Abstract
Controversy exists as to whether a fall in the intracellular Ca2+ concentration ([Ca2+]i) is a requisite element of the vasodilatory response to nitric oxide (NO).
We studied the effect of NO on the coupling between [Ca2+]i and vasoconstriction in arterial segments loaded with the [Ca2+]i-sensitive, intracellular dye fura-2. As data interpretation is equivocal when fura-2 is loaded into both endothelial and smooth muscle cells, we compared results from in vitro experiments on segments of the rat tail artery in which fura-2 and noradrenaline were applied on the luminal or adventitial side, and endothelium was removed ‘physically’ (rubbing or air) or ‘functionally’ (Nω-nitro-l-arginine methyl ester). The use of air perfusion to remove endothelium is of considerable benefit since it allows paired observations in a single tissue.
Fura-2 loaded into endothelial cells but endothelial ‘contamination’ of the smooth muscle cell [Ca2+]i signal was minimal.
Endogenous NO decreased vasoconstrictor responses to noradrenaline but had no effect on [Ca2+]i.
Nitroglycerine decreased vasoconstrictor responses in a concentration-dependent fashion but had no effect on [Ca2+]i.
In conclusion, NO causes vasodilatation via a mechanism which is downstream of [Ca2+]i mobilization.
The intracellular calcium concentration ([Ca2+]i) plays an important role in regulating vascular smooth muscle contractility through calmodulin and the myosin light chain kinase which activates the contractile apparatus (Kamm & Stull, 1985). However, there may not be a direct relationship between [Ca2+]i and contractility as factors downstream of [Ca2+]i mobilization such as the [Ca2+]i sensitivity of myosin phosphorylation are known to vary (Rembold, 1990). Thus, vasodilators could produce smooth muscle cell relaxation by lowering [Ca2+]i, [Ca2+]i sensitivity of myosin phosphorylation, or [Ca2+]i sensitivity of contraction.
Endothelium-derived or exogenous nitric oxide (NO) increases the concentration of cGMP in vascular smooth muscle cells and this is an essential step in relaxation (Murad, 1987; Moncada & Biggs, 1995). In cultured smooth muscle cells high concentrations of an exogenous NO donor (sodium nitroprusside) and 8-bromoguanosine 3′,5′ cyclic monophosphate block extracellular Ca2+ entry (Magliola & Jones, 1990; Blatter & Wier, 1994). In addition to a reduction in [Ca2+]i, endogenous and exogenous NO may cause dilatation via [Ca2+]i-independent mechanisms (Abe, Kanaide & Nakamura, 1990; Chen & Rembold, 1996).
We describe a series of experiments designed to test the hypothesis that NO causes vasodilatation by lowering the [Ca2+]i sensitivity of force. We used the fura-2 perfusion-loaded rat tail artery (Capdeville-Atkinson, Oster, Thorin-Trescases, Robert, Boutinet & Atkinson, 1993; Capdeville-Atkinson, Oster, Thorin-Trescases, Robert, Corman & Atkinson, 1995) and a technique we developed to remove endothelium by air perfusion (Atkinson, Tatchum-Talom & Capdeville-Atkinson, 1994).
When arterial segments are loaded intraluminally with fura-2, dye may be taken up by both the endothelial and smooth muscle cells. Changes in [Ca2+]i are the sum of those occurring in the two cell types. Agonists such as noradrenaline may stimulate one receptor subtype on the vascular smooth muscle cells leading to contraction and another subtype on the endothelial cells leading to release of NO (Cocks & Angus, 1983). The mechanical response depends on two opposing influences, direct constriction of the muscle by noradrenaline and concurrent dilatation due to noradrenaline-induced NO release from the endothelium. Endothelial cells may also act as a barrier to the dye or agonist (Lew & Duling, 1992).
One way of overcoming the problems posed by the endothelium (incorporation of dye and barrier) is to load the dye or apply the agonist from the adventitial side. In order to separate out the three main variables - dye-loading route, route of noradrenaline application and endothelial status - several protocols were used. To investigate the impact of the dye-loading route, segments were loaded by the intraluminal (in the presence or absence of endothelium) or adventitial route (with endothelium intact). To investigate the impact of the route of noradrenaline application, noradrenaline was applied from the luminal or the adventitial side (in the presence or absence of endothelium). Finally, to evaluate the impact of endothelial status arterial segments were challenged with noradrenaline in the presence or absence of endothelium or the nitric oxide synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (L-NAME).
METHODS
Animals
Adult male outbred Wistar rats (524 ± 12 g; Iffa-Credo, L'Arbresle, France) were housed under standard conditions (temperature, 21 ± 1°C; humidity, 60 ± 10 %; lights on, 08.00- 20.00 h) and given a standard rodent diet (code number A04; UAR, Villemoisson sur Orge, France) and water ad libitum for 1 week before each experiment. In each specific protocol (see below) six arterial segments, each from a different rat, were used.
[Ca2+]i-vasoconstriction coupling
This technique has been described in detail elsewhere (Capdeville-Atkinson et al. 1993, 1995). The tail artery (intima, 20 ± 2 μm; media, 91 ± 5 μm; adventitia, 50 ± 2 μm; internal diameter, 350 ± 15 μm and wall thickness/internal diameter, 0.04 ± 0.02; calculated volume: intima, 0.232 mm3; media, 1.375 mm3; adventitia, 0.977 mm3; n= 6) was dissected out under sodium pentobarbitone anaesthesia (60 mg kg−1, i.p.). Animals were killed with an overdose of sodium pentobarbitone (100 mg kg−1, i.p.). The proximal end of the tail artery was cannulated with polyethylene tubing (0.4 mm i.d. and 0.8 mm o.d.; Jencons, Leighton Buzzard, Bedfordshire, UK) and perfused in situ with a physiological salt solution (PSS; (mM): NaCl, 140; KCl, 5; CaCl2, 1.5; MgCl2, 1; glucose, 6; and Hepes, 10; pH, 7.40 ± 0.01) at a rate of 4 ml min−1 and an intraluminal pressure of 50 mmHg.
