Abstract
Electrophysiological (whole-cell clamp) techniques were used to study the effect of NO synthase (NOS) inhibitors on guinea-pig ventricular calcium current (ICa), and biochemical measurements (Western blot and citrulline synthesis) were made to investigate the possible mechanisms of action.
The two NOS inhibitors, NG-monomethyl-L-arginine (L-NMMA, 1 mM) and NG-nitro-L-arginine (L-NNA, 1 mM), induced a rapid increase in ICa when applied to the external solution. D-NMMA (1 mM), the stereoisomer of L-NMMA, which has no effect on NOS, did not enhance ICa.
Western blot experiments gave no indication of the presence of inducible NOS protein (iNOS) in our cell preparation, neither immediately after dissociation nor after more than 24 h. Statistically, there was no significant difference between electrophysiological experiments performed on freshly dissociated cells and experiments performed the next day. Moreover cells prepared and kept in the presence of dexamethasone (3 μM), to inhibit the expression of iNOS, gave the same response to L-NMMA as control cells.
The stimulatory effect of L-NMMA (1 mM) on basal ICa was reversed by competition with higher doses (5 mM) of externally applied L-arginine, the natural substrate of NOS. The effect of L-NMMA was also eliminated by L-arginine in the patch pipette solution.
Intracellular perfusion with GDPβS (0.5 mM), which stabilizes the G-proteins in the inactive state, did not affect the L-NMMA-induced stimulation of ICa.
Carbachol (1 μM) reduced the ICa previously stimulated by L-NMMA, and intracellular cGMP (10 μM) prevented L-NMMA enhancement.
Simultaneous treatment with L-NMMA and isoprenaline (1 μM) induced a non-cumulative enhancement of ICa that could not be reversed by carbachol (1 μM).
NO synthesis, measured by the formation of [3H]citrulline from L-[3H]arginine during a 15 min incubation, showed a relatively high basal NO production, which was inhibited by L-NMMA but not affected by carbachol.
These results suggest that inhibitors of NOS are able to modulate the basal ventricular ICa in the absence of a receptor-mediated pathway, and that NO might be required for the muscarinic reduction of ICa under isoprenaline stimulation, even if NO production is not directly controlled by the muscarinic pathway.
Since its discovery as a bioregulator (Nathan, 1992), nitric oxide (NO) has been shown to be produced by a variety of cell types and to be involved in many fundamental regulatory processes (Moncada, Palmer & Higgs, 1991; Lowenstein & Snyder 1992; Schmidt, Lohmann & Walter, 1993; Schmidt & Walter, 1994). Among its proposed roles are the endothelial regulation of smooth muscle (Palmer, Ferrige & Moncada, 1987), and a contribution to skeletal muscle relaxation (Kobzik, Reid, Bredt & Stamler, 1994).
Most of the work on the NO pathway has been performed by applying exogenous NO, by eliminating the messenger with haemoglobin as a scavenger, or by inhibiting its synthesis (Moncada et al. 1991). Arginine derivatives (Rees, Schulz, Hodson, Palmer & Moncada, 1990) which can inhibit the production of NO by competing with L-arginine or by blocking the NO synthase (NOS) enzymes (Marletta 1994; Nathan & Xie, 1994) have been widely used.
Several groups have also investigated the possible roles of NO in cardiac regulation (for a review see Kelly, Balligand & Smith, 1996), and both the inducible isoform (Balligand et al. 1994) and the endothelial isoform (Balligand, Kelly, Marsden, Smith & Michel, 1993) of NOS have been detected. NO can contribute to the regulation of L-type calcium current (ICa) of cardiomyocytes by at least one mechanism, the regulation of cGMP production by guanylyl cyclase (Méry, Pavoine, Belhassen, Pecker & Fischmeister, 1993; Levi, Alloatti, Penna & Gallo, 1994; Wahler & Dollinger, 1995).
The complex effects of cGMP on the calcium current have been attributed either to mediation by phosphodiesterase regulation or to cGMP-dependent protein kinase activation (Fischmeister & Hartzell, 1987; Levi, Alloatti & Fischmeister, 1989; Ono & Trautwein, 1991; Méry, Lohmann, Walter & Fischmeister, 1991; Lohman, Fischmeister & Walter, 1991; Mubagwa, Shirayama, Moreau & Pappano, 1993), depending, among other factors, on the species studied.
A cGMP contribution to calcium current regulation by muscarinic receptors has also been proposed, and thus a role for NO has been suggested in this pathway (Balligand et al. 1993; Han, Shimoni & Giles, 1994; Wang & Lipsius, 1995), probably mediated by phosphodiesterase regulation (Mubagwa et al. 1993; Han, Shimoni & Giles, 1995). It has been suggested that in mammalian preparations NO could act as an obligatory mediator (Han, Shimoni & Giles, 1994, 1995; Balligand et al. 1995) in the muscarinic inhibition of ICa.
In the present report we show a surprising effect of two different NOS inhibitors (NG-methyl-L-arginine, L-NMMA and NG-nitro-L-arginine, L-NNA), namely a rapid enhancement of ICa in isolated ventricular cells of adult guinea-pig in the absence of induction of iNOS. This response is suppressed by L-arginine (both intra- and extracellular) and cannot be obtained with the stereoisomer NG-methyl-D-arginine (D-NMMA). Further, we show the absence of an effect of carbachol on the synthesis of NO, as measured by synthesis of labelled L-citrulline.
Our results suggest that the cross-talk between the nitric oxide pathway and the regulation of calcium current is still a complicated puzzle and that probably NO and NOS inhibitors can interfere with ICa in several ways.
METHODS
Isolation of ventricular cardiomyocytes
Cells from guinea-pig ventricles were enzymatically dispersed with methods based on those described by Gallo and colleagues (Gallo, Alloatti, Eva, Oberto & Levi, 1993). Young adult guinea-pigs (200-350 g) of either sex were killed by stunning and cervical dislocation after ether anaesthesia. The hearts were removed, washed in modified Tyrode solution (for composition of this and other solutions see Solutions) and cannulated via the aorta. All the following operations were carried out under a laminar flow hood. The heart was perfused with Tyrode solution at a constant rate of 10 ml min−1 with a peristaltic pump for approximately 10 min to wash away the blood and restore a regular beat. Afterwards the heart was perfused for 3 min with a low Ca2+-low Na+ solution then with 50 ml of the first enzymatic medium (collagenase and trypsin). The heart was detached from the cannula and the ventricles cut away and minced. The fragments were collected in the second enzymatic medium (pronase) and gently stirred with a plastic blade connected to an electric motor. The medium was collected and replaced every 10 min, this step was repeated up to nine times. After the second repetition the collected supernatant contained viable cells (more than 50 % rod-shaped cells) and was diluted in Tyrode solution supplemented with penicillin G (60 mg l−1) and streptomycin (100 mg l−1). Isolated cells were stored at room temperature (22-25°C) in the same Tyrode solution and electrophysiological experiments were performed within 48 h, while measurement of citrulline synthesis was performed within less than 8 h of dissociation. During this period the cells retained the typical brick shape and clear striations of freshly dissociated cardiomyocytes but were not beating spontaneously. Control experiments established that no change developed in the amplitude or shape of stimulated action potentials nor in the currents during the 48 h storage period. As described in Results, in some dissociations dexamethasone (3 μM) was added to all solutions from the initial perfusion solution to the Tyrode storage medium to inhibit expression of the inducible form of NO synthase (Kelly et al. 1996).
