Abstract
Excitation-contraction coupling in mouse cardiac muscle remains poorly characterized, despite the fact that the mouse is the mammalian species of choice for genetic manipulation. In this study, we characterized the relationship between internal calcium concentration ([Ca2+]i) and contraction in intact mouse ventricular muscle loaded with fura-2 salt at 20–22°C.
Both Ca2+ transient amplitude and twitch force increased monotonically as external Ca2+ concentration ([Ca2+]o) was increased up to 8.0 mm, with no changes in diastolic levels or in the times to peak of either Ca2+ transients or force. The decay of Ca2+ transients was accelerated as [Ca2+]o increased, while relaxation was prolonged. Both Ca2+ transient amplitude and twitch force increased as stimulation rate increased from 0.2 to 4 Hz, but the increase in force was much greater than the underlying increase in [Ca2+]i.
The steady-state force-[Ca2+]i relationship revealed an [Ca2+]i required for 50% of maximal activation (Ca50) of 0.95 ± 0.08 μm, a Hill coefficient of 9.9 ± 2.6, and a maximal Ca2+-activated force (Fmax) of 60 ± 5 mN mm−2.
Unlike rat ventricular myocardium, mouse cardiac muscle resists supraphysiological [Ca2+]o. The strong positive force-frequency relationship in mouse cardiac muscle, with increases of force disproportionate to the increases in Ca2+ transients, suggests frequency-dependent ‘sensitization’ of the myofilaments. During steady-state activation, mouse muscle exhibits decreased Ca2+ responsiveness relative to other species, but high co-operativity.
These physiological features of mouse cardiac muscle merit consideration when interpreting the phenotypic consequences of genetic manipulations in this species.
Mice have become the most popular mammals for transgenesis, in which certain genes are either over-expressed or selectively knocked out, and mutagenesis in which selected portions of a given gene are mutated or replaced. In cardiac muscle physiology, transgenic (or mutant) mice provide unique tools to probe the role of a given gene in a clear-cut way. For example, ablation of the phospholamban gene was found to enhance the overall performance of the heart (Luo et al. 1994), while over-expression of phospholamban depresses contraction (Kadambi et al. 1996). The results of these (and many other) studies are important but cannot necessarily be interpreted at face value, given that little is known regarding the physiological properties of excitation-contraction coupling in the mouse heart. Electrophysiologically, it has been found that both neonatal and adult mouse cardiac cells have short action potentials (Nilius, Boldt & Benndorf, 1986; Binah, Arieli, Beck, Rosen & Palti, 1987; Nuss & Marban, 1994) due to a large transient K+ current (Nuss & Marban, 1994). In contrast, few measurements of [Ca2+]i and contraction are available (Hampton, Kranias & Morgan, 1996).
The paucity of information about the relationship between [Ca2+]i and force development in adult mouse cardiac muscle led us to characterize these properties. We find that adult mouse cardiac muscle is remarkably tolerant to calcium loading and exhibits a strong positive force-frequency relationship. Furthermore, the myofilaments are less responsive to Ca2+ but more co-operative than in rat or ferret ventricle.
METHODS
Mouse muscle preparations
Adult male mice (SVEV strain, 10-13 weeks, 25-35 g, Taconic, German Town, NY, USA) were anaesthetized by intra-abdominal injection of sodium pentobarbitone (≈10-20 mg), and the hearts rapidly excised via midsternal thoracotomy. The hearts were retrogradely perfused with modified Krebs-Henseleit (KH) buffer with high K+ (20 mM), gassed with 95 % O2-5 % CO2 gas mixture, in a dissection dish at room temperature (20-22°C). Only ≈30 % of the mouse hearts examined had long thin trabeculae which were technically suitable for force measurements and fura-2 microinjection. When encountered, such trabeculae (mm: 1.01 ± 0.22 long, 0.17 ± 0.07 wide, and 0.09 ± 0.02 thick; means ±s.d., n= 10) were quickly dissected from the right ventricle and mounted between a force transducer and a micromanipulator in a perfusion bath. The trabeculae were superfused with KH buffer equilibrated with 95 % O2- 5 % CO2. The KH buffer was composed of (mM): 142 Na+, 5 K+, 1.2 Mg2+, 127.4 Cl−, 2 PO4−, 20 HCO3− and 1.0 CaCl2, pH 7.35-7.4. The perfusion rate was ≈10 ml min−1 and the preparations were stimulated at 0.5 Hz unless otherwise indicated. All experiments were performed at room temperature. While several experiments were attempted at 37°C, such experiments were technically inadequate due to rapid loss of the Ca2+ indicator and deterioration of contractile function. Force and sarcomere length (SL) were measured as described previously (Gao, Liu, Mellgren & Marban, 1996). Diastolic SL was measured by laser diffraction, set to 2.1-2.2 μm, and monitored throughout the experiments. The preparations were field stimulated with 5 ms pulses (Grass S44 Stimulator). Tetanization was achieved as described previously for rat muscle (Gao et al. 1996), with variations of [Ca2+]i and force effected by changes in [Ca2+]o (usually 0.5-15 mM).
Measurement of intracellular [Ca2+] with fura-2
[Ca2+]i was measured as described previously (Gao, Backx, Azan-Backx & Marban, 1994; Backx, Gao, Azan-Backx & Marban, 1995). Fura-2 potassium salt was microinjected iontophoretically into one cell and allowed to spread throughout the muscle via gap junctions. The loading did not affect force development. The small size of the preparations enabled loading and equilibration to be achieved within 10-20 min, as compared with 40-60 min in rat trabeculae (Gao et al. 1994; Backx et al. 1995). However, the small size also rendered the preparations quite fragile, such that only ≈60 % of the muscles tolerated the impalement without injury (hypercontracture or depolarization). Thus, coupled with the low frequency of geometrically suitable preparations, the overall success rate of the experiments was 15-20 %.
