Abstract
Our primary purpose was to test the hypothesis that the tongue protrudor (genioglossus, GG) and retractor (styloglossus, SG and hyoglossus, HG) muscles are co-activated when respiratory drive increases, and that co-activation will cause retraction of the tongue. This was addressed by performing two series of experiments using a supine, anaesthetized, tracheotomized rat in which tongue muscle force and the neural drive to the protrudor and retractor muscles could be measured during spontaneous breathing. In the first series of experiments, respiratory drive was increased progressively by occluding the tracheal cannula for thirty respiratory cycles; in the second series of experiments, the animals were subjected to hyperoxic hypercapnia and poikilocapnic hypoxia.
Airway occlusion for thirty breaths caused progressive, quantitatively similar increases in efferent motor nerve activity to protrudor and retractor tongue muscles. Net tongue muscle force was always consistent with tongue retraction during occlusion, and peak force rose in parallel with the neural activites. When airway occlusion was repeated following section of the lateral XIIth nerve branch (denervation of retractor muscles) the tongue either protruded (15/21 animals; 10 ± 2 mN at the 30th occluded breath) or retracted weakly (6/21 animals; 6 ± 2 mN at 30th occluded breath).
To ensure that our findings were not the result of damage to the muscle nerves, occlusion experiments were also done in eight animals in which GG EMG activity was recorded instead of nerve activities. Changes in peak integrated GG electryomyogram (EMG) activity and peak retraction force during occlusion were highly correlated (r2= 0.86, slope = 1.05).
In separate experiments in fourteen rats, we found that hyperoxic hypercapnia and poikilocapnic hypoxia also result in parallel increases in the respiratory-related EMG activity of the GG and HG muscles. Also, as in the occlusion experiments, augmentations of protrudor and retractor muscle EMG activities were associated with parallel changes in tongue retraction force.
These studies in anaesthetized rats demonstrate that tracheal occlusion and independent stimulation of central or peripheral chemoreceptors results in inspiratory-related co-activation of the protrudor and retractor muscles, and proportional changes in tongue retraction force. These observations also demonstrate that recording GG EMG activity in isolation could lead to erroneous conclusions about respiratory-related movements of the tongue.
Tongue position is controlled primarily by the mechanical actions of the extrinsic tongue muscles, which are innervated by separate branches of the hypoglossal (XIIth) nerve. The genioglossus (GG) muscle, which causes tongue protrusion, is innervated by the medial XIIth branch, while the hyoglossus (HG) and styloglossus (SG) muscles, which cause retraction of the tongue, are innervated by the lateral XIIth branch. A recent study showed that stimulating the XIIth nerve before its bifurcation into medial and lateral branches (i.e. co-activating the tongue protrudor and retractor muscles) caused tongue retraction in human subjects (Eisele, Smith, Alam & Schwartz, 1997). Consistent with these data, Gilliam & Goldberg (1995), using a rat model, found that the retractor muscles produce 10-20 times more force than the GG muscle, which explains why co-activation of both muscles results in strong tongue retraction. Thus, in both humans and rats, when protrudor and retractor muscle groups are activated maximally and simultaneously by electrical stimulation of the whole hypoglossal nerve, the tongue will retract. It is not clear, however, if the retractor and protrudor muscles are co-activated during spontaneous breathing, or how the activity of these antagonist muscles influences respiratory-related tongue movements.
A recent study in anaesthetized dogs (Yasui et al. 1993) showed phasic, inspiratory-related SG muscle EMG activity during hypoxia and hypercapnia, and found that the apnoeic threshold for SG activity was below that for the GG. Accordingly, the present experiments were designed to test the hypothesis that progressive increases in respiratory drive are associated with co-activation of the extrinsic tongue muscles, and retraction of the tongue. This was done by adapting the model of Gilliam & Goldberg (1995), which allowed us to make simultaneous measurements of tongue muscle force and the neural drive to both tongue protrudor and retractor muscles during breathing in anaesthetized, tracheotomized rats. Our hypothesis was confirmed, and the significance of these observations for the maintenance of pharyngeal airway patency during spontaneous breathing is discussed.
METHODS
Animals and surgical procedure
Male Sprague-Dawley rats weighing 194-494 g were used for all but three experiments, which were performed on cats (3.3-3.6 kg). All procedures adhered to the guidelines established by the Institutional Animal Care and Use Committee at the University of Arizona. Animals were initially anaesthetized with 2-4 % halothane (balance O2). Following halothane anaesthesia, rats were intraperitoneally injected with urethane (1.3 g kg−1) and atropine sulphate (0.5 mg kg−1). Subsequent doses of urethane (0.3 g kg−1) were administered as needed. For the cats, halothane was slowly withdrawn while a bolus of α-chloralose (35 mg kg−1) and urethane (200 mg kg−1) was slowly injected into a femoral vein, as described previously (Fregosi & Mitchell, 1994). Supplemental doses of α-chloralose (5 mg kg−1) and urethane (20 mg kg−1) were given as needed. No surgical procedures were performed until animals were unresponsive to intense pressure applied with haemostats to the paws and tail. During all surgical and experimental procedures animals were supine with limbs secured to the operating table. At the conclusion of all experiments, animals were killed with sodium pentobarbitone (200 mg kg−1).
Rectal temperature was monitored with a thermistor (Yellow-Springs Instruments) and maintained at 37°C with the use of a servo-controlled heating lamp. The trachea was cannulated caudal to the larynx to ensure airway patency during the surgery and to permit subsequent tracheal occlusions. The GG and HG muscles, and XIIth nerves were exposed bilaterally using a ventral approach (Gilliam & Goldberg, 1995). Following isolation, the XIIth nerves were bathed in mineral oil. A suture was passed through the tip of the tongue at the mid-line frenulum for subsequent measurements of the direction and force of tongue movements (see ‘Tongue force measurements’). The upper jaw was secured to the surgery table using a wire frame; this prevented the head from moving without interfering with the measurement of tongue muscle force (see Fig. 1).
