Abstract
Aldosterone- and adrenaline-induced K+ secretion were investigated in rat late distal colon using conductance scanning and Ussing chamber techniques. K+ secretion was unmasked by the K+ channel blocker tetraethylammonium (TEA). Electrogenic Na+ absorption was inhibited by amiloride. Rb+ net fluxes consistently measured about 80 % of K+ secretion estimated using change in short-circuit current (ΔISC) measurements.
Partial block of K+ absorption by mucosal ouabain did not change TEA-sensitive K+ secretion. Thus, K+ absorption and K+ secretion are not coupled.
Additivity of Rb+ fluxes as well as ΔISC caused by 3 nM aldosterone (6 h in vitro incubation) and, subsequently, adrenaline suggested additivity of aldosterone-induced and cAMP-mediated K+ secretion in the presence of amiloride.
Conductance scanning under control conditions revealed a small TEA-sensitive K+ conductivity in surface epithelium (0.3 ± 0.2 mS cm−2) but not in crypts, as well as a small basal K+ secretion in surface epithelium (ΔISC = 0.3 μmol h−1 cm−2), which increased during sham incubation.
Aldosterone (3 nM, 6 h in vitro incubation) resulted, after correction for the basal K+ secretion, in a K+ secretion of ΔISC = 0.9 μmol h−1 cm−2. Aldosterone induced a TEA-sensitive conductivity of 1.1 ± 0.3 mS cm−2 in surface epithelium, but not in crypts.
Adrenaline (5 μm) caused, in fresh tissue, a K+ secretion of ΔISC = 1.2 μmol h−1 cm−2 and equal conductivity changes in crypts (0.7 ± 0.2 mS cm−2) and surface epithelium (0.7 ± 0.1 mS cm−2).
We conclude that K+ secretion induced by aldosterone in physiological concentration is restricted to surface epithelium, whereas cAMP-mediated K+ secretion is located equally in crypts and surface epithelium.
Although renal potassium transport provides the basis for K+ homeostasis, the distal large intestine contributes to fine tuning of the K+ content of the body. In order to meet this function, the distal colon shows both active K+ absorption and active K+ secretion (Hayslett & Binder, 1982; Binder & Sandle, 1994). The latter can be stimulated by aldosterone as well as by 3′,5′-cyclic monophosphate (cAMP) (Foster, Sandle, Hayslett & Binder, 1983; Foster, Hayslett & Binder, 1984; McCabe, Smith & Sullivan, 1984; Smith & McCabe, 1984). Localization of K+ secretion in rat late distal colon is of interest, because functional differences between the epithelium of the flat surface of the colon mucosa and that of the tubular crypts extending to the muscularis mucosae have been demonstrated.
Sodium absorption induced by physiological (i.e. nanomolar) aldosterone concentration is confined to surface epithelium (Köckerling, Sorgenfrei & Fromm, 1993), but cAMP-induced Cl− secretion occurs not only in the crypts but also in the surface epithelium (Köckerling & Fromm, 1993). There is a controversy, however, about the location of active K+ secretion in the colon. For a long time, it has been attributed to Cl−-secretory crypt cells (Welsh, Smith, Fromm & Frizzell, 1982). This point of view could be maintained for aldosterone-induced K+ secretion, because short-circuit current recordings in mammalian colon show K+ secretion independent of Na+ absorption (Foster et al. 1983, 1984; McCabe et al. 1984; Halm & Frizzell, 1986; Rechkemmer & Halm, 1989), whereas in turtle colon K+ secretion is coupled to Na+ absorption (Halm & Dawson, 1984). An elaborate micropuncture study in rat distal colon challenged this view, finding aldosterone-induced K+ secretion in 82 % of surface cells, but only in 50 % of accessible crypt cells (Lomax & Sandle, 1994; Lomax, McNicholas, Lombès & Sandle, 1994). Micropuncture studies, however, lacking access to deeper crypt cells, do not allow a quantification of the contribution of the whole crypt. The location of cAMP-induced K+ secretion is still obscure. The finding that adrenaline and aldosterone non-additively induce the same amount of K+ secretion in guinea-pig colon suggests that both secretagogues may act on the same transport pathway (Rechkemmer & Halm, 1989), but the site of action has not been determined.
In the present study, aldosterone- and adrenaline-induced K+ secretion in rat late distal colon were assessed in both crypt and surface epithelium by a new method, an improvement of the voltage scanning technique (Frömter, 1972). It is based on the analysis of local differences in current density, which are recorded in the supra-epithelial bath solution with a stepping and scanning glass electrode during application of a transepithelial clamp current (Köckerling & Fromm, 1993; Köckerling et al. 1993). Since the locally differentiated conductivity of an epithelium is evaluated, the method is called conductance scanning. In combination with conventional measurements of short-circuit current and ion fluxes, conductance scanning allowed for localization of transepithelial K+ transport: aldosterone-induced K+ secretion is, like Na+ absorption, confined to surface epithelium, whereas adrenaline-induced K+ secretion can be found in both surface epithelium and crypts.
METHODS
Tissue preparation
Late distal colon was obtained from male albino Wistar rats (250–290 g) which had free access to normal rat laboratory diet (Altromin 1320; Altromin, Lage, Germany) and tap water. The animals were killed by 10 min inhalation of a saturated atmosphere of diethylether and, in the interest of absolute safety, subsequent opening of the heart. The keeping and the killing of the animals were in agreement with the guidelines of the Society of Laboratory Animal Science (GV-SOLAS). The colon was removed, rinsed with Ringer solution and ‘totally’ stripped of serosa and muscle layers, as described previously (Schulzke, Fromm & Hegel, 1986; Fromm, Schulzke & Hegel, 1993).