[Ca2+]i was determined by measuring the epifluorescence of fura-2 in the cells excited, using ultraviolet light at 380 nm and 340 nm. The fluorescent light was collected at 510 nm by a photomultiplier tube (R1527, Hamamatsu, Bridgeport, NJ, USA). The output of the photomultiplier tube was filtered at 100 Hz, collected by an A/D converter and stored in the computer for later analysis.
Intracellular [Ca2+] was given by the following equation (after subtraction of the autofluorescence of the muscle):
| (1) |
where R is the observed ratio of fluorescence (340 nm/380 nm), Kd is the apparent dissociation constant, Rmax is the ratio of 340 nm/380 nm at saturating [Ca2+], and Rmin is the ratio of 340 nm/380 nm at zero [Ca2+]. The values for Kd, Rmax and Rmin were 3.5, 7.20 and 0.47 μM, respectively, as determined by in vivo calibrations performed in a group of mouse trabeculae dedicated to this purpose (see Fig. 1), using methods described previously for rat (Backx et al. 1995; Gao et al. 1996). The Kd found here is similar to the Kd of 2.9-3.2 μM reported in rat cardiac muscle.
Figure 1. In vivo calibration of fura-2 in mouse trabeculae.
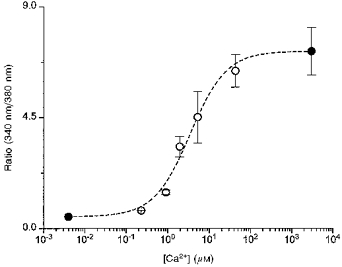
After loading fura-2 by micro-iontophoresis, trabeculae were superfused with (mM): KCl, 140; Hepes, 25; MgCl2, 1.2; NaCN, 2; and iodoacetic acid, 0.5; at pH 7.4 and room temperature. Fifteen minutes after full rigor development, the muscles were bathed in solution containing (mM): K2EGTA, 10; KCl, 80; Hepes, 25; MgCl2, 0.75; NaCN, 2; iodoacetic acid, 0.5; and 50 μM ionomyocin, with pH held at 7.2 to permeabilize the membrane to Ca2+. [Ca2+] was varied by mixing K2EGTA and CaEGTA proportionally. Data are means ±s.e.m.•, n= 7; ○,n= 2.
Statistics
Student's t test and analysis of variance were used for statistical analysis of the data (Snedecor & Cochran, 1989; Winer, 1989). A value of P < 0.05 was considered to indicate significant differences between groups. Unless otherwise indicated, pooled data are expressed as means ±s.e.m.
RESULTS
Force development at varied [Ca2+]o
Figure 2 shows three individual records of twitch force (right) and the corresponding Ca2+ transients (left) at different [Ca2+]o. Both force and Ca2+ transients increased as [Ca2+]o increased, even at [Ca2+]o as high as 8.0 mM. Figure 3 shows pooled data for [Ca2+]i (left) and force (right) from 5-7 muscles. Ca2+ transients and force increased monotonically as [Ca2+]o increased up to 8.0 mM, with no significant changes in the diastolic values. A continued rise in force development over such a wide range of [Ca2+]o, without aftercontractions or other manifestations of Ca2+ overload, was unanticipated; certainly rats, which mice are often assumed to resemble, tolerate increases of [Ca2+]o only over a narrow range before developing calcium overload (Lappe & Lakatta, 1980). Thus, mouse cardiac muscle is more resistant to high Ca2+, and in this regard resembles rabbit ventricular muscle (Kort & Lakatta, 1984, 1988).
Figure 2. Changes of Ca2+ transients and twitch force in a representative mouse ventricular trabeculum at 2.0, 4.0 and 8.0 mM [Ca2+]o.

The muscle was superfused with modified KH buffer and stimulated at 0.5 Hz. Increases in [Ca2+]o from 2.0 to 8.0 mM increased systolic [Ca2+]i from 0.61 to 1.51 μM and twitch force from 9.8 to 42 mN mm−2. There were no significant changes in diastolic [Ca2+]i or force.
Figure 3. Effect of [Ca2+]o on [Ca2+]i and twitch force of mouse cardiac muscle.
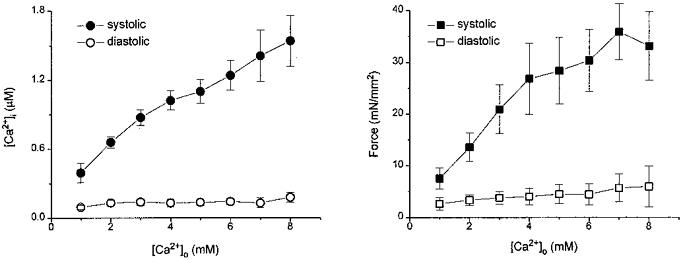
Pooled data for systolic and diastolic [Ca2+]i (left) and force (right) from 5-8 traceculae were plotted at varied [Ca2+]o. Both systolic [Ca2+]i and force increased at higher [Ca2+]o with no changes in diastolic [Ca2+]i and force. All muscles were stimulated at 0.5 Hz. Data are means ±s.e.m.
