Abstract
The effects of intracellular pH changes on the acetylcholine (ACh) release and cytoplasmic Ca2+ concentration at developing neuromuscular synapses were studied in Xenopus nerve-muscle co-cultures.
Spontaneous and evoked ACh release of motoneurons was monitored by using whole-cell voltage-clamped myocytes. Intracellular alkalinization with 15 mm NH4Cl slightly reduced the frequency of spontaneous synaptic currents (SSCs). However, cytosolic acidification following withdrawal of extracellular NH4Cl caused a marked and transient increase in spontaneous ACh release.
Another method of cytosolic acidification was used in which NaCl in Ringer solution was replaced with weak organic acids. The increase in spontaneous ACh release paralleled the level of intracellular acidification resulting from addition of these organic acids. Acetate and propionate but not isethionate, methylsulphate and glucuronate, caused an increase in intracellular pH and a marked increase in spontaneous ACh release.
Impulse-evoked ACh release was slightly augmented by intracellular alkalinization and inhibited by cytosolic acidification.
Cytosolic acidification was accompanied by an elevation in the cytoplasmic Ca2+ concentration ([Ca2+]i), resulting from both external Ca2+ influx and intracellular Ca2+ mobilization. In contrast, the increase in [Ca2+]i induced by high K+ was inhibited by cytosolic acidification.
We conclude that cytosolic acidification regulates spontaneous and evoked ACh release differentially in Xenopus motoneurons, increasing spontaneous ACh release but inhibiting evoked ACh release.
Cytosolic pH (pHi) is tightly regulated and changes in pHi tend to exert profound effects on the properties of cells (Moody, 1984). Enzymes and ion channels are clearly affected by changes in pHi. For example, an alkalinization of only 0.1 pH units increases the rate of the in vitro enzymatic activity of phosphofructokinase from close to zero to almost full activity (Trivedi & Danforth, 1966). During the earliest responses to neural induction in Xenopus, the cytosolic alkalinization may participate in changes in gene expression associated with the induced differentiative pathway (Sater, Alderton & Steinhardt, 1994). Ion channels of the gap junction complex are notable for their sensitivity to physiologically relevant shifts in intracellular pH. The conductance of the gap junction can display a steep rise with alkalinization of around 0.1 units (Spray, Harris & Bennett, 1981), while in the developing rat neocortex intracellular acidification reduced gap junction coupling (Rorig, Klausa & Sutor, 1996). NMDA-mediated synaptic transmission may be particularly sensitive to shifts in extracellular pH. In mammalian central neurons, NMDA-evoked currents were significantly enhanced by extracellular alkaline shifts of a few tenths of a pH unit (Tang, Dichter & Morad, 1990) and inhibited by extracellular acidification (Traynelis & Cull-Candy, 1990). On the other hand, inhibitory currents mediated by GABAA receptors are increased by external protons (Pasternack, Bountra, Voipio & Kaila, 1992).
The pHi in frog motoneurons is decreased after prolonged neuronal activity (Chesler & Kaila, 1992). Brain ischaemia has been reported to lower extracellular pH (pHo) and pHi (Nedergaard, Kraig, Tanabe & Pulsinelli, 1991). Hypoxia and/or glucose depletion in hippocampal slices have also been shown to result in a decrease in pHi within 5 min of exposure (Fujiwara, Abe, Endoh, Warashina & Shimoji, 1992). Many cellular processes in neurons are controlled by changes in the extracellular or intracellular pH (Busa & Nuccitelli, 1984). In acidic media, the evoked release of ACh at the frog neuromuscular junction is reduced (del Castillo, Nelson & Sanchez, 1962; Landau & Nachshen, 1975). In contrast, the spontaneous release of transmitter at frog and rat neuromuscular junctions is increased by extracellular acidification (Hubbard, Jones & Landau, 1968; Landau & Nachshen, 1975; Cohen & van der Kloot, 1976). The nature of these opposite effects of extracellular pH on evoked and spontaneous ACh release is unknown. There have also been numerous studies of the effects of extracellular pH shifts on neuronal activity and ion channels, but comparatively little is known of the effect of these shifts. In the current study, we explored the effects and mechanisms of action of both extracellular and intracellular pH changes on transmitter release in developing motoneurons. We found that cytosolic acidification exerted more pronounced effects than extracellular acidification on both spontaneous and evoked ACh release. Cytosolic acidification increased spontaneous but inhibited impulse-evoked ACh release in motoneurons. In contrast, cytosolic alkalinization slightly reduced spontaneous transmitter secretion and augmented evoked ACh release.
METHODS
Cell culture
Xenopus nerve-muscle cultures were prepared as reported previously (Tabti & Poo, 1991). Briefly, the neural tube and the associated myotomal tissues of 1-day-old Xenopus embryos (stages 20-22) were dissociated in Ca2+- and Mg2+-free Ringer solution supplemented with EDTA. The cells were plated onto clean glass coverslips and were used for experiments after 24 h at room temperature (20-22°C). The culture medium consisted of 50 % (v/v) Ringer solution (115 mM NaCl, 2 mM CaCl2, 1.5 mM KCl, 10 mM Hepes, pH 7.6), 49 % L-15 Leibovitz medium (Sigma), 1 % fetal bovine serum (Gibco) and antibiotics (100 units ml−1 penicillin and 100 μg ml−1 streptomycin).
Electrophysiology
Whole-cell patch-clamp recording methods followed those described by Hamill, Marty, Neher, Sakmann & Sigworth (1981). Patch pipettes were pulled with a two-stage electrode puller (pp-83, Narishige, Japan) and the tips were polished immediately before the experiment using a microforge (MF-83, Narishige, Japan). Synaptic currents were recorded from innervated myocytes by whole-cell recording in the voltage-clamp mode. Recordings were made at room temperature in Ringer solution. For whole-cell recordings, the solution inside the recording pipette contained 150 mM KCl, 1 mM NaCl, 1 mM MgCl2 and 10 mM Hepes (pH 7.2). The extent of the potentiation was measured by the frequency ratio of spontaneous synaptic currents (SSCs), which is defined as the ratio of SSC frequency observed during or after application of drugs compared with the mean frequency observed before drug treatment. Evoked synaptic currents (ESCs) were elicited by stimulating presynaptic neurons at the soma with heat-polished glass microelectrodes (tip opening, 1-2 μm) filled with Ringer solution at a frequency of 0.1 Hz. For suprathreshold stimulation of the neuron, a square current pulse of 0.3 ms in duration and 2-4 μA in amplitude was applied through the pipette. Such currents usually induce twitch contraction of the muscle cell when applied to the soma of the innervating neuron. The membrane currents passing through the patch pipette were recorded with a patch-clamp amplifier (Dagan 8900, Minneapolis, MN, USA). The data were digitized using Neuro-corder (Neuro Data DR 390, New York, USA) and stored on a videotape for later playback onto a storage oscilloscope (Tektronix 5113, Beaverton, OR, USA) or an oscillographic recorder (Gould RS3200, Valley View, OH, USA). The Data 6100 waveform analyser (Data Precision, Danvers, MA, USA) was used to analyse the frequency of the spontaneous synaptic currents and the SCAN computer program (Dagan, Minneapolis, MN, USA) was used to analyse the current amplitude and decay time. Data are expressed as means ±s.e.m. Statistical significance was evaluated by Student's t test.
