Abstract
The transverse brainstem slice preparation containing the pre-Bötzinger complex (PBC) was used in mice to study developmental changes of the response of the in vitro respiratory network to hypoxia. This preparation generates at different postnatal stages (postnatal days (P) 0–22) spontaneous rhythmic activity in hypoglossal (XII) rootlets that occur in synchrony with periodic bursts of neurones in the PBC.
In slices from P0–4 mice, hypoxia did not significantly affect the amplitude of rhythmic synaptic drive potentials in four of five inspiratory neurones. Hypoxia reduced, but did not suppress, the amplitude of synaptic drive potentials in only one inspiratory neurone. Spike discharge and phasic ‘inspiratory’ hyperpolarizations of six expiratory neurones were suppressed during hypoxia revealing a phasic ‘inspiratory’ depolarization.
The coupling between rhythmic activity in PBC neurones and XII bursts occurred under control conditions in preparations from P0–4 mice in a 1:1 manner (n = 11) and from mice older than P5 in a 3:1 manner (n = 9). During hypoxia, PBC and XII activity were linked in a 1:1 manner in all slices.
In six of fourteen inspiratory PBC neurones, the amplitude of synaptic drive potentials of slices from mice older than P8 was increased during the period of augmentation, reduced during the period of depression and suppressed during a hypoxic response which we refer to as central apnoea. Augmentation led to a weak-to-moderate membrane depolarization which on average was 4.8 ± 3.7 mV. This depolarization was followed by a hyperpolarization of 6.2 ± 4.1 mV only in four inspiratory neurones. In the majority of neurones (n = 9), however, membrane depolarization remained stable and was not followed by hyperpolarization. In expiratory neurones (n = 12) from this age group hypoxia suppressed phasic hyperpolarizations that occurred in synchrony with XII bursts. As similarly seen in inspiratory neurones, membrane potentials were depolarized by 5.1 ± 4.1 mV during the period of hypoxic augmentation.
The hypoxic response of respiratory neurones within the pre-Bötzinger complex resembles the response of neurones that were previously described under in vivo conditions. Thus we conclude that the ‘transverse rhythmic slice’ is a good model for studying the hypoxic response of the respiratory network under in vitro conditions.
It is well established that the mammalian respiratory system responds to hypoxia in a biphasic manner (Cherniack, Edelman & Lahiri 1971; Haddad & Mellins, 1984; Bureau, Zinman, Foulon & Begin, 1984; St John & Bianchi, 1985; Fregosi, Knuth, Ward & Bartlett, 1987; Maruyama, Yoshida & Fukuda, 1989; Neubauer, Melton & Edelman, 1990; Richter, Bischoff, Anders, Bellingham, Windhorst, 1991, 1993; Richter & Ballanyi, 1996). An initial increase in the frequency and amplitude of ventilation (augmentation) is followed by a secondary depression during which the ventilatory frequency and strength is decreased. During prolonged hypoxic conditions depression can terminate in a cessation of ventilation (central apnoea). As described in another paper (Ramirez, Quellmalz & Wilken, 1997), a biphasic response to hypoxia can be demonstrated in the rhythmic hypoglossal activity spontaneously generated in the transverse slice preparation of mice at all postnatal stages (postnatal day (P) 0–22) (Funk, Smith & Feldman, 1994; Ramirez, Quellmalz & Richter, 1996). It is assumed that this XII rhythmicity originates from the pre-Bötzinger complex (PBC) (Smith, Ellenberger, Ballanyi, Richter & Feldman, 1991). In this study we examine how hypoxia affects the activity of neurones within the pre-Bötzinger complex, the presumed site of respiratory rhythm generation (Smith et al. 1991; Smith, Funk, Johnson & Feldman, 1995). We expected to get a better understanding of the ontogenetic changes of the direct hypoxic effects on the respiratory network itself. In fact, a direct analysis of the hypoxic effect on pre-Bötzinger neurones was necessary to complement our previous study, since some effects on hypoglossal neuronal activity may reflect not only the response of the respiratory network, but also hypoglossal specificity (Haddad & Donnelly, 1990). The hypoglossal nerve does not just serve a respiratory function, it therefore exhibits certain properties that are different from those of the respiratory network. These differences include, for example, the tonic activation of the hypoglossal nerve during hypoxia (Haddad & Donnelly, 1990; Jiang, Xia & Haddad, 1992; Ballanyi, Völker & Richter, 1995; Ramirez et al. 1997). Such tonic activation is not seen in recordings from the phrenic nerve (Richter et al. 1991), a nerve which is devoted primarily to respiratory functions.
Thus, intracellular recordings were obtained from spontaneously active neurones localized within the pre-Bötzinger complex. These neurones were functionally identified as inspiratory, if their rhythmic activity was in phase with the extracellularly recorded hypoglossal burst. In contrast, neurones that were inhibited prior to and during hypoglossal activity will be defined as expiratory neurones. This functional identification refers to the fact, that the hypoglossal nerve in vivo discharges rhythmically in phase with inspiratory activity (Withington-Wray, Mifflin & Spyer, 1988; Smith, Greer, Liu & Feldman, 1990). Using this approach we also demonstrate at the single cell level that the biphasic response generated by the transverse slice preparation has many similarities to the response of the intact respiratory control system. The hypoxic response as revealed e.g. from both inspiratory and expiratory neurones was similar to the previously published hypoxic response which was recorded under in vivo conditions from equivalent neurones (e.g. Richter et al. 1991; England, Melton, Douse & Duffin, 1995).
