Abstract
In frog pituitary melanotrophs, GABA induces a transient stimulation followed by prolonged inhibition of hormone secretion. This biphasic effect is inconsistent with the elevation of cytosolic calcium and the inhibition of electrical activity also provoked by GABA in single melanotrophs. In the present study, standard patch-clamp configurations and gramicidin-perforated patches were used to investigate the physiological GABAA receptor-mediated response and intracellular chloride concentration ([Cl−]i) in cultured frog melanotrophs.
In the gramicidin-perforated patch configuration, 1 μM GABA caused a depolarization associated with an action potential discharge and a slight fall of membrane resistance. In contrast, at a higher concentration (10 μm) GABA elicited a depolarization accompanied by a transient volley of action potentials, followed by a sustained inhibitory plateau and a marked fall of membrane resistance. Isoguvacine mimicked the GABA-evoked responses, indicating a mediation by GABAA receptors.
In gramicidin-perforated cells, the depolarizing excitatory effect of 1 μm GABA was converted into a depolarizing inhibitory action when 0.4 μm allopregnanolone was added to the bath solution.
After gaining the whole-cell configuration, the amplitude and/or direction of the GABA-evoked current (IGABA) rapidly changed before stabilizing. After stabilization, the reversal potential of IGABA followed the values predicted by the Nernst equation for chloride ions when [Cl−]i was varied.
In gramicidin-perforated cells, the steady-state I–V relationships of 10 μm GABA- or isoguvacine-evoked currents yielded reversal potentials of −37.5 ± 1.6 (n= 17) and −38.6 ± 2.0 mV (n= 8), respectively. These values were close to those obtained by using a voltage-ramp protocol in the presence of Na+, K+ and Ca2+ channel blockers. The current evoked by 1 μm GABA also reversed at these potentials.
We conclude that, in frog pituitary melanotrophs, chloride is the exclusive charge carrier of IGABA. In intact cells, the reversal potential of IGABA is positive to the resting potential because of a relatively high [Cl−]i (26.5 mm). Under these conditions, GABA induces a chloride efflux responsible for a depolarization triggering action potentials. However, GABA at a high concentration or in the presence of the potentiating steroid allopregnanolone exerts a concomitant shunting effect leading to a rapid inhibition of the spontaneous firing.
In the adult central nervous system, γ-aminobutyric acid (GABA) is widely recognized as the major inhibitory neurotransmitter. Employed by 17-30 % of synapses (Bloom & Iversen, 1971), GABA inhibits the release of transmitters from nerve terminals throughout the brain, principally via the activation of ionotropic GABAA or metabotropic GABAB receptors (Mody, De Koninck, Otis & Soltesz, 1994; Kaupmann et al. 1997). In contrast, in the pituitary, GABAA receptors mediate an excitatory action on the secretory activity. In the rat anterior hypophysis, GABAA receptor agonists increase the secretion of growth hormone, adrenocorticotrophic hormone (Anderson & Mitchell, 1986a) and luteinizing hormone (Anderson & Mitchell, 1986a; Virmani, Stojilkovic & Catt, 1990). Moreover GABAA receptor agonists exert a biphasic effect, i.e. a transient stimulation followed by a prolonged inhibition, on the basal release of prolactin (Anderson & Mitchell, 1986b) and the stimulated secretion of thyroid-stimulating hormone (Tapia-Arancibia, Roussel & Astier, 1987).
In the pituitary intermediate lobe of mammalian and non-mammalian vertebrates, the melanotrophs which receive GABAergic inputs originating from the hypothalamus possess GABAA receptors as well (De Rijk, van Strien & Roubos, 1992; Tonon, Bosler, Stoeckel, Pelletier, Tappaz & Vaudry, 1992). In the rat and porcine pars intermedia, GABAA receptor agonists stimulate α-melanocyte-stimulating hormone (α-MSH) secretion (Tomiko, Taraskevich & Douglas, 1983; Demeneix, Taleb, Loeffler & Feltz, 1986). In the African clawed toad Xenopus laevis and frog Rana ridibunda, GABA induces a biphasic effect. Both phases are mimicked by GABAA receptor agonists and are blocked by bicuculline (Adjeroud et al. 1986; Desrues, Vaudry, Lamacz & Tonon, 1995; Buzzi, Bemelmans, Roubos & Jenks, 1997). In the amphibians, the stimulatory and inhibitory actions of GABA can thus be ascribed to the activation of GABAA receptors. The secretion of α-MSH requires calcium entry through voltage-gated calcium channels (Shibuya & Douglas, 1993; Desrues et al. 1995; Buzzi et al. 1997; Shibuya, Kongsamut & Douglas, 1997). It has recently been shown that the phasic elevations of cytosolic calcium in Xenopus laevis melanotrophs are triggered by action potentials (Valentijn & Valentijn, 1997). However, extracellular recordings of the spontaneous electrical activity have only revealed a marked inhibitory effect of GABA attributed to a membrane hyperpolarization (Taraskevich & Douglas, 1982; Louiset, Valentijn, Vaudry & Cazin, 1992). Hence, the mechanism of action of GABA in the melanotrophs remains unclear.
The GABAA receptor subtype is composed of the association of five subunits lining an integral chloride-selective ion channel (Mody et al. 1994). As a result, the reversal potential of the GABA-evoked current (Vrev,GABA) corresponds to the chloride equilibrium potential (ECl) (Louiset et al. 1990, 1992; Le Foll, Castel, Louiset, Vaudry & Cazin, 1997). It thus appears that the intracellular chloride concentration ([Cl−]i) plays a key role in the GABA response. So far, accurate measurement of Vrev,GABA has been coming up against technical difficulties which hampered prediction of whether GABA depolarizes or hyperpolarizes the cells in physiological conditions. Conventional electrophysiological methods using cell impalement with sharp electrodes may alter [Cl−]i because of the cell injury and ion diffusion from and into the electrode. Evaluation of [Cl−]i by tight-seal whole-cell recording is not suitable either, because of the rapid chemical equilibration between the pipette solution and cell compartments (Marty & Neher, 1995). Herein, we took advantage of the recently developed gramicidin-perforated patch method to overcome these limitations (Ebihara, Shirato, Harata & Akaike, 1995). Contrary to the ionophores (i.e. amphotericin B or nystatin) commonly used to perforate the cell membrane (Rae, Cooper, Gates & Watsky, 1991), gramicidin creates pores which are completely impermeable to anions (Hladky & Haydon, 1984). In this respect, the gramicidin-perforated patch technique allows patch-clamp recordings with intact [Cl−]i. The aim of the present study was to determine the physiological effect of GABA in the modulation of the bioelectrical activity of cultured frog melanotrophs.
METHODS
Animals
Adult male frogs (Rana ridibunda; body weight, 30-40 g) were obtained from a commercial supplier (Couétard, Saint-Hilaire de Riez, France). Frogs were housed in a temperature-controlled room (8°C) under an established photoperiod of 12 h of light-day (lights on from 06.00-18.00 h). The animals had free access to running water and were maintained in these conditions for at least 1 week before use. Animal manipulations were performed according to the recommendations of the French Ethical Committee and under the supervision of authorized investigators.
Reagents
GABA, MS-222, allopregnanolone (5α-pregnan-3α-ol-20-one), gramicidin D and Leibovitz L-15 medium were purchased from Sigma. Isoguvacine was from Research Biochemicals.