Changes in dynamics of twitches and Ca2+ transients at varied [Ca2+]o
Increases in [Ca2+]o did not affect the time to peak of Ca2+ transients, while the time-to-peak force increased slightly for [Ca2+]o up to 8.0 mM (Fig. 4, upper right panel, P > 0.05 by ANOVA). Further increases in [Ca2+]o decreased the time-to-peak force. The decay of Ca2+ transients accelerated as [Ca2+]o was raised (Fig. 4, lower left panel, P < 0.01 by ANOVA). Relaxation of twitch force, on the other hand, showed an initial acceleration, then paradoxically slowed down at higher [Ca2+]o and returned to rates comparable with those at 1.0 mM [Ca2+]o (Fig. 4). This delay of relaxation of force in the presence of accelerated relaxation of Ca2+ transients may be the result of force-dependent changes in the affinity of cardiac troponin C for Ca2+ at higher levels of force development, as described previously in ferret ventricular muscle (Kurihara, 1994).
Figure 4. Effects of [Ca2+]o on dynamics of Ca2+ transients and force.
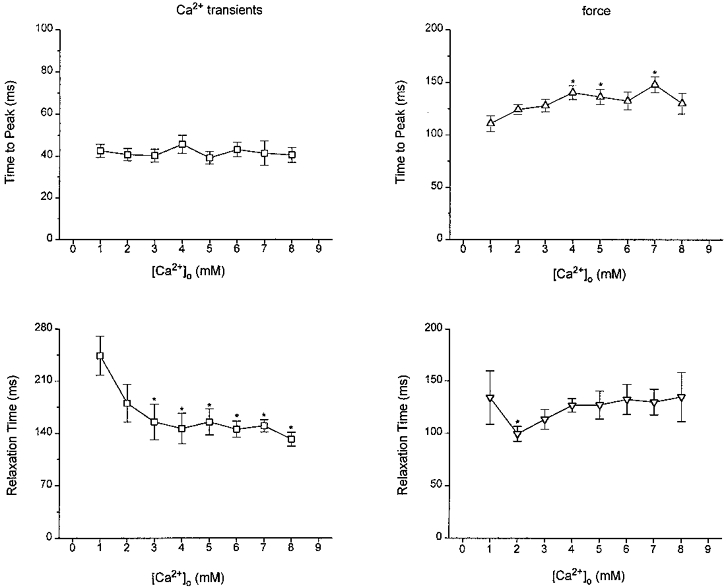
Pooled data for time to peak (upper panels) and time from peak to 50% relaxation (lower panels) of Ca2+ transients (left panels) and twitch force (right panels) (n= 5-8). *P < 0.05vs. 1.0 mM (see text for details).
Effects of stimulation frequency on [Ca2+]i and contraction
Increasing stimulation frequency had profound effects on both Ca2+ transients and contractions. Figure 5 shows raw records of [Ca2+]i and corresponding force at four different stimulation rates. Systolic [Ca2+]i increased from 0.63 to 0.97 μM and force from 1.9 to 3.5 mN mm−2 when stimulation rate was increased from 0.5 to 1.0 Hz. There was an additional 60 % increase of systolic [Ca2+]i, but a ≈5-fold increase in force when stimulation rate was increased from 1.0 to 3.0 Hz. Overall, the amplitude of Ca2+ transients increased about 3-fold, while force increased 7-fold over the range from 0.2 to 4.0 Hz (Fig. 6). The increase of force is much more pronounced than the increase in Ca2+ transients, especially at the higher frequencies. The relatively constant Ca2+ transient amplitude is not caused by saturation of the fura-2 signal based on the calibration of fura-2 in mouse muscles (see Fig. 1). The time-to-peak force decreased as stimulation rate increased (P < 0.05) while the time-to-peak Ca2+ did not change. There was a significant acceleration of relaxation of both force and Ca2+ transients (P < 0.01), with relaxation of Ca2+ transients being faster than that of force (Fig. 6). Although the changes in the peak values of Ca2+ transients and force suggest ‘frequency-induced’ sensitization, the acceleration of the relaxation time of both seems to oppose this conclusion. Nevertheless, increases in [Ca2+]i also augment the activity of the sarcoplasmic reticulum (SR) Ca2+ pump, thus making it difficult to assess changes in myofilament Ca2+ sensitivity using only the relaxation time.
Figure 5. Changes of [Ca2+]i and twitch force at stimulation frequencies of 0.5, 1.0, 2.0 and 4.0 Hz of a representative mouse trabeculum.
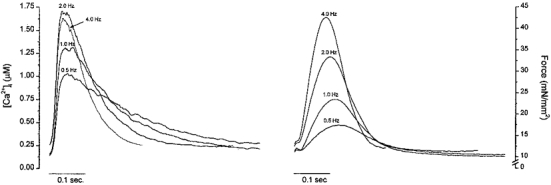
[Ca2+]o was 2.0 mM. Note the dramatic increase in systolic force when stimulation rate was increased from 1.0 to 4.0 Hz relative to increases in systolic [Ca2+]i.
Figure 6. Force-frequency relationship of mouse cardiac muscle.