The artery was thus removed from the tail under constant perfusion in order to minimize damage to endothelial cells, and placed in PSS at 22°C.
A 1 cm segment was cannulated at both ends (intravascular portion of cannula, 0.2 mm) and mounted in a perfusion-cuvette system placed in a dual-wavelength spectrofluorometer (Fluorolog F1 T11; SPEX, Edison, NJ, USA). A longitudinal tension of 0.5 g was used to restore the segment to its in situ length. Changes in longitudinal tension from 0.2 to 1 g (Atkinson, Fouda & Sonnay, 1986) or from 0.5 to 2.5 g (N. N. P. Tran & C. Capdeville-Atkinson, unpublished results) have no effect on baseline perfusion pressure or increases in perfusion pressure produced by noradrenaline.
Arteries were perfused with PSS saturated with 100 % O2 (1.5 ml min−1); the cuvette was filled with oxygenated PSS. Noradrenaline- or KCl-elicited increases in perfusion pressure were measured by a pressure transducer (Baxter; Bentley Laboratories Europe, Uden, Netherlands) placed between the peristaltic pump and the arterial segment; the transducer was linked to a signal processing system (MacLab/MacBridge; World Precision Instruments Inc., Sarasota, FL, USA). All pressure values were corrected by subtraction of the pressure generated by the resistance of the tubing (15 mmHg).
Following a 30 min equilibration period, baseline perfusion pressure and autofluorescence (AF360; excitation wavelength, 360 nm; emission wavelength, 510 nm; global average, 1.03 × 106± 0.02 × 106 counts s−1, n= 48) were determined.
The arterial segment was exposed to a high-potassium depolarizing solution (80 mM KCl, 2 min) and then to noradrenaline (3 μM, 2 min) in order to evaluate its reactivity (Test; Fig. 1).
Figure 1. Specific experimental protocols.
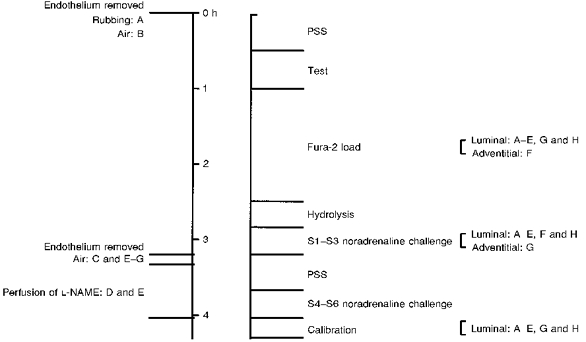
Protocol A, endothelium denuded by rubbing at the beginning. Protocol B, endothelium denuded by air at the beginning. Protocol C, endothelium denuded by air in the middle. Protocol D, L-NAME perfusion. Protocol E, L-NAME perfusion plus endothelium denuded by air. Protocol F, fura-2 adventitial loading. Protocol G, noradrenaline adventitial challenge. Protocol H, time control (with endothelium). n= 6 per protocol.
In preliminary experiments pD2 (-logEC50) and maximum pressure for noradrenaline were 4.92 ± 0.06 and 145 ± 16 mmHg in the presence of endothelium, and 5.33 ± 0.11 and 152 ± 20 mmHg in the absence of endothelium, respectively (n= 6). In the absence of endothelium vasoconstriction was related to [Ca2+]i (Fig. 2).
Figure 2.
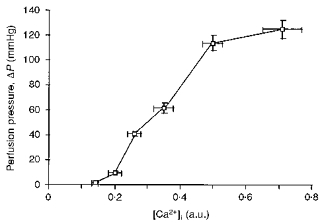
Relationship between [Ca2+]i and vasoconstriction produced by noradrenaline (0.1-30 μM, 2 min at each concentration); n= 6.
Segments whose vasoconstrictor responses to KCl or noradrenaline were outside the range 30-60 mmHg were discarded. The segment was perfused with PSS containing fura-2 AM (acetoxymethyl ester form of fura-2; 5 μM, 90 min), Pluronic F127 (0.06 %), and bovine serum albumin (0.01 %). Loading was followed by wash-out with PSS (20 min), at the end of which fluorescence at the isoemissive point (F360) was measured in order to calculate loading efficiency (F360/AF360).
The segment was illuminated at excitation wavelengths of 340 or 380 nm with a change from one to the other every second; emission fluorescence (510 nm) was measured with a photomultiplier. The ratio (R'340/380) was determined from the background corrected fluorescence at each excitation wavelength before (baseline) and during perfusion of noradrenaline. [Ca2+]i was calculated using a modification (Capdeville-Atkinson et al. 1995) of the equations of Grynkiewicz, Poenie & Tsien (1985) and Scanlon, Williams & Fay (1987):
in arbitrary units (a.u.), where R'=R'340/380; R'max is the ratio of fluorescence in the presence of a saturating concentration of calcium (CaCl2, 4 mM) and the calcium ionophore ionomycin (10 μM, 5 min); R'min is the ratio of fluorescence in the presence of Ca2+-free PSS containing EGTA (10 mM) and ionomycin (10 μM, 7 min); and β' is the ratio of F'380 at zero and saturating calcium concentrations. The absence of [Ca2+]i-insensitive, fluorescent metabolites of fura-2 was checked by perfusing with Ca2+-free PSS containing manganese chloride (1 mM) and ionomycin (10 μM) at the end of the experiment for 3 min. FMn values, which were not significantly different from AF values, were used for background fluorescence. FMn is useful in determining the extent of fura-2 AM de-esterification since manganese binds to fura-2 and so quenches fura-2-[Ca2+]i fluorescence (Scanlon et al. 1987). In all protocols except protocol F calibration parameters were determined using intraluminal perfusion. In protocol F calibration solutions were superfused on the adventitial side for the same length of time.
