Abstract
A total of twelve synaptic connections between pairs of pyramidal neurones in layer 2/3 of slices of rat visual cortex maintained in vitro was investigated using whole-cell voltage recordings under visual control. The connections varied widely in strength, with the mean peak amplitudes of the resulting excitatory postsynaptic potentials (EPSPs) ranging between approximately 40 μV and 2 mV at 23°C. The smaller mean amplitudes included a substantial proportion of apparent failures of transmission.
The properties of these EPSPs were examined over a range of temperatures between 13 and 36°C. All the connections became more reliable, in that they showed fewer apparent failures of transmission, and showed less trial-to-trial variability at the higher temperatures. These changes appeared to be due primarily to an increase in the mean number of transmitter quanta released per presynaptic action potential.
At 36°C most connections were relatively reliable, with a mean failure rate of only 16%. Five connections showed virtually no failures (1% or fewer) at this temperature.
We conclude that quantal transmitter release is temperature dependent at these synapses, and that experiments performed at room temperature could lead to an exaggerated impression of the unreliability of transmission at central excitatory synapses.
At the vertebrate neuromuscular junction, it has been well documented that quantal transmitter release is depressed at low temperatures (e.g. Barrett, Barrett, Botz, Chang & Mahaffey, 1978). However, many recent studies of synaptic transmission at central synapses have been performed using in vitro preparations held at temperatures well below physiological temperatures. Indeed, since the widespread application of the whole-cell recording technique to brain slices, a high proportion of published experimental results has been obtained at room temperature. This may be relevant to the study of excitatory synaptic transmission in higher brain areas such as the hippocampus and neocortex, which is a highly controversial area of research with widely disparate results reported. It is commonly stated that the probability of transmitter release is generally very low at synapses in higher brain areas (e.g. Stevens, 1994). For example, the overwhelming majority of excitatory synapses in the CA1 region of the hippocampus have been reported to have release probabilities of < 0.1 (Hessler, Shirke & Malinow, 1993) and several studies have highlighted the high incidence of transmission failure (Allen & Stevens, 1994; Stevens & Wang, 1994, 1995). Most of these studies, however, were performed at room temperature. On the other hand, some studies performed at more physiological temperatures have reported a much lower incidence of failures at pyramid-to-pyramid connections in neocortex (Thomson, Deuchars & West, 1993a; Markram, Lübke, Frotscher, Roth & Sakmann, 1997) and moderately high release probabilities in hippocampus (Larkman, Jack & Stratford, 1997). These are important issues, because the reliability of synaptic transmission will have profound implications for the way in which signals could be transmitted, and for the styles of computation that higher brain areas might use (Stevens, 1994; Smetters & Zador, 1996; Lisman, 1997). It has been reported that changing temperature has no effect on the reliability of central synapses (Allen & Stevens, 1994) and so cannot be a factor in this controversy. However, in this and many other studies in this field, the technique of minimal extracellular stimulation was used, so it is difficult to exclude the possibility that some failures of transmission were due to failures of axonal stimulation.
In this study, we have used simultaneous paired recordings and investigated the effects of changing temperature on the reliability and variability of local synaptic transmission between pyramidal cells in layer 2/3 of the visual cortex of the rat at low frequencies of action potential firing.
METHODS
Sprague-Dawley rats (20-22 days old) were killed by cervical dislocation and slices (400 μm thick) of visual cortex were prepared by conventional methods (Larkman et al. 1997). The slices were maintained in artificial cerebrospinal fluid (ACSF) containing (mM): 119 NaCl, 3.5 KCl, 1 NaH2PO4, 2.5 CaCl2, 1 MgSO4, 26 NaHCO3 and 10 glucose. The ACSF reservoir was bubbled with 95 % O2 and 5 % CO2 at room temperature (21-25°C) throughout. Closely adjacent pairs of pyramidal neurones were selected by infrared differential interference contrast video microscopy (Dodt & Zieglgänsberger, 1990) using an upright microscope, and whole-cell voltage recordings were obtained from their cell bodies using the ruptured patch technique. Recording pipettes contained (mM): 110 potassium gluconate, 10 KCl, 2 MgCl2, 2 Na2ATP, 10 EGTA and 10 Hepes, adjusted to pH 7.3 and 270 mosmol. On-line spike-triggered averaging was used to detect EPSPs produced in one cell by action potentials induced in the other. We always inspected individual traces and averaged at least fifty trials to maximize the chance of identifying even weak and unreliable connections. Once a connection had been identified, single presynaptic spikes were usually induced by 100 ms pulses repeated at 0.5 Hz. Individual records were low-pass filtered (usually at 2 kHz), digitized (usually at 5 kHz) and stored on disk for analysis off-line.