Solutions
The control Tyrode solution contained (mM): 154 NaCl; 4 KCl; 2 CaCl2; 1 MgCl2; 5.5 D-glucose; 5 Hepes; pH 7.35 adjusted with NaOH. The low Ca2+-low Na+ medium contained (mM): 33.6 NaCl; 22 D-glucose; 132 sucrose; 10 KCl; 1.1 KH2PO4; 5 MgSO4; 50 taurine; 10 Hepes; pH 7.3 adjusted with KOH. Caesium Tyrode solution contained (mM): 138 NaCl; 20 CsCl; 2 CaCl2; 1 MgCl2; 5.5 D-glucose; 5 Hepes; pH 7.35 adjusted with NaOH.
The first enzymatic solution comprised the low Ca2+-low Na+ solution with the following additions per 50 ml: 7-15 mg collagenase (Type V, ∼140 units ml−1, amount depending on enzyme activity), 10 mg trypsin (Type III) and 50 mg bovine serum albumin (fraction V; Boehringer Mannheim). The second enzymatic solution comprised the low Ca2+-low Na+ solution with the sole addition of 2.5 mg (per 50 ml) pronase (Boehringer).
The pipette solution used to dialyse the cells contained (mM): 133 CsCl; 5 EGTA free acid; 5 Na2ATP; 5 disodium phosphocreatine; 5 Hepes; 3 MgCl2; 0.4 Na2GTP; pH 7.3 adjusted with CsOH. In some experiments, caesium was replaced equimolarly with potassium. All drug-containing solutions were freshly prepared before the experiments. Where not specified all chemicals and drugs used in the experiments were purchased from Sigma.
Electrophysiological measurements
Voltage clamp of cardiomyocytes was performed using the conventional whole-cell patch-clamp procedure. All the experiments were performed at approximately 35°C under thermostatic control. To measure L-type Ca2+ current (ICa), the cell was depolarized every 5 s from -100 to -40 mV for 50 ms, to inactivate both fast Na+ current and T-type ICa, and then for 300 ms to different potentials in the range -90 to +50 mV.
The choice of using a brief prepulse to inactivate the Na+ current instead of keeping the cells throughout the experiments at -40 mV was adopted to reduce run-down of the Ca2+ current, which can be accelerated by prolonged depolarizations. In some cells the only potential tested was +10 mV, the potential at which in most cells ICa is maximal; in other cells ICa was measured repeatedly with stimulation of the cell through the full range of potentials (-100 to +50 mV) of the current-voltage relationship. The latter experiments were performed to establish that no significant voltage shifts in the current-voltage relationship were present after drug-induced modification of ICa. Warm (35°C) Tyrode or caesium Tyrode solution was constantly perfused into the bath with a peristaltic pump. Calcium current was measured as the difference between the peak of the inward current and the current at the end of the 300 ms pulse. The steady-state current at -100 mV and the fast Na+ current (INa) were monitored to control the stability of the preparation and the validity of the voltage clamp. No compensation or subtraction of cell capacitance was performed, while the series resistance of the cell cytoplasm and electrode was analogically compensated for by the built-in control of the patch-clamp amplifier (Bio-Logic, France). Series resistance compensation was performed according to the manufacturer's instructions, by adjusting the controls to a point close to the spontaneous oscillation of the current.
To exchange external solutions the pipette with the cell attached was moved into the tip of one of a series of six silicon plastic tubes (250 μM diameter) connected to gravity-fed reservoirs containing the test solutions. The experiments were conducted and analysed using AxoData 1, AxoGraph 3 (Axon Instruments, CA, USA) and KaleidaGraph (Synergy Software, PA, USA) software, on Apple Macintosh computers (Apple Computer, CA, USA).
Detection of iNOS protein by Western blot
Isolated cells were directly lysed in boiling Laemmli buffer (62.5 mM Tris-HCl pH 6.8, 10 % glycerol, 2 % SDS, 5 %β-mercaptoethanol) either immediately after cell dispersion from the hearts (time 0), or after 24 h. The protein content of cell lysate was assessed with the BCA kit from Pierce (Rockford, IL, USA). Aliquots equivalent to 30 μg protein for each of these experimental conditions were separated by SDS-PAGE (8 %). Thereafter proteins were transferred to nitrocellulose sheets and probed with a monoclonal antibody anti-murine macrophage iNOS (anti-iNOS, directed against a protein fragment of 183 amino acids, diluted 1 : 500; Transduction Laboratories, Lexington, KY, USA) and detected by enhanced chemiluminescence (Amersham, Bucks, UK). Lane 0 and lane 24 indicate lysate from cells lysed at time 0 and after 24 h, respectively, and lane C indicates positive control (lysate of murine macrophages previously treated with interferon-γ and lipopolysaccharide for 12 h, included in the detection kit). Electrophoresis reagents were from Bio-Rad Laboratories.
Measurement of citrulline synthesis as a marker of NO production
NOS transforms L-arginine into NO, a free radical with a half-life of few seconds, and the more stable L-citrulline, with a 1 : 1 stoichiometry; citrulline can be easily separated from arginine by ion exchange chromatography. Citrulline synthesis was measured by a radiometric method modified from a technique described previously (Ghigo et al. 1993). A cell suspension (150 ml) was centrifuged (5 min, 800 g) and resuspended at a high density in 400 μl of oxygenated Tyrode solution. Aliquots (100 μl, 0.27 ± 0.1 mg cell protein) were incubated at 37°C with 7.5 μCi L-[3H]arginine (L-[2,3,4,5-3H]arginine monohydrochloride, 62 Ci mmol−1; Amersham International, Bucks, UK) and 10 μM L-arginine for 15 min, together with L-NMMA and carbachol where used. The reaction was stopped by washing cells with cold phosphate-buffered saline (PBS: 150 mM NaCl, 10 mM Na2HPO4, pH 7.4) containing 5 mM L-arginine and 4 mM EDTA. After the supernatant had been removed, 0.5 ml ethanol was added to each monolayer and allowed to evaporate, then 2 ml of 20 mM Na-Hepes (adjusted to pH 6.0 with HCl) was added. After 5 min and an additional centrifugation the supernatant was collected and applied to 2 ml columns of Dowex AG50WX-8 (Na+ form) and eluted with 4 ml water. The radioactivity in 6 ml eluate corresponding to [3H]citrulline was measured by liquid scintillation counting. Citrulline synthesis was expressed as picomoles of citrulline per 15 min per milligram cell protein.