Cell cultures
Primary cultures of frog pituitary melanotrophs were prepared as previously described (Le Foll et al. 1997). Briefly, after anaesthetization by immersion in 1 % MS-222, the animals were killed by cervical dislocation and decapitated. Neurointermediate lobes were carefully dissected and washed in Leibowitz L-15 culture medium adjusted to frog osmolality (f-L-15) and supplemented with 1 % (v/v) kanamycin and antibiotic-antimycotic solutions (Boehringer Mannheim). Pars intermedia cells were then dissociated by enzymatic digestion in f-L-15 containing 0.15 % protease Type IX and 0.15 % collagenase Type IA for 15 min at room temperature. After mechanical disaggregation by gentle aspiration in a fire-polished siliconized Pasteur pipette, the cells were centrifuged (60 g, 5 min) and resuspended four times in f-L-15 supplemented with 10 % fetal calf serum (Boehringer Mannheim) and antibiotics. The cells were then plated at a density of 10 000 cells per 35 mm culture dish (Costar, Cambridge, MA, USA). Cultured cells were incubated at 22°C in a humidified atmosphere.
Electrophysiological recordings and analysis
Patch-clamp recordings were performed at room temperature on 5- to 10-day-old cultured melanotrophs. Culture dishes were placed on the stage of an inverted microscope (Wilovert, Leitz). Unless otherwise indicated, the culture medium was replaced with standard extracellular solution containing (mM): 112 NaCl, 2 KCl, 2 CaCl2, 15 Hepes (adjusted to pH 7.4 with NaOH). The cells were allowed to adapt to this new medium for 30 min. Conventional whole-cell and gramicidin-perforated patch recordings were made using soft glass patch electrodes fabricated from thin-walled microhaematocrit tubes on a two-step vertical pipette puller (L/M-3P-A, List Medical). The whole-cell and the cell-attached pipette solution contained (mM): 100 potassium glutamate, 1 CaCl2, 2 MgCl2, 10 Hepes, 10 EGTA, 2 K2ATP (adjusted to pH 7.4 with KOH). The gigaseal formation was induced by slowly placing the electrodes (3-5 MΩ) down onto the cell surface until a resistance increase was detected (10-20 MΩ). A suction pulse was then applied to form a tight seal (> 4 GΩ). To establish whole-cell recording, additional suction was performed to rupture the patch membrane. The gramicidin-perforated patch pipette solution contained (mM): 100 KCl, 10 Hepes (adjusted to pH 7.4 with KOH). In some experiments, KCl was replaced with equimolar CsCl. Gramicidin was first dissolved in methanol to a concentration of 10 mg ml−1 and then diluted in the pipette solution to a final concentration of 100 μg ml−1 just before use (Ebihara et al. 1995). No filtering was employed, in order to avoid any trapping by the filter of antibiotic in suspension in the pipette solution. Before backfilling the electrode with the gramicidin-containing solution, the tip of the electrode was always loaded with a small volume of an antibiotic-free pipette solution. Perforated-patch recordings were obtained using the same procedure as for whole-cell recordings, except mechanical rupture of the plasma membrane was omitted. After getting a gigaseal, the command potential was set at -50 mV, so that, before membrane perforation, the transpatch potential was close to 0 mV and, after perforation, the cell was roughly at resting potential. The progress of perforation was monitored by evaluating the access resistance deduced from the amplitude of the capacitive transients in response to repeated 10 mV hyperpolarizing steps. Usually, the access resistance decreased and the apparent input capacitance increased to stabilize within a delay of 10 min after establishment of the seal. The access resistance and input capacitance reached 13.6 ± 2.0 MΩ (n= 16) and 16.4 ± 0.7 pF (n= 14), respectively. These values were close to those (11.8 ± 0.6 MΩ and 19.7 ± 0.6 pF, n= 72) obtained with the conventional whole-cell technique. During perforation, the noise of the current trace gradually augmented. Concomitant stabilization of both membrane potential and action potentials occurred within 2 min of recording. Drugs were applied after the access resistance was below 20 MΩ. Currents were acquired using an Axopatch 200A amplifier (Axon Instruments) interfaced to a Digidata 1200 (Axon Instruments) and directly digitized with pCLAMP 6 software for further off-line analysis. Spontaneous activity signals were stored on a DTR1200 digital tape recorder (Biologic, Claix, France) and later replayed on a 2200S chart recorder (Gould). The liquid junction potential between the bath and the pipette potassium glutamate solutions was corrected before either the seal formation or data analysis. Quantitative data are expressed as means ±s.e.m., and Student's t test was used for statistical analysis.
Drug application
GABA was dissolved in the standard extracellular solution and focally applied by pressure ejection (2-4 p.s.i.) through a micropipette (1-2 μm tip diameter). To avoid uncontrolled drug leakage, the ejection pipette was only brought near the recorded cell (10-20 μm) just before microejection. To establish steady-state I-V relationships, families of currents were evoked by successive applications of GABA at membrane potentials varying from -80 to +80 mV by 20 mV steps at 2 min intervals. Only one I-V curve per cell was established, in order to minimize a possible influence of the GABA-evoked currents on the internal chloride concentration and subsequent changes in Vrev,GABA. The extracellular solution was continuously superfused at a flow rate of 3 ml min−1 using a gravity-fed pipe (inner diameter, 500 μm) positioned approximately 500 μm away from the recorded cell. The excess bathing solution was continuously aspirated via a suction needle.
RESULTS
The action of GABAA receptor agonists on the electrical activity of cultured frog melanotrophs was studied in the cell-attached, whole-cell and gramicidin-perforated patch configurations in a total of 223 cells.
Effects of GABAA receptor agonists on membrane potential and spontaneous electrical activity of frog melanotrophs
After establishment of the whole-cell configuration with a low chloride concentration in the pipette solution ([Cl−]i, 6 mM), 75 % of the melanotrophs studied in the current-clamp mode emitted spikes from resting potential varying between -30 and -68 mV (mean, -45.6 ± 1.0 mV; n= 79). All the spiking cells responded to GABA (10 μM) by a total arrest of the action potentials. The more positive the membrane potential, the more pronounced the hyperpolarization induced by GABA (Fig. 1A). The voltage response amplitude-membrane potential (V-V) relationship yielded a reversal potential of -72 mV, a value very close to the chloride equilibrium potential (ECl, -75 mV) predicted by the Nernst equation in the present experimental conditions (Fig. 1B). To investigate the response to GABA with unaltered [Cl−]i in melanotrophs, the perforated patch recording technique with gramicidin D as an ionophore was used. In the gramicidin-perforated patch configuration, resting potential varied from cell to cell between -36 and -68 mV. The mean value (-46.3 ± 1.5 mV, n= 29) did not differ from that measured in the whole-cell configuration (P > 0.5). In both configurations, the action potential threshold ranged between -35 and -65 mV. Figure 1C and D illustrates the voltage response amplitude-membrane potential relationship of the action of GABA (10 μM) in the gramicidin-perforated patch configuration. The V-V curve crosses the 0 mV response amplitude at -30 mV, revealing that at potentials flanking the mean resting potential of the melanotrophs, GABA triggers a depolarization. At these potentials (-40 to -70 mV), the effect of GABA on the electrical activity appeared to be biphasic, consisting first of a transient volley of action potentials at the onset of the depolarization followed by a sustained inhibitory plateau.
Figure 1. Relationships between GABA-evoked voltage responses and membrane potential in cultured frog melanotrophs.