The temperature of the preparation was monitored by a small thermocouple probe placed in the recording chamber close to the slice, and altered by heating or cooling the incoming ACSF using a water jacket around the inlet tube. We waited until a stable temperature within ± 1°C of the target temperature had been achieved and typically a hundred or more trials were recorded at each temperature. The usual temperature sequence was 23, 30, 36, 18 and 13°C, although in some cases not all temperatures could be tested before some recording instability occurred. We did not monitor the pH of the ACSF during recording, but in control experiments we found that if the ACSF was gassed at room temperature, no changes in pH occurred on heating or cooling over the range 13-36°C within the time period (less than 2 min) that the ACSF was in contact with the slices. Resting membrane potentials were -67 ± 7 mV at room temperature and were held approximately constant during the temperature changes, using injected current if necessary. Most neurones showed a tendency to depolarize during cooling.
EPSP amplitudes and shape indices were analysed using a computer optimizing routine that fitted the sum of two exponential functions to each record. The onset of the EPSP and the time interval over which the fitting was to be performed were determined by eye. The fit interval was chosen to include the rising phase and as much of the falling phase of the EPSP as possible before contamination by noise or spontaneous synaptic events became apparent. The EPSP latency was measured from the peak of the presynaptic action potential to the onset of the EPSP. The EPSP peak amplitude, 10-90 % rise time, width at half-amplitude and time integral were measured from the fitted line. Apparent failures of transmission were identified by eye. The level of the contaminating recording noise is difficult to quantify by this method, but our calibration using injected current pulses suggests that it is low compared with conventional methods involving the comparison of average voltages within brief time windows. Estimates of EPSP variability have therefore not been corrected for noise, and the amplitudes of failures have been taken to be zero.
The locus of the change in synaptic strength was investigated using graphs of the (coefficient of variation)−2 of the EPSP against its mean. It has been suggested that, for a range of statistical models of transmitter release, including Poisson and binomial quantal models (Malinow & Tsien, 1990), the trajectory of such graphs will be close to or steeper than the diagonal if the change is due to an altered number of quanta released per trial. However, if the change is due to an alteration in the size of the effect produced by each quantum, the trajectory will be close to horizontal. There are situations in which these plots can be misleading, but when applied to paired recordings, when it is known that a single presynaptic axon is stimulated reliably, their interpretation is much more straightforward and unequivocal (Faber & Korn, 1991).
Spontaneous miniature EPSPs (mEPSPs) were recorded from a sample of ten pyramidal neurones in the presence of 1 μM tetrodotoxin (TTX) and 100 μM picrotoxin. One hundred sweeps of data, each 400 ms in length, were recorded to disk and analysed off-line. The mEPSPs were identified by eye on the basis of their rapid rise and slow decay phases and their amplitudes were measured using the same fitting procedure as for evoked EPSPs. Samples of 125 mEPSPs were analysed at each temperature for each neurone.
Measurements of changes in neuronal input resistance and membrane time constant with temperature were obtained from the averaged responses to brief pulses (1 ms) of hyperpolarizing current injected into a further sample of six pyramidal neurones.
Values quoted in the text are given as means ± standard deviation; the error bars in the figures show standard error of the mean.