METHODS
Preparation
Male and female mice (MRI-1 and Bahabor, P0-22) were deeply anaesthetized with ether and decapitated at the C3-C4 spinal level. All steps to obtain functional slice preparations have been published elsewhere (Ramirez et al. 1996), and shall only briefly be summarized in this study. The brainstem was isolated in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): 128 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 24 NaHCO3, 0.5 NaH2PO4 and 30 D-glucose equilibrated with carbogen (95 % O2 and 5 % CO2) to pH 7.4. Secured in a vibratome with the rostral end up, thin slices were sectioned serially from rostral to caudal until reaching the rostral boundary of the pre-Bötzinger complex. The pre-Bötzinger complex was recognized by cytoarchitectonic landmarks, such as inferior olive (IO), nucleus of the solitary tract (NTS), hypoglossal nucleus (XII) and nucleus ambiguus (NA); however, the slice did not contain the facial nucleus. The rhythmic slice which was contained within 650–700 μm caudal of this rostral boundary (Fig. 1) was immediately transferred into a recording chamber. Submerged under a stream of ACSF (temperature, 29°C; flow rate, 10 ml min−1), the preparation was stabilized for 30 min in ACSF. The potassium concentration of the ACSF was raised over a further period of 30 min and maintained at 8 mm to keep rhythmic activity regular for up to 13 h. Hypoxia was induced by bubbling the ACSF with 95 % N2 and 5 % CO2 (pH 7.4). In this study, hypoxia was always maintained for 30 min. To avoid O2 diffusion all tubing except that within the roller pump were made of stainless steel. The hypoxic conditions resulted in anoxia at all depths of the transverse slice preparation (Ramirez et al. 1997).
Figure 1. Schematic representation of the transverse brainstem slice obtained from mice together with an intracellular recording from a rhythmically active neurone.
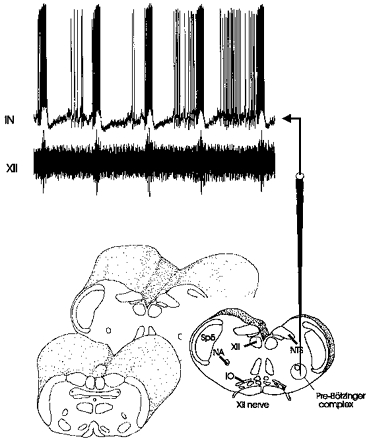
Transverse slice, encircled area indicates the location of the pre-Bötzinger complex (PBC). The arrow above the schematic electrode points toward a whole-cell patch recording from an inspiratory neurone (IN) recorded in the pre-Bötzinger complex of a P15 mouse (upper trace) and simultaneously with hypoglossal (XII) rootlet activity using a suction electrode (lower trace). Abbreviations: IO, inferior olive; NA, nucleus ambiguus; NTS, nucleus of the tractus solitarius; Sp5, spinal trigeminal nucleus; XII, hypoglossal motor nucleus; XII nerve, hypoglossal rootlet.
Recording and data analysis
The activity from the peripheral end of cut XII rootlets was recorded extracellularly with suction electrodes (Fig. 1, lower trace) and integrated electronically with a leaky resistance-capacitance (RC) circuit (for example see Fig. 2, lower traces). Whole-cell patch recordings were obtained from the pre-Bötzinger complex, a region which was identified using anatomical landmarks clearly visible under a dissection microscope (see Fig. 1A). Somatic recordings were distinguished from intra-axonal recordings by the shape of the action potential and presence of spontaneous synaptic activity. Recordings were obtained with unpolished patch electrodes with an outer tip diameter of 1–3 μm. These electrodes were manufactured from filamented borosilicate glass (Clarke GC150F) and had a resistance of 7–8 MΩ when filled with a solution containing (mm): 140 D-gluconic acid (potassium salt), 1 CaCl2, 10 EGTA, 2 MgCl2, 4 Na2ATP, 10 Hepes (pH 7.3–7.4). Respiratory neurones were recorded with the blind-patch technique using an intracellular amplifier (NPI SEC-10L). Before establishing a seal, neurones were identified as respiratory, if the extracellularly recorded rhythmic activity coincided with the rhythmic activity recorded from the hypoglossal nerve (for details see Ramirez et al. 1996).
Figure 2. Response to hypoxia in an inspiratory neurone of a P1 mouse.
