for the Na+-H+ exchangers of the proximal tubule (Kinsella & Aronson, 1981; Nagami, 1988; Preisig & Alpern, 1990). Because the inward gradients for Na+ exceed the inward gradients for NH4+ at both surfaces of the MTAL, one would predict that exchange of internal NH4+ ((NH4+)i) for external Na+ (Na+o) on these transporters might actually lead to opposite effects on transcellular NH4+ absorption. However, no study has demonstrated unambiguously that the exchangers of the MTAL can transport NH4+, and consequently their possible contribution to transcellular NH4+ absorption remains to be established. Recent studies (Good & Watts, 1996) have shown that luminal amiloride in the isolated perfused rat MTAL has no effect on net ammonium absorption, whereas preliminary results from the same laboratory (Watts & Good, 1993) demonstrated that addition of amiloride to the bath inhibited ammonium absorption. These results were interpreted to indicate that luminal Na+-H+ exchange is not important for NH4+ absorption, whereas basolateral Na+-H+ exchange may be involved in the absorption of this cation, possibly by mediating H+ extrusion in parallel with passive NH3 diffusion. However, given the multiple pathways for NH4+/NH3 absorption through transcellular and paracellular routes in the MTAL, it is difficult to establish or refute with certainty secondary active NH4+ transport on the Na+-H+ antiporters of this nephron segment by measuring transepithelial NH4+ fluxes.
To address this issue, we have exploited a new technique recently developed in our laboratory which allows the simultaneous isolation of highly purified luminal and basolateral plasma membrane vesicles (LMVs and BLMVs) from rat MTALs (Attmane-Elakeb et al. 1996). Plasma membrane vesicles permit direct and separate investigations of the interactions of NH4+ with the external and internal transport sites of the apical and basolateral Na+-H+ exchangers. We have found that both the apical and basolateral membrane vesicles can mediate the exchange of internal NH4+ for external Na+ on the amiloride-sensitive Na+-H+ antiporters. Thus, our data are compatible with the possibility that operation of the BLMV antiporter on the NH4+-Na+ mode may represent an important pathway for transepithelial NH4+ absorption, whereas apical NH4+-Na+ exchange may serve as a feedback mechanism that would limit the maximum medullary interstitial ammonium concentration.
METHODS
Preparation of MTAL tubules
The tubule isolation procedure was similar to that described by Attmane-Elakeb et al. (1996). Male Sprague-Dawley rats weighing 250-300 g were anaesthetized with pentobarbitone sodium (50 mg (kg body wt)−1, i.p.), kidneys were rapidly removed, and the animals were killed with an overdose of anaesthetic (300 mg (kg body wt)−1). Kidneys were decapsulated and sliced sagitally. Slices were transferred to Hanks’ modified medium (composition (mM):145 NaCl, 0.4 MgSO4, 0.5 MgCl2, 0.4 KH2PO4, 0.3 Na2HPO4, 25 NaHCO3, 10 Hepes, 4 KCl, 1.2 CaCl2, 5 glucose, 5 L-leucine, and 1 mg ml−1 bovine serum albumin (BSA); pH 7.40, bubbled with 95 % O2-5 % CO2). The inner strip of the outer medulla was carefully excised under stereomicroscopic control. The resulting tissue was subjected at 37°C to successive 10 min periods of collagenase digestion (0.40 g l−1), which minimized the time of exposure of the tubules to collagenase. In the final suspensions, most of the tubules (> 95 %) proved to be MTAL in origin, based on immunofluorescence staining for Tamm-Horsfall protein (Attmane-Elakeb et al. 1996), a specific marker for the thick ascending limb (Hoyer & Seiler, 1979).
Isolation of plasma membranes
Typically, the preparation began with 15-20 mg protein of MTAL tubules obtained from ten rats. Both types of plasma membrane vesicles were isolated simultaneously by a combination of Ca2+ aggregation and differential- and density-gradient centrifugations, as recently described in detail (Attmane-Elakeb et al. 1996). Compared with the homogenate, Na+,K+-ATPase, a basolateral marker, was enriched > 9-fold in the BLMVs and only 0.5-fold in the LMVs, whereas γ-glutamyltransferase, a LMV marker, was enriched ∼10-fold in the LMVs and 2-fold in the BLMVs. Western blot analysis, using specific NHE isoform antibodies (Attmane-Elakeb et al. 1996), indicated that NHE-3 was markedly enriched only in the apical fraction, and NHE-1 markedly enriched only in the basolateral membrane fraction. More recent Western blot analysis of BLMVs and LMVs isolated from the rat MTAL demonstrated that the NHE-2 antibody detected an 85 kDa protein predominantly in LMVs. NHE-2 expression in the apical membrane was confirmed by labelling experiments carried out on sections of paraformaldehyde-fixed rat kidney embedded in paraffin (Chambrey et al. 1997).
Vesicle integrity and membrane sidedness of the luminal and basolateral fractions
The specific activities of bafilomycin A1 (BAF)-sensitive ATPase and Na+,K+-ATPase were assayed using [γ-32P]ATP under several different conditions, as described previously (Boumendil-Podevin & Podevin, 1983; Chambrey, Paillard & Podevin, 1994), in order to estimate the membrane vesicle integrity and membrane sidedness of the LMVs and BLMVs, respectively. The estimate of sidedness of the LMV fraction was based on evidence that the catalytic site of the vacuolar ATPase is localized on the cytoplasmic face, and that BAF in the nanomolar range is a specific inhibitor of this ATPase (Bowman, Siebers & Altendorf, 1988). In these series of experiments, it was assumed that ATP does not diffuse across LMVs. The LMVs were permeabilized with 0.5 % octyl-β-D-glucopyranoside in the presence of 8 mg ml−1 BSA for 15 min at 30°C. As illustrated in Fig. 1A (a vs. c), 90 % of the BAF-sensitive ATPase activity was latent, indicating that most of the vesicles were sealed. In the absence of detergent pretreatment, the BAF-sensitive ATPase activity of inside-out vesicles can additionally be measured in the presence of nigericin and K+, added to collapse the proton electrochemical gradient which would otherwise build up and inhibit the H+-ATPase activity in sealed vesicles. In the presence of the appropriate ionophore and K+, however, the activity of the BAF-sensitive ATPase (Fig. 1A; b vs. a) was not increased significantly. These results suggest that most of the luminal vesicles (84.9 ± 7.17 %) were sealed and oriented right-side-out.