Following fura-2 loading, arteries were stimulated 3 times (S1, S2 and S3) with noradrenaline (3 μM, 2 min, intra- or extraluminally) with 5 min PSS perfusion between each stimulation. Arteries were then perfused with PSS for 30 min and challenged again with noradrenaline (S4, S5 and S6). At each stimulation, increases in [Ca2+]i and perfusion pressure were measured and [Ca2+]i-vasoconstriction coupling efficiency was calculated: maximal vasoconstriction/peak [Ca2+]i mobilization (mmHg/a.u.).
Specific protocols (Fig. 1)
Rubbing and air perfusion
In order to compare the air perfusion technique for removal of endothelium with that previously used in our laboratory (rubbing), the endothelium was removed by passing a wire (external diameter, 0.3 mm) 3 times through the lumen of the segment before mounting (Rubbing; protocol A; n= 6; Fig. 1). Results were compared with those obtained in protocol B in which endothelium was removed by perfusing the segment with air (0.4 ml min−1) plus PSS (1.5 ml min−1) for 10 min at an intraluminal pressure of 40 ± 10 mmHg at the beginning of the equilibration period (Air; protocol B; n= 6; Fig. 1). This intraluminal air perfusion technique for the removal of endothelium is based on that previously developed in our laboratory (Atkinson et al. 1994).
Air perfusion before and after fura-2 loading
In an attempt to separate the barrier and vasomotor effects of endothelium, in protocol C (n= 6) segments were perfused with air (as described above) following fura-2 loading and the first series of noradrenaline stimulations (S1-S3). This protocol reveals a key feature of the air perfusion technique, viz. that changes in [Ca2+]i and perfusion pressure can be monitored before, during and after removal of endothelium without the interruption produced by dismounting and remounting the tissue if endothelium is removed by rubbing. The perfusate was collected at 2 min intervals during air perfusion and excitation spectra performed on the fractions.
Inhibition of NOS by L-NAME
In order to gain insight into the possibility that the effects of endothelium on [Ca2+]i-vasoconstriction coupling were mediated by release of NO, in protocol D (n= 6) the second series of noradrenaline stimulations (S4-S6) were performed in the presence of L-NAME (10 μM; Vo, Reid & Rand, 1992). In order to evaluate the endothelial origin of the NO involved, this protocol was repeated following destruction of the endothelium by air perfusion (protocol E; n= 6; Fig. 1). In a preliminary experiment we observed that L-NAME (10 μM) had no effect on the excitation spectrum of fura-2 in the absence or presence of CaCl2 (1.5 mM) (results not shown).
Adventitial loading of fura-2
In order to separate the barrier effect of endothelium from that produced by loading of fura-2 into endothelial cells, fura-2 was loaded from the adventitial side of the segment by replacing the PSS in the cuvette by PSS plus fura-2 AM (5 μM, 90 min; protocol F; n= 6; Fig. 1). A peristaltic pump (flow rate, 1.5 ml min−1) assured that the loading solution was replaced every minute. This recirculation rate is 35 times lower than that obtained with intraluminal loading. Segments were stimulated with noradrenaline (S1-S3), endothelium was removed by air perfusion and noradrenaline stimulation (S4-S6) was repeated. [Ca2+]i calibration was performed by adventitial perfusion.
Adventitial noradrenaline challenge
In order to test whether the endothelium acts as a barrier to noradrenaline, segments were challenged with noradrenaline from the adventitial side before (S1-S3) and after (S4-S6) removal of endothelium by air. This was designated as protocol G (n= 6).
Nitroglycerine following noradrenaline pre-contraction
In order to evaluate the effects of an exogenous NO donor a protocol similar to protocol C was used with the following difference. Instead of separate challenges of noradrenaline, arteries (n= 6) were continuously perfused with noradrenaline (3 μM for 24 min) to which nitroglycerine was added (0.01, 1 and 100 μM at 7 min intervals). Control experiments revealed that the vasoconstrictor response to noradrenaline was stable for 30 min both before and after removal of endothelium but there was a slight (5 %) fall in the R'340/380 signal (results not shown).
Histomorphometry of endothelium
Following fura-2 calibration, the presence of endothelial cells was assessed using the silver nitrate en face staining technique (Caplan, Gerrity & Schwartz, 1974). Endothelial surface area (n= 6, protocol D; and n= 6, protocol H) was measured with an image analysis algorithm (Saisam®; Microvision, Evry, France). The mathematical formula used was:
where S is surface area, L is length and p is perimeter.
The endothelial cell surface was assessed at three random points each with an area of 9 mm2 at × 40 magnification.
Substances
Fura-2 AM, bovine serum albumin, noradrenaline bitartrate, Hepes, ionomycin, EGTA, L-NAME and nitroglycerine (glyceryl trinitrate) were purchased from Sigma. Pluronic F127 was purchased from Calbiochem. NaCl, KCl, CaCl2, MgCl2 and glucose were purchased from Merck. The concentrations of drugs given in the text refer to salts where appropriate.
Statistical analysis
Results are expressed as means ±s.e.m. Differences between means (both paired and independent groups) were determined by analysis of variance (ANOVA) followed by the Bonferroni test. The null hypothesis was rejected at a probability level of P < 0.05. Protocol H (n= 6) was a time control.
RESULTS
Time controls (protocol H)
Baseline [Ca2+]i (0.27 ± 0.03 a.u.) and perfusion pressure (15 ± 1 mmHg) were stable throughout and were similar in all nine protocols (results not shown).