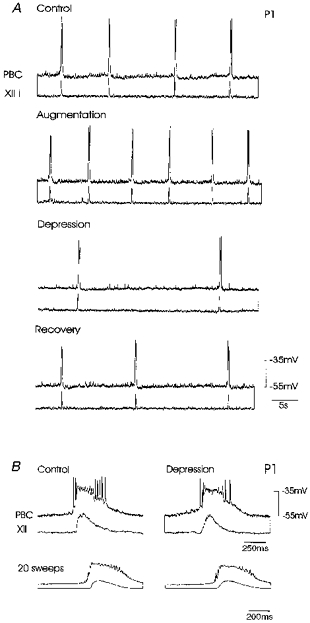
A, hypoxia initially induced an increase in the frequency of rhythmic activity (augmentation, second panel) recorded intracellularly in a PBC neurone and hypoglossal activity which was integrated (XII i) (here and in subsequent figures paired as upper and lower traces, respectively). Augmentation was followed by a decrease in frequency of rhythmic activity (depression, third panel). Five minutes after hypoxia (recovery, lower panel) frequency of rhythmic activity was increased, but control frequency values were not reached (upper panel). B, the shape of rhythmic depolarizations in the inspiratory neurone (upper trace pairs) was not affected as shown in the average of twenty bursts (lower trace pairs) obtained during the control (left panel) and depression (right panel). Note, that action potentials inactivated during the peak of the burst, since the neurone was current clamped at a depolarized level of -55 mV.
To better analyse neuronal activity, data were evaluated on- and off-line with a software program written for personal computers. Data were digitized using a Labmaster interface (Scientific Solutions, Inc.). All quantitative data are given as means ± s.e.m. Significances (P < 0.05) were determined using Student's t test. This study is based on forty-six intracellular recordings from respiratory neurones.
RESULTS
Throughout this paper hypoxic ‘augmentation’ is defined as the initial phase of the hypoxic response. This phase was characterized by an increase in the frequency of rhythmic discharges in neurones within the pre-Bötzinger complex as well as in burst discharges within the XII nerve. ‘Depression’ refers to the second phase of the hypoxic response, which was characterized by a decrease in the frequency of rhythmic discharges in neurones within the pre-Bötzinger complex and XII. This phase led in more mature preparations (> P8) to a cessation of rhythmic discharge in neurones of the pre-Bötzinger complex and XII nerve, referred to as ‘central apnoea’.
Hypoglossal activity was used as a marker to define functional types of respiratory neurones. Two types of rhythmically active neurones were evaluated: (1) ‘inspiratory’ neurones, which discharged in phase with hypoglossal activity (n = 25) and (2) ‘expiratory’ neurones, which were rhythmically inhibited prior to and during the hypoglossal bursts (n = 21). Other subtypes of neurones were not considered in this study.
PBC neurones in P0–4 slices
Inspiratory neurones
The most obvious hypoxic effect on inspiratory neurones obtained from mice aged P0–4 was a change in the frequency of occurrence of rhythmic depolarizing potentials (Fig. 2A, upper traces). In four of five inspiratory neurones neither membrane potentials during interburst intervals nor the amplitude of rhythmic drive potentials (on average 21 ± 1.7 mV under control conditions, 18 ± 2.5 mV during augmentation and 22 ± 1.4 mV during depression) were significantly affected by hypoxia (Fig. 2B, upper panel). This is also evident in the average of twenty rhythmic drive potentials obtained from the same neurone in Fig. 2A under control conditions (Fig. 2B, lower left panel) and during ‘depression’ of respiratory activity (Fig. 2B, lower right panel). One of five neurones was hyperpolarized by 3 mV and depolarizing drive potentials decreased from 21 ± 0.7 to 11 ± 1 mV during augmentation and to 3.5 ± 0.4 mV during depression. Rhythmic activity was maintained in inspiratory neurones throughout the entire duration of hypoxia (examined always for 30 min) without leading to a ‘central apnoea’.
Expiratory neurones
Neurones that were inactive during XII burst activity (Fig. 3, upper left panel) were referred to as expiratory neurones. In neonates, the inhibition, started before the XII burst. However, note that this early inhibition was not as obvious, compared with the situation in more mature mice (see for example Fig. 7). Since these neurones exhibited a weak inhibition prior to the onset of the hypoglossal burst and no inhibition after the hypoglossal burst, these neurones do not appear to be equivalent to the in vivo E2-neurones (e.g. Richter et al. 1991). The in vivo E2 neurones show typically an inhibition following inspiration. The depolarization pattern of the expiratory neurones described in this study ressemble neurones that are under in vivo conditions called ‘post-inspiratory neurones’ (Schwarzacher, Smith & Richter, 1995). The in vitro expiratory neurones became tonically active during the period of augmentation (n = 6, Fig. 3, middle left panel). Later during depression an excitatory component became apparent that occurred in phase with XII bursts (Fig. 3, lower left panel). To document this change we averaged eighteen sweeps of neuronal activity using the onset of hypoglossal activity as a trigger. The inspiratory hyperpolarization of 1.6 ± 0.4 mV in the expiratory neurone (Fig. 3, upper right panel), disappeared during late augmentation and the beginning of depression, revealing a slight phasic depolarization (Fig. 3, middle right panel). This depolarization was not seen in the left panel, since it is an example which was obtained at the beginning of the augmentation phase. During depression, action potential discharge occurring during the interburst intervals ceased in expiratory neurones due to a steady membrane hyperpolarization of 4.1 ± 2.3 mV. However, the amplitude of phasic inspiratory depolarizing input increased, being 3.7 ± 0.4 mV at the beginning of depression and 5.4 ± 1 mV (n = 6) after 15 min of depression. As depicted in the lower panel of Fig. 3, this depolarizing input caused the neurones to discharge in phase with XII rootlet activity. It should be emphasized that the recordings were obtained in the whole-cell mode with 1–3 μm tipped patch electrodes containing low chloride concentrations (4 mm). It is therefore unreasonable to assume that there was reversal of IPSPs due to increase in intracellular chloride and a shift in chloride equilibrium potential. The increase in the amplitude of the inspiratory depolarizing drive potential is seen as the average (Fig. 3, lower right panel), which was obtained from eighteen sweeps of consecutive bursts of the neurone shown in the left panel. This ‘inspiratory activity’ was maintained throughout the entire duration of hypoxia (30 min).
