Abstract
Taurine, prominently concentrated in glial cells in the supraoptic nucleus (SON), is probably involved in the inhibition of SON vasopressin neurones by peripheral hypotonic stimulus, via activation of neuronal glycine receptors. We report here the properties and origin of the osmolarity-dependent release of preloaded [3H]taurine from isolated whole SO nuclei.
Hyposmotic medium induced a rapid, reversible and dose-dependent increase in taurine release. Release showed a high sensitivity to osmotic change, with a significant enhancement with less than a 5 % decrease in osmolarity. Hyperosmotic stimulus decreased basal release.
Evoked release was independent of extracellular Ca2+ and Na+, and was blocked by the Cl− channel blockers DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid) and DPC (N-phenylanthranilic acid), suggesting a diffusion process through volume-sensitive Cl− channels.
Evoked release was transient for large osmotic reductions (≥ 15 %), probably reflecting regulatory volume decrease (RVD). However, it was sustained for smaller changes, suggesting that taurine release induced by physiological variations in osmolarity is not linked to RVD.
Basal and evoked release were strongly inhibited by an incubation of the tissue with the glia-specific toxin fluorocitrate, but were unaffected by a neurotoxic treatment with NMDA, demonstrating the glial origin of the release of taurine in the SON.
The high osmosensitivity of taurine release suggests an important role in the osmoregulation of the SON function. These results strengthen the notion of an implication of taurine and glial cells in the regulation of the whole-body fluid balance through the modulation of vasopressin release.
Taurine, an amino acid present at high concentration in the brain, has been attributed an important role in the regulation of intracellular osmolarity (Wade, Olson, Samson, Nelson & Pazdernik, 1988; Solís, Herranz, Herreras, Lerma & Martín del Río, 1988; see reviews by Huxtable, 1992; Oja & Saransaari, 1996; Pasantes-Morales & Schousboe, 1997). Indeed, neurones and glial cells swell upon a decrease in external osmolarity, and release taurine apparently as part of the regulatory volume decrease undergone by cells in the process of re-equilibration of osmotic pressure (Pasantes-Morales, Alavez, Sánchez-Olea & Morán, 1993; Morán, Maar & Pasantes-Morales, 1994; Vitarella, DiRisio, Kimelberg & Aschner, 1994). In astrocytes, the mechanism of osmolarity-dependent release of taurine has been extensively studied in cultured preparations (Martin, Madelian, Seligmann & Shain, 1990; Pasantes-Morales, Morán & Schousboe, 1990; Kimelberg, Goderie, Higman, Pang & Waniewski, 1990; see review by Oja & Saransaari, 1996). It has been correlated with cell swelling (Pasantes-Morales et al. 1993; Vitarella et al. 1994), has been shown to be independent of extracellular Na+ (Kimelberg et al. 1990; Sánchez-Olea, Morán, Schousboe & Pasantes-Morales, 1991) and to be sensitive to Cl− channel blockers (Pasantes-Morales et al. 1990; Sánchez-Olea, Peña, Morán & Pasantes-Morales, 1993), suggesting passive diffusion through volume-activated Cl− channels (Roy, 1995; see reviews by Strange & Jackson, 1995; Pasantes-Morales & Schousboe, 1997). However, these properties have not been verified in situ.
Recently, we provided evidence that taurine may have an extended role in osmoregulation, being involved in the regulation of the whole-body fluid balance through the modulation of neurones secreting the antidiuretic hormone vasopressin in the blood circulation (Hussy, Deleuze, Pantaloni, Desarménien & Moos, 1997b). These vasopressin magnocellular neurones are located in the hypothalamic supraoptic (SON) and paraventricular nuclei, and their electrical and neurosecretory activity depends strongly upon plasma osmolarity, being excited and inhibited by hyperosmotic and hyposmotic stimuli, respectively (for review see Renaud & Bourque, 1991; Bourque, Oliet & Richard, 1994). The SON exhibits high levels of taurine (Palkovits, Elekes, Láng & Patthy, 1986), which has been reported to be located prominently in glial cells (Decavel & Hatton, 1995). We have shown that hyposmotic stimulus selectively enhances the release of endogenous taurine from SON in vitro, and that taurine activates preferentially strychnine-sensitive glycine receptors on magnocellular neurones (Hussy et al. 1997b). Moreover, in vivo, the inhibitory influence of peripheral hyposmotic stimuli (i.p. injection of distilled water) on the activity of vasopressin neurones can be partly suppressed by local application of low concentrations of strychnine, pointing to an involvement of glycine receptors in this regulation (Hussy et al. 1997b). These data suggest that a decrease in plasma osmotic pressure stimulates the release of taurine in the SON, presumably from glial cells, to inhibit the activity of vasopressin neurones through the activation of neuronal glycine receptors. This glial regulatory loop would complement other inhibitory influences, involving peripheral and central osmoreceptors as well as stretch-inactivated channels present on SON magnocellular neurones (see review by Bourque et al. 1994), to decrease vasopressin release by the neurohypophysis. Interestingly, a recent report also demonstrated a high level of taurine in neurohypophysial glial cells, the release of which can be stimulated by hyposmotic medium (Miyata, Matsushima & Hatton, 1997).