Statistical analysis
Results are presented as means ±s.e.m.; Student's t test or analysis of variance (as indicated) were used for statistical analysis. P values less than 0.05 were considered significant.
RESULTS
L-NMMA and L-NNA stimulation of ICa
The calcium current in guinea-pig ventricular cells was measured with the whole-cell patch-clamp technique. The first aim was to investigate the effect of the NOS inhibitor, L-NMMA, on the basal ICa of ventricular cells (Fig. 1). Our results show that L-NMMA induces a large increase in the calcium current, and the same stimulatory effect is obtained with L-NNA, another well-known inhibitor of NOS. L-NMMA was used at concentrations between 0.1 and 1 mM, and L-NNA at 1 mM; both drugs were applied externally to the cell a few minutes after the start of the experiments and the passage to whole-cell configuration.
Figure 1. L-NMMA and L-NNA stimulation of basal calcium current.
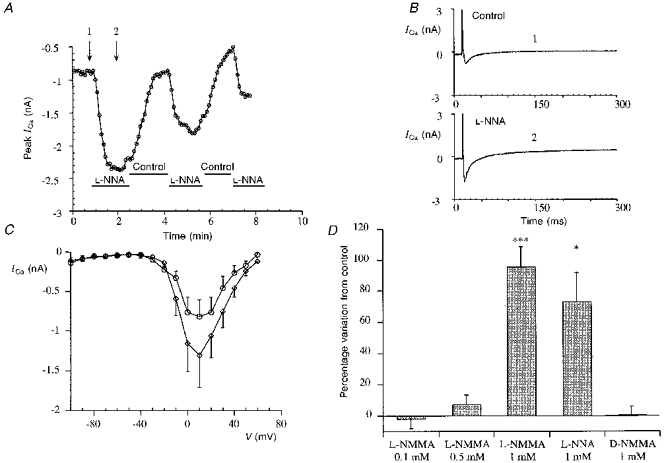
A, time course of the peak ICa (+10 mV, measured as difference between the initial peak and the end of the pulse) in a representative experiment with L-NNA (1 mM) on basal calcium current. The cell was stimulated every 5 s from -100 to -40 mV (prepulse) for 50 ms and then for 300 ms from -40 to +10 mV to record the peak of the current. Control indicates caesium Tyrode solution. Application of the drug was repeated to test the reversibility of the L-NNA effect. B, single traces of ICa recorded at the time indicated by the arrows in A, in control conditions (1) and during L-NNA application (2). C, average of the current-voltage relationships (error bars represent s.e.m.) between -80 and +60 mV in 4 typical experiments of L-NMMA stimulation of basal calcium current. In this experiment the cell was repeatedly stimulated every 5 s from -100 to -40 mV to inactivate both fast Na+ current and T-type calcium current and then with steps to the different potentials. The two I-V relationships were recorded under control conditions (○) and after L-NMMA (1 mM application; ⋄). D, bar graph comparing percentage increase (error bars represent s.e.m.) of the basal calcium current induced by L-NMMA (0.1 mM), L-NMMA (0.5 mM), L-NMMA (1 mM) (***P < 0.001 vs. control), L-NNA (1 mM) (*P < 0.05 vs. control) and D-NMMA (1 mM).
The effect of L-NNA (1 mM) on the time course of calcium current is presented in Fig. 1A, the current increases very rapidly and after removal of the drug returns to the basal level; repetitive applications of L-NNA are still effective though the response diminishes. Single tracings from the same experiment in control and during L-NNA stimulation show no marked changes in the time course of the current (Fig. 1B), with in most experiments a small reduction of the time to peak (data not shown).
Figure 1C presents the mean and s.e.m. of current-voltage (I-V) relationships (from -100 to +60 mV) of ICa from five typical experiments in control solution and with L-NMMA (1 mM) stimulation of calcium current. The increase of ICa is not accompanied by significant shifts in the voltage-dependence of the activation. In Fig. 1D a bar graph summarizes the effect on basal calcium current of L-NMMA (0.1, 0.5 and 1 mM); L-NNA (1 mM) and D-NMMA (1 mM). The percentage increases induced by 1 mM L-NMMA and 1 mM L-NNA are comparable (respectively 95.8 ± 12.2 %, n= 18, P < 0.001; 72.7 ± 19.3 %, n= 7, P < 0.05); L-NMMA at 0.1 and 0.5 mM produced very little effect on ICa (respectively -2.1 ± 5.8 %, n= 4; 7.4 ± 5.7 %, n= 4); D-NMMA (1 mM), a stereoisomer of L-NMMA known to be inactive on NOS, did not influence the basal calcium current (0.5 ± 5.4 %, n= 4).
Possible involvement of inducible NO synthase (iNOS)
The increase in ICa induced by NO synthase inhibitors may represent a new role for the constitutive NOS in the regulation of calcium current. Since the electrophysiological experiments do not exclude the involvement of iNOS in the effects described nor its induction by the dissociation procedure or by cell storage, we tested its presence directly.
To investigate whether our data were complicated by the presence of the inducible form of NOS, we performed Western blot analysis on isolated ventricular cells with a monoclonal antibody directed against the murine iNOS. A representative experiment is shown in Fig. 2A, neither lane 0 obtained from the cell lysate prepared soon after dissociation nor lane 24 obtained from the lysate prepared after 24 h show the 130 kDa protein corresponding to the murine iNOS; the band is present in the control lane (C, lysate of murine macrophages stimulated with interferon-γ and LPS).
Figure 2. Absence of involvement of iNOS in the L-NMMA enhancement of ICa.
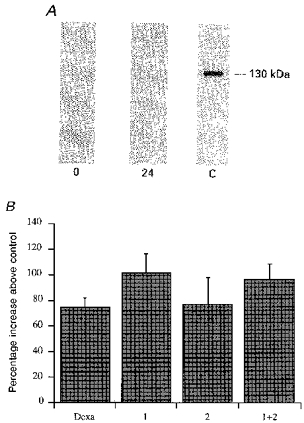
A, Western blot experiment showing the absence of iNOS protein on isolated ventricular cells. Cells were lysed soon after, or 24 h after, dissociation, as described in Methods. The probe used was a monoclonal antibody anti-murine macrophage inducible NOS. 0 = lysate at time 0, 24 = lysate at 24 h and C = positive control (lysate of murine macrophages previously treated with interferon-γ and LPS for 12 h). B, bar graph showing no substantial variation in the L-NMMA (1 mM) effect in cells used both soon after (1, within 5-6 h), and the day after dissociation (2). The bar labelled 1 + 2 is the effect on all cells tested, and Dexa represents the cells isolated and kept in the presence of 3 μM dexamethasone in the Tyrode solution from the start of the procedure and throughout the storage period.