Figure 3. Response to hypoxia in an expiratory neurone of a P0 mouse.
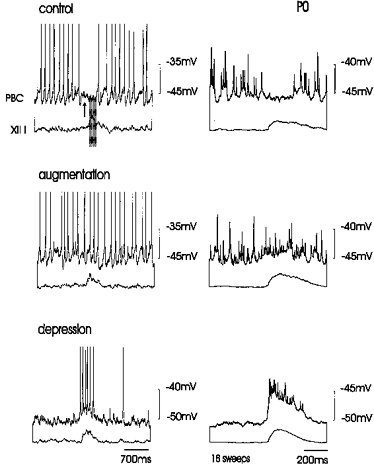
The phasic hyperpolarization in the expiratory PBC neurone (Control, upper panel), occurred prior to (arrow) and during integrated XII activity (I for inspiration). This phasic hyperpolarization was suppressed during hypoxia-induced augmentation (augmentation, second panel) and a phasic depolarization was seen during depression (upper traces, lower panel). Left panels show original recordings, right panels illustrate the averaged activity obtained from eighteen sweeps.
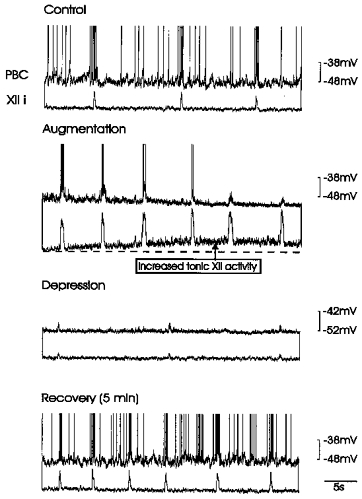
Figure 7. The effect of hypoxia on expiratory neurones in a P10 mouse.
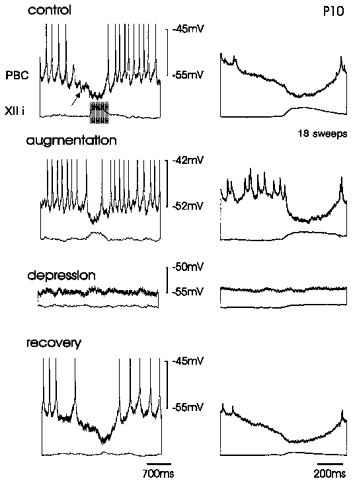
The rhythmic hyperpolarizations occurring prior to and during integrated XII activity were, compared with control conditions (upper panel), decreased during augmentation (second panel) and suppressed during depression (third panel). The hyperpolarization consisted of two components as evident in the average of eighteen sweeps (right panels). Note that hypoxia had a differential effect on the two components of phasic hyperpolarization.
PBC neurones in P4-7 slices and the effect of hypoxia on the coupling ratio
As described previously in Ramirez et al. (1996), the coupling between rhythmic bursts recorded from XII rootlets and rhythmic bursts of inspiratory neurones (n = 25) changed at the age of 4 days. This property is also seen in Fig. 4A (upper panel). In slices older than P4, a burst in the hypoglossal rootlets occurred only every 3rd to 5th burst in the pre-Bötzinger complex neurone. Although this weak coupling between neuronal activity in the PBC and XII is typical for slices obtained from more mature mice, we found that the hypoxic responses of slices (n = 6) obtained between P4 and P7 were still characterized by neonatal features: (1) the augmentation was restricted to a change in frequency without a significant effect on the amplitude of hypoglossal activity (see for example Fig. 4A, second panel) and (2) the depression, which was mainly characterized by a decrease in frequency, was sustained without terminating in apnoea (Fig. 4A, third panel). At the beginning of hypoxia, i.e. during the augmentation phase, neurones in the pre-Bötzinger complex revealed an increase in spike discharge and in the amplitude of respiratory drive potentials coinciding with hypoglossal bursts (Fig. 4A, second panel). This was the case for all six neurones examined (on average drive potentials increased from 7.5 ± 0.8 to 10 ± 1 mV). During depression, which was characterized by a decrease in frequency of rhythmic XII activity, the membrane potential was hyperpolarized by approximately 3.3 ± 2 mV and the amplitude of drive potentials was reduced to 5.9 ± 1 mV (Fig. 4A, third panel). Also reduced was the phasic hyperpolarization following each drive potential (control, 4 ± 1 mV; during depression, 2.4 ± 0.4 mV). In five of these six neurones a depression, i.e. a reduction in rhythmic drive potentials, started before an amplitude depression was obvious in the extracellularly recorded XII nerve.
