Abstract
Long-term potentiation (LTP) and depression (LTD) were investigated at synapses formed by pairs of monosynaptically connected CA3 pyramidal cells in rat hippocampal slice cultures.
An N-methyl-D-aspartate (NMDA) receptor-mediated component of the unitary EPSP, elicited at the resting membrane potential in response to single action potentials in an individual CA3 cell, could be isolated pharmacologically.
Associative LTP was induced when single presynaptic action potentials were repeatedly paired with 240 ms postsynaptic depolarizing pulses that evoked five to twelve action potentials or with single postsynaptic action potentials evoked near the peak of the unitary EPSP. LTP induction was prevented by an NMDA receptor antagonist.
Associative LTD was induced when single presynaptic action potentials were repeatedly elicited with a certain delay after either 240 ms postsynaptic depolarizing pulses or single postsynaptic action potentials. The time window within which presynaptic activity had to occur for LTD induction was dependent on the amount of postsynaptic depolarization. LTD was induced if single pre- and postsynaptic action potentials occurred synchronously.
Homosynaptic LTD was induced by 3 Hz tetanization of the presynaptic neuron for 3 min and was blocked by an NMDA receptor antagonist.
Depotentiation was produced with stimulation protocols that elicit either homosynaptic or associative LTD.
Recurrent excitatory synapses between CA3 cells display associative potentiation and depression. The sign of the change in synaptic strength is a function of the relative timing of pre- and postsynaptic action potentials.
In order to account for the associative formation of memory, Hebb (1949) postulated that if cell A ‘repeatedly and consistently takes part’ in firing cell B, then some ‘change takes place in one or both cells such that A's efficiency, as one of the cells firing cell B, is increased.’ Although it is imprecise, this statement is usually interpreted to mean that if A and B fire synchronously, then the synapses between them will become stronger (for review see Brown, Kairess & Keenan, 1990). The synapses formed between CA3 pyramidal cells by their recurrent axon collaterals may be a particularly important site of associative memory formation because CA3 cells form a matrix-like organization which appears to be well adapted for this task (Marr, 1971; McNaughton & Morris, 1987; Treves & Rolls, 1994).
Long-term potentiation (LTP) is a particularly attractive cellular mechanism to account for Hebbian increases in synaptic strength because associative interactions greatly facilitate its induction and are required in many induction protocols (Barrionuevo & Brown, 1983; Debanne, Gähwiler & Thompson, 1996). Indeed, there is evidence that potentiation of synchronously active CA3 pyramidal cells plays a crucial role in spatial learning (Wilson & McNaughton, 1994). Associative long-term depression (LTD) at CA3-CA3 synapses may also contribute to spatial learning (Willshaw & Dayan, 1990; Debanne, 1996), for example, by weakening synaptic contacts between cells that are firing asynchronously because they have spatially unrelated place fields (e.g. O'Keefe & Dostrovsky, 1971).
Homosynaptic LTP within area CA3 is induced at both mossy fibre and recurrent excitatory synapses with high frequency presynaptic tetanization (Harris & Cotman, 1986; Jaffe & Johnston, 1990; Zalutsky & Nicoll, 1990). N-methyl-d-aspartate (NMDA) receptor activation is required for LTP induction at recurrent excitatory synapses but not at mossy fibre synapses (Harris & Cotman, 1986; Zalutsky & Nicoll, 1990). Synchronous pairing of presynaptic activation and large postsynaptic depolarizations has also been shown to induce LTP in CA3 pyramidal cells (Zalutsky & Nicoll, 1990), but there has been no direct experimental demonstration that LTP results from Hebbian, synchronized action potential discharge in identified pre- and postsynaptic CA3 neurons.
Homosynaptic and associative LTD have also been well characterized in area CA1 (Dunwiddie & Lynch, 1978; Dudek & Bear, 1992; Mulkey & Malenka, 1992; Debanne, Gähwiler & Thompson, 1994), in the dentate gyrus (see Christie, Kerr & Abraham, 1994, for review), and at mossy fibre-CA3 synapses (Battistin & Cherubini, 1994; Derrick & Martinez, 1996; Kobayashi, Manabe & Takahashi, 1996; Urban & Barrionuevo, 1996), but these forms of depression have not yet been well studied at recurrent excitatory synapses in area CA3. For example, although associative depression has been reported to occur at commissural/associative synapses (Chattarji, Stanton & Sejnowski, 1989), the requirements for its induction have not been established.
The use of paired recordings from identified CA3 pyramidal cells permits a good characterization of recurrent synapses because there is no ‘contamination’ from mossy fibre synapses. We have investigated the role of the temporal relation between pre- and postsynaptic activity in determining the strength of synapses between CA3 pyramidal cells in hippocampal slice cultures. Because CA3 cells in vivo discharge both single spikes and bursts of spikes (e.g. McNaughton, Barnes & O'Keefe, 1983), we have compared the effectiveness of single postsynaptic action potentials or bursts of action potentials as conditioning stimuli. In addition, we have examined whether NMDA receptor activation is required for induction of LTP and homosynaptic and associative LTD at these synapses.