Figure 1. Vesicle integrity and membrane sidedness of the luminal and basolateral membrane vesicles isolated from rat MTAL tubules.
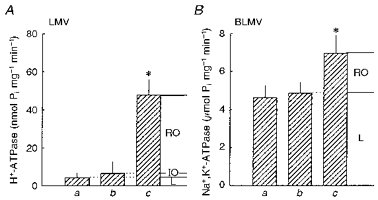
BAF-sensitive ATPase (H+-ATPase) and Na+,K+-ATPase activities in LMVs (A) and BLMVs (B) are given under different experimental conditions. a, in the presence of the appropriate standard media with no additions. For H+-ATPase assays, the standard medium consisted of (mM): 300 mannitol, 2 EGTA, 0.3 Na3VO4, 1 NaN3, 3 ouabain, 5 MgCl2, 5 Na2ATP, 20 Tris-Hepes (pH 7.40), a trace amount of [γ-32P]ATP, and 8 mg ml−1 BSA. For Na+,K+-ATPase assays, the standard medium consisted of (mM): 100 NaCl, 5 KCl, 2 EDTA, 8 MgCl2, 8 Na2ATP, 20 Tris-Hepes (pH 7.40), a trace amount of [γ-32P]ATP, and 0.6 mg ml−1 BSA. b, as in a but with either 2 μM nigericin and 10 mM KCl for LMV, or with 2 μM monensin, 2 μM valinomycin and 100 μM digitoxigenin for BLMV. c, as in a after permeabilization of both types of plasma membrane vesicles, as described in Methods. For all experimental situations, the final concentration of ethanol was 0.8 %. RO, right-side-out; IO, inside-out; L, leaky. Percentages of vesicles in each orientation and of leaky vesicles were calculated as follows: RO = (c - b)/c) × 100; IO = (b - a)/c× 100; and L = (a/c) × 100. Values are means ±s.d. of six determinations from three different LMV and BLMV preparations. *P < 0.01 vs. respective a and b values by Student's paired, two-tailed t test. Pi, inorganic phosphate.
The BLMV integrity and membrane sidedness were estimated by determination of the Na+,K+-ATPase latency, as previously described (Boumendil-Podevin & Podevin, 1983) with modifications. The rationale for this method was based on evidence that the catalytic site of Na+,K+-ATPase is localized on the cytoplasmic side, whereas ouabain binds on the opposite membrane face. In these series of experiments, it was assumed that both ATP and ouabain do not diffuse across BLMVs. The BLMVs were permeabilized by treatment for 10 min at 25°C with sodium dodecyl sulphate (7 μg (μg protein)−1) in the presence of 0.6 mg ml−1 BSA. The increase in the Na+,K+-ATPase activity by detergent pretreatment (Fig. 1B; c vs. a; 34 %) is a measure of the sealed vesicles. In the absence of detergent pretreatment, the Na+,K+-ATPase activity of inside-out vesicles can additionally be measured using digitoxigenin, a permeant inhibitor of the Na+,K+-ATPase. In this series of experiments, the ionophores monensin and valinomycin were used to collapse monovalent cation electrochemical gradients which would otherwise build up and inhibit Na+,K+-ATPase activity in sealed vesicles. Under these conditions, however, no significant stimulation of the digitoxigenin-sensitive Na+,K+-ATPase activity (b vs. a in Fig. 1B) was detected. From these data, it was estimated that only 34 % of the vesicles were sealed and that most of the vesicles (> 95 %) were oriented right-side-out.
Transport measurements
Na+-H+ antiporter activity was assayed by measurement of 22Na+ uptake at 20-25°C by a rapid filtration technique (Attmane-Elakeb et al. 1996). BLMVs and LMVs were equilibrated at room temperature for 2 h for loading with desired constituents. For each experiment, the specific conditions are given in the figure legends. In general, a 10 μl aliquot of either BLMVs or LMVs (15-30 μg protein) was added to an appropriate reaction medium containing 0.5-1 μCi ml−1 of 22Na+. Incubation periods of 4 or 9 s were used to estimate initial rates. Uptake was terminated by the addition of 1.5 ml of an ice-cold stop solution containing 20 mM Tris-Hepes, pH 7.4, and the desired LiCl concentration to maintain isosmolality (equilibration medium, incubation medium, and stop solution were always kept isosmotic). This suspension was rapidly filtered on the centre of a 0.45 μm prewetted HAWP cellulose filter (Millipore, Bedford, MA, USA) and washed with an additional 15 ml of the same ice-cold stop solution. For all experiments, non-specific isotopic binding to the filter was measured with appropriate blanks and subtracted from values of the incubated samples. The filters were dissolved in 3 ml of scintillant (Filter-Count, Packard), and radioactivity was measured in a liquid scintillation spectrometer. The stop solution contained Li+ because it is a trans inhibitor of 22Na+ efflux (Kinsella & Aronson, 1981).