Figure 4. Effect of hypoxia on the coupling between an inspiratory neurone and the XII rootlets in a P6 mouse.
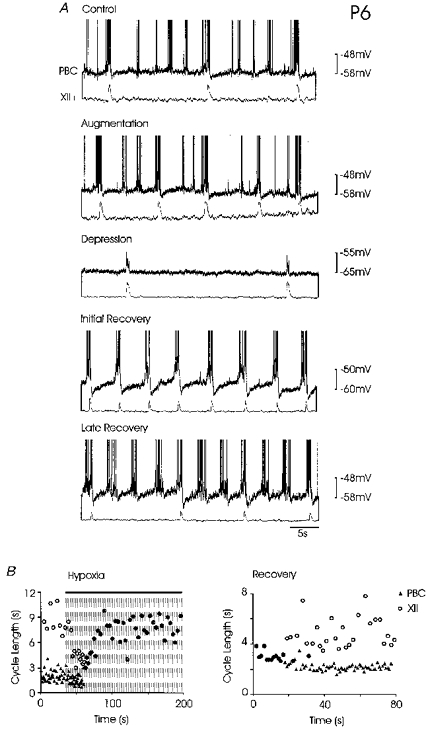
Hypoxia initially induced an increase in the frequency of rhythmic activity (Augmentation, second panel) recorded in a PBC neurone and integrated hypoglossal activity. Augmentation was followed by a decrease in frequency of rhythmic activity (Depression, third panel). Five minutes after hypoxia (Recovery, lower panel) frequency of rhythmic activity was increased. Note that the coupling between the activity in the PBC neurone and the XII rootlet changed during hypoxia. During control, augmentation and late recovery, bursts occurred in the PBC neurone also in the interval between XII bursts. A 1:1 coupling is seen during depression and early recovery. B, sequential histograms demonstrating the effect of hypoxia on the coupling of a PBC neurone (▴) and XII rootlet activity (○) at the onset of hypoxia (left histogram) and during recovery (right histogram). Note, when coupling is 1:1, i.e. PBC and XII neuronal activity coincide, the superimposition of the filled triangles and the open circles appears as filled circles.
The drive potentials occurring in the interval between two XII bursts were always smaller in amplitude than the bursts occurring in phase with XII bursts. Thus, due to a steady depression of network interaction, only the strongest bursts remained visible and ‘interphase bursts’ were suppressed during hypoxic depression. Due to this depressing effect there was also a change in the coupling between the neurone in the PBC and XII rootlet activity. This is documented in sequential event histograms taken from one individual neurone (Fig. 4B) where cycle length of rhythmic PBC neuronal activity (Fig. 4B, triangles) and XII rootlet activity (Fig. 4B, open circles) are plotted against time. As seen in the left graph of Fig. 4B, there was a decrease in XII cycle length (augmentation). During the peak of augmentation, cycle length values of the XII were similar to those of PBC neuronal activity. At the time, just prior to the peak of augmentation a slight decrease in PBC neuronal cycle length was also apparent, but not as obvious as for XII activity. Augmentation was followed by a depression during which cycle lengths of XII and PBC neuronal activity coincided in a 1:1 manner. After re-establishment of normoxic conditions, cycle length of XII and PBC activity were decreased (Initial recovery, Fig. 4A) and their burst activity coupled in a 1:1 manner (Fig. 4A, fourth panel). This period of increased activity was accompanied by an enhanced amplitude of drive potentials (on average for the examined neurones, control, 8.5 ± 1.5 mV; recovery, 12.4 ± 0.9 mV, n = 6). The phasic hyperpolarizations following each burst in the PBC neurone were also more pronounced compared with control conditions (control, 3.7 ± 1.3 mV; recovery, 7.4 ± 0.59 mV). The coupling between the activity in the PBC neurone and the XII rootlet weakened again later during recovery (late recovery, Fig. 4A, lower panel) which is also graphically shown in the right sequential histogram (Fig. 4B).
The coupling ratio between the XII activity and inspiratory neurones was identical with the ratio between the XII and expiratory neurones. Thus, it was not unexpected that the hypoxic changes as described for inspiratory neurones were the same in expiratory neurones (n = 3). During depression, the phasic hyperpolarizations occurring during the pre-inspiratory interval were suppressed before the hyperpolarizations occurring in phase with XII bursts.
PBC neurones in slices older than 8 days
Inspiratory neurones
The hypoxia-induced increase in rhythmic XII discharge (initial augmentation) was accompanied by an increase in the frequency of excitatory drive potentials and a steady membrane depolarization of 4.8 ± 3.7 mV in inspiratory neurones (n = 14). A feature which was always seen in inspiratory neurones (n = 14) was a transient increase in postsynaptic noise activity (Fig. 6B, middle panel). During later periods of augmentation some inspiratory neurones (n = 4) were hyperpolarized by 6.2 ± 4.1 mV (relative to the control membrane potential) before a depression was manifested in a decreased frequency of XII rootlet activity (Fig. 5, second panel). These four neurones remained hyperpolarized throughout the apnoea period which was characterized not only by the absence of rhythmic activity in the XII nerve but also by the absence of rhythmic drive potentials in respiratory neurones recorded within the PBC. Although apnoea was observed in all examined inspiratory neurones of this age group (n = 14), the hyperpolarization was not seen in all neurones. Hyperpolarization was either missing (n = 9) or not sustained (n = 1) throughout the hypoxic response which was in this study always maintained for 30 min. In these cases a depolarization of 2–10 mV persisted throughout the apnoea period (Fig. 7).
