Abstract
The functions of ipsilateral cutaneous reflexes were studied with short trains of stimuli presented pseudorandomly to the sural nerve during human walking. Electromyograms (EMG) of lower (tibialis anterior (TA), soleus, lateral (LG) and medial (MG) gastrocnemius) and upper leg (vastus lateralis and biceps femoris) muscles were recorded, together with ankle, knee and hip joint angles. Net reflex EMG responses were quantified in each of the sixteen parts of the step cycle. The kinematic measurements included ankle eversion- inversion, and ankle, knee and hip flexion-extension.
The function of the sural reflexes depended upon the part of the step cycle in which the nerve was stimulated and the intensity of stimulation. During stance, reflexes in MG and TA muscles in response to a medium intensity of stimulation (1.9 × radiating threshold, × RT) were closely associated with ankle eversion and dorsiflexion responses, respectively. These responses could assist in accommodation to uneven terrain that applies pressure to the lateral side of the foot (sural innervation area). Non-noxious, high intensity (2.3 × RT) stimulation resulted in strong suppression of LG and MG during stance which was correlated to a small reduction in ankle plantarflexion. At this higher intensity the response would function to prevent the foot from moving more forcefully onto a potentially harmful obstacle.
During swing, ankle dorsiflexion increased and was significantly correlated to the net TA EMG response after both medium and high intensity stimulation. Knee flexion was increased throughout swing at both intensities of stimulation. These responses may serve in an avoidance response in which the swing limb is brought past an obstacle without destabilizing contact.
The net EMG and kinematic responses suggest that cutaneous reflexes stabilize human gait against external perturbations produced by an uneven surface in stance or obstacles encountered during swing.
Non-noxious electrical stimulation of cutaneous nerves during human locomotion produces reflexes that are task dependent (e.g. phasic locomotor vs. tonic maintained activity; Lisin, Frankstein & Rechtmann, 1973; Kanda & Sato, 1983; Duysens, Tax, Trippel & Dietz, 1993), intensity dependent (e.g. noxious vs. non-noxious stimulation; Bélanger & Patla, 1984; Crenna & Frigo, 1984; Duysens, Trippel, Horstmann & Dietz, 1990), and phase dependent (e.g. swing vs. stance; Yang & Stein, 1990; Duysens et al. 1990; Duysens, Tax, Trippel & Dietz, 1992; De Serres, Yang & Patrick, 1995; Zehr, Komiyama & Stein, 1997). Additionally, cutaneous reflexes have contralateral as well as ipsilateral effects (Tax, Van Wezel & Dietz, 1995; Van Wezel, Ottenhoff & Duysens, 1997). Local sign, in which the anatomical innervation area of the stimulated nerve determines the reflex, is also important, as revealed by experiments in which nerves with different cutaneous fields have been studied (Zehr et al. 1997; Van Wezel et al. 1997). The majority of the experiments have concerned the sural nerve, which innervates the lateral margin of the foot, although the tibial nerve, which innervates the plantar surface of the foot, has also been studied (Yang & Stein, 1990; Duysens et al. 1990, 1992; Zehr et al. 1997; Van Wezel et al. 1997). Recently, reflexes from the superficial peroneal nerve (SP; innervates the foot dorsum) have been examined (Zehr et al. 1997; Van Wezel et al. 1997).
Most of the human experiments have relied solely upon information obtained from electromyography (EMG) to delineate reflex function; rarely have measurements of movement kinematics been included in human studies examining cutaneous reflexes after electrical stimulation. Thus, evaluating the function of cutaneous reflexes during human walking has been difficult. We previously described kinematic and EMG responses to superficial peroneal (SP) and tibial nerve stimulation and incorporated these into a simple framework in which cutaneous reflexes function to stabilize gait in the face of perturbation (Zehr et al. 1997). Alterations in ankle and knee flexion and extension at reflex latencies were reported after stimulation of the sural nerve by Duysens et al. (1992). Van Wezel et al. (1997) also reported kinematic changes in the saggital plane associated with sural nerve stimulation. However, these results from the sural nerve, unlike those from SP and tibial nerve stimulation, have been difficult to interpret in a functional framework.
From experiments in the cat, Nichols (1994) suggested that the anatomical linkage and lines of force generated by a muscle or muscle group participating in cutaneous or other reflexes may determine reflex function. He also suggested that reflexes may play a powerful role in responses outside of the saggital plane (such as inversion-eversion at the ankle). To date only limited data on ankle and knee flexion and extension and none on inversion-eversion are available for cutaneous reflexes during human walking. Therefore the function of sural nerve reflexes during walking remains uncertain.
