Abstract
The composition of myosin heavy chains (MHCs) was investigated in young (1- to 8-week-old) and mature (9- to 26-week-old) guinea-pigs using two monoclonal antibodies directed specifically against α-MHC and β-MHC. In addition, maximum force and the rate of ATP consumption during isometric contraction were measured in chemically skinned trabeculae taken from the same hearts.
An age-dependent shift in the MHC composition was found. The α-MHC fraction decreased from 0.17 ± 0.02 (mean ± s.e.m.; n = 24) in young to 0.04 ± 0.01 (n = 43) in mature hearts. This shift was correlated with a decrease in tension cost (i.e. ATP consumption per second per trabecula volume/force per cross-sectional area) from 4.1 ± 0.2 mmol kN−1 m−1 s−1 (n = 23) in young to 2.5 ± 0.1 mmol kN−1 m−1 s−1 (n = 57) in mature hearts.
From the results it follows that the slow β-MHC isoform, which predominates in hearts of mature guinea-pigs, is about 5 times more economical than the fast α-MHC isoform. Calcium sensitivity of force and ATP consumption decreased with age, but stabilized within a few weeks after birth. The pronounced dependence of cardiac energetics on MHC composition should be taken into account in long-term studies of cardiac overload.
In the heart the expression of different proteins of the contractile apparatus changes during development and under pathological conditions (Swynghedauw, 1986). Such changes in protein expression have important consequences for cardiac energetics. Usually these changes are adaptations to new functional demands of the heart. For instance, during cardiac hypertrophy, an improved economy of contraction (Alpert & Mulieri, 1982) and a decrease in maximal velocity of shortening (Schwartz, Lecarpentier, Martin, Lompré, Mercadier & Swynghedauw, 1981; Ebrecht, Rupp & Jacob, 1982) and in the rate of tension recovery after quick stretch (Ventura-Clapier, Mekhfi, Oliviero & Swynghedauw, 1988) have been observed.
Myosin is one of the main proteins of the contractile apparatus. Together with actin, myosin takes part in the mechanism by which chemical energy of adenosine triphosphate (ATP) is converted to mechanical work. Hoh, McGrath & Hale (1977) have shown that two different myosin heavy chain isoforms exist in the heart: α-myosin heavy chain (α-MHC) and β-myosin heavy chain (β-MHC). The MHCs carry the site for the ATPase activity. In association with the myosin light chains, the two MHC isoforms give rise to three different isomyosins: the homodimers V1, composed of two α-MHCs, and V3, composed of two β-MHCs, and a heterodimer of α-MHC and β-MHC, named V2. It has been observed in vitro that V1 has the highest myosin ATPase activity and V3 the lowest. V2 has an ATPase activity intermediate between V1 and V3 (Pope, Hoh & Weeds, 1980).
In adult rats V1 is present almost exclusively in the heart ventricles. During cardiac hypertrophy, a reduced ATPase activity is found, which is correlated with a shift from the fast isomyosin V1 to the slow isomyosin V3 (Mercadier et al. 1981; Gorza, Pauletto, Pessina, Sartore & Schiaffino, 1981).
We have studied the ventricular MHC composition, maximum force and rate of ATP consumption (i.e. ATPase activity) during isometric heart contraction in guinea-pigs, because the MHC composition in their hearts is similar to the MHC composition in human hearts. The distribution of α-MHC and β-MHC was examined in both young (1- to 8-week-old) and mature (9- to 26-week-old) guinea-pigs using two specific monoclonal antibodies directed against α-MHC and β-MHC in an enzyme-linked immunosorbent assay (ELISA). To relate the MHC isoforms expressed with the economy of contraction, the maximum isometric force and the rate of ATP consumption were measured in chemically skinned trabeculae isolated from right and left ventricles. In the method used, the resynthesis of ATP is enzymatically coupled to the oxidation of reduced nicotinamide-adenine dinucleotide (NADH), which can be quantified photometrically. An advantage of this method is that it allows determination of the contractile and energetic properties simultaneously. Moreover, by standardization of the conditions (i.e. composition of the intracellular medium and sarcomere length) disturbing factors present in the intact heart (i.e. hormonal factors and variable calcium concentrations) are minimized. Since the heart normally works under submaximal circumstances the calcium sensitivity of force production and ATPase activity were also determined.