Measurement of pHi
Measurement of pHi has been described in detail elsewhere (Wu, Tsai & Tseng, 1994). In brief, cultures were loaded with 5 μM of the acetoxymethyl ester form of 2,7-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF AM; Molecular Probes, Eugene, OR, USA) for 5-10 min at room temperature in Ringer solution and then washed with the same solution three times. The density of culture in some areas was low enough to allow a proper separation of single cells. A single soma was isolated by adjusting the slit width of the photomultiplier tube and excited by alternate flashes of 490 and 440 nm wavelength light, using a filter wheel (Cairn Research, Kent, England) rotating at 32 Hz. The excitation light was transmitted to the cell under study using a 510 nm dichroic mirror under the microscope nosepiece, and the resulting fluorescence was collected via a × 40 oil-immersion lens. The overall sampling rate was 0.5 Hz. The 490 nm/440 nm emission ratio from the intracellular BCECF was calculated and converted to a linear pH scale using in situ calibration data obtained at the end of the experiment by the nigericin technique as described elsewhere (Rink, Tsien & Pozzan, 1982). A calibration curve similar to that used by Wu et al. (1994) was established by measuring the fluorescence ratio values between pHi 4.5 and pHi 9.5. Between pHi 5.5 and pHi 9.0, the response is linear and fits the equation:
where R is the ratio of 530 nm fluorescence at 490 nm excitation to 530 nm fluorescence at 440 nm excitation. Rmax and Rmin are the maximum and minimum ratio values from the data curve and pK is the dissociation constant for the dye, taken as 55 nM.
Measurement of intracellular Ca2+ levels
The intracellular concentration of free Ca2+ ([Ca2+]i) in the soma was measured using the Ca2+-sensitive fluorescent dye fura-2. Cultures plated onto a 25 mm coverslip (No. 1001; Assistent, Germany) were loaded with 2 μM fura-2 AM (the acetoxymethyl ester form of fura-2; Molecular Probes) in Ringer solution for 1 h at room temperature. The cells were then washed with the same solution. The fluorescence of a single soma was measured as indicated above except that the cell was excited by alternate flashes of 340 and 380 nm wavelength light. The 340 nm/380 nm emission ratio from the intracellular fura-2 was calculated and converted to a linear Ca2+ scale by in situ calibration at the end of the experiment using the Ca2+ ionophore, ionomycin (5 μM; Sigma). The following equation (Grynkiewicz, Poenie & Tsien, 1985) was used to convert the fluorescence ratio into the intracellular Ca2+ concentration:
where R is the ratio of 510 nm fluorescence at 340 nm excitation to 510 nm fluorescence at 380 nm excitation. Rmax (2 mM Ca2+) and Rmin (10 mM EGTA in Ca2+-free Ringer solution) are the maximum and minimum ratio values from the data curve. Kd is the dissociation constant for the dye, taken as 135 nM at room temperature, and Sf2/Sb2 is the ratio of the 380 nm signals determined at Rmin and Rmax.
Chemicals
The following chemicals were used: BAPTA AM, NH4Cl, sodium acetate, sodium glucuronate, sodium isethionate and nifedipine (Sigma), sodium methylsulphate (Aldrich), and sodium propionate (Hayashi, Osaka, Japan).
RESULTS
Potentiation of spontaneous synaptic activity by NH4Cl prepulse
In nerve-muscle cultures prepared from 1-day-old Xenopus embryos, synaptic contacts were established, forming natural synapses between dissociated spinal neurons and myocyte within the first day of culture. Spontaneous synaptic currents (SSCs) were readily detectable from the innervated myocytes by whole-cell voltage-clamp recording. These currents have been shown to be caused by spontaneous ACh secretion from the neuron, since they are abolished by bath application of d-tubocurarine and unaffected by tetrodotoxin (Xie & Poo, 1986). At constant pHo (7.6), the ammonium prepulse technique (Roos & Boron, 1981) was used to induce cytosolic pH (pHi) transients in cultured Xenopus spinal neurons. Figure 1A depicts the recording of SSCs from an innervated myocyte. Perfusion of 15 mM NH4Cl for 5-10 min caused a slight decrease in SSC frequency (the SSC frequency ratio was 0.5 ± 0.1; n= 8). Following wash-out of NH4Cl with standard Ringer solution, the SSC frequency increased by more than 100-fold (the peak SSC frequency ratio was 122.7 ± 56.5; n= 4). The time course- response curves are shown in Fig. 1B. Some motoneurons in the cultures remained isolated and these were termed ‘naive neurons’ (Fig. 1C). The change in cytosolic pH in a single soma of a naive neuron was monitored using the BCECF fluorescence method and Fig. 1D shows the shift in pHi following NH4Cl application. The baseline pHi value was 7.17 ± 0.03 (n= 46). Exposure of the soma to 15 mM NH4Cl resulted in a rapid increase in pHi to a peak value of 7.67 ± 0.14 (n= 8). Once external NH4Cl was removed, there was a rebound intracellular acidification and pHi decreased to 6.50 ± 0.12 (n= 8). The initial alkalinization seen during the first moments of exposure to NH4+ is presumably caused by the rapid, passive entry of NH3 and its subsequent hydration to form NH4+ and OH−. Removal of external NH4+ causes an efflux of NH3, leaving the H+ of the accumulated NH4+ behind and resulting in intracellular acidification (Roos & Boron, 1981). Cytosolic acidification by NH4Cl was concentration dependent, as was the increase in SSC (Fig. 2A and B). Although withdrawal of 1.5 and 5 mM NH4Cl from the bathing solution caused abrupt pHi changes of ≈0.2 and ≈0.5 units, repectively, the SSC frequency did not increase. Bath application of 15 mM NH4Cl caused a greater pHi change of 0.85-1.0 units and an increase in SSC frequency (Fig. 2B). The mean SSC amplitudes after cytosolic alkalosis and acidosis, using the 15 mM NH4Cl prepulse technique, were 248.1 ± 74.0 and 183.5 ± 63.9 pA, respectively (n= 5) (the control SSC amplitude was 225.7 ± 36.5 pA; n= 8). The decay times (90 %) of SSC during intracellular alkalinization and acidification were 6.6 ± 1.0 and 5.1 ± 0.4 ms (n= 5), respectively (control, 8.4 ± 0.7 ms; n= 9).