The purpose of the present paper was, therefore, to test the functional effect of non-noxious electrical stimulation of the sural nerve during treadmill walking. This was done by examining net reflex changes in the EMG activity and in the kinematics about the hip (flexion-extension), knee (flexion- extension), and ankle (flexion-extension and inversion- eversion). We interpreted these reflexes in terms of possible functional roles, such as Forssberg's (1979) stumbling corrective responses in the cat. Portions of this work have previously been published in abstract form (Zehr, Komiyama & Stein, 1996).
METHODS
Subjects and general procedure
Twelve subjects (10 male and 2 female), 24-55 years old, participated in the experiments with informed, written consent and according to the Declaration of Helsinki. All experiments were conducted under an approved protocol for human subjects at the University of Alberta. During each session, subjects walked on the treadmill at speeds of 2 and 4 km h−1 for approximately 7-10 min at each speed. Approximately 400-600 steps were collected for each speed (including stimulated and control unstimulated steps). Two stimulation intensities were employed (6 subjects participated in each condition) and the data collected varied between the two conditions (see details below).
Nerve stimulation
The sural nerve was stimulated using either a Grass SD9 isolated constant voltage stimulator (Grass Instruments, Quincey, MA, USA) or a custom-designed constant current stimulator with trains of five pulses at 200 Hz with a pulse width of 1.0 ms. The sural nerve was stimulated on the lateral surface of the ankle, just posterior to the lateral malleolus. At this location subjects reported strong radiating parasthaesia in the cutaneous receptive field of the sural nerve, i.e. at the lateral margin of the foot and towards the heel. Flexible 1 cm disposable Electrotrace (Jason, Huntington Beach, CA, USA) Ag-AgCl surface EMG electrodes were used for cathodal stimulation. The threshold of stimulation in terms of the radiating threshold (RT; defined as a clear radiating parasthaesia in the cutaneous field), was determined in all subjects. The perceptual threshold (PT), defined as the lowest stimulation that was just detectable by the subject, was also determined. The PT probably represents local activation of cutaneous receptors lying immediately underneath the recording electrodes, whereas the RT represents electrical activation of fascicles in the underlying cutaneous nerve. Two stimulus intensities were used, medium and high, and six subjects were tested at each. The medium stimulation intensities were on average 1.9 × RT and the high intensity 2.3 × RT. These corresponded to between 2.5 × PT and 3.6 × PT. For the high intensity, the intensity was varied somewhat for each subject to obtain the strongest stimulation possible that was described as non-noxious by the subject and was subthreshold for evoking a flexion reflex (a generalized withdrawal of the limb by flexion at the ankle, knee and hip) while standing. In our most recent publication (Zehr et al. 1997) we were able to use small motor responses to ascertain that the stimulation intensity was constant throughout the step cycle; this check is unavailable for the sural nerve. However, our stimulus delivery is quite similar to methodology previously described (see Yang & Stein, 1990; Duysens, Tax, Nawijn, Berger, Prokop & Altenmuller, 1995) and variation in stimulus intensity throughout the step cycle probably played a very small role in results observed here.
The stimulator was driven by a pseudorandom pulse generator with a minimal repeat time approximately equal to the step cycle duration and a maximum that was twice this. In general this procedure resulted in a stimulus delivered once in a 3-4 s period. We were thus able to collect many unstimulated steps between stimulated steps and no step had more than one stimulus train. Outputs from both the trigger pulse generator and the Grass stimulator were sent to a 12 bit A/D converter and then into a 486 66 MHz microcomputer running Axoscope (Axon Instruments) data acquisition software.
Electromyography
After light abrasion and cleansing of the skin with alcohol, disposable Electrotrace (Jason) Ag-AgCl surface EMG electrodes were applied in bipolar configuration longitudinal to the predicted path of the muscle fibres (≈2 cm interelectrode distance) over the soleus (Sol), lateral (LG) and medial gastrocnemii (MG), tibialis anterior (TA), vastus lateralis (VL), and biceps femoris (BF) muscles. Sol electrodes were placed distal to the termination of the gastrocnemius muscles, whereas MG and LG electrodes were placed over the medial and lateral heads of the gastrocnemius, respectively. TA electrodes were placed over the largest girth of the tibialis anterior muscle. For VL, the distal electrode was placed approximately 4-6 cm proximal to the lateral margin of the patella and for BF placement was over the muscle belly at approximately 1/3 of the distance from the knee to the hip. Ground electrodes were placed over electrically neutral tissue. EMG signals were sequentially pre-amplified, high-pass filtered (100 Hz), full-wave rectified (thus yielding components down to DC), and then low-pass filtered (100 Hz). The processed output was sent to a 12 bit A/D converter and then to a microcomputer running Axoscope (Axon Instruments) data acquisition software.