Solutions and drugs
The standard bathing solution contained (mm): Na+, 140; Cl−, 123.8; K+, 5.4; Ca2+, 1.2; Mg2+, 1.2; HPO42−, 2.4; H2PO4−, 0.6; HCO3−, 21; D(+)-glucose, 10; D(+)-mannose, 10; glutamine, 2.5; β-hydroxybutyrate, 0.5. For flux measurements, the solutions contained 5.4 mm Rb+ instead of K+. The solutions were oxygenated with 95 % O2 and 5 % CO2. The pH was 7.4 at 37°C, which was the temperature in the perfusion chamber during all experiments. Cyclic AMP-dependent K+ secretion was stimulated by 5 × 10−6 M adrenaline (serosal and mucosal side). Mineralocorticoid-dependent K+ secretion was stimulated by 6 h incubation with 3 × 10−9 M aldosterone. The tissue was incubated either in the Ussing chamber for flux measurements, or in a water bath prior to usage in the scanning chamber. Apical K+ conductances were blocked by 2 × 10−2 M TEA, osmotically balanced by 4 × 10−2 M mannitol on the serosal side. In order to show that the small Cl− gradient present during TEA block did not change short-circuit current or transepithelial voltage, in a control experiment, basal K+ secretion was blocked utilizing the standard procedure (TEA-mannitol). Then, the solutions on both sides of the tissue were exchanged, filling 20 mm TEA-Cl to the mucosal side again and 20 mm choline chloride to the serosal side, thereby eliminating the Cl− gradient. This exchange step was repeated several times, switching between mannitol and choline chloride on the serosal side. No significant changes in short-circuit current or transepithelial voltage were observed.
In order to enhance the resolution of the conductance scanning method, the ‘background conductance’ of apical Na+ channels was blocked by 10−4 M amiloride in the scanning experiments and, for the sake of comparability, also in the Ussing experiments. In some experiments, apical K+-H+-ATPase was blocked by 10−3 M ouabain. All blockers were added to the mucosal compartment only.
Flux measurements
Potassium transport was characterized by bi-directional flux measurements with 86Rb+, assuming identical pathways of these ions. These measurements were performed in standard Ussing-type chambers (Fromm et al. 1993). Each flux period had a duration of 15 min. After addition of an effector, we waited at least two periods before a second effector was added. The first period after addition of an effector was discarded. For calculation of net fluxes, tissues were paired for resistance. Tissues showing a resistance of less than 120 Ω cm−2 30 min after mounting were discarded. When pairing tissues for flux measurements, their resistances differed by 13–23 %.
Conductance scanning
A 1 cm2 piece of late distal colon supported by fine nylon mesh (in order to prevent vertical movement of the epithelium) was mounted horizontally (mucosal-side up) in an Ussing-type chamber, which allowed simultaneous measurements of transepithelial electrical parameters by the conventional 4-electrode technique and of local conductances by a scanning microelectrode. The exposed tissue area was 0.3 cm2. The scanning chamber was attached to the stage of an upright light microscope (Zeiss Axioplan) and continuously perfused at 37°C. Total tissue conductivity (Gt) was measured with the help of direct current (DC) pulses of ± 70 μA cm−2. Short-circuit current (ISC) was calculated from Gt (corrected for bath resistance) and from transepithelial voltage (Ve). The method of conductance scanning was used as described previously (Köckerling et al. 1993). In brief, a 30 Hz alternating current (100–200 μA cm−2) passing through the tissue allowed determination of local conductances. As current flows through two conductors in parallel (i.e. crypts and surface epithelium) according to their respective conductances, a typical pattern of voltage deflections is induced above the tissue surface. A glass microelectrode was placed either 400 μm above the surface (for determination of total epithelial conductivity) or directly above the surface epithelium (for determination of surface conductivity). From this start position, the electrode was moved 30 μm up and down by a piezoelectric driver at a frequency of 0.7 Hz. A stationary potential reference was provided by a second microelectrode placed 100 μm above the tissue. Signals collected at forward and backward positions of the stepping electrode were locked into the external 30 Hz AC and then subtracted from each other in synchrony with the 0.7 Hz frequency of the piezo driver. With this synchronous demodulation procedure, noise levels were effectively reduced and a resolution of 0.5 μV was obtained.
First, scanning signals were sensed under control conditions. Then, after stimulation by secretagogues and again after block of K+ channels by TEA, scanning signals were measured in the same sites. Calculation of total epithelial conductivity (Gt) and the conductivity of surface epithelium (Gs) from the scanning signals (ΔV/Δx) was performed with the equation:
| (1) |
where Δx is the excursion of the scanning electrode (30 μm), ΔV is the voltage drop along Δx, ρ is the specific resistivity of the bathing solution, and Ve is the transepithelial voltage, corrected for the contribution produced by the voltage drop across the saline and the mesh supporting the tissue. All conductivities are referred to the gross tissue area. Since crypts and surface epithelium are conductors in parallel, crypt conductivity (Gc) is the difference between total and surface conductivity, Gt - Gs. In some experiments crypt conductivity, Gc, was also calculated directly from crypt scanning signals, measured in the centre of a crypt opening, using a mathematical model of supraepithelial current distribution, as described by Köckerling et al. (1993). No significant difference between Gc values derived by the two methods was observed under any experimental condition.
Statistical analysis
Results are given as means ± s.e.m. Student's unpaired t test was used to determine the significance of differences. P < 0.05 was considered significant. If more than two successive values were obtained from one tissue, P values were subjected to the Bonferroni correction.
RESULTS
The conductance scanning technique allows the detection of conductivity changes during an experiment. Applying the K+ channel blocker TEA to the tissue leads to changes in conductivity and in short-circuit current which are indicative of K+ secretion. In order to link TEA-sensitive short-circuit currents and local conductivity changes to K+ secretion, 86Rb+ flux measurements were performed. Mediation of ouabain-sensitive K+ absorption was studied to ensure that observed changes in conductivity can be attributed to changes in K+ secretion without disturbing influences of K+ absorption on ISC and conductivity. As the flux experiments showed that K+ secretion can be monitored by recording the TEA-dependent ISC, conductance scanning experiments could be performed in order to localize the TEA-sensitive K+ conductances, and thus the sites of K+ secretion stimulated by aldosterone and cAMP were determined. In order to determine basal (unstimulated) K+ secretion in Ussing chamber experiments and with conductance scanning, two sets of control experiments were performed: one (‘fresh controls’) for a short incubation time (45 min), as used for examination of cAMP-mediated K+ secretion, and a second one for a long incubation time (6 h), which corresponds to the duration of preincubation in aldosterone experiments.
Rb+ fluxes and short-circuit current
Basal K+ secretion
Unstimulated K+ secretion was studied in epithelia after 6 h sham incubation (Table 1). In the control condition, the net Rb+ flux of +1.0 μmol h−1 cm−2 indicated a net K+ absorptive state of the late distal colon. After mucosal addition of 10−4 M amiloride, a small drop in ISC could be observed (-0.4 ± 0.1 μmol h−1 cm−2) without any significant change in net Rb+ flux (JRb,net). Therefore, the change in ISC represents spontaneous electrogenic Na+ absorption. Half an hour later, 2 × 10−2 M TEA was added to the mucosal side. The potassium channel blocker caused a rise in ISC of +0.8 ± 0.1 μmol h−1 cm−2, and in JRb,net of +0.6 ± 0.1 μmol h−1 cm−2, the difference between the flux with amiloride present and the flux with amiloride and TEA present, representing inhibition of a basal K+ secretory process. As the change in ISC correlated well with the change in Rb+ flux, changes in conductivity caused by TEA are attributable to K+ secretion.
