Abstract
Glycine activated strychnine-sensitive chloride conductances at both the dendrites and the axonal telodendria of most bipolar cells in the salamander retina.
The chloride equilibrium potential of bipolar cells was found to be negative to -50 mV, indicating that glycinergic synapses on bipolar cells are inhibitory.
Some bipolar cells exhibited discrete, strychnine-sensitive, chloride-mediated inhibitory postsynaptic currents (IPSCs). These were elicited by focal application of glutamate at the inner plexiform layer (IPL). Glycinergic synapses were localized using simultaneous focal application of calcium to retinal slices bathed in calcium-free media. Both dendritic and telodendritic glycinergic IPSCs were observed.
The decay of the telodendritic IPSCs was well fitted by a single exponential with a time constant of 17.7 ± 8.7 ms. Similar kinetics were observed for dendritic IPSCs in some cells, but in one class of on-centre bipolar cell the decay of the dendritic IPSCs was better fitted by a sum of two exponentials with time constants 9.9 ± 4.3 and 51.3 ± 24.3 ms.
The dendritic IPSCs were best driven by application of glutamate at the distal IPL (the off sublamina), while the telodendritic IPSCs were driven best by application near the telodendria. These results suggest that bipolar cell dendrites receive inhibitory glycinergic inputs from interplexiform cells that are excited by off-centre bipolar cells, whereas bipolar cell telodendria receive glycinergic amacrine cell inputs that are antagonistic to the photoreceptor inputs.
Both inputs could be elicited in the presence of tetrodotoxin (TTX), but the dendritic IPSCs were sometimes abolished by TTX, suggesting that sodium-dependent spikes play an important role in the transmission of interplexiform cell signals to the outer plexiform layer.
Bipolar cells are non-spiking neurons involved in the integration of signals at both synaptic layers of the retina (Werblin & Dowling, 1969). At the outer plexiform layer (OPL), excitatory conductances on bipolar cell dendrites are modulated by photoreceptor synapses. Light hyperpolarizes photoreceptors and causes a reduction of transmitter release at these synapses. On-centre bipolar cells are depolarized by illumination of their receptive field centres, because the photoreceptor transmitter, glutamate, deactivates excitatory dendritic conductances in these cells (Slaughter & Miller, 1981; Attwell, Mobbs, Tessier-Lavigne & Wilson, 1987). Off-centre bipolar cells are hyperpolarized by illumination of their receptive field centres, because for these cells glutamate activates excitatory dendritic conductances (Slaughter & Miller, 1983; Maple, Werblin & Wu, 1994). Bipolar cells are the first cells in the visual system to exhibit centre-surround receptive field organization (Werblin & Dowling, 1969). The surround antagonism of their receptive fields is thought to result primarily from presynaptic inhibition of photoreceptors by GABAergic horizontal cells (Wu, 1992), although horizontal cell inputs to bipolar cell dendrites may also play a role (Yang & Wu, 1991; Hare & Owen, 1992). In addition, bipolar cell dendrites receive synapses from interplexiform cells (Smiley & Yazulla, 1990), but little is known about the role of these inputs in the generation of bipolar cell receptive fields.
At the inner plexiform layer (IPL) bipolar cell axonal processes make excitatory glutamatergic synapses on ganglion cells (the output neurons of the retina), on amacrine cells (responsible for lateral inhibition at the IPL), and on interplexiform cells (Maguire, Lukasiewicz & Werblin, 1990; Mittman, Taylor & Copenhagen, 1990, Dixon & Copenhagen, 1992; Marc, Murray & Basinger, 1995). In the salamander retina, bipolar cell axons give rise to many fine telodendria, which ramify laterally in IPL. The bipolar cell telodendria and associated output synapses are segregated in the IPL according to bipolar cell type. Off- centre bipolar cells have telodendria that ramify and synapse on amacrine and ganglion cell dendrites in the distal IPL (sublamina A), while on-centre bipolar cell telodendria ramify and synapse on amacrine and ganglion cell dendrites in the proximal IPL (sublamina B) (Famiglietti & Kolb, 1976; Famiglietti, Kaneko & Tachibana, 1977; Nelson, Famiglietti & Kolb, 1978; Maple & Wu, 1996). Bipolar cells also receive inhibitory synaptic inputs from amacrine cells (Wong-Riley, 1974; Marc, 1992; Maple & Wu, 1996). Much of this inhibition is probably GABAergic, since in the salamander retina a large fraction of amacrine cells accumulate GABA (Wu, 1991) and GABA activates large chloride conductances localized at the telodendria of bipolar cells (Lukasiewicz & Werblin, 1994; Maple & Wu, 1996).
In contrast to the considerable body of research that has been devoted to GABAergic circuits in the retina, relatively little attention has been given to the role of glycinergic circuitry. Immunocytochemical studies have shown that in the salamander retina, glycine is accumulated by subpopulations of amacrine cells and interplexiform cells, but not by horizontal cells (Wu, 1991). It is possible, therefore, that bipolar cells might receive synaptic inputs from glycinergic interplexiform cells at the OPL and/or glycinergic amacrine cells at the IPL. This article presents a systematic study of glycinergic inputs to retinal bipolar cells and provides the first direct electrophysiological evidence for glycinergic synapses from both amacrine cells and interplexiform cells. It begins with an examination of the spatial distribution of glycine-activated chloride conductances on bipolar cells. This is followed by a description of strychnine-sensititive inhibitory postsynaptic currents (IPSCs) observed in bipolar cells. A method is demonstrated for localizing the sites of these synaptic inputs through the focal application of calcium to a retinal slice. Based on the results of experiments combining focal stimulation of the retina with focal application of calcium, we present a detailed model for the circuitry of glycinergic synaptic inputs to one class of on-centre bipolar cell in the salamander retina.
METHODS
The retina of the larval tiger salamander (Ambystoma tigrinum) was used these studies. Animal care and tissue preparation protocols adhered to Association for Research in Vision and Ophthalmology (ARVO) and National Institutes of Health (NIH) guidelines and were approved by the Committee on Animal Research of Baylor College of Medicine, Houston, Texas. Experiments were performed on light-adapted retinae except were otherwise noted. Bipolar cells were voltage-clamped in retinal slices (Werblin, 1978) with patch electrodes. The input resistances of the cells were were usually 500 MΩ to 2 GΩ, and series resistances were typically 10-20 MΩ (uncompensated). Bipolar cell glycine receptors were directly activated by iontophoresis of 1 M glycine (pH 1.0, with 50 ms, 10 nA pulses) or by focal pressure ejection of 100 μM glycine from pipettes with a lumen of about 1 μm. Light responses were obtained using diffuse, white-light stimuli. Inhibitory synaptic inputs to bipolar cells were also elicited by focal pressure ejection of 100 μM glutamate, which depolarized presynaptic neurons. The bipolar cells were visualized using Lucifer Yellow fluorescence, and characterized by the level at which their axonal telodendria ramified in the inner plexiform layer (IPL). This level was quantified as the distance from the distal margin of the IPL to the telodendria as a fraction of the IPL thickness, which we defined as one inner plexiform unit (IU). The cells could be identified as on-centre or off-centre according to the level of ramification, since in this species the off-centre bipolar cells stratify in the distal 55 % of the IPL (0-0.55 IU), while the on-centre bipolar cells stratify within the proximal 45 % of the IPL (0.55-1.0 IU) (Maple & Wu, 1996). Fluorescing cells were photographed on colour reversal film, and the resulting images were digitally scanned and enhanced. The images presented here are grey scale negatives of the original fluorescence images. In some Figures the boundaries of the IPL are denoted by cropped images of cell bodies present at the margins of the IPL.