RESULTS
The proportion of connected neuronal pairs (Fig. 1A) was quite high (approximately one in every seven pairs tested) and a total of twelve connections was investigated. The connections varied widely in their strength, with EPSP mean peak amplitudes ranging from approximately 40 μV to 2 mV at 23°C. Failures of transmission could be distinguished with reasonable certainty (Fig. 1B), and the proportion of apparent failures was also highly variable (ranging between 0 and 0.81 at 23°C). Large EPSPs showed fewer failures than small ones (Fig. 1C), suggesting that the mean number of quanta of transmitter released per action potential (m) was an important factor in determining the average size of the EPSP produced at a given connection.
Figure 1. General properties of synaptic connections.
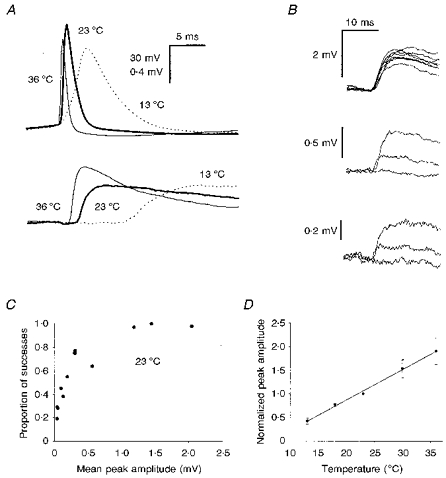
A, paired recording showing presynaptic action potentials (upper trace) and averaged postsynaptic responses (lower trace; averages of 50 trials); those shown by the thick lines were recorded at 23 °C, thin lines at 36 °C and dotted lines at 13 °C. B, examples of superimposed consecutive single trials from EPSPs of different mean amplitudes, recorded at 23 °C: upper traces, strong connection showing no failures; middle traces, connection of moderate strength, showing one failure; lower traces, weak connection. Note that the failure can be distinguished from the successes, even though the peak amplitude of the smaller success is only approximately 170 μV. C, graph showing the proportion of transmission successes as a function of the EPSP mean peak amplitude, recorded at 23 °C and showing that large EPSPs failed less often than small ones. Each point represents an individual connection and values were calculated for epochs of 100 trials. D, graph showing how the mean EPSP amplitude for the twelve connections tested increased with temperature. Because the EPSPs differed greatly in size, the mean amplitudes for each were normalized to their mean amplitude at 23 °C before pooling. Means were calculated for epochs of 50 or 100 trials. The continous line was fitted by linear regression (r= 0.996).
We investigated the properties of the EPSP over a range of temperatures between 13 and 36°C. In every case, the EPSP mean peak amplitude increased with temperature. When the EPSP amplitudes were normalized to their amplitude at 23°C and pooled, there was a roughly linear increase in amplitude with temperature (Fig. 1D), but individual connections differed in their behaviour. The weaker connections showed dramatic increases right across the temperature range tested. The stronger connections showed less increase, especially over the higher temperature range, with some actually showing a decrease between 30 and 36°C. The proportional increase in amplitude between 23 and 36°C was inversely related to the amplitude at 23°C (r= 0.737, P < 0.01; data not shown). The group mean peak amplitude at 36°C was 618 ± 497 μV, very much in line with an earlier microelectrode study of similar connections at a similar temperature (550 ± 490 μV; Mason, Nicoll & Stratford, 1991).
In every case the reliability of transmission improved with increasing temperature (Fig. 2A). The group mean failure rate decreased from 0.56 ± 0.32 at 13°C to 0.16 ± 0.18 at 36°C (Fig. 2B). In five of the twelve connections the proportion of failures was 1 % or less at 36°C. As the reliability of transmission increased with temperature, so the trial-to-trial variability decreased, from a mean coefficient of variation (CV) of 2.33 ± 2.01 at 13°C to 0.61 ± 0.37 at 36°C (Fig. 2C).
Figure 2. Changes in transmission reliability and variability with temperature.