Table 1.
Short-circuit current and Rb+ fluxes; K+ secretion not stimulated
| ISC | JRb,sm | JRb,ms | JRb,net | ΔJRb,net/ΔISC | |
|---|---|---|---|---|---|
| Control | 0.62 ± 0.12 | −0.46 ± 0.07 | 1.36 ± 0.17 | 0.98 ± 0.14 | — |
| +Amiloride | 0.20 ± 0.06**† | −0.56 ± 0.06 n.s. | 1.58 ± 0.15 n.s. | 1.09 ± 0.12 n.s. | — |
| +TEA | 1.05 ± 0.06***† | −0.21 ± 0.06**† | 1.79 ± 0.14n.s. | 1.65 ± 0.12**† | 0.66 ± 0.07 |
| +Ouabain | 1.22 ± 0.07n.s. | −0.24 ± 0.07n.s. | 0.50 ± 0.05***† | 0.27 ± 0.09***† | — |
ISC, short-circuit current (μmol h−1 cm−2, n = 22). JRb,sm, Rb+ flux in serosa to mucosa direction; JRb,ms, Rb+ flux in mucosa to serosa direction; JRb,net, Rb+ net flux (all fluxes in μmol h−1 cm−2, n = 11). All drugs were added successively to the same tissue with all drugs present at the final step. ΔJRb,net and ΔISC, changes in JRb,net and ISC in comparison with preceding condition. Significances always refer to the preceding condition; *P < 0.05
P < 0.01
P < 0.001; n.s., not significantly different
value subjected to Bonferroni correction, if significant. Values are given as means ± s.e.m.
Blocking the apical K+-H+-ATPase with 10−3 M ouabain did not cause any significant change in ISC, but caused a decrease in JRb,net of -1.4 ± 0.1 μmol h−1 cm−2. This means that changes in K+ absorption do not affect ISC, and therefore do not disturb interpretation of the scanning data. The serosal to mucosal Rb+ flux (JRb,sm) remained unchanged after addition of ouabain, which is not surprising in the presence of TEA. JRb,net (0.3 μmol h−1 cm−2) was still different to ISC (1.2 μmol h−1 cm−2), which suggests some additional anion secretion.
Adrenaline-induced K+ secretion
Cyclic AMP-induced K+ secretion was investigated by recording changes in ISC and JRb,net after addition of adrenaline (Table 2). Sodium absorption was blocked by amiloride. Mucosal ouabain (10−3 M) was added after 6 h of sham incubation. As expected, because ouabain does not change electrogenic K+ secretion but electroneutral K+ absorption, this did not result in any significant change in ISC, but did result in a drop in JRb,net of -1.1 μmol h−1 cm−2. Half an hour later, 5 × 10−6 M adrenaline was added to both sides of the epithelium. This resulted in a decrease in ISC of -0.6 μmol h−1 cm−2 and in JRb,net of -0.5 μmol h−1 cm−2. Subsequent addition of TEA to the mucosal side revealed an increase in ISC of 1.5 μmol h−1 cm−2 and in JRb,net of +1.2 μmol h−1 cm−2. Again, the effectors adrenaline and TEA caused corresponding changes in ISC and JRb,net, whereas ouabain only influenced JRb,net.
Table 2.
Short-circuit current and Rb+ fluxes; adrenaline-induced K+ secretion
| ISC | JRb,sm | JRb,ms | JRb,net | ΔJRb,net/ΔISC | |
|---|---|---|---|---|---|
| Amiloride | 0.03 ± 0.08 | −0.67 ± 0.09 | 1.24 ± 0.10 | 0.57 ± 0.12 | — |
| +Ouabain | 0.14 ± 0.08n.s. | −0.91 ± 0.09n.s. | 0.42 ± 0.03***† | −0.49 ± 0.09***† | — |
| +Adrenaline | −0.42 ± 0.07***† | −1.32 ± 0.08**† | 0.32 ± 0.02**† | −1.00 ± 0.09*** | 0.89 ± 0.15 |
| +TEA | 1.15 ± 0.07***† | −0.18 ± 0.02***† | 0.41 ± 0.02** | 0.23 ± 0.03***† | 0.79 ± 0.07 |
ISC, n = 18. JRb, n = 9. See Table 1 for further descriptions.
Then, the ISC measurements were repeated without previous addition of ouabain (n = 9). After 6 h of sham incubation, amiloride was added. The changes in ISC caused by adrenaline (-0.6 ± 0.1 μmol h−1 cm−2) and subsequent addition of TEA (+1.5 ± 0.1 μmol h−1 cm−2) did not differ significantly from the results presented above. Hence, it is possible to state that changes in K+ absorption, do not have any electrical influence on K+ secretion, i.e. the processes are not coupled. After 6 h of sham incubation, the effect of TEA was more than twice as great as that of adrenaline. The difference (ΔISC = 1.0 ± 0.1 μmol h−1 cm−2; JRb,net = 0.7 ± 0.2 μmol h−1 cm−2) corresponds to the basal K+ secretion described before.
In order to demonstrate a time course of the effect of adrenaline, the ISC measurement was repeated with a short time of sham incubation (45 min). In contrast to the results obtained above, addition of adrenaline after amiloride yielded a change in ISC of -1.7 ± 0.2 μmol h−1 cm−2 (n = 12). This shows that the K+ secretion elicited by addition of adrenaline decreases with the incubation time. On the other hand, the basal K+ secretion found at the beginning of the incubation time (ΔISC = 0.6 ± 0.1 μmol h−1 cm−2) was smaller than that found after 5–6 h sham incubation. This means that adrenaline-induced K+ secretion diminishes with the increase of basal K+ secretion, suggesting the same site of action.