The age-dependent changes observed are important, not only because they might alter cardiac performance and interfere with the interpretation of changes in cardiac performance after cardiac overload, but also because they reflect a steep relation between energetic properties and MHC composition, which might be partly responsible for conflicting findings in the literature on myosin ATPase activity of human myocardium in vitro (e.g. Alpert & Gordon, 1962; Mercadier et al. 1983; Alousi, Grant, Etzler, Cofer, Van der Bel-Kahn & Melvin, 1990).
METHODS
Preparations
The adult guinea-pigs (Dunkin Hartley, female) studied were divided into two groups: young (150–450 g, 1–8 weeks old; n = 16) and mature (500–900 g, 9–26 weeks old; n = 27). The guinea-pigs were anaesthetized with xylazine (5 mg kg−1i.m.), ketamine (60 mg kg−1i.m.) and pentobarbitone (50 mg kg−1i.p.), and the hearts were rapidly excised. After excision, the heart was immediately perfused with Tyrode solution (mm: NaCl, 128.3; KCl, 4.7; CaCl2, 1.36; MgCl2, 1.05; NaHCO3, 20.2; NaH2PO4, 0.42; d-(+)-glucose monohydrate, 11.1) containing 20 mm 2,3-butanedione monoxime. Unbranched trabeculae were carefully dissected from the walls of the right and left ventricles (RVs and LVs, respectively). After dissection, the trabeculae were transferred to a dish containing standard relaxing solution to which 1 % (v/v) Triton X-100 was added in order to chemically permeabilize the preparation. The composition of this standard relaxing solution was (mm): Na2ATP, 6.13; MgCl2, 6.56; EGTA, 20; phosphocreatine, 10; Bes, 100; pH 7.1, adjusted with KOH; ionic strength, 200 mm, adjusted with potassium propionate. The preparations were left in this solution for 2 h at about 5°C to allow solubilization of virtually all membranous structures. Next, the skinned trabecula was attached to aluminium T-clips and mounted in the experimental set-up.
The dimensions of the preparations from young animals were 1.68 ± 0.11 and 1.51 ± 0.11 mm in length, 239 ± 18 and 181 ± 14 μm in width, and 241 ± 18 and 172 ± 14 μm in thickness (means ± s.e.m.) for, respectively, RVs (n = 11) and LVs (n = 12), measured at a resting sarcomere length of 2.2 μm. The dimensions of the right (n = 24) and left (n = 33) ventricular preparations from mature animals were, respectively, 2.01 ± 0.12 and 1.76 ± 0.11 mm in length, 215 ± 10 and 211 ± 10 μm in width, and 203 ± 10 and 200 ± 10 μm in thickness.
Experimental apparatus
The skinned trabecula was attached to a force transducer element (AE 801; SensoNor, Horten, Norway) at one end, and to a fixed hook at the other end. The natural frequency of the force transducer was about 2 kHz. The muscle preparation could be transferred manually between several baths to expose the trabecula to various solutions. The measuring bath used for the ATPase assay (volume, 30 μl) had thin glass windows to allow transmission of near-UV light (340 nm) for the measurement of NADH absorbance. The contents of this bath were stirred continuously by motordriven vibration (frequency about 3 Hz) of a membrane positioned at the bottom of the bath. The temperature inside the bath was kept at 20 ± 1°C. The absorbance and force signals were filtered at 2.5 Hz (-12 dB per octave) and recorded on an Olivetti M280 personal computer after A/D conversion at a sampling frequency of 5 Hz. The sarcomere length of the preparation was measured in relaxing solution by means of He-Ne laser diffraction (Model 1125; Uniphase).