Kinematics and step-cycle kinetics
Data on movement kinematics (ankle and knee joint angle) and dynamics (vertical ground reaction force measured under the ipsilateral, or stimulated, foot) were recorded. The angular position of the leg was recorded with custom-made potentiometric electrogoniometers as well as bi-axial goniometers (Penny + Giles Biometrics Ltd, Cwemfellinfach, Gwent, UK) placed over the joint and secured with plastic tape and fabric straps. For the ankle joint, the bi-axial goniometers were placed with the distal end on the heel of the subject's shoe and the proximal end fixed to the lower leg along the midline. Signals obtained from custom-made force sensors located in the insole of the subject's shoe were used to establish step cycle parameters (e.g. heel contact, toe-off). This technique, based on force-sensing resistors (FSRs), has been previously described (Zehr, Stein, Komiyama & Kenwell, 1995b). Angle and force signals were pre-amplified at the subject (the subject wore a small pouch and belt to hold the amplifiers) and then sent directly to the Axoscope-computer interface.
Data acquisition and analysis
The data were sampled continuously at 500 Hz and stored on hard disk for off-line analysis. Custom-written software programs were used to separate the step cycle into sixteen equal parts, beginning with heel contact. The stimuli occurred randomly throughout the step cycle. All responses to stimuli occurring in the same part of the step cycle were averaged together and aligned to stimulus delivery within that part of the step cycle. The averages of unstimulated steps were subtracted from the corresponding values obtained for each of the sixteen parts of the step cycle after stimulation.
EMG analysis
Stimulus artifacts were digitally removed and then the EMGs were filtered with an equally weighted five point digital moving average filter. The evoked EMGs for each subject were analysed for the net reflex effect using the average cumulative reflex EMG up to 150 ms (ACRE150; see Zehr et al. 1997). Using the subtracted EMGs, the post-stimulus data were integrated and any significant facilitation or suppression was identified as a positive or negative deflection, respectively. The value obtained at 150 ms after stimulation was then divided by the time of integration to measure a net reflex (i.e. negative values indicate overall suppression and positive values overall facilitation). A 150 ms post-stimulus interval was chosen since it preceded any significant voluntary activation (see Zehr, Komiyama & Stein, 1995a;Komiyama, Zehr & Stein, 1995). To facilitate comparison between subjects, the ACRE150 values for each subject were normalized to the peak EMG value (averaged over a 40 ms time window) occurring during the step cycle for each muscle at each walking speed and expressed as percentages.
Kinematic analysis
Subtracted values for angular changes were obtained as described above. The maximum change observed in these smoothed (n= 5 point filtering) signals over an interval of 140-220 ms post stimulation was calculated. This window has been previously employed by us (Zehr et al. 1997) and was chosen to take into account the delays between an EMG response and the peak mechanical change in moving muscles (see Stein, Hunter, Lafontaine & Jones, 1995). As with the EMG, these values were then normalized to the maximal range of motion recorded for each subject during the step cycle and expressed as percentages.
Statistics
In all instances, analysis was conducted on averaged values of each subject from each part of the step cycle. Significant differences from zero for the net reflex effects were determined by calculation of t ratios for each part of the step cycle. Statistics were calculated on the combined data from both walking speeds yielding eleven degrees of freedom. Linear least-squares regression analysis was used to evaluate correlation between EMG indices and changes in ankle and knee joint angle at each part of the step cycle. Descriptive statistics included means ±s.e.m. and statistical significance was set at P≤ 0.05.
RESULTS
High intensity stimulation
Lower leg responses
High intensity sural nerve stimulation produced significant suppression of the LG and MG during the middle parts of the stance phase (see Fig. 1B and C), but no significant effect on Sol (Fig. 1A; in all such figures filled symbols indicate significant differences at P < 0.05) during stance. At this time in the step cycle there were some small changes in plantarflexion force (Fig. 1D) which were related to MG and LG suppression. Also, a significant reduction in ankle PF (seen as a change in the dorsiflexion (DF) direction in Fig. 1F) occurred during mid-swing. These changes in the gastrocnemi muscles were correlated to the kinematic changes. Regression data from mid-stance (part 5) has been plotted in Fig. 2 (top panel) for MG muscle and ankle flexion-extension. Further, there were some periods of significant facilitation throughout swing for the triceps surae. However, the prominent feature was the LG and MG suppression and associated mechanical change during stance.