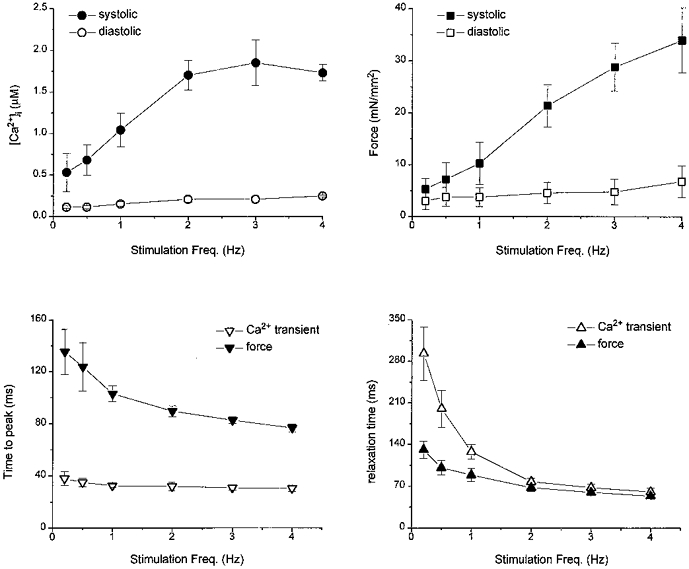
Pooled data of [Ca2+]i (top left) and twitch force (top right) from 3-5 mouse trabeculae are plotted at varied stimulation frequencies at [Ca2+]o of 2.0 mM. Note the profound increases in systolic twitch force as opposed to systolic [Ca2+]i when stimulation frequency increased. Changes of time to peak of both Ca2+ transients and force as stimulation rates increased is shown bottom left. The changes in time-to-peak force are significant: P < 0.05, ANOVA). Bottom right shows acceleration of relaxation time of both force and Ca2+ transient as stimulation rate increases. These changes are significant (P < 0.05). See text for details.
To check this point further, we plotted [Ca2+]ivs. force over the entire trajectory of the twitch. We have previously used such phase-plane analysis to illustrate differences between the force-[Ca2+]i relationship during steady-state activation and during relaxation of twitch contractions (Backx et al. 1995; Gao, Atar, Backx & Marban, 1995). In phase-plane analysis, [Ca2+]i and force are linked on a point-to-point basis over the entire trajectory of the twitch. The force-[Ca2+]i relationship during a twitch generally falls to the left of the steady-state force-[Ca2+]i relationship, indicating that the intrinsic properties of the myofilaments, and not Ca2+ removal from the cytosol, dominate relaxation. However, if relaxation is accelerated and/or the dynamics of Ca2+ handling slowed, the force-[Ca2+]i relationship during the twitch would coincide more closely with that of the steady state (Backx et al. 1995; Dobrunz, Backx & Yue, 1995). Thus, the degree of divergence, which is represented by the difference between [Ca2+]i at 50 % of twitch relaxation and [Ca2+]i at the same level of steady-state activation, reflects changes in cross-bridge kinetics and/or dynamics of Ca2+ handling. Figure 7 shows typical phase-plane plots of twitches at four different stimulation frequencies and the steady-state [Ca2+]i-force relationship in the same muscle. The midpoint of relaxation is highlighted in each phase-plane loop by a filled circle. Increases in stimulation frequency increased force development, but at the same time shifted the relaxation phase of each loop further to the left relative to the steady-state relationship. Since increases in stimulation frequency accelerated the decay of Ca2+ transients, a slowing in the kinetics of force relaxation must be responsible for the increased divergence. So, as stimulation frequency increases, the kinetics of cross-bridge cycling become increasingly rate limiting, consistent with the idea of frequency-dependent myofilament sensitization. Such behaviour was consistently seen in all muscles (n= 5) in which force-frequency relations were studied. Thus, the ‘frequency-induced’ sensitization appears to represent an unusual physiological property of mouse cardiac muscle.
Figure 7. Phase-plane analysis of relaxation.
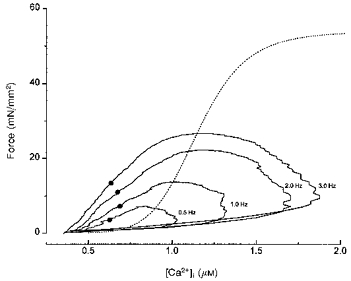
Twitch forces vs. corresponding [Ca2+]i from a mouse trabeculum at different stimulation frequencies ([Ca2+]o, 2.0 mM) are plotted to generate phase-plane plots. The steady-state force-[Ca2+]i relation of this muscle is superimposed (dotted line). The points (•) indicate 50 % of peak force during relaxation. Please note that the distance between the points and the corresponding levels of force of the superimposed steady-state force -[Ca2+]i relation (designated as Δ[Ca2+]i (Gao et al. 1995)) increases at higher stimulation frequencies.
Steady-state force-[Ca2+]i relationship in mouse cardiac muscle
Steady-state activation of intact cardiac muscle offers a direct assessment of the relation between calcium and force development in vivo. In cardiac muscle, steady-state activation is usually achieved by stimulating the muscle at high frequency (10 Hz) in the presence of either ryanodine or cyclopiazonic acid (CPA) (Gao, Backx, Azan-Backx & Marban, 1994; Backx et al. 1995). Figure 8 shows the steady-state tetanized force and [Ca2+]i at different levels of activation from a mouse trabecula. The behaviour of the tetanized [Ca2+]i and force looks superficially similar to that of other species (e.g. rat (Gao et al. 1994), ferret (Yue, Marban & Wier, 1986)), but careful inspection reveals some distinctive features: the initial rising phase is slow for [Ca2+]i and even slower for force; at higher activation levels, small increases in [Ca2+]i resulted in large increases in tetanized force. Figure 8C shows pooled data for the steady-state force-[Ca2+]i relations from six muscles. Maximal Ca2+- activated force (Fmax) is 60 ± 5 mN mm−2, Ca50 is 0.95 ± 0.08 μM, and the Hill coefficient is 9.9 ± 2.6. Overall myofilament responsiveness is lower than in rat (Gao et al. 1994; Backx et al. 1995) or ferret (Yue et al. 1986; Okazaki, Suda, Hongo, Koshi & Kurihara, 1990) ventricular muscle, but co-operativity appears to be unusually high in the mouse.