Fluorescence studies
In studies with the pH indicator acridine orange, H+ influx rates were determined when gradients of acetate or sulphate salts of NH4+ were imposed. Fluorescence was recorded at 20-25°C using a Jobin-Yvon spectrofluorometer (excitation, 492 nm; emission, 523 nm). Proton fluxes were calculated as the rate of change in fluorescence as a percentage of the baseline signal.
Chemicals
Carrier-free 22NaCl was obtained from Amersham. BAF was a generous gift from Professor K. Altendorf (Universitat Osnabruck, Germany). 5-(N,N-dimethyl)-amiloride (DMA) was obtained from Sigma, and dissolved in Me2SO at a concentration of 0.05 M.
Statistical analyses
Unless stated otherwise, data are presented as means ±s.e.m. Comparisons between groups were generally carried out by two-way ANOVA or Student's t test. For all analyses, statistical significance was accepted as P < 0.05.
RESULTS
Effect of external NH4+ on Na+ influx
We first compared the effect of external NH4+ ((NH4+)o) on Na+ uptake stimulated by outwardly directed H+ gradients into BLMVs and LMVs prepared simultaneously. As illustrated in Fig. 2, the BLMV and LMV titration curves differed markedly: the BLMV antiporter had an apparent half-maximal inhibition (K0.5) of 3.39 ± 1.02 mM, whereas the luminal K0.5 value was 28.80 ± 8.80 mM (P < 0.05). These data suggest that NH4+ directly interacts with the external transport sites of both the LMV and BLMV Na+-H+ exchangers, and that NH4+ has a greater affinity for the BLMV exchanger.
Figure 2. Effect of external NH4+ on Na+-H+exchange in BLMVs (×) and LMVs (○).
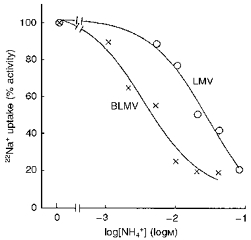
Both types of vesicles were loaded with a pH 6.0 medium consisting of 200 mM mannitol, 50 mM tetramethylammonium hydroxide (TMA)- nitrate, 3 mM EGTA, 50 mM Tris-Mes. Uptake of 0.5 mM 22Na+ at 4 s was measured by incubating the LMVs or BLMVs in medium containing 200 mM mannitol, 50 mM TMA-nitrate, 3 mM EGTA, 50 mM Tris-Hepes, pH 8.0, and increasing concentrations of external (NH4)2SO4. Tetramethylammonium sulphate was added as appropriate to maintain constant osmolality. Values are means of six determinations from three different LMV and BLMV preparations.
The above observations could also be explained by alkalinization of the intravesicular spaces of the vesicles due to rapid diffusion of NH3. The resulting collapse of the outward H+ gradient would then inhibit Na+ uptake. As shown in Fig. 3, however, we found that inhibition of Na+ uptake by (NH4+)o (10 mM) was not significantly modified by increasing the intravesicular buffer concentration from 25 to 100 mM. It would be expected that the effect of an inward NH4+ gradient, to collapse the pH gradient, would be reduced when the buffering capacity is increased. These data thus indicate that (NH4+)o interacts directly with the BLMV exchanger. This figure also shows that the effect of 10 mM (NH4+)o on Na+ uptake, in relatively low (25 mM Tris-Mes) or high (100 mM Tris-Mes) buffer concentrations, was not significantly modified whether the accompanying anion was acetate or SO42−, two salts that differ in their effects on the internal pH (pHi) of renal cortical membrane vesicles (Beck & Sacktor, 1975; Kinsella & Aronson, 1981). Similar results were obtained in LMVs (Fig. 3), the cis effects of NH4+ (20 mM) on Na+ uptake were independent of whether sulphate or acetate salts were used. These data, therefore, indicate that the intravesicular buffer capacity used in our transport experiments was more than adequate to prevent alterations in pHi.
Figure 3. Effects of inward NH4+ gradients on pH-gradient stimulated Na+ uptake into BLMVs and LMVs.
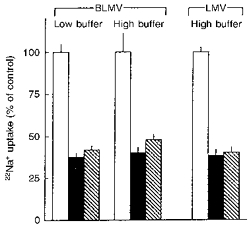
Uptake of 0.1 mM 22Na+ into BLMVs was assayed as described for Fig. 2 except that the concentration of Tris-Mes was either 25 or 100 mM in the equilibration media, and the concentration of Tris-Hepes in the respective incubation media was 25 or 100 mM. Uptake was determined in the presence of sucrose (control; □) or with isosmotic replacement of sucrose by 10 mM ammonium acetate ( ) or 5 mM (NH4)2SO4 (▪). Uptake of 0.1 mM 22Na+ into LMVs was as described above with high (100 mM) buffer concentration except that the concentrations of ammonium acetate and (NH4)2SO4 were 20 and 10 mM, respectively. Values are means ±s.e.m. of six determinations on two different BLMV and LMV preparations.
) or 5 mM (NH4)2SO4 (▪). Uptake of 0.1 mM 22Na+ into LMVs was as described above with high (100 mM) buffer concentration except that the concentrations of ammonium acetate and (NH4)2SO4 were 20 and 10 mM, respectively. Values are means ±s.e.m. of six determinations on two different BLMV and LMV preparations.