Figure 1. Schematic diagram of model used to study the behaviour and mechanical actions of the extrinsic tongue muscles in the rat.
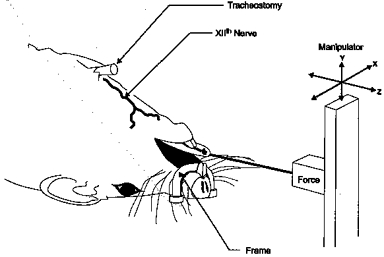
The model allows us to stimulate or record the activity of the XIIth nerves or their branches, to perform tracheal airway occlusion, to control inspired gases, to measure protrusive or retractive tongue forces, and to record EMG activity of the extrinsic tongue muscles (see text for explanation).
Delivery of inspired gases and monitoring of expired and blood gases
Mixtures of O2, CO2 and N2 were delivered to the animal by connecting the outflow port of a rotometer to the tracheostomy cannula with a t-tube. The rate of airflow was set about 3 times greater than the ventilatory rate of the animal. This system allows rapid and precise adjustments of the CO2 and O2 concentrations of the inspired air in the spontaneously breathing animal (Fregosi, 1994). End-tidal CO2 (ETCO2) concentrations were monitored with a rapidly responding CO2 analyser (model 1265, Novametrix) placed on the expired side of the t-tube. Femoral artery blood samples were taken immediately before and after a thirty breath tracheal occlusion in one rat, and the partial pressures of O2 (PO2) and CO2 (PCO2) were determined with a blood gas analyser (model BGM, Cameron Instruments).
Electromyogram (EMG) and electroneurogram (ENG) recordings
EMG activities of the GG, HG and diaphragm (Dia) were recorded by inserting two fine wire (diameter, 0.125 mm; Formvar, California Fine Wire) electrodes into each muscle (Fregosi, 1994). The wires were insulated except for the terminal 2 mm. If GG and HG EMG activities were not abolished after section of the medial and lateral XIIth nerve branches, respectively, we assumed poor electrode placement and discarded the data. ENGs were recorded by placing the medial and lateral XIIth nerve branches across bipolar, stainless-steel hook electrodes (tip diameter, 0.5 mm). The EMG and ENG signals were amplified and filtered (30-3000 Hz) with AC coupled differential amplifiers (model 7P511K, Grass), rectified, and moving time averages obtained with a time constant of 100 ms (model S76-01, Coulbourn Instruments).
Tongue force measurements
Tongue force was measured by attaching the tongue to an isometric force transducer (model TRN001, Kent Scientific or model FT03, Grass) via the tongue suture (Gilliam & Goldberg, 1995; Fig. 1). With this system tongue retraction pulls, or loads, the transducer and protrusion releases, or unloads, the transducer. The force transducer was mounted on a micromanipulator that allowed precise control of the position of the force transducer in three dimensions (Fig. 1). To record both protrusion and retraction of the tongue, tension must be placed on the line connecting the tongue to the force transducer. In an effort to standardize the line tension, the relationship between muscle twitch force and line tension for both protrudor and retractor muscles was initially established in all experiments, although these data were only recorded in eleven animals. The line tension was first set at zero (i.e. a ‘slack’ line), and the transducer was then moved away from the animal in 1 mm increments. The peak muscle twitch force and the passive line tension were recorded at each length. In this manner the optimal line tension is determined for both protrudor and retractor muscles, and used throughout each experiment. If the optimal line tension for the protrudor and retractor muscle twitch forces differed, the tension was set at the average of the two values. As shown in Fig. 2, the relationships between line tension and twitch force for protrudor and retractor muscles are entirely consistent with the length-twitch force relationships obtained for various limb muscles, which increases our confidence in the measurements of tongue force.
Figure 2. Representative twitch forces, and the relationship between line tension and protrudor and retractor muscle twitch force.
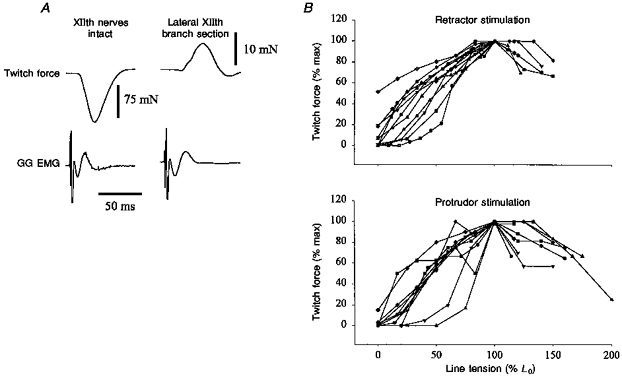
A, representative twitch forces with XIIth nerves intact and following lateral XIIth branch section (i.e. retractor muscle denervation). The retractive force produced by intact XIIth nerve stimulation was much greater than the protrusive force that resulted from XIIth nerve stimulation following lateral branch section (note the difference in force calibration between the two twitches). B, relationship of line tension and twitch force for the retractor and protrudor muscles. The tongue was pulled out of the mouth in 1 mm increments and the twitch force of the protrudor and retractor muscles was recorded at each increment, beginning with the tongue completely unloaded (i.e. a ‘slack line’). Twitches were evoked by either direct stimulation of the GG and HG or the medial and lateral XIIth nerve branches. The top panel shows the response for retractor muscle stimulation while the bottom panel demonstrates the results of protrudor stimulation. The tension in the line connecting the tongue to the force transducer is expressed as a percentage of the tension that yielded the peak twitch force (L0). The twitch forces are expressed as a percentage of the peak force obtained at any point in the experiment.