Figure 1. Percentage changes in overall reflex effects in EMG (A-C and E), dynamics (D) and movement kinematics (F) (see Methods) in the lower leg after high intensity (2.3 × RT) sural nerve stimulation.
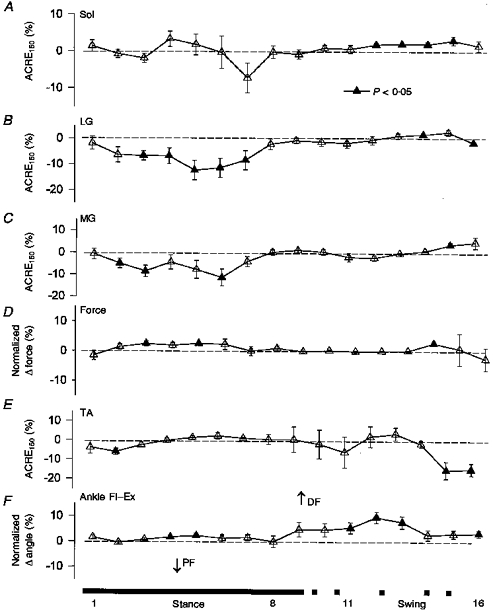
For details of data presentation see Methods. Values are means ±s.e.m. averaged across all subjects. Filled symbols indicate values significantly different from no effect (dashed line) at P < 0.05. Sol, soleus; LG, lateral gastrocnemius; MG, medial gastrocnemius; TA, tibialis anterior; PF, plantarflexion; DF, dorsiflexion; Fl, flexion; Ex, extension. The bar below indicates when the foot is on the ground (stance, continuous section) and off the ground (swing, dashed section). Numbers indicate portions of the step cycle.
Figure 2. Regression plots between MG (top) and TA (bottom) and ankle kinematics after high intensity stimulation for all subjects.
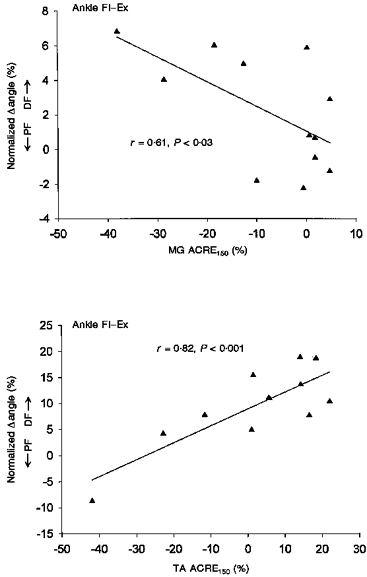
MG data is from mid-stance (part 5) and TA is from mid-swing (part 12). Significant regression is as indicated by the continuous lines.
TA showed only small net effects on average (see Fig. 1E) during most of the step cycle. However, during swing the Pearson r values were quite high and significant correlation between TA and ankle movement was observed. Regression data from swing (part 12) during 4 km h−1 walking have been plotted in Fig. 2 (see bottom panel). The reason for the high value for the correlation despite the small mean value was that some subjects showed net facilitation and others net suppression and the angle changes followed these effects closely. With this intersubject variability (see error bars), the average effects were not significantly different from zero. The reason for the variability is unknown, but the receptive field of the sural nerve is on the lateral border of the foot. Thus, movements in other planes such as inversion or eversion may be more important, but such angular changes were not measured at this intensity (see below). Additionally, other muscles, such as peroneous longus, may have contributed to the reflex but were not recorded here.
Upper leg responses
Significant flexion of the knee with sural nerve stimulation was seen particularly during mid- to late swing (Fig. 3C). This was associated with increased VL activity which would be expected to extend the knee (Fig. 3A). However, while the responses were generally facilitatory, no significant effect was observed in BF (see Fig. 3B). Thus, more flexion was associated with higher VL activity, so this activity appears to be braking flexion that is produced elsewhere (e.g. secondarily to hip flexion). However, the correlation Pearson r values were generally not significant between either VL or BF and knee angle after high intensity stimulation.
Figure 3. Mean data from the upper leg after high intensity stimulation for all subjects.