Figure 1. Potentiation of spontaneous ACh release by NH4Cl prepulse.
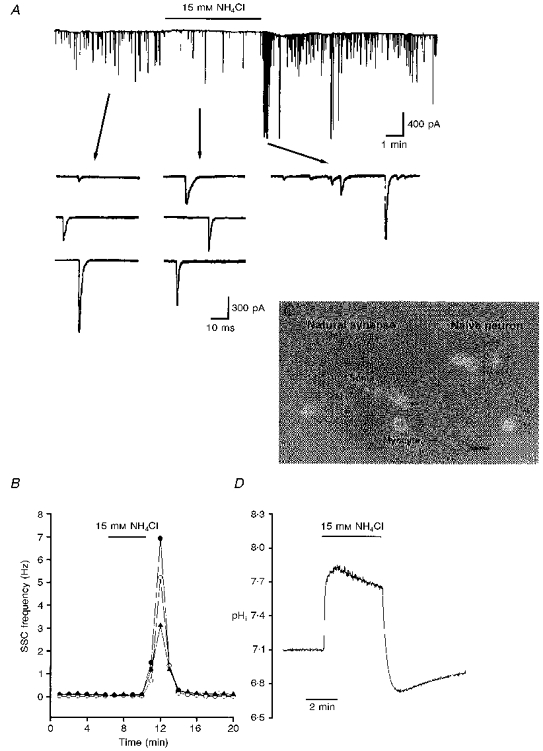
A, continuous trace depicting membrane currents measured by the whole-cell recording method from an innervated myocyte in a 1-day-old Xenopus culture. The myocyte was voltage clamped at -65 mV. Spontaneous synaptic currents (SSCs) appear as random downward deflections (filtered at 150 Hz). NH4Cl was present during the time marked by the horizontal line. Samples of synaptic currents are shown below the trace at a higher time resolution (filtered at 10 kHz). Note the increase in the frequency of SSCs after withdrawal of external NH4Cl. B, time course of the change in SSC frequency. Each curve represents data collected from one synapse. C, cultures were loaded with BCECF AM and ratio fluorimetric measurements of intracellular pH were made on a single soma of a naive neuron outlined by the dotted box. Scale bar, 50 μm. D, cytosolic alkalinization following addition of external NH4Cl and the rebound acidification after withdrawal of NH4Cl.
Figure 2. Concentration-dependent action of NH4Cl.
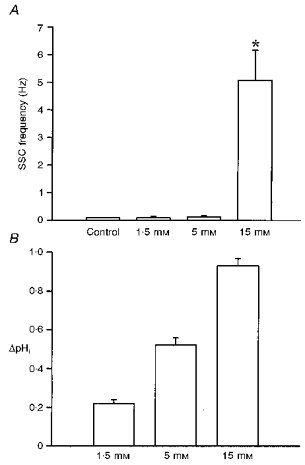
A, after a control period of 10 min, NH4Cl was applied at different concentrations to 1-day-old Xenopus nerve-muscle co-cultures. The peak level of SSC frequency was obtained after withdrawal of external NH4Cl. B, change in pHi after withdrawal of different concentrations of external NH4Cl was measured in the neuronal soma. *P < 0.05 compared with control.
Potentiation of SSC by weak organic acids
Another method of cytosolic acidification was the use of weak organic acid substitution. Weak organic acids with pKa values in the range of 4-6 might allow selective acidification of cells in an acidic environment. The protonated forms of such weak acids are more membrane permeable than their charged anionic form and dissociate intracellularly to liberate H+. Exposure of cells to weak acids is thus expected to result in an acute acid load to the cytoplasm, which increases at lower pHo, when a higher proportion of acid is in the protonated and uncharged form. For these experiments, all the extracellular NaCl in pH 6.6 Ringer solution was replaced by the sodium salts of organic acids. Substitution with either acetate or propionate markedly increased SSC frequency (peak SSC frequency ratios were 250.5 ± 62.6 and 120.7 ± 53.7, respectively (n= 4) for acetate and propionate; Fig. 3A and B). However, substitution of NaCl with other organic acids such as glucuronate, methylsulphate and isethionate resulted in no significant increase in SSC frequency (SSC frequency ratios were 1.1 ± 0.1, 1.0 ± 0.1 and 1.2 ± 0.1, respectively, for glucuronate, methylsulphate and isethionate (n= 3); Fig. 3C, D and E). The time course-response curves are shown in Fig. 4. Note that the SSC frequency increased markedly and then declined rapidly when acetate and propionate were applied (Fig. 4A and B). After wash-out of either acetate or propionate, the SSC frequency returned to basal values in a few minutes. The change in extracellular pH from 7.6 to 6.6 did not by itself significantly affect the frequency of spontaneous ACh secretion (SSC frequency ratio was 1.2 ± 0.1; n= 4), indicating that a change in pHo of 1 unit has no significant effect on spontaneous ACh release. When the pHi of the soma of a naive neuron was measured, a rapid intracellular acidification occurred after exposure to either acetate (Fig. 5A) or propionate (Fig. 5B). The pHi reached peak values of 5.76 ± 0.12 (n= 9) with acetate and 5.50 ± 0.15 (n= 9) with propionate. The intracellular fluid revealed an acidic shift of 1.2-1.6 pH units. The acidification declined slightly to a more sustained value over the period of acid exposure. Removal of acetate or propionate resulted in a rebound cytosolic alkalinization. External acidification of the standard solution from pHo 7.6 to pHo 6.6 only caused a slight cytosolic acidification of 0.22 ± 0.06 units (n= 4). On the other hand, the peak values of pHi after exposure to glucuronate (Fig. 5C), methylsulphate (Fig. 5D) and isethionate (Fig. 5E) were 6.86 ± 0.08 (n= 4), 7.02 ± 0.09 (n= 3) and 7.03 ± 0.08 (n= 3), respectively. The decline in pHi is similar to that seen in pH 6.6 Ringer solution alone. Replacement of one- or two-thirds of the NaCl with sodium acetate or sodium propionate also decreased intracellular pH by 0.7-1.1 units, while the SSC frequency was either not affected or only slightly increased (Table 1).