Figure 8. Steady-state force-[Ca2+]i relationship of mouse cardiac muscle.
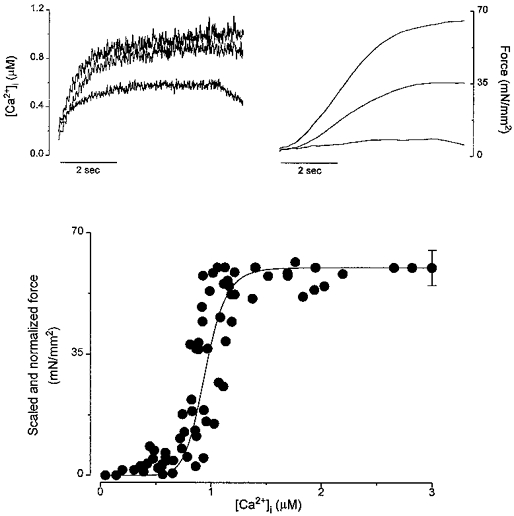
Recordings of [Ca2+]i (top left) and force (top right) at different levels of activations from a representative muscle. Tetanizations were achieved by stimulating the muscle at 10 Hz in the presence of cyclopiazonic acid (CPA, 50 μM) at varied [Ca2+]o to obtain different levels of tetanizations. Pooled data for steady-state force-[Ca2+] relationships of six mouse trabeculae are shown at bottom. The absolute Fmax is shown as mean ±s.e.m. and is plotted at highest [Ca2+]i. All other force levels were normalized with respect to their own maximal values. The continuous line is the Hill fit (Gao et al. 1994; Backx et al. 1995) based on the means of Ca50 and Hill coefficient (n= 6).
DISCUSSION
Effect of [Ca2+]o on contraction in mouse cardiac muscle
We find that excitation-contraction coupling in mouse cardiac muscle exhibits several unusual and distinctive features. First of all, murine ventricular muscle is very tolerant to high [Ca2+]o. The amplitudes of both Ca2+ transients and force increased monotonically at rising [Ca2+]o up to 8.0 mM (Fig. 3) without Ca2+ overload. In some muscles, progressive increases in [Ca2+]o up to 10-12 mM augmented force development even further (results not shown). Muscles from other mammalian species, particularly the rat, cannot tolerate [Ca2+]o as high as 2 mM under the same experimental conditions without developing aftercontractions and a decline in systolic force generation due to asynchronous contractile activation (Kort, Capogrossi & Lakatta, 1985; Capogrossi, Suarez-Isla & Lakatta, 1986; Capogrossi, Stern, Spurgeon & Lakatta, 1988). This upstream regulation by, and tolerance to, Ca2+ has to be maintained by powerful Ca2+ removal systems. Mouse muscle has a diastolic [Ca2+]i of 50-110 nM which is identical to that in other species. The resistance to Ca2+ overload reflects either a small amount of Ca2+ release during each twitch relative to the rat or unusually efficient Ca2+ removal mechanisms after each contraction. The former may be true at low [Ca2+]o, since the Ca2+ transients were indeed smaller than in rat (e.g. 0.66 μM on average vs. 1.10-1.50 μM at [Ca2+]o= 2.0 mM (Gao et al. 1995; Gao et al. 1996)). However, at high [Ca2+]o, efficient removal of Ca2+ must play a dominant role since the release of Ca2+ was clearly increased. This may involve either a greater Ca2+-ATPase activity of the SR, more calcium buffering within the SR, and/or more powerful sarcolemmal extrusion mechanisms than in other commonly used mammalian species. Interestingly, mouse cardiac SR has an initial maximal Ca2+ uptake capability of ≈1.0 mM (kg wet wt)−1 (Luo et al. 1994, 1996), which is over 2-fold faster than in other species (e.g. rat and guinea-pig (McCollum, Besch, Entman & Schwartz, 1972; Levitsky, Benevolensky, Levchenko, Smirnov & Chazov, 1981; Wu & Feher, 1995)).
Effect of stimulation rate on contraction
Another distinctive feature of mouse cardiac muscle is its positive force-frequency relationship (Fig. 5). Species with short action potentials are generally assumed to exhibit a negative force-frequency relationship (Bers, 1993); clearly this is not the case for the mouse. Particularly notable is the dramatic increase (3.5-fold) in force from 1.0 to 4.0 Hz, a range over which Ca2+ transients increase only modestly (1.7-fold). The positive force-frequency relation at low stimulation rates (0.2-1.0 Hz) can be rationalized by increased activator Ca2+ availability (upstream regulation by Ca2+), since increases in force followed closely the increases in systolic [Ca2+]i. Mouse myocytes are known to have short action potentials as compared with other species (Binah et al. 1987; Nuss & Marban, 1994). This permits augmented Ca2+ entry per unit time at high stimulation frequencies without refractoriness. The SR may also facilitate the positive force-frequency relationship by quickly taking up the increased Ca2+ and releasing it subsequently. At higher stimulation rates (1.0-4.0 Hz), increases in twitch force cannot be explained simply by increases in Ca2+ transients, especially at frequencies of 2.0-4.0 Hz over which force continued to rise despite a fairly constant Ca2+ transient amplitude. Release of catecholamines at high stimulation rates may increase force, thus complicating the force-frequency relationship. However, such an effect was unlikely to account for the present results given the relatively constant Ca2+ transient amplitudes and the absence of acceleration of relaxation which would be expected in response to sympathetic stimulation (Okazaki et al. 1990; Dobrunz et al. 1995).