Aldosterone- plus adrenaline-induced K+ secretion
In connection with aldosterone-induced K+ secretion we examined (i) the interdependence of changes in ISC and JRb,net, and (ii) the additivity of aldosterone- and adrenaline-induced K+ secretion. In this set of experiments (Table 3), 3 × 10−9 M aldosterone was added after mounting the colon and, 6 h later, Na+ absorption was blocked by amiloride. This resulted in a decrease in ISC of -8.7 ± 0.7 μmol h−1 cm−2 and a change in JRb,net of +1.0 ± 0.2 μmol h−1 cm−2. This change in K+ secretion after addition of amiloride was not observed in control experiments (Table 1) and, therefore, may represent a specific aldosterone effect.
Table 3.
Short-circuit current and Rb+ fluxes; aldosterone- and adrenaline-induced K+ secretion
| ISC | JRb,sm | JRb,ms | JRb,net | ΔJRb,net/ΔISC | |
|---|---|---|---|---|---|
| Aldosterone | 8.46 ± 0.68 | −1.36 ± 0.19 | 1.28 ± 0.10 | −0.07 ± 0.20 | — |
| +Amiloride | −0.18 ± 0.06***† | −0.75 ± 0.06*† | 1.65 ± 0.11*† | 0.89 ± 0.06**† | — |
| +Adrenaline | −0.79 ± 0.12*** | −1.00 ± 0.08* | 1.46 ± 0.12n.s. | 0.45 ± 0.12** | 0.71 ± 0.18 |
| +TEA | 1.25 ± 0.07***† | 0.05 ± 0.03***† | 2.14 ± 0.17*† | 2.09 ± 0.16**† | 0.83 ± 0.09 |
ISC, (n = 18). JRb, (n = 9). See Table 1 for further descriptions.
Subsequent addition of 5 × 10−6 M adrenaline yielded a decrease in ISC of -0.6 ± 0.1 μmol h−1 cm−2 and in JRb,net of -0.4 ± 0.1 μmol h−1 cm−2. These changes were similar to those observed with adrenaline alone after 6 h sham incubation (Table 2), leading to the conclusion that, under these experimental conditions (i.e. 6 h incubation time, in the presence of amiloride), aldosterone- and adrenaline-induced K+ secretion are fully additive.
TEA block leads to changes in ISC of 2.0 ± 0.1 μmol h−1 cm−2 and in JRb,net of 1.6 ± 0.2 μmol h−1 cm−2 (Table 3). As these values show the combined K+ secretion triggered by aldosterone and adrenaline, the adrenaline-induced K+ secretion (ΔISC = 0.6 ± 0.1 μmol h−1 cm−2; ΔJRb,net = 0.4 ± 0.1 μmol h−1 cm−2; Table 3) has to be subtracted from the TEA-induced changes, yielding changes in ISC of 1.4 ± 0.1 μmol h−1 cm−2 and in JRb,net of 1.2 ± 0.2 μmol h−1 cm−2 (Table 3). This value has to be corrected for basal K+ secretion found after 6 h sham incubation (ΔISC = 0.8 ± 0.1 μmol h−1 cm−2; ΔJRb,net = 0.6 ± 0.1 μmol h−1cm−2; Table 1), leaving ΔISC = 0.6 ± 0.3 μmol h−1 cm−2 and ΔJRb,net = 0.6 ± 0.3 μmol h−1 cm−2 for K+ secretion exclusively induced by aldosterone in this experiment.
In order to calculate the complete aldosterone-induced K+ secretion, that component of aldosterone-induced K+ secretion already inhibited by addition of amiloride has to be added to the value obtained by TEA block corrected for adrenaline-induced and basal K+ secretion. This produces an estimated aldosterone-induced K+ secretion of 1.6 μmol h−1 cm−2. It has to be emphasized that a partial block of aldosterone-induced K+ secretion by amiloride is irrelevant to the detection of the location of this type of K+ secretion by conductance scanning, because amiloride influences the driving force for K+ secretion rather than blocking K+ conductances per se.
Conductance scanning
Adrenaline-induced K+ secretion
Prior to the investigation of adrenaline-induced K+ secretion, it is important to know the changes in conductivities taking place in rat distal colon without any further stimulation. Basal K+ secretion was determined in unstimulated tissue 45 min after mounting the epithelium (Fig. 1; n = 10). Electrogenic Na+ absorption was blocked by mucosal 10−4 M amiloride. This led to a decrease in surface conductivity (Gs) of -36 ± 7 % and no change in crypt conductivity (ΔGc = -7 ± 12 %; n.s.). This is in good accordance with the fact that electrogenic Na+ absorption takes place in surface epithelium and not in crypts. Subsequent addition of TEA yielded a decrease in Gs of -31 ± 11 % (P = 0.058) and no significant change in Gc (ΔGc = +7 ± 9 %; n.s.). Thus, the epithelia showed a small basal K+ current which may be localized in surface epithelium rather than in crypts. The corresponding rise in short-circuit current (ΔISC) after TEA block measured +0.3 ± 0.1 μmol h−1 cm−2, which was somewhat lower than in the Ussing chamber measurements.
Figure 1. Basal K+ secretion and conductivity in rat late distal colon.

Short-circuit current (ISC) and the conductivity of surface epithelium (Gs) and crypts (Gc), and the total conductivity (Gt) were determined by conductance scanning. Amiloride (100 μm) and TEA (20 mm), added successively and additively to the mucosal side, unmasked Na+ absorption and K+ secretion and the conductivities of surface epithelium and crypts. A small TEA-sensitive ISC was found. Results are means ± s.e.m. of n = 10 tissues. n.s., not significantly different; **P < 0.01; ***P < 0.001 (compared with the preceding condition); †, value subjected to Bonferroni correction, if significant.
For the localization of cAMP-mediated K+ secretion, 5 × 10−6 M adrenaline was administered 45 min after mounting the epithelium (Fig. 2; n = 13). This increased Gs by +33 ± 4 % (P < 0.01) and Gc by +37 ± 5 % (P < 0.01). The corresponding ΔISC was -1.1 ± 0.1 μmol h−1 cm−2. Application of TEA (2 × 10−2 M) decreased Gs by -36 ± 5 % (P < 0.01) and Gc by -22 ± 4 % (P < 0.05), accompanied by a ΔISC of +1.2 ± 0.1 μmol h−1 cm−2. Thus the effects of adrenaline and TEA are very similar in size.
Figure 2. Adrenaline-induced K+ secretion and conductivity.