METHODS
Slice culture preparation
Hippocampal slice cultures were prepared and maintained as described previously (Gähwiler, 1981; Gähwiler, Thompson, Audinat & Robertson, 1991). In brief, the hippocampi were dissected from 5- to 7-day-old rat pups killed by decapitation, and 400 μm-thick transverse slices were cut and attached to glass coverslips with clotted chicken plasma. The coverslip and slice were placed in individual sealed test-tubes containing semi-synthetic medium and maintained on a roller drum in an incubator at 36°C for 2-4 weeks. The culture medium consisted of 50 % Eagle's basal medium, 25 % balanced salt solution with either Hanks' or Earle's salts, 25 % heat-inactivated horse serum, 33.3 mM D-glucose and 0.1 mM glutamine. Media were purchased from Life Technologies, Paisley, UK. For electrophysiological recordings, cultures were transferred to a recording chamber mounted on an inverted microscope and continuously superfused with a warmed (32°C) saline containing (mM): Na+, 149; Cl−, 149; K+, 2.7; Ca2+, 2.8; Mg2+, 2.0; HCO3−, 11.6; H2PO4−, 0.4; glucose, 5.6; and Phenol Red, 10 mg l−1, at pH 7.4.
Electrophysiological recordings
CA3 pyramidal cells were impaled in stratum pyramidale using sharp microelectrodes filled with 1 M potassium methylsulphate. Changes in the input resistance of the postsynaptic cell were monitored throughout the experiment with short hyperpolarizing current pulses (40 ms duration, -0.1 nA). The analog signals from the two electrodes were amplified × 100 (Axoclamp-2A, Axon Instruments), digitized at 18 kHz, and recorded on videotape. Off-line acquisition of 200 ms sequences was performed on an IBM PC with a digitization rate of 8 kHz (Acquis1, Bio-logic, Claix, France).
For extracellular stimulation experiments, EPSPs were evoked using patch pipettes filled with 155 mM NaCl placed in stratum radiatum. Stimuli (0.1 ms, -5 to -15 mA) were always delivered at 0.3 Hz. The criteria for establishing that EPSPs between cell pairs were monosynaptic included relatively short and invariant onset latencies, as described in detail elsewhere (Debanne, Guérineau, Gähwiler & Thompson, 1995). EPSP amplitudes were always measured at a fixed latency from the presynaptic action potential. The mean resting membrane potential of the cells in which LTP and LTD induction with single postsynaptic action potentials was attempted was -62.5 ± 0.8 mV (n= 16). Values of potentiation and depression given in the text are based on averages of EPSPs over 2-3 min taken 10 min after the end of the pairing procedure or the tetanus, and are presented as means ±s.e.m. D-2-Amino-5-phosphonovalerate (AP5) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were purchased from Tocris Cookson (Bristol, UK), prepared as a frozen aqueous stock solution, and applied by bath perfusion.
RESULTS
Unitary NMDA receptor-mediated EPSPs
Because many forms of synaptic plasticity are NMDA receptor dependent, we asked whether single action potentials in CA3 pyramidal cells could produce a detectable activation of synaptic NMDA receptors in other monosynaptically excited CA3 pyramidal cells. We first examined the effects of the NMDA receptor antagonist AP5 (40 μM) on unitary EPSPs with the postsynaptic cell at its resting membrane potential (-58 ± 1 mV, n= 4 cells). When the unitary EPSP in the presence of AP5 was subtracted from the control EPSP, it was clear that a slowly rising EPSP component had been blocked (Fig. 1). The AP5-sensitive component of the EPSP had a time to peak of 32 ± 5 ms, and a mean amplitude of 0.4 ± 0.1 mV (n= 4). Similarly, in the presence of the non-NMDA receptor antagonist CNQX (40 μM), a slow EPSP was recorded having a time to peak of 25 ± 3 ms and an amplitude of 0.4 ± 0.1 mV (n= 3) at the resting membrane potential. Unitary NMDA receptor-mediated EPSPs between CA3 cells thus had an amplitude at the resting potential that was roughly one-third of the total control EPSP amplitude (≈1 mV), and were essentially identical to unitary NMDA receptor-mediated EPSPs at Schaffer collateral connections in hippocampal slice cultures (Debanne et al. 1995).
Figure 1. The NMDA receptor-mediated component of unitary EPSPs.
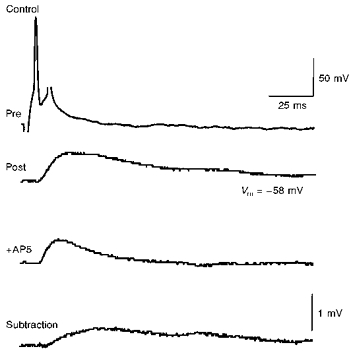
A single presynaptic action potential in one CA3 pyramidal cell (Pre) elicited a unitary EPSP in a second CA3 pyramidal cell (Post) at its resting membrane potential (-58 mV). Application of 40 μM AP5 decreased the duration of the unitary EPSP (middle trace). Digital subtraction of the unitary EPSP in the presence of AP5 from the control EPSP revealed the component of the EPSP blocked by AP5 (bottom trace). This NMDA receptor-mediated component had a time to peak of 27 ms and an amplitude of 0.5 mV.