Measurement of ATP consumption
ATP consumption of the skinned trabeculae was measured by an enzyme-coupled assay as described in detail previously (Ebus, Stienen & Elzinga, 1994). After ATP hydrolysis into adenosine diphosphate (ADP) and inorganic phosphate (Pi), the ADP formed was resynthesized to ATP. This reaction was enzymatically coupled to the oxidation of NADH to NAD+. This reaction sequence was catalysed by pyruvate kinase and lactate dehydrogenase. The breakdown of NADH was determined photometrically by measuring the absorbance of 340 nm near-UV light obtained from a 75 W xenon lamp, which was projected through the bath just beneath the preparation. Calibration of the NADH absorbance signal was carried out by injecting 0.05 μl of 10 mm ADP (i.e. 0.5 nmol) into the measuring bath.
Solutions
Three solutions were used, a relaxing solution, a pre-activating solution with low-calcium buffering capacity, and an activating solution, which contained, respectively (mm): MgCl2, 8.13, 7.69 and 7.57; Na2ATP, 5.82, 5.82 and 5.94; EGTA, 20, 0.5 and 0; HDTA, (hexan-diaminotetraacetic acid), 0, 19.5 and 0; CaEGTA, 0, 0 and 20; KOOCH2CH2CH3 (potassium propionate), 39.4, 40.3 and 40. CaEGTA was made by dissolving equimolar amounts of CaCO3 and EGTA. In addition, all solutions contained 0.9 mm NADH, 100 mm Bes, 5 mm sodium azide, 10 mm phosphoenolpyruvate, 4 mg ml−1 pyruvate kinase (500 U mg−1), 0.24 mg ml−1 lactate dehydrogenase (870 U mg−1), 10 μm oligomycin B, and 0.2 mm P1,P5-di(adenosine-5′)pentaphosphate. The experiments were performed at pH 7.0 (adjusted with KOH) and 20°C. The compositions were calculated as described by Fabiato (1981). The free Mg2+ and MgATP concentrations were 1 and 5 mm, respectively. Solutions with lower free Ca2+ concentrations were obtained by appropriate mixing of the activating and relaxing solutions, assuming an apparent stability of the CaEGTA complex of 106.58. All chemicals were of the highest purity available (Sigma).
Experimental protocol
During the experiments, the trabeculae were incubated in relaxing solution for at least 3 min, in pre-activating solution for at least 3 min, in activating solution for 1–5 min, and then returned to the relaxing solution. Before the first activation-relaxation cycle the sarcomere length of the preparation, as measured in relaxing solution, was adjusted to 2.2 μm. Then, following a first activation at saturating calcium concentration (pCa (-log[Ca2+]), 4.5), the sarcomere length was readjusted to 2.2 μm if necessary, and the length of the trabecula between the clips as well as the width and the thickness were measured (at × 50 magnification). It was found that after this readjustment, the resting sarcomere length remained stable throughout the experiment. The second contracture served as a first force and ATP consumption rate reference at a pCa of 4.5. The next two to three contractions were carried out at different pCa values (> 4.5). These measurements were followed by a control measurement at saturating Ca2+ concentration. This procedure was repeated until the full force-pCa and ATPase-pCa curves were obtained or until the isometric force of a control measurement was less than or equal to 80 % of the first control contraction. Usually eight to fifteen contractions could be performed. To correct for the relatively small deterioration of force development and ATPase activity, values were normalized to the nearest control values.
ATPase activity was derived from linear regression analysis of the absorbance signal. To correct for bleaching of NADH under the intense UV light and contaminant ATPase activity in the enzymes used, the slope of the absorbance signal measured after the trabecula had been returned to the relaxing solution was subtracted from the slope of the absorbance signal measured during the contraction. The Ca2+-activated ATPase activity was corrected for basal ATPase activity measured in relaxing solution (pCa 9) (Ebus et al. 1994). Basal activity amounted to about 20 % of the maximal ATPase activity.