[Ca2+]i mobilization, vasoconstriction and [Ca2+]i-vasoconstriction coupling following S1-S6 were stable (values following S1 were 0.32 ± 0.02 a.u., 34 ± 4 mmHg and 105 ± 8 mmHg/a.u., respectively; Fig. 3, top panel). Calibration values were: F360/AF360, 3.5 ± 0.3; R'max, 10.1 ± 1.7; R'min, 0.88 ± 0.06; and β', 2.9 ± 0.4.
Figure 3. F340 and F380, [Ca2+]i and vasoconstriction following noradrenaline (S1-S6) in protocols H (top), A (middle) and B (bottom).
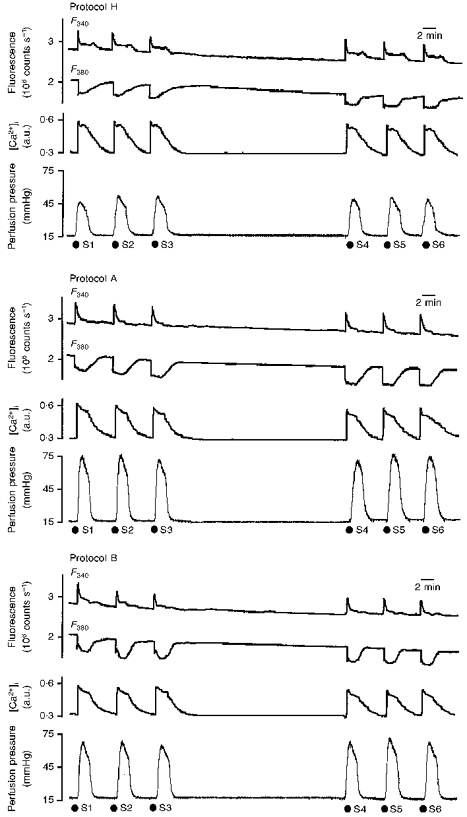
Each panel shows original recordings of changes in fluorescence (excitation wavelengths, 340 nm (F340) and 380 nm (F380)) of the intracellular [Ca2+]i-sensitive dye fura-2 (upper records), calculated [Ca2+]i (middle records), and perfusion pressure at constant flow rate (bottom records) following intraluminal perfusion of noradrenaline (S1-S6, 3 μM, 2 min).
Rubbing (protocol A) and air perfusion (protocol B)
Both techniques produced an increase in the vasoconstrictor response with no change in [Ca2+]i mobilization and an increase in coupling efficiency; values for [Ca2+]i mobilization, vasoconstriction and [Ca2+]i-vasoconstriction, respectively, following S1 for protocol A were 0.36 ± 0.05 a.u., 57 ± 5 mmHg and 165 ± 14 mmHg/a.u., and for protocol B, 0.37 ± 0.07 a.u., 59 ± 5 mmHg and 174 ± 19 mmHg/a.u. Responses were stable (Fig. 3, middle and bottom panels) and following S6 [Ca2+]i-vasoconstriction coupling was 163 ± 12 mmHg/a.u. Calibration values were similar to those obtained in protocol H (results not shown).
Air perfusion before (protocol B) and after (protocol C) fura-2 loading
Before air perfusion in protocol C, [Ca2+]i mobilization, vasoconstriction and coupling efficiency following S1-S3 noradrenaline challenges were similar (Fig. 4, top panel) to those observed in time controls (protocol H; Fig. 3, top panel).
Figure 4. F340 and F380, [Ca2+]i and vasoconstriction following S1-S6 noradrenaline challenges in protocols C (top), D (middle) and E (bottom).
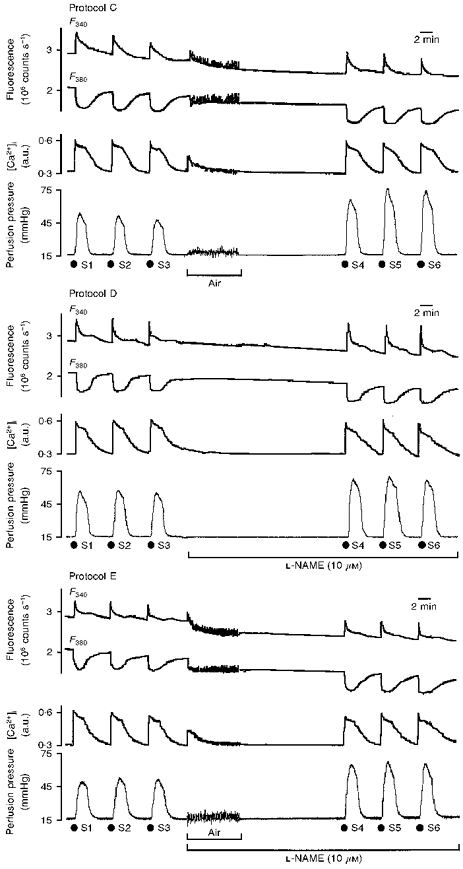
Original recordings (see Fig. 3) showing the effect of intraluminal perfusion of noradrenaline (3 μM, 2 min) on [Ca2+]i mobilization and perfusion pressure before (S1-S3) and after (S4-S6) intraluminal perfusion of air or L-NAME (10 μM). During air perfusion there was a marked increase in the variability of the baseline fluorescence and perfusion pressure signals.