To test the glial origin of taurine release in the SON, and to investigate its osmotic dependence and mechanisms, we studied the release of pre-loaded tritiated taurine in vitro from isolated SON.
METHODS
Dissection procedure
SON were isolated from adult male Wistar rats (150–250 g) as previously described (Hussy et al. 1997b). Rats were decapitated without anaesthesia, their brain rapidly removed and placed in oxygenated Locke solution (mm: NaCl, 132; KCl, 5; CaCl2, 2; MgCl2, 2; KH2PO4, 1.2; Hepes, 10; glucose, 10; pH 7.4; osmolarity, 300 mosmol l−1) at 4°C. Two thin translucid strips of tissue located lateral to the optic chiasm, corresponding to the SON (dimension 1.2 mm × 0.4 mm × 0.5 mm), were carefully dissected and freed from any residual optic tracts and blood vessels. Tissues were incubated for 40 min in oxygenated Locke solution supplemented with 400–600 nM [3H]taurine (Amersham, Les Ulis, France) in a shaking incubator at 35°C, washed three times, and placed in parallel perfusion chambers (two nuclei per chamber). In the experiments involving long-term incubation of the tissues (5–8 h), the dissection was performed in a Locke solution in which all Na+ was replaced by an equiosmotic amount of sucrose. Nuclei were then incubated in an oxygenated normal Locke medium containing the antioxidants ascorbic acid and thiourea. This procedure has been reported to enhance the long-term viability of the tissues (Stern & Armstrong, 1995). In experiments using fluorocitrate, the glial toxin (300 μm) was added to the incubation medium just after the dissection and was present throughout the incubation as well as during the sample collection period. The neuronal excitotoxic treatment consisted of an incubation with 300 μm NMDA for 2 h in a Mg2+-free Locke solution. Neuronal death was first attested by Nissl staining in hypothalamic slices. Coronal slices (400 μm thick) were cut with a vibratome (Campden Instruments Ltd) at 4°C. The NMDA treatment was followed by a 6 h incubation in a Locke solution to allow for the detection of cell death. Slices were fixed with 4 % formol in phosphate-buffered saline (PBS) for 24 h, followed by incubation in PBS with 30 % sucrose for another 24 h. Tissues were then frozen and 30 μm slices were cut with a cryostat (Leica, Rueil-Malmaison, France), stained with 0.1 % Cresyl Violet, rinsed, dehydrated, mounted in Entellan media (Merck, Nogent-sur-Marne, France), and viewed on a Reichert microscope. To estimate the impact of neuronal death on the release of taurine, the same protocol (2 h with NMDA, 6 h in control solution) was applied to isolated SON; the tissues were then allowed to incorporate [3H]taurine before being processed for release measurements. Control experiments were systematically done in parallel, with an equivalent number of SON (or slices) going through the same procedure except for the presence of the drug.
Measurement of taurine release
Chambers (250 μl, made according to Korpi & Oja, 1984) were perfused with oxygenated solution at 35°C, at a rate of 250 μl min−1 using a peristaltic pump (Gilson). The perfusate during the first 15 min was discarded; samples were then collected every 2 min using a sample collector (LKB, Orsay, France) and the radioactivity in each sample was estimated by scintillation counting. The amount of radioactivity left in the tissue was measured at the end of each experiment. Basal release of taurine decreased with time in a monoexponential manner (Fig. 1A). Therefore, a single exponential function was fitted to the baseline, and data points were divided by the fit to express release relative to basal level (Fig. 1B). Hyposmotic solutions were obtained by removing the appropriate amount of NaCl, and the hyperosmotic solution was a Locke medium with added sucrose. Half of the chambers were systematically used as control, and the effects of test solutions were compared with these controls.
Figure 1. Normalization of the release of [3H]taurine evoked by hyposmotic medium.
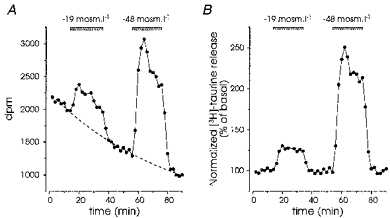
A, release evoked by successive applications of medium of reduced osmolarity (-19 and -48 mosmol l−1), expressed as number of disintegrations per minute (d.p.m.). Basal release could be fitted with a single exponential function (dashed line). B, data in A were divided by the fit in order to express them as a per cent of basal release.
Analysis and fits were performed with Origin software (Microcal Software, Northampton, MA, USA). All results were obtained with at least two different preparations. Results are expressed as means ± s.e.m. All drugs were purchased from Sigma (France) or RBI (Natick, MA, USA). DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid) and DPC (N-phenylanthranilic acid) were dissolved in DMSO, with a final concentration of DMSO not exceeding 0.3 %. The lack of effect of DMSO was attested by the systematic addition of the same amount of DMSO to test and control solutions. Fluorocitrate was purchased as the barium salt, and Ba2+ was precipitated before the experiments according to Paulsen, Contestabile, Villani & Fonnum (1987).