Quantification of α- and β-myosin heavy chains in guinea-pig heart ventricles
Guinea-pigs were weighed on the day of heart removal. After dissection of the trabeculae, the atria and ventricles were separated and subsequently freeze dried. Dry weights were determined and the tissues were stored at -80°C. Heart weights were normalized to body weight.
In rat ventricular tissue the fast (α) and slow (β) MHC isoforms can be separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and quantified by subsequent laser densitometry. However, as found by others (Lompréet al. 1981), this separation was not successful for guinea-pig. To determine the amount of α- and β-MHC in guinea-pig ventricular tissue, an ELISA was performed, using two monoclonal antibodies directed specifically against α- and β-MHCs (mAb 249-5A4 and mAb 169-1D5, respectively). The production of these antibodies has been described previously (De Groot, Lamers & Moorman, 1989).
ELISA
Ninety-six-well plates (Greiner) were coated overnight at room temperature with homogenized ventricular tissue (1 μg well−1), diluted in 100 μl phosphate-buffered saline (PBS; 146 mm NaCl, 8 mm Na2HPO4 and 2 mm NaH2PO4; pH 7.35) containing 3 % bovine serum albumin (BSA). After coating, the plates were incubated for 1 h at 37°C with PBS-BSA to reduce non-specific binding. Next, the plates were incubated for 1 h at 37°C with the monoclonal antibodies (100 μl). All samples were applied in duplicate for incubation with the antibody directed against α-MHC (1:50, diluted in PBS-BSA) and also with the antibody against β-MHC (1:100). Following this step, antibody binding was detected using goat anti-mouse-HRP (horseradish peroxidase, Sigma; 100 μl; 1:800, diluted in PBS-BSA). Again the incubation took place for 1 h at 37°C. Between each step in the protocol the plates were washed with PBS-Tween 20 (0.05 % v/v). The most sensitive chromogenic substrate for detection of HRP-labelled antibodies is tetramethylbenzidine (TMB). Thus, to visualize the immunocomplex formed in the wells, the plates were incubated with 100 μl of 0.1 mg ml−1 TMB (Sigma) and 0.01 % hydrogen peroxide in 0.11 M sodium acetate (pH 5.5) for 30 min in the dark. The colour reaction was stopped by adding 50 μl 1 n H2SO4. The plates were read at 450 nm relative to a blank (PBS-BSA added to the wells instead of the antigen in the first step; following steps were the same). The duplicated results were reproducible within 5 %. Averaged results were used in the analysis.
Calibration of the ELISA was performed using hypothyroid and euthyroid rat ventricles. The samples were a kind gift from Dr P. P. de Tombe (Chicago, IL, USA). Rats were made hypothyroid by adding 0.8 g l−1 propylthiouracil (PTU) to the drinking water for a period of at least 6 weeks, while euthyroid control rats were housed under the same conditions without PTU. MHC composition in the rat ventricles was determined by SDS-PAGE and subsequent densitometry, as described below. Hypothyroid rat myocardium proved to be 100 %β-MHC. The euthyroid control animals contained a mixture of 95 %α-MHC and 5 %β-MHC. The calibration curves for the ELISA were obtained from the optical density (OD) readings from hypothyroid and euthyroid tissue and from mixtures of these two samples (see Fig. 1). From the curves fitted to the data points the amounts of α- and β-MHC present in the guinea-pig ventricles were calculated. The non-linearity of the relations obtained most probably reflects the binding characteristics of the primary antibodies to the myosin isoforms, because the curvature observed was similar to that found when the total protein concentration of the samples applied was varied. The degree of curvature of the relation between OD and total protein concentration from rat and guinea-pig were very similar. This strongly suggests that the binding characteristics of the two specific antibodies with the MHCs were the same for rat and guinea-pig. Using this method, the α-MHC and β-MHC content could be determined as a fraction of the total MHC content.
Figure 1. Calibration curves for the ELISA.