The normal Ringer solution consisted of (mM): NaCl, 120; KCl, 2.5; CaCl2, 2; MgCl2, 1; Hepes, 5; and glucose, 5 adjusted to pH 7.7 with NaOH. Cobalt Ringer solution was prepared by equimolar substitutions of CoCl2 for CaCl 2 and BaCl2 for MgCl2. The barium in this solution promoted seal stability in the absence of calcium, but was not sufficient to enable synaptic transmission when presynaptic neurons were depolarized by glutamate application (see Fig. 4B). The standard intracellular solution (for a chloride equilibrium potential of -50 mV) consisted of (mM): Cs2SO4, 69; CsCl, 15.7; MgCl2, 1; BaCl2, 0.1; EGTA, 2; Hepes, 1; Na2ATP, 1; Na3GTP, 0.5; and Lucifer Yellow CH (lithium salt), 0.4; adjusted to pH 7.2 with CsOH. In some experiments the chloride equilibrium potential (ECl) was set to -40 mV (with 63 mM Cs2SO4 and 24.3 mM CsCl) or to -60 mV (using 72.9 mM Cs2SO4 and 9.85 mM CsCl). The liquid junction potential between the Ringer solution and standard internal solution was compensated for by a command offset of 9.5 mV. The sites of inhibitory synapses were localized by stimulating slices in Co2+ Ringer solution while simultaneously applying normal Ca2+-containing Ringer solution to different regions of the bipolar cell. In these experiments the stimulating pipette contained 100 μM glutamate dissolved in Co2+ Ringer solution. In experiments involving a single stimulating pipette in a normal Ringer solution bath, the puff pipette contained glutamate dissolved in normal Ringer solution.
Figure 4. Strychnine-sensitive IPSCs elicited by stimulation with glutamate.
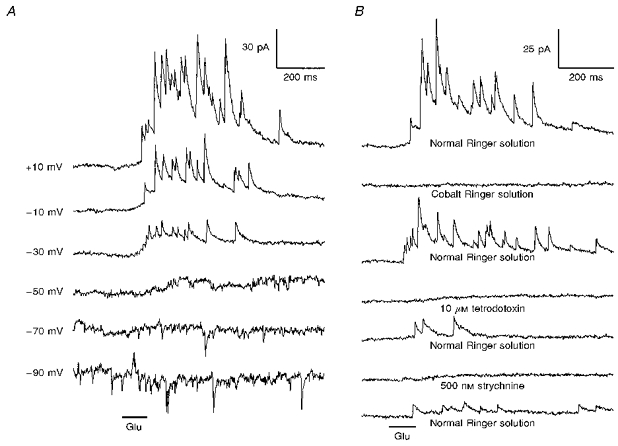
A, responses of an on-centre (0.7 IU) bipolar cell to focal pressure ejection of 100 μM glutamate (Glu) at the distal region of the IPL. The holding potential for each record is indicated on the left. The IPSCs reversed near ECl (set to -50 mV in this experiment). B, the IPSCs were reversibly abolished by substitution of Co2+ for Ca2+, and by the addition of 10 μM tetrodotoxin or 500 nM strychnine to the bath. The holding potential was -10 mV.
Amplifier signals were lowpass filtered at 2 kHz with a 4-pole Bessel filter and digitized at 10 kHz. Filtering by the series resistance limited the actual bandwidth of the data and caused significant attenuation of frequencies over 500 Hz (Attwell et al. 1987). The data shown here were further filtered to a 500 Hz -3 db lowpass cut-off frequency using a Gaussian-windowed digital filter and assuming that prior Bessel filtration was approximately Gaussian. The time course of IPSCs was modelled as a difference of exponentials with either one exponential of decay,
or two exponentials of decay:
In these equations τ1 approximately describes the rate of rise for the IPSC, while τ2 and τ3 describe the rate(s) of IPSC decay. The constant C1 is related to the IPSC amplitude, and it is proportional to the amplitude for any fixed set of time constants. The constant C2 in eqn (2) describes the relative weighting given to the two exponentials of decay: it is the fractional weight given to the exponential governed by τ3. The IPSCs were fitted to Gaussian-window filtered forms of eqns (1) and (2), where each exponential was replaced by a filtered exponential given by:
where
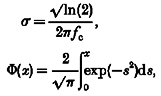 |
and fc= low-pass cut-off frequency.
Fitting was performed by χ2 minimization using the Levenberg- Marquardt method (Press, Flannery, Teukolsky & Vetterling, 1989). Experiments were performed at 20-21°C.
RESULTS
Responses of bipolar cells to focally applied glycine
Glycine was ionotophoretically applied to thirty bipolar cells in retinal slices bathed in Co2+ Ringer solution. Figure 1A shows current responses obtained by applying glycine at the telodendria of an on-centre bipolar cell held at several different potentials. The responses reversed near ECl (-50 mV), and they were consistent with a chloride conductance increase. Glycine sensitivity was carefully mapped out as illustrated in Fig. 1B, which shows the spatial amplitude profile for an array of responses obtained for one cell. Glycine activated a chloride conductance increase at both the dendrites and the telodendria in 93 % of the bipolar cells studied. In some cells, weak responses were obtained at sites near the cell body or near axonal swellings, suggesting that glycinergic synapses might be present at these locations. But the vast majority of glycine receptors were clearly located on the dendrites and telodendria. Figure 1C gives the mean peak current responses, as a function of holding potential, for 10 cells subjected to both dendritic and telodendritic glycine application. The mean interpolated reversal potential was -48.4 ± 3.0 mV for the dendritic responses and -48.8 ± 1.8 mV for the telodendritic responses (here and elsewhere results are given as means ±s.d.). The dendritic responses were always outwardly rectifying and were usually larger than the telodendritic responses. The telodendritic responses, on average, showed less outward rectification and in some cases were inwardly rectifying. The variability of the current rectification suggests that bipolar cells possess at least two types of glycine-activated channels, each with different current-voltage relations. Detailed evidence for multiple glycine receptors on bipolar cells will be presented in a separate communication (B. R. Maple & S. M. Wu, in preparation). As illustrated by the open symbols in Fig. 1C, both the dendritic and telodendritic responses were completely abolished by 1 μM strychnine, an antagonist for glycine receptors that activate chloride channels (Olsen, 1981).
Figure 1. Voltage clamp responses of bipolar cells to iontophoretic application of glycine.