Air perfusion had two effects. Firstly, the variability of the fluorescence and pressure signals increased substantially throughout the perfusion period. Secondly, there was an initial transient increase in the [Ca2+]i signal. Excitation spectra performed on the five fractions collected during air perfusion revealed a maximum at 340 nm; values were: 0-2 min, 4.9 × 104± 1.2 × 104 counts s−1; 2-4 min, 2.6 × 104± 1.0 × 104 counts s−1; 4-6 min, 1.7 × 104± 1.1 × 104 counts s−1; 6-8 min, 1.6 × 104± 0.7 × 104 counts s−1; and 8-10 min, 1.5 × 104± 0.6 × 104 counts s−1. Following air perfusion the arterial F340 and F380 signals fell by 12 % but baseline R340/380 remained unchanged. In the absence of endothelium the vasoconstrictor response to noradrenaline increased (S4, 51 ± 4 mmHg; S1, 37 ± 4 mmHg; P < 0.05). This gave a 50-60 % increase in [Ca2+]i-vasoconstriction coupling (S4, 157 ± 18 mmHg/a.u.; S1, 110 ± 9 mmHg/a.u.; P < 0.05). This effect was stable up to S6 (164 ± 25 mmHg/a.u.; Fig. 4, top panel).
Inhibition of NOS by L-NAME
Perfusion of L-NAME in the presence of endothelium (protocol D) produced an increase in the vasoconstrictor response and in coupling efficiency (S6, 183 ± 27 mmHg/a.u.) with no change in [Ca2+]i mobilization (Fig. 4, middle panel).
Perfusion of L-NAME in the absence of endothelium (protocol E) produced no further increase in the vasoconstrictor response or in coupling efficiency (S6, 187 ± 30 mmHg/a.u.) above that observed by the removal of endothelium (Fig. 4, lower panel).
Adventitial loading of fura-2
Noradrenaline-evoked vasoconstriction before air perfusion (S1-S3) was similar to that of time controls; air perfusion produced an increase in vasoconstriction (S3, 42 ± 5 mmHg; S6, 60 ± 7 mmHg; P < 0.05; Fig. 5, upper panel). Calibration values were low (F360/AF360, 1.7 ± 0.3; R'max, 2.0 ± 0.4; R'min, 0.9 ± 0.4; β', 1.2 ± 0.3).
Figure 5. F340 and F380, R340/380 or [Ca2+]i, and vasoconstriction following S1-S6 noradrenaline challenges in protocols F (top) and G (bottom).
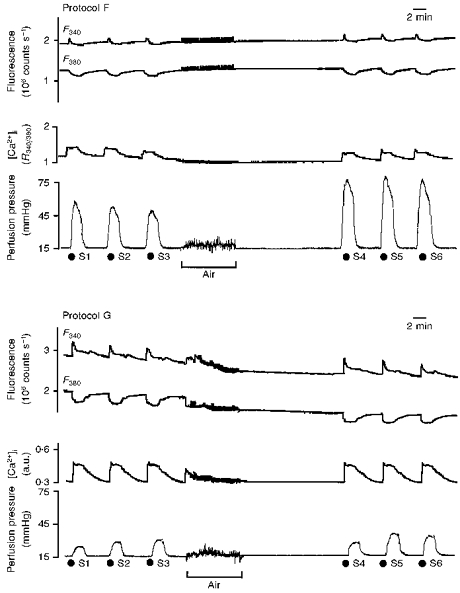
Original recordings (see Fig. 3) showing the effect of noradrenaline (3 μM, 2 min) on [Ca2+]i mobilization (R340/380 (top panel) or a.u. (bottom panel)) and perfusion pressure before (S1-S3) and after (S4-S6) intraluminal perfusion of air. [Ca2+]i signals in protocol F are represented as R340/380 as calibration values were low (see Discussion).
Adventitial noradrenaline challenge
When noradrenaline was applied from the adventitial side (protocol G), [Ca2+]i mobilization, vasoconstriction and coupling efficiency were reduced (Fig. 5, lower panel). Air perfusion produced a slight increase in vasoconstriction and in coupling efficiency (S6, 18 ± 3 mmHg and 124 ± 14 mmHg/a.u. versus S3, 13 ± 2 mmHg and 92 ± 10 mmHg/a.u.; both P < 0.05).
Nitroglycerine following noradrenaline pre-contraction
Nitroglycerine (0.01, 1 and 100 μM) produced a concentration-dependent fall in vasoconstriction of 74 ± 3 % at 0.01 μM, 56 ± 5 % at 1 μM and 24 ± 4 % at 100 μM. Nitroglycerine had no effect on [Ca2+]i mobilization (Fig. 6). After removal of the endothelium by air, noradrenaline-induced vasoconstriction and coupling efficiency were amplified (52 ± 5 mmHg and 159 ± 4 mmHg/a.u. versus 40 ± 2 mmHg and 115 ± 4 mmHg/a.u.; both P < 0.05). Nitroglycerine (0.01, 1 and 100 μM) produced the same effects as those observed in the presence of endothelium.
Figure 6. F340 and F380, [Ca2+]i and vasoconstriction in arteries pre-contracted by noradrenaline and exposed to nitroglycerine.
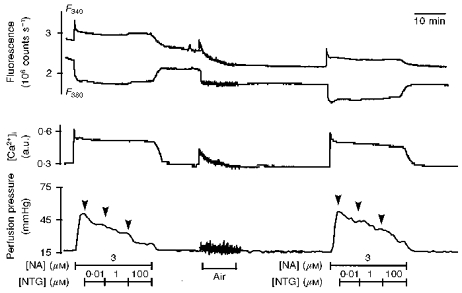
Original recordings (see Fig. 3) showing the effect of intraluminal perfusion of noradrenaline (NA; 3 μM, 21 min) and of nitroglycerine (NTG; 0.01, 1 and 100 μM; 7 min) on [Ca2+]i mobilization and perfusion pressure before and after intraluminal perfusion of air.
Histomorphometry of endothelium
Silver nitrate en face staining revealed the typical cobblestone pattern of endothelium in arterial segments from protocol H (Fig. 7, top panel). Air perfusion completely denuded the endothelium (protocol C; Fig. 7, middle panel) whereas L-NAME (protocol D; Fig. 7, bottom panel) was without effect. L-NAME had no effect on cell morphology, average endothelial cell surface area was 134 ± 6 μm2 in protocol H and 133 ± 2 μm2 in protocol D (P > 0.05).