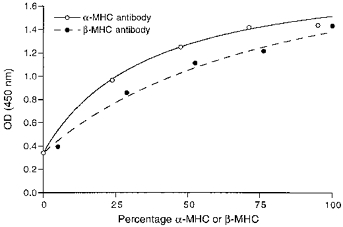
Hypothyroid (100 %β-MHC) and euthyroid (95 %α-MHC and 5 %β-MHC) rat heart samples were mixed in the same proportions as in Fig. 2B to obtain standard samples with known percentages of α- and β-MHC. The percentages of α- and β-MHC in the hypothyroid and euthyroid rat hearts were quantified by separating the MHCs in SDS-PAGE and subsequent densitometric analysis of the bands (see Methods). Hyperbolic relations were fitted to the data points as described in the text.
SDS-PAGE
SDS-PAGE was performed as described previously (Giulian, Moss & Greaser, 1983), using an acrylamide to bis-acrylamide ratio of 200:1 in the separating gel (12 % acrylamide; pH 9.3) and of 20:1 in the stacking gel (3.5 % acrylamide; pH 6.8). A Protean II xi cell was used (Bio-Rad). The gel dimensions were: width, 16 cm; length, 20 cm; and thickness, 0.75 mm. The stacking gel was 2.5 cm in length. Samples of the ventricles were dissolved in sample buffer containing 62.5 mm Tris (pH 6.8), 15 mm dithiothreitol, 0.1 mm phenylmethylsulphonyl fluoride, 0.5 mm leupeptin, 1 % (w/v) SDS, 0.01 % (w/v) bromophenol blue and 15 % (v/v) glycerol. Comparable protein amounts (0.8 μg) were loaded in each gel lane. The samples were run at constant current (24 mA) for a total of 5 h (approximately 1800 Volt hours). Silver staining was performed as described by Giulian et al. (1983). In Fig. 2, examples are shown which indicate that in rat myocardium the α-and β-MHCs were nicely separated, whereas, as has been found by others (Lompréet al. 1981), in guinea-pig ventricular muscle the separation was not sufficient to allow a quantitative analysis.
Figure 2. Protein analysis of guinea-pig and rat samples.
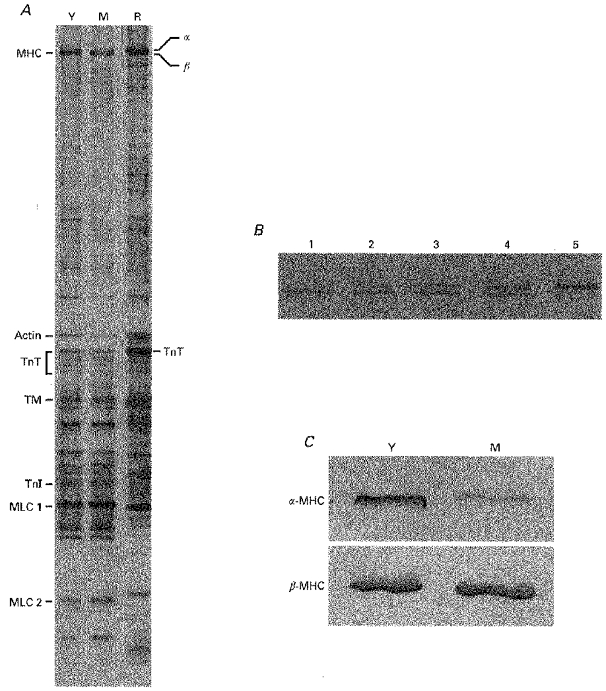
A, silver-stained SDS-polyacrylamide gel from young (Y) and mature (M) guinea-pig hearts and from euthyroid rat heart (R) (0.8 μg protein per lane). Contractile proteins were identified by Western immunoblotting with specific antibodies, as indicated in the text: MHC, myosin heavy chain; TnT, troponin T; TM, tropomyosin; TnI, troponin I; MLC, myosin light chain. B, separation of the two MHC isoforms in rat heart samples. Lane 1, hypothyroid; lane 5, euthyroid; lanes 2–4, 3:1, 1:1 and 1:3 mixtures of hypo- and euthyroid rat samples, respectively. C, although in guinea-pig ventricular muscle the α- and β-MHCs were not separated by SDS-PAGE (A, lanes Y and M), the presence of α-MHC and β-MHC in young and mature guinea-pig hearts could be visualized with Western immunoblotting using two monoclonal antibodies directed specifically against α-MHC and β-MHC (0.8 μg protein was loaded in each lane).