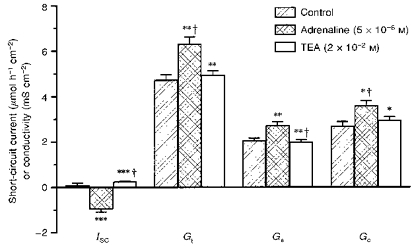
Effects of adrenaline (5 μm) and mucosal TEA (added subsequently) on ISC and tissue conductivities (G) (n = 10). Changes in ISC and conductivities triggered by adrenaline or TEA can be equally attributed to surface epithelium and crypts. n.s., not significantly different; *P < 0.05; **P < 0.01; ***P < 0.001 (compared with the preceding condition); †, value subjected to Bonferroni correction, if significant.
The change of conductivity found in surface epithelium after TEA block may be corrected for basal K+ secretion (Fig. 1), which accounted for about 25–30 % of the TEA effect in this time range. A decrease in Gs of about 25 % remained that was exclusively attributed to the adrenaline effect. This means that adrenaline (i) induced K+ secretion and (ii) increased the TEA-sensitive K+ conductivity in both crypts and surface epithelium. These findings suggest strongly that cAMP-mediated K+ secretion is located in both crypts and surface epithelium.
Aldosterone-induced K+ secretion
For localization of aldosterone-induced K+ secretion, stripped colon was pre-incubated in a water bath with 3 × 10−9 M aldosterone for 6 h (Fig. 3; n = 10). Amiloride revealed electrogenic Na+ absorption by a ΔISC of -7.8 ± 1.0 μmol h−1 cm−2 and decreases in Gs of -42 ± 4 % (P < 0.01) and Gc of -10 ± 3 % (n.s.). Thus, at nanomolar aldosterone concentration, Na+ absorption was found predominantly in surface epithelium. Mucosal addition of TEA yielded a decrease in Gs of -46 ± 7 % (P < 0.01) and no change in Gc (-4 ± 4 %; n.s.), accompanied by a ΔISC of +1.5 ± 0.2 μmol h−1 cm−2. Absence of TEA-sensitive conductances in crypts and opening of TEA-sensitive conductances in surface epithelium indicated that aldosterone-induced K+ secretion was confined to surface epithelium.
Figure 3. Aldosterone-induced K+ secretion and conductivity (after 6 h).
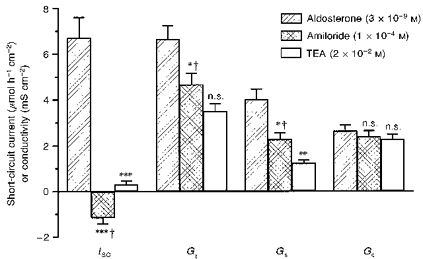
Effects of mucosal amiloride and TEA (added subsequently) after 6 h incubation with aldosterone (3 nM), on ISC and tissue conductivities (G) (n = 10). Blocking of TEA-sensitive ISC was accompanied by a drop in Gs, indicating that the aldosterone-induced conductivity was restricted to surface epithelium. n.s., not significantly different; *P < 0.05; **P < 0.01; ***P < 0.001 (compared with the preceding condition); †, value subjected to Bonferroni correction, if significant.
From Ussing chamber experiments (Table 1) it was known that basal K+ secretion after 6 h sham incubation can be expected to be much higher than found in control experiments performed with freshly mounted epithelium with the conductance scanning technique (Fig. 1). Therefore, another set of control experiments was done, in order to determine basal K+ secretion in the absence of aldosterone after 6 h of sham incubation (Fig. 4; n = 7), i.e. with the same incubation time as used in aldosterone experiments. Addition of TEA yielded no significant changes in any of the measured parameters (ISC, Gt, Gc) except Gs, which decreased by -29 ± 5 %. This drop in surface conductivity is not statistically different from that found after TEA block with aldosterone-incubated tissue.
Figure 4. Basal K+ secretion and conductivity after 6 h.
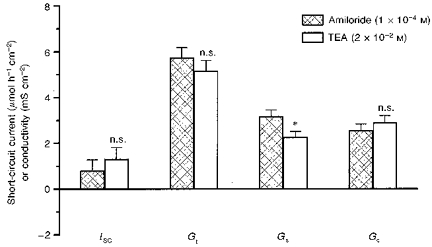
Mucosal TEA following amiloride after 6 h sham incubation revealed a basal TEA-sensitive conductivity in the surface epithelium (Gs; n = 7). *P < 0.05; n.s., not significantly different from amiloride group.
Thus, after incubation with aldosterone, an increase in a TEA-blockable K+ conductivity together with K+ secretion (represented by a ΔISC) was found, whereas after sham incubation only an increase in K+ selective conductivity was observed, without a similar rise in ISC. This means that the effect of aldosterone cannot be explained by an increase in K+ selective conductivity (i.e. an opening of K+ channels) alone, because this was shown not to be sufficient for K+ secretion (Fig. 4). This result probably represents the rise in driving force by activation of the Na+-K+-ATPase.
DISCUSSION
Potassium absorption in rat distal colon is electroneutral, sodium independent (Foster et al. 1984; Sweiry & Binder, 1990), at least in part, chloride independent (Foster, Sandle, Hayslett & Binder, 1986), and independent of the basolateral Na+-K+-ATPase (Sweiry et al. 1990; Del Castillo, Rajendran & Binder, 1991). It is driven by H+-K+-ATPase(s) in the apical membrane of the enterocyte, showing some characteristics of both Na+-K+-ATPase and parietal cell H+-K+-ATPase (for a recent review see Binder & Sandle, 1994). As demonstrated with Rb+ fluxes, this H+-K+-ATPase can be blocked by mucosal ouabain (Pandiyan, Rajendran & Binder, 1992). In the present study only two-thirds of the Rb+ flux in the mucosa to serosa direction (JRb,ms) was inhibited by mucosal ouabain, which may be explained by the presence of a second, ouabain-insensitive H+-K+-ATPase (Abrahamse, de Jonge, Bimdels & van Os, 1995; Lee, Rajendran, Mann, Kashgarian & Binder, 1995). In a very recent paper, the ouabain-insensitive H+-K+-ATPase could be allocated to the surface epithelium, where its expression could be upregulated by dietary Na+ depletion but not by dietary K+ depletion (Sangan, Rajendran, Mann, Kashgarian & Binder, 1997).
Electrical and flux measurements in the present study confirm the electroneutral character of K+ absorption and its independence from K+ secretion. Therefore, changes in K+ absorption do not interfere with measurements of short-circuit current (ISC) or conductance changes that are sensitive to mucosal application of tetraethylammonium (TEA).