Nevertheless, a major concern is that the mechanical actions of the tongue muscles were influenced by the method used to record tongue force, primarily for three reasons. First, during all experiments, the mouth was held open with the use of a small retractor because the lower jaw of the animal often prevented the tongue from protruding fully during medial XIIth nerve branch stimulation. Second, retraction of the tongue, which loads the transducer, results in an isometric contraction of the retractor muscles in this system. In contrast, protrusion of the tongue unloads the transducer and results in concentric contractions of the GG. Therefore, based on the skeletal muscle force-velocity relationship, our force data are probably biased towards the retractor muscles (i.e. these muscles are on a more advantageous portion of their force-velocity curve). Third, we have noted tongue depression at times during both XIIth nerve stimulation and tracheal occlusion. Since depression of the tongue will load the force transducer, it will be read as a retraction force; this action may overestimate the real tongue retraction force. Because of these concerns we conducted separate experiments in which we watched movements of the tongue during XIIth nerve stimulation and airway occlusion under conditions where no manipulation of the tongue or mouth was performed. In these experiments we observed the same retractive and protrusive movements described in Results. In support of these observations, Hellstrand (1981) has shown that hypoglossal nerve stimulation-induced tongue muscle contractile properties are similar whether they are measured with the tongue attached to a transducer, or with the tongue unattached and measured by light reflection.
We were also concerned that the intrinsic tongue muscles may have influenced our force recordings. Axons of the intrinsic tongue muscle motoneurones run in both the medial and lateral branches of the XIIth nerve (Gilliam & Goldberg, 1995), so it was impossible to avoid stimulating these muscles. The intrinsic tongue muscles can alter the size and shape of the tongue, and may contribute to protrusive tongue movements (Smith & Kier, 1989). Although the contribution of the intrinsic tongue muscles cannot be discounted, we feel that the tongue forces measured reflect primarily activation of the extrinsic tongue muscles. We say this because the twitch force evoked by direct GG stimulation and medial XIIth nerve branch stimulation are not significantly different (D. Fuller, J. Sullivan & R. F. Fregosi, unpublished observations). Thus, although our methods may influence the quantitative assessment of tongue muscle force, our ability to determine whether the tongue is protruding or retracting is not hindered by the measurement apparatus.
Nerve stimulation
Both XIIth nerves were placed across bipolar, stainless-steel hook electrodes (separated by approximately 2 mm) with a tip diameter of 0.5 mm (Fregosi & Mitchell, 1994). The electrodes were mounted on a micromanipulator and connected to a constant current stimulus isolation unit (SIU; model PSIU6, Grass) and stimulator (model S48, Grass). The nerves were placed on the electrodes proximal to their bifurcation into medial and lateral branches. In all experimental protocols the duration of the stimulus pulse was 0.1 ms, and the applied current was supramaximal (i.e. 10-25 % above the level needed to evoke a maximal twitch). This resulted in currents that ranged from 40 to 80 μA.
Experimental protocol
Series 1: tracheal occlusion
After approximately 1 min of control data were obtained, the tracheal cannula was occluded at end-expiration for thirty to thirty-five respiratory efforts. Once the XIIth ENG activities returned to baseline levels the lateral branches of the XIIth nerves were cut (i.e. retractor muscle denervation), and tracheal occlusion was repeated. The remaining branches of both XIIth nerves were then sectioned, and the occlusion test was done a third time. This last experiment served as an important control for force artifacts induced by muscles not innervated by the XIIth nerve (see below). Following all nerve sections, the central cut end of each nerve branch was left on the recording electrodes, to allow efferent ENG activity to be monitored. Tracheal occlusion experiments were performed on a total of twenty-one animals; tongue force was recorded in all twenty-one, and XIIth nerve recordings were made in nine rats. In eight rats, GG EMG activity was recorded in place of XIIth ENG activity to insure that the force measurements were not influenced by the nerve manipulations.
Series 2: hypoxia and hypercapnia
In these experiments tongue force and EMG activity of the GG, HG and Dia muscles were recorded. Two minutes of control data were obtained with the animals breathing oxygen. The animals were then subjected to four different levels of hypoxia (FI,O2 (inspired oxygen fraction) = 1.0, 0.21, 0.15 and 0.10) or hyperoxic hypercapnia (FI,CO2 (inspired carbon dioxide fraction) = 0, 0.04, 0.07 and 0.10; balance O2). Each inspired gas level was held until EMG activities were stable (approximately 3 min), and the inspirate was then changed. In all cases the inspired gas levels were applied successively, without intervening periods of hyperoxia. The order of presentation of the hypoxic and hypercapnic trials was randomized. At the conclusion of the hypoxic and hypercapnic trials, a two to three breath airway occlusion was performed and the response was used to define the maximum EMG activity of the GG, HG and Dia. These experiments were completed in fourteen rats.
Series 3: measurement of tongue muscle contractile properties
In fifteen rats, five supramaximal shocks were delivered to the whole XIIth nerves while tongue force was monitored; the lateral XIIth nerve branches were then sectioned and nerve stimulation was repeated.
Data analysis
General
Tongue force, EMG and ENG signals were recorded on VCR tapes following pulse code modulation (model 4000, Vetter). The tongue force, ETCO2 and integrated EMG and ENG activities were recorded directly onto a Grass model 79 polygraph. Muscle contractile properties (twitch force, time-to-peak force (TTP), and half-relaxation time (T½)) were analysed using CODAS computer software (Dataq Instruments, Akron, OH, USA). Peak tongue force, peak integrated GG, HG, and Dia EMG activities, peak integrated XIIth nerve ENG activities, breathing frequency, and ETCO2 were analysed from the polygraph tracings. The neural minute ventilation was calculated by multiplying the breathing frequency (breaths min−1) by the peak diaphragm EMG activity (% max). For all airway occlusion experiments, data from each of the first thirty occluded respiratory efforts were analysed. For the hypoxic and hypercapnic experiments, all breaths in the final 30 s of each inspired gas level were analysed.