Figure 7.

Silver nitrate en face staining of representative arterial segments from protocols H (top), C (middle) and D (bottom). Scale bar, 25 μm.
DISCUSSION
Our results suggest that NO attenuates noradrenaline-induced vasoconstriction by lowering the [Ca2+]i sensitivity of force. Before discussing this hypothesis in more detail we will first deal with several methodological aspects. We and others have developed techniques to study [Ca2+]i-vasomotion coupling in vascular smooth muscle cells using perfused, fura-2-loaded, intact segments in vitro. This is a powerful tool. The final intracellular messenger ([Ca2+]i) and the physiological response can be measured simultaneously. Coupling is studied in a ‘physiological’ context in which factors such as static and phasic wall and shear stress can be measured or set at a given level. However, this technique has a potential drawback. The intraluminal loading of the [Ca2+]i-sensitive dye and intraluminal challenge with agonists involves the endothelium. To gain further insight into this problem we developed a technique to remove endothelium by transient air perfusion, which allows paired observations in a single tissue.
Results obtained in protocol A in which endothelium was removed by our ‘old’ technique, viz. rubbing the intimal surface with a wire, and those of protocol B in which endothelium was removed by coperfusion of air were very similar, suggesting that air coperfusion is an efficient way to remove endothelium. It is also an easier technique to handle. Providing that care is taken to dissect out segments under pressure, a first series of pharmacological or other challenges can be performed in the presence of an intact, functional endothelium, the endothelium can then be removed by air without dismounting the segment, with constant monitoring of fluorescence and perfusion pressure.
Does fura-2 load into endothelial cells?
The fura-2 loading ratio (F360/AF360) was similar in protocols in which endothelium was removed before loading (A and B) and the protocol in which endothelium was intact (H). This suggests either that endothelial cells do not take up fura-2, or that endothelial cells have similar autofluorescence characteristics to those of smooth muscle cells and take up a similar amount of fura-2 per unit volume.
When air perfusion was performed in protocols (C, E and G) in which segments were previously perfused intraluminally with fura-2, there was a transient increase in R'340/380 and fluorescence with an excitation peak at 340 nm was detected in the perfusate. F340 and F380 values of the arterial segment then fell. The fall in fluorescence (-12 %) was equal to the relative volume of the intima (12 %). This series of observations could be explained on the basis of the hypothesis that endothelial cells and medial smooth muscle cells take up a similar amount of fura-2 per unit volume and that air perfusion causes endothelial cells to burst so releasing fura-2 into the perfusate where it chelates Ca2+. Such events were not observed when segments were loaded with fura-2 from the adventitial side before air perfusion. The apparent transient increase in [Ca2+]i following the onset of air perfusion could also reflect the release of endothelium-derived vasoactive factors. This is unlikely. There was no increase in perfusion pressure suggesting that endothelium-derived contracting factors were not released and the air-induced [Ca2+]i transient was not modified by L-NAME. Overall our results indicate that, when intact segments are intraluminally perfusion-loaded with fura-2, endothelial cells are capable of taking up the dye.
Does the endothelium act as a barrier?
The fura-2 loading ratio (F360/AF360) was similar in segments loaded with fura-2 in the presence (protocol H) or absence (protocol A and B) of endothelium. This suggests that endothelium does not act as a barrier for the passage of fura-2 AM into medial smooth muscle cells.
In the case of noradrenaline the results are less clear cut. The fact that vasoconstrictor responses increased following removal of endothelium suggests that the latter may be acting as a physical or metabolic barrier preventing access to medial smooth muscle cells. However, noradrenaline challenge in the presence of L-NAME and of an intact endothelium (which under 2-D histomorphometric analysis, at least, appeared structurally undamaged) produced a similar increase in the vasoconstrictor response and in pharmacomechanical coupling. This argues against the endothelium acting as a barrier to noradrenaline. In endothelium-denuded arteries (modified protocol B) the responses to noradrenaline in the presence (S4-S6) or absence (S1-S3) of L-NAME (10 μM) were similar (results not shown).
Does the adventitia act as a barrier?
The adventitia does act as a barrier to both fura-2 AM (protocol F) and to noradrenaline (protocol G). This is presumably linked to factors such as its composition, thickness and location relative to medial smooth muscle cells, and the presence of a neuronal uptake mechanism.
In protocol F in which fura-2 was loaded from the outside and noradrenaline was delivered from the inside, pressure responses and the impact of the removal of endothelium on pressure responses were unchanged but [Ca2+]i signals were low. These observations suggest that the endothelium does not stop noradrenaline from reaching medial muscle cells (see above) but that full activation of these cells produces a weak response. This is probably due to poor loading with fura-2 when the latter is presented from the adventitial side. Using the calibration values for this protocol an increase in [Ca2+]i of 0.36 ± 0.15 a.u. for an increase in perfusion pressure of 47 ± 4 mmHg (for S1) was obtained. Such data are compatible with those presented in Fig. 2.
In protocol G in which noradrenaline was applied from the adventitial side there was a parallel decrease in [Ca2+]i and pressure. The results for the noradrenaline-induced increases in [Ca2+]i and pressure are compatible with the data presented in Fig. 2. This suggests that there is a barrier which prevents noradrenaline applied from the outside from reaching the medial smooth muscle cells. This problem has been extensively studied by de la Lande and co-workers who showed that in the perfused isolated central artery of the rabbit ear the vascular sensitivity to noradrenaline applied from the inside was much greater compared with that applied from the outside and that the sensitivity to noradrenaline applied from the outside could be greatly increased by the neuronal uptake blocker cocaine (de la Lande, Frewin & Waterson, 1967). Using histamine (which is resistant to neuronal uptake) as agonist, the inside:outside ratio was 2 and was unaffected by cocaine whereas that for noradrenaline was lowered from 10 to 1-2 with cocaine. Similar experiments in the perfused rat tail artery (Venning & de la Lande, 1984, 1985) led de la Lande and co-workers to suggest that in perfused muscular artery segments there is a barrier located at the adventitia-media border, which is composed of sympathetic nerve endings and limits the access of noradrenaline applied from the outside to the media.