Associative induction of LTP
LTP was induced in CA3 pyramidal cells of hippocampal slice cultures when extracellular stimulation in stratum radiatum was synchronously paired with a 240 ms postsynaptic depolarizing pulse, and this pairing was repeated 15-50 times at 0.3 Hz. The potentiation was to 168 ± 14 % of the control EPSP amplitude (n= 11 cells) and could be followed more than 20 min after the synchronous pairing procedure (Fig. 2A). Sharp microelectrode recordings were made simultaneously from pairs of CA3 pyramidal neurons because the recruitment of mossy fibres, which use a different mechanism for LTP induction (Harris & Cotman, 1986; Zalutsky & Nicoll, 1990), cannot be totally excluded when using extracellular stimulation. LTP of unitary EPSPs between monosynaptically coupled neurons was induced when single presynaptic action potentials were paired with long bursts of five to ten postsynaptic action potentials using pulses having an arbitrary length of 240 ms, and this pairing was repeated 15-50 times at 0.3 Hz (Fig. 2B). On average, unitary CA3-CA3 EPSPs were potentiated to 319 ± 75 % of the control EPSP amplitude (n= 16 pairs). In the presence of the NMDA receptor antagonist AP5 (40 μM), repeated, synchronous pairing of single presynaptic action potentials and bursts of postsynaptic action potentials failed to elicit any significant change in the amplitude of unitary EPSPs (mean amplitude after pairing: 101 ± 6 % of the control amplitude, n= 3).
Figure 2. Potentiation of EPSPs between CA3 cells.
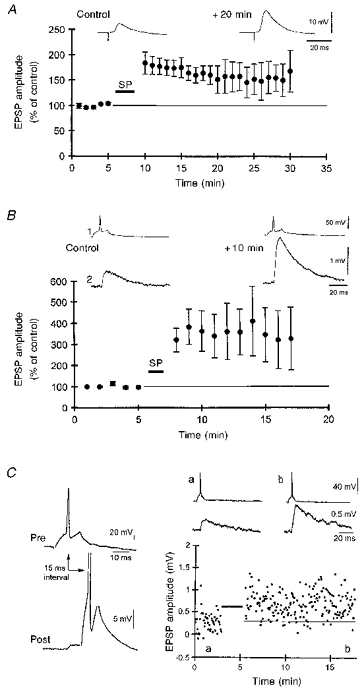
A, synchronous pairing (SP) of low frequency (at 0.3 Hz) extracellular stimuli and postsynaptic depolarization (240 ms duration, 0.5-2.5 nA) induced a long-lasting potentiation of the amplitude and initial slope of compound EPSPs evoked in CA3 pyramidal cells (n= 11 cells). A representative example is shown above. B, pooled data illustrating the potentiation of unitary CA3-CA3 EPSPs induced with the synchronous pairing of single presynaptic action potentials and postsynaptic depolarizations (240 ms duration, 30-80 repetitions) which produced 7-12 action potentials. Traces from a representative experiment are shown above the graph. C, potentiation induced by the pairing (indicated by bar in graph) of a single presynaptic action potential elicited 15 ms before a single postsynaptic action potential (repeated × 50 at 0.3 Hz). Averaged traces from the labelled time points are illustrated above the graph.
CA3 neurons in vivo fire not only bursts of action potentials; single action potentials at low frequency (0.1-10 Hz) can also be recorded commonly in freely moving rats (McNaughton et al. 1983). We therefore investigated whether pairing of unitary EPSPs with single postsynaptic action potentials would also be sufficient to induce LTP. Surprisingly, perfectly synchronous pairing of single pre- and postsynaptic action potentials led to a depression in unitary EPSP amplitude (see below). If, however, the delay between the pre- and postsynaptic action potentials was adjusted to 15 ms, so that the postsynaptic action potential occurred near the peak of the unitary EPSP (Fig. 1), then lasting potentiation was induced following fifty to eighty repetitions of this pairing protocol (Fig. 2C). On average, unitary EPSPs were potentiated by this procedure to 155 ± 26 % of the control amplitude (n= 6 pairs). The potentiation was long lasting (> 10 min, Fig. 2C), but not saturating. An additional fifty to eighty synchronous pairings of 240 ms depolarizing pulses with the unitary EPSP resulted in a potentiation of a further 71 ± 27 % (n= 3 pairs).
Associative induction of LTD
Associative LTD of Schaffer collateral synapses is induced when presynaptic stimulation repeatedly follows a strong postsynaptic depolarization (5-12 action potentials) with a delay of 0.5-2 s (Debanne et al. 1994). This asynchronous pairing between presynaptic activity and postsynaptic depolarization (240 ms pulse, 800 ms delay, 100 repetitions) also resulted in long-lasting depression of extracellularly evoked EPSPs in area CA3 to 69 ± 11 % of the control amplitude, which lasted for > 20 min (n= 3 cells; Fig. 3A). In order to ensure that this associative depression occurred at CA3-CA3 synapses, and not at mossy fibre synapses, we recorded from pairs of monosynaptically connected CA3 pyramidal cells. Significant depression of unitary CA3-CA3 EPSPs was indeed induced when single presynaptic action potentials repeatedly followed large postsynaptic depolarizations by 800 ms (to 64 ± 4 % of the control EPSP amplitude, n= 6 pairs; Fig. 3B).
Figure 3. Associative depression of EPSPs between CA3 cells.