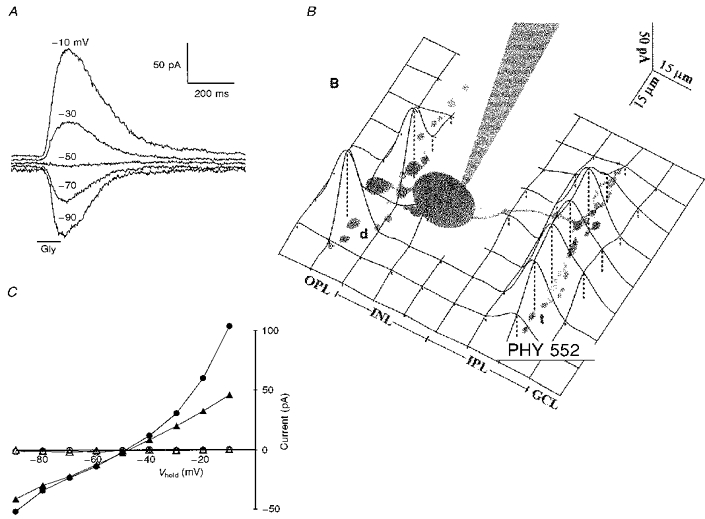
A, responses of a bipolar cell to application of glycine (Gly) at the axonal telodendria. B, a spatial profile for glycine sensitivity generated by applying glycine to many sites with the bipolar cell held at -10 mV. The height of the surface (indicated by dashed lines) gives the magnitude of the current response at each location (d, dendrites; t, telodendria; OPL, outer plexiform layer; IPL, inner plexiform layer; INL, inner nuclear layer; GCL, ganglion cell layer). C, the mean current response as a function of holding potential for dendritic application (○, •) and telodendritic application (▵, ▴) of glycine to 10 cells (5 on-centre and 5 off-centre). The filled symbols indicate responses obtained in Co2+ Ringer solution. The open symbols indicate responses for the same cells in Co2+ Ringer solution plus 1 μM strychnine. (For 5 cells glycine was applied first at the dendrites, then at the telodendria, then again at the same telodendritic site after strychnine had been applied. For the other five cells the order was reversed and strychnine was applied while the iontophoretic pipette was positioned at the dendrites.) The chloride equilibrium potential was set to -50 mV in these experiments.
Estimation of the chloride equilibrium potential
The normal physiological ECl for bipolar cells was estimated by measuring the reversal potential for the dendritic glycine response immediately after establishing a whole cell recording configuration. Figure 2 compares the responses of an on-centre bipolar cell at the start of recording (Fig. 2A) with those obtained from the same cell after 10 min of intracellular perfusion, when the response reversed close to the calculated ECl of -40 mV (Fig. 2B). Similar data for fifteen cells is summarized in Fig. 2C, which gives the mean reversal potential as a function of time for three different pipette chloride concentrations. While the steady-state reversal potentials (obtained after the cells were well perfused internally) were close to ECl, the initial reversal potential measured around -55 mV for both on-centre and off-centre bipolar cells, largely independent of the chloride concentration in the pipette. Similar results have been obtained for the application of GABA to bipolar cell telodendria (B. R. Maple & S. M. Wu, unpublished observations). The reversal potentials measured at early times, before the cells were well internally perfused, were more positive than the true value for ECl at these times, due to a liquid junction potential between the pipette solution and the cytoplasm. The exact value for this junction potential was unknown, but it caused the actual intracellular potential to be as much as 10 mV negative to the command potential (Marty & Neher, 1983). Although we could not establish a precise value for the native ECl, it is clear that it was negative to -50 mV. This is negative to the resting potential of bipolar cells in darkness (near -40 mV) and in a potential range at which bipolar cell synaptic calcium currents are not significantly activated (Maguire, Maple, Lukasiewicz & Werblin, 1989b). Therefore, we conclude that glycinergic inputs to bipolar cells are inhibitory.
Figure 2. Estimation of the normal physiological ECl for bipolar cells.
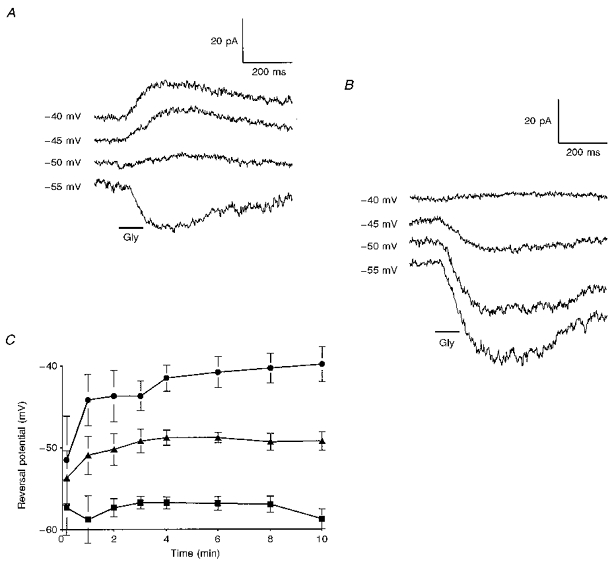
A, voltage clamp responses of a bipolar cell to pressure ejection of 100 μM glycine (Gly) at the OPL immediately after the voltage clamp was established. B, responses from the same cell after 10 min of internal perfusion. (ECl was set to -40 mV in this experiment). C, the mean reversal potential as a function of time (obtained by interpolation between the smallest positive and negative responses) for ECl= -40 mV (•), ECl= -50 mV (▴), and ECl= -60 mV (▪). Data from 8 on-centre cells, 3 off-centre cells, and 4 axotomized or multistratified cells.
Strychnine-sensitive IPSCs in bipolar cells
Some bipolar cells exhibited spontaneous inhibitory postsynaptic currents. These are illustrated in Fig. 3A, which shows light responses of a bipolar cell in a dark-adapted retinal slice. In darkness this cell displayed IPSCs that reversed near ECl (set to -40 mV in this experiment). The IPSCs were suppressed by a diffuse light stimulus, and then strongly elicited at light off. In the presence of 2 μM strychnine the discrete IPSCs were completely abolished (Fig. 3B), leaving an enhanced on-off inhibition that was not obviously composed of discrete events. These results suggest that the discrete IPSCs were generated by the activation of chloride channels at glycinergic synapses. Spontaneous IPSCs similar to those depicted in Fig. 3A were completely abolished by 1-2 μM strychnine in all six bipolar cells (5 on-centre and 1 off-centre) tested under dark-adapted conditions. The strychnine-resistant inhibition in Fig. 3B was not pharmacologically identified, but it may represent GABAergic input from amacrine cells to the bipolar cell telodendria (Lasansky, 1992; Lukasiewicz & Werblin, 1994; Maple & Wu, 1996). Perhaps this inhibition became enhanced because strychnine removed inhibition that GABAergic amacrine cells normally receive from glycinergic amacrine cells. Although there was little photoreceptor input evident in the light responses of Fig. 3, on the basis of axon morphology this 0.7 IU cell was identified as an on-centre bipolar cell. (It was not unusual for photoreceptor inputs to on-centre bipolar cells to be weak under the conditions of these experiments. Responses to application of glutamate at the dendrites also tended to be weak in these cells (Maple & Wu, 1996). Perhaps this reflects a lability of the second messenger system that mediates the effect of glutamate on these cells (Nawy & Jahr, 1990).) Since photoreceptors depolarize on-centre bipolar cells when illuminated, the light-induced suppression of the strychnine-sensitive IPSCs depicted in Fig. 3A was synergistic with the photoreceptor input this cell would normally receive.