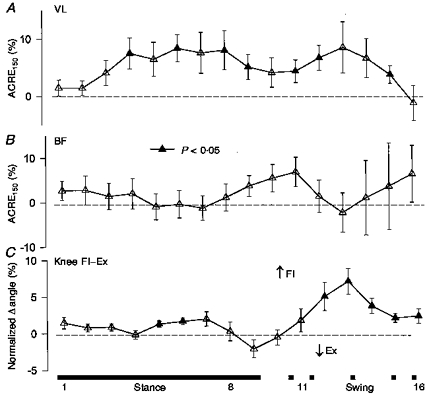
Values are means ±s.e.m. Filled symbols indicate values significantly different from no effect (dashed line) at P < 0.05. VL, vastus lateralis; BF, biceps femoris. The bar below indicates periods of stance and swing. Numbers indicate portions of the step cycle.
Medium intensity stimulation
Lower leg responses
Both MG and TA showed significant facilitatory responses to sural stimulation; the largest effects were observed during mid- to late stance (see Fig. 4A and C). MG also had some significant facilitation during swing phase. These results should be contrasted with those from high intensity stimulation in which suppression of MG was the prominent feature. Associated with these EMG changes were significant increases in ankle eversion (Fig. 4B) and dorsiflexion (Fig. 4D), again during late stance and at the stance to swing transition. Regressions plotted in Fig. 5 (top panel) are mid-stance (part 4) data for MG and ankle inversion-eversion at 2 km h−1 and plotted at figure bottom are data from late stance (part 9) for TA and ankle flexion- extension at 4 km h−1.
Figure 4. Grouped lower leg data after medium intensity (1.9 × RT) sural nerve stimulation.
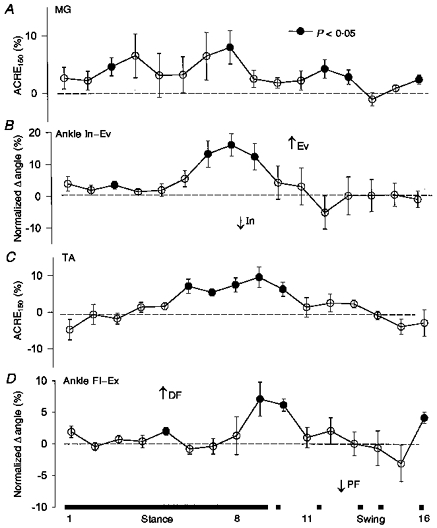
Format is the same as Fig. 1. Ev, eversion; In, inversion.
Figure 5. Regression plots for MG and ankle eversion (top) and TA and ankle dorsiflexion (bottom) during stance after medium intensity stimulation.

Data for MG are from mid-stance (part 4) and for TA from late stance (part 9).
Upper leg responses
Medium intensity sural stimulation produced significant facilitation in VL and BF throughout the step cycle, particularly during stance (see Fig. 6A and B). For VL, these responses are quite similar to those observed after high intensity stimulation (see above and Fig. 3, top panel). However, significant facilitation was observed in BF which was absent after high intensity stimulation. This facilitation in BF was associated with and correlated to knee flexion (Fig. 6C) and hip extension (Fig. 6D) (highest r= 0.92 and 0.97, respectively). VL was also correlated to knee flexion (r= 0.79) during swing. These anomolous results would require coactivation with other hip muscles that we did not record from and could arise from mechanical linkage secondary to movement at the hip joint.
Figure 6. Grouped upper leg data after medium intensity sural stimulation.
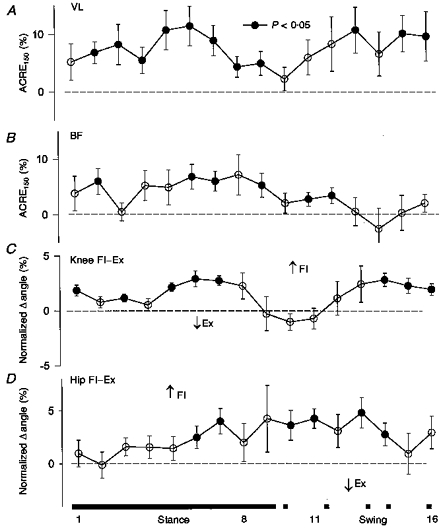
Format is similar to Fig. 3.
DISCUSSION
In this study we have shown significant effects of sural nerve stimulation on many muscles in both the upper and lower leg during treadmill walking. Changes in kinematics were associated with and correlated to the EMG responses at both high and medium intensity stimulation. New findings include: (1) the correlation between reflex facilitation of MG and ankle eversion during stance, (2) the association of BF facilitation with knee and hip flexion, and (3) the widespread facilitation of VL responses throughout the step cycle.