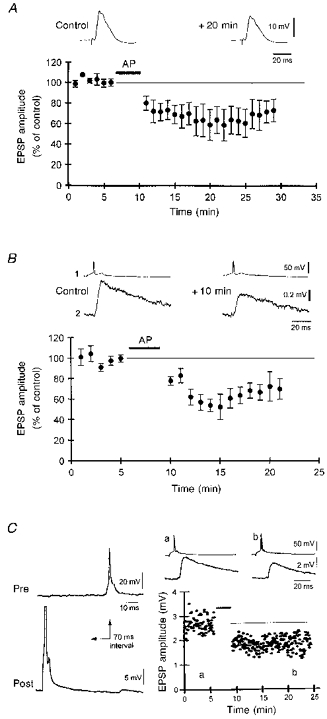
A, asynchronous pairing (AP) of low frequency extracellular stimuli (at 0.3 Hz) and postsynaptic depolarization (240 ms, preceding the EPSP by 800 ms, repeated × 100) induced a long-lasting depression of both amplitude and slope of compound EPSPs recorded in CA3 pyramidal neurons (n= 3 cells). A representative example is shown above. B, pooled data illustrating associative LTD of 6 unitary CA3-CA3 EPSPs following the asynchronous pairing of single presynaptic action potentials and postsynaptic depolarization (240 ms duration, 7-12 action potentials, 100 repetitions) delayed by 800 ms. C, depression induced by the asynchronous pairing (indicated by bar in graph in C) of a single presynaptic action potential elicited 70 ms after a single postsynaptic action potential (repeated × 100 at 0.3 Hz). Averaged traces from the labelled time points are illustrated above the graph.
As at Schaffer collateral synapses, NMDA receptor activation was found to be required for induction of associative LTD at CA3-CA3 synapses. No significant depression was induced when the asynchronous pairing procedure was performed in the presence of the NMDA receptor antagonist AP5 (40 μM; mean EPSP amplitude after asynchronous pairing: 92 ± 6 % of the control amplitude, n= 3 pairs). In two of two cell pairs tested, LTD was induced successfully using asynchronous pairing upon washout of AP5 (not shown).
Associative LTD could also be induced with the asynchronous pairing of single pre- and postsynaptic action potentials. Single postsynaptic action potentials were elicited 15-200 ms before single presynaptic action potentials, and this pairing procedure was repeated 100 times. A typical example of LTD, induced with a 70 ms delay between the post- and presynaptic action potentials, is illustrated in Fig. 3C. On average, the depression produced by this protocol was to 68 ± 7 % of the control amplitude (n= 5 pairs) when the delay was 70 ms (Fig. 4). Asynchronous pairing of single action potentials with a delay of 15 ms resulted in a depression of comparable magnitude (Fig. 4). The depression induced with both intervals was of similar magnitude to that produced by an asynchronous pairing of 240 ms depolarizing pulses with an extracellular stimulus after an 800 ms delay (see above). In contrast, no statistically significant depression was induced by the asynchronous pairing of single action potentials when the delay between the post- and presynaptic action potentials was 200 ms (89 ± 3 % of control amplitude, n= 3 pairs, P > 0.1, Mann-Whitney U test; Fig. 4). Inconsistent with the classical interpretation of Hebb's postulate, repeatedly (× 100) eliciting pre- and postsynaptic action potentials in perfect synchrony at 0.3 Hz resulted in a depression in the amplitude of the unitary EPSP to 71 ± 3 % of the control amplitude (n= 3 pairs).
Figure 4. Changes in synaptic efficacy as a function of the time interval between pre- and postsynaptic action potentials.
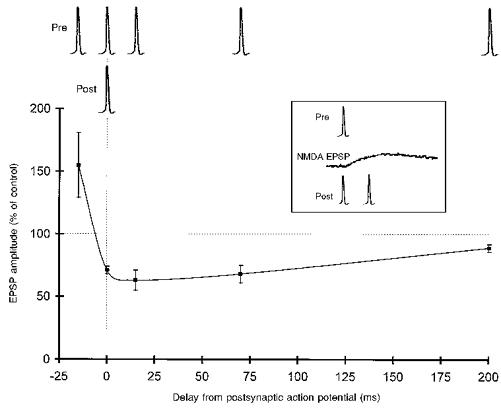
Potentiation was induced only when the presynaptic action potential preceded the postsynaptic action potential (-15 ms of delay). Depression was induced for synchronous (0 ms) and asynchronous pairing (15-70 ms). The change in unitary EPSP amplitude observed when the time interval was increased to 200 ms was not significant (P > 0.01, Mann-Whitney U test). The inset illustrates the timing of the NMDA receptor-mediated component of the unitary EPSP (from Fig. 1) relative to the pre- and postsynaptic action potentials when they are synchronous or when the delay is -15 ms.
A decrease in the amount of postsynaptic activity (one action potential versus a burst of several action potentials) thus shifts the time window for LTD induction towards shorter intervals. Moreover, whether a synapse becomes potentiated or depressed is determined by a very small change in the amount of synchronization between pre- and postsynaptic action potentials.
Induction of homosynaptic LTD
Whether homosynaptic depression of recurrent connections between CA3 cells can be induced with low frequency stimulation (e.g. Dunwiddie & Lynch, 1978) is not known. We found that tetanization within area CA3 at 3 Hz for 3 min resulted in a depression in the amplitude of extracellularly evoked EPSPs to 64 ± 3 % of the control amplitude (n= 5 cells), which persisted for > 20 min (Fig. 5A). Homosynaptic depression of unitary EPSPs between pairs of monosynaptically connected CA3 pyramidal cell pairs was also induced by eliciting single presynaptic action potentials at 3 Hz for 3 min. Such low frequency tetani induced a stable depression which could be followed for as long as the two impalements could be maintained (> 15 min; Fig. 5B). The depression of unitary CA3-CA3 synapses (to 58 ± 5 % of the control amplitude, n= 5 pairs; Fig. 5C) was not significantly different in magnitude from the depression of extracellularly evoked EPSPs produced by the same tetani (P > 0.1; Mann-Whitney U test).