Fluorescence studies
We next investigated, using the pH-sensitive fluorescent dye acridine orange, whether application of NH4+ gradients as the acetate or SO42− salts also caused distinct effects on the pHi of plasma membrane vesicles of the MTAL. To increase the sensitivity of the assays, we monitored the pH of a poorly buffered intravesicular solution (5 mM Tris-Mes). In BLMVs (Fig. 4, left panel), the imposition of an outward NH4+ gradient of 150 mM : 4.16 mM with SO42− (a) as the accompanying anion caused a more rapid rate of quenching of acridine orange fluorescence, and thus of intravesicular acidification, than did the same NH4+ gradient with acetate (b) as the accompanying anion (5.5 ± 0.5 vs. 1.5 ± 0.3 % s−1, n=8, P < 0.01). Figure 4 also shows that maximum acridine orange quenching (percentage quench) 15 s after BLMV addition was increased 5-fold in BLMVs equilibrated with (NH4)2SO4 compared with those equilibrated with ammonium acetate (38.1 ± 0.74 vs. 7.6 ± 0.53 %, n=8, P < 0.01). In the absence of NH4+ outward gradients, with either SO42− (c) or acetate (d) as the accompanying anion, no quenching of the fluorescence developed. As can be seen in the right panel of Fig. 4, qualitatively similar results were obtained in studies using LMVs. Imposition of an outward NH4+ gradient with SO42− (a) as the accompanying anion also caused a more rapid rate of intravesicular acidification than did the same NH4+ gradient with acetate (b) as the accompanying anion (P < 0.01). When the above-described experiments were repeated at a higher pH value (pH 8.0 instead of pH 6.4), i.e. a condition that would favour NH3 diffusion and minimize CH3COOH diffusion, imposition of an outward NH4+ gradient with SO42− as the accompanying anion also caused a more rapid rate of quenching than did the same NH4+ gradient with acetate (7.7 ± 0.17 vs. 2.0 ± 0.12 % s−1 for LMV, n=6, P < 0.001; and 9.8 ± 0.2 vs. 2.4 ± 0.12 % s−1 for BLMV, n=6, P < 0.001). For both of these preparations, therefore, these results are consistent with the hypothesis that application of outward (NH4)2SO4 gradients acidified the internal space by liberating H+ as NH3 diffused down its concentration gradient. On the other hand, imposition of the same NH4+ gradients with acetate as the accompanying anion markedly reduced intravesicular acidification, as undissociated free acid diffused outward down its concentration gradient in parallel with NH3 (Kinsella & Aronson, 1981). The small intravesicular acidification that was detected in both plasma membrane vesicles in the presence of outward ammonium acetate gradients may be due either to a slightly greater permeability for NH3 than for acetic acid, or to the possible operation of these exchangers on the (NH4+)i-external H+ (H4+) mode.
Figure 4. NH4+ gradient-stimulated H+ influx in BLMVs (left panel) and LMVs (right panel) monitored fluorometrically using acridine orange.
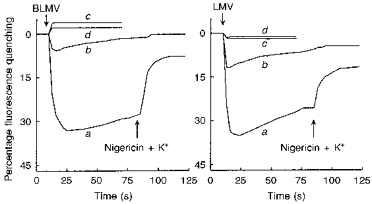
Vesicles were equilibrated for 120 min at ambient temperature (20-25 °C) in a medium consisting of 100 mM mannitol, 3 mM EGTA, 60 mM N-methyl-D-glucamine (NMG)-nitrate, 5 mM Tris-Mes, pH 6.4, which either contained 150 mM ammonium acetate, or 75 mM (NH4)2SO4 plus 75 mM mannitol. Twenty microlitres of either BLMVs or LMVs (25 μg of membrane protein) pre-equilibrated with either (NH4)2SO4 (trace a) or ammonium acetate (trace b) were added (first arrow) to 700 μl of a stirred solution containing 10 μM acridine orange, 100 mM mannitol, 3 mM EGTA, 60 mM NMG-nitrate, 150 mM TMA-gluconate, and 5 mM Tris-Mes, pH 6.4. Control experiments (absence of NH4+ gradient) were performed by addition of 20 μl of vesicles (25 μg of protein) pre-equilibrated with either 75 mM (NH4)2SO4 or 150 mM ammonium acetate to 700 μl of the corresponding incubation medium (traces c and d, respectively). At the time indicated by the second arrow, pH gradients were collapsed by addition of 3 μM nigericin plus 10 mM potassium gluconate. Fluorescence is represented as a percentage of the baseline signal. The results are representative of experiments performed in quadruplicate on two separate membrane preparations.
Lastly, acridine orange studies were carried out to ensure that a 2 h preincubation in different buffers (5 vs. 100 mM Tris-Mes pH 6.40) actually resulted in significant changes in the intravesicular buffering capacity. As expected, imposition of an outward ammonium acetate gradient of 150 mM : 4.16 mM with high internal buffer concentrations (100 mM) inhibited the rate of quenching of acridine orange fluorescence, and thus of intravesicular acidification, by 82 % in BLMVs (2.11 ± 0.50 vs. 0.38 ± 0.30 % s−1, n=6, P < 0.001) and by 61 % in LMVs (1.78 ± 0.08 vs. 0.70 ± 0.06 % s−1, n=6, P < 0.001). It should be noted that the rate of quenching of acridine orange fluorescence observed in response to application of ammonium acetate gradients at high buffer concentrations were negligible, being less than 10 % of those observed at low buffer concentrations using (NH4)2SO4 gradients (Fig. 4).
Both the apical and basolateral antiporters can mediate exchange of intravesicular NH4+ for extravesicular Na+
We next evaluated whether the BLMV and LMV antiporters can mediate the exchange of (NH4+)i for Na+o. In these experiments, vesicles were preincubated with high (125 mM) buffer concentrations, and two different methods were used to prevent possible alterations in pHi due to NH3 diffusion. In the first approach, we evaluated the effects of outwardly directed ammonium acetate gradients on 22Na+ uptake, taking advantage of the observation in Fig. 4 that, even in the presence of a very low intravesicular buffer capacity (5 mM Tris-Mes), imposition of an outward NH4+ gradient with acetate as the accompanying anion caused only marginal intravesicular acidification. The results of these experiments are shown in Fig. 5. Imposition of outwardly directed ammonium acetate gradients stimulated 22Na+ uptake by 52.4 ± 9.32 and 72.0 ± 12.7 % in BLMVs and LMVs, respectively (P < 0.01). As expected, this NH4+ gradient-stimulated Na+ uptake on both membranes was completely abolished by 0.5 mM DMA.