Figure 3. The effect of light on the frequency of spontaneous strychnine-sensitive IPSCs.
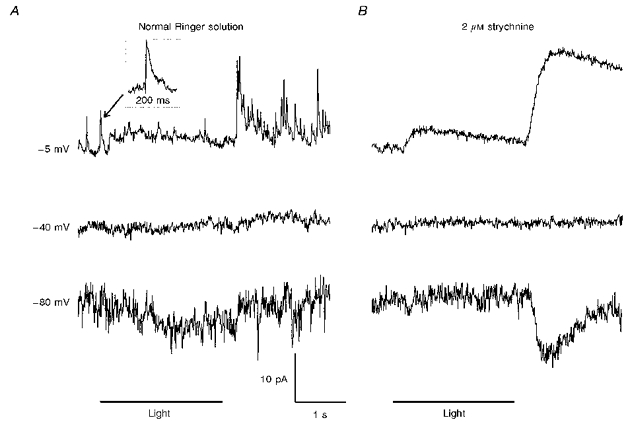
A, responses of an on-centre (0.7 IU) bipolar cell to diffuse illumination. Discrete IPSCs, suppressed by light and strongly elicited by light off, reversed near ECl (set to -40 mV in this experiment). B, 2 μM strychnine abolished the discrete IPSCs, leaving an enhanced on-off inhibition that was not obviously composed of discrete events.
In an attempt to dissect the circuitry associated with this putative glycinergic input, we used focal application of glutamate as a method of driving the IPSCs by depolarizing presynaptic glycinergic neurons. Figures 4 and 5A present examples of this for one on-centre bipolar cell. Responses to focal application of 100 μM glutamate at the IPL are illustrated in Fig. 4, where panel A shows synaptic currents elicited at different holding potentials and panel B shows how the responses were affected by a series of pharmacological treatments. The IPSCs reversed near ECl (-50 mV) and were reversibly abolished by 500 nM strychnine, again consistent with representing the glycinergic activation of a synaptic chloride conductance. Similar discrete IPSCs were completely abolished by 500 nM to 2 μM strychnine in all fourteen bipolar cells (10 on-centre and 4 off-centre) tested under these conditions. Looking more closely at the voltage dependence of the response, note that in Fig. 4A the response was larger at -10 mV than at -90 mV, although the magnitude of the driving force for chloride current flow was the same at these two potentials. The extent of this outward rectification varied among different cells. It was qualitatively consistent with the outward rectification of the glycine responses shown in Fig. 1, and it may have resulted from voltage-dependent rectification inherent to the glycine-activated channels. It may also partially reflect effects of the voltage clamp on transmitter release from presynaptic glycinergic neurons however, as evidenced by the fact that the frequency of spontaneous IPSCs was sometimes affected by holding potential. For the cell of Fig. 4 the rate of spontaneous IPSCs was greater at -90 mV than at more positive potentials. In some other bipolar cells (both on-centre and off-centre) depolarization was observed to increase IPSC frequency (Maple & Werblin, 1986). These feedback effects were not characterized in detail, because they were seen only occasionally and disappeared rapidly as the bipolar cells were internally perfused. A depolarization-induced increase of IPSC frequency can be easily explained, since depolarization of a bipolar cell might excite amacrine cells that feed back onto the same bipolar cell. A hyperpolarization-induced increase of IPSC frequency as shown in Fig. 4A must involve a more complicated circuit, but it might arise if, for example, hyperpolarization of a bipolar cell removes tonic excitation of GABAergic amacrine cells that inhibit glycinergic neurons synapsing on the same bipolar cell.
Figure 5. Characterization of dendritic glycinergic IPSCs.
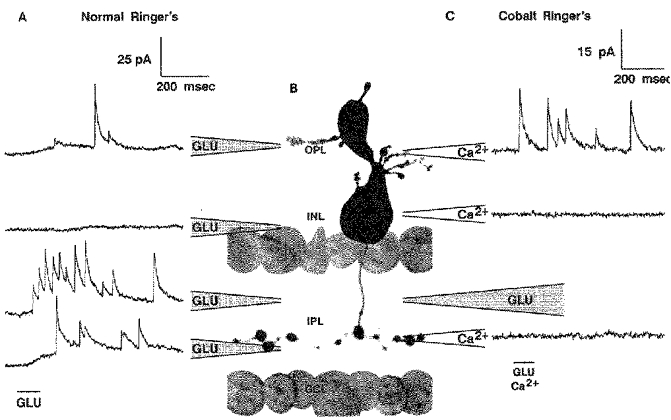
A, a spatial profile for the glutamate (GLU) response of the cell of Fig. 4. The IPSCs were best elicited by application of glutamate at the distal IPL, although a weaker response was also isolated to the OPL. B, a photograph of another bipolar cell with morphology and glutamate response profile similar to that of the one in A. C, responses of the cell depicted in B to application of glutamate at the distal IPL. In this case the cell was bathed in Co2+ Ringer solution and Ca2+ Ringer solution was simultaneously applied focally to different regions of the slice. IPSCs were elicited only when calcium was applied at the OPL. The holding potential was -10 mV for all traces.
Two additional pharmacological observations are illustrated in Fig. 4B. First, the IPSC response was reversibly abolished by substitution of cobalt for calcium in the bathing medium. Spontaneous strychnine-sensitive IPSCs, prominent in some bipolar cells, were also completely abolished in Co2+ Ringer solution (not shown). These findings are consistent with the idea that the IPSCs represent Ca2+-dependent synaptic transmission from presynaptic neurons that were depolarized by glutamate. Secondly, in this cell the IPSC response was reversibly abolished by 10 μM tetrodotoxin, which blocks sodium channel activation and has been shown to block both somatic and dendritic spikes in retinal amacrine cells (Miller, 1979). This suggests that presynaptic sodium-dependent spikes may be required for generation of the IPSCs. (In some bipolar cells the glutamate-elicited IPSCs were not abolished by tetrodotoxin. This will be addressed later in the results.) Taken together, the results of Fig. 4 suggest that glutamate depolarized a spiking, glycinergic neuron that synapsed on this bipolar cell and generated chloride-mediated IPSCs.