Figure 3. Potentiation of spontaneous ACh secretion by weak organic acids.
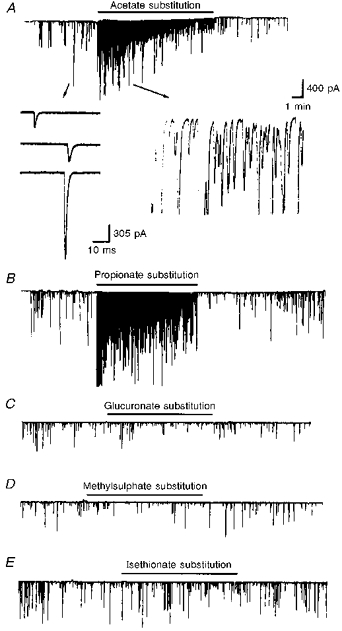
The continuous traces depict the membrane currents recorded from the innervated myocytes before and after bath application of various organic acids (filtered at 150 Hz). Downward deflections are SSCs (holding potential, -65 mV). All of the NaCl in pH 7.6 Ringer solution was replaced by the sodium salts of the organic acids in pH 6.6 Ringer solution for the periods indicated by the horizontal lines. Note that acetate (A) and propionate (B) but not glucuronate (C), methylsulphate (D) and isethionate (E) potentiated SSC frequency. Samples of synaptic currents are shown below trace A at a higher time resolution.
Figure 4. Time-dependent action of weak organic acids.
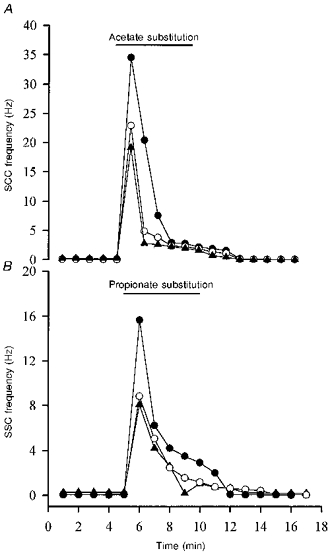
The spontaneous synaptic currents (SSCs) were measured by the whole-cell recording method from an innervated myocyte in a 1-day-old Xenopus culture. All of the NaCl in pH 7.6 Ringer solution was replaced by the sodium salts of organic acids in pH 6.6 Ringer solution for the period indicated by the horizontal lines. Note that SSC frequency increased markedly and then declined rapidly following application of acetate (A) and propionate (B). Each curve represents data collected from one synapse.
Figure 5. Cytosolic pH changes induced by organic acids in single soma.
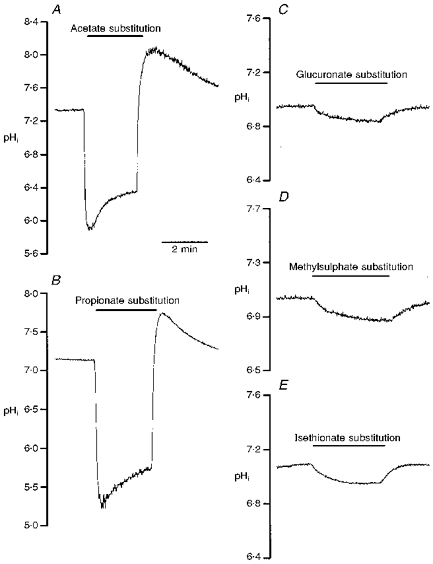
One-day-old Xenopus nerve-muscle co-cultures were loaded with BCECF AM and ratio fluorimetric measurements of intracellular pH were made on a single soma. All of the NaCl in pH 7.6 Ringer solution was replaced by the sodium salts of organic acids in pH 6.6 Ringer solution for the period indicated by the horizontal lines. Note that acetate (A) and propionate (B) induced a marked cytosolic acidification and a rebound alkalinization after withdrawal of external weak organic acids. Glucuronate (C), methylsulphate (D) and isethionate (E) only slightly reduced pHi.
Table 1.
Concentration-dependent effect of organic acids on pHi changes and enhancement of SSC frequency
| Fraction of NaCl replaced | pHi | ΔpHi | SSC frequency (Hz) | |
|---|---|---|---|---|
| Control | 7.17 ± 0.03 (46) | 0.09 ± 0.01 | ||
| Sodium acetate substitution | 1/3 | 6.32 ± 0.06 (6)* | 0.78 ± 0.13 | 0.23 ± 0.02 (3) |
| 2/3 | 5.99 ± 0.08 (11)* | 1.07 ± 0.15 | 0.48 ± 0.02 (3)* | |
| 100 % | 5.76 ± 0.12 (9)* | 1.44 ± 0.26 | 25.55 ± 4.62 (6)* | |
| Sodium propionate substitution | 1/3 | 6.32 ± 0.07 (4)* | 0.81 ± 0.06 | 0.80 ± 0.02 (3) |
| 2/3 | 6.04 ± 0.07 (7)* | 1.13 ± 0.05 | 1.16 ± 0.08 (4)* | |
| 100 % | 5.58 ± 0.15 (9)* | 1.51 ± 0.13 | 10.86 ± 2.4 (5)* |
SSC frequency was obtained from an innervated myocyte, which was whole-cell voltage clamped at −65 mV. Different fractions of NaCl were replaced by either sodium acetate or sodium propionate in pH 6.6 Ringer solution. The peak level of SSC frequency was measured after organic acid application. pHi represents the absolute value of cytosolic pH and ΔpHi represents the changes of pHi in the soma after organic acid application. Data are presented as means ±s.e.m. with number of experiments given in parentheses.