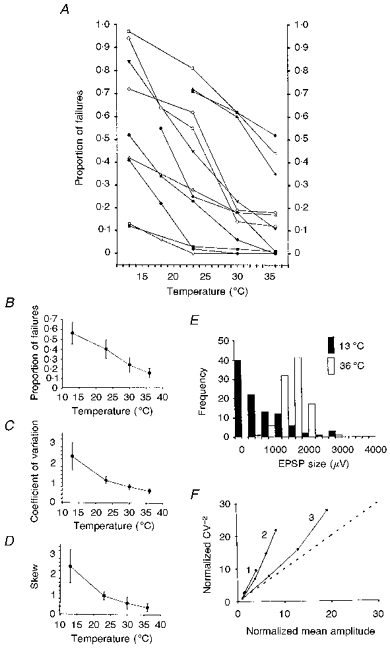
Values were calculated for epochs of 50 or, more commonly, 100 trials at each temperature. A, graph showing the reduction in the incidence of failures with increasing temperature for each of the twelve connections. B, as A, but showing group mean values. C, reduction in EPSP CV with temperature. D, the group mean skew of EPSP amplitude distributions decreases with temperature. E, histograms of EPSP amplitudes (100 trials in each case) for one connection recorded at different temperatures. At 13 °C (▪), the histogram shows pronounced positive skew (skew = 2.24), but at 36 °C (□) it is roughly symmetrical (skew = -0.22), consistent with a release probability of approximately 0.5 at the higher temperature. F, relationship between CV−2 and mean amplitude for three example EPSPs. As the mean amplitude increased with temperature, CV−2 also increased, and in these, and in all nine other cases, the trajectory was steeper than the line of identity (dotted), consistent with an increase in m.
The locus of the change
The reduction in failure rate with increasing temperature suggests that a major effect is an increase in the mean number of transmitter quanta released in response to an action potential (m). Three other lines of argument support this view.
(1) Changes in skew of the EPSP amplitude distribution. At low temperatures, the distributions of EPSP amplitudes were generally strongly positively skewed. With increasing temperature, the amount of positive skew decreased, from 2.41 ± 2.46 at 13°C to 0.36 ± 0.68 at 36°C (Fig. 2D and E). A reduction in positive skew is again consistent with an increase in m.
(2) Changes in the CV of the EPSP. CV−2 against mean graphs can be used to probe the locus of changes in synaptic strength (Malinow & Tsien, 1990). We prepared graphs of CV−2 against mean amplitude as it changed with temperature for each connection, and in every case the trajectory was steeper than the diagonal (Fig. 2F). The steep trajectories of these graphs are consistent with an increase in m rather than in quantal size.
(3) No increase in peak amplitude of miniature EPSPs with temperature. For a further sample of ten pyramidal neurones, we looked at the peak amplitudes of spontaneous miniature EPSPs (mEPSPs) at different temperatures, recorded in the presence of tetrodotoxin (TTX), a specific blocker of sodium channels that prevents action potential activity. The mEPSP frequency increased dramatically with increasing temperature (from 3.4 ± 2.0 Hz at 18°C to 14.6 ± 6.9 Hz at 36°C; Fig. 3A), but the mean mEPSP peak amplitude did not increase (Fig. 3B). Of course, the mEPSPs can arise from any of the large number of excitatory synapses distributed over the dendrites, but the simplest interpretation of this result is that the quantal size did not increase with warming.
Figure 3. Further changes in the properties of mEPSPs and connections with temperature.