Figure 5. Trans-stimulation of 22Na+ influx by NH4+ in BLMVs (left panel) and LMVs (right panel).
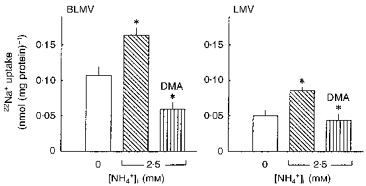
Both types of vesicles were loaded for 120 min at ambient temperature in a medium consisting of 100 mM mannitol, 3 mM EGTA, 60 mM NMG-nitrate, 40 mM NMG-gluconate, 125 mM Tris-Mes, pH 6.6, and with either 0 or 2.5 mM ammonium acetate. Osmolarity was maintained constant with sucrose. Membrane vesicles were diluted 1 : 41 in a NH4+-free medium consisting of 0.5 mM 22Na+, 100 mM mannitol, 3 mM EGTA, 60 mM NMG-nitrate, 40 mM NMG-gluconate, and 125 mM Tris-Mes, pH 6.6, and incubated for 9 s. DMA (0.5 mM) was added to the incubation medium where indicated. Values are means ±s.e.m. of nine determinations on three different BLMV and LMV preparations. *P < 0.01for 2.5 mM (NH4+)ivs. 0 mM (NH4+)i; and for 2.5 mM (NH4+)i+ DMA vs. 2.5 mM (NH4+)i, by ANOVA.
In the second approach, the effects of outwardly directed (NH4)2SO4 gradients on 22Na+ uptake were evaluated in the absence of an NH3 gradient. This was achieved by diluting appropriately acidic vesicles (pHi, 6.60) into incubation media at pH 8.26. As illustrated in Fig. 6, imposition of an outward NH4+ gradient of 4.98 mM : 0.109 mM, while [NH3]i and [NH3]o were fixed at 12.5 μM, significantly stimulated Na+ uptake by 28.0 ± 9.5 and 66.6 ± 12.6 % in BLMVs and LMVs, respectively (P < 0.05). In these experiments, Na+ uptake into acidic membrane vesicles preloaded with NH4+ could occur by both Na+-H+ and Na+-NH4+ exchange. As shown in Fig. 6, 0.5 mM DMA inhibited the internal H+ (H+i)- and (NH4+)i-stimulated Na+ uptake by 71.7 ± 3.8 and 76.0 ± 5.0 % in BLMVs and LMVs, respectively (P < 0.001). Taken together, these experiments provide strong support for the concept that internal NH4+ is a substrate for the apical and basolateral Na+-H+ exchangers of rat MTAL cells.
Figure 6. Trans-stimulation of 22Na+ influx by NH4+ in the absence of transmembrane NH3 gradient.
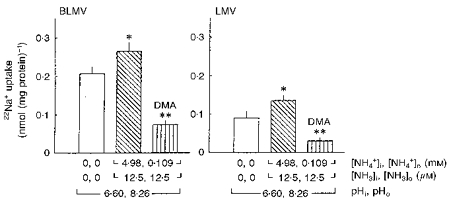
BLMVs (left panel) and LMVs (right panel) were loaded for 120 min at ambient temperature in a medium consisting of 100 mM mannitol, 3 mM EGTA, 60 mM NMG-nitrate, 40 mM NMG-gluconate, 125 mM Tris-Mes, pH 6.60, and with either 2.5 mM (TMA)2SO4 or 2.5 mM (NH4)2SO4. The membrane vesicles were then diluted 1 : 41 in a medium containing 0.5 mM 22Na+, 100 mM mannitol, 3 mM EGTA, 60 mM NMG-nitrate, 40 mM NMG-gluconate, and 125 mM Tris-Hepes, pH 8.26. DMA (0.5 mM) was added to the incubation medium where indicated. Incubations were for 9 s at 25 °C. Intravesicular and extravesicular concentrations of NH4+ and NH3 were calculated with the use of a pKa value (-log of the dissociation constant) for the NH3-NH4+ buffer of 9.20 at 25 °C. Values are means ±s.e.m. of nine determinations from three different BLMV and LMV preparations. *P < 0.05 for 4.98 mM (NH4+)ivs. 0 mM (NH4+)i; and **P < 0.001 for 4.98 mM (NH4+)i+ DMA vs. 4.98 mM (NH4+)i, by ANOVA.
pHi dependence of the BLMV and LMV Na+-H+ exchangers
Lastly, we examined the kinetics of the BLMV and LMV exchangers with respect to H+i, using a wide pHi range (5.5-7.5). As can be seen in Fig. 7, the BLMV and LMV exchangers displayed a similar pHi dependence with half-maximal H+i activation values (pK) of 6.58 ± 0.048 and 6.52 ± 0.002, respectively. Kinetic analysis demonstrated that Na+-H+ exchange rates, for pHi values ranging from 5.5 to 7.5, fitted a sigmoidal curve with an apparent affinity (napp) of 1.9 for the two antiporters, suggesting an allosteric H+i-binding site.
Figure 7. pHi dependence of apical and basolateral Na+-H+ antiporters.