Figure 5. Homosynaptic depression of EPSPs between CA3 cells.
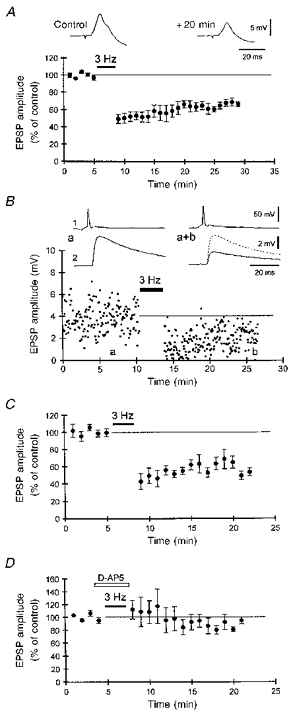
A, low frequency tetanus (3 Hz, 3 min) resulted in a persistent depression of the amplitude and the initial slope (not shown) of compound EPSPs (inset) evoked with an extracellular stimulation in the area CA3 (n= 5 cells). B, example of homosynaptic LTD of a unitary CA3-CA3 EPSP following 3 Hz tetanization of the presynaptic cell for 3 min. Averaged traces from the labelled time points are illustrated above the graph. C, pooled data illustrating homosynaptic LTD of 5 unitary CA3-CA3 EPSPs after 3 Hz tetanization for 3 min. D, no LTD of unitary CA3-CA3 EPSPs was induced when the NMDA receptor antagonist AP5 (40 μM) was present during the 3 Hz tetanus (n= 4 cell pairs).
In area CA1, NMDA receptor activation is required for induction of homosynaptic LTD (Dudek & Bear, 1992; Mulkey & Malenka, 1992). We therefore investigated whether activation of NMDA receptors was also necessary for homosynaptic LTD induction at unitary CA3-CA3 connections. Indeed, no significant depression was observed when the 3 Hz tetanus was delivered in the presence of the NMDA receptor antagonist AP5 (40 μM; mean EPSP amplitude after tetanization: 94 ± 10 % of the control amplitude, n= 4 pairs; Fig. 5D). In three of three cell pairs tested, LTD could be induced successfully with 3 Hz tetanization upon washout of AP5 (not shown).
Bidirectional plasticity between individual CA3 pyramidal cells
We next investigated whether unitary CA3-CA3 EPSPs could be potentiated and then depotentiated. Potentiation was first induced with the synchronous pairing procedure using 240 ms depolarizing pulses, as described above. The asynchronous pairing protocol (as defined in Fig. 3B) was found to reverse previously established LTP. On average, the amount of depotentiation induced by asynchronous pairing was -39 ± 8 % from the potentiated amplitude (n= 3 pairs; Fig. 6A). Depotentiation of unitary EPSPs could also be induced with 3 Hz tetanization of the presynaptic cell for 3 min (not shown). On average, the amount of depotentiation produced by 3 Hz tetanization was -42 ± 5 % from the potentiated amplitude (n= 3 pairs; Fig. 6B). These results demonstrate that the strength of the synapses between two individual CA3 pyramidal neurons can be alternatively up- and downregulated.
Figure 6. Potentiation and depotentiation of a unitary CA3-CA3 EPSP induced with synchronous and asynchronous pairing.
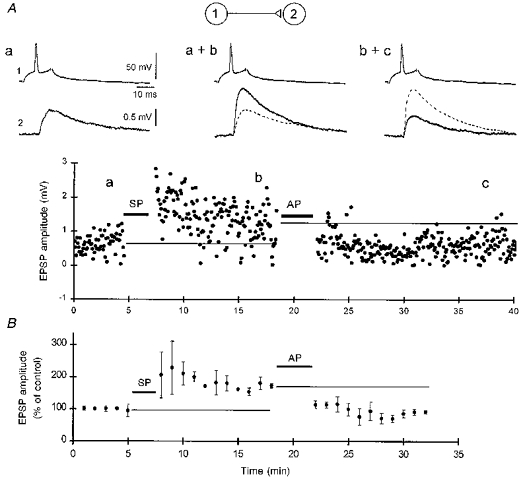
A, a 61 ± 8 % potentiation (all responses 5-10 min after the pairing) over the control amplitude was first induced by a synchronous pairing (SP) of 240 ms postsynaptic depolarizing pulses and single action potentials in the presynaptic cell (repeated × 30). After stabilization, the asynchronous pairing (AP) of 240 ms postsynaptic depolarizing pulses followed 800 ms later by single presynaptic action potentials (repeated × 100) resulted in a 58 ± 5 % decrease in amplitude relative to the potentiated level (measured 5-10 min after the AP), which persisted for 20 min. Averaged traces from the labelled time points are illustrated above the graph. B, pooled data illustrating potentiation of 3 unitary CA3-CA3 EPSPs using the SP protocol, followed by depotentiation using the AP protocol. The large standard error bars for the mean responses immediately after SP reflect the variable occurrence of the transient, short-term potentiation in some cells, such as the example in A.