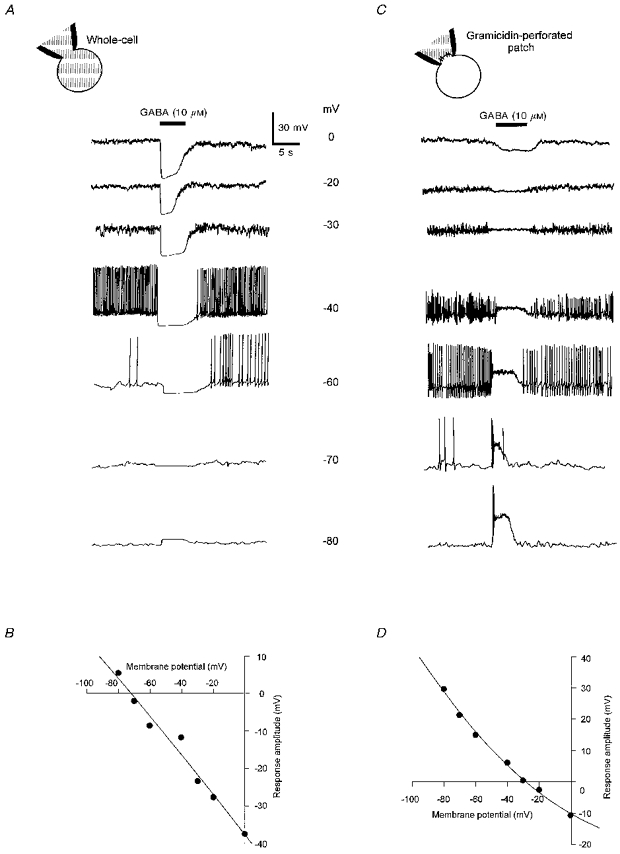
A, voltage responses to GABA (10 μM) recorded in the whole-cell configuration from a single cell held at different membrane potentials as indicated beside the traces. Chloride concentration in the patch pipette solution was 6 mM (ECl= -75 mV). Applications of GABA (10 μM, 5 s duration, filled bar) hyperpolarized the cell at potentials more positive than -70 mV. At membrane potentials allowing spontaneous firing, GABA concomitantly inhibited the action potentials. B, amplitude of the GABA-evoked voltage responses shown in A, plotted against membrane potential. The deduced reversal potential (-72 mV) corresponded to ECl. C, voltage responses to GABA (10 μM) recorded in the gramicidin-perforated patch configuration from another melanotroph. Chloride concentration in the patch pipette solution was 115 mM (ECl= -0.6 mV). GABA was pressure-ejected at various membrane potentials as in A. GABA depolarized the cell at potentials more negative than -30 mV. At potentials where activity was infrequent or absent (-70 and -80 mV), GABA produced first a volley of action potentials followed by a sustained inhibitory plateau. D, V-V curve corresponding to the traces shown in C, displaying a reversal potential of -30 mV.
The effects of GABA on the spontaneous electrical activity of the melanotrophs were further studied by using either low (0.1-1 μM) or high (5-10 μM) GABA concentrations. In the cell-attached configuration, spontaneous biphasic action currents were observed in 68 % of the melanotrophs (n= 38). The action current amplitude was highly variable (5-20 pA) from cell to cell. At a low concentration (0.1 μM), GABA increased the action current frequency in six out of eleven spontaneously active cells (Fig. 2A). In the other cells, the firing pattern was apparently not modified (not shown). In melanotrophs which did not exhibit any electrical activity (n= 12), 0.1 μM GABA induced bursts of action currents (Fig. 2A). In extremely active cells recorded in the gramicidin-perforated patch configuration, 1 μM GABA provoked slight depolarizations responsible for an increase in spike frequency (Fig. 2B). During GABA exposure, the action currents or action potentials were weaker in amplitude than those occurring in control conditions. Moreover, the spike amplitude gradually declined throughout the GABA application. In cells which were less active, GABA (1 μM) elicited bursts of action potentials without any apparent tachyphylaxis (Fig. 2B). The depolarization from resting potential varied between 4 and 15 mV (6.9 ± 1.5 mV, n= 8; Fig. 2C) and was accompanied by a slight fall in the input resistance. The input resistances measured before (2.5 ± 0.4 GΩ) and during GABA exposure (1.8 ± 0.4 GΩ) did not significantly differ (n= 8, P > 0.2, Fig. 2D).
Figure 2. Effects of low GABA concentrations on the bioelectrical activity of frog melanotrophs with intact internal chloride concentration.
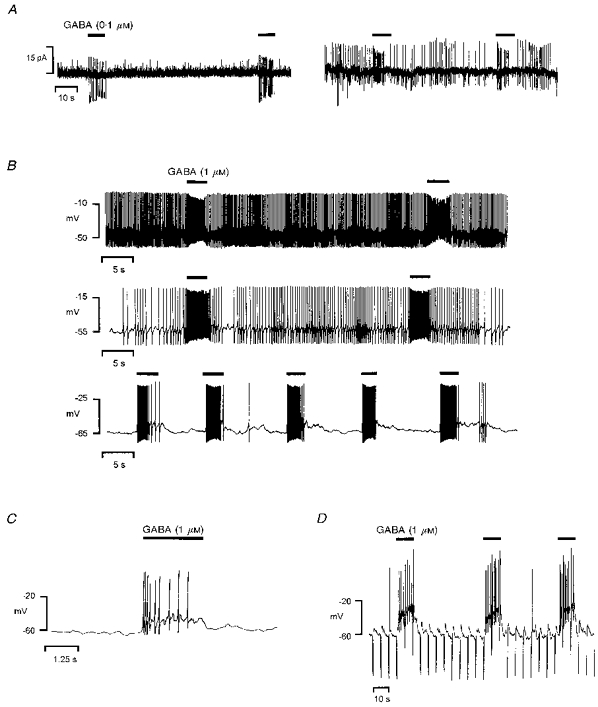
GABA was pressure-ejected in the vicinity of the cells as indicated by the filled bars above the traces. A, recordings obtained from two distinct melanotrophs in the cell-attached configuration. Left, in a cell which did not exhibit spontaneous firing, repeated applications of 0.1 μM GABA elicited action current discharges. Right, in a spontaneously spiking cell, 0.1 μM GABA increased the action current frequency. Note the decrease of the action current amplitude during GABA exposure. B-D, effects of 1 μM GABA in three melanotrophs recorded in the gramicidin-perforated patch configuration. B, membrane potential was adjusted from the resting value (-49 mV, top trace) to -55 mV (middle trace) and -65 mV (bottom trace) in order to modify the firing rate of the cell. For each distinct discharge pattern, an increase in the spike frequency persisted throughout the application of GABA. C, fast sweep exhibiting a GABA (1 μM)-induced depolarization associated with a burst of action potentials. To hamper spontaneous spiking activity, the cell was hyperpolarized from resting potential (-47 mV) to -60 mV. D, the cell input resistance was monitored by hyperpolarizing pulses (-20 pA, 400 ms, 0.2 Hz) superimposed to the holding current. The GABA (1 μM)-evoked depolarization was accompanied by a slight fall in the membrane resistance.
At a higher concentration (10 μM), GABA inhibited the action currents in a large majority (13:15) of spontaneously spiking cells recorded in the cell-attached configuration (Fig. 3A). In gramicidin-perforated melanotrophs, 5 μM GABA induced a depolarization associated with a blockage of the action potentials at potentials where spontaneous activity was present (Fig. 3B). Typically, the inhibitory plateau was preceded by one to three spikes of a weak amplitude (Fig. 3B). The depolarization from resting potential reached 8-16 mV (8.4 ± 1.0 mV, n= 9) and was marked by an immediate and significant collapse of the input resistance from 2.2 ± 0.3 to 0.4 ± 0.2 GΩ (n= 7, P < 0.001; Fig. 3C). In order to investigate the possible contribution of other ionic conductances in the changes of membrane potential and input resistance, the effects of GABA were studied in the presence of voltage-gated ion channel blockers in the extracellular saline (TEA, TTX and Co2+) and pipette solution (CsCl). In such conditions, the reversal of voltage response to GABA and the fall of membrane resistance were similar to those obtained in normal salines. The absolute input resistance in the presence of 10 μM GABA appeared to be independent of membrane potential (Fig. 3D).