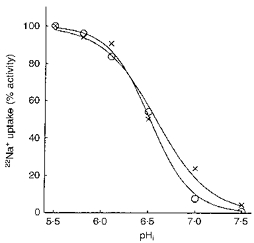
Both types of vesicles were equilibrated for 120 min at ambient temperature in a medium consisting of 300 mM mannitol, 40 mM TMA-nitrate, 3 mM EGTA, and with either 100 mM Tris-Mes at pH 5.50, 5.80, 6.10, 6.50, or with 100 mM Tris-Hepes at pH 7.00 and 7.50. The membrane vesicles were then diluted 1 : 9 and incubated for 9 s at 25 °C in 0.1 mM 22Na+, 300 mM mannitol, 40 mM TMA-nitrate, 3 mM EGTA, and 100 mM Tris-Hepes, pH 7.5. Data were normalized as a percentage of the maximal rate of 22Na+ uptake at pH 5.50. Levels of background influx that were not inhibitable by 0.5 mM DMA were subtracted from the total influx. Values represent the mean of nine experiments on three separate BLMV (×) and LMV (○) preparations.
DISCUSSION
Work from several groups suggests that the Na+-H+ exchanger of the proximal tubule can function in multiple exchange modes involving Na+, H+, Li+ and NH4+ (for review, see Aronson, 1985). Regarding NH4+, exchange of internal Na+ for external NH4+ has been demonstrated in renal brush border membrane vesicles (Kinsella & Aronson, 1981).
The present study shows that NH4+ directly interacts with the apical and basolateral membrane Na+-H+ exchangers of the rat MTAL. This study also shows for the first time that both of these transport systems can mediate the physiologically relevant exchange of internal NH4+ for external Na+. We have ruled out the possibility that both the cis and trans effects of NH4+ on Na+ uptake are indirect, i.e. secondary to alterations in pHi via non-ionic diffusion of NH3. Indeed, we have provided direct evidence that, although sulphate and acetate differ markedly in their effects on pHi, they elicited identical cis inhibitory effects on Na+ uptake in both BLMVs and LMVs. Moreover, we also demonstrated that alteration in pHi by ammonium acetate was negligible when vesicles were equilibrated with high buffer concentrations. Conversely, two series of experiments, conducted with vesicles preincubated with high buffer (125 mM), suggest that the NH4+ gradient-stimulated Na+ uptake in both types of plasma membrane vesicles does not result from acidification of the intravesicular space. First, an outwardly directed ammonium acetate gradient stimulated Na+ uptake in both preparations (Fig. 5). In this circumstance, parallel diffusion of NH3 and CH3COOH down their concentration gradients should produce no change in pHi in the presence of high internal buffer concentrations. Second, (NH4+)i stimulated Na+ uptake in the absence of an NH3 gradient (Fig. 6) when alteration in pHi via NH3 diffusion could not occur. Inhibition of all the (NH4+)i-activated 22Na+ uptake by DMA, a Na+-H+ exchange inhibitor, provides further evidence for NH4+-Na+ exchange in both of these vesicles. When taken together, the aforementioned results provide strong evidence that the cis and trans effects of NH4+ on Na+ uptake by both apical and basolateral membrane vesicles are direct, and not secondary to alteration in pHi.
In BLMVs and LMVs, which were found to have a right-side orientation, NH4+-activated Na+ influxes were observed with NH4+ and Na+ gradients in the physiological orientations and at the normal acidic pHi (6.60) of MTAL cells. These results, together with our observations in Fig. 7 that H+i is not saturating for the BLMV and LMV Na+-H+ exchangers at pHi 6.60 under hyperosmotic conditions (450 mosmol (kg H2O)−1), indicate that unoccupied carriers can operate on the (NH4+)i-Na+o exchange mode under physiological circumstances. Parenthetically, the pK value of 6.58 for the BLMV exchanger is close to the pK value of 6.75 reported for rat NHE-1 expressed in Na+-H+ exchanger-deficient cell lines (Orlowski, 1993). On the other hand, the pK value of 6.52 reported here for the LMV exchanger and of 6.75 for the apical Na+-H+ exchanger of the rat MTAL perfused in vitro in hyperosmotic conditions (Watts & Good, 1994b) probably represented contributions of NHE-3 and NHE-2. Indeed, preliminary studies have demonstrated that NHE-2 is also expressed predominantly at the apical site of the rat MTAL (Sun et al. 1996; Chambrey et al. 1997), and heterologous expression studies have shown that the rat NHE-2 exhibited a higher apparent affinity for H+i than NHE-3, with apparent pK values of 6.90 and 6.45, respectively (Orlowski, 1993; Yu, Shull & Orlowski, 1993).
Based on current data on Na+-H+ exchanger isoforms in the rat MTAL (Amemiya, Loffing, Lötscher, Kaissling, Alpern & Moe, 1995; Attmane-Elakeb et al. 1996; Sun et al. 1996), NHE-1 and both NHE-3 and NHE-2 are the most likely candidates to mediate Na+-NH4+ transport on the basolateral and apical membranes, respectively. On the basolateral antiporter (NHE-1), exchange of (NH4+)i for Na+o energized by the basolateral Na+ gradient may represent an important pathway mediating the final step of ammonia reabsorption. On the apical membrane, it is expected that the apical (NH4+)i-Na+o exchanger (NHE-3 and/or NHE-2) and the Na+-K+(NH4+)-2Cl− cotransporter would counteract one another. This would give the MTAL cell the option to limit NH4+ absorption when the medullary interstitial ammonium concentration rises. Consistent with the latter possibility, it has been shown that increasing the NH4+ concentration in lumen and bath diminished net ammonium absorption in the rat MTAL (Good, 1994).