RESULTS
Dose dependence of hyposmolarity-evoked [3H]taurine release
Application of hyposmotic medium induced a rapid increase in the release of [3H]taurine in isolated SON. The response reached a peak within 6 min after the onset of application, was rapidly reversible upon restoration of isosmotic medium, and could be repeated (Fig. 1). The amount of taurine released depended on the osmolarity of the external medium, a significant enhancement (11 ± 2 %, P < 0.01, Student's t test) being already detectable for osmotic decreases as small as 14 mosmol l−1 (Fig. 2A). The induced release was specific to a decrease in osmolarity, because application of media with similar reductions in NaCl concentration, but with osmolarity readjusted to 300 mosmol l−1 by addition of an appropriate amount of sucrose, abolished or strongly reduced the response (Fig. 2A). Interestingly, hyperosmotic medium induced a decrease in basal taurine release, demonstrating a significant osmolarity-dependent level of release in isosmotic conditions. We constructed the dose-response relationship by plotting the peak amplitude of the response as a function of osmolarity, which showed that the level of basal release lay within an active range, and could be modulated both by hypotonic and hypertonic stimulation (Fig. 2B).
Figure 2. Release of taurine shows a high osmosensitivity.
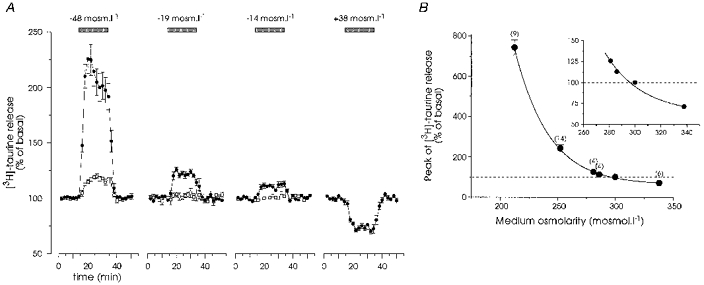
A, responses to solutions of decreased or increased osmolarity (•). Each trace represents the mean of 4–14 experiments. Error bars are shown when exceeding the size of the symbols. Hyposmolarity was obtained by removing an appropriate amount of NaCl. In control experiments, NaCl was replaced by an isosmotic amount of sucrose (□). Note that isosmotic substitution of up to 19 mosmol l−1 NaCl had no effect on taurine release, whereas replacement of 48 mosmol l−1 NaCl evoked a significant enhancement in release. B, peak amplitude of taurine release was plotted as a function of osmolarity. The relationship was modelled with a sigmoid curve (Boltzmann equation, continuous line). Inset, the same plot on a larger scale indicates the high sensitivity of the release to small changes in osmolarity.
The time course of the release also appeared to depend upon the magnitude of the change in osmolarity (Fig. 2A). We studied this dependence by long applications (1 h) of solutions of decreasing osmolarity (Fig. 3). For small changes in osmolarity (-19 mosmol l−1) the evoked release was sustained during the whole application (n = 4; Fig. 3A), whereas larger changes were accompanied by a progressive reduction in the level of release (Fig. 3B and C), possibly reflecting regulatory volume compensation (Pasantes-Morales & Schousboe, 1988). The time course of this decay was best fitted with double exponential functions, with time constants of 5.3 ± 0.7 and 36.1 ± 7.1 min for a reduction of 48 mosmol l−1 (n = 4) and 5.6 ± 1.6 and 33.0 ± 13.7 min for a reduction of 88 mosmol l−1 (n = 3). The fit indicated that the decay was incomplete, with extrapolated minima of 128 ± 7 and 116 ± 8 % of the basal release, respectively.
Figure 3. Time course of hyposmolarity-induced taurine release.
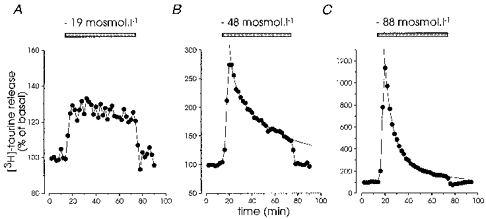
Hypotonic media of decreasing osmolarity were applied for 1 h to estimate the kinetics of release. With a small reduction (19 mosmol l−1), taurine release was sustained over the entire duration of application (A), whereas larger changes (-48 and -88 mosmol l−1) induced a transient response characterized by a rapid peak followed by a slowly decaying biexponential component (B and C), with time constants of 3.5 and 34.4 min (-48 mosmol l−1, B) and 6.2 and 24.3 min (-88 mosmol l−1, C).
Ionic sensitivity of hyposmolarity-evoked taurine release
Both the basal release of [3H]taurine and that induced by a reduction in osmotic pressure of 48 mosmol l−1 were completely insensitive to the absence of extracellular Ca2+, with Ca2+ replacement by an equimolar concentration of Mg2+ and the addition of 1 mm EGTA (n = 4; Fig. 4A). The dependence upon Na+ ions was studied by replacing all external Na+ by N-methyl-D-glucamine (NMDG+, Fig. 4B). Application of an isosmotic NMDG+ solution induced a strong, slowly rising and sustained increase in basal release of [3H]taurine, which could reflect the inhibition of a Na+-dependent reuptake of taurine. However, the absence of Na+ did not affect the release evoked by the hypotonic solution (Fig. 4B).