Figure 3. Effects of high GABA concentrations on the bioelectrical activity of frog melanotrophs with intact internal chloride concentration.
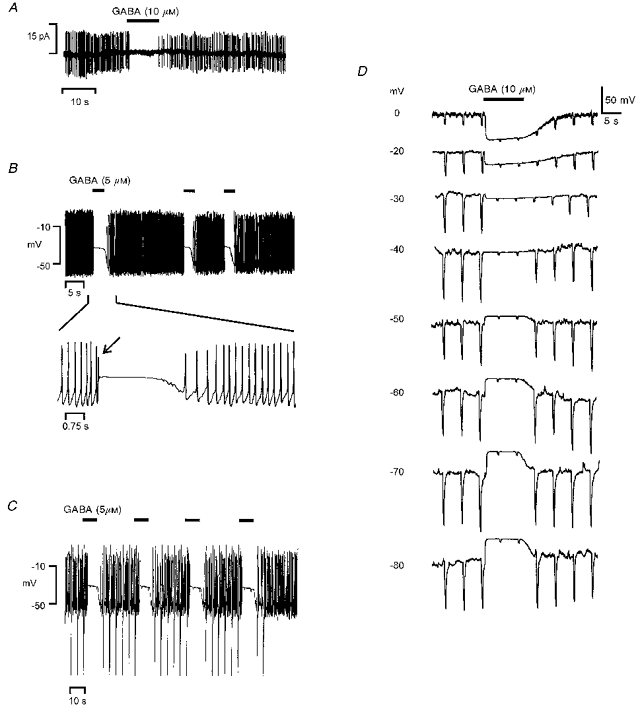
A, cell-attached recording obtained from a spontaneously spiking cell. GABA (10 μM, filled bar) provoked a reversible arrest of action current discharges. B-D, gramicidin-perforated patch recordings performed in three other melanotrophs. B, resting potential was -44 mV. At a concentration of 5 μM, GABA induced a marked depolarization associated with an arrest of firing. The fast sweep shows the initial depolarization-induced spike (arrow) preceding a sustained inhibitory plateau. C, resting potential was -49 mV. The cell input resistance was monitored by hyperpolarizing pulses (-20 pA, 400 ms, 0.2 Hz) superimposed to the holding current. Note that the depolarization induced by 5 μM GABA was accompanied by a dramatic drop in the membrane resistance. D, voltage and input resistance responses to 10 μM GABA at various membrane potentials in the presence of voltage-gated ion channel blockers. The bath solution contained (mM): 92 NaCl, 20 TEA-Cl, 15 Hepes, 2 CoCl2 and 0.001 TTX. The pipette was filled with a solution containing (mM): 100 CsCl and 15 Hepes. During GABA applications the membrane resistance, monitored as in C, fell markedly to reach a level that did not appear to depend on the membrane potential.
In very much the same way as GABA, isoguvacine (10 μM) depolarized the melanotrophs. At 1 μM concentration, isoguvacine triggered action potentials without any modification of the input resistance (Fig. 4A and B). At 10 μM concentration, isoguvacine exerted a depolarizing effect associated with a steep drop in the input resistance, and an arrest of spike discharges at membrane potentials allowing spontaneous activity (Fig. 4C and D).
Figure 4. Isoguvacine-evoked membrane potential and input resistance changes recorded from gramicidin-perforated patches in melanotrophs.

Isoguvacine (1 or 10 μM) was administrated as indicated by the filled bars above the traces. A, the cell was hyperpolarized from resting potential (-54 mV) to -65 mV. Isoguvacine (1 μM) induced slight depolarizations accompanied by action potential discharges. B, the cell input resistance was monitored by hyperpolarizing pulses (-20 pA, 400 ms, 0.2 Hz) superimposed to the holding current. In the presence of isoguvacine (1 μM), spike discharges were evoked by the hyperpolarizing pulses without any apparent modification of the membrane resistance. C, recording obtained from another melanotroph. Resting potential was -41 mV. At a higher concentration (10 μM), isoguvacine induced a depolarization accompanied by an arrest of firing. D, the cell input resistance was monitored as in B. The depolarization induced by 10 μM isoguvacine was associated with a pronounced reduction in the membrane resistance.
Modulation of the response to GABA by allopregnanolone
To determine the role of the input resistance decrease in the abolition of the pacemaker activity, cells (n= 8) were exposed to 1 μM GABA in the absence or presence of allopregnanolone (5α-pregnan-3α-ol-20-one), a neurosteroid which potentiates the GABA-evoked current (Fig. 5). In cells exhibiting a spiking activity at resting potential, application of 1 μM GABA in the presence of 0.4 μM allopreganolone resulted in a response pattern corresponding to the effect of a high GABA concentration (5-10 μM), i.e. an initial volley of action potentials followed by an inhibitory plateau. Typically, the inhibitory phase persisted over a short period after the GABA application (Fig. 5A). The biphasic effect of 1 μM GABA occurring in the presence of allopregnanolone was underlain by an intense collapse of the input resistance (Fig. 5B).
Figure 5. Effect of allopregnanolone on the GABA-evoked voltage responses recorded in the gramicidin-perforated patch configuration in melanotrophs.
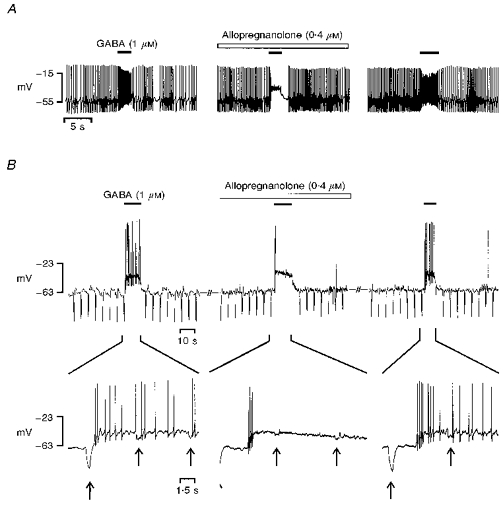
GABA (1 μM) was pressure-ejected as indicated by the filled bars above the traces. A, resting potential was -54 mV. GABA provoked a transient increase in the action potential frequency (left). During bath perfusion with 0.4 μM allopregnanolone (open bar), GABA induced a depolarization associated with an early volley of action potentials, followed by an inhibitory plateau (middle). Recovery of the control response was obtained after washout (right). B, the cell was hyperpolarized from resting potential (-51 mV) to -63 mV, to hamper spontaneous action potentials. Hyperpolarizing pulses (-6 pA, 400 ms, 0.2 Hz) were superimposed to the holding current to monitor the cell input resistance. A fast sweep is presented below each trace in order to detail both the evoked action potentials and the voltage jumps (arrows) caused by the hyperpolarizing current pulses. GABA induced a clear-cut depolarization accompanied by a burst of action potentials (left). The membrane resistance fell from 4.5 GΩ in control to 2.1 GΩ during GABA administration. In the presence of allopregnanolone (0.4 μM, open bar), the GABA-evoked depolarization triggered a brief flurry of action potentials followed by a complete arrest of firing (middle). Membrane resistance throughout depolarization was 0.6 GΩ. After washout, recovery of the control response was obtained (right).