Microperfusion studies have demonstrated that the luminal and basolateral Na+-H+ exchangers of the mammalian MTAL cells are under separate regulatory control by arginine vasopressin (AVP) and osmolality (Good, 1992; Sun et al. 1992). Sun et al. (1992) have established that AVP in the mouse MTAL stimulates the basolateral, while inhibiting the apical, Na+-H+ exchanger. Hyperosmolality inhibits the luminal Na+-H+ exchanger in the rat MTAL (Watts & Good, 1994b), and the effects of AVP (via cyclic AMP) and hypertonicity are additive (Good, 1992), resulting in an almost complete (∼90 %) inhibition of net HCO3− absorption. These results are in contrast to findings suggesting that AVP and hypertonicity stimulate basolateral Na+-H+ exchange activity in the mouse MTAL via a two-step process (Sun, Saltzberg, Kikeri & Hebert, 1990). These findings, however, are not inconsistent with operation of the apical exchanger on either the Na+-H+ or the Na+-NH4+ mode during antidiuresis. Indeed, AVP and hypertonicity also stimulate collecting duct and medullary interstitial cells to produce prostaglandin E2 (Schlondorff & Ardaillou, 1986; Conrad & Dunn, 1992; Skorecki, Brown, Ercolani & Ausiello, 1992), which in turn largely reverses AVP inhibition of HCO3− absorption in the rat MTAL (Good & George, 1996), presumably through stimulation of apical Na+-H+ exchange.
Preliminary studies (Watts & Good, 1993) in the rat MTAL, perfused under isotonic conditions, have shown that addition of amiloride to the basolateral solution inhibited ammonia absorption. These investigators concluded that basolateral Na+-H+ exchange may be involved in NH4+ absorption by the combination of H+ extrusion and passive NH3 efflux. From such studies, however, it is difficult to establish with certainty the mechanism underlying amiloride inhibition of net ammonium absorption. In light of our results, it is likely that the effect they observed was due, at least in part, to inhibition of the BLMV exchanger operating on the (NH4+)i-Na+o mode. On the other hand, Good & Watts (1996) recently found that 1 mM luminal amiloride in the isolated perfused rat MTAL had no effect on ammonium absorption. On the basis of these data, it was concluded that the apical membrane Na+-H+ exchanger is not important for transepithelial NH4+ absorption. The latter microperfusion studies were performed in isosmotic solutions, i.e. under conditions in which the luminal Na+-H+ exchanger is relatively unresponsive to changes in pHi over the physiological pHi range (6.5-7.2) (Watts & Good, 1994b). If the internal transport site of this exchanger is actually fully saturated with H+ in isotonic media, then Na+-NH4+ exchange would be very low, unless the exchanger can mediate Na+-NH4+ exchange faster than it can mediate Na+-H+ exchange, as it is indeed the case for the renal microvillus membrane Na+-H+ antiporter (Kinsella & Aronson, 1981). In fact, it should be noted that the amiloride studies of Good & Watts (1996) do not eliminate luminal Na+-NH4+ exchange as a possible pathway for NH4+ secretion. Indeed, the lack of effect of luminal amiloride on NH4+ absorption may be because inhibition of the antiporter is associated with direct and/or indirect effects of this drug to alter luminal NH4+ uptake by other transport pathways. Of note, the feasibility of Na+-NH4+ exchange contributing to NH4+ secretion is supported by physiological studies demonstrating that inhibition of the Na+-K+(NH4+)-2Cl− cotransporter by luminal furosemide (frusemide) eliminated NH4+ absorption and converted net absorption to net secretion in five of seven isolated perfused rat MTALs (Good et al. 1984).
In summary, these experiments demonstrate that both the apical and basolateral Na+-H+ exchangers of the rat MTAL can also mediate the physiologically relevant exchange of internal NH4+ for external Na+ at the normal acidic pHi of the cells of this nephron segment. NHE-1 in BLMVs may contribute to NH4+ reabsorption. NHE-3 (and possibly NHE-2) in LMVs may provide a feedback mechanism that limits net NH4+ absorption as the interstitial NH4+ concentration increases.
Acknowledgments
The authors thank F. Pezy for excellent technical assistance. We also thank Professor K. Altendorf for the generous gift of bafilomycin A1.