P < 0.05 compared with control.
Ca2+-dependent action
It is well known that the [Ca2+]i level exerts a dominant effect on the rate of spontaneous transmitter release. We therefore investigated the source of Ca2+ contributing to the SSC-potentiating effect of cytosolic acidification. L-type Ca2+ channels in the nerve terminals of developing motoneurons are involved in the SSC-potentiating action of ATP and glutamate (Fu & Huang, 1994; Fu, Liou, Lee & Liou, 1995). Compared with that in normal Ringer solution (Fig. 6A), treatment with nifedipine 10 μM) significantly reduced SSC potentiation by intracellular acidification after removal of 15 mM NH4Cl (Fig. 6B). Treatment with nifedipine (10 μM) in normal Ca2+ Ringer solution also reduced SSC enhancement by intracellular acidification following acetate substitution (the peak SSC frequency ratio was 158.5 ± 28.7; n= 5), indicating that L-type Ca2+ channels are also involved in the cytosolic acidosis-induced SSC-potentiating action. When Ca2+ was removed from Ringer solution (by replacement with equimolar Mg2+), intracellular acidification with either 15 mM NH4Cl (Fig. 6C and E) or sodium acetate (Fig. 6F) still produced an increase in SSC but to a lesser extent (peak SSC frequency ratios were 61.9 ± 46.1 (n= 7) and 140.5 ± 51.5 (n= 6), respectively, in NH4Cl and sodium acetate). Pretreatment with 20 μM BAPTA AM for 30 min completely inhibited the SSC-potentiating action following withdrawal of external NH4Cl in Ca2+-free Ringer (Fig. 6D and E). These results suggest that a rise of [Ca2+]i is crucial for the SSC-potentiating action of cytosolic acidification, and both external Ca2+ and intracellular Ca2+ stores contribute to the rise in [Ca2+]i levels.
Figure 6. Ca2+-dependent increase in spontaneous synaptic currents by cytosolic acidification.
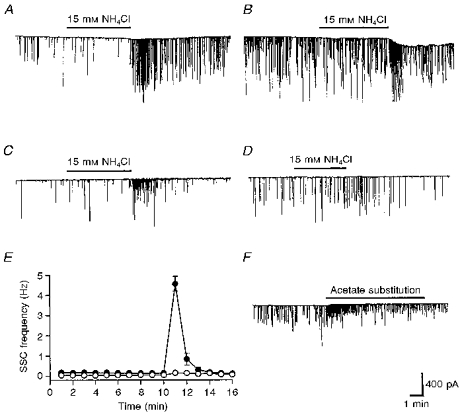
A, continuous trace depicting membrane currents, measured by the whole-cell recording method from an innervated myocyte in a 1-day-old Xenopus culture in normal Ringer solution. Spontaneous synaptic currents (SSCs) appear as random downward deflections. SSC frequency increased after withdrawal of external NH4Cl. B, pretreatment with 10 μM nifedipine inhibited the SSC-potentiating action of intracellular acidification by withdrawal of external NH4Cl. C, SSC-potentiating action after withdrawal of external NH4Cl in Ca2+-free Ringer solution. D, pretreatment with 20 μM BAPTA AM for 30 min inhibited the SSC-potentiating action after withdrawal of external NH4Cl in Ca2+-free Ringer solution. E, time course-response curves. Data are shown as means ±s.e.m. (n= 4). •, Ca2+-free Ringer solution; ○, Ca2+-free Ringer solution + BAPTA AM. F, all of the NaCl was replaced by sodium acetate in pH 6.6 Ca2+-free Ringer solution. Note that acetate substitution increased SSC frequency in the absence of external Ca2+.
Suppression of evoked synaptic currents by cytosolic acidification
The effect of intracellular acidosis on spontaneous ACh release is in striking contrast to its inhibitory action on impulse-evoked ACh release. Evoked synaptic currents (ESCs) were recorded from the innervated muscle cells in 1-day-old Xenopus cultures by the whole-cell voltage-clamp method. The presynaptic neurons were stimulated extracellularly at the soma to initiate action potentials at a frequency of 0.1 Hz. As shown in Fig. 7A and C, cytosolic alkalinization with 15 mM NH4Cl increased the amplitude of ESCs. However, cytosolic acidification following withdrawal of NH4Cl from the bathing solution reduced the amplitude of ESCs (data not shown). The frequency of spontaneous ACh release, as reflected by the SSCs, increased during inhibition of the ESCs (Fig. 7A). Substitution of one-third of the NaCl in pH 6.6 Ringer solution with the organic acid sodium acetate also inhibited the amplitude of the ESCs (Fig. 7B and C). The pHo change in Ringer solution from 7.6 to either 6.6 or 8.6 did not by itself significantly affect the ESC amplitude (Fig. 7C).
Figure 7. Opposite effects of cytosolic acidification on the evoked and spontaneous ACh release.
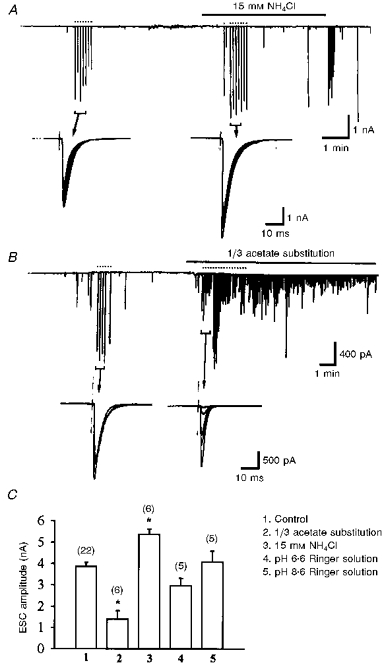
A, presynaptic neurons were stimulated with an extracellular microelectrode at the soma to initiate action potentials as indicated by the dots above the trace. The membrane currents of the innervated myocyte were monitored to record ESCs and SSCs, which appeared as downward deflections in the current trace. Note that the amplitude of ESCs increased upon application of NH4Cl. B, similar to A except that 1/3 of the NaCl in pH 6.6 Ringer solution was replaced by sodium acetate. Note that intracellular acidification decreased the amplitude of ESCs and increased the SSC frequency. C, summary of the action of both external and internal pH changes on the ESCs. Data are presented as means ±s.e.m. with number of experiments indicated in parentheses above the columns. *P < 0.05 compared with control.