Another way to exclude the possibility of iNOS contamination was to dissociate, and to keep, the ventricular cells in the presence of dexamethasone (Dexa) to inhibit gene induction.
Dexamethasone (3 μM) was present in the perfusion medium from the start of dissociation; the dissociated cells were stored in Tyrode solution with dexamethasone (3 μM) for 12 h and then used for the experiment on ICa stimulation by L-NMMA (1 mM). Under these conditions L-NMMA stimulation of calcium current was not statistically different (Student's t test, 74.3 ± 7.7 %, n= 4) from the stimulation obtained in cells dissociated and kept in the standard solution. As a final test to show that cell storage in Tyrode solution did not modify the response to L-NMMA we compared the L-NMMA-induced increase in calcium current in experiments conducted soon after, and the day after, cell dissociation, to confirm the absence of any difference that could be attributed to putative iNOS induction. The results of this statistical analysis (Fig. 2B) show there is no difference in the increase in ICa between cells used on the day of dissociation (bar 1, 101.3 ± 14.5 %, n= 14) and the cells used on the following day (bar 2, 76.5 ± 20.9 %, n= 4); for comparison bar 2 + 1 shows the results obtained from all the cells tested (95.8 ± 12.2 %, n= 18).
Reversal by intracellular or external L-arginine of the L-NMMA stimulatory effect on ICa
To explore the possibility of a non-specific effect of L-NMMA and L-NNA, independent of their inhibitory actions on NOS, we performed a series of experiments in which we tested the competition between L-NMMA and L-arginine, the natural substrate of NOS, in the stimulation of the calcium current.
We used L-arginine both in the external solution or in the perfusion pipette, at a higher concentration (5 mM) than L-NMMA, to accumulate an excess of the natural substrate and to displace the inhibitor.
In Fig. 3A a typical experiment with external L-arginine is shown: the cell is pre-treated with L-arginine for some minutes and when L-NMMA (1 mM) is added to the bath solution no increase of ICa can be observed. An increase in ICa develops only after several minutes following washout of L-arginine in the continued presence of L-NMMA. In Fig. 3B we present from the same experiment single traces of ICa in the presence of L-arginine alone, and with L-NMMA.
Figure 3. Effects of L-arginine competition with L-NMMA on calcium current.
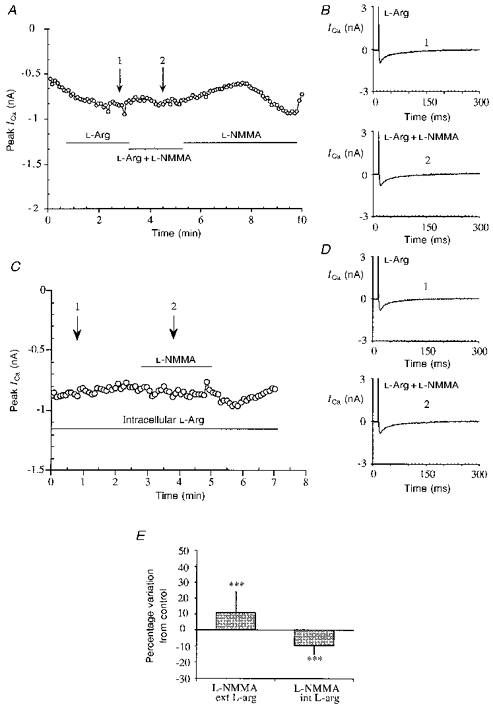
A, typical experiment showing the lack of effect of L-NMMA (1 mM) in the presence of external L-arginine (L-arg) (5 mM). Stimulation protocol as in Fig. 1A, single traces at the times indicated by the arrows in A, during L-arginine treatment (1) and in the presence of both L-arginine and L-NMMA (2). C, time course of ICa in a representative cell internally perfused with 5 mM L-arginine and then stimulated with 1 mM L-NMMA. L-arginine was applied directly in the perfusion pipette from the beginning and L-NMMA stimulation started after a few minutes. D, single traces of ICa recorded at the time indicated by the arrows in C, during L-arginine perfusion alone (1) and after L-NMMA application (2). E, bar graph showing the absence of significant effect of L-NMMA (1 mM) on basal ICa in the presence of external (ext) (***P < 0.001 vs. L-NMMA on basal ICa) and internal (int) (***P < 0.001 vs. L-NMMA on basal ICa) L-arginine (5 mM).
The experiments with external L-arginine demonstrate competition between the two substrates, but do not exclude the possibility of an effect at receptor level or at a common transporter site; to bypass the membrane-limited steps we examined cells perfused with L-arginine (5 mM) intracellularly from the patch electrode and then stimulated with external L-NMMA. L-NMMA was applied 3-4 min after the start of the experiments, to allow diffusion of the drug from the pipette to the cell. With this experimental protocol L-NMMA was again unable to stimulate the calcium current.
In Fig. 3C the time course of a representative experiment with L-arginine in the patch electrode is shown, and in Fig. 3D single traces of ICa from the same experiment in the absence and in the presence of L-NMMA are shown. Figure 3E summarizes the lack of effect of L-NMMA on basal ICa in the presence of extracellular L-arginine (10.7 ± 13.3 %, n= 4, P < 0.001 vs. L-NMMA alone) and intracellular L-arginine (-9.5 ± 5.4 %, n= 4, P < 0.001 vs. L-NMMA alone).
Intracellular perfusion with GDPβS failed to block the stimulatory effect of L-NMMA
The results obtained in the presence of L-arginine in the patch electrode suggest that intracellular mechanisms are involved in the L-NMMA enhancement of ICa. Further experiments were devised to exclude a membrane receptor mechanism, and specifically the involvement of a G-protein-dependent receptor system.
In these experiments we dialysed the cells intracellularly with GDPβS, an analogue of GDP that is not exchanged with GTP and blocks G-proteins in the inactive state. The intracellular solution contained 0.5 mM GDPβS but not Na2GTP.
The time course of a representative experiment is shown in Fig. 4A. L-NMMA (1 mM) was applied 3-5 min after rupture of the patch to permit diffusion of GDPβS. In the presence of L-NMMA, ICa rapidly increased in a reversible manner. The effect was comparable to that in other experiments performed in the presence of the standard intracellular medium (containing 0.4 mM Na2GTP).
Figure 4. Effects of L-NMMA and isoprenaline (Iso) on basal ICa in the presence of internal GDPβS.