A spatial profile for the glutamate response of this same cell is shown in Fig. 5A. Here the cell was held at -10 mV, near the reversal potential for currents generated by glutamate receptors on the bipolar cell dendrites. Weak responses were obtained when glutamate was applied at the outer plexiform layer, but much larger responses were elicited by application of glutamate to the IPL. Furthermore, the glycinergic IPSCs were elicited most strongly, and with the shortest latency, when glutamate was applied to the distal IPL (sublamina A), where the off-centre bipolar cells make their output synapses. Application of glutamate at the proximal IPL (sublamina B) near the telodendria of this cell elicited far fewer glycinergic IPSCs, with a longer latency, riding on top of a more graded inhibition that was not obviously composed of discrete IPSCs.
Identification of dendritic IPSCs
At the IPL, glutamate depolarizes amacrine cells (Maguire, Lukasiewicz & Werblin, 1989a) and interplexiform cells (Maguire et al. 1990), both of which are spiking neurons that may release glycine. Given this and our finding that the glycine receptors of bipolar cells are principally located on the dendrites and telodendria, the strychnine-sensitive IPSCs elicited by IPL glutamate application most likely represent glycinergic synaptic inputs from amacrine cells to the bipolar cell telodendria and/or from interplexiform cells to the bipolar cell dendrites. In order to determine whether the IPSCs were of dendritic or telodendritic origin, we utilized the Ca2+ dependence of synaptic transmission at these synapses. The experiment of Fig. 5A was repeated in a Co2+ bath, but this time with simultaneous focal application of Ca2+ Ringer solution at various locations in order to locally enable synaptic transmission. Figure 5C shows particularly significant records from one such experiment, which involved the same type of cell as described in Figs 4 and 5A. For this cell glutamate puffs at the distal IPL elicited IPSCs when calcium was applied near the dendrites, but not when calcium was applied near the telodendria. This result was consistent with the presence of Ca2+-dependent glycinergic synapses at the bipolar cell dendrites. Calcium sensitivity was definitely restricted to the OPL, as no responses were obtained when calcium was applied at the mid INL, contrary to what would be expected if the IPSCs occurred at amacrine cell synapses on the bipolar cell body. In these experiments we could never rule out the existence of synapses at any site, because even when the calcium pipette was positioned close to a glycinergic synapse there was no guarantee that the associated glycinergic neuron would have glutamate receptors near the surface of the slice, where they would be accessible to the glutamate pipette. But it is clear that IPSCs were generated at the dendrites of this bipolar cell. As for the cell of Figs 4 and 5A, the dendritic IPSCs in this cell were reversibly abolished by 500 nM strychnine and best elicited by glutamate puffs at the distal IPL (not shown). (Due to geometric constraints associated with the simultaneous manipulation of three micropipettes, the effect of glutamate at the OPL was not routinely examined. However, the cell of Fig. 5B, with identified dendritic IPSCs, was so tested, and the IPSCs were not elicited by OPL glutamate application under these conditions (not shown).) Dendritic IPSCs were observed in only 16 % of the forty-four cells studied in this manner. These synaptic inputs were identified in both on- and off-centre bipolar cells, but were most prominent in centrally ramifying on-centre bipolar cells (0.6-0.8 IU), where they were observed in 42 % of the twelve cells studied. In all these cells they were best, or exclusively, elicited by distal IPL stimulation. These results are summarized in Table 1. In all three bipolar cells tested (2 on-centre and 1 off-centre) the dendritic IPSCs were completely abolished by 500 nM strychnine.
Table 1.
Summary of the data for dendritic IPSCs
| Region of IPL where glutamate was an effective stimulus (number of cells) | |||||
|---|---|---|---|---|---|
| Bipolar cell type | Number of cells tested | Number of cells exhibiting dendritic IPSCs | Distal IPL | Proximal IPL | Both distal and proximal IPL |
| Off-centre | 16 | 2 | 1 | 0 | 1 |
| Centrally ramifying on-centre (0.56–0.80 IU) | 12 | 5 | 5 | 0 | 0 |
| Proximally ramifying on-centre (0.81–1.0 IU) | 10 | 0 | — | — | — |
| Cells ramifying in both on and off sublaminae | 4 | 0 | — | — | — |
| Axotomized cells | 2 | 0 | — | — | — |
These data strongly suggest that some centrally ramifying on-centre bipolar cells receive a dendritic glycinergic input from an interplexiform cell that is excited by off-centre bipolar cells in sublamina A of the IPL. This is also consistent with the light-induced suppression of the strychnine-sensitive IPSCs in Fig. 3, since off-centre bipolar cells are hyperpolarized by light. It is also consistent with the IPSC activity elicited by glutamate application at the OPL in Fig. 5A, since glutamate activates excitatory conductances at the dendrites of off-centre bipolar cells, and this would be expected to indirectly excite the interplexiform cells. Finally, it is not inconsistent with the effect of holding potential on IPSC frequency shown in Fig. 4A. Hyperpolarization of on-centre bipolar cells might cause hyperpolarization of postsynaptic amacrine cells involved in cross-laminar inhibition of the glycinergic interplexiform cells.
Characterization of telodendritic IPSCs
In 69 % of forty-two bipolar cells with intact axons it was possible to identify telodendritic IPSCs. Figure 6 illustrates this for another centrally ramifying on-centre cell. In this case no dendritic IPSCs were observed (Fig. 6A), but telodendritic IPSCs were elicited by proximal, but not by distal IPL glutamate application (Fig. 6B). (The inward current response in Fig. 6B is a Ca2+ current, often observed when calcium was applied very close to the telodendria. This will be described in a separate communication (B. R. Maple & S. M. Wu, in preparation)). The telodendritic IPSCs were also reversibly abolished by 500 nM strychnine (Fig. 6C). Most likely, this cell received input from glycinergic amacrine cells that were postsynaptic to on-centre bipolar cells in sublamina B of the IPL.
Figure 6. Characterization of telodendritic glycinergic IPSCs.
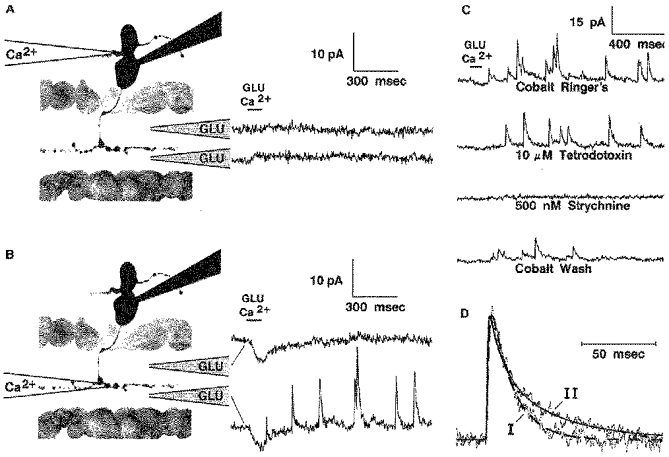
Responses are shown for another on-centre bipolar cell stimulated by simultaneous focal application of glutamate (Glu) and calcium in a Co2+ Ringer solution bath. In this case no IPSCs were elicited when calcium was applied to the dendrites (A). IPSCs were observed when calcium was applied to the telodendria, but only when glutamate was also applied at the proximal IPL (B). The telodendritic IPSCs were relatively unaffected by 10 μM TTX, but were reversibly abolished by 500 nM strychnine (C). In D the time course of a telodendritic IPSC from this cell (I) is compared with that of a dendritic IPSC (II) from the cell of Fig. 5B. The broken line gives the best fit of the telodendritic IPSC by eqn (1) with τ1= 1.2 ms and τ2= 15.1 ms. The continuous line gives the best fit of the dendritic IPSC to eqn (2) with τ1= 0.39; τ2= 9.4 and τ3= 40.5 ms; C2= 0.54. The amplitudes of the IPSCs were normalized for purposes of comparison.