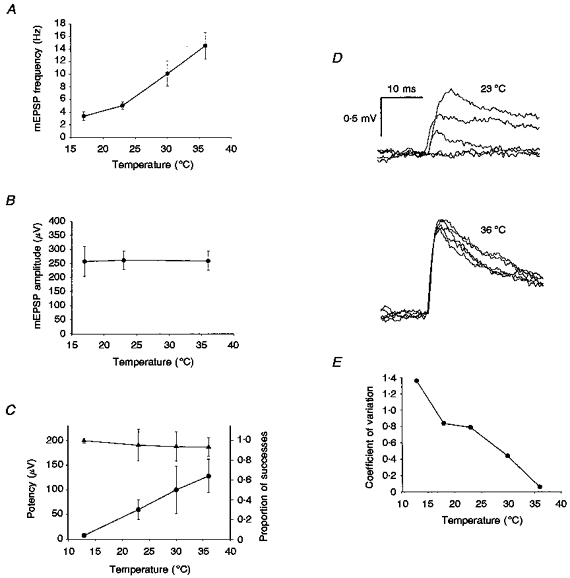
A, the average frequency of spontaneous mEPSPs from ten individual pyramidal neurones recorded in TTX increased with temperature; 125 mEPSPs were analysed for each cell at each temperature. B, the mean peak amplitudes of the mEPSPs did not change with temperature. C, properties of the four connections apparently mediated by just a single release site. With increasing temperature, the mean proportion of successes (•) increased dramatically, but the mean potency of the connections (the mean amplitude of the successes; ▴) did not increase. The mean success rate at 36 °C was 0.64, implying a mean release probability of 0.64 for these connections at this temperature. D, examples of five consecutive trials from a strong, putative multi-site connection. At 23 °C this connection showed failures, latency jitter and substantial amplitude fluctuations (upper traces). At 36 °C, however (lower traces), it did not fail and showed little more amplitude variability than expected from the background noise. E, graph showing the fall in CV with temperature for the connection shown in D, calculated for epochs of 50 trials. At 36 °C, the CV was only 0.06, consistent with a release probability close to 1 at this temperature.
Taken together, these data strongly suggest that m increases with temperature, or, put another way, that low temperatures depress the release of transmitter.
Number of release sites and release probability
For eight of the twelve connections, as the temperature was increased, not only did the proportion of apparent failures decrease, but the mean size of the successes (sometimes called the ‘potency’ of the connection) increased. This suggests that these connections were mediated by more than one transmitter release site. For the other four connections, the mean amplitude of the successes did not increase as the failure rate decreased (Fig. 3C) and the simplest explanation is that these connections were each mediated by only a single release site.
The probability of transmitter release at 36°C appeared to be quite high. For many models of transmitter release, release probabilities < 0.5 will yield amplitude distributions showing positive skew, while release probabilities > 0.5 will produce negative skew. If the release probabilities are close to 0.5, the distributions will be roughly symmetrical. Generally, the amplitude distributions we recorded at 36°C showed only slight skew (range 1.68 to -0.33, mean 0.36 ± 0.68; Fig. 2E). Five distributions showed negative skew and nine distributions gave small skew values of between 0.5 and -0.33. These relatively unskewed amplitude distributions suggest release probabilities of the order of 0.5 on average. Additionally, for those four connections apparently mediated by only a single release site, the failure rate gives a direct indication of release probability. At 36°C, these connections showed only a moderate proportion of failures (ranging from 0.12 to 0.52), suggesting that their release probabilities ranged between 0.48 and 0.88, with a mean of 0.64 ± 0.18.
One of the putative multi-site connections was of particular interest (Fig. 3D). At 23°C or below, it showed both amplitude fluctuations and transmission failures. At 30°C it fluctuated but did not fail, and at 36°C it not only showed no failures but also showed little more amplitude variability than would be expected from the background noise (CV = 0.06; Fig. 3E). This behaviour is most easily explained by the probability of transmitter release at this connection increasing to close to 1 at 36°C.