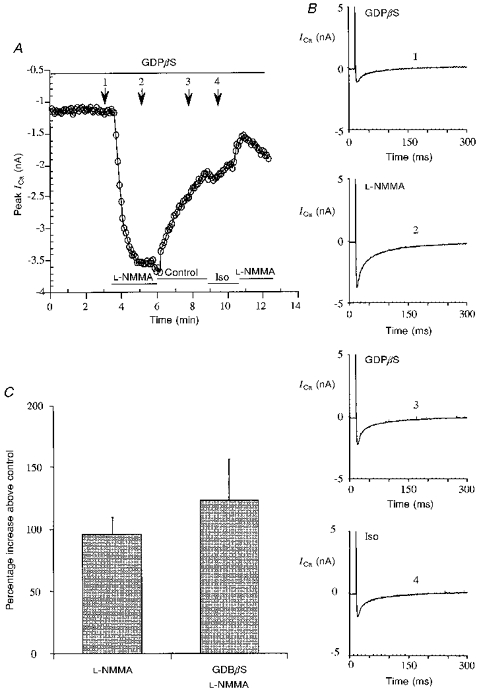
A, calcium current time course in a typical cell internally perfused with 0.5 mM GDPβS and exposed to L-NMMA and Iso. L-NMMA induces an increment of ICa, while Iso is almost completely ineffective. Experimental protocol as in the previous figures. The pipette solution was as described in Methods, but contained 0.5 mM GDPβS and no Na2GTP. B, single traces of ICa recorded at the times indicated by the arrows in A: control 1 (1), L-NMMA (1 mM) (2), control 2 (3), Iso (1 μM) (4). C, bar graph comparing the experiments with L-NMMA (1 mM) in the absence or presence of internal GDPβS (0.5 mM), and showing that no significant difference can be observed.
To verify the efficacy of the treatment with GDPβS, β-adrenergic stimulation was also tested. Exposure of the cell to 1 μM isoprenaline did not enhance ICa while a second stimulation with L-NMMA was still effective. In Fig. 4B the single traces of ICa corresponding to the different treatments are presented. Figure 4C summarizes the results with L-NMMA in the presence of GDPβS (122 ± 33.2 %, n= 6), showing that they are comparable to those with L-NMMA in standard medium, i.e. in the presence of GTP, without GDPβS (95.8 ± 12.2 %, n= 18).
Carbachol and intracellular cGMP abolish the L-NMMA stimulation of the calcium current
To investigate the intracellular mechanism involved in L-NMMA-mediated stimulation of the calcium current, we first considered the muscarinic receptor cascade.
We therefore performed a series of experiments to test possible cross-talk between the NOS and the muscarinic pathways. As described in several papers carbachol alone has no effect on the basal ICa of guinea-pig ventricular cardiomyocytes (Gallo et al. 1993), but can reduce the calcium current only after stimulation with β-adrenergic or other agonists such as histamine (Levi & Alloatti, 1988). The following experiments show that carbachol was able to reverse the increase in the calcium current induced by the NOS inhibitors. In these experiments calcium current was first stimulated with L-NMMA, and then with L-NMMA plus carbachol (1 μM). A few seconds after the addition of carbachol the L-NMMA-enhanced current returned toward the basal level. After removal of carbachol, L-NMMA was still able to increase the calcium current (typical experiment and single traces of the current in Fig. 5A and B).
Figure 5. Carbachol reversion of the L-NMMA stimulatory effect on basal ICa.
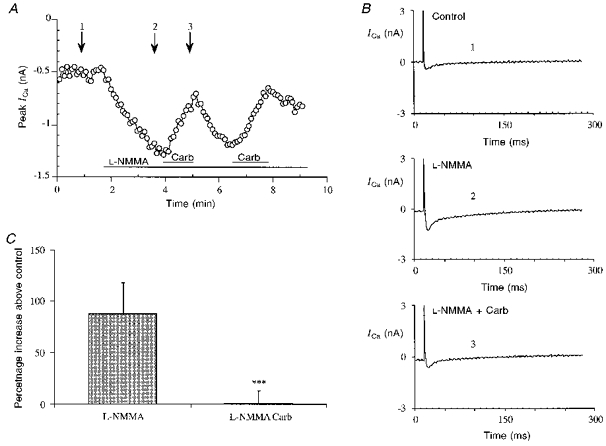
A, typical calcium current time course in a cell stimulated with L-NMMA (1 mM) and then with L-NMMA plus carbachol (Carb, 1 μM). Experimental protocol as in the previous figures. The carbachol application was repeated to show the reversibility of the effects. B, single traces of ICa at the times indicated by the arrows in A: control (1), L-NMMA (1 mM) (2), L-NMMA (1 mM) and Carb (1 μM) (3). C, bar graph showing the increase of basal ICa stimulated by L-NMMA and the return close to control with L-NMMA and carbachol (***P < 0.001 vs. L-NMMA alone).
In Fig. 5C we show a bar graph summarizing the abolition of the effect of L-NMMA by carbachol (1.0 ± 12.3 % of control, n= 5) compared with the increase in ICa induced by L-NMMA on the basal calcium current (87.7 ± 30.3 %, n= 5).
A guanylate cyclase-mediated mechanism has been proposed for the muscarinic inhibition of cardiac ICa. Guanylate cyclase is one of the intracellular targets for NO, and cGMP has also been shown to be involved in cardiac ICa regulation. We therefore considered whether the cGMP-dependent pathway was involved in the effect of carbachol on the ICa previously stimulated by L-NMMA. In a previous paper we have shown that Methylene Blue, an inhibitor of NO-dependent guanylate cyclase is ineffective on basal current, but can block muscarinic regulation (Levi et al. 1994).
We used cells perfused intracellularly with cGMP (10 μM) and observed that under these conditions L-NMMA failed to increase the ICa; this is shown in Fig. 6A. Figure 6B from the same experiment shows single traces of ICa before L-NMMA stimulation and a few minutes after its application. A bar graph summarizing this group of experiments is given in Fig. 6C; in cGMP-perfused cells L-NMMA does not modify ICa (-3.9 ± 6.3 %, n= 7).
Figure 6. Lack of effect of L-NMMA in the presence of intracellular perfusion with cGMP.
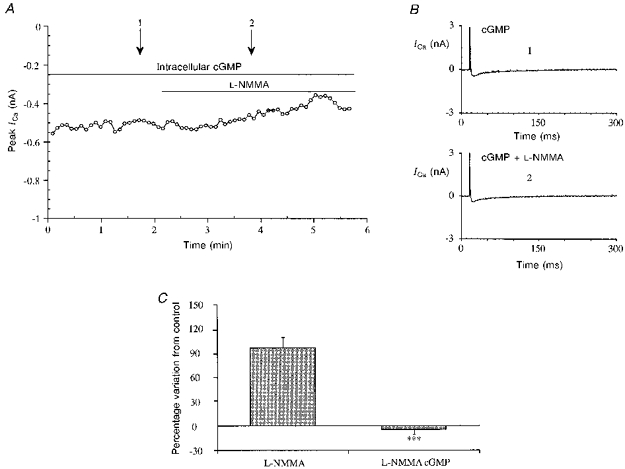
A, time course of ICa from a typical experiment with L-NMMA (1 mM) in the presence of cGMP. Stimulation protocol as in the previous figures. The patch electrode contained the standard intracellular solution described in the Methods section with 10 μM cGMP. B, single traces of ICa at the times indicated by the arrows in A: control (1) and L-NMMA (1 mM) (2). C, bar graph showing the lack of effect of L-NMMA in the presence of cGMP (10 μM, ***P < 0.0005 vs. no cGMP) compared with the effect in its absence.