Estimation of SG, HG and GG muscle force
The innervation of the extrinsic tongue muscles allows for relatively simple measurement of extrinsic tongue muscle force production during XIIth nerve stimulation. Stimulation of the whole XIIth nerve proximal to its bifurcation activates the GG, SG, HG and intrinsic tongue muscles (Hellstrand, 1981). We therefore were able to assess the effects of co-activating these muscles on the direction and magnitude of tongue force. When these experiments were repeated following lateral XIIth branch section (i.e. denervation of the retractive musculature), only the GG and intrinsic tongue muscles were activated (Hellstrand, 1981) and this enabled the estimation of the protrusion force produced by the GG muscle (see above). The same method was used to estimate tongue muscle force during spontaneous breathing, although we had to account for movement artifacts that occurred during tracheal occlusion. Despite our efforts to stabilize the animal, we noted that postural movements introduced a varying degree of artifact during airway occlusion. In an effort to quantify this artifact, we repeated the protocol following complete bilateral XIIth nerve section at the conclusion of all experiments. Any tongue force subsequently measured was classified as an artifact induced by actions of muscles other than the extrinsic tongue muscles. Therefore, we estimated the GG protrusion force to be the difference between the force recorded with lateral and whole XIIth nerve section. Similarly, the retractive force of the SG and HG was calculated as the difference between the force recorded with XIIth nerves intact and following lateral branch section.
Statistics
Contractile properties of the protrudor and retractor muscles were compared with a paired t test. The influence of sustained tracheal occlusion on force, EMG and ENG activities was analysed by subjecting each of the thirty occluded breaths to one-way analysis of variance. The effect of nerve section on the response to tracheal occlusion was analysed using a paired t test with the Bonferroni correction factor for multiple comparisons. The relationship between changes in GG EMG activity and changes in the direction and magnitude of tongue force was assessed with linear regression analysis. The influence of hypoxia and hypercapnia on all parameters was tested using a two-way analysis of variance. All data are presented as the mean ±s.e.m. The level of significance was set at P < 0.05.
RESULTS
Series 1: tracheal occlusion
In all rats, parallel increases in the respiratory-related activity of the medial and lateral XIIth nerve branches were observed during end expiratory airway occlusion as shown in Figs 3 and 4 (left panels). Also note that co-activation of motoneurones to protrudor and retractor muscles resulted in progressively greater retractions of the tongue as the occlusion persisted. The mean XIIth ENG and tongue force responses to airway occlusion (Fig. 5) are consistent with the records shown in Figs 3 and 4, in that progressive increases in medial and lateral nerve branch activities were associated with parallel increases in tongue retraction force.
Figure 3. Sample record showing the typical response of tongue force, and medial and lateral XIIth ENG activities to end-expiratory tracheal occlusion.
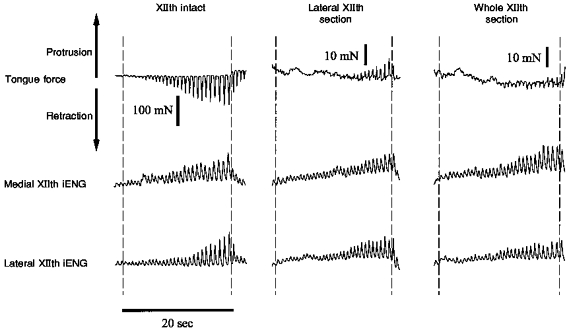
Three airway occlusion tests were performed: (1) with XIIth nerves intact (left panel); (2) following lateral XIIth nerve section (middle panel); and (3) following whole XIIth nerve section (right panel). Following lateral or medial XIIth nerve branch section (middle and right panels), the central end of the cut nerve was placed on the electrodes to enable recording of efferent motor nerve activity. In each panel, the dashed lines demarcate the period of tracheal occlusion. Occlusion produced forceful tongue retraction with XIIth nerves intact, and weak tongue protrusion following lateral XIIth nerve branch section. Note the parallel increases in activity of the medial and lateral XIIth nerve branches under all three conditions, indicating co-activation of the tongue retractor and protrudor muscles during airway occlusion. iENG, integrated electroneurogram.
Figure 4. Sample recording showing the alternate response of tongue force to end-expiratory tracheal occlusion.
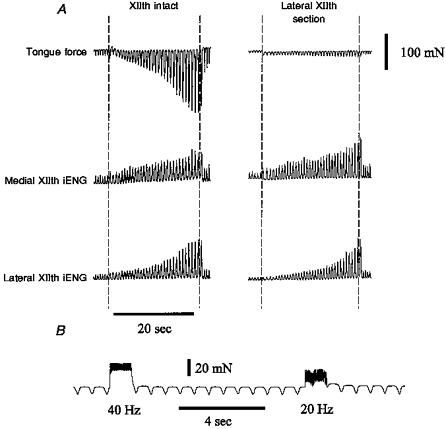
A, in a few animals, airway occlusion following lateral XIIth branch section did not result in protrusion of the tongue. Nevertheless, nerve section essentially abolished the tongue force response to occlusion. B, tongue force trace showing the effects of medial XIIth nerve branch stimulation in the same animal. The medial XIIth nerve branches were stimulated at 40 and 20 Hz to show that the tongue was capable of protruding during the occlusion experiment shown in A.
Figure 5. Mean tongue force and medial and lateral XIIth nerve branch activity over thirty occluded breaths with XIIth nerves intact.
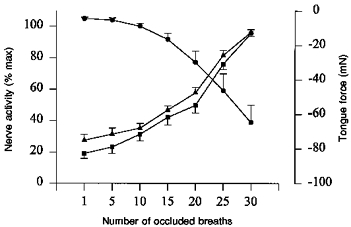
•, tongue force; ▴ and ▪, medial and lateral XIIth nerve branch activity, respectively. Retraction of the tongue is indicated by a negative tongue force. Note the similar increases in the activity of the medial and lateral XIIth nerve branches over the course of the airway occlusion (n= 9).