Figure 6. Effect of hypoxia on the depolarizations of an inspiratory neurone in a P12 mouse.
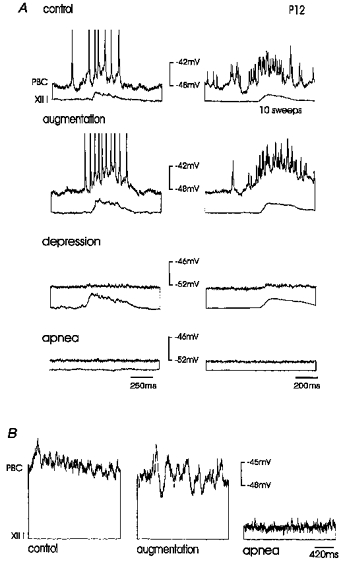
A, the rhythmic drive potentials occurring in phase with integrated XII activity were suppressed during depression (third panel) and abolished during apnoea (fourth panel). Left panels, original recording. Right panels, recordings averaged from ten sweeps. B, the membrane potential recorded during the interburst intervals was compared with control conditions (left panel) characterized by increased synaptic noise during augmentation (middle panel), followed by a decreased level of synaptic activity during apnoea (right panel).
Figure 5. Effect of hypoxia on an inspiratory neurone in a P12 mouse.
The inspiratory neurone recorded simultaneously with integrated XII activity was, compared with control conditions (top panel), hyperpolarized during augmentation (second panel) and depression (third panel).
An increase in the amplitude of depolarizing drive potentials was observed in six of fourteen neurones (on average for all examined inspiratory neurones: control, 5.7 ± 1.2 mV; augmentation, 8.3 ± 2 mV; n = 14) which led to an increased discharge frequency of action potentials (Fig. 5, second trace, first burst; Fig. 6A, left second panel). The enhanced amplitude of drive potentials is further documented in the average of ten sweeps of neuronal activity triggered from the onset of XII bursts (Fig. 6A, right second panel). It must be emphasized that the response of inspiratory neurones was not uniform and eight of the fourteen neurones exhibited no alteration in the amplitude of drive potentials (n = 4.8 ± 1 mV; augmentation, 5.1 ± 2.8 mV) despite an increase in the frequency of rhythmic drive potentials.
However, in all inspiratory neurones the amplitudes of depolarizing drive potentials decreased gradually (Fig. 5, second and third panel) until they disappear completely before (n = 9) or in parallel with rhythmic XII activity (n = 5; Fig. 6A, lower panel; Fig. 6B, right panel).
Five to ten minutes after reintroducing carbogen (95 % O2 and 5 % CO2) to the bath, rhythmic depolarizing drive potentials reappeared in all inspiratory neurones, sometimes (n = 7) preceeding a recovery of rhythmic activity in hypoglossal rootlets.
Expiratory neurones
As described for expiratory neurones recorded in neonatal slices, augmentation was initially accompanied by a depolarization (on average 5.1 ± 4.1 mV, n = 12), an increased synaptic noise and a reduction of phasic inhibition occurring in phase with XII activity (control, 7.3 ± 3.1 mV; early augmentation, 4.7 ± 1.5 mV). The hyperpolarizing input to expiratory neurones consisted of two very distinct components: (1) a ‘pre-inspiratory’ or ‘presumably expiratory’ component preceding the XII bursts and (2) an ‘inspiratory’ component coinciding with XII bursts (Fig. 7, upper panel). As documented by the average of sixteen sweeps triggered from the onset of XII bursts, the slopes of the two components were different, being slower in the ‘pre-inspiratory’ and faster in the ‘inspiratory’ component. The two components were differentially affected by hypoxia. The pre-inspiratory component was supressed 1 min after the onset of augmentation, while the inspiratory component was maintained during augmentation. All phasic hyperpolarizations in expiratory neurones were finally suppressed during depression and apnoea (Fig. 7, third panel). As demonstrated for expiratory neurones in neonatal slices, some expiratory neurones of more mature slices (n = 5) exhibited during depression a phasic depolarizing input occurring in phase with hypoglossal activity.