Table 2 summarizes the data obtained for telodendritic IPSCs in all the cells studied. In 52 % of the twenty-seven monostratified bipolar cells with identified telodendritic IPSCs, these inputs were best, or exclusively, elicited by glutamate stimuli at the sublamina that the telodendria ramified within. This was the case for all but one of the seven centrally ramifying (0.6-0.8 IU) on-centre bipolar cells studied. In this class of on-centre bipolar cell the telodendritic IPSCs were probably generated mostly by on amacrine cells. In another 41 % of the cells with identified telodendritic IPSCs, glutamate sensitivity was distributed fairly uniformly over both sublamina A and sublamina B. The IPSCs observed in this population of cells were probably generated by glycinergic amacrine cells that were postsynaptic to both on-centre and off-centre bipolar cells. Only in 7 % of the cells were telodendritic responses elicited best by glutamate stimuli at the sublamina that did not contain the bipolar cell telodendria. Glutamate was applied at the dendrites of two bipolar cells with identified telodendritic IPSCs (1 on-centre and 1 off-centre), but no responses were obtained for dendritic glutamate application. In all fifteen bipolar cells tested (8 on-centre, 5 off-centre, and two that stratified in both the on and off sublaminae) the telodendritic IPSCs were completely abolished by 500 nM strychnine.
Table 2.
Summary of the data for telodendritic IPSCs
| Region of IPL where glutamate was an effective stimulus (number of cells) | |||||
|---|---|---|---|---|---|
| Bipolar cell type | Number of cells tested | Number of cells exhibiting telo-dendritic IPSCs | Distal IPL | Proximal IPL | Both distal and proximal IPL |
| Off-centre | 16 | 13 | 6 | 1 | 6 |
| Centrally ramifying on-centre (0.56–0.80 IU) | 12 | 7 | 0 | 6 | 1 |
| Proximally ramifyingon-centre (0.81–1.0 IU) | 10 | 7 | 1 | 2 | 4 |
| Cells ramifying in both on and off sublaminae | 4 | 2 | 0 | 0 | 2 |
Effects of tetrodotoxin on the strychnine-sensitive IPSCs
Tetrodotoxin slightly decreased the number of telodendritic IPSCs elicited by IPL glutamate application, as illustrated in Fig. 6C, but in all ten cells tested the telodendritic response persisted strongly in 10 μM TTX. This is in marked contrast to the large effect of TTX shown on the unidentified, but presumed dendritic IPSCs in Fig. 4B. TTX had a more variable effect on the dendritic inputs. In two out of three tested bipolar cells with identified dendritic IPSCs (1 on-centre and 1 off-centre), the IPSCs were totally abolished in 10 μM TTX, yet in another on-centre cell they persisted strongly. Evidently both dendritic and telodendritic glycinergic IPSCs can be generated in the absence of presynaptic sodium spikes.
Kinetics of the strychnine-sensitive IPSCs
Figure 6D illustrates the fitting of strychnine-sensitive IPSCs with a difference of exponentials and compares the time course (I) of a typical telodendritic IPSC from the cell in Fig. 6B with the time course (II) of a typical dendritic IPSC from the cell in 5B. Most of the telodendritic IPSCs exhibited type I kinetics, with a decay well fitted by a single exponential (eqn (1)). The mean time constants for the strychnine-sensitive telodendritic IPSCs obtained from fifteen cells were: τ1= 1.7 ± 1.5 and τ2= 17.7 ± 8.7 ms (mean of 159 IPSCs with amplitude 10.0 ± 6.2 pA). Only 5 % of these IPSCs were significantly better fitted by eqn (2), with two exponentials of decay, and these represented a small minority of the IPSCs in each cell where they were observed. In four bipolar cells (2 on-centre and 2 off-centre) the dendritic IPSCs were also well fitted by eqn (1) and displayed similar kinetics: τ1= 1.0 ± 0.60 and τ2= 15.8 ± 7.4 ms (26 IPSCs with mean amplitude 9.7 ± 5.2 pA). For three morphologically similar (0.7 IU) on-centre bipolar cells, however, the dendritic IPSCs exhibited type II kinetics, and these were fitted much better by eqn (2), with two exponentials of decay. These IPSCs were best fitted with τ1= 0.43 ± 0.31; τ2= 9.9 ± 4.3 and τ3= 51.3 ± 24.3 ms; C2= 0.44 ± 0.15 (29 IPSCs with mean amplitude 21.7 ± 7.9 pA). In summary, there appears to be no general way to distinguish dendritic IPSCs from telodendritic IPSCs based solely on their kinetics. Kinetics may, on the other hand, distinguish dendritic IPSCs from telodendritic IPSCs in specific types of bipolar cells.
It is worth noting that the type II IPSCs, with the slower late component of decay, also had the fastest rise time, as indicated by the relatively small mean value for τ1. While electrical filtering could conceivably account for the relatively long rise time of the telodendritic IPSCs, it certainly cannot account for the slower late phase present in the decay of type II dendritic IPSCs. The difference in the decay of type I and type II IPSCs probably results from differences in the kinetics of postsynaptic glycine receptors and/or presynaptic differences affecting the time course of transmitter release.
Under all the conditions we have examined, IPSCs with qualitatively similar kinetics, both spontaneous and stimulus-induced, were completely abolished by strychnine at concentrations of 500 nM or greater (38 cells). Also at concentrations of 100-200 nM, strychnine qualitatively appeared to decrease the amplitude of these IPSCs (6 cells). In two cells where the latter effect was quantified the mean amplitude of spontaneous IPSCs was decreased by 51 ± 3 % in the presence of 200 nM strychnine.
DISCUSSION
These experiments have provided strong evidence that bipolar cells receive glycinergic inputs both at dendritic synapses in the OPL and at telodendritic synapses in the IPL. Based on dual stimulus experiments, in which the sites of glycinergic synapses were correlated with the sites of glutamate receptors on the corresponding presynaptic glycinergic neurons, we propose the scheme depicted in Fig. 7. Glycinergic interplexiform cells, which are excited by off-centre bipolar cells in sublamina A, inhibit bipolar cells at dendritic synapses in the OPL. Glycinergic amacrine cells, which are excited by off-centre bipolar cells in sublamina A and/or on-centre bipolar cells in sublamina B, feed back onto and inhibit these same bipolar cells (or other bipolar cells ramifying at the same level(s)). For the on-centre bipolar cell depicted in Fig. 7, illumination of the receptive field centre would cause a decrease of inhibitory input from glycinergic interplexiform cells and increased inhibition by glycinergic on amacrine cells. Since illumination causes a depolarizing effect at photoreceptor synapses on on-centre bipolar cells, the interplexiform cell input is synergistic with the photoreceptor input, while the amacrine cell input is antagonistic.