Two tongue force responses were seen following section of the lateral XIIth nerve branch (retractor muscle denervation). In the majority of experiments (5/9), occlusion following lateral XIIth section resulted in tongue protrusion after roughly ten occluded efforts (Fig. 3, middle panel), whereas in the remaining experiments, (4/9) weak tongue retraction forces were observed (Fig. 4, right panel). Medial XIIth nerve branch stimulation produced clear tongue protrusion in the latter experiments (Fig. 4B), indicating that the medial branches were not damaged and that the tongue was capable of protruding during the occlusion. Nevertheless, the main point is that denervation of the retractor muscles greatly decreased the respiratory-related tongue forces recorded during airway occlusion, whether or not occlusion produced protrusion or weak retraction following lateral XIIth nerve section.
To ensure that manipulation of the hypoglossal nerve branches did not influence the recording of tongue force, in separate experiments we recorded both tongue force and GG EMG activity in response to tracheal occlusion in ten animals, although EMG data were discarded in two of these animals. With XIIth nerves intact, end expiratory tracheal occlusion resulted in retractive tongue movements and progressive increases in GG EMG activity over the course of the occluded inspiratory efforts (Fig. 6, left panel). When we repeated the occlusion following lateral XIIth branch section we observed similar increases in the GG EMG, but the force produced by the tongue was greatly reduced and in most animals (8/10) the tongue protruded (Fig. 6, middle panel). As with the nerve recording experiments, a few (2/10) of the animals demonstrated tongue retraction forces after lateral branch section. In all occlusion experiments, section of all XIIth nerve branches resulted in small artifactual tongue forces during occluded respiratory efforts (Figs 3 and 6; right panels).
Figure 6. Sample record showing the effects of end-expiratory tracheal occlusion on tongue force and GG EMG.
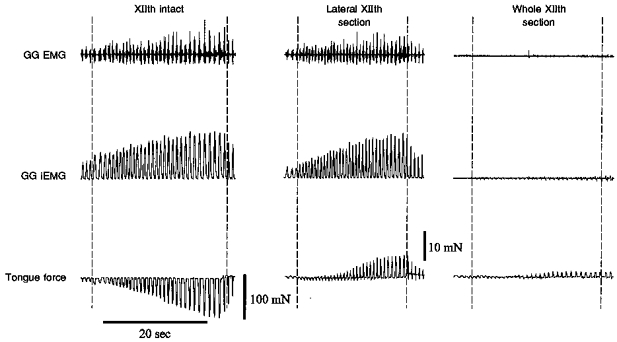
The occlusion experiment was performed with XIIth nerves intact (left panel), following lateral XIIth nerve branch section (middle panel), and following whole XIIth nerve section (right panel). In each panel, the trachea was occluded between the dashed lines. With XIIth nerves intact, airway occlusion produced retractive tongue movements despite increases in GG EMG activity. Following lateral XIIth branch section (i.e. retractive muscle denervation) tongue force was primarily protrusive. Complete XIIth nerve section abolished the GG EMG response, but small force fluctuations remained (see text).
The relationship between GG EMG activity and tongue force during tracheal occlusion is shown in Fig. 7. During tracheal occlusion with XIIth nerves intact the GG EMG activity and the estimated retraction force of the SG and HG muscles were linearly related (top panel of Fig. 7). However, after lateral branch section, a similar relationship was observed (bottom panel of Fig. 7). In other words, GG EMG activity was correlated with tongue force during airway occlusion whether or not the tongue was retracting (top panel) or protruding (bottom panel).
Figure 7. Relationship between respiratory-related GG EMG activity and extrinsic tongue muscle force over thirty occluded breaths.
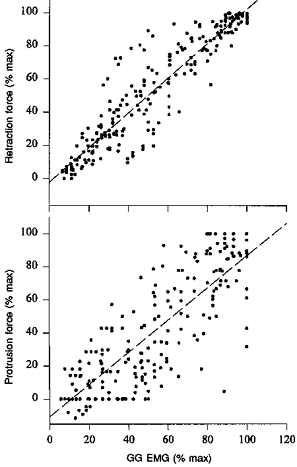
The top panel demonstrates the association between tongue retraction force and peak GG EMG activity. The bottom panel shows the relationship between tongue protrusion force and peak GG EMG activity. Peak GG EMG activity and tongue muscle force are expressed as a percentage of the maximum activity observed during airway occlusion. Respiratory-related GG EMG activity correlated well with both retraction force (r2= 0.86, slope of regression line = 1.05) and protrusion force (r2= 0.70, slope of regression line = 0.98). The data are pooled from eight rats. All force values were corrected for artifact, as described in Methods.
In an effort to determine the likely arterial blood gas levels that were present at the end of thirty occluded breaths, we repeated the tracheal occlusion study in one rat in which arterial blood gases were measured immediately before and after the occlusion trial. The Pa,O2 decreased from 70 to 17 mmHg, and Pa,CO2 increased from 30 to 47 mmHg in this animal.
Series 2: hypercapnia and hypoxia
Both poikilocapnic hypoxia and hyperoxic hypercapnia evoked parallel recruitment of the GG and HG, yet the net tongue force was always retractive. An example of the changes in tongue force and GG and HG EMG activities in response to hypercapnia is shown in Fig. 8; mean responses are given in Fig. 9. Note that hypercapnia caused increases in drive to the GG and HG muscles, and that tongue muscle retraction force increased in parallel. Breathing frequency increased non-significantly from 84 ± 3 to 87 ± 5 breaths min−1 as FI,CO2 was increased from 0 to 0.10, but the ‘neural minute ventilation’ (frequency × Dia EMG activity (% max)) increased from 5010 ± 305 to 6204 ± 652 (P < 0.05), and PET,CO2 increased from 44 ± 1 to 55 ± 1 Torr (P < 0.05).