Comparison with the literature
As well as the novel findings, we confirmed and extended previous studies that investigated sural nerve reflexes during walking. Ankle joint changes mirrored the trends described previously (Duysens et al. 1992; Van Wezel et al. 1997). In this paper we also observed a differential response to high intensity sural nerve stimulation within the triceps surae. This was previously observed after superficial peroneal nerve (SP) stimulation (Zehr et al. 1997) and a separation within the gastrocnemi after sural stimulation has been described by Duysens, van Wezel, Prokop & Berger (1996). Also the general facilitation of BF muscles has been observed previously, most recently by Van Wezel et al. (1997). With regard to upper leg kinematics, Duysens et al. (1992) also reported knee flexion, and a reversal to extension at end swing, after both high and low intensity sural nerve stimulation. We observed a predominant knee flexion response after both high and medium intensity stimulation. In another study of upper leg responses, Tax et al. (1995) studied three different stimulus intensities and found BF facilitation to be a robust feature. However, knee kinematics were not reported in that study.
One difference is that we did not observe the phenomenon of ‘reflex reversal’ as distinctly here as in other papers. This is most probably due to the fact that our EMG method, ACRE150 (see Methods), is by its nature less sensitive to small fluctuations at a discrete latency (i.e. the middle latency P2 response; see Duysens & Tax, 1994) which do seem to be amenable to phasic reversals. Our methodology is well suited to investigating reflex function and providing a useful index for comparison to kinematics. However, we did observe that TA muscle which has often shown such reversals (see Yang & Stein, 1990) from facilitatory responses during stance to suppressive during swing, showed net suppressive responses at end swing and either facilitation or no significant net effect during stance at both stimulus intensities (see Figs 1E and 4C). Thus, our results are mainly similar to previously published reports, but need integration into a functional framework for sural nerve reflexes during human walking.
Reflex function during swing phase
The responses during swing are the most straightforward to interpret, particularly those reflexes generated by high intensity stimulation. Stimulation resulted in increased dorsiflexion at the ankle and flexion at the knee. There was a very tight correlation between the TA and ankle DF and VL and knee flexion. Activation of the sural nerve cutaneous field would occur during swing if an obstacle was encountered or if the foot prematurely touched the ground and the lateral foot margin were compressed. If this were to occur, then the most efficient and stable response would be to increase ankle DF and flex the knee slightly to avoid the obstacle and clear the ground. Hip flexion was also observed at medium intensity. Thus, ground clearance and obstacle avoidance would both be achieved, as observed here in the upper and lower leg responses (see schematic diagram in Fig. 7A). The angular trajectory of the swing limb is changed to avoid the obstacle.
Figure 7. Schematic illustration indicating proposed functional roles of sural reflexes during walking.
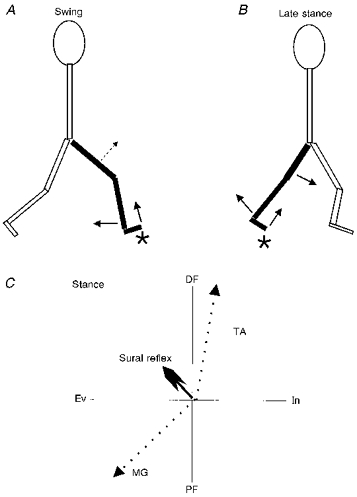
A, swing phase in which knee flexion and ankle DF predominate. B, late stance in which hip and knee flexion as well as ankle DF are key features. C, net effect of lower leg responses during stance (large arrowhead), wherein MG and TA responses act in concert to evert (Ev) and DF the foot. The putative contributions of MG to PF, and Ev and TA to DF and inversion (In) are shown by the dotted lines. ⋆ Activation of the sural nerve cutaneous field.
The VL response (i.e. increased activation) may seem somewhat puzzling as it occurred in concert with increased knee flexion. Although the simplest explanation for knee flexion would have been increased BF activation, this facilitation was not significant here. However, flexion at a more proximal joint (i.e. the hip) would cause flexion at the knee as well. Thus, the VL response can serve to control and prevent excessive knee flexion. As described after SP nerve stimulation (Zehr et al. 1997), activation of VL during swing may also serve as a ‘fail safe’ if the ankle response fails to overcome the perturbation. Then, stumbling and increased loading of the swing limb could occur and VL activation, by increasing knee stiffness, would help reduce the likelihood of limb collapse and a consequent fall.