Effects of pHi changes on neuronal cytoplasmic Ca2+
The intracellular concentration of free Ca2+ ([Ca2+]i) in the soma was measured using the Ca2+-sensitive fluorescent dye fura-2. The basal neuronal [Ca2+]i value was 70.2 ± 4.6 nM (n= 32). Superfusion with a solution containing weak organic acids (pH 6.6 Ringer solution in which 100 % of the NaCl was replaced with sodium acetate) caused a rapid increase in intracellular Ca2+ ([Ca2+]i) of 481.4 ± 168.9 nM (n= 7) (Fig. 8A). After 2 min, the rise of [Ca2+]i gradually returned to a lower plateau level. However, the pH change in the Ringer solution, from 7.6 to 6.6, by itself slightly reduced [Ca2+]i by 18.1 ± 3.7 nM (n= 6). Experiments similar to that described above were performed in the absence of external Ca2+ (replacement with Mg2+) (Fig. 8B). The basal [Ca2+]i levels were lower in cells assayed in the absence of external Ca2+ (32.1 ± 4.7 nM, n= 11) than those in the presence of external Ca2+ ions. Superfusion with a solution in which 100 % of the NaCl was replaced with sodium acetate still exerted a large increase in [Ca2+]i by 220.2 ± 93.7 nM (n= 4) (Fig. 8B). The recovery profiles were similar to those seen when external Ca2+ was present. These results indicate that acidification of intracellular fluid caused the release of Ca2+ from internal stores, but that external Ca2+ also contributed to the effect. Exposure to a high external concentration of K+ depolarizes the membrane of the soma. The possibility that Ca2+ movement was sensitive to membrane potential was explored. As shown in Fig. 8C, high K+ (20 mM) increased the [Ca2+]i within 1 min by 202.4 ± 38.0 nM (n= 12). Pretreatment with the weak acid sodium acetate (substitution of one-third of the NaCl in pH 6.6 Ringer solution with sodium acetate) attenuated the high K+-induced increase in [Ca2+]i.
Figure 8. Effects of intracellular acidification on the cytoplasmic Ca2+ in the soma.
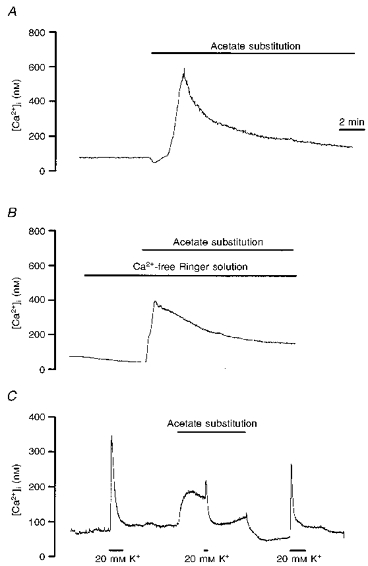
A, Xenopus nerve-muscle culture was loaded with fura-2 AM and ratio fluorimetric measurement of intracellular Ca2+ was made on a single soma. All of the NaCl in pH 7.6 Ringer solution was replaced by sodium acetate in pH 6.6 Ringer solution for the period indicated by the horizontal line. B, similar to A except that the measurement was made in the absence of external Ca2+. C, high K+-induced increase in cytosolic Ca2+ was inhibited by intracellular acidification by replacement of 1/3 of the NaCl in pH 6.6 Ringer solution with sodium acetate.
DISCUSSION
Presynaptic potentiation evoked by cytosolic acidification
In excitable cells, changes in pHi are known to influence the electrical activity of the cell membrane by affecting the properties of ion channels (Moody, 1984). A shift in pHi induces changes in the concentration of intracellular ions in both nerve and muscle. We have observed that exposure of cultured Xenopus spinal neurons to NH4Cl induces a relatively rapid and concentration-dependent cytosolic alkalinization. The frequency of spontaneous synaptic currents (SSCs) was slightly inhibited by cytosolic alkalinization. An abrupt cytosolic acidification caused by the withdrawal of external NH4Cl, however, induced a marked and transient increase in SSC frequency. The increase in SSC frequency seems to be related to the degree of change in pHi (ΔpHi): an absolute pHi change of around 0.2-0.6 units upon application and withdrawal of 1.5 and 5 mM NH4Cl caused little change in SSC frequency, whereas SSC frequency increased markedly if ΔpHi was larger than 1 pH unit following a 15 mM NH4Cl prepulse. Another method of intracellular acidification was used to investigate the effect of pHi change on the release of ACh. It has long been known that weak acids can readily cross cell membranes in their uncharged forms and then dissociate, thereby acidifying the cell interior. Therefore, the rate of permeation and distribution of weak acids across cell membranes is dependent on extra- and intracellular pH. A decrease in pHo enhances the effects of weak acids on pHi. The presumed mechanism for the increase in intracellular acidosis by weak acids at lower pHo is an increased diffusion of protonated and uncharged forms of the weak acids. Once inside the cell, the pHi is well above the pKa, leading to dissociation of the protonated form and acidification (Karuri, Dobrowsky & Tannock, 1993). Therefore, the anions of weak acids (pKa > 4.5) cause a large internal acidification and the anions of strong acids (pKa < 2.6) cause little or no change in pHi (Sharp & Thomas, 1981). Our results showed that, in pH 6.6 Ringer solution, methylsulphate (pKa < 1.0), isethionate (pKa < 1.25) and glucuronate (pKa 3.18) induced little pHi change in neurons, whereas acetate (pKa= 4.75) and propionate (pKa= 4.87) caused a larger intracellular acidification. Our data are comparable to those of Sharp & Thomas (1981). The increase in SSC frequency paralleled the levels of cytosolic acidification by these organic acids. Cl− substitution alone did not affect spontaneous ACh release, as evidenced by the lack of effect on SSC frequency when Cl− was replaced by methylsulphate, isethionate or glucuronate. Since lowering the pHo to 6.6 did not significantly affect the SSC frequency, a decrease in pHi is the most likely reason that organic acids produce an increase in spontaneous ACh secretion. It seems that to produce an increase in SSC frequency weak organic acids must produce a larger pHi change (around 1.4-1.6 pH units) than an NH4Cl prepulse. This probably results from the unknown effect of the simultaneous pHo reduction from 7.6 to 6.6 which occurs on weak acid perfusion.