DISCUSSION
In this study we recorded from respiratory neurones within the pre-Bötzinger complex and showed that they exhibited under in vitro conditions a biphasic response to hypoxia. An initial augmentation during which respiratory neurones were depolarized was accompanied by an increased synaptic noise. Inspiratory neurones exhibited an increased frequency of rhythmic depolarizing drive potentials, expiratory neurones an increased frequency of rhythmic hyperpolarizations occurring in phase with hypoglossal activity. Augmentation was followed by a secondary depression during which some neurones were hyperpolarized, while others remained depolarized. During prolonged phases of hypoxia drive potentials and rhythmic hyperpolarizing inputs were suppressed in slices from mice older than P8. Thus, our in vitro study is consistent with in vivo studies, where it has been demonstrated that the biphasic hypoxic response in the phrenic nerve corresponds to a biphasic response of neurones recorded within the ventral respiratory group (Cherniack et al. 1971; Richter et al. 1991; England et al. 1995). The finding that in more mature slices neurones within the pre-Bötzinger complex cease to discharge rhythmically at later stages of the hypoxic depression was an important finding. It suggests that the hypoxic cessation of rhythmicity as seen in the transverse slice preparation resembles the in vivo central apnoea, where neurones within the ventral respiratory group also cease to discharge rhythmically (Richter et al. 1991). A central apnoea is further suggested, since it is assumed that neurones located within the pre-Bötzinger complex are essential for respiratory rhythm generation (Smith et al. 1991, 1995). It contains all known functional types of respiratory neurones in vivo (Schwarzacher et al. 1995) and deletion of this area abolishes respiratory rhythmic activity, both in vitro (Smith et al. 1991) and in vivo (Ramirez et al. 1994; Koshiya & Guyenet, 1996; Pierrefiche, Schwarzacher & Richter, 1997).
In this study many characteristic cellular changes were described that have previously been published for neurones within the respiratory network of in vivo adult cats (St John & Bianchi, 1985; Richter et al. 1991, 1993). As described for the in vivo respiratory system we found that: (i) respiratory augmentation was accompanied by a decline in the activity of several inspiratory neurones and a decline in the amplitude of phasic hyperpolarizations in expiratory neurones; (ii) respiratory depression was preceded by a phase of increased synaptic noise; and (iii) apnoea was associated in some neurones with a membrane hyperpolarization and disappearance of phasic synaptic transmission in neurones of the pre-Bötzinger complex; central apnoea was not due to a failure to generate action potentials, since pre-Bötzinger complex neurones continued to generate action potentials on several occassions also during apnoea.
In this study we found for expiratory neurones a complete suppression of a pre-inspiratory, presumably expiratory hyperpolarizing component and a reduction in the amplitude of an inspiratory component. A similar reduction of phasic hyperpolarizations can be achieved by blocking glycinergic receptors in the transverse slice with strychnine (Ramirez et al. 1996) suggesting that hypoxia causes the suppression of glycinergic chloride-mediated synaptic transmission. In this previous study we have also demonstrated that blockade of glycinergic inhibition reveals excitatory components that could alter the phasic discharge of expiratory neurones. Here, we found that hypoxia induced a similar change in the phasic discharge of neonatal expiratory neurones. Further investigations will be necessary to test the hypothesis that hypoxia may indeed block more effectively glycinergic inhibition. Since the rhythmic discharge pattern of expiratory neurones depends highly on phasic glycinergic inputs, while inspiratory neurones can generate a rhythmic discharge pattern also following blockade of glycinergic inhibition (Ramirez et al. 1996), we would expect that the discharge pattern of expiratory neurones is more affected than inspiratory neurones.
Although the physiological significance remains unclear, we demonstrated in this study, that in slices from mice older than P8 rhythmic activity in XII rootlets and the PBC was not always coupled in a 1:1 manner. As previously described (Ramirez et al. 1996) several inspiratory neurones receive three to four rhythmic depolarizing drive potentials during the interval between two consecutive hypoglossal bursts. These ‘interval-drive potentials’ were always smaller in amplitude than those occurring in phase with the hypoglossal bursts (Fig. 4, Ramirez et al. 1996) and disappeared during hypoxic depression prior to drive potentials occurring in phase with hypoglossal bursts (Fig. 4). Following the recovery from hypoxia the coupling was still 1:1 and no extra bursts were generated in the PBC (Fig. 4, fourth trace). The physiological relevance of these bursts may reflect the special situation of the reduced slice preparation. It nevertheless reveals that hypoxia clearly affected the coupling between bursts in neurones of the pre-Bötzinger complex and the bursts recorded in the XII rootlets. The hypoxia-induced effect which led to a 1:1 coupling between the neurones in the PBC and the XII motor output may be interpreted as an increased excitability of the respiratory system and/or the hypoglossal network during the initial phase of hypoxia.
Although some aspects of the hypoxic response may reflect the in vitro nature of the slice preparation (e.g. the hypoxic effect on the coupling), our study has indicated that most hypoxic changes in the transverse slice are very similar to those reported for the intact respiratory system. This is an important finding, since it clearly indicates that the neuronal structures captured by the 600–700 μm thick transverse slice are sufficient to generate the essential features at the cellular and systems level which have previously been described for the intact respiratory system at both the motor output level (Ramirez et al. 1997) and within the respiratory network (shown in this study). Of particular interest was the demonstration of apnoea, i.e. the cessation of rhythmic discharge in pre-Bötzinger complex neurones, which appeared at P8 and was not visible in neonatal preparations as shown in this and previous studies (Ballanyi et al. 1995; Völker, Ballanyi & Richter, 1995). In the future, this will enable us to investigate in further detail the cellular mechanisms underlying the generation of central apnoea and its maturational development.