Reflex function during stance phase
The function of the reflexes during stance is somewhat more complex. Nichols (1994) advocated an anatomical approach to understand reflex function in the cat. In particular, he suggested that the lines of force generated by the muscles as prime movers and as synergists be closely examined. Parsons (1894) initially made the observation that the MG muscle fibres pass obliquely outwards and over the Achilles' tendon and that the tendon itself twists around to insert on the lateral margin of the calcaneous. In this way the outer part of the tendon is largely formed by MG while LG and Sol make up the inner part. Indeed, this was found to be a common feature in many mammalian species examined (Parsons, 1894). Thus, in addition to causing plantarflexion, MG will also evert the foot. Ankle eversion during stance did occur here and was correlated to MG facilitation after medium intensity stimulation. In contrast, TA was facilitated at this time which would produce DF and inversion. The resultant vector of these and other muscles not recorded could be flexion and eversion, as schematically illustrated in Fig. 7C. Recall that the sural innervation area could be strongly activated by contact or pressure on the lateral foot surface and near the heel. This would produce ankle extension (PF) and inversion, which, in principle, would be balanced by the resultant effect of TA and MG. The diagram of Fig. 7C is merely for illustrative purposes and would have to be tested by measuring the force vectors of each of the major ankle muscles. Interestingly, it has recently been suggested that MG can contribute significantly to isometric torque production in the sagittal plane after medial perturbation at the knee joint (Buchanan & Lloyd, 1997). This could, in an unrestrained subject, generate eversion at the ankle.
Excitatory responses in the upper leg in BF and VL muscles and flexion at the knee and hip also occurred during stance (refer again to Fig. 7B). The net effect of these reflexes would be to increase stiffness at the knee while still allowing for the perturbation at the foot (i.e. foot lift-inversion) to be associated with knee flexion. The addition of hip flexion would also assist by lowering the centre of body mass and the net effect is accommodation in the stance limb to the encountered perturbation. Further, the BF activation with hip flexion could serve the same purpose as described above for VL and knee flexion; excessive hip movement (as could be caused by sudden loading) would be countered by BF activation. Although the BF effects were not significant during high intensity stimulation, the knee joint kinematics were quite similar to those observed after medium intensity stimulation. Lastly, if the BF responses during stance (Fig. 6B) contribute to exorotation of the femur (see Tax et al. 1995) then it is conceivable that this might generate a torque at the foot that could cause eversion, as recorded here (Fig. 4B). However, rotation of the femur was not directly recorded and awaits further study.
Effect of stimulus intensity
The subtle differences between high and medium intensity stimulation probably result from the application of two slightly different strategies. With high intensity stimulation the feeling of tingling or pressure on the foot margin is quite strong. The responses in the lower leg are therefore suppressive and allow for accommodation at the proximal segments (i.e. knee and hip flexion) with some dorsiflexion but no excitatory MG response. During swing, the foot withdraws from the encountered object to continue on through to heel contact. However, with medium intensity stimulation, the reflexes function to stabilize gait during stance and play a more minor role during swing.
To summarize, our data on the sural nerve indicate that non-noxious reflexes function to stabilize human gait, both during stance and swing, so that unimpeded locomotion can continue. The effect of a perturbation will be minimized by accommodation of the limb to uneven terrain during stance and avoidance of an obstacle during swing. In this way, the results extend our previous interpretation of SP and tibial nerve reflexes during walking in a logical way (Zehr et al. 1997). In addition, the present results describe functional responses during stance as well as swing. Gradually, the roles of cutaneous reflexes are emerging. Further work is still needed in relation to contralateral responses, more natural stimulation (i.e. contact with real objects), and pathological gait.
Acknowledgments
This work was funded by a Medical Research Council of Canada grant to R. B. S. E. P. Z. was supported by studentships from the Natural Sciences and Engineering Research Council of Canada and the Alberta Heritage Foundation for Medical Research. T. K. was funded by a grant from the Japanese Ministry of Education as a visiting scientist in Edmonton. We thank Dr S. J. De Serres for the modification and use of the phase-averaging software.