Increase in SSC frequency depends on the rise of cytoplasmic Ca2+
It has been reported that internal acidification results in Ca2+-independent release of neurotransmitters in rat brain synaptosomes (Drapeau & Nachshen, 1988). The current study demonstrates that cytosolic acidification increases intracellular Ca2+ and spontaneous ACh release. The absence of an effect on SSC frequency in BAPTA AM-treated cultures during cytosolic acidification (see Fig. 6D) indicates that a rise in [Ca2+]i level is essential for the enhancement of ACh release. We show here that, in cultured spinal neurons, rapid cytosolic acidosis causes a prompt increase in [Ca2+]i as a result of Ca2+ mobilization from internal stores as well as from extracellular Ca2+. The gradual decrease in SSC frequency during the maintained acid load mainly results from a transient peak increase in [Ca2+]i after acid load in either normal Ca2+ or Ca2+-free Ringer solutions. The exact mechanism by which changes in pHi affect [Ca2+]i is not clear. There are at least four possible mechanisms which could account for the observed rise in [Ca2+]i associated with rapid pHi changes: (i) increased Ca2+ entry via the plasma membrane, (ii) Ca2+ release from intracellular stores, (iii) decreased uptake into intracellular stores and (iv) reduced Ca2+ extrusion via the plasma membrane. Our previous works (Fu & Huang, 1994; Fu et al. 1995) show that L-type Ca2+ channels are involved in the regulation of spontaneous ACh release at developing neuromuscular synapses in Xenopus cultures. We have shown here that nifedipine partially inhibited a cytosolic acidification-induced increase in SSCs, suggesting that cytosolic acidification may depolarize nerve terminals and open L-type Ca2+ channels, resulting in an increase in intracellular Ca2+ and spontaneous ACh release. Mobilization of Ca2+ from internal stores as a result of intracellular acidification also contributes to the increase in SSCs. The SSC frequency was slightly inhibited by cytosolic alkalinization, which paralleled a slight decrease in the intracellular Ca2+ levels (data not shown). Cytosolic alkalosis reduces the intracellular Ca2+ concentration, probably by enhancing sequestration of Ca2+ (OuYang, Mellergard, Kristian, Kristianova & Siesjo, 1994).
Opposite action on evoked ACh release by cytosolic acidification
It is widely known that cerebral acidosis depresses, while alkalosis enhances, neuronal excitability. Sensitivity of Ca2+ currents to extracellular H+ has been described in a variety of cell types (Irisawa & Sato, 1986; Prodhom, Pietrobon & Hess, 1989; Tytgat, Nilius & Carmeliet, 1990). In hippocampal CA1 neurons, Ca2+ channels were reversibly depressed by moderate extracellular acidosis (pH 6.0-6.9) and enhanced slightly by alkaline exposure (pH 8.0) (Tombaugh & Somjen, 1996). On the other hand, alkaline intracellular pH enhanced and acidic intracellular pH inhibited vascular and cardiac L-type Ca2+ currents by affecting the channel availability and/or channel open probability (Kaibara & Kameyama, 1988; Iino, Hayashi, Saito, Tokuno & Tomita, 1994). However, T-type Ca2+ channel activity was decreased by pHo acidification, but not affected by internal protons (Tytgat et al. 1990). Therefore, differences in pHi sensitivity among high voltage-activated Ca2+ channel types may exist. We observed here that a reduction in pHo from 7.6 to 6.6 did not significantly affect the impulse-evoked ACh release. However, intracellular acidification with either an NH4Cl prepulse or weak organic acids markedly inhibited the evoked responses. Since the amplitude of SSCs was only slightly inhibited by intracellular acidification, the inhibition of evoked synaptic currents may result mainly from presynaptic events. We found previously that impulse-evoked ACh release from developing motoneurons in Xenopus cultures resulted mainly from the activation of N-type Ca2+ channels, since ω-conotoxin at 1 μM completely inhibited the evoked responses (data not shown). Therefore, cytosolic acidification may have an inhibitory action on presynaptic N-type Ca2+ channels, resulting in the inhibition of evoked synaptic currents. On the other hand, cytosolic alkalinization with NH4Cl slightly enhanced the evoked ACh release, probably as a result of the enhancement of N-type Ca2+ currents. It has been shown that the miniature endplate current amplitude of frog neuromuscular junctions is not affected, or is only slightly reduced, when the bathing pH is reduced from 7 to 5.5 (Mallart & Molgo, 1978; Landau, Gavish, Nachshen & Lotau, 1981), depending on the species examined. However, the decay time of the miniature endplate current is prolonged in acidic pHo and shortened in alkaline pHo (≈pH 9.0; Mallart & Molgo, 1978). Our results show that cytosolic acidification shortens the decay time of SSCs, indicating that the ACh receptor channel is also sensitive to intracellular pH changes.
In conclusion, a moderate decline in cytosolic pH increased spontaneous ACh release but inhibited impulse-evoked ACh secretion in Xenopus motoneurons. Mobilization of internal Ca2+ stores and Ca2+ influx from external solution may contribute to the potentiating effect, whereas the inhibition of N-type Ca2+ channels may be involved in the suppressive action. Since nerve cells may accumulate H+ after prolonged activity (Ahmed & Connor, 1980), cytosolic acidification may thus have a regulatory role in transmitter release under physiological or pathological conditions. In addition, cytosolic acidification has been postulated to contribute to ischaemic- and glutamate-induced neurotoxicity (Siesjo, 1992; Hartley & Dubinsky, 1993), and therefore a rise in cytoplasmic Ca2+ levels following intracellular acidosis may further enhance excitotoxic damage under some pathological conditions.
Acknowledgments
This work was supported by National Science Council Research Grant NSC 86-2314-B002-299.