Figure 8. Sample record showing the effects of hypercapnia on tongue force, HG, GG and Dia EMG, and PET,CO2.
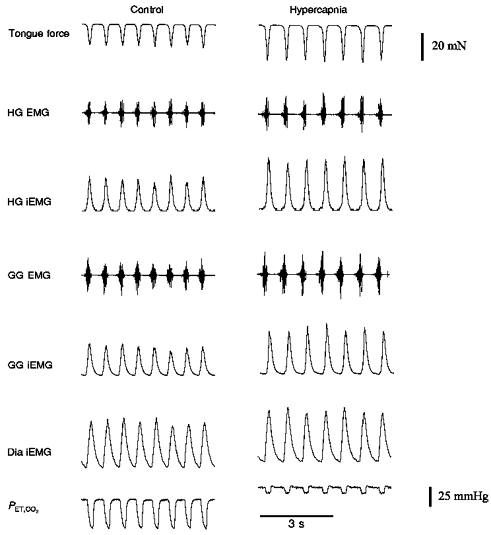
In all animals, hypercapnia caused parallel increases in the neural drive to both protrudor and retractor muscles. Control, FI,O2= 1.0; hypercapnia, FI,CO2= 0.07 (balance O2).
Figure 9. Mean GG, HG, and Dia EMG activities and tongue force during progressive hyperoxic hypercapnia.
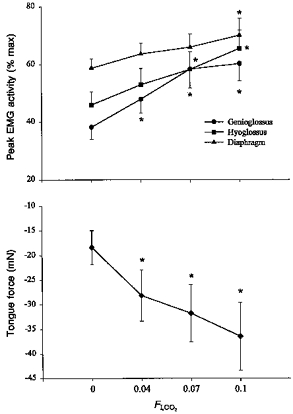
Note that increasing FI,CO2 from 0 to 0.10 resulted in co-activation of GG and HG muscles, and tongue retraction. * Different from FI,CO2= 0.
Poikilocapnic hypoxia had little effect on GG and HG EMG activities until FI,O2 fell to 0.10. This severe hypoxic level evoked parallel increases in GG and HG EMG activities, and strong tongue retractions (Fig. 10). Breathing frequency increased non-significantly from 85 ± 4 to 95 ± 5 breaths min−1 as FI,O2 fell from 1.0 to 0.10, while neural minute ventilation increased from 4794 ± 497 to 6636 ± 841 (P < 0.05). The PET,CO2 fell from 44 ± 1 to 42 ± 2 Torr, but the change was not significant.
Figure 10. Mean GG, HG, and Dia EMG activities and tongue force during progressive poikilocapnic hypoxia.
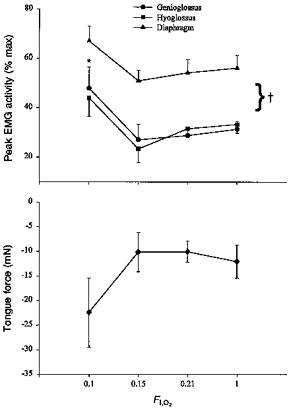
As FI,O2 was lowered from 1.0 to 0.10, the GG and HG responded in parallel, and the tongue retracted. No significant changes were seen until FI,O2 reached 0.10. * Different from FI,O2= 1.0; † different from Dia EMG response.
Series 3: tongue muscle conractile properties
An example of the tongue muscle twitch force evoked by bilateral XIIth nerve stimulation is shown in Fig. 2A. Note that the force produced by intact whole XIIth nerve stimulation (co-activation of protrudor and retractor muscles) caused tongue retraction, producing a twitch force of 93 ± 7 mN, a time-to-peak force (TTP) of 19.6 ± 0.7 ms, and a half-relaxation time (T½) of 12.5 ± 0.4 ms. Following lateral XIIth branch section, twitch force was protrusive (5.6 ± 0.9 mN; P < 0.05), and TTP and T½ averaged 15.5 ± 1.0 ms and 12.5 ± 0.4 ms, respectively.
Given that the majority of the published data on tongue muscle activities and pharyngeal airway mechanics have been done in the cat, we wished to insure that our observations and those of Gilliam & Goldberg (1995) were not unique to the rat. Accordingly, we examined the influence of selective hypoglossal nerve branch stimulation on tongue muscle contractile properties in three cats using the same system developed for the rat (Fig. 1). Although the absolute tongue forces were higher and the twitch kinetics slower in the cat, the data are qualitatively similar in that bilateral stimulation of the XIIth nerves evoked powerful tongue retraction (twitch force = 594.7 ± 197.6 mN, TTP = 49.9 ± 0.9 ms; T½= 30.7 ± 2.4 ms), and stimulation after lateral XIIth nerve section resulted in weak tongue protrusion (twitch force = 110 ± 37.8 mN, P < 0.05; TTP = 43.4 ± 7.8 ms; T½= 28.4 ± 1.9 ms).
DISCUSSION
Our major finding is that the muscles that protrude and retract the tongue are co-activated when respiratory drive is increased, and that co-activation is always associated with tongue retraction force during inspiration. Our twitch force data show that the retractor muscles produce more force than the GG in both rat and cat, which confirms recent reports in the rat (Gilliam & Goldberg, 1995) and human (Eisele et al. 1997).