Figure 4. Hyposmolarity-evoked taurine release is independent of extracellular Ca2+ and Na+.
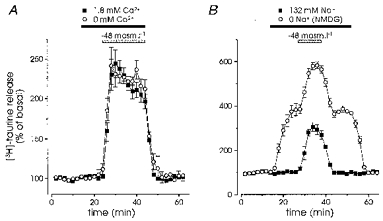
A, both the basal and the release induced by a reduction of 48 mosmol l−1 in osmolarity were unaffected by the replacement of all external Ca2+ by an equimolar concentration of Mg2+ and addition of 1 mm EGTA (n = 4). B, basal release was strongly increased by complete replacement of extracellular Na+ by NMDG+. However, the hyposmolarity-induced release was similar in the presence or absence of Na+ (n = 6)
We tested the effect of the Cl− channel and Cl− transport inhibitor DIDS, as well as of the more specific anion channel blocker DPC. Application of DIDS alone slightly decreased the basal release of [3H]taurine, and markedly inhibited the hyposmolarity-induced release by 41 ± 8 and 55 ± 4 % at 0.3 and 1 mm, respectively (n = 3 and 4; Fig. 5A and C). DPC had similar effects both on basal and on hyposmotic-induced taurine release with 32 ± 6 and 51 ± 2 % inhibition at 0.1 and 0.3 mm, respectively (n = 4 and 6; Fig. 5B and C).
Figure 5. Taurine release is inhibited by Cl− channel blockers.
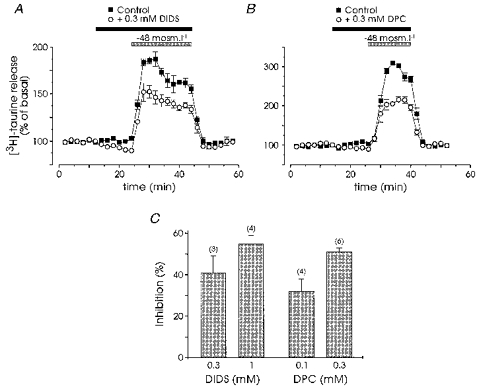
Both basal taurine release and that evoked by a reduction of 48 mosmol l−1 in osmolarity were reduced in the presence of 0.3 mm DIDS (A, n = 3) and of 0.3 mm DPC (B, n = 6). C, percentage inhibition of evoked release by the two Cl− channel blockers at the different concentrations indicated. Number of observations are indicated in parentheses.
The osmolarity-evoked release is of glial origin
To test the glial specificity of the uptake and release of [3H]taurine, we used the selective glial metabolic toxin fluorocitrate, an inhibitor of aconitase specifically taken up by glial cells, which blocks the Krebs cycle and thus interferes with the energy metabolism (Paulsen et al. 1987; Paulsen & Fonnum, 1989). Before incubation with [3H]taurine, SON were exposed to 0.3 mm fluorocitrate for 5 or 8 h, treatments that have been used to establish glial mediation of physiological effects (Paulsen et al. 1987; Paulsen & Fonnum, 1989; Berg-Johnsen, Paulsen, Fonnum & Langmoen, 1993).
To estimate the effect of fluorocitrate on the uptake of taurine by SON, we added the amount of radioactive taurine released during the experiments to that remaining in the tissue. In the presence of fluorocitrate, the incorporation of taurine was reduced by 31 ± 7 % for a 5 h incubation and by 49 ± 4 % for an 8 h incubation (n = 4 each, data not shown). On the other hand, these treatments markedly blocked both the basal and hyposmolarity-induced release (Fig. 6). Basal release, estimated as the integral of the exponential fit of the baseline over the duration of the experiment, was blocked by 69 ± 4 % (5 h, n = 4) and 74 ± 2 % (8 h, n = 4; Fig. 6A). Stimulated release was suppressed by 63 ± 9 % (5 h, n = 4) and 78 ± 2 % (8 h, n = 4; Fig. 6B). Interestingly, when expressed as a percentage of basal level, the amplitude and time course of the hyposmolarity-evoked release in control and fluorocitrate-treated SON were almost identical (Fig. 6C), suggesting a similar mechanism of release in the two experimental conditions. This would be expected if release came from a single taurine pool, the uptake and/or release being incompletely blocked by the toxin.
Figure 6. Inhibition of glial cell metabolism blocks taurine release.