DISCUSSION
The CA3 region is a critical link in the circuitry of the limbic system, and is remarkable for the extent of the recurrent axonal collaterals formed by the pyramidal cells. The plasticity of the excitatory synapses formed by these collaterals with other CA3 cells has received relatively little attention until now, and in this paper we have attempted to define the conditions under which LTP and LTD might become induced.
Associative LTP between CA3 pyramidal cells
Associative LTP can be induced either by pairing an extracellularly evoked EPSP with a postsynaptic depolarization or by tetanizing another input to the postsynaptic cell. Induction of LTP in area CA3 in vitro using these techniques has been reported previously (Chattarji et al. 1989; Zalutsky & Nicoll, 1990). We have used paired recordings from monsynaptically coupled pairs of CA3 pyramidal cells in hippocampal slice cultures to ensure that only synapses formed by the recurrent axon collaterals have been studied. Potentiation of unitary EPSPs between CA3 cells has been reported earlier as the result of simultaneous tetanization of other afferent pathways using extracellular stimulation (Miles, 1988). It was not established in this study, however, whether the activation of the presynaptic neuron producing the unitary EPSP was essential for LTP induction, i.e. whether the potentiation was Hebbian.
We have demonstrated that co-activation of two CA3 pyramidal neurons leads to a long-lasting potentiation of the strength of their connections. Bursts of action potentials in the postsynaptic cell were not required for this potentiation to occur. LTP was also induced when individual postsynaptic action potentials occurred 15 ms after single presynaptic action potentials, i.e. during the rising phase of the NMDA receptor-mediated component of the unitary EPSP (Figs 2 and 4). This result is accounted for by the recent demonstration that the simultaneous occurrence of single, directly evoked postsynaptic action potentials and NMDA receptor activation results in a supralinear summation of voltage-dependent and NMDA receptor-mediated Ca2+ influx in postsynaptic dendrites (Magee & Johnston, 1997).
Surprisingly, potentiation was not induced when the pre- and postsynaptic action potentials were perfectly synchronous (0 ms delay), but rather depression. Under these conditions, the postsynaptic action potential preceded the NMDA receptor-mediated component of the unitary EPSP because of the time required for conduction in the presynaptic axon, synaptic delay, and NMDA receptor activation. These data indicate that the key parameter for LTP induction is not the temporal coincidence of the pre- and postsynaptic action potentials, but rather the coincidence of the NMDA receptor-mediated EPSP and the postsynaptic depolarization achieved by back-propagating action potentials.
The failure of the synapses formed between synchronously active cells to display potentiation suggests that the classical formulation of Hebb's postulate does not necessarily apply to pre- and postsynaptic action potential discharge. The cellular ‘learning rules’ illustrated in Fig. 4 should be taken into account when designing realistic simulations of synaptic plasticity in neuronal networks (e.g. Muller & Stead, 1996).
Similar data have been obtained recently for unitary EPSPs between layer V neocortical pyramidal cells (Markram, Lübke, Frotscher & Sakmann, 1997b). These authors have also observed that coincidence of postsynaptic action potentials and EPSPs results in potentiation, whereas EPSPs preceded by postsynaptic action potentials result in depression. The effects of pairing synchronous pre- and postsynaptic action potentials were not tested, however.
Associative LTD between CA3 pyramidal cells
Asynchronous activation of pre- and postsynaptic CA3 cells resulted in a persistent depression of their recurrent excitatory synapses if the presynaptic activity followed postsynaptic discharge within a restricted period of time. This depression was associative because the magnitude of the depression was decreased as the delay between pre- and postsynaptic activity was increased (Fig. 4). We have previously reported that Schaffer collateral synapses display an identical form of LTD and that inactive synapses are not depressed by postsynaptic activity (Debanne et al. 1994). NMDA receptor activation was necessary for the induction of associative LTD of unitary CA3-CA3 EPSPs. The association between (i) voltage-dependent Ca2+ influx, elicited by single action potentials or bursts of action potentials, and (ii) NMDA receptor-mediated Ca2+ influx thus appears to be critical in the induction of this form of LTD in area CA3, as at CA3-CA1 (Debanne et al. 1994; Debanne & Thompson, 1996) and neocortical (Markram et al. 1997b) synapses.
This form of depression may be especially important in area CA3 because the interconnections between CA3 cells result in a matrix-like organization which favours associative interactions. The functional role of such associative interactions remains to be determined experimentally in the living brain. It may be proposed, for example, that connections between spatially unrelated place cells would become depressed during spatial learning tasks because their discharge would be asynchronous (see Debanne, 1996). Induction of associative depression would thus enhance the ‘contrast’ between spatially unrelated place cells.
Homosynaptic LTD at recurrent CA3-CA3 synapses
Homosynaptic LTD is induced in area CA1 when low frequency stimuli are delivered to presynaptic fibres (Dunwiddie & Lynch, 1978) and its induction requires NMDA receptor activation (Dudek & Bear, 1992; Mulkey & Malenka, 1992). In contrast, recent studies have demonstrated that homosynaptic LTD at mossy fibre-CA3 synapses requires metabotropic glutamate, but not NMDA, receptor activation (Kobayashi et al. 1996). We provide evidence here that low frequency tetanization results in the induction of LTD of recurrent excitatory synapses in area CA3, and have shown that NMDA receptor activation is also required for its induction. Furthermore, low frequency discharge of a single cell is sufficient to produce a decrease in the strength of that cell's synapses with its postsynaptic target cells, as previously demonstrated for unitary Schaffer collateral EPSPs (Debanne et al. 1996). The number of NMDA receptors activated at unitary CA3-CA3 connections at the resting membrane potential (Fig. 1) thus permits a sufficient level of Ca2+ influx to induce LTD.