Critique of methods
Before discussion of the major findings, there are two methodological issues that should be addressed. First, tracheal occlusion in this preparation removes lung volume feedback, which has been shown to have an inhibitory influence on upper airway motor activity (Kuna, 1986; Gauda, Carrol, Schwartz, Smith & Fitzgerald, 1994). We have recently examined the effects of lung volume feedback on the respiratory-related EMG activities of the GG and HG in the rat and found that lung inflation causes proportional inhibition of the drive to both muscles (Fregosi & Fuller, 1997). Thus, it is likely that the increase in neural drive to the tongue muscles observed within the first few occluded respiratory cycles was due, in part, to removal of phasic volume feedback in these vagally intact animals. However, this influence probably had little impact on the activity recorded on the 3rd to the 30th occluded cycles; rather, the progressive increase in drive to the tongue muscles as the occlusion persisted was probably the result of increases in central and peripheral chemoreceptor stimulation as the animals became increasingly more hypercapnic and hypoxic.
Second, during intact XIIth nerve recordings, the ENG signal could represent both efferent and afferent activity. O'Reilly & Fitzgerald (1990) examined the fibre composition of the hypoglossal nerve in the rat and found that a small number of afferent fibres travel in the medial and lateral XIIth nerve branches; therefore afferent activity probably contributed at least some power to the recorded ENG signal. For example, in some animals the ENG activity was lower following nerve section, which is consistent with loss of the afferent signal following nerve section. However, the mean medial and lateral XIIth ENG responses to tracheal occlusion after lateral and whole XIIth nerve section were not significantly different from those recorded with the XIIth nerves intact. Therefore, we are confident that the majority of the XIIth ENG activity that we recorded was the result of XIIth motoneural activities.
Response to tracheal occlusion
To our knowledge, the present study is the first to examine changes in tongue muscle force during spontaneous breathing or airway occlusion. In most animals, we observed tongue retraction force during eupnoeic inspirations (Fig. 8). Tracheal occlusion caused progressive increases in inspiratory-related tongue retraction force, and parallel augmentation of the activity of both the medial and lateral XIIth nerve branches. These data are consistent with the observation that electrical stimulation of the whole XIIth nerve produces a retraction force in rats, cats and humans (see below), and indicate that the retractor muscles overwhelm the protrusive actions of the GG when both muscle groups are activated simultaneously.
Hypoxia and hypercapnia
Tracheal occlusion is a very severe respiratory stimulus, and increases in upper airway muscle activity during occlusion are probably due to changes in blood gases as well as removal of inhibitory volume-related afferent feedback from the lungs (see above). Therefore, the independent effects of peripheral and central chemoreceptor stimulation were examined by subjecting the animals to hyperoxic hypercapnia and poikilocapnic hypoxia. Both stimuli evoked parallel increases in the EMG activity of the GG and HG, and progressive increases in tongue retraction force as the level of respiratory drive increased. These observations are consistent with the tracheal occlusion experiments, and emphasize further the phenomenon of co-activation of the protrudor and retractor muscles during hyperpnoea.
Relationship between GG EMG activity and tongue force
Measurements of GG EMG activity are widely used as an index of upper airway motor activity in animal and human subjects. Genioglossus EMG activity increases with hypoxia, hypercapnia (Onal, Lopata & Connor, 1981a, b) and tracheal occlusion (Brouliette & Thach, 1979). Moreover, decreases in GG EMG activity during sleep have been implicated in the pathogenesis of obstructive sleep apnoea (OSA) (Remmers, DeGroot, Sauerland & Anch, 1978). The usual interpretation of the correlation between decreased GG EMG activity and increased airflow resistance is that when GG EMG activity decreases, the tongue moves posteriorly and occludes the pharyngeal airway. Similarly, increases in the respiratory-related activity of the GG are thought to reflect tongue protrusion, and dilatation of the pharyngeal airway. We measured GG EMG activity during airway occlusion and found that it correlated with tongue force, whether or not the force was retractive (as in the intact animal) or protrusive (following lateral XIIth branch section). Recent experiments in goats (Brennick, Parisi & England, 1997) have examined the relationship between GG muscle length and GG EMG activity during breathing. Interestingly, these experiments have shown that increases in GG muscle length can be accompanied by increases in the GG EMG, demonstrating that increased drive to the GG does not necessarily result in shortening of the muscle. These data fit well with our observation that increases in GG EMG activity can be associated with either retraction or protrusion of the tongue. It is suggested, therefore, that changes in GG EMG activity should not be used as an independent predictor of changes in tongue muscle force or the direction of tongue movements during spontaneous breathing.
XIIth nerve stimulation
Electrical stimulation of the whole XIIth nerve caused strong retraction of the tongue in both rats and cats. Therefore, the net mechanical result of simultaneously stimulating the protrudor and retractor muscles is retraction of the tongue. In an effort to determine the contribution of the GG muscle to the measured tongue force, we repeated the XIIth nerve stimulation following lateral XIIth branch section (i.e. retractive muscle denervation) in the same animals. Following lateral branch section, XIIth nerve stimulation resulted in weak tongue protrusion. These results suggest that during whole XIIth nerve stimulation, the mechanical actions of the retractor muscles are dominant. Our results are consistent with recent reports in both humans (Eisele et al. 1997) and rats (Gilliam & Goldberg, 1995) showing that whole XIIth nerve stimulation results in tongue retraction. Therefore, co-activation of the GG and HG/SG muscles during spontaneous breathing will favour tongue retraction, presumably because the retractor muscles are stronger than the GG.
Physiological significance
The functional significance of co-activating tongue retractor and protrudor muscles has not been established. However, recent evidence from both animal models and human subjects shows that tongue muscle co-activation improves pharyngeal airway mechanics. Schwartz et al. (1993) found that whole XIIth nerve stimulation increased the maximal rate of inspiratory flow and decreased collapsibility of the isolated feline upper airway. In a similar experiment (Hida et al. 1995) it was shown that whole XIIth nerve stimulation decreased the compliance of the isolated canine upper airway from 4 to 3 ml cmH2O−1. In both of these experiments the XIIth nerve was stimulated proximal to its bifurcation into medial and lateral branches, thereby co-activating the protrudor and retractor tongue muscles. Based on the present experiments, whole XIIth nerve stimulation was most certainly associated with tongue retraction in the experiments of Schwartz et al. (1993) and Hida et al. (1995), indicating that improved pharyngeal mechanics and tongue retraction are somehow linked.