References
- Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of the rat renal proximal tubule and thick ascending limb. Kidney International. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- Aronson PS. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annual Review of Physiology. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Attmane-Elakeb A, Chambrey R, Tsimaratos M, Leviel F, Blanchard A, Warnock DG, Paillard M, Podevin R-A. Isolation and characterization of luminal and basolateral plasma membrane vesicles from the medullary thick ascending loop of Henle. Kidney International. 1996;50:1051–1057. doi: 10.1038/ki.1996.408. [DOI] [PubMed] [Google Scholar]
- Beck JC, Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush-border membrane vesicles. Journal of Biological Chemistry. 1975;250:8674–8680. [PubMed] [Google Scholar]
- Boumendil-Podevin EF, Podevin R-A. Isolation of basolateral and brush-border membranes from the rabbit kidney cortex. Vesicle integrity and membrane sidedness of the basolateral fraction. Biochimica et Biophysica Acta. 1983;735:86–94. doi: 10.1016/0005-2736(83)90263-8. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proceedings of the National Academy of Sciences of the USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MB. Background and development of microperfusion technique. Kidney International. 1982;22:417–424. doi: 10.1038/ki.1982.194. [DOI] [PubMed] [Google Scholar]
- Chambrey R, Mandet C, Bélair M-F, Podevin R-A, Warnock DG, Bruneval P, Bariety J, Paillard M. Immunolocalization of the Na/H exchanger isoform NHE2 in rat kidney. Journal of the American Society of Nephrology. 1997;8:4A. doi: 10.1152/ajprenal.1998.275.3.F379. [DOI] [PubMed] [Google Scholar]
- Chambrey R, Paillard M, Podevin R-A. Enzymatic and functional evidence for adaptation of the vacuolar H+-ATPase in the proximal tubule apical membrane from rats with chronic metabolic acidosis. Journal of Biological Chemistry. 1994;269:3243–3250. [PubMed] [Google Scholar]
- Conrad KP, Dunn MJ. Renal prostaglandins and other eicasanoids. In: Windhager EE, editor. Handbook of Physiology, section 8, Renal Physiology. II. Bethesda, MD, USA: American Physiological Society; 1992. pp. 1707–1757. chap 35. [Google Scholar]
- Good DW. Effects of osmolality on bicarbonate absorption by medullary thick ascending limb of the rat. Journal of Clinical Investigation. 1992;89:184–190. doi: 10.1172/JCI115560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DW. Ammonium transport by the thick ascending limb of Henle's loop. Annual Review of Physiology. 1994;56:623–647. doi: 10.1146/annurev.ph.56.030194.003203. 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- Good DW, George T. Regulation of HCO3− absorption by prostaglandin E2 and G proteins in rat medullary thick ascending limb. American Journal of Physiology. 1996;270:F711–717. doi: 10.1152/ajprenal.1996.270.5.F711. [DOI] [PubMed] [Google Scholar]
- Good DW, George T, Watts BA., III Basolateral membrane Na+/H+ exchange enhances HCO3− absorption in rat medullary thick ascending limb: Evidence for coupling between basolateral and apical membrane Na+/H+ exchangers. Proceedings of the National Academy of Sciences of the USA. 1995;92:12525–12529. doi: 10.1073/pnas.92.26.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DW, Knepper MA, Burg MB. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. American Journal of Physiology. 1984;247:F35–44. doi: 10.1152/ajprenal.1984.247.1.F35. [DOI] [PubMed] [Google Scholar]
- Good DW, Watts BA., III Functional roles of apical membrane Na+/H+ exchange in rat medullary thick ascending limb. American Journal of Physiology. 1996;270:F691–699. doi: 10.1152/ajprenal.1996.270.4.F691. [DOI] [PubMed] [Google Scholar]
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney International. 1979;16:279–289. doi: 10.1038/ki.1979.130. [DOI] [PubMed] [Google Scholar]
- Kinsella JL, Aronson PS. Interaction of NH4+ and Li+ with the renal microvillus membrane Na+-H+ exchanger. American Journal of Physiology. 1981;241:C220–226. doi: 10.1152/ajpcell.1981.241.5.C220. [DOI] [PubMed] [Google Scholar]
- Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. Journal of Clinical Investigation. 1988;81:159–164. doi: 10.1172/JCI113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski J. Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low affinity (NHE-3) isoforms of the rat Na/H exchanger. Journal of Biological Chemistry. 1993;268:16369–16377. [PubMed] [Google Scholar]
- Preisig PA, Alpern RJ. Pathways for apical and basolateral membrane NH3 and NH4+ movement in rat proximal tubule. American Journal of Physiology. 1990;259:F587–593. doi: 10.1152/ajprenal.1990.259.4.F587. [DOI] [PubMed] [Google Scholar]
- Schlondorff D, Ardaillou R. Prostaglandins and other arachidonic acid metabolites in the kidney. Kidney International. 1986;29:108–119. doi: 10.1038/ki.1986.13. [DOI] [PubMed] [Google Scholar]
- Skorecki KL, Brown D, Ercolani L, Ausiello DA. Molecular mechanisms of vasopressin action in the kidney. In: Windhager EE, editor. Handbook of Physiology, section 8, Renal Physiology. II. Bethesda, MD, USA: American Physiological Society; 1992. pp. 1185–1218. chap. 26. [Google Scholar]
- Sun AM, Kikeri D, Hebert SC. Vasopressin regulates apical and basolateral Na+/H+ exchangers in mouse medullary thick ascending limbs. American Journal of Physiology. 1992;262:F241–247. doi: 10.1152/ajprenal.1992.262.2.F241. [DOI] [PubMed] [Google Scholar]
- Sun AM, Saltzberg SN, Kikeri D, Hebert SC. Mechanisms of cell volume regulation by the mouse medullary thick ascending limb of Henle. Kidney International. 1990;38:1019–1029. doi: 10.1038/ki.1990.308. [DOI] [PubMed] [Google Scholar]
- Sun AM, Yip KP, Liu Y, Centracchio JN, Tse CM, Donowitz M, Dworkin LD. NHE-2 is present in the apical membrane of medullary thick ascending limb (MTAL) Journal of the American Society of Nephrology. 1996;7:1261. [Google Scholar]
- Watts BA, III, Good DW. Basolateral membrane Na+/H+ exchange enhances transepithelial HCO3− and NH4+ absorption in rat medullary thick ascending limb. Journal of the American Society of Nephrology. 1993;4:849. [Google Scholar]
- Watts BA, III, Good DW. Effects of ammonium on intracellular pH in rat medullary thick ascending limb. Mechanisms of apical membrane NH4+ transport. Journal of General Physiology. 1994a;103:917–936. doi: 10.1085/jgp.103.5.917. 10.1085/jgp.103.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts BA, III, Good DW. Apical membrane Na+/H+ exchange in rat medullary thick ascending limb. pHi-dependence and inhibition by hyperosmolality. Journal of Biological Chemistry. 1994b;269:20250–20255. [PubMed] [Google Scholar]
- Yu FH, Shull GE, Orlowski J. Functional properties of the rat Na/H exchanger NHE-2 isoform expressed in Na/H exchanger-deficient chinese hamster ovary cells. Journal of Biological Chemistry. 1993;268:25536–25541. [PubMed] [Google Scholar]