The phase-plane analysis at different frequencies reveals a leftward shift of the force-[Ca2+]i relations during twitch relaxation (Fig. 7). Previously, it has been shown that accelerating the kinetics of relaxation (i.e. increasing cross-bridge detachment rate and/or decreasing attachment rate) or slowing the decay of Ca2+ transients would tend to superimpose the force-[Ca2+]i relations during the twitch and during steady-state activation (Backx, Gao, Azan-Backx & Marban, 1994; Backx et al. 1995; Dobrunz et al. 1995). The kinetics of force relaxation seemed to be slowed at higher rates, as gauged by the phase-plane analysis (Fig. 7). The mechanism for this finding is not clear. One possible explanation would be the notion that mouse myocardium also demonstrates a strong downstream regulation of contraction, i.e. a rate-dependent increase in the force-generating ability of the cross-bridges after Ca2+ binding to troponin C. Increases in [Ca2+]i have been shown to increase levels of phosphorylation of myosin light chain II, which facilitates force generation in a positive feedback mechanism (Morano, 1993). It is possible, but as yet untested, that the Ca2+-calmodulin-dependent myosin light chain kinase plays a major role in the rate-dependent increase in force development we have observed in mouse cardiac trabeculae.
Our results are inconsistent with those of Wolska & Solaro (1996) who showed a negative shortening-frequency relationship in isolated mouse myocytes. This may be related to trauma in the process of enzymatic isolation of single cells, or to differences between unloaded cell shortening and loaded muscle contractions. With regard to the former possibility, enzymatically isolated cells are more prone to Ca2+ overload than is intact myocardium (Marban et al. 1989). Regarding the latter possibility, it is worth noting that, after potentiation, the recovery of twitch force usually follows an exponential course in the muscle (results not shown here, but see Wier & Yue, 1986, for examples in ferret muscle), while such a feature is virtually absent in these cells (Fig. 3 of Wolska & Solaro, 1996).
Steady-state force-[Ca2+]i relationship in mouse cardiac muscle
The steady-state force-[Ca2+]i relationship in mouse ventricular muscle differs significantly from that in other mammalian species (Fig. 8). Before speculating on the mechanism(s) underlying these differences, it is worth considering how the intracellular constituents are altered during tetanic contraction and how such alterations might affect the force-[Ca2+]i relationship. During prolonged, maximally-activated tetani in perfused ferret hearts at 30°C, Kusuoka, Weisfeldt, Zweier, Jacobus & Marban (1986) found a < 2.0 mM increase in inorganic phosphate, and no consistent changes in ATP concentration or intracellular pH. The increase in inorganic phosphate concentration was implicated in the ‘fatigue’ they observed during maintained contractions. In the present study, we did not observe any fatigue, i.e. force remained steady over the 4-6 s typically used to achieve tetanic stimulation. In any case, if changes comparable with those reported by Kusuoka et al. (1986) were operative here, they would be in the desensitizing direction, tending to minimize the highly co-operative activation which we have observed.
In intact mouse cardiac muscle, steady-state force-[Ca2+]i is shifted towards the left and is more co-operative than in skinned murine cardiac cells (Metzger et al. 1993). The differences, as demonstrated directly in our previous study in rat cardiac muscle (Gao et al. 1994), are genuine and are probably due to loss of endogenous sensitizers and/or changes in lattice spacing of the cross-bridges as a result of skinning.
Mouse cardiac muscle is less sensitive to Ca2+ when compared with rat and other mammalian species, with lower Fmax and higher Ca50 (Yue et al. 1986; Okazaki et al. 1990; Backx et al. 1995). Although the mechanism underlying these differences is not understood at present, a low sensitivity to Ca2+ may be physiologically advantageous to the mouse heart in vivo. Given the fact that the heart rate of the mouse is very high (≈500 beats min−1, perhaps the fastest among mammals (Farrell, 1991)), conventional myofilament properties might produce a ‘hypercontractile state’ at baseline with little room for further increases. The observed low sensitivity to Ca2+ would tend to offset the demands of the high stimulation rate, facilitating relaxation and increasing contractile reserve. This may well be reflected by the strong positive force- frequency relation seen at higher stimulation frequencies. Mouse muscle is also more co-operative after activation (e.g. Hill coefficient ≈10 vs.≈5 in rat (Gao et al. 1994; Backx et al. 1995)). This high co-operativity may explain, at least partially, the steeply positive force-frequency relationship.
Summary and implications for genetically altered mouse models
This study represents the first characterization of the relationship between force and intracellular calcium, both during twitch contractions and at steady-state, in mouse cardiac muscle. We have found that mouse ventricular myocardium responds to a wide range of [Ca2+]o, with no evidence of Ca2+ overload even at high [Ca2+]o. Mouse trabeculae also respond positively to increasing stimulation frequency, especially at frequencies greater than 1 Hz. The myofilaments are less sensitive to Ca2+ (as compared with other species) but more co-operative. This combination of physiological features of excitation-contraction coupling appears unique to mouse cardiac muscle.