The results of these studies in anaesthetized animals are supported by a recent study in patients with OSA. Eisele et al. (1997) showed that co-activating the tongue muscles by whole XIIth nerve stimulation caused visible tongue retraction and an increase in maximal inspiratory flow in their patients. Moreover, the increases in flow observed during co-activation were not different from the increase observed with selective stimulation of the medial XIIth branch, during which clear tongue protrusion was observed. These data are particularly interesting in light of the data of Schwartz et al. (1996), demonstrating that selectively stimulating the retractor muscles causes tongue retraction and a reduction in airflow in human subjects. Taken together, these two studies suggest that co-activating the protrudor and retractor muscles can improve pharyngeal mechanics despite net tongue retraction, whereas selective retractor muscle activation will narrow the airway and reduce flow. Although further experiments are needed to determine how tongue muscle co-activation and retraction of the tongue can lead to improved pharyngeal airway mechanics, our working hypothesis is that co-contraction serves to stiffen the tongue as the protrudor and retractor muscle fibres work against one another (Fregosi & Fuller, 1997). This would make the tongue more resistant to posterior displacement in the face of the negative hypo-pharyngeal pressures that are generated during inspiration. However, because co-contraction is accompanied by tongue retraction, pharyngeal narrowing may co-exist with the increase in tongue stiffness and inertia. Nevertheless, the recent studies in human subjects, cited above, strongly support our hypothesis, and suggest that enhanced tongue stiffness is more important than tongue position in improving pharyngeal airflow mechanics.
Acknowledgments
We would like to thank Dr Edwin Gilliam for his technical assistance. This study was supported by NIH grants HL 51053 and 41790.
References
- Brouliette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. Journal of Applied Physiology:Respiratory, Environmental and Exercise Physiology. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnoea. Archives of Otolaryngology: Head and Neck Surgery. 1997;123:57–61. doi: 10.1001/archotol.1997.01900010067009. [DOI] [PubMed] [Google Scholar]
- Fregosi RF. Influence of hypoxia and carotid sinus nerve stimulation on abdominal muscle activities in the cat. Journal of Applied Physiology. 1994;76:602–609. doi: 10.1152/jappl.1994.76.2.602. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respiratory Physiology. 1997;110:295–306. doi: 10.1016/s0034-5687(97)00095-9. 10.1016/S0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. The Journal of Physiology. 1994;477:469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Carroll TP, Schwartz AR, Smith PL, Fitzgerald RS. Mechano- and chemoreceptor modulation of respiratory muscles in response to upper airway negative pressure. Journal of Applied Physiology. 1994;76:2656–2662. doi: 10.1152/jappl.1994.76.6.2656. [DOI] [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: Effects of hypoglossal nerve and extracellular motoneuron stimulation in the rat. Journal of Neurophysiology. 1995;74:547–555. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- Hellstrand E. Contraction times of the cat's tongue muscles measured by light reflection: innervation of individual tongue muscles. Acta Physiologica Scandinavica. 1981;111:417–423. doi: 10.1111/j.1748-1716.1981.tb06757.x. [DOI] [PubMed] [Google Scholar]
- Hida W, Kurosawa H, Okabe S, Kikuchi Y, Midorikowa J, Chung Y, Takishami T, Shirato K. Hypoglossal nerve stimulation affects the pressure-volume behaviour of the upper airway. American Journal of Respiratory and Critical Care Medicine. 1995;151:455–460. doi: 10.1164/ajrccm.151.2.7842206. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative pressure in man. The Journal of Physiology. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, Innes JA, McIvor J, Cheesman AD, Guz A. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. Journal of Applied Physiology. 1996;80:1595–1604. doi: 10.1152/jappl.1996.80.5.1595. [DOI] [PubMed] [Google Scholar]
- Kuna ST. Inhibition of inspiratory upper airway motoneuron activity by phasic volume feedback. Journal of Applied Physiology. 1986;60:1373–1379. doi: 10.1152/jappl.1986.60.4.1373. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BR. Genioglossus muscle responses to upper airway pressure changes: afferent pathways. Journal of Applied Physiology:Respiratory, Environmental and Exercise Physiology. 1982;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal EMG responses to isocapnic hypoxia in humans. American Review of Respiratory Disease. 1981;124:215–217. doi: 10.1164/arrd.1981.124.3.215. [DOI] [PubMed] [Google Scholar]
- Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. Journal of Applied Physiology. 1981;50:1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- O'Reilly PMR, Fitzgerald MJT. Fibre composition of the hypoglossal nerve in the rat. Journal of Anatomy. 1990;172:227–243. [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. Journal of Applied Physiology:Respiratory, Environmental and Exercise Physiology. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Thut DC, Tuss B, Seelagy M, Yuan X, Brower RG, Permutt S, Wise RA, Smith PL. Effect of electrical stimulation of the hypoglossal nerve on airflow mechanics in the isolated upper airway. American Review of Respiratory Disease. 1993;147:1144–1150. doi: 10.1164/ajrccm/147.5.1144. [DOI] [PubMed] [Google Scholar]
- Smith KK, Kier WM. Trunks, tongues, and tentacles: moving with skeletons of muscle. American Scientist. 1989;77:29–35. [Google Scholar]
- Yasui Y, Kogo M, Iida S, Hamaguchi M, Koizumi H, Kohara H, Matsuya T. Respiratory activities in relation to external glossal muscles. Journal of the Osaka University Dental School. 1993;33:27–33. [PubMed] [Google Scholar]