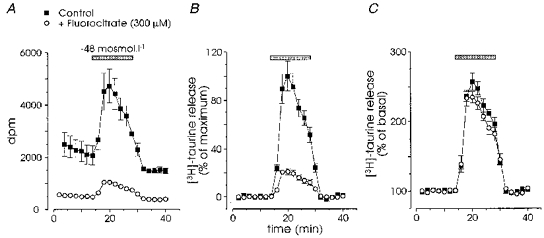
Incubation (8 h) with the specific glial toxin fluorocitrate (300 μm) strongly inhibited both basal and hyposmolarity-induced release. A, raw data were expressed in number of disintegrations per minute (dpm), which showed the large block of basal release by the toxin (n = 4). B, to compare evoked releases, we subtracted the fit of the basal release from the data shown in A in order to null the baseline, and expressed the release in each experiment as the percentage of the maximal release measured in the absence of fluorocitrate. The toxin inhibited evoked release by about 80 %. C, when the stimulated release was normalized to the basal release as in Fig. 1, control and test responses showed similar amplitude and time course.
Since an inhibition of the metabolism of glial cells may also affect neuronal function, we checked whether taurine uptake and release was altered when neurones were killed with a neurotoxic treatment. As magnocellular neurones express NMDA receptors (Hussy, Boissin-Agasse, Richard & Desarménien, 1997a), we assessed the neurotoxicity of a 2 h treatment with 300 μm NMDA in the absence of extracellular Mg2+ on SON neurones, in a hypothalamic slice preparation. Nissl staining of control slices revealed that most neurones were stained prominently in their cytoplasm and showed a large, homogeneous, clearer nucleus (Fig. 7A). In contrast, virtually all neurones treated with NMDA presented a shrunken nucleus, heavily and irregularly labelled, with dense spots mostly concentrated on the periphery, indicative of the chromatin aggregation that takes place in the process of cell death (Fig. 7B). The same protocol was applied to isolated SON, which were subsequently incubated with [3H]taurine. NMDA treatment did not alter the uptake of tritiated taurine (data not shown), nor did it affect the basal and hyposmolarity-evoked release (n = 4; Fig. 7C and D). The strong inhibitory effect of the glial toxin, as well as the lack of effect of the elimination of magnocellular neurones, demonstrate that taurine is specifically taken up and released by glial cells in the SON.
Figure 7. Elimination of magnocellular neurones does not affect taurine release.
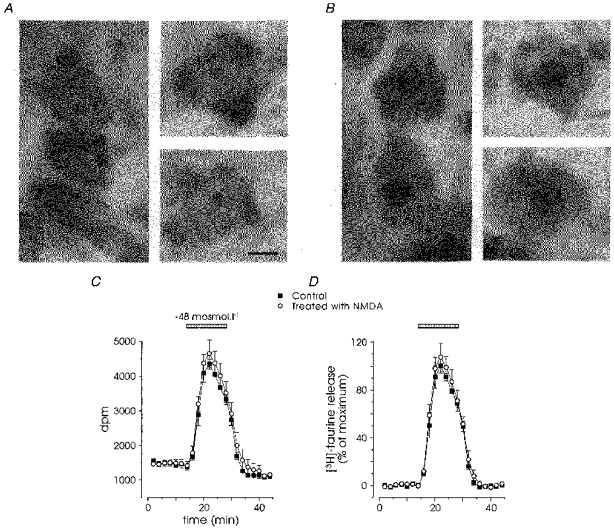
A and B, Nissl staining of supraoptic neurones in control conditions (A) and after a 2 h treatment with 300 μm NMDA (B). Control neurones showed a large nucleus, less densely stained than the cytoplasm. Cell death of NMDA-treated neurones was attested by shrunk, heavily stained nuclei, with dense spots often arranged as a circle on the periphery of the nucleus. Scale bar, 5 μm. C and D, release of taurine in control (▪) and NMDA-treated (○) isolated SON, expressed as raw data (n = 4; C) or after subtraction of the baseline fit and normalization to the maximal control release (D). Note the lack of impact of neuronal death on the basal and hyposmolarity-evoked release.
DISCUSSION
We previously demonstrated a hyposmolarity-dependent release of endogenous taurine from isolated SON (Hussy et al. 1997b), and detail here the osmosensitivity and properties of this release using [3H]taurine. The large blockade of taurine uptake and release by the selective glial toxin fluorocitrate and their insensitivity to the presence of neuronal elements demonstrate that taurine comes essentially from glial cells, in agreement with the marked preferential localization of endogenous taurine in SON astrocytes (Decavel & Hatton, 1995). The high sensitivity of taurine release to osmotic change was shown by the significant enhancement of release upon reductions in osmolarity by as little as 14 mosmol l−1, corresponding to less than a 5 % decrease. Interestingly, basal release was inhibited by hyperosmotic solutions, indicating that there is a significant osmosensitive release of taurine in isosmotic conditions, which lies in an active range where any small variation in osmotic pressure will result in a modulation of the rate of taurine release. These results strengthen the importance of taurine in the osmotic regulation of magnocellular neurones. Similarly high sensitivity to hypo- and hypertonicity has been reported in cultured astrocytes (Martin, Madelian & Shain, 1989; Martin et al. 1990; Pasantes-Morales et al. 1990; Sánchez-Olea, Morán & Pasantes-Morales, 1992).