Our results have important practical implications for the interpretation of phenotypic changes in genetically altered mice. Knockout of phospholamban has been reported to increase cardiac contraction and to accelerate relaxation, with no adverse long-term consequences (Luo et al. 1994). Likewise, overexpression of β2 receptors in transgenic mice boosts contractile force generation, with no indication of deleterious effects attributable to calcium overload (Rockman, Hamilton, Jones, Milano, Mao & Lefkowitz, 1996; Du, Vincan, Woodcock, Milano, Dart & Woodcock, 1996). The fact that mouse cardiac muscle is unusually resistant to calcium overload means that caution is merited in extrapolating the results of such studies directly to other species, particularly to humans. Our results give reason to believe that interventions which appear salutary in the mouse may, in some cases, turn out to be detrimental (or even dangerous) in patients. The distinctively low myofilament Ca2+ responsiveness of wild-type mouse ventricle also points out the need for careful controls when evaluating the phenotype of mutant or transgenic mice designed to express altered contractile proteins (cf. Geisterfer-Lowrance et al. 1996).
Acknowledgments
This work was supported by NIH (RO1 HL 44065) and by a fellowship from the CONICET of Argentina (N. G. P.).
References
- Backx PH, Gao WD, Azan-Backx MD, Marban E. Mechanism of force inhibition by 2,3-butanedione monoxime in rat cardiac muscle: roles of [Ca2+]i and cross-bridge kinetics. The Journal of Physiology. 1994;476:487–500. doi: 10.1113/jphysiol.1994.sp020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx PH, Gao WD, Azan-Backx MD, Marban E. The relationship between contractile force and intracellular [Ca2+] in intact rat cardiac trabeculae. Journal of General Physiology. 1995;105:1–19. doi: 10.1085/jgp.105.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers MD. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kluwer Academic Publishers; 1993. [Google Scholar]
- Binah O, Arieli R, Beck R, Rosen MR, Palti Y. Ventricular electrophysiological properties: is interspecies variability related to thyroid state? American Journal of Physiology. 1987;252:H1265–1274. doi: 10.1152/ajpheart.1987.252.6.H1265. [DOI] [PubMed] [Google Scholar]
- Capogrossi MC, Stern MD, Spurgeon HA, Lakatta EG. Spontaneous Ca2+ release from the sarcoplasmic reticulum limits Ca2+-dependent twitch potentiation in individual cardiac myocytes. A mechanism for maximum inotropy in the myocardium. Journal of General Physiology. 1988;91:133–155. doi: 10.1085/jgp.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogrossi MC, Suarez-Isla BA, Lakatta EG. The interaction of electrically stimulated twitches and spontaneous contractile waves in single cardiac myocytes. Journal of General Physiology. 1986;88:615–633. doi: 10.1085/jgp.88.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Backx PH, Yue DT. Steady-state [Ca2+]i-force relationship in intact twitching cardiac muscle: direct evidence for modulation by isoproterenol and EMD 53998. Biophysical Journal. 1995;69:189–201. doi: 10.1016/S0006-3495(95)79889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XJ, Vincan E, Woodcock DM, Milano CA, Dart AM, Woodcock EA. Response to cardiac sympathetic activation in transgenic mice overexpressing beta 2-adrenergic receptor. American Journal of Physiology. 1996;271:H630–636. doi: 10.1152/ajpheart.1996.271.2.H630. [DOI] [PubMed] [Google Scholar]
- Farrell AP. Circulation of body fluids. In: Prosser CL, editor. Environmental and Metabolic Animal Physiology-Comparative Animal Physiology. New York: Wiley-Liss, Inc.; 1991. pp. 509–558. [Google Scholar]
- Gao WD, Atar D, Backx PH, Marban E. Relationship between intracellular calcium and contractile force in stunned myocardium. Direct evidence for decreased myofilament Ca2+ responsiveness and altered diastolic function in intact ventricular muscle. Circulation Research. 1995;76:1036–1048. doi: 10.1161/01.res.76.6.1036. [DOI] [PubMed] [Google Scholar]
- Gao WD, Backx PH, Azan-Backx M, Marban E. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circulation Research. 1994;74:408–415. doi: 10.1161/01.res.74.3.408. [DOI] [PubMed] [Google Scholar]
- Gao WD, Liu Y, Mellgren R, Marban E. Intrinsic myofilament alterations underlying the decreased contractility of stunned myocardium. A consequence of Ca2+-dependent proteolysis? Circulation Research. 1996;78:455–465. doi: 10.1161/01.res.78.3.455. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance AA, Christe M, Conner DA, Ingwall JS, Schoen FJ, Seidman CE, Seidman JG. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996;272:731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- Hampton TG, Kranias EG, Morgan JP. Simultaneous measurement of intracellular calcium and ventricular function in the phospholamban-deficient mouse heart. Biochemistry and Biophysics Research Communications. 1996;226:836–841. doi: 10.1006/bbrc.1996.1437. 10.1006/bbrc.1996.1437. [DOI] [PubMed] [Google Scholar]
- Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. Journal of Clinical Investigation. 1996;97:533–539. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort AA, Capogrossi MC, Lakatta EG. Frequency, amplitude, and propagation velocity of spontaneous Ca2+-dependent contractile waves in intact adult rat cardiac muscle and isolated myocytes. Circulation Research. 1985;57:844–855. doi: 10.1161/01.res.57.6.844. [DOI] [PubMed] [Google Scholar]