Effects of cooling on synaptic integration
Temperature can influence many aspects of chemical synaptic transmission (see Fig. 1A). For example, we found that EPSP latency was strongly influenced by temperature (mean at 13°C, 6.6 ± 1.6 ms; at 23°C, 2.5 ± 0.9 ms; at 36°C, 1.1 ± 0.4 ms). The time course of EPSPs was also dramatically affected. For the twelve connections, EPSP 10-90 % rise times were 2.32 ± 0.62 ms at 36°C, but 11.1 ± 4.1 ms at 13°C (Fig. 4A). Similarly, EPSP half-widths increased from 15.0 ± 6.2 to 66.5 ± 21.3 ms. For mEPSPs in TTX, whose peak amplitude hardly changed with temperature, their area (or time integral) increased from 4.04 ± 2.60 mV ms at 36°C to 14.2 ± 10.5 mV ms at 13°C. These changes in EPSP time course can be largely explained by changes in the passive electrical properties of the postsynaptic neurones. For a sample of six pyramidal neurones, we found that input resistance and membrane time constant increased from 135 ± 83 MΩ and 16.2 ± 5.9 ms, respectively, at 36°C, to 613 ± 400 MΩ and 68.6 ± 22 ms at 13°C (Fig. 4B).
Figure 4. The time course of EPSPs was prolonged at low temperature.
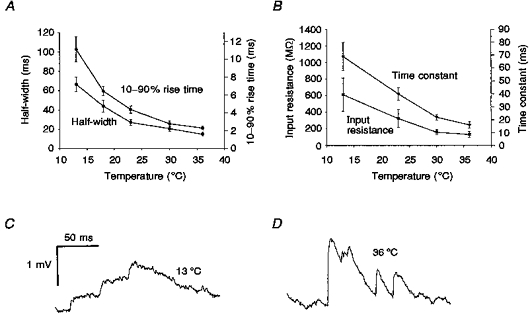
A, the 10-90 % rise time and the width at half-amplitude of the EPSPs from the twelve connections decreased with temperature. B, the membrane time constants and neuronal input resistances obtained from a further sample of six pyramidal neurones decreased with temperature. C and D, EPSPs showed more effective temporal summation at low temperatures. Three spontaneous EPSPs arriving within an 80 ms period showed effective summation at 13 °C (C), while EPSPs at 36 °C, although generally having larger peak amplitudes and occurring at a higher frequency, showed less summation (D).
These changes in the time courses of EPSPs will mean that those EPSPs that do occur at low temperatures will summate much more effectively (Fig. 4C and D). Thus although cooling tends to depress transmitter release, its effects on the activity of a network of neurones may be complex.
DISCUSSION
The most important conclusion from this study is that excitatory synaptic transmission between local pairs of pyramidal neurones is substantially more reliable and less variable at 36°C than at room temperature or below. This seems to be brought about by an increase in the mean number of quanta released per trial at the higher temperatures, which in turn probably reflects an increase in the probability of transmitter release. A similar effect of temperature on release has been reported for the vertebrate neuromuscular junction (e.g. Barrett et al. 1978). However, these findings are not consistent with those of Allen & Stevens (1994) who reported that changing between room temperature and 32-37°C had no effect on the reliability of transmission in hippocampal slices. The age of the animals used in both studies was similar and we think it unlikely that the temperature dependence of a process like transmitter release would differ greatly between hippocampus and neocortex, so the reason for the discrepancy between these findings is not clear. We can only point to the fact that Allen & Stevens used minimal extracellular stimulation while we used paired recording, and so we could observe the presynaptic action potential directly.
It is widely held that excitatory synaptic transmission in higher brain areas is very unreliable, with the majority of individual presynaptic action potentials failing to produce any postsynaptic response (Allen & Stevens, 1994; Stevens & Wang, 1994, 1995). For these brain areas to function reliably, it might be expected that their circuitry would show a high degree of redundancy (Allen & Stevens, 1994), or that signals might be conveyed using trains or bursts rather than single action potentials (Lisman, 1997). The unreliability of transmission is thought to be due to two main factors. First, the probability of transmitter release at individual release sites is thought to be low. Hessler et al. (1993) estimated that 85 % of hippocampal synapses have release probabilities of 0.06 ± 0.01 at 22°C. Recently, Murthy, Sejnowski & Stevens (1997) concluded that the majority had release probabilities < 0.2 (their Fig. 2B) and commented that they might have underestimated the number of synapses with very low probabilities. Second, it is thought that most connections, at least in the hippocampus, are mediated by only a single transmitter release site (Raastad, Storm & Andersen, 1992; Bolshakov & Siegelbaum, 1995) that releases either zero or one quantum (Schikorski & Stevens, 1997).