Both basal and evoked releases were independent of extracellular Ca2+. Insensitivity of taurine release to Ca2+ has generally been found in cultured astrocytes (Martin et al. 1989; O'Connor & Kimelberg, 1993), and indicates a non-synaptic type of mechanism. However, Ca2+ depletion-induced increase in taurine release has also been reported (Holopainen, Kontro & Oja, 1985; Takuma, Matsuda, Kishida, Asano, Azuma & Baba, 1996). We have shown that basal release was strongly enhanced by removal of extracellular Na+, indicative of the presence of a Na+-dependent taurine uptake. In contrast, hyposmolarity-evoked release was insensitive to the presence of external Na+, as found in cultured glial cells (Kimelberg et al. 1990; Pasantes-Morales et al. 1990), which suggests the absence of involvement of the taurine transport system. Lastly, the release was found to be sensitive to DIDS and DPC, inhibitors of Cl− channels. Sensitivity of taurine release to Cl− channel blockers also appears as a general observation in cultured astrocytes (Pasantes-Morales et al. 1990; Sánchez-Olea et al. 1993), although DPC was reported ineffective in the latter study. Our results are in agreement with passive diffusion of taurine through swelling-activated, amino acid-permeable Cl− channels (Roy, 1995; for review see Strange & Jackson, 1995; Pasantes-Morales & Schousboe, 1997), and show that the properties of the release from glial cells in situ are similar to those reported on cultured astrocytes.
We found that the time course of hyposmolarity-evoked taurine release was dependent upon the magnitude of the osmotic change. For small decreases (19 mosmol l−1, i.e. 6.3 %), the release was sustained over at least 1 h, whereas larger changes induced slowly decaying releases with similar kinetics for decreases in osmolarity of 48 mosmol l−1 (16 %) and 88 mosmol l−1 (29 %). This transient nature of taurine release has generally been attributed to the regulatory volume decrease (RVD) associated with cell swelling in hypotonic media. Indeed, RVD and taurine release show similar kinetics and pharmacology (Pasantes-Morales & Schousboe, 1988; Pasantes-Morales, Murray, Lilja & Morán, 1994a; Pasantes-Morales, Murray, Sánchez-Olea & Morán, 1994b;Vitarella et al. 1994), and both excess of extracellular taurine and depletion of intracellular taurine inhibit RVD (Pasantes-Morales et al. 1994b;Morán et al. 1994). On these grounds, taurine has been postulated to play a crucial role in RVD. However, these studies have involved large changes in osmolarity (≥ 15 %), which are unlikely to be encountered in non-pathological situations. The picture may be different with more physiological variations in osmotic pressure. The sustained release evoked by small osmolarity decreases observed in this study would suggest a lack of significant volume regulation of glial cells in these conditions. Indeed, the absence of RVD in cases of limited osmotic stimuli as well as incomplete volume regulation have been documented in cultured astrocytes (Medrano & Gruenstein, 1993; Pasantes-Morales et al. 1993). These observations raise the question of the role of the release of taurine from glial cells under physiological conditions.
As first postulated by Hatton and collaborators (Hatton, 1990; Decavel & Hatton, 1995; Miyata et al. 1997), our data in the SON support the notion of an involvement of taurine in the osmotic regulation of vasopressin secretion. We have recently shown that taurine is an agonist of strychnine-sensitive glycine receptors in the SON and that the in vivo basal activity of vasopressin neurones is enhanced by local applications of a low concentration of strychnine (Hussy et al. 1997b). Moreover, we have shown that the inhibitory effect of a peripheral hyposmotic stimulus on the activity of vasopressin neurones is mediated, at least in part, via the activation of SON glycine receptors (Hussy et al. 1997b). Although taurine was not demonstrated as the mediator of these actions, several observations support its involvement in the activation of these glycine receptors: (i) the absence of a demonstrated glycine afferent pathway to the SON (Rampon, Luppi, Fort, Peyron & Jouvet, 1996); (ii) the significant osmosensitive basal release of taurine that could probably account for the basal activation of glycine receptors on magnocellular neurones in vivo (Hussy et al. 1997b); (iii) the specificity of the hyposmolarity-evoked release of endogenous taurine compared with the other glycine receptor agonists glycine and β-alanine (Hussy et al. 1997B); (iv) the high sensitivity of taurine release to small variations in external medium tonicity; and (v) the sustained nature of this release that is compatible with the sustained activation of glycine receptors by peripheral hypotonic stimulus (Hussy et al. 1997b). Interestingly, taurine also appears to be released in an osmotic-dependent manner by glial cells in the neurohypophysis, e.g. at the level of the axon terminals of SON and PVN vasopressin and oxytocin neurones (Miyata et al. 1997). This may suggest that taurine also acts directly on the terminals to regulate hormone secretion. It will be important to check for the presence of glycine receptors on these terminals, as well as for the local effect of taurine on the release of the two hormones.