- Kort AA, Lakatta EG. Calcium-dependent mechanical oscillations occur spontaneously in unstimulated mammalian cardiac tissues. Circulation Research. 1984;54:396–404. doi: 10.1161/01.res.54.4.396. [DOI] [PubMed] [Google Scholar]
- Kort AA, Lakatta EG. Spontaneous sarcoplasmic reticulum calcium release in rat and rabbit cardiac muscle: relation to transient and rested-state twitch tension. Circulation Research. 1988;63:969–979. doi: 10.1161/01.res.63.5.969. [DOI] [PubMed] [Google Scholar]
- Kurihara S. Regulation of cardiac muscle contraction by intracellular Ca2+. [Review] Japanese The Journal of Physiology. 1994;44:591–611. doi: 10.2170/jjphysiol.44.591. [DOI] [PubMed] [Google Scholar]
- Kusuoka H, Weisfeldt ML, Zweier JL, Jacobus WE, Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circulation Research. 1986;59:270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Lappe DL, Lakatta EG. Intensity fluctuation spectroscopy monitors contractile activation in ‘resting’ cardiac muscle. Science. 1980;207:1369–1371. doi: 10.1126/science.7355295. [DOI] [PubMed] [Google Scholar]
- Levitsky DO, Benevolensky DS, Levchenko TS, Smirnov VN, Chazov EI. Calcium-binding rate and capacity of cardiac sarcoplasmic reticulum. Journal of Molecular and Cellular Cardiology. 1981;13:785–796. doi: 10.1016/0022-2828(81)90236-4. 10.1016/0022-2828(81)90236-4. [DOI] [PubMed] [Google Scholar]
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circulation Research. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- Luo W, Wolska BM, Grupp IL, Harrer JM, Haghighi K, Ferguson DG, Slack JP, Grupp G, Doetschman T, Solaro RJ, Kranias EG. Phospholamban gene dosage effects in the mammalian heart. Circulation Research. 1996;78:839–847. doi: 10.1161/01.res.78.5.839. [DOI] [PubMed] [Google Scholar]
- McCollum WB, Besch HR, Jr, Entman ML, Schwartz A. Apparent initial binding rate of calcium by canine cardiac-relaxing system. American Journal of Physiology. 1972;223:H608–614. doi: 10.1152/ajplegacy.1972.223.3.608. [DOI] [PubMed] [Google Scholar]
- Marban E, Robinson SW, Wier WG, Kitakaze M, Pike MM, Yue DT, Chacko VP. Calcium metabolism in experimentally dissociated rat heart cells and in intact perfused ferret hearts. In: Sideman S, Beyar R, editors. Analysis and Simulation of The Cardiac System-Ischemia. Boca Raton, FL, USA: CRC Press Inc.; 1989. pp. 422–435. [Google Scholar]
- Metzger JM, Parmacek SM, Barr E, Pasyk K, Lin W, Cochrane KL, Field LJ, Leiden JM. Skeletal troponin C reduces contractile sensitivity to acidosis in cardiac myocytes from transgenic mice. Proceedings of the National Academy of Sciences of the USA. 1993;90:9036–9040. doi: 10.1073/pnas.90.19.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano I. Myosin light chain phosphorylation and myosin isoenzyme expression regulate cardiac calcium sensitivity by modulation of cross-bridge cycling kinetics. In: Lee JA, Allen DG, editors. Modulation of Cardiac Calcium Sensitivity. A New Approach to Increasing the Strength of the Heart. Oxford: Oxford University Press Inc.; 1993. pp. 178–196. [Google Scholar]
- Nilius B, Boldt W, Benndorf K. Properties of aconitine-modified sodium channels in single cells of mouse ventricular myocardium. General Physiology and Biophysics. 1986;5:473–484. [PubMed] [Google Scholar]
- Nuss HB, Marban E. Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. The Journal of Physiology. 1994;479:265–279. doi: 10.1113/jphysiol.1994.sp020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki O, Suda N, Hongo K, Konishi M, Kurihara S. Modulation of Ca2+ transients and contractile properties by beta-adrenoceptor stimulation in ferret ventricular muscles. The Journal of Physiology. 1990;423:221–240. doi: 10.1113/jphysiol.1990.sp018019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Hamilton RA, Jones LR, Milano CA, Mao L, Lefkowitz RJ. Enhanced myocardial relaxation in vivo in transgenic mice overexpressing the beta 2-adrenergic receptor is associated with reduced phospholamban protein. Journal of Clinical Investigation. 1996;97:1618–1623. doi: 10.1172/JCI118587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, IA, USA: Iowa University Press; 1989. [Google Scholar]
- Wier WG, Yue DT. Intracellular calcium transients underlying the short-term force-interval relationship in ferret ventricular myocardium. The Journal of Physiology. 1986;376:507–530. doi: 10.1113/jphysiol.1986.sp016167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York, NY: McGraw-Hill Inc.; 1989. [Google Scholar]
- Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. American Journal of Physiology. 1996;271:H1250–1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- Wu QY, Feher JJ. Effect of ischemia and ischemia-reperfusion on ryanodine binding and Ca2+ uptake of cardiac sarcoplasmic reticulum. Journal of Molecular and Cellular Cardiology. 1995;27:1965–1975. doi: 10.1016/0022-2828(95)90018-7. 10.1016/0022-2828(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Yue DT, Marban E, Wier WG. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. Journal of General Physiology. 1986;87:223–242. doi: 10.1085/jgp.87.2.223. 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