NO production is not modulated by muscarinic agonists
Recent data in the literature have shown that in some cardiac cell preparations antagonists of NO synthesis are able to block the muscarinic inhibition of ICa prestimulated with isoprenaline (Han, Shimoni & Giles, 1994, 1995; Balligand et al. 1995), we have now confirmed the absence of an inhibitory effect of carbachol in the simultaneous presence of L-NMMA and isoprenaline.
In these experiments the cells were treated from the start of the protocol with L-NMMA (1 mM) and isoprenaline (1 μM) with subsequent addition of carbachol (1 μM); a typical time course is presented in Fig. 7A, and in Fig. 7B single traces of the calcium current from the same experiment are presented. In these conditions we observed an increase in calcium current comparable to the β-adrenergic stimulation, as observed in experiments with isoprenaline (1 μM) alone. The subsequent application of carbachol (1 μM) had no effect on ICa. We also performed the same experiment with different concentrations of L-NMMA (0.5, 1 and 5 mM) and observed that concentrations below 1 mM failed almost completely to inhibit the muscarinic effect (data not shown).
Figure 7. L-NMMA-dependent block of the muscarinic inhibition of ICa and NO production measurement in the presence of muscarinic agonists.
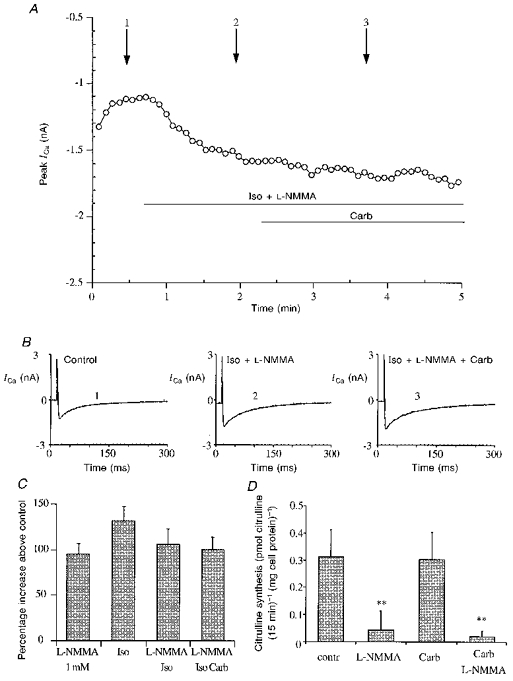
A, calcium current time course from a representative experiment with Iso (1 μM), L-NMMA (1 mM) and carbachol (1 μM), showing the absence of the Carb effect on Iso and L-NMMA together. Experimental protocol as in the previous figures. The cell was stimulated with both Iso and L-NMMA and after few minutes Carb was added. B, single traces of ICa at the time indicated by the arrows in A: control (1), Iso and L-NMMA (2), Iso, L-NMMA and Carb (3). C, bar graph summarizing the increase of ICa in the presence of: L-NMMA, Iso, L-NMMA and Iso, L-NMMA, Iso plus Carb (1 μM), ANOVA showed no significant difference between groups. D, nitric oxide production in isolated ventricular cardiocytes, measured as synthesis of radiolabelled [3H]citrulline from L-[3H]arginine: carbachol does not modulate the synthesis (no significant difference from control), while L-NMMA almost completely suppresses it, also in the presence of carbachol (0.1 mM) (P < 0.01 in both cases vs. control).
The bar graph in Fig. 7C summarizes the increase of ICa in the presence of: L-NMMA (1 mM) (95.8 ± 12.2 %, n= 18), Iso (1 μM) (132.4 ± 15.8 %, n= 5), L-NMMA and Iso (107.0 ± 16.5 %, n= 12), L-NMMA plus Iso plus carbachol (0.1 mM) (101.0 ± 14.4 %, n= 7).
To investigate the mechanism whereby NO is involved in the muscarinic modulation of ICa and in the NO effects on basal ICa, we verified whether NO synthesis was regulated by muscarinic agonists.
To detect NO synthesis in short time periods, the formation of radiolabelled [3H]citrulline from L-[3H]arginine was measured during a 15 min incubation, the results are summarized in Fig. 7D. Basal citrulline levels (0.31 ± 0.10 pmol citrulline (15 min)−1 (mg cell protein)−1; n= 11) of isolated ventricular cells were comparable to those measured in human endothelial cells (Ghigo et al. 1993). Citrulline synthesis was completely inhibited when cells were incubated in the presence of 1 mM L-NMMA (0.04 ± 0.07 pmol citrulline (15 min)−1 (mg cell protein)−1; n= 11). Addition of carbachol (0.1 mM) to the incubation medium did not affect either citrulline production (0.30 ± 0.10 pmol citrulline (15 min)−1 (mg cell protein)−1; n= 6) or the L-NMMA inhibitory effect (0.02 ± 0.02 pmol citrulline (15 min)−1 (mg cell protein)−1; n= 5).
DISCUSSION
The results presented in this paper demonstrate that two different inhibitors of nitric oxide synthesis can increase the cardiac calcium current, in the absence of other chemical stimuli. This effect is relatively large and is comparable to that produced by β-adrenergic stimulation, but is elicited only by very high concentrations of the NOS inhibitors. The concentrations required are in the order of 1 mM, which is much higher than the nanomolar to micromolar concentrations required to interfere with NO synthesis in other experimental models (Moncada et al. 1991; Schmidt et al. 1995). These concentrations are, however, closer to those used, with a 30 min or longer preincubation, by Han, Shimoni & Giles (1994, 1995) and by Balligand et al. (1995) in experiments showing the interference of NOS inhibitors with cardiac muscarinic regulation. One hypothesis to explain the requirement for high concentrations could be that the rapid effect shown in our experiments (a few seconds) requires a higher external concentration of arginine analogues, since the limiting step could be the transport of the inhibitors into the cells (Schmidt, List, Klatt & Mayer, 1995).
We have performed several experiments to try to test the effective specificity of the mechanism involved in the action of NOS inhibitors on ICa. The D-enantiomer of NMMA was devoid of any effect on ICa, while L-arginine, the natural substrate of NOS, acted competitively and was able to block the increase of ICa induced by L-NMMA, when applied either extra- or intracellularly. Western blot experiments show the lack of iNOS expression both in freshly dissociated cells and after 24 h, thus showing that neither preparation of the cells, nor the solutions used, act as inducers of the iNOS isoform. These experiments tend to exclude a role of iNOS and complement the observation of the absence of a statistical difference between electrophysiological experiments performed during the first hours after cell isolation and at later times, and also between cells isolated under control conditions or in the presence of dexamethasone (a drug widely used to prevent induction of iNOS, see Stein, Frank, Schmitz, Scholz & Thoenes, 1996).