Potassium secretion is initiated by basolateral uptake via Na+-K+-2Cl− cotransport, which is driven by Na+-K+-ATPase. Apical K+ efflux requires a K+ conductance, which can be induced or activated by aldosterone and intracellular cAMP, but is blocked by TEA (Foster et al. 1983, 1984; Halm & Dawson, 1984; Binder, McGlone & Sandle, 1989; Rajendran, Kashgarian & Binder, 1989; Rechkemmer & Halm, 1989; Sweiry & Binder, 1989). Chloride has to recycle across the basolateral membrane. This process may be electrogenic (Halm & Frizzell, 1986), so that it contributes to the current measured during K+ secretion. This appears to be a possible explanation for the result of this and of other studies that adrenaline- or TEA-induced K+-related ISC changes are up to 30 % larger than the corresponding changes in Rb+ fluxes.
Aldosterone-induced K+ secretion
The first study to demonstrate intestinal K+ secretion after in vitro incubation with aldosterone in mammalian epithelium (guinea-pig colon) was that of Rechkemmer & Halm (1989), who regularly applied a concentration of 1 μm aldosterone. A concentration of 3 nM aldosterone, similar to that observed in vivo after stimulation is, however, sufficient to induce K+ secretion (Fromm et al. 1993) as well as Na+ absorption (Fromm, Oelkers & Hegel, 1983).
In our study, the observed aldosterone-induced K+ secretion divides into two separate components: one part linked to Na+ absorption and, therefore, inhibitable by amiloride, and a second part revealed by TEA block and, therefore, accessible by the conductance scanning method.
Aldosterone-induced electrogenic K+ secretion in rat distal colon has been shown to be independent of Na+ absorption, since inhibition of Na+ absorption by mucosal amiloride did not change unidirectional K+ fluxes in adrenalectomized rats (Foster et al. 1984). However, under certain circumstances (e.g. following inhibition of the basolateral Na+-K+-2Cl− cotransport system), part of the K+ secretory flux is linked to Na+ absorption (Sweiry & Binder, 1989), which we have confirmed in the present study. We propose two possible explanations for the apparent partial linking of K+ secretion to Na+ absorption, both of which involve a decrease in the driving force for K+ secretion. First, the observed Na+ absorption judged by ISC in our study (8.5 ± 0.7 μmol h−1 cm−2) was nearly twice that reported by Foster et al. (1984) (4.5 ± 0.7 μmol h−1 cm−2). Consequently, the decrease in driving force for K+ secretion produced by amiloride in the present study was also probably twice that produced in the study of Foster et al. (1984). Second, while the values for JRb,sm before addition of amiloride in our study and in the study of Foster et al. (1984) with adrenalectomized rats were similar (1.4 ± 0.2 and 1.5 ± 0.1 μmol h−1 cm−2, respectively), those for JRb,ms were more than twice as high in our study (1.3 ± 0.1 and 0.6 ± 0.1 μmol h−1 cm−2, respectively). Thus, we predict that during our experiments with aldosterone, the inhibition of K+ absorption was less pronounced than in adrenalectomized rats, and in our study the basolateral K+ conductance was probably much higher, resulting in a lower driving force for K+ secretion across the apical membrane.
The second component of aldosterone-induced K+ secretion was unmasked by the application of TEA. In fresh controls, ISC blocked by mucosal TEA was relatively small (Fig. 1), but after 6 h of incubation with 3 nM aldosterone the values of ISC and JRb,sm were large and TEA-sensitive, both in the Ussing experiments (Table 3) and during conductance scanning (Fig. 3). By contrast, incubation for the same time in the absence of aldosterone did not evoke a similar K+ secretion (Table 1 and Fig. 4). Thus, K+ secretion induced by aldosterone may have reflected a specific mineralocorticoid action, similar to that seen in rat distal colon in response to secondary hyperaldosteronism induced by dietary sodium depletion (Foster et al. 1984).
Localization of aldosterone-induced K+ secretion
Until recently, potassium secretion has been assigned to the crypts (Halm & Frizzell, 1991) because it persists after inhibition of electrogenic Na+ absorption, which is located in the surface epithelium. Furthermore, apical membrane vesicles isolated from surface cells of aldosterone-treated rats do not possess a significant K+ conductance (Rajendran et al. 1989), which may reflect the loss of an additional factor involved in K+ channel activation. Recent micropuncture studies performed in rat late distal colon give the first direct evidence that aldosterone-induced K+ secretion may be located predominantly in surface cells (Lomax et al. 1994; Lomax & Sandle, 1994).
Conductance scanning after 6 h incubation with 3 nM aldosterone indicated that a TEA-blockable apical conductance was absent in crypt cells. As crypts do not exhibit apical K+ secretion, it must take place in the surface epithelium. Accordingly, a large TEA-blockable apical conductance was found in the surface epithelium. We conclude that aldosterone-induced K+ secretion is confined to enterocytes of the surface epithelium, which contain TEA-sensitive K+ channels in their apical membrane. It should be noted that after 6 h of sham incubation without aldosterone, a significant TEA-blockable K+ conductance was also found in the surface epithelium, but K+ secretion was negligible (Fig. 4). Therefore, the main effect of aldosterone on K+ secretion is not chiefly an increase in apical K+ conductance but a rise in driving force for K+ outflow.
The partial linkage of K+ secretion to Na+ absorption found in our study is consistent with the localization of K+ secretion as determined by conductance scanning. Our results suggest that aldosterone-induced K+ secretion occurs in surface epithelium, which is also the site of aldosterone-induced Na+ channels (Köckerling et al. 1993) and amiloride-blockable electrogenic Na+ absorption.