Acknowledgments
We would like to thank Drs Jörg Schmidt and Keir G. Pearson for writing the software which was essential for the analysis of the data presented in this study. We also appreciate the excellent technical assistance by Anja Weiße-Blanke and Werner Ochotzki. This study is part of a doctoral thesis of U. J. A. Q. This study was supported by grants from the DFG (Ra 573/4–1, SFB 406 A4 (J. M. R.) and B6 (D. W. R.)).
References
- Ballanyi K, Völker A, Richter DW. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. NeuroReport. 1995;6:165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Bureau MA, Zinman R, Foulon P, Begin R. Diphasic ventilatory response to hypoxia in the newborn lamb. Journal of Applied Physiology. 1984;56:84–90. doi: 10.1152/jappl.1984.56.1.84. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Edelman NH, Lahiri S. Hypoxia and hypercapnia as respiratory stimulants and depressants. Respiratory Physiology. 1971;11:113–126. doi: 10.1016/0034-5687(70)90107-6. [DOI] [PubMed] [Google Scholar]
- England SJ, Melton JE, Douse MA, Duffin J. Activity of respiratory neurons during hypoxia in the chemodenervated cat. Journal of Applied Physiology. 1995;78:856–861. doi: 10.1152/jappl.1995.78.3.856. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Knuth SL, Ward DK, Bartlett D., Jr Hypoxia inhibits abdominal respiratory nerve activity. Journal of Applied Physiology. 1987;63:211–220. doi: 10.1152/jappl.1987.63.1.211. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JF, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. Journal of Neurophysiology. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Donnelly DF. O2 deprivation induces a major depolarisation in brainstem neurones in the adult but not in the neonatal rat. The Journal of Physiology. 1990;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG, Mellins RB. Hypoxia and respiratory control in early life. Annual Review of Physiology. 1984;46:629–643. doi: 10.1146/annurev.ph.46.030184.003213. 10.1146/annurev.ph.46.030184.003213. [DOI] [PubMed] [Google Scholar]
- Jiang C, Xia Y, Haddad GG. Role of ATP-sensitive K+ channels during anoxia: major differences between rat (newborn and adult) and turtle neurons. The Journal of Physiology. 1992;448:599–612. doi: 10.1113/jphysiol.1992.sp019060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. The Journal of Physiology. 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama R, Yoshida A, Fukuda Y. Differential sensitivity to hypoxic inhibition of respiratory processes in the anesthetized rat. Japanese The Journal of Physiology. 1989;39:857–871. doi: 10.2170/jjphysiol.39.857. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Melton JE, Edelman NH. Modulation of respiration during brain hypoxia. Journal of Applied Physiology. 1990;68:441–449. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Pierrefiche P, Schwarzacher SW, Richter DW. Functional significance of inhibitory synaptic mechanisms in respiratory rhythm generation of in vivo cat. Pflügers Archiv. 1997;433:R122. [Google Scholar]
- Ramirez JM, Pierrefiche O, Schwarzacher SW, Filloux F, McIntosh JM, Olivera BM, Richter DW. The influence of N-type calcium channel blockers on the respiratory network of adult cats. Society for Neuroscience Abstracts. 1994;20:1755. [Google Scholar]
- Ramirez JM, Quellmalz UJA, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. The Journal of Physiology. 1996;491:799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJA, Wilken B. Developmental changes in the hypoxic response of the hypoglossus respiratory motor output in vitro. Journal of Neurophysiology. 1997;78:383–392. doi: 10.1152/jn.1997.78.1.383. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballanyi K. Response of the medullary respiratory network to hypoxia: A comparative analysis of neonatal and adult mammals. In: Haddad GG, Lister G, editors. Tissue Oxygen Deprivation: Developmental, Molecular and Integrated Function. New York: Marcel Dekker, Inc.; 1996. pp. 751–777. [Google Scholar]
- Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. The Journal of Physiology. 1991;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Modulation of respiratory patterns during hypoxia. In: Speck DF, Dekin MS, Revelette WR, Frazier DT, editors. Respiratory Control, Central and Peripheral Mechanisms. Lexington, Kentucky: University Press; 1993. pp. 21–28. [Google Scholar]
- St John WM, Bianchi AL. Responses of bulbospinal and laryngeal respiratory neurones to hypocapnia and hypoxia. Journal of Applied Physiology. 1985;59:1201–1207. doi: 10.1152/jappl.1985.59.4.1201. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. Journal of Neurophysiology. 1995;73:1452–1459. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger H, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Funk GD, Johnson SM, Feldman JL. Cellular and synaptic mechanisms generating respiratory rhythm: insights from in vitro and computational studies. In: Trouth CO, Mils R, Kiwull-Schone H, Schlaefke M, editors. Ventral Brainstem Mechanisms and Control of Respiration and Blood Pressure. New York: Marcel Dekker; 1995. pp. 463–496. [Google Scholar]
- Smith JC, Greer J, Liu G, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. Journal of Neurophysiology. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Völker A, Ballanyi K, Richter DW. Anoxic disturbance of the isolated respiratory network of neonatal rats. Experimental Brain Research. 1995;103:9–19. doi: 10.1007/BF00241960. [DOI] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]