Further experiments have tested the possibility that the effect could be due to a non-specific activation of a G-protein-coupled receptor. It has been shown that another analogue of arginine used as a NOS inhibitor, L-NAME, binds to muscarinic receptors and acts as a cholinergic inhibitor (Buxton, Cheek, Eckman, Westfall, Sanders & Keef, 1993). We have excluded the possibility of an activation of β-adrenergic receptors, by the inability of propranolol to block the L-NMMA effect (data not shown), and more generally, as shown above, by the inability of GDPβS to counteract the NOS inhibitor-induced potentiation of ICa. This GTP analogue should stabilize all GTP-dependent proteins in their inactive form thus excluding all effects mediated by G-protein-coupled receptors. The efficacy of the latter treatment was confirmed by the absence of β-adrenergic response after isoprenaline stimulation in cells treated with GDPβs. The other experiments performed were aimed at the recently much discussed question of NO involvement in muscarinic regulation of the calcium current. The most surprising finding is the inhibition by carbachol of L-NMMA stimulation of calcium current. It is interesting to compare the results of these experiments with those presented by Han et al. (1994, 1995) and Balligand et al. (1995) which show the block by L-NMMA of muscarinic inhibition of isoprenaline-enhanced calcium current. The conclusion by the latter groups was of an obligatory role of NO production in the muscarinic inhibitory pathway. Interestingly no evidence could be found in amphibians for a role of nitric oxide as a mediator of muscarinic inhibition (Méry, Hove-Madsen, Chesnais, Hartzell & Fischmeister, 1996) and at least two reports have confuted the involvement of NOS- or muscarinic-stimulated synthesis of cGMP in mammalian cardiomyocytes (Stein, Drogemuller, Mulsch, Schmitz & Scholz, 1993; MacDonell, Tibbits & Diamond, 1995). Our data show that carbachol can inhibit the stimulation produced by NO synthesis inhibitors themselves, thus muscarinic receptors are able to modulate ICa in the presence of the blockade of the NO synthesis pathway. This experiment suggests moreover that the pathway leading to ICa stimulation by L-NMMA shares many of the components of the well-studied mechanisms that lead for instance to β-adrenergic stimulation. The block by internal cGMP (10 μM) of the L-NMMA enhancement of ICa is interesting when compared with the effects of NO donors and cGMP described in several papers (Fischmeister & Hartzell, 1987; Levi et al. 1989, 1994; Méry et al. 1993).
The observation in our previous work (Levi et al. 1994) that Methylene Blue was unable to modulate basal ICa is not easy to explain, since the present experiments would suggest a positive modulation of basal current by this drug. One possible explanation for this anomaly could be the lack of specificity of Methylene Blue. In some preparations it has indeed been shown to inhibit calcium current (Méry et al. 1993). Moreover the concentration of Methylene Blue used, while sufficient to prevent cGMP-dependent modulation of cAMP-enhanced current, might have been too low to modulate the basal current.
The set of experiments in which we measured labelled citrulline production in living cells shows that NO is indeed produced by unstimulated ventricular cells, as suggested by Balligand et al. (1993). Moreover these experiments show that L-NMMA is able to suppress the production almost completely. Only one concentration (1 mM) of inhibitor was tested in our present experiments, as the major issue to be addressed was the involvement of the muscarinic pathway in the regulation of NO, and it can be expected that lower concentrations of L-NMMA could have, as reported in other models, an effect on citrulline synthesis. The lack of any modulation of NO synthesis by carbachol indicates, instead, either that the modulation of NO production by the muscarinic pathway is too small to be biochemically detectable or that NO is involved in the regulation of basal ICa but is not itself increased during muscarinic stimulation.
Our finding that in the presence of L-NMMA, carbachol is unable to decrease isoprenaline-stimulated ICa, confirms those of Han et al. (1995) and Balligand et al. (1995). One possible interpretation of these results is that, for some unexplained reason, the summation of the stimulation of ICa by β-adrenergic receptors and L-NMMA, is not subject to muscarinic inhibition, while stimulations by either isoprenaline or L-NMMA alone are largely suppressed by carbachol.
In conclusion, the data presented in this paper suggest that care must be taken when interpreting experiments based on the effect of NOS inhibition by arginine-derived antagonists. Several hypotheses could be considered in interpreting the paradoxical increase of ICa by either L-NMMA or L-NNA. The first, and maybe more attractive idea could be that these substances remove a constant basal inhibition of ICa by endogenous NO synthesized by constitutive NOS. The presence of NOS has been shown at the molecular level by Balligand et al. (1993) and its activity in intact cells is evident from our experiments. The basal production of NO could influence ICa in several ways, not only through cGMP production as suggested by Han et al. (1995), and the high reactivity of nitric oxide could lead to functional modifications in any of the numerous proteins involved in the modulation of calcium current amplitude by thiol nitrosylation (Molina y Vedia et al. 1992) or other reactions. The block of muscarinic modulation could then be a simple consequence of the simultaneous activation of ICa channels by the removal of inhibition by NO, and the activation by cAMP-dependent phosphorylation whose synergy could displace the inhibitory dose-response curve to the right (thus requiring much higher concentrations of muscarinic agonist) or render the activation insensitive to muscarinic regulation. Interestingly L-NMMA stimulation of ICa itself is susceptible to muscarinic inhibition, thus suggesting a relative independence of the muscarinic pathway from the one in which NOS inhibitors act.
New complications have been introduced by the complex pattern of effects of specific NOS knockout in mice reported by Huang, Dawson, Bredt, Snyder & Fishmann (1993), Laubach, Shesely, Smithies & Sherman, (1995), Huang et al. (1995) and Nelson et al. (1995). Nevertheless these results might help in further investigations of the role of NOS subtypes in cardiac regulation.
Acknowledgments
We wish to thank Drs R. Fischmeister and P. F. Méry for useful discussion of our results. We thank Professor D. R. Milne for proof reading the English manuscript. This work was partly supported by grants from Ministero dell'Università e Ricerca Scientifica e Tecnologica, Istituto di Fisica della Materia and from Telethon-Italy.