The scanning experiments demonstrated that more than 90 % of the electrogenic K+ secretion induced by physiological (i.e. nanomolar) concentrations of aldosterone is confined to the surface epithelium. The results of microelectrode studies (Lomax et al. 1994; Lomax & Sandle, 1994) demonstrating aldosterone-induced electrogenic K+ secretion in cells of the upper half of the crypt may reflect the chronic administration of a high dose (70 μg (100 g body weight)−1 day−1) of aldosterone, which resulted in the recruitment of receptors that do not bind at physiological aldosterone concentrations. Alternatively, the relatively differentiated cells of the upper crypt segment may not be representative of the entire crypt. On the other hand, the studies of Lomax et al. (1994) and Lomax & Sandle (1994) show that the TEA-sensitive apical K+ conductance induced by aldosterone in upper crypt cells was relatively small compared with that induced in surface cells. If the component of aldosterone-induced K+ secretion in crypts does not exceed 10 %, it will probably not be detected by conductance scanning. Therefore, our results fit well with the data from the microelectrode studies cited and allow us to quantify the contribution of the whole crypts to the total aldosterone-induced K+ secretion.
cAMP-mediated K+ secretion
Potassium secretion is stimulated by intracellular cAMP or agents which increase cAMP (e.g. forskolin and adrenaline). Forskolin increases intracellular cAMP much more than adrenaline, thereby also triggering Cl− secretion by cAMP-dependent phosphorylation and opening of apical Cl− channels (Diener, Hug, Strabel & Scharrer, 1996). In the present study, cAMP-induced K+ secretion was investigated using adrenaline, which does not stimulate Cl− secretion (Halm & Frizzell, 1986; Plass, Gridl & Turnheim, 1986; Smith & McCabe, 1986). A rise in intracellular cAMP may enhance K+ transport by several mechanisms (Diener et al. 1996): (i) stimulation of K+ uptake via the Na+-K+-2Cl− cotransporter in the basolateral cell membrane; (ii) increase of the electrochemical gradient for K+ across the apical membrane due to depolarization caused by opening of Cl− channels; and (iii) decrease of the basolateral membrane/apical membrane K+ conductance ratio (thus increasing mucosal and decreasing serosal efflux of K+ from the cell).
In the latter mechanism, basolateral K+ channels close, which leads to a diminished total K+ conductance of the cell, but until now it was not certain whether or not apical channels open. In the present experiments, adrenaline elicited increases in the TEA-sensitive conductance of surface epithelium and crypts. This result shows that cAMP opens apical K+ channels. Furthermore, an elevated intracellular cAMP level is known to attenuate K+ absorption (Diener et al. 1996). This was also observed in the present experiments (see Table 2), although the effect was very small (0.1 μmol h−1 cm−2). Possibly, higher concentrations of cAMP are needed than for the opening of K+ channels.
In the flux experiments performed in the time range needed for establishing the aldosterone effect (6 h), adrenaline, applied after amiloride, induced the same amount of K+ secretion in the absence (Table 2) and presence (Table 3) of aldosterone. This means that under these experimental conditions the effects of aldosterone and adrenaline are fully additive. As aldosterone-induced K+ secretion is restricted to surface epithelium, it is evident that at least the component of adrenaline action directed towards crypts and the effect of aldosterone are additive. These findings are in contrast to the results obtained with guinea-pig colon (Rechkemmer & Halm, 1989), where the effects of aldosterone and adrenaline in the presence of amiloride were found to be non-additive, and, therefore, the same site of action is claimed for both effectors. In this pioneering in vitro study very high concentrations of aldosterone were used (1 μm) which might lead to additional effects. However, the Na+ absorption observed in the study cited is only half that of our study, while the effect of adrenaline on K+ secretion is larger (2.2 μmol h−1 cm−2). In a recent study of Rechkemmer, Frizzell & Halm (1996), the authors showed that the high amount of K+ secretion observed in guinea-pig distal colon linked this K+ secretory process to K+ absorption, as shown by a partial block of K+ secretion following the application of mucosal ouabain, in contrast to the finding in our study. Therefore, there may be a partial link of adrenaline-induced K+ secretion to aldosterone-induced K+ secretion in guinea-pig colon.
Localization of cAMP-mediated K+ secretion
After application of adrenaline, conductance scanning revealed the presence of cAMP-induced K+ conductances in both surface epithelium and crypts. Co-localization of TEA-sensitive K+ secretion and heightened K+-conductance in both crypts and surface epithelium suggests that adrenaline-induced K+ secretion, unlike aldosterone-induced K+ secretion, resembles cAMP-induced Cl− secretion in its distribution to both the crypts and the surface epithelium (Köckerling & Fromm, 1993).
In summary, we demonstrated that the site of active K+ secretion in rat late distal colon depends on the mechanism of activation. While cAMP-dependent K+ secretion occurs in both the surface epithelium and the crypts, aldosterone-induced K+ secretion is confined to the surface epithelium.
Acknowledgments
This study was supported by grants of the Deutsche Forschungsgemeinschaft (DFG Fr 652/3–3) and the Sonnenfeld-Stiftung.
References
- Abrahamse SI, de Jonge HR, Bimdels RJ, van Os CH. Two distinct K(+)-ATPase activities in rabbit distal colon. Biochemical and Biophysical Research Communications. 1995;207:1003–1008. doi: 10.1006/bbrc.1995.1284. 10.1006/bbrc.1995.1284. [DOI] [PubMed] [Google Scholar]
- Binder HJ, McGlone F, Sandle GI. Effects of corticosteroid hormones on the electrophysiology of rat distal colon: implications for Na+ and K+ transport. The Journal of Physiology. 1989;410:425–441. doi: 10.1113/jphysiol.1989.sp017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3. Vol. 2. New York: Raven Press; 1994. pp. 2133–2171. [Google Scholar]
- Del Castillo JR, Rajendran VM, Binder HJ. Apical localization of ouabain-sensitive K(+)-activated ATPase activities in rat distal colon. American Journal of Physiology. 1991;261:G1005–1011. doi: 10.1152/ajpgi.1991.261.6.G1005. [DOI] [PubMed] [Google Scholar]