Western immunoblotting
The presence of α- and β-MHC in guinea-pig hearts was also confirmed in Western immunoblots. This technique was also used to determine troponin T (TnT) and troponin I (TnI) content and to investigate TnT and TnI isoform distribution. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (0.45 μm pore size) using the Bio- Rad TransBlot Cell. After transfer, the nitrocellulose membrane was stained with Ponceau S to demonstrate protein bands. After destaining the membrane in PBS-Tween 20 (0.5 %), the nitrocellulose membrane was incubated in 3 % BSA in PBS-Tween 20 for 1 h, washed 3 times and incubated for 1 h with various primary antibodies, diluted in BSA-PBS-Tween 20. After removing the primary antibody and washing 3 times, the primary antibody binding was detected using alkaline phosphatase-labelled goat anti-mouse antibody (Promega), diluted 1:7500 in BSA-PBS-Tween 20. Finally, nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Sigma) were used to visualize the specific immunocomplex formed on the nitrocellulose membrane. The primary MHC antibodies used were the same as those used for ELISA, i.e. α-MHC antibody (mAb 249-5A4, 1:50) and β-MHC antibody (mAb 169-1D5, 1:100). To detect TnT and TnI, TnT antibody (clone JLT-12, 1:200; Sigma) and TnI antibody (mAb 1691, 1:200; Chemicon, Temecula, CA, USA) were used. For further identification of the protein bands on the gels actin antibody (clone C4, 1:100; Boehringer Mannheim), myosin light chain antibody (clone MY-21, 1:200; Sigma) and tropomyosin antibody (clone TM311, 1:400; Sigma) were used.
Data analysis
Sigmoidal force-pCa and ATPase-pCa relations were fitted by a non-linear fit procedure to a modified Hill equation:
where P is steady-state force (or ATPase activity). P0 denotes the steady isometric force (or ATPase activity) at saturating Ca2+ concentration, nH represents the steepness of the relationship, and K (or pK) represents the Ca2+ concentration (or pCa) at which force (or ATPase activity) = 0.5 ×P0, i.e. the mid-point of the force-pCa (ATPase-pCa) relation. P0 was determined from the first reference activation at a pCa of 4.5. The calibration curves for the ELISA were fitted by means of a hyperbolic function:
where x is the percentage of α- or β-MHC of the total MHC content and a, b and c are constants.
Values are given as means ± s.e.m. of n experiments. Differences were tested by means of Student's t test at a 0.05 or 0.001 level of significance (P < 0.05, P < 0.001). The linear regression lines shown in the figures were calculated treating the parameter plotted along the x-axis as an independent variable. Differences between the slopes of regression lines, obtained with results from left and right ventricles, were tested using a multiple slope comparison (Sokal & Rohlf, 1981).
RESULTS
ATP consumption and force production in chemically skinned trabeculae were measured during isometric (fixed ends) contraction. Original recordings obtained during a contraction-relaxation cycle are shown in Fig. 3. In this figure, the isometric force production (A) and ATPase activity (B) at a sarcomere length of 2.2 μm are shown. Following transfer of the trabecula to the activating solution, active isometric force developed and the NADH absorbance started to decrease. After the isometric contraction, the trabecula was returned to the relaxing solution. Recording of NADH absorbance in the measuring bath was continued for some time to allow determination of the background decline in NADH absorbance.
Figure 3. Recordings of force production (A) and NADH absorbance (B) during a contraction-relaxation cycle.