Effects of GABAA receptor agonists on membrane current
In whole-cell recordings, internal chloride re-equilibration occurs very rapidly after membrane rupture. Recordings of GABA-evoked current (IGABA) immediately after gaining whole-cell access indicated that cell dialysis resulted in a substantial alteration of physiological [Cl−]i in intact melanotrophs (n= 10; Fig. 6A). The time course of the peak current was monitored within the first 25 s following the membrane rupture. At a holding potential of -40 mV, IGABA was first inwardly directed and reversed after a period of 2-6 s recording. At a holding potential of -80 mV, the initial IGABA was inwardly directed, while at -35 mV it was outwardly directed. The GABA-evoked current gradually stabilized to a level depending on the holding potential. The more negative the holding potential, the larger the difference between the initial and the stabilized currents. Monoexponential curves fitted to the data gave a time constant of 3.4 ± 0.7 s (n= 6) for stabilization of IGABA. To verify the effectiveness of the gramicidin-perforated patch technique in keeping intact the physiological [Cl−]i, in some cells the perforated patch recording was converted into a whole-cell recording. Equimolar chloride concentrations were used in the pipette and bath solutions. As shown in Fig. 6B, in the gramicidin-perforated patch configuration, the isoguvacine-evoked current was outwardly directed at -20 mV and inwardly directed at -50 mV. In contrast, in the whole-cell configuration isoguvacine induced an inward current at both -20 and -50 mV.
Figure 6. Currents evoked by GABAA receptor agonists in the whole-cell and gramicidin-perforated patch configurations.

A, time course of the GABA-evoked current amplitude within the first seconds following whole-cell access. Chloride concentration in the patch pipette solution was 6 mM (ECl= -75 mV). Left, the arrow indicates the onset of whole-cell access. Holding potential was -40 mV. Repeated 3 s pulses of GABA (10 μM, filled bar) were delivered 1, 6, 11, 16 and 21 s after membrane rupture. Note that the first pulse of GABA induced an inward current while the following pulses generated outward currents. Right, time course of the GABA-evoked current amplitude in three cells clamped at holding potentials of -80 (○), -40 (□) or -35 mV (▵). The data were fitted to monoexponential functions yielding time constants of 5.2, 4.6 and 4.3 s, respectively. Note that the initial GABA-evoked current was inwardly directed at -80 and -40 mV, whilst it was outwardly directed at -35 mV. B, chloride concentration in the patch pipette solution was 115 mM (ECl= -0.6 mV). Isoguvacine (10 μM) was pressure-ejected (5 s, filled bar) in the vicinity of a melanotroph clamped at -20 (top traces) or -50 mV (bottom traces). The perforated patch recording (left) was subsequently converted into a whole-cell recording (right) by a suction pulse-induced rupture of the underlying plasma membrane. Isoguvacine-induced currents were studied within the first minute after gaining whole-cell access to avoid gramicidin perforation of the whole-cell membrane. In the gramicidin-perforated patch recording, the currents evoked by isoguvacine at -20 and -50 mV were opposite, whilst in the whole-cell configuration they both remained inwardly directed.
In order to assess the intracellular chloride concentration, a series of experiments was conducted in voltage-clamped melanotrophs. To avoid desensitization and possible changes in [Cl−]i, currents were elicited by brief (2.5 s) microejections of 10 μM GABA or isoguvacine onto cells held at different holding potentials (Fig. 7). In the whole-cell configuration with 6 mM chloride in the pipette solution, IGABA reversed at -74.7 ± 1.5 mV (n= 16), a value which corresponds to the chloride equilibrium potential according to the Nernst equation (Fig. 7A). When KCl in the pipette solution was substituted in different amounts by potassium glutamate, reversal potential was shifted by 56.3 mV per 10-fold change in internal chloride concentration, as expected for a chloride-specific conductance (Fig. 7B). In the gramicidin-perforated patch configuration, the steady-state I-V curves yielded reversal potentials of -37.5 ± 1.6 (n= 17) and -38.6 ± 2.0 mV (n= 8) for the responses evoked by GABA or isoguvacine, respectively (Fig. 7C and D). These values did not differ significantly (P > 0.5). In the present experimental conditions (118 mM external chloride), assuming that chloride was the only charge carrier of the GABA-evoked current, the intracellular chloride concentration computed from the Nernst equation was 26.5 mM.
Figure 7. Steady-state I-V relationships of currents evoked by GABAA receptor agonists in melanotrophs.
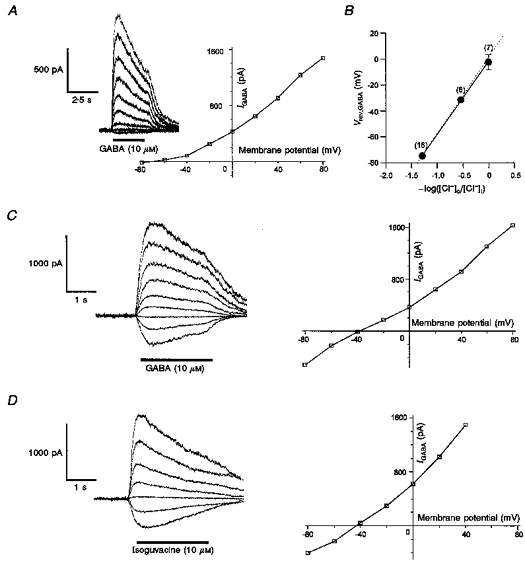
A, I-V relationships of GABA-evoked currents recorded in the whole-cell configuration. Chloride concentration in the patch pipette was 6 mM (ECl= -75 mV). The amplitude of the currents evoked by GABA (10 μM) at potentials varying from -80 to +80 mV by 20 mV steps (inset) was measured at the peak and plotted against the membrane potential. The curve displayed an outward rectification. Reversal potential (-71 mV) was very close to ECl. B, chloride dependence of the reversal potential of the current evoked by GABA. Intracellular chloride was varied by partial replacement of KCl with potassium glutamate. All reversal potentials were determined by interpolation from I-V relationships of the type illustrated in A. Data points are means ±s.e.m. and values indicated in parentheses are numbers of melanotrophs examined. The straight line, fitted to the data by linear regression, has a slope of 56.3 mV. The dotted line corresponds to ECl values calculated from the Nernst equation. C, I-V relationship of the GABA-evoked current recorded in the gramicidin-perforated patch configuration. Left, family of currents evoked by GABA as in A. Right, the corresponding I-V curve yielded a reversal potential of -39 mV. D, same as in C, GABA being replaced by isoguvacine (10 μM). The deduced reversal potential was -42 mV.
The I-V relationships of the currents evoked by 1 or 10 μM GABA were also determined by applying short voltage ramps (Fig. 8). To minimize current desensitization throughout the voltage ramp, a fast sweep rate (150 mV s−1) was used. In these experiments, the contribution of voltage-gated currents was ruled out by including channel blockers in the internal and external solutions. The current evoked by the voltage ramp in the absence of GABA was subtracted from that recorded in the presence of GABA (1.5 s). The instantaneous currents reversed at -35.4 ± 1.8 mV (n= 5) or -39.8 ± 2.5 (n= 11) for 1 or 10 μM GABA, respectively. These values did not significantly differ (P > 0.3).
Figure 8. Instantaneous I-V relationships of currents evoked by GABA recorded in the gramicidin-perforated patch configuration in melanotrophs.
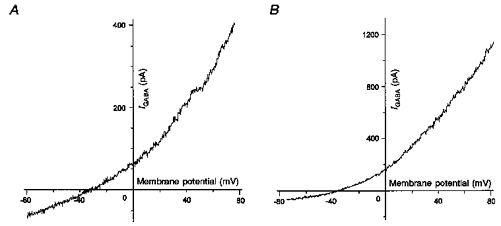
The bath solution contained (mM): 92 NaCl, 20 TEA-Cl, 15 Hepes, 2 CoCl2 and 0.001 TTX. The pipette was filled with a solution containing (mM): 100 CsCl and 15 Hepes. The cells were submitted to depolarizing voltage ramps (150 mV s−1) from -80 to +80 mV. The instantaneous current recorded in the absence of the agonist was subtracted from the current obtained in the presence of 1 (A) or 10 μM (B) GABA. Reversal potentials were measured as the x-intercept value of the resulting current. The deduced reversal potentials were -32 (A) and -35 mV (B) for 1 and 10 μM GABA, respectively.