Thus, physiological variations in plasma osmolarity would modulate the release of taurine by SON glial cells, and consequently the level of activation of glycine receptors, which would complement the effects mediated by mechanosensitive stretch-inactivated channels present on these cells (see Bourque et al. 1994; Bourque & Oliet, 1997). The presence of a basal release of taurine highly sensitive to both hypo- and hyperosmolarity should be considered in parallel to the basal level of activity of mechanoreceptors, also modulated by both osmotic stimuli (see Bourque et al. 1994). This suggests that the level of activity of magnocellular neurones in isosmotic conditions would depend on the balance between these two excitatory (mechanoreceptors) and inhibitory (glycine receptors) influences. Since both factors would be regulated in an opposite direction by changes in osmotic pressure, their effect on neuronal excitability would be synergistic, which should confer to the system a high sensitivity to even minute osmotic variations. This intrinsic osmosensitivity of the SON, in conjunction with the indirect influence coming from other osmosensitive regions (see Bourque et al. 1994; Bourque & Oliet, 1997), would be a major determinant of the regulation of the activity pattern of vasopressin neurones, and therefore of the secretion of vasopressin in the blood (see Dyball, 1988), implicating taurine and glial cells in the regulation of the whole-body fluid balance.
Acknowledgments
We are grateful to Line Boissin for her help in the histology experiments, and to Michel G. Desarménien, Françoise Moos, Alain Rabié and Philippe Richard for constructive discussions and critical reading of the manuscript.
References
- Berg-Johnsen J, Paulsen RE, Fonnum F, Langmoen IA. Changes in evoked potentials and amino acid content during fluorocitrate action studied in rat hippocampal cortex. Experimental Brain Research. 1993;96:241–246. doi: 10.1007/BF00227104. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SHR. Osmoreceptors in the central nervous system. Annual Review of Physiology. 1997;59:601–619. doi: 10.1146/annurev.physiol.59.1.601. 10.1146/annurev.physiol.59.1.601. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SHR, Richard D. Osmoreceptors, osmoreception, and osmoregulation. Frontiers in Neuroendocrinology. 1994;15:231–274. doi: 10.1006/frne.1994.1010. [DOI] [PubMed] [Google Scholar]
- Decavel C, Hatton GI. Taurine immunoreactivity in the rat supraoptic nucleus: prominent localization in glial cells. Journal of Comparative Neurology. 1995;354:13–26. doi: 10.1002/cne.903540103. [DOI] [PubMed] [Google Scholar]
- Dyball REJ. The importance of bursting in determining secretory response: how does a phasic firing pattern influence peptide release from neurohypophysial vasopressin terminals. In: Leng G, editor. Pulsatility in Neuroendocrine Systems. Boca Raton, FL, USA: CRC Press; 1988. pp. 181–196. [Google Scholar]
- Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Progress in Neurobiology. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. 10.1016/0301-0082(90)90017-B. [DOI] [PubMed] [Google Scholar]
- Holopainen I, Kontro P, Oja SS. Release of preloaded taurine and hypotaurine from astrocytes in primary culture: stimulation by calcium-free media. Neurochemical Research. 1985;10:123–131. doi: 10.1007/BF00964777. [DOI] [PubMed] [Google Scholar]
- Hussy N, Boissin-Agasse L, Richard Ph, Desarménien MG. NMDA receptor properties in rat supraoptic magnocellular neurons: characterization and postnatal development. European Journal of Neuroscience. 1997a;9:1439–1449. doi: 10.1111/j.1460-9568.1997.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarménien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. The Journal of Physiology. 1997b;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiological Reviews. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. Journal of Neuroscience. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Oja SS. Comparison of two superfusion systems for study of neurotransmitter release from rat cerebral cortex slices. Journal of Neurochemistry. 1984;43:236–242. doi: 10.1111/j.1471-4159.1984.tb06701.x. [DOI] [PubMed] [Google Scholar]
- Martin DL, Madelian V, Seligmann B, Shain W. The role of osmotic pressure and membrane potential in K+-stimulated taurine release from cultured astrocytes and LRM55 cells. Journal of Neuroscience. 1990;10:571–577. doi: 10.1523/JNEUROSCI.10-02-00571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DL, Madelian V, Shain W. Spontaneous and beta-adrenergic receptor-mediated taurine release from astroglial cells do not require extracellular calcium. Journal of Neuroscience Research. 1989;23:191–197. doi: 10.1002/jnr.490230209. [DOI] [PubMed] [Google Scholar]
- Medrano S, Gruenstein E. Mechanisms of regulatory volume decrease in UC-11MG human astrocytoma cells. American Journal of Physiology. 1993;264:C1201–1209. doi: 10.1152/ajpcell.1993.264.5.C1201. [DOI] [PubMed] [Google Scholar]
- Miyata S, Matsushima O, Hatton GI. Taurine in rat posterior pituitary: localization in astrocytes and selective release by hypoosmotic stimulation. Journal of Comparative Neurology. 1997;381:513–523. [PubMed] [Google Scholar]