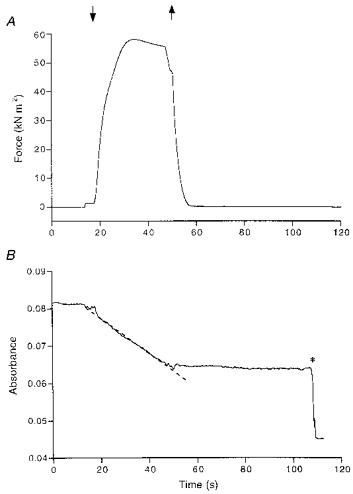
When the trabecula was transferred to the activating solution (downward arrow), active isometric force developed, and the NADH absorbance signal started to decrease. The rate of decline is a measure of ATP consumption. ATP consumption was determined by calculation of the slope of the dashed regression line fitted to a selected part of the absorbance signal. After returning the trabecula into the relaxing solution (upward arrow), the NADH absorbance baseline was recorded. At the time indicated by the asterisk, 0.5 nmol ADP was injected into the measuring bath to calibrate the absorbance signal. The zero level of the absorbance signal was arbitrarily chosen.
The Ca2+ sensitivity of force and ATPase activity
The Ca2+ sensitivity of the contractile apparatus of the hearts of very young (1- to 2.5-week-old; n = 7) and mature (9- to 19-week-old; n = 5) guinea-pigs was determined by exposing the trabeculae to different Ca2+ concentrations and measuring the isometric force and ATPase activity. The mean force-pCa and ATPase-pCa relationships are shown in Fig. 4A and B, respectively. The isometric force and ATPase activity values were fitted to the Hill equation. The steepness of the force-pCa and ATPase-pCa relations did not differ significantly between very young (respectively, 3.26 ± 0.49 and 2.96 ± 0.37) and mature (respectively, 4.01 ± 0.57 and 3.43 ± 0.34) guinea-pigs. The pK values representing the mid-points of the force-pCa and ATPase-pCa relations were considerably higher (0.16 pCa units) in very young guinea-pigs (respectively, 5.74 ± 0.02 and 5.73 ± 0.02 pCa units) than in mature animals (respectively, 5.58 ± 0.02 and 5.57 ± 0.01 pCa units), indicating a decreased calcium responsiveness in mature guinea-pigs. In a separate study (second group), the calcium sensitivity of isometric force and ATPase activity in young guinea-pigs (4–8 weeks old; n = 9) and mature guinea-pigs (9–26 weeks old; n = 22) were compared. No differences were found between these groups. This indicates that the decline in calcium responsiveness stabilizes quickly after birth.
Figure 4. Isometric force and ATPase activity at different free Ca2+ concentrations.
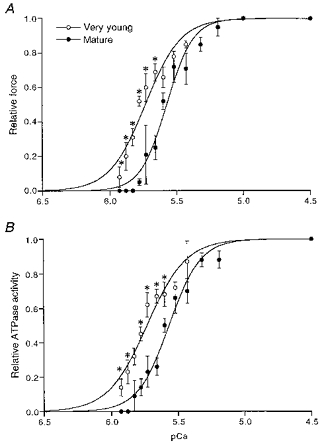
Isometric force (A) and ATPase activity (B) for each trabecula are normalized to the control force and ATPase activity found at saturating Ca2+ concentration (pCa = 4.5). Mean values are shown ± s.e.m.*P < 0.05, significantly different from mature guinea-pigs. The Hill curves were fitted to the data points as indicated in the text.
It should be noted that the results from the first group (very young and mature animals) and from the second group (young and mature animals) were obtained with two different batches of EGTA. Although a correction was made for differences in EGTA purity, the results of calcium sensitivity in the mature groups differed slightly, probably due to minor differences in the free calcium concentration. However, this does not affect the results obtained at saturating calcium concentration presented below.