DISCUSSION
In pituitary melanotrophs, the presence of GABAA receptors is now well documented. Studies using perifusion experiments have shown that GABAA receptor agonists cause either an increase in α-MSH release or a biphasic response consisting of an early stimulation followed by a prolonged inhibition of hormone secretion (Tomiko et al. 1983; Demeneix et al. 1986; Adjeroud et al. 1986; Desrues et al. 1995; Buzzi et al. 1997). These observations are inconsistent with electrophysiological data revealing that GABA induces an immediate abolition of the spontaneous action currents (Taraskevich et al. 1982; Louiset et al. 1992). The present report provides the first evidence that, in intact frog melanotrophs, GABA provokes a depolarization underlain by a chloride efflux from the intracellular compartment that maintains an unusually high [Cl−]i.
Responses mediated by GABAA receptors in frog melanotrophs
When recorded in the whole-cell configuration, both the voltage and current responses to GABA reversed at a potential corresponding exactly to the calculated chloride equilibrium potential (ECl). Moreover, by varying the intracellular chloride concentration, we have demonstrated that Vrev,GABA followed the values predicted by the Nernst equation for ECl. Thus, it can be assumed that the current evoked by GABA (IGABA) was a ‘pure’ chloride flux, predominantly mediated through the GABAA receptor- chloride channel complex. These data are in a good agreement with previous pharmacological studies showing that in rat (Demeneix et al. 1986; Kehl, Hughes & McBurney, 1987) and frog melanotrophs (Louiset et al. 1990, 1992; Le Foll et al. 1997), IGABA was mimicked by muscimol or isoguvacine, totally blocked by picrotoxin, bicuculline or SR95531, and influenced by benzodiazepines, barbiturates or neuroactive steroids. In addition, it is well-established that, in these cells, the GABAB receptor agonist baclofen fails to elicit any electrical response (Demeneix et al. 1986; Kehl et al. 1987; Louiset et al. 1990). We conclude that, in the present experimental conditions, chloride was the exclusive charge carrier of IGABA. This implies that the polarity of the response to GABA only depended on the chloride driving force.
The frog melanotrophs exhibit spontaneous electrical activity involving neurone-like ionic conductances (Louiset, Cazin, Lamacz, Tonon & Vaudry, 1988). Herein, by using the non-invasive approach of extracellular recording in the cell-attached configuration, we have observed opposite effects of GABA. While at a high concentration, GABA arrested autonomous activity, at a low concentration it often caused a flurry of action currents of declining amplitude. This latter discharge pattern is reminiscent of K+-induced depolarizations marked by an initial brief burst of action potentials preceding a plateau, as previously described in our cell model (Louiset et al. 1988). The present results are also in accordance with recent data obtained in rat chromaffin cells in which the biphasic effect of GABA on the action current discharge was associated with a brief rise in cytosolic calcium concentration ([Ca2+]i) followed by a reversible inhibition of spontaneous [Ca2+]i oscillations (Busik, Nakamura, Abe, Shibuya & Kanno, 1996). In frog melanotrophs, calcium measurements have shown that GABAA receptor activation increased [Ca2+]i through nifedipine-sensitive voltage-gated calcium channels (Desrues et al. 1995). In Xenopus laevis melanotrophs that exhibit [Ca2+]i oscillations, GABAA receptor agonists induced an initial peak and a subsequent arrest of [Ca2+]i pulsing (Shibuya & Douglas, 1993; Buzzi et al. 1997; Shibuya et al. 1997). Altogether these findings strongly suggest that, in pituitary melanotrophs, GABAA-receptors mediate an early membrane depolarization. However, the ionic mechanisms leading to the biphasic effect of GABA on hormone secretion, [Ca2+]i and bioelectrical activity had until now never been investigated in cells in which [Cl−]i was unaltered.
After whole-cell access with the potassium glutamate solution in the patch pipette, IGABA rapidly changed before stabilizing. At -80, -40 or -35 mV holding potentials, the stabilized currents were determined by the corresponding theoretical chloride driving forces (-5, 35 or 40 mV). Since the initial currents were distinct in amplitude and/or direction, it is suggested that the chloride driving force gradually developed from the native level to a value imposed by the chloride exchange between the pipette solution and cell compartments. The time constant for chloride equilibration was 3.4 s, a value very close to that found by Marty & Neher (1995) for small diffusible particles. In their study, chloride diffusion was supposed to be still negligible at 1 s of pipette-cell communication - the time at which reversal of the initial current occurred between -40 and -35 mV. This narrow range of potentials provides an evaluation of the native ECl, which appears to be positive to the resting potential. As a consequence, GABA is expected to depolarize the cells by generating an outward chloride current. Depolarizing effects of GABA have previously been described from recordings performed with sharp electrodes in mammalian and non-mammalian immature neurones (Ben-Ari, Cherubini, Corradetti & Gaiarsa, 1989; Rohrbough & Spitzer; 1996) and in glial cells (MacVicar, Tse, Crichton & Kettenmann, 1989). In the rat melanotrophs, GABA has been shown to induce a depolarization or hyperpolarization, depending on whether the intracellular electrode was filled with KCl or potassium acetate solutions (Taraskevich & Douglas, 1983; Williams, MacVicar & Pittman, 1989). These controversial data may be attributed to cell damage and anion leakage that cause drifts of ECl after cell impalement. To solve these discrepancies, in the present study we have employed the gramicidin-perforated patch approach, known to keep the physiological [Cl−]i intact (Ebihara et al. 1995). The reversal of the isoguvacine-evoked current observed immediately after conversion of the perforated patch into a whole-cell recording strongly suggests that the native transmembrane chloride gradient was indeed preserved in gramicidin-perforated cells.
Evidence for a depolarizing excitatory or inhibitory effect of GABA provided by the gramicidin-perforated patch approach
The data obtained from gramicidin-perforated patches reveal that, as expected, GABAA receptor agonists exert a depolarizing effect in the frog melanotrophs. Such an effect of GABA has recently been demonstrated in embryonic neurones by using the perforated patch approach (Chen, Trombley & van den Pol, 1996; Rohrbough & Spitzer, 1996). In the present work, we have considered Vrev,GABA to be equivalent to ECl. We did not find any difference between Vrev,GABA deduced from the I-V relationships established during repeated GABA applications at various steady-state membrane potentials or during a single GABA administration in cells submitted to a brief voltage ramp. This observation suggests that the currents evoked by GABA did not produce any noticeable change in [Cl−]i. Therefore, the I-V plots permitted us to determine that the physiological [Cl−]i was 26.5 mM, a concentration allowing an efflux of chloride at resting potential. Alternatively, the reversal potential for GABAA receptor-mediated responses could be influenced by other permeant anions. In particular, it has been shown that, in adult cortical neurones, the GABA-mediated depolarization was, at least in part, underlain by bicarbonate passing through the GABAA receptor-channel complex (Kaila, Voipio, Paalasmaa, Pasternack & Deisz, 1993; Staley, Soldo & Proctor, 1995). Since we have employed salines containing Hepes buffer without CO2 equilibration, a possible involvement of bicarbonate was excluded. Thus, in the present experimental conditions, the depolarizing effect of GABA can be ascribed to the relatively high physiological [Cl−]i.