References
- Balligand JL, Kelly RA, Marsden PA, Smith TW, Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signalling system. Proceedings of the National Academy Sciences of the USA. 1993;90:347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand JL, Kobzik L, Han X, Kaye DM, Belhassen L, O'Hara DS, Kelly RA, Smith TW, Michel T. Nitric oxide-dependent parasympathetic signalling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. Journal of Biological Chemistry. 1995;270:14582–14586. doi: 10.1074/jbc.270.24.14582. 10.1074/jbc.270.24.14582. [DOI] [PubMed] [Google Scholar]
- Balligand JL, Ungureanu-Longrois D, Simmons WW, Pimental D, Malinski TA, Kapturczak M, Taha Z, Lowenstein CJ, Davidoff AJ, Kelly RA, Smith TV, Michel T. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Journal of Biological Chemistry. 1994;269:27580–27588. [PubMed] [Google Scholar]
- Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KF. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circulation Research. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Hartzell CH. Cyclic guanosine 3′,5′-monophosphate regulates the calcium current in single cell from frog ventricle. The Journal of Physiology. 1987;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo MP, Alloatti G, Eva C, Oberto A, Levi RC. M1 muscarinic receptors increase calcium current and phosphoinositide turnover in guinea-pig ventricular cardiocytes. The Journal of Physiology. 1993;471:41–60. doi: 10.1113/jphysiol.1993.sp019890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo D, Alessio P, Foco A, Bussolino F, Costamagna C, Heller R, Garbarino G, Pescarmona GP, Bosia A. Nitric oxide synthesis is impaired in glutathione-depleted human umbilical vein endothelial cells. American Journal of Physiology. 1993;265:C728–732. doi: 10.1152/ajpcell.1993.265.3.C728. [DOI] [PubMed] [Google Scholar]
- Han X, Shimoni Y, Giles WR. An obligatory role for nitric oxide in autonomic control of mammalian heart rate. The Journal of Physiology. 1994;476:309–314. doi: 10.1113/jphysiol.1994.sp020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Shimoni Y, Giles WR. A cellular mechanism for nitric oxide-mediated cholinergic control of mammalian heart rate. Journal of General Physiology. 1995;106:45–66. doi: 10.1085/jgp.106.1.45. 10.1085/jgp.106.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishmann MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–243. doi: 10.1038/377239a0. 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circulation Research. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Shesely EG, Smithies O, Sherman PA. Proceedings of the National Academy Sciences of the USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi RC, Alloatti G. Histamine modulates calcium current in guinea pig ventricular myocytes. Journal of Pharmacology and Experimental Therapeutics. 1988;246:377–383. [PubMed] [Google Scholar]
- Levi RC, Alloatti G, Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea-pig ventricular myocytes. Pflügers Archiv. 1989;426:419–426. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Levi RC, Alloatti G, Penna C, Gallo MP. Guanylate-cyclase-mediated inhibition of cardiac ICa by carbachol and sodium nitroprusside. Pflügers Archiv. 1994;413:685–687. doi: 10.1007/BF00388305. [DOI] [PubMed] [Google Scholar]
- Lohmann SM, Fischmeister R, Walter U. Signal transduction by cGMP in the heart. Basic Research Cardiology. 1991;86:503–514. doi: 10.1007/BF02190700. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Snyder S. Nitric oxide, a novel biological messenger. Cell. 1992;70:705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- MacDonell KL, Tibbits GF, Diamond J. cGMP elevation does not mediate muscarinic agonist-induced negative inotropy in rat ventricular cardiomyocytes. American Journal of Physiology. 1995;269:H1905–1912. doi: 10.1152/ajpheart.1995.269.6.H1905. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Méry PF, Hove-Madsen L, Chesnais JM, Hartzell CH, Fischmeister R. Nitric oxide synthase does not participate in the negative inotropic effect of acetylcholine in the frog heart. American Journal of Physiology. 1996;270:H1178–1188. doi: 10.1152/ajpheart.1996.270.4.H1178. [DOI] [PubMed] [Google Scholar]
- Méry PF, Lohmann SM, Walter U, Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proceedings of the National Academy Sciences of the USA. 1991;88:1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méry PF, Pavoine C, Belhassen L, Pecker F, Fischmeister R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl ciclase activation. Journal of Biological Chemistry. 1993;268:26286–26295. [PubMed] [Google Scholar]
- Molinay Vedia L, McDonald B, Reep B, Brüne B, Di Silvio M, Billiar TM, Lapetina E. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. Journal of Biological Chemistry. 1992;267:24929–24932. [PubMed] [Google Scholar]
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43:109–142. [PubMed] [Google Scholar]
- Mubagwa K, Shirayama T, Moreau M, Pappano AJ. Effects of PDE inhibitors and carbachol on the L-type Ca current in guinea-pig ventricular myocytes. American Journal of Physiology. 1993;264:H1353–1363. doi: 10.1152/ajpheart.1993.265.4.H1353. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB Journal. 1992;6:3051–3064. [PubMed] [Google Scholar]
- Nathan C, Xie Q. Nitric Oxide synthases: roles, tolls and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishmann MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Ono K, Trautwein W. Potentiation by cyclic GMP of β-adrenergic effect on Ca2+ current in guinea-pig ventricular cells. The Journal of Physiology. 1991;443:387–404. doi: 10.1113/jphysiol.1991.sp018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rees DD, Schulz R, Hodson HF, Palmer RM, Moncada S. Identification of some novel inhibitors of the vascular nitric oxide synthase in vivo and in vitro. In: Moncada S, Higgs EA, editors. Nitric Oxide from L-arginine: a Bioregulatory System. Amsterdam: Elsevier; 1990. pp. 485–487. [Google Scholar]
- Schmidt HHHW, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochimica et Biophysica Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schmidt HHHW, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schmidt K, List BM, Klatt P, Mayer B. Characterization of neuronal amino acid transporters: uptake of nitric oxide synthase inhibitors and implications for their biological effects. Journal of Neurochemistry. 1995;64:1469–1475. doi: 10.1046/j.1471-4159.1995.64041469.x. [DOI] [PubMed] [Google Scholar]
- Stein B, Drogemuller A, Mulsch A, Schmitz W, Scholz H. Ca2+-dependent constitutive nitric oxide synthase is not involved in the cyclic GMP-increasing effects of carbachol in ventricular cardiomyocytes. Journal of Pharmacology and Experimental Therapeutics. 1993;266:919–925. [PubMed] [Google Scholar]
- Stein B, Frank P, Schmitz W, Scholz H, Thoenes M. Endotoxin and citokines induce direct cardiodepressive effects in mammalian cardiomyocytes via induction of nitric oxide synthase. Journal of Molecular and Cellular Cardiology. 1996;28:1631–1639. doi: 10.1006/jmcc.1996.0153. [DOI] [PubMed] [Google Scholar]
- Wahler GM, Dollinger SJ. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. American Journal of Physiology. 1995;268:C45–54. doi: 10.1152/ajpcell.1995.268.1.C45. [DOI] [PubMed] [Google Scholar]
- Wang YG, Lipsius SL. Acetylcholine elicits a rebound stimulation of Ca2+ current mediated by pertussis toxin-sensitive G protein and cAMP-dependent protein kinase A in atrial myocytes. Circulation Research. 1995;76:634–644. doi: 10.1161/01.res.76.4.634. [DOI] [PubMed] [Google Scholar]