Is the fura-2 signal generated from smooth muscle cells that contribute to the pressure response?
Smooth muscle cells of perfused, cylindrical arterial segments can develop force in three directions: radial, longitudinal and circumferential. This could be due to the presence of longitudinal smooth muscle in the adventitial layer or a helical arrangement of the medial smooth muscle cells. We surmise that the fura-2 loads mainly into cells producing a radial contraction and that [Ca2+]i modulates pressure. That this is so is illustrated in Fig. 2 which shows that increasing concentrations of noradrenaline (0.1-30 μM) produced parallel changes in pressure and [Ca2+]i. We also attempted to evaluate the contribution of longitudinal contraction by measuring longitudinal force with an isometric transducer (modified protocol H; n= 6). There was no significant change in longitudinal force during the increase in perfusion pressure but during the decrease in pressure there was a minor (2 %) fall in longitudinal force. The possible involvement of circumferential contraction, which may alter the stiffness of the wall without necessarily modifying pressure, has not been investigated in this preparation. Using a similar technique (common carotid artery segment and pulsatile perfusion) we showed that [Ca2+]i plays a minor role in the determination of wall rigidity (Boutinet, Giummelly, Atkinson & Capdeville-Atkinson, 1994).
Endothelium-derived NO attenuates vascular smooth muscle cell [Ca2+]i-vasoconstriction coupling via a mechanism downstream of [Ca2+]i mobilization
When endothelium was removed - either at the beginning or in the middle of the experiment - subsequent challenges with noradrenaline produced a greater vasoconstrictor response with an increase in [Ca2+]i-vasoconstriction coupling. The endothelial factor involved appears to be NO as inhibition of NOS with L-NAME produced the same effect. Challenge in the presence of L-NAME following air-induced destruction of endothelium did not produce any additional effect, suggesting that the NOS involved is of endothelial origin.
Our hypothesis is that NO has no effect on noradrenaline-induced [Ca2+]i mobilization in smooth muscle cells. However, one could argue that [Ca2+]i mobilization does not change following removal of endothelium because in the presence of endothelium the global [Ca2+]i signal is the sum of [Ca2+]i mobilization involved in NO release (Graier, Sturek & Kukovetz, 1994) and a smooth muscle cell signal. If we postulate that the noradrenaline-induced [Ca2+]i mobilization in smooth muscle cells is attenuated by NO, then following removal of this endothelial ‘brake’ there would be an increase in the smooth muscle cell [Ca2+]i signal. This increase would compensate the fall in the global signal which would otherwise have occurred following ‘removal’ of the endothelial signal. This hypothesis depends upon [Ca2+]i being involved in NO release. If the loss of the endothelial signal were to exactly counterbalance any increase in the signal from smooth muscle, however, endothelial [Ca2+]i would have to rise by an amount several times greater than the concomitant rise in smooth muscle [Ca2+]i to compensate for its much smaller volumetric contribution to the signal, assuming similar dye loading in each tissue. Furthermore, we have to assume that L-NAME is able to block endothelial [Ca2+]i mobilization. Certain authors (e.g. Buchan & Martin, 1991) do not agree with the latter statement.
Our observation that the exogenous NO donor produced a substantial reduction in pressure with no significant change in [Ca2+]i adds strength to our argument that NO has a vasodilator action which is independent of any effect on vascular wall [Ca2+]i.
In summary, we put forward the working hypothesis that the main vasodilator mechanism of NO is a decrease in the [Ca2+]i sensitivity of force. This hypothesis agrees with that for sodium nitroprusside proposed by Karaki, Sato, Ozaki & Murakami (1988) and that for nitroglycerine proposed by Yanagisawa, Kawada & Taira (1989) and Chen & Rembold (1996). The latter authors used the rat tail artery and concluded that ‘physiological’ relaxation involved primarily a decrease in the [Ca2+]i sensitivity of tension. Changes in [Ca2+]i sensitivity can also be obtained with entirely different agents such as carbachol (Sato, Ozaki & Karaki, 1990) and L-NAME (Dietrich, Kimura & Dacey, 1994).
Acknowledgments
This work was supported by grants from the French Ministry of Education and Research (JE250, DRED), from Rhône-Poulenc Rorer, Paris and from Lipha Pharmaceuticals, Lyon, France. We wish to thank Ms Catherine Vandeputte and Thérèse Colas for their excellent technical help with the histomorphometric analysis. The laboratory is part of the BIOMED EureCa network.