The voltage responses observed in gramicidin-perforated cells profoundly differed according to GABAA receptor agonist concentrations. Herein, we show that, at a low concentration, GABA or isoguvacine generated a sustained action potential discharge. In contrast, at a higher concentration both agonists elicited a voltage jump that triggered a few spikes before reaching a plateau. In our study, the voltage-clamp experiments clearly indicated that Vrev,GABA did not depend on the GABA concentration. Therefore, the excitatory action of low GABA concentrations as well as the biphasic effect of higher GABA concentrations resulted from a GABAA receptor-mediated chloride efflux. The arrest of firing occurring in the biphasic effect was due to the intense collapse of the membrane resistance to less than 20 % of its resting value, shunting the ionic conductances responsible for the spontaneous activity. Our data also demonstrated that the degree of depolarization and the drop of membrane resistance induced by a high GABA concentration were unchanged in the absence or presence of voltage-gated ion channel blockers. This result indicates that the participation of other active conductances in the effect mediated by the GABAA receptor remained negligible. Additional evidence for the involvement of a shunting effect of GABA was provided by the observation that allopregnanolone switched the excitatory effect of GABA into an inhibitory action, without any apparent modification in the amplitude of the GABA-induced depolarization. This finding signifies that the ionic currents underlying action potentials (in particular voltage-gated Na+ channels) did not inactivate when the membrane was depolarized to Vrev,GABA. In a recent study, we showed that allopregnanolone exerted a potent enhancement of IGABA in frog melanotrophs (Le Foll et al. 1997). We now demonstrate that, in the presence of the neurosteroid, the additional drop in membrane resistance resulting from the potentiation of the GABA-evoked current rendered the membrane ionic currents unable to generate subsequent voltage changes. As a consequence, membrane potential was clamped at Vrev,GABA. Similar strong shunting effects of GABA have also been described in neurones (Soltesz & Mody, 1994; Chen et al. 1996).
The above data shed new light on the understanding of the biphasic effect of GABA on α-MSH release from the frog pituitary pars intermedia observed in vitro in perifusion experiments (Adjeroud et al. 1986; Desrues et al. 1995). Melanotrophs, like other endocrine cells of the hypophysis, display all-or-none action potentials and possess voltage-gated calcium channels. In these cells, the frequency and amplitude of [Ca2+]i transients are supposed to be dependent on the membrane potential. Action potential-driven calcium delivery is thought to be directly responsible for the basal and stimulated hormone secretions (Louiset et al. 1988; Shibuya & Douglas, 1993; Desrues et al. 1995; Shibuya et al. 1997; Buzzi et al. 1997; Valentijn & Valentijn, 1997). Thus, the early transient stimulation of α-MSH release is probably due to the GABA-induced depolarization leading to the activation of voltage-operated sodium and calcium channels. The delayed inhibitory phase of the secretory response could originate from the shunting effect causing cessation of firing. In addition, during prolonged exposure to GABA, transient voltage-dependent conductances would gradually inactivate, the lack of membrane repolarization preventing removal of inactivation. This supposed mechanism of action of GABA could also support the biphasic effects of this neurotransmitter on the hormone release from other anterior pituitary cells, as described for prolactin (Anderson & Mitchell, 1986b) and thyroid-stimulating hormone secretion (Tapia-Arancibia, Roussel & Astier, 1987).
Functional implications
The physiological effect of GABA on α-MSH release is probably linked to the dynamics of GABA release in neuroendocrine synapses. Several lines of evidence indicate that very high concentrations of GABA (0.5-1 mM), sufficient to saturate the postsynaptic receptors, are delivered for very short periods (clearance half-time 50-200 μs) in the synaptic cleft (Mody et al. 1994; Clements, 1996). The quantal release of GABA at the synapses formed between hypothalamic nerve terminals and pituitary melanotrophs has been demonstrated (Schneggenburger & Konnerth, 1992). Interestingly, conventional tight-seal whole-cell recordings of GABAergic inhibitory postsynaptic currents (IPSCs) from rat hypothalamic neurones and pituitary intermediate lobe cells in co-culture (Poisbeau, Rene, Egles, Felix, Feltz & Schlichter, 1996) indicated that a quantum of GABA may activate eight to nine channels. It should be noted that these experiments were conducted using non-physiological [Cl−]i (ECl, -2 mV; holding potential, -60 mV) and probably provide an overestimation of the amplitude of the IPSCs occurring in melanotrophs in vivo. Assuming a mean unitary conductance of 20 pS for GABAA receptor-chloride channels, GABAergic IPSCs correspond to a maximum increase of the input membrane conductance of less than 200 pS, a value not sufficient to induce any shunting effect. In such a case, GABA probably causes a stimulation of α-MSH secretion in vivo. Alternatively, if a larger number of GABAA receptors are simultaneously gated because of intense synchronous synaptic activity, or if enhancing agents such as neurosteroids or benzodiazepines merge IPSCs by prolonging their decay time course (Harrison, Vicini & Barker, 1987; Mody et al. 1994), the fall in membrane resistance accompanying the depolarization is supposed to shunt voltage-dependent conductances and inhibit α-MSH secretion.
Acknowledgments
This work was supported by grants from INSERM (U413), the European Union (Human Capital and Mobility Programme; ERBCHRXCT920017) and the Conseil Régional de Haute-Normandie. F. L. F. was a recipient of a doctoral fellowship from La Direction Générale de la Recherche et de la Technologie. The authors wish to thank Mrs Catherine Buquet for excellent technical assistance.