Our present findings are at odds with both these points. The majority of our connections behaved as though they were mediated by multiple release sites, in line with other recent neocortical paired recording studies (Thomson et al. 1993a; Markram et al. 1997). At 36°C, the amplitude frequency distributions of most of our connections showed only slight skew, indicating release probabilities of the order of 0.5. The combination of moderate release probabilities and multiple release sites meant that most of our connections were quite reliable; nine of the twelve showed < 20 % failures and five showed essentially no failures at 36°C with the low rates of stimulation that we used in our experiments. Similarly low failure rates have been reported previously for pyramid-to-pyramid connections recorded at close to physiological temperature (Thomson et al. 1993a; Markram et al. 1997), although some pyramid-to-interneurone connections have low release probabilities (Thomson, Deuchars & West, 1993b). Reliable connections could not only allow cortical circuitry to operate economically but could also permit information to be encoded in the precise timing of single action potentials even at low firing frequencies. Transmission reliability obviously varies even among connections of a given type and will also depend on the frequency of action potential firing and the recent history of activity (Tsodyks & Markram, 1997). Nevertheless, it may be that experiments performed at low temperatures have contributed to an exaggerated impression of synaptic unreliability in some higher brain areas.
Finally, cooling has been used to inactivate local regions of cortex to investigate their role in complex processes such as visual receptive field generation (e.g. Ferster, Chung & Wheat, 1996). Our slice data suggest that this approach should be used with caution. Although the reliability of transmission falls on cooling, the large increases in EPSP time integral mean that those EPSPs that do occur will summate much more effectively than at body temperature. Thus the effects of cooling on network activity could be complex.
Acknowledgments
N. R. H. holds an MRC Postgraduate Studentship and A. U. L. is a Royal Society University Research Fellow. Additional support was provided by The Wellcome Trust (Programme Grant 034204).
References
- Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proceedings of the National Academy of Sciences of the USA. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN, Botz D, Chang DB, Mahaffey D. Temperature-sensitive aspects of evoked and spontaneous transmitter release at the frog neuromuscular junction. The Journal of Physiology. 1978;279:253–273. doi: 10.1113/jphysiol.1978.sp012343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- Dodt H-U, Zieglgänsberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Research. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. 10.1016/0006-8993(90)90380-T. [DOI] [PubMed] [Google Scholar]
- Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophysical Journal. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Larkman AU, Jack JJB, Stratford KJ. Quantal analysis of excitatory synapses in hippocampal CA1 in vitro during low-frequency depression. The Journal of Physiology. 1997;505:443–456. doi: 10.1111/j.1469-7793.1997.457bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends in Neurosciences. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. The Journal of Physiology. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. Journal of Neuroscience. 1991;11:72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. 10.1016/S0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Raastad M, Storm JF, Andersen P. Putative single quantum and single fibre excitatory postsynaptic currents show similar amplitude range and variability in rat hippocampal slices. European Journal of Neuroscience. 1992;4:113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. Journal of Neuroscience. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetters DK, Zador A. Synaptic transmission: noisy synapses and noisy neurons. Current Biology. 1996;6:1217–1218. doi: 10.1016/s0960-9822(96)00699-9. 10.1016/S0960-9822(96)00699-9. [DOI] [PubMed] [Google Scholar]
- Stevens CF. What form should a cortical theory take? In: Koch C, Davis JL, editors. Large-Scale Neuronal Theories of the Brain. Cambridge, MA, USA: The MIT Press; 1994. pp. 239–255. [Google Scholar]
- Stevens CF, Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994;371:704–707. doi: 10.1038/371704a0. 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–801. doi: 10.1016/0896-6273(95)90223-6. 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. Journal of Neurophysiology. 1993a;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993b;54:347–360. doi: 10.1016/0306-4522(93)90257-g. 10.1016/0306-4522(93)90257-G. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proceedings of the National Academy of Sciences of the USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]