References
- Abe S, Kanaide H, Nakamura M. Front-surface fluorometry with fura-2 and effects of nitroglycerin on cytosolic calcium concentrations and on tension in the coronary artery of the pig. British Journal of Pharmacology. 1990;101:545–552. doi: 10.1111/j.1476-5381.1990.tb14118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Fouda AK, Sonnay M. Vasoconstriction of the perfused rat caudal artery is independent of longitudinal tension and flow rate. British Journal of Pharmacology. 1986;88:437. abstract P. [Google Scholar]
- Atkinson J, Tatchum-Talom R, Capdeville-Atkinson C. Reduction of endothelial function with age in the mesenteric arterial bed of normotensive rat. British Journal of Pharmacology. 1994;111:1184–1188. doi: 10.1111/j.1476-5381.1994.tb14870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter LA, Wier WG. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium. 1994;15:122–131. doi: 10.1016/0143-4160(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Boutinet S, Giummelly P, Atkinson J, Capdeville-Atkinson C. Simultaneous measurement of intracellular calcium levels and the damping capacity in the rat common carotid artery with a new in vitro pulsatile system. British Journal of Pharmacology. 1994;112:584. abstract P. [Google Scholar]
- Buchan KW, Martin W. Modulation of agonist-induced calcium mobilisation in bovine aortic endothelial cells by phorbol myristate acetate and cyclic AMP but not GMP. British Journal of Pharmacology. 1991;104:361–366. doi: 10.1111/j.1476-5381.1991.tb12436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdeville-Atkinson C, Oster L, Thorin-Trescases N, Robert A, Boutinet S, Atkinson J. Intracellular free Ca2+ and vasoconstriction determined simultaneously in the perfused rat tail artery. American Journal of Physiology. 1993;265:C1689–1702. doi: 10.1152/ajpcell.1993.265.6.C1689. [DOI] [PubMed] [Google Scholar]
- Capdeville-Atkinson C, Oster L, Thorin-Trescases N, Robert A, Corman B, Atkinson J. Effect of chronic ANG I-converting enzyme inhibition on aging processes. V. Intracellular calcium-vasoreactivity coupling. American Journal of Physiology. 1995;268:R1394–1400. doi: 10.1152/ajpregu.1995.268.6.R1394. [DOI] [PubMed] [Google Scholar]
- Caplan BA, Gerrity RG, Schwartz CJ. Endothelial cell morphology in focal areas of in vivo Evans blue uptake in the young pig aorta. Experimental and Molecular Pathology. 1974;21:102–117. doi: 10.1016/0014-4800(74)90082-3. [DOI] [PubMed] [Google Scholar]
- Chen XL, Rembold CM. Nitroglycerin relaxes rat tail artery primarily by lowering Ca2+ sensitivity and partially by repolarization. American Journal of Physiology. 1996;271:H962–968. doi: 10.1152/ajpheart.1996.271.3.H962. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–629. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- de la Lande IS, Frewin D, Waterson JG. The influence of sympathetic innervation on vascular sensitivity to noradrenaline. British Journal of Pharmacology and Pharmacotherapy. 1967;31:82–93. doi: 10.1111/j.1476-5381.1967.tb01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich HH, Kimura M, Dacey RG., Jr Nω-nitro-L-arginine constricts cerebral arterioles without increasing intracellular calcium levels. American Journal of Physiology. 1994;266:H1681–1686. doi: 10.1152/ajpheart.1994.266.4.H1681. [DOI] [PubMed] [Google Scholar]
- Graier WF, Sturek M, Kukovetz WR. Ca2+ regulation and endothelial vascular function. Endothelium. 1994;1:223–236. [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annual Review of Pharmacology and Toxicology. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Karaki H, Sato K, Ozaki H, Murakami K. Effects of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. European Journal of Pharmacology. 1988;156:259–266. doi: 10.1016/0014-2999(88)90329-9. 10.1016/0014-2999(88)90329-9. [DOI] [PubMed] [Google Scholar]
- Lew MJ, Duling BR. Access of blood-borne vasoconstrictors to the arteriolar smooth muscle. Journal of Vascular Research. 1992;29:341–346. doi: 10.1159/000158949. [DOI] [PubMed] [Google Scholar]
- Magliola L, Jones AW. Sodium nitroprusside alters Ca2+ flux components and Ca2+-dependent fluxes of K+ and Cl− in rat aorta. The Journal of Physiology. 1990;421:411–424. doi: 10.1113/jphysiol.1990.sp017952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Biggs EA. Nitric oxide in the cardiovascular system. In: Cuello AC, Collier B, editors. Pharmacological Sciences: Perspectives for Research & Therapy in the Late 1990s. Basel: Birkhauser; 1995. pp. 347–353. [Google Scholar]
- Murad F. The mechanism of relaxation of EDRF: similarities of the effects of EDRF and nitrovasodilators on cyclic GMP formation and vascular relaxation. In: Rand MJ, Raper C, editors. Pharmacology. Amsterdam: Excerpta Medica; 1987. pp. 355–357. [Google Scholar]
- Rembold CM. Modulation of the [Ca2+] sensitivity of myosin phosphorylation in intact swine arterial smooth muscle. The Journal of Physiology. 1990;429:77–94. doi: 10.1113/jphysiol.1990.sp018245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Ozaki H, Karaki H. Differential effects of carbachol on cytosolic calcium levels in vascular endothelium and smooth muscle. Journal of Pharmacology and Experimental Therapeutics. 1990;255:114–119. [PubMed] [Google Scholar]
- Scanlon M, Williams DA, Fay FS. Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Journal of Biological Chemistry. 1987;262:6308–6312. [PubMed] [Google Scholar]
- Venning MG, de la Lande IS. Effect of uptake and surface of entry on the responses of the rat caudal artery to noradrenaline, adrenaline and methoxamine. Blood Vessels. 1984;21:149–155. doi: 10.1159/000158507. [DOI] [PubMed] [Google Scholar]
- Venning MG, de la Lande IS. Effect of inhibition of neuronal uptake on the flux of extraluminal noradrenaline into the lumen of the rat tail artery. Blood Vessels. 1985;22:244–246. doi: 10.1159/000158608. [DOI] [PubMed] [Google Scholar]
- Vo PA, Reid JJ, Rand MJ. Attenuation of vasoconstriction by endogenous nitric oxide in rat caudal artery. British Journal of Pharmacology. 1992;107:1121–1128. doi: 10.1111/j.1476-5381.1992.tb13417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T, Kawada M, Taira N. Nitroglycerin relaxes canine coronary arterial smooth muscle without reducing intracellular Ca2+ concentration measured with fura-2. British Journal of Pharmacology. 1989;98:469–482. doi: 10.1111/j.1476-5381.1989.tb12620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