References
- Bélanger M, Patla AE. Corrective responses to perturbation applied during walking in humans. Neuroscience Letters. 1984;49:291–295. doi: 10.1016/0304-3940(84)90304-5. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Lloyd DG. Muscle activation at the human knee during isometric flexion-extension and varus-valgus loads. Journal of Orthopaedic Research. 1997;15:11–17. doi: 10.1002/jor.1100150103. [DOI] [PubMed] [Google Scholar]
- Crenna P, Frigo C. Evidence of phase-dependent nociceptive reflexes during locomotion in man. Experimental Neurology. 1984;85:336–345. doi: 10.1016/0014-4886(84)90144-4. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Yang JF, Patrick SK. Mechanism for reflex reversal during walking in human tibialis anterior motor units. The Journal of Physiology. 1995;488:249–258. doi: 10.1113/jphysiol.1995.sp020963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J, Tax T. Interlimb reflexes during gait in cats and humans. In: Swinnen SP, Heuer H, Massion J, Casaer P, editors. Interlimb Coordination: Neural, Dynamical, and Cognitive Constraints. San Diego, CA: Academic Press; 1994. pp. 97–126. [Google Scholar]
- Duysens J, Tax AAM, Nawijn S, Berger W, Prokop T, Altenmuller E. Gating of sensation and evoked potentials following foot stimulation during human gait. Experimental Brain Research. 1995;105:423–431. doi: 10.1007/BF00233042. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AAM, Trippel M, Dietz V. Phase-dependent reversal of reflexly induced movements during human gait. Experimental Brain Research. 1992;90:404–414. doi: 10.1007/BF00227255. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AAM, Trippel M, Dietz V. Increased amplitude of cutaneous reflexes during human running as compared to standing. Brain Research. 1993;613:230–238. doi: 10.1016/0006-8993(93)90903-z. 10.1016/0006-8993(93)90903-Z. [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Experimental Brain Research. 1990;82:351–358. doi: 10.1007/BF00231254. [DOI] [PubMed] [Google Scholar]
- Duysens J, van Wezel BMH, Prokop T, Berger W. Medial gastrocnemius is more activated than lateral gastrocnemius in sural nerve induced reflexes during human gait. Brain Research. 1996;727:230–232. doi: 10.1016/0006-8993(96)00525-2. 10.1016/0006-8993(96)00525-2. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. Journal of Neurophysiology. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Kanda K, Sato H. Reflex responses of human thigh muscles to non-noxious sural stimulation during stepping. Brain Research. 1983;288:378–380. doi: 10.1016/0006-8993(83)90123-3. 10.1016/0006-8993(83)90123-3. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Zehr EP, Stein RB. A novel method for evaluating the overall effect of nerve stimulation in humans. Electroencephalography and Clinical Neurophysiology. 1995;97:S179. [Google Scholar]
- Lisin VV, Frankstein SI, Rechtmann MB. The influence of locomotion on flexor reflex of the hind limb in cat and man. Experimental Neurology. 1973;38:180–183. doi: 10.1016/0014-4886(73)90019-8. 10.1016/0014-4886(73)90019-8. [DOI] [PubMed] [Google Scholar]
- Nichols TR. A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anatomica. 1994;151:1–13. doi: 10.1159/000147637. [DOI] [PubMed] [Google Scholar]
- Parsons FG. On the morphology of the tendo-achillis. Journal of Anatomy and Physiology. 1894;28:414–418. [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Hunter W, Lafontaine SR, Jones LA. Analysis of short-latency reflexes in human elbow flexor muscles. Journal of Neurophysiology. 1995;73:1900–1911. doi: 10.1152/jn.1995.73.5.1900. [DOI] [PubMed] [Google Scholar]
- Tax AAM, Van Wezel BMH, Dietz V. Bipedal reflex coordination to tactile stimulation of the sural nerve during human running. Journal of Neurophysiology. 1995;73:1947–1964. doi: 10.1152/jn.1995.73.5.1947. [DOI] [PubMed] [Google Scholar]
- Van Wezel BMH, Ottenhoff FAM, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. Journal of Neuroscience. 1997;17:3804–3814. doi: 10.1523/JNEUROSCI.17-10-03804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. Journal of Neurophysiology. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Evaluating the overall electromyographic effect of cutaneous nerve stimulation in humans. Society for Neuroscience Abstracts. 1995a;21:681. [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Functional antagonism of cutaneous reflexes in the human tibialis anterior (TA) muscle during walking. Proceedings of Canadian Society for Biomechanics 9th Biennial Meeting. 1996:170–171. [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. Journal of Neurophysiology. 1997;77:3311–3325. doi: 10.1152/jn.1997.77.6.3311. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB, Komiyama T, Kenwell Z. Linearization of force sensing resistors (FSRs) for force measurement during gait. Proceedings of the 17th Annual International Conference IEEE Engineering in Medicine and Biology Society. 1995b:300–301. [Google Scholar]