References
- Adjeroud S, Tonon MC, Lamacz M, Leneveu E, Stoeckel ME, Tappaz ML, Cazin L, Danger JM, Bernard C, Vaudry H. GABA-ergic control of α-melanocyte-stimulating hormone (α-MSH) release by frog neurointermediate lobe in vitro. Brain Research Bulletin. 1986;17:717–723. doi: 10.1016/0361-9230(86)90206-6. 10.1016/0361-9230(86)90206-6. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Mitchell R. Effects of γ-aminobutyric acid receptor agonists on the secretion of growth hormone, luteinizing hormone, adrenocorticotrophic hormone and thyroid stimulating hormone from the rat pituitary gland in vitro. Journal of Endocrinology. 1986a;108:1–8. doi: 10.1677/joe.0.1080001. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Mitchell R. Biphasic effect of GABAA receptor agonists on prolactin secretion: evidence for two types of GABAA receptor complex on lactotrophs. European Journal of Pharmacology. 1986b;124:1–9. doi: 10.1016/0014-2999(86)90118-4. 10.1016/0014-2999(86)90118-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. The Journal of Physiology. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F, Iversen LL. Localizing [3H]GABA in nerve terminals of rat cerebral cortex by electronmicroscopic autoradiography. Nature. 1971;229:628–630. doi: 10.1038/229628a0. [DOI] [PubMed] [Google Scholar]
- Busik J, Nakamura M, Abe Y, Shibuya I, Kanno T. Effects of GABA on spontaneous [Ca2+]c dynamics and electrical properties of rat adrenal chromaffin cells. Brain Research. 1996;739:97–103. doi: 10.1016/s0006-8993(96)00814-1. [DOI] [PubMed] [Google Scholar]
- Buzzi M, Bemelmans FJ, Roubos EW, Jenks BG. Neuroendocrine γ-aminobutyric acid (GABA): functional differences in GABAA versus GABAB receptor inhibition of the melanotrope cell of Xenopus laevis. Endocrinology. 1997;138:203–212. doi: 10.1210/endo.138.1.4886. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. The Journal of Physiology. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD. Transmitter time course in the synaptic cleft: its role in central synaptic function. Trends in Neurosciences. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Demeneix BA, Taleb O, Loeffler JP, Feltz P. GABAA and GABAB receptors on porcine pars intermedia cells in primary culture: functional role in modulating peptide release. Neuroscience. 1986;17:1275–1285. doi: 10.1016/0306-4522(86)90094-1. 10.1016/0306-4522(86)90094-1. [DOI] [PubMed] [Google Scholar]
- De Rijk EPCT, van Strien FJC, Roubos EW. Demonstration of coexisting catecholamine (dopamine), amino acid (GABA), and peptide (NPY) involved in inhibition of melanotrope cell activity in Xenopus laevis: a quantitative ultrastructural, freeze-substitution immunocytochemical study. Journal of Neuroscience. 1992;12:864–871. doi: 10.1523/JNEUROSCI.12-03-00864.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrues L, Vaudry H, Lamacz M, Tonon MC. Mechanism of action of γ-aminobutyric acid on frog melanotrophs. Journal of Molecular Endocrinology. 1995;14:1–12. doi: 10.1677/jme.0.0140001. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. The Journal of Physiology. 1995;484:77–86. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. Journal of Neuroscience. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky SB, Haydon DA. Ion movements in gramicidin channels. Current Topics in Membranes and Transport. 1984;21:327–371. [Google Scholar]
- Kaila K, Voipio J, Paalasmaa P, Pasternack M, Deisz RA. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. The Journal of Physiology. 1993;464:273–289. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bishoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froesti W, Bettler B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Kehl SJ, Hughes D, McBurney RN. A patch-clamp study of γ-aminobutyric acid (GABA)-induced macroscopic currents in rat melanotrophs in cell culture. British Journal of Pharmacology. 1987;95:573–585. doi: 10.1111/j.1476-5381.1987.tb11359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll F, Castel H, Louiset E, Vaudry H, Cazin L. Multiple modulatory effects of the neuroactive steroid pregnanolone on GABAA receptor in frog pituitary melanotrophs. The Journal of Physiology. 1997;504:387–400. doi: 10.1111/j.1469-7793.1997.387be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louiset E, Cazin L, Lamacz M, Tonon MC, Vaudry H. Patch-clamp study of the ionic currents underlying action potentials in cultured frog pituitary melanotrophs. Neuroendocrinology. 1988;48:507–515. doi: 10.1159/000125057. [DOI] [PubMed] [Google Scholar]
- Louiset E, Valentijn JA, Vaudry H, Cazin L. Central-type benzodiazepines modulate GABAA receptor chloride channels in cultured pituitary melanotrophs. Molecular Brain Research. 1992;12:1–6. doi: 10.1016/0169-328x(92)90062-g. 10.1016/0169-328X(92)90062-G. [DOI] [PubMed] [Google Scholar]
- Louiset E, Van De Put FHMM, Tonon MC, Basille C, Jenks BG, Vaudry H, Cazin L. Electrophysiological evidence for the existence of GABAA receptors in cultured frog melanotrophs. Brain Research. 1990;517:151–156. doi: 10.1016/0006-8993(90)91020-h. 10.1016/0006-8993(90)91020-H. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Tse FW, Crichton SA, Kettenmann H. GABA-activated Cl− channels in astrocytes of hippocampal slices. Journal of Neuroscience. 1989;9:3577–3583. doi: 10.1523/JNEUROSCI.09-10-03577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Neher E. Tight-seal whole-cell recording. In: Sakmann B, Neher E, editors. Single-channel Recording. New York: Plenum Press; 1995. pp. 31–52. [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends in Neurosciences. 1994;17:517–524. doi: 10.1016/0166-2236(94)90155-4. 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, René F, Egles C, Félix J-M, Feltz P, Schlichter R. Characterization of functional GABAergic synapses formed between rat hypothalamic neurons and pituitary intermediate lobe cells in coculture: Ca2+ dependence of spontaneous IPSCs. Journal of Neuroscience. 1996;16:4835–4845. doi: 10.1523/JNEUROSCI.16-16-04835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recrodings using amphotericin B. Journal of Neuroscience Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. 10.1016/0165-0270(91)90017-T. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Spitzer N. Regulation of intracellular Cl− levels by Na+-dependent Cl− cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor-mediated responses in spinal neurons. Journal of Neuroscience. 1996;16:82–91. doi: 10.1523/JNEUROSCI.16-01-00082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Konnerth A. GABA-mediated synaptic transmission in neuroendocrine cells: a patch-clamp study in a pituitary slice preparation. Pflügers Archiv. 1992;421:364–373. doi: 10.1007/BF00374225. [DOI] [PubMed] [Google Scholar]
- Shibuya I, Douglas WW. Spontaneous cytosolic calcium pulsing detected in Xenopus melanotrophs: modulation by secreto-inhibitory and stimulant ligands. Endocrinology. 1993;132:2166–2175. doi: 10.1210/endo.132.5.8386613. [DOI] [PubMed] [Google Scholar]
- Shibuya I, Kongsamut S, Douglas WW. Both GABAA and GABAB receptors participate in suppression of [Ca2+]i pulsing in toad melanotrophs. European Journal of Pharmacology. 1997;321:241–246. doi: 10.1016/s0014-2999(96)00936-3. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Mody I. Patch-clamp recordings reveal powerful GABAergic inhibition in dentate hilar neurons. Journal of Neuroscience. 1994;14:2365–2376. doi: 10.1523/JNEUROSCI.14-04-02365.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Roussel JP, Astier H. Evidence for a dual effect of γ-aminobutyric acid on thyrotropin (TSH)-releasing hormone-induced TSH release from perifused rat pituitary. Endocrinology. 1987;121:980–986. doi: 10.1210/endo-121-3-980. [DOI] [PubMed] [Google Scholar]
- Taraskevich PS, Douglas WW. GABA directly affects electrophysiological properties of pituitary pars intermedia cells. Nature. 1983;299:733–734. doi: 10.1038/299733a0. [DOI] [PubMed] [Google Scholar]
- Tomiko SA, Taraskevich PS, Douglas WW. GABA acts directly on cells of pituitary pars intermedia to alter hormone output. Nature. 1983;301:706–707. doi: 10.1038/301706a0. [DOI] [PubMed] [Google Scholar]
- Tonon MC, Bosler O, Stoeckel ME, Pelletier G, Tappaz M, Vaudry H. Colocalization of tyrosine hydroxylase, GABA and neuropeptide Y within axon terminals innervating the intermediate lobe of the frog Rana ridibunda. Journal of Comparative Neurology. 1992;319:599–605. doi: 10.1002/cne.903190409. [DOI] [PubMed] [Google Scholar]
- Valentijn JA, Valentijn K. Two distinct Na+ currents control cytosolic Ca2+ pulsing in Xenopus laevis pituitary melanotrophs. Cell Calcium. 1997;21:241–251. doi: 10.1016/s0143-4160(97)90048-8. [DOI] [PubMed] [Google Scholar]
- Virmani MA, Stojilkovic SS, Catt KJ. Stimulation of luteinizing hormone release by γ-aminobutyric acid (GABA) agonists: mediation by GABAA-type receptors and activation of chloride and voltage-sensitive calcium channels. Endocrinology. 1990;126:2499–2505. doi: 10.1210/endo-126-5-2499. [DOI] [PubMed] [Google Scholar]
- Williams PJ, MacVicar BA, Pittman QJ. Identification of a GABA-activated chloride-mediated synaptic potential in rat pars intermedia. Brain Research. 1989;483:130–134. doi: 10.1016/0006-8993(89)90043-7. [DOI] [PubMed] [Google Scholar]