- Diener M, Hug F, Strabel D, Scharrer E. Cyclic AMP-dependent regulation of K+ transport in the rat distal colon. British Journal of Pharmacology. 1996;118:1477–1487. doi: 10.1111/j.1476-5381.1996.tb15563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ES, Hayslett JP, Binder HJ. Mechanism of active potassium absorption and secretion in the rat colon. American Journal of Physiology. 1984;246:G611–617. doi: 10.1152/ajpgi.1984.246.5.G611. [DOI] [PubMed] [Google Scholar]
- Foster ES, Sandle GI, Hayslett JP, Binder HJ. Cyclic adenosine monophosphate stimulates active potassium secretion in the rat colon. Gastroenterology. 1983;84:324–330. [PubMed] [Google Scholar]
- Foster ES, Sandle GI, Hayslett JP, Binder HJ. Dietary potassium modulates active potassium absorption and secretion in rat distal colon. American Journal of Physiology. 1986;251:G619–626. doi: 10.1152/ajpgi.1986.251.5.G619. [DOI] [PubMed] [Google Scholar]
- Fromm M, Oelkers W, Hegel U. Time course of aldosterone and corticosterone plasma levels in rats during general anesthesia and abdominal surgery. Pflügers Archiv. 1983;399:249–254. doi: 10.1007/BF00652747. [DOI] [PubMed] [Google Scholar]
- Fromm M, Schulzke JD, Hegel U. Control of electrogenic Na+ absorption in rat late distal colon by nanomolar aldosterone added in vitro. American Journal of Physiology. 1993;264:E68–73. doi: 10.1152/ajpendo.1993.264.1.E68. [DOI] [PubMed] [Google Scholar]
- Frömter E. Route of passive ion movement through the epithelium of Necturus gallbladder. Journal of Membrane Biology. 1972;8:259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Halm DR, Dawson DC. Potassium transport by turtle colon: active secretion and active absorption. American Journal of Physiology. 1984;246:C315–322. doi: 10.1152/ajpcell.1984.246.3.C315. [DOI] [PubMed] [Google Scholar]
- Halm DR, Frizzell RA. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. American Journal of Physiology. 1986;251:C252–267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Halm DR, Frizzell RA. Ion transport across the large intestine. In: Field M, Frizzell RA, editors. Handbook of Physiology, Intestinal Absorption and Secretion, The Gastrointestinal System, section 6. IV. Bethesda, MD, USA: American Physiological Society; 1991. pp. 257–273. [Google Scholar]
- Hayslett JP, Binder HJ. Mechanism of potassium adaptation. American Journal of Physiology. 1982;243:F103–112. doi: 10.1152/ajprenal.1982.243.2.F103. [DOI] [PubMed] [Google Scholar]
- Köckerling A, Fromm M. Origin of cAMP dependent Cl− secretion from both crypts and surface epithelia of rat intestine. American Journal of Physiology. 1993;264:C1294–1301. doi: 10.1152/ajpcell.1993.264.5.C1294. [DOI] [PubMed] [Google Scholar]
- Köckerling A, Sorgenfrei D, Fromm M. Electrogenic Na+ absorption of rat distal colon is confined to the surface epithelium. A voltage scanning study. American Journal of Physiology. 1993;264:C1285–1293. doi: 10.1152/ajpcell.1993.264.5.C1285. [DOI] [PubMed] [Google Scholar]
- Lee J, Rajendran VM, Mann AS, Kashgarian M, Binder HJ. Functional expression and segmental localization of rat colonic K-adenosine triphosphatase. Journal of Clinical Investigation. 1995;96:2002–2008. doi: 10.1172/JCI118247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax RB, McNicholas CM, Lombès M, Sandle GI. Aldosterone-induced apical Na+ and K+ conductances are located predominantly in surface cells in rat distal colon. American Journal of Physiology. 1994;266:G71–82. doi: 10.1152/ajpgi.1994.266.1.G71. [DOI] [PubMed] [Google Scholar]
- Lomax RB, Sandle GI. Comparison of aldosterone- and RU-28362-induced apical Na+ and K+ conductances in rat distal colon. American Journal of Physiology. 1994;267:G485–493. doi: 10.1152/ajpgi.1994.267.3.G485. [DOI] [PubMed] [Google Scholar]
- McCabe RD, Smith PL, Sullivan LP. Ion transport by rabbit descending colon: mechanism of transepithelial potassium transport. American Journal of Physiology. 1984;246:G594–602. doi: 10.1152/ajpgi.1984.246.5.G594. [DOI] [PubMed] [Google Scholar]
- Pandiyan V, Rajendran VM, Binder HJ. Mucosal ouabain and Na+ inhibit active Rb+ (K+) absorption in normal and sodium-depleted rat colon. Gastroenterology. 1992;102:1846–1853. doi: 10.1016/0016-5085(92)90304-h. [DOI] [PubMed] [Google Scholar]
- Plass H, Gridl A, Turnheim K. Absorption and secretion by rabbit descending colon. Pflügers Archiv. 1986;406:509–519. doi: 10.1007/BF00583375. [DOI] [PubMed] [Google Scholar]
- Rajendran VM, Kashgarian M, Binder HJ. Aldosterone induction of electrogenic sodium transport in the apical membrane vesicles of rat distal colon. Journal of Biological Chemistry. 1989;264:18638–18644. [PubMed] [Google Scholar]
- Rechkemmer G, Frizzell RA, Halm DR. Active potassium transport across guinea-pig distal colon: action of secretagogues. The Journal of Physiology. 1996;493:485–502. doi: 10.1113/jphysiol.1996.sp021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechkemmer G, Halm DR. Aldosterone stimulates K secretion across mammalian colon independent of Na absorption. Proceedings of the National Academy of Sciences of the USA. 1989;86:397–401. doi: 10.1073/pnas.86.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangan P, Rajendran VM, Mann AS, Kashgarian M, Binder HJ. Regulation of colonic H-K-ATPase in large intestine and kidney by dietary Na depletion and dietary K depletion. American Journal of Physiology. 1997;272:C685–696. doi: 10.1152/ajpcell.1997.272.2.C685. [DOI] [PubMed] [Google Scholar]
- Schulzke JD, Fromm M, Hegel U. Epithelial and subepithelial resistance of rat large intestine: segmental differences, effect of stripping, time course, and action of aldosterone. Pflügers Archiv. 1986;407:632–637. doi: 10.1007/BF00582644. [DOI] [PubMed] [Google Scholar]
- Smith PL, McCabe RD. Mechanism and regulation of transcellular potassium transport by the colon. American Journal of Physiology. 1984;247:G445–446. doi: 10.1152/ajpgi.1984.247.5.G445. [DOI] [PubMed] [Google Scholar]
- Smith PL, McCabe RD. Potassium secretion by rabbit descending colon: effects of adrenergic stimuli. American Journal of Physiology. 1986;250:G432–439. doi: 10.1152/ajpgi.1986.250.4.G432. [DOI] [PubMed] [Google Scholar]
- Sweiry JH, Binder HJ. Characterization of aldosterone-induced potassium secretion in rat distal colon. Journal of Clinical Investigation. 1989;83:844–851. doi: 10.1172/JCI113967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiry JH, Binder HJ. Active potassium absorption in rat distal colon. The Journal of Physiology. 1990;423:155–170. doi: 10.1113/jphysiol.1990.sp018016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ, Smith PL, Fromm M, Frizzell RA. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982;218:1219–1221. doi: 10.1126/science.6293054. [DOI] [PubMed] [Google Scholar]