Figure 7. A model for the circuitry of glycinergic inputs to centrally-ramifying on-centre bipolar cells.
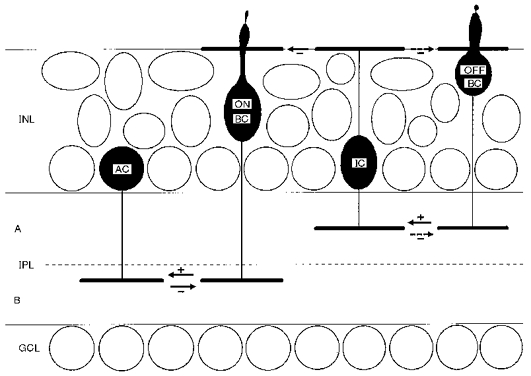
The border between sublaminae A and B is indicated by the dashed line. Excitatory synapses are indicated by arrows with a (+) sign. Inhibitory (chloride-mediated) synapses are indicated by arrows with (-) signs. AC, amacrine cell; BC, bipolar cell; GCL, ganglion cell layer; IC, interplexiform cell; INL, inner nuclear layer; IPL, inner plexiform layer.
We have chosen a centrally ramifying on-centre bipolar cell as the central element in Fig. 7, because these cells displayed the strongest glycinergic interplexiform cell input and the most consistent glutamate response profiles in the dual stimulus experiments. As depicted for the on-centre cell in Fig. 7, most bipolar cells exhibited antagonistic glycinergic amacrine cell inputs, but different glutamate response profiles were sometimes observed in other bipolar cell types. A large fraction of off-centre bipolar cells and proximally ramifying on-centre bipolar cells apparently receive inhibition from glycinergic amacrine cells that are excited by bipolar cells in both sublamina A and sublamina B. These bipolar cells most likely receive on-off glycinergic inhibition. For the sake of simplicity, the amacrine cell inputs to off-centre bipolar cells and proximally-ramifying on-centre bipolar cells have not been illustrated in Fig. 7. Although we have some evidence for dendritic glycinergic inputs to off-centre bipolar cells, we do not know specifically which off-centre bipolar cells excite the glycinergic interplexiform cells. For this reason the interplexiform cell input to the off-centre bipolar cell dendrites in Fig. 7 has been marked more tenuously by an arrow with a dashed line. It is possible that some of the telodendritic IPSCs observed in off-centre bipolar cells were generated by glycinergic interplexiform cells, and this has also been indicated by an arrow with a dashed line.
These conclusions are based on the assumption that the glutamate responses observed involved principally monosynaptic connections to bipolar cells and not more complex polysynaptic circuits. This is likely to be the case, particularly for the experiments performed in Co2+ media, where the transmission elicited was very weak and very localized. In fact, in the dual stimulus experiments in Co2+ Ringer solution, strychnine-resistant transmission appeared to be particularly strongly suppressed. In normal Ca2+-containing media, responses to glutamate application at the IPL tended to be dominated by a large chloride current that was not composed of the glycinergic IPSCs described in this paper. This is only mildly apparent in the bottom trace of Fig. 5A, but more typical large responses without discrete IPSCs have been shown previously (Maple & Wu, 1996). In contrast, glycinergic IPSCs were the dominant form of synaptic transmission observed in the dual stimulus experiments performed in Co2+ Ringer solution. Under these conditions very little other inhibition was observed.
It should be emphasized that the discrete IPSCs described in this paper were always abolished by low concentrations of strychnine, and we have taken this as evidence that they were always glycinergic. In other retinal neurons GABAergic IPSCs with similar kinetics have been described (Gao, Yang & Wu, 1997), and since GABA receptors are present on the telodendria of retinal bipolar cells, this raises the question of why similar strychnine-resistant IPSCs were not observed in bipolar cells. The answer may lie in the fact that the bipolar cell receptors are bicuculline-resistant GABAC receptors (Lukasiewicz, Maple & Werblin, 1994). The miniature synaptic currents associated with these receptors might be too small and slow to be readily observed as discrete events. The graded strychnine-resistant inhibition shown in Fig. 3B may well be composed of such GABAergic minature currents. Based on our experiments with strychnine, it appears that glycinergic inputs to salamander bipolar cells can be distinguished from other inhibitory inputs based solely on the size and kinetics of the IPSCs associated with glycinergic synaptic transmission.
It should be noted that the peak conductance associated with the glycinergic IPSCs is similar to that of miniature excitatory synaptic currents generated at bipolar cell dendrites (Maple et al. 1994). In the case of the excitatory synaptic currents it has been argued that they are miniature currents, based on the observation that they can be observed at a low rate in a Co2+ bath, where transmitter release should be uncoupled from presynaptic voltage fluctuations. This claim cannot be made for the glycinergic IPSCs, since given prolonged superfusion with Co2+ Ringer solution the rate of spontaneous glycinergic IPSCs went essentially to zero. We have shown here, however, that glycinergic IPSCs can still be observed when presynaptic sodium channels are blocked by tetrodotoxin. In fact TTX had no obvious effect on the amplitude of the IPSCs observed in these experiments. While we cannot rule out the possibility that presynaptic spikes are generated by some mechanism not involving TTX-sensitive sodium channels, it appears likely that the glycinergic IPSCs represent the basic unit of transmitter release at these synapses and that discrete presynaptic voltage excursions are not required for the generation of these events. At the same time it should be noted that TTX had a powerful blocking effect on interplexiform cell inputs to some bipolar cells. This suggests that sodium spikes may play an important role in the transmission of interplexiform cell signals to the outer plexiform layer.