Bidirectional plasticity between individual CA3 pyramidal cells
A major function of synaptic depression may be to reverse previously established increases in synaptic strength at connections that are no longer functionally relevant. Depotentiation of previously potentiated EPSPs has been reported in area CA1 (Barrionuevo, Shottler & Lynch, 1980; Mulkey & Malenka, 1992; Debanne et al. 1994; Debanne, Gähwiler & Thompson, 1997), in the dentate gyrus (Christie et al. 1994), and at the mossy fibre-CA3 cell synapse (Kobayashi et al. 1996). Alternative potentiation and depotentiation of extracellularly stimulated commissural/associative synapses has also been reported in area CA3 (Chattarji et al. 1989). We significantly strengthen this observation with the demonstration that the synapses formed by two identified CA3 pyramidal cells can also express bidirectional changes in synaptic strength. Furthermore, depotentiation of recurrent excitatory synapses can be elicited using either homosynaptic and associative stimulation protocols.
In conclusion, LTP and LTD induction at recurrent connections between CA3 pyramidal cells require NMDA receptor activation, but not at the mossy fibre-CA3 synapse (Harris & Cotman, 1986; Zalutsky & Nicoll, 1990; Kobayashi et al. 1996). In this regard, CA3-CA3 synapses appear to be identical to CA3-CA1 synapses, not only in their basic properties (Debanne et al. 1995), but also in the mechanisms underlying the induction of synaptic plasticity.
Consequences of associative plasticity on network properties
We show here that the precise timing between pre- and postsynaptic activity determines the sign of the change in synaptic strength. Potentiation was only induced when postsynaptic action potentials occurred 15 ms after presynaptic action potentials. Depression was induced when the postsynaptic action potential occurred either 15-70 ms before, or perfectly synchronized with, the presynaptic action potential. Such differential effects on synaptic efficacy could have particularly relevant consequences in the case of bidirectional connections, which are found between one-third of the CA3 pyramidal cells in hippocampal slice cultures (Debanne et al. 1995), 28 % of layer V pyramidal neurons in acute slices of somatosensory cortex from young rats (Markram, Lübke, Frotscher, Roth & Sakmann, 1997a), and 2.3 % of layer II/III pyramidal neurons in acute slices of visual cortex (Mason, Nicoll & Stratford, 1991). If two such reciprocally connected cells exhibit perfectly synchronous activity (e.g. when activated by a common input), no reinforcement of their connection would occur. In contrast, if one of the two neurons is repeatedly firing 15-75 ms after the other, we predict the induction of asymmetrical changes in synaptic efficacy of their reciprocal connection: potentiation of the positively correlated connection and depression of the negatively correlated connection. Generalized to a large number of cells, this may result in the establishment of preferred ‘directions’ in synaptic excitation, as observed in disinhibited hippocampal slices (Traub, Jefferys & Miles, 1993).
Neuronal activity, temporal precision and synaptic efficacy
In order to account for Hebbian-like synaptic plasticity in the developing visual cortex (for review see Frégnac & Shulz, 1994), Bienenstock, Cooper & Munro (1982) have proposed that there is a modification threshold, which represents a critical level of postsynaptic activity that determines the sign of the change in synaptic efficacy (LTP or LTD) induced by a given pattern of synaptic activity. Experimental evidence for such a threshold has been provided in the visual cortex and hippocampus with the use of homosynaptic (Artola, Bröcher & Singer, 1990; Dudek & Bear, 1992) and associative protocols of synaptic plasticity (Frégnac, Shulz, Thorpe & Bienenstock, 1988; Frégnac & Shulz, 1994; Debanne et al. 1994). The modification threshold depends critically on both the amount of postsynaptic activity and its temporal relation to presynaptic activity. When single postsynaptic action potentials were used as the conditioning stimulus for induction of plasticity, the relative timing of pre- and postsynaptic activity was found to be critical. A variation of as little as 15 ms will have extremely important consequences on the sign and magnitude of the change in synaptic efficacy, as shown in Fig. 4. This finding implies that single action potential discharge in pyramidal cells will permit greater temporal accuracy in the discrimination and stabilization of neuronal inputs than will bursts of action potentials (see also Gerstner, Kempter, van Hemmen & Wagner, 1996).
Acknowledgments
We thank L. Heeb, L. Rietschin, H. Kasper, R. Kägi and R. Schöb for excellent technical assistance, and Dr J.-C. Poncer for helpful discussions and for critically reading the manuscript. This study was supported by the Fyssen and Dr Eric Slack-Gyr Foundations, and the Swiss National Science Foundation (grant 31-42174.94).