References
- Ahmed Z, Connor JA. Intracellular pH changes induced by calcium influx during electrical activity in molluscan neurons. Journal of General Physiology. 1980;75:403–426. doi: 10.1085/jgp.75.4.403. 10.1085/jgp.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa WB, Nuccitelli R. Metabolic regulation via intracellular pH. American Journal of Physiology. 1984;246:R409–438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila A. Modulation of pH by neuronal activity. Trends in Neurological Sciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Cohen I, van der Kloot W. The effects of pH changes on the frequency of miniature end plate potentials at the frog neuromuscular junction. The Journal of Physiology. 1976;262:401–414. doi: 10.1113/jphysiol.1976.sp011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo J, Nelson TE, Sanchez V. The mechanism of increased acetylcholine sensitivity of skeletal muscle in low pH solutions. Journal of Cellular and Comparative Physiology. 1962;59:35–44. doi: 10.1002/jcp.1030590105. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Nachshen DA. Effects of lowering extracellular and cytosolic pH on calcium fluxes, cytosolic calcium levels, and transmitter release in presynaptic nerve terminals isolated from rat brain. Journal of General Physiology. 1988;91:305–315. doi: 10.1085/jgp.91.2.305. 10.1085/jgp.91.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WM, Huang FL. L-type Ca2+ channel is involved in the regulation of spontaneous transmitter release at developing neuromuscular synapses. Neuroscience. 1994;58:131–140. doi: 10.1016/0306-4522(94)90160-0. 10.1016/0306-4522(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Fu WM, Liou JC, Lee YH, Liou HC. Potentiation of neurotransmitter release by activation of presynaptic glutamate receptors at developing neuromuscular synapses of Xenopus. The Journal of Physiology. 1995;489:813–823. doi: 10.1113/jphysiol.1995.sp021094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukiwara N, Abe T, Endoh H, Warashina A, Shimoji K. Changes in intracellular pH of mouse hippocampal slices responding to hypoxia and/or glucose depletion. Brain Research. 1992;572:335–339. doi: 10.1016/0006-8993(92)90496-v. 10.1016/0006-8993(92)90496-V. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartley Z, Dubinsky JM. Changes in intracellular pH associated with glutamate excitotoxicity. Journal of Neuroscience. 1993;13:4690–4699. doi: 10.1523/JNEUROSCI.13-11-04690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JO, Jones SF, Landau EM. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. The Journal of Physiology. 1968;194:355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S, Hayashi H, Saito H, Tokuno H, Tomita T. Effects of intracellular pH on calcium currents and intracellular calcium ions in the smooth muscle of rabbit portal vein. Experimental Physiology. 1994;79:669–680. doi: 10.1113/expphysiol.1994.sp003799. [DOI] [PubMed] [Google Scholar]
- Irisawa H, Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circulation Research. 1986;59:348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Kaibara M, Kameyama M. Inhibition of the calcium channel by intracellular proton in single ventricular myocytes of the guinea-pig. The Journal of Physiology. 1988;403:621–640. doi: 10.1113/jphysiol.1988.sp017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuri AR, Dobrowsky E, Tannock IF. Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. British Journal of Cancer. 1993;68:1080–1087. doi: 10.1038/bjc.1993.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau EM, Gavish B, Nachshen DA, Lotau I. pH dependence of the acetylcholine receptor channel: A species variation. Journal of General Physiology. 1981;77:647–666. doi: 10.1085/jgp.77.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau EM, Nachshen DA. The interaction of pH and divalent cations at the neuromuscular junction. The Journal of Physiology. 1975;251:775–790. doi: 10.1113/jphysiol.1975.sp011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A, Mologo J. The effects of pH and curare on the time course of endplate currents at the neuromuscular junction of the frog. The Journal of Physiology. 1978;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W., Jr Effects of intracellular H+ on the electrical properties of excitable cells. Annual Review of Neuroscience. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. American Journal of Physiology. 1991;260:R581–588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OuYang YB, Mellergard P, Kristian T, Kristianova V, Siesjo BK. Influence of acid-base changes on the intracellular calcium concentration of neurons in primary culture. Experimental Brain Research. 1994;101:265–271. doi: 10.1007/BF00228746. [DOI] [PubMed] [Google Scholar]
- Pasternack M, Bountra C, Voipio J, Kaila K. Influence of extracellular and intracellular pH on GABA-gated chloride conductance in crayfish muscle fibers. Neuroscience. 1992;47:921–929. doi: 10.1016/0306-4522(92)90040-9. [DOI] [PubMed] [Google Scholar]
- Prodhom B, Pietrobon D, Hess P. Interactions of protons with single open L-type calcium channels. Journal of General Physiology. 1989;94:23–42. doi: 10.1085/jgp.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink TJ, Tsien RY, Pozzan T. Cytosolic pH and free Mg2+ in lymphocytes. Journal of Cell Biology. 1982;95:189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Boron F. Intracellular pH. Physiological Reviews. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Rorig B, Klausa G, Sutor B. Intracellular acidification reduced gap junction coupling between immature rat neocortical pyramidal neurons. The Journal of Physiology. 1996;490:31–49. doi: 10.1113/jphysiol.1996.sp021125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sater AK, Alderton JM, Steinhardt RA. An increase in intracellular pH during neural induction in Xenopus. Development. 1994;120:433–442. doi: 10.1242/dev.120.2.433. [DOI] [PubMed] [Google Scholar]
- Sharp AP, Thomas RC. The effects of chloride substitution on intracellular pH in crab muscle. The Journal of Physiology. 1981;312:71–80. doi: 10.1113/jphysiol.1981.sp013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo BK. Pathology and treatment of focal cerebral ischemia. II. Mechanisms of damage and treatment. Journal of Neurosurgery. 1992;77:337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AC, Bennett MVL. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Tabti N, Poo MM. Culturing Xenopus nerve and muscle cells. In: Banker G, Goslin K, editors. Cultures of Nerve Cells. Boston, MA, USA: MIT Press; 1991. pp. 139–153. [Google Scholar]
- Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proceedings of the National Academy of Sciences of the USA. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons. The Journal of Physiology. 1996;493:719–732. doi: 10.1113/jphysiol.1996.sp021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis S, Cull-Candy S. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. Journal of Biological Chemistry. 1966;241:4110–4114. [PubMed] [Google Scholar]
- Tytgat J, Nilius B, Carmeliet E. Modulation of the T-type cardiac Ca channel by changes in proton concentration. Journal of General Physiology. 1990;96:973–990. doi: 10.1085/jgp.96.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ML, Tsai ML, Tseng YZ. DIDS-sensitive pHi-regulation in single cardiac myocytes in nominally HCO3-free conditions. Circulation Research. 1994;75:123–132. doi: 10.1161/01.res.75.1.123. [DOI] [PubMed] [Google Scholar]
- Xie Z, Poo MM. Initial events in the formation of neuromuscular synapses. Proceedings of the National Academy of Sciences of the USA. 1986;83:7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]