- Morán J, Maar TE, Pasantes-Morales H. Impaired cell volume regulation in taurine deficient cultured astrocytes. Neurochemical Research. 1994;19:415–420. doi: 10.1007/BF00967318. [DOI] [PubMed] [Google Scholar]
- O'Connor ER, Kimelberg HK. Role of cacium in astrocyte volume regulation and in the release of ions and amino acids. Journal of Neuroscience. 1993;13:2638–2650. doi: 10.1523/JNEUROSCI.13-06-02638.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja SS, Saransaari P. Taurine as osmoregulator and neuromodulator in the brain. Metabolic Brain Disease. 1996;11:153–164. doi: 10.1007/BF02069502. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Elekes I, Láng T, Patthy A. Taurine levels in discrete brain nuclei of rats. Journal of Neurochemistry. 1986;47:1333–1335. doi: 10.1111/j.1471-4159.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Alavez S, Sánchez-Olea R, Morán J. Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochemical Research. 1993;18:445–452. doi: 10.1007/BF00967248. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Morán J, Schousboe A. Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia. 1990;3:427–432. doi: 10.1002/glia.440030514. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Lilja L, Morán J. Regulatory volume decrease in cultured astrocytes. I. Potassium- and chloride-activated permeability. American Journal of Physiology. 1994a;266:C165–171. doi: 10.1152/ajpcell.1994.266.1.C165. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Sánchez-Olea R, Morán J. Regulatory volume decrease in cultured astrocytes. II. Permeability pathway to amino acids and polyols. American Journal of Physiology. 1994b;266:C172–178. doi: 10.1152/ajpcell.1994.266.1.C172. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. Journal of Neuroscience Research. 1988;20:505–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Schousboe A. Role of taurine in osmoregulation in brain cells: mechanisms and functional implications. Amino Acids. 1997;12:281–292. [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. Journal of Neurochemistry. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Fonnum F. Role of glial cells for the basal and Ca2+-dependent K+-evoked release of transmitter amino acids investigated by microdialysis. Journal of Neurochemistry. 1989;52:1823–1829. doi: 10.1111/j.1471-4159.1989.tb07263.x. [DOI] [PubMed] [Google Scholar]
- Rampon C, Luppi PH, Fort P, Peyron C, Jouvet M. Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience. 1996;75:737–755. doi: 10.1016/0306-4522(96)00278-3. 10.1016/0306-4522(96)00278-3. [DOI] [PubMed] [Google Scholar]
- Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Progress in Neurobiology. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Roy G. Amino acid current through anion channels in cultured human glial cells. Journal of Membrane Biology. 1995;147:35–44. doi: 10.1007/BF00235396. [DOI] [PubMed] [Google Scholar]
- Sánchez-Olea R, Morán J, Pasantes-Morales H. Changes in taurine transport evoked by hyperosmolarity in cultured astrocytes. Journal of Neuroscience Research. 1992;32:86–92. doi: 10.1002/jnr.490320111. [DOI] [PubMed] [Google Scholar]
- Sánchez-Olea R, Morán J, Schousboe A, Pasantes-Morales H. Hypoosmolarity-activated fluxes of taurine in astrocytes are mediated by diffusion. Neuroscience Letters. 1991;130:233–236. doi: 10.1016/0304-3940(91)90404-h. 10.1016/0304-3940(91)90404-H. [DOI] [PubMed] [Google Scholar]
- Sánchez-Olea R, Peña C, Morán J, Pasantes-Morales H. Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl− transport in cultured astrocytes. Neuroscience Letters. 1993;156:141–144. doi: 10.1016/0304-3940(93)90458-w. 10.1016/0304-3940(93)90458-W. [DOI] [PubMed] [Google Scholar]
- Solís JM, Herranz AS, Herreras O, Lerma J, Martín del, Río R. Does taurine act as an osmoregulatory substance in rat brain? Neuroscience Letters. 1988;91:53–58. doi: 10.1016/0304-3940(88)90248-0. 10.1016/0304-3940(88)90248-0. [DOI] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. The Journal of Physiology. 1995;488:701–708. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Jackson PS. Swelling-activated organic osmolytes efflux: a new role for anion channels. Kidney International. 1995;48:994–1003. doi: 10.1038/ki.1995.381. [DOI] [PubMed] [Google Scholar]
- Takuma K, Matsuda T, Kishida Y, Asano S, Azuma J, Baba A. Ca2+ depletion facilitates taurine release in cultured rat astrocytes. Japanese Journal of Pharmacology. 1996;72:75–78. doi: 10.1254/jjp.72.75. [DOI] [PubMed] [Google Scholar]
- Vitarella D, DiRisio DJ, Kimelberg HK, Aschner M. Potassium and taurine release are highly correlated with regulatory volume decrease in neonatal primary rat astrocyte cultures. Journal of Neurochemistry. 1994;63:1143–1149. doi: 10.1046/j.1471-4159.1994.63031143.x. [DOI] [PubMed] [Google Scholar]
- Wade JV, Olson JP, Samson FE, Nelson SR, Pazdernik TL. A possible role for taurine in osmoregulation within the brain. Journal of Neurochemistry. 1988;51:740–745. doi: 10.1111/j.1471-4159.1988.tb01807.x. [DOI] [PubMed] [Google Scholar]