References
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Brown TH. Associative long-term potentiation in hippocampal slices. Proceedings of the National Academy of Sciences of the USA. 1983;80:7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo G, Shottler F, Lynch G. The effects of repetitive low frequency stimulation on control and ‘potentiated’ synaptic responses in the hippocampus. Life Sciences. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Battistin T, Cherubini E. Developmental shift from long-term depression to long-term potentiation at the mossy fibre synapses in the rat hippocampus. European Journal of Neuroscience. 1994;6:1750–1755. doi: 10.1111/j.1460-9568.1994.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Bienenstock E, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. Journal of Neuroscience. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, Kairiss EW, Keenan CL. Hebbian synapses: biophysical mechanisms and algorithms. Annual Review of Neuroscience. 1990;13:475–511. doi: 10.1146/annurev.ne.13.030190.002355. [DOI] [PubMed] [Google Scholar]
- Chattarji S, Stanton PK, Sejnowski TJ. Commissural synapses, but not mossy fiber synapses, in hippocampal field CA3 exhibit associative long-term potentiation and depression. Brain Research. 1989;495:145–150. doi: 10.1016/0006-8993(89)91228-6. [DOI] [PubMed] [Google Scholar]
- Christie BR, Kerr DS, Abraham WC. Flip side of synaptic plasticity: long-term depression mechanisms in the hippocampus. Hippocampus. 1994;4:127–135. doi: 10.1002/hipo.450040203. [DOI] [PubMed] [Google Scholar]
- Debanne D. Associative synaptic plasticity in hippocampus and visual cortex: cellular mechanisms and functional implications. Reviews in the Neurosciences. 1996;7:29–46. doi: 10.1515/revneuro.1996.7.1.29. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proceedings of the National Academy of Sciences of the USA. 1994;91:1148–1152. doi: 10.1073/pnas.91.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Cooperative interactions in the induction of long-term potentiation and depression of synaptic excitation between hippocampal CA3-CA1 cell-pairs in vitro. Proceedings of the National Academy of Sciences of the USA. 1996;93:11225–11230. doi: 10.1073/pnas.93.20.11225. 10.1073/pnas.93.20.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Bidirectional associative plasticity of unitary CA3-CA1 EPSPs in the rat hippocampus in vitro. Journal of Neurophysiology. 1997;77:2851–2855. doi: 10.1152/jn.1997.77.5.2851. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Physiology and pharmacology of unitary synaptic connections between pairs of cells in areas CA3 and CA1 of rat hippocampal slice cultures. Journal of Neurophysiology. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- Debanne D, Thompson SM. Associative long-term depression in the hippocampus in vitro. Hippocampus. 1996;6:9–16. doi: 10.1002/(SICI)1098-1063(1996)6:1<9::AID-HIPO3>3.0.CO;2-M. 10.1002/(SICI)1098-1063(1996)6:1<9::AID-HIPO3>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Martinez JL. Associative, bidirectional modifications at the hippocampal mossy fibre-CA3 synapse. Nature. 1996;381:429–434. doi: 10.1038/381429a0. 10.1038/381429a0. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proceedings of the National Academy of Sciences of the USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T, Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. The Journal of Physiology. 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y, Shulz D. Models of synaptic plasticity and cellular analogs of learning in the developing and adult vertebrate visual cortex. In: Casagrande V, Shinkman P, editors. Advances in Neural and Behavioral Development. Vol. 4. Norwood, NJ, USA: Neural Ablex Publ.; 1994. pp. 97–109. [Google Scholar]
- Frégnac Y, Shulz D, Thorpe S, Bienenstock E. A cellular analogue of visual cortical plasticity. Nature. 1988;333:367–370. doi: 10.1038/333367a0. 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic monolayer cultures of nervous tissue. Journal of Neuroscience Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Thompson SM, Audinat E, Robertson RT. Organotypic slice cultures of neural tissue. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, MA, USA: MIT Press; 1991. pp. 397–411. [Google Scholar]
- Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383:76–78. doi: 10.1038/383076a0. 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neuroscience Letters. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Jaffe D, Johnston D. Induction of long-term potentiation at hippocampal mossy-fiber synapses follows a Hebbian rule. Journal of Neurophysiology. 1990;64:948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Experimental Brain Research. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987;10:408–415. 10.1016/0166-2236(87)90011-7. [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick-tufted pyramidal neurones in the developing rat neocortex. The Journal of Physiology. 1997a;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997b;275:213–215. doi: 10.1126/science.275.5297.213. 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philosophical Transactions of the Royal Society. 1971;B 262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. Journal of Neuroscience. 1991;11:72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R. Plasticity of recurrent excitatory synapses between CA3 hippocampal pyramidal cells. Society for Neuroscience Abstracts. 1988;18:19. [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. 10.1016/0896-6273(92)90248-C. [DOI] [PubMed] [Google Scholar]
- Muller RU, Stead M. Hippocampal place cells connected by Hebbian synapses can solve spatial problems. Hippocampus. 1996;6:709–719. doi: 10.1002/(SICI)1098-1063(1996)6:6<709::AID-HIPO13>3.0.CO;2-4. 10.1002/(SICI)1098-1063(1996)6:6<709::AID-HIPO13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JGR, Miles R. Analysis of the propagation of disinhibition-induced after-discharges along the guinea-pig hippocampal slice in vitro. The Journal of Physiology. 1993;472:267–287. doi: 10.1113/jphysiol.1993.sp019946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Urban NN, Barrionuevo G. Induction of Hebbian and non-Hebbian mossy fiber long-term potentiation by distinct patterns of high-frequency stimulation. Journal of Neuroscience. 1996;16:4293–4299. doi: 10.1523/JNEUROSCI.16-13-04293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willshaw D, Dayan P. Optimal plasticity from matrix memories: what goes up must come down. Neural Computation. 1990;2:85–93. [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]