Finally, several points should be mentioned concerning the role of glycinergic inputs in the generation of bipolar cell receptive fields. Since inputs from glycinergic amacrine cells apparently tend to be antagonistic to the photoreceptor inputs, it is possible that they contribute to the surround antagonism observed in bipolar cells. This might particularly be the case for antagonism generated by surround transients, since amacrine cells are thought to mediate such antagonism at the inner plexiform layer (Werblin & Copenhagen, 1974). At the same time, our finding that the inputs from glycinergic interplexiform cells are synergistic with the photoreceptor inputs to on-centre bipolar cells suggests that glycinergic interplexiform cells are probably not involved in the generation of surround antagonism, at least for on-centre bipolar cells. More likely these interplexiform cells are involved in the local processing of visual information. It should be emphasized that we have observed the strongest interplexiform cell inputs in the centrally ramifying on-centre bipolar cells, which are thought to be cone-dominated cells (S. M. Wu & B. R. Maple, unpublished observations). In these cells central illumination evidently elicits a substantial interplexiform cell-mediated chloride-conductance decrease at the dendrites, in addition to the cationic-conductance increase generated by photoreceptor synapses. The difference between the conductance mechanisms used by these two synapses may hold some significance for the light sensitivity of these bipolar cells. In the absence of an interplexiform cell input, the current response to a small conductance change at photoreceptor synapses should become smaller in bright light, because both the cell input resistance and driving force for synaptic current will decrease with illumination. The interplexiform cell synapses, on the other hand, should behave in the opposite manner: the driving force for the synaptic chloride current should increase with illumination, while the chloride conductance decreases. This interplexiform cell input might increase the light sensitivity of a cone-dominated on-centre bipolar cell in bright light, relative to what it would be in the absence of this input. Of course, how the glycinergic interplexiform cells release transmitter under different conditions is probably of greater relevance to how they may modulate bipolar cell light sensitivity. A better understanding of the role of glycinergic transmission in the generation of bipolar cell receptive fields will require further study of the light responses of bipolar cells under voltage clamp. These studies should be facilitated by our finding that glycinergic inputs to salamander bipolar cells can be discerned purely on the basis of the kinetics of the associated IPSCs, without the necessity of employing pharmacological means.
Acknowledgments
This work was supported by grants from NIH EY 04446, the Retina Research Foundation (Houston), and the Research to Prevent Blindness, Inc.
References
- Attwell D, Mobbs P, Tessier-Lavigne M, Wilson M. Neurotransmitter-induced currents in retinal bipolar cells of the axolotl, Ambystoma mexicanum. The Journal of Physiology. 1987;387:125–161. doi: 10.1113/jphysiol.1987.sp016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DB, Copenhagen DR. Two types of glutamate receptors differentially excite amacrine cells in the tiger salamander retina. The Journal of Physiology. 1992;449:589–606. doi: 10.1113/jphysiol.1992.sp019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kaneko A, Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977;198:1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Gao F, Yang J-H, Wu SM. Miniature inhibitory postsynaptic currents in retinal ganglion cells. Investigative Ophthalmology and Visual Science. 1997;38:S51. abstract 242. [Google Scholar]
- Hare WA, Owen WG. Effects of 2-amino-4-phosphonobutyric acid in the distal layers of the tiger salamander's retina. Journal of Neurophysiology. 1992;445:741–757. doi: 10.1113/jphysiol.1992.sp018948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Properties of depolarizing bipolar cell responses to central illumination in salamander retinal slices. Brain Research. 1992;576:181–196. doi: 10.1016/0006-8993(92)90679-4. 10.1016/0006-8993(92)90679-4. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Maple BR, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. Journal of Neuroscience. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. Journal of Neuroscience. 1994;14:1213–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire GW, Lukasiewicz PD, Werblin FS. Amacrine cell interactions underlying the response to change in the tiger salamander retina. Journal of Neuroscience. 1989a;9:726–735. doi: 10.1523/JNEUROSCI.09-02-00726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G, Lukasiewicz P, Werblin F. Synaptic and voltage-gated currents in interplexiform cells of the tiger salamander retina. Journal of General Physiology. 1990;95:755–770. doi: 10.1085/jgp.95.4.755. 10.1085/jgp.95.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G, Maple B, Lukasiewicz P, Werblin F. γ-Aminobutyric acid type B receptor modulation of L-type calcium current at bipolar cell terminals in the retina of the tiger salamander. Proceedings of the National Academy of Sciences of the USA. 1989b;86:10 144–10 147. doi: 10.1073/pnas.86.24.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple B, Werblin F. Inhibitory feedback to bipolar cells in the tiger salamander retina. Society for Neuroscience Abstracts. 1986;12:634,, 175,, 175.6. [Google Scholar]
- Maple BR, Werblin FS, Wu SM. Miniature excitatory postsynaptic currents in bipolar cells of the tiger salamander retina (letter) Vision Research. 1994;34:2357–2362. doi: 10.1016/0042-6989(94)90281-x. 10.1016/0042-6989(94)90281-X. [DOI] [PubMed] [Google Scholar]
- Maple BR, Wu SM. Synaptic inputs mediating bipolar cell responses in the tiger salamander retina. Vision Research. 1996;36:4015–4023. doi: 10.1016/s0042-6989(96)00126-5. 10.1016/S0042-6989(96)00126-5. [DOI] [PubMed] [Google Scholar]
- Marc RE. Structural organization of GABAergic circuitry in ectotherm retinas. (Review) Progress in Brain Research. 1992;90:61–92. doi: 10.1016/s0079-6123(08)63609-2. [DOI] [PubMed] [Google Scholar]
- Marc RE, Murray RF, Basinger SF. Pattern recognition of amino acid signatures in retinal neurons. Journal of Neuroscience. 1995;15:5106–5129. doi: 10.1523/JNEUROSCI.15-07-05106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Neher E. Tight-seal whole cell recording. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1983. pp. 107–122. [Google Scholar]
- Miller RF. The neuronal basis of ganglion cell receptive field organization and the physiology of amacrine cells. In: Schmidt FO, Worden FG, editors. The Neuroscience Fourth Study Program. Cambridge, MA, USA: MIT Press; 1979. pp. 227–245. [Google Scholar]
- Mittman S, Taylor WR, Copenhagen DR. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. The Journal of Physiology. 1990;428:175–197. doi: 10.1113/jphysiol.1990.sp018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. Journal of Neurophysiology. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Olsen RW. GABA-benzodiazepine-barbiturate receptor interactions. Journal of Neurochemistry. 1981;37:1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes: the Art of Scientific Computing. Cambridge, UK: Cambridge University Press; 1989. pp. 523–528. [Google Scholar]
- Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science. 1983;219:1230–1232. doi: 10.1126/science.6131536. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Yazulla S. Glycinergic contacts in the outer plexiform layer of the Xenopus laevis retina characterized by antibodies to glycine, GABA and glycine receptors. Journal of Comparative Neurology. 1990;299:375–388. doi: 10.1002/cne.902990309. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Transmission along and between rods in the tiger salamander retina. The Journal of Physiology. 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Copenhagen DR. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. Journal of General Physiology. 1974;63:88–110. doi: 10.1085/jgp.63.1.88. 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. Journal of Neurophysiology. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M T. T. Synaptic organization of the inner plexiform layer in the retina of the tiger salamander. Journal of Neurocytology. 1974;3:1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]
- Wu SM. Signal transmission and adaptation-induced modulation of photoreceptor synapses in the retina. Progress in Retinal Research. 1991;10:27–44. 10.1016/0278-4327(91)90007-O. [Google Scholar]
- Wu SM. Feedback connections and operation of the outer plexiform layer of the retina. (Review) Current Opinion in Neurobiology. 1992;2:462–468. doi: 10.1016/0959-4388(92)90181-j. 10.1016/0959-4388(92)90181-J. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Feedforward lateral inhibition in retinal bipolar cells: input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proceedings of the National Academy of Sciences of the USA. 1991;88:3310–3313. doi: 10.1073/pnas.88.8.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]