Since the difference in calcium sensitivity between very young and mature guinea-pigs might be due to changes in the troponin complex (Godt, Fogaça & Nosek, 1990; Reiser, Westfall, Schiaffino & Solaro, 1994), the composition of TnT and TnI were compared in very young and mature guinea-pigs, using SDS-PAGE and Western immunoblotting. Actin was used as an internal standard in these determinations. The silver-stained gels and immunoblots both showed that the molecular weights and ratios of TnT and TnI to actin did not vary significantly between very young and mature guinea-pigs. The ratios of the different TnT isoforms, TnT1, TnT2, TnT3 and TnT4, and of TnI to actin were, respectively, in very young guinea-pigs: 0.49 ± 0.02, 0.20 ± 0.01, 0.16 ± 0.01, 0.10 ± 0.01, and 0.36 ± 0.01, and in mature guinea-pigs: 0.54 ± 0.02, 0.21 ± 0.02, 0.15 ± 0.02, 0.10 ± 0.01 and 0.44 ± 0.05.
Force and ATPase activity versus body weight
For all trabeculae measured in this study, maximum force per cross-sectional area and ATPase activity per trabecula volume were calculated. To determine whether these parameters are age dependent, results were presented in Fig. 5 as a function of body weight. Body weight is a reliable index of age because the growth curves of the animals show little individual variation. Since the number of suitable trabeculae per heart on which measurements could be performed varied, the results of all trabeculae were plotted as a function of body weight. Plotting the mean data as a function of body weight yielded very similar results. Both force per cross-sectional area and ATPase activity per volume gave rise to a scattered pattern of values. Part of this scatter may be due to inaccuracy in the determination of the cross-sectional area of the trabeculae. The regression coefficients (r) for the relation between ATPase activity per volume and body weight were 0.65 and 0.47 for LV and RV, respectively (Fig. 5A). The slopes of both regression lines were both significantly different from zero (P < 0.001). The regression coefficients for the relation between maximum force per cross-sectional area and body weight for LV and RV were 0.27 and 0.17, respectively (Fig. 5B); the slopes of both regression lines were not significantly different from zero. Tension cost was calculated for each trabecula by dividing the ATPase activity per volume by the force per cross-sectional area. The tension cost informs us about the rate of ATP splitting necessary for maintenance of isometric force. It is a measure of muscle economy, which has the advantage that it is not influenced by the inaccuracy in the determination of cross-sectional area. The relation between tension cost of the trabeculae and body weight of the guinea-pigs is shown in Fig. 5C. Statistical analysis revealed highly significant correlations (r = 0.78 for LV and 0.74 for RV; P < 0.001) between tension cost and body weight. This figure clearly shows that the tension cost of cardiac contraction declines with age.
Figure 5. Dependence of ATP consumption, isometric force and tension cost on body weight.
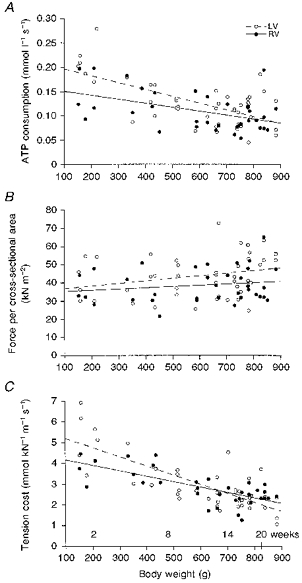
A, rate of ATP consumption per volume; B, isometric force per cross-sectional area; C, tension cost. BW, body weight; LV, left ventricular trabeculae; RV, right ventricular trabeculae. Regression lines shown: LV: ATPase activity per volume = (0.21 ± 0.02) - (14.0 ± 2.3) × 10−5× BW (r = 0.65; P < 0.001), force per cross-sectional area = (35.4 ± 4.9) + (14.0 ± 7.6) × 10−3× BW (r = 0.27), tension cost = (5.65 ± 0.35) - (4.4 ± 0.6) × 10−3× BW (r = 0.78; P < 0.001). RV: ATPase activity per volume = (0.16 ± 0.02) - (8.2 ± 2.7) × 10−5× BW (r = 0.47; P < 0.001), force per cross-sectional area = (34.5 ± 4.5) + (6.8 ± 6.9) × 10−3× BW (r = 0.17), tension cost = (4.44 ± 0.27) - (2.6 ± 0.4) × 10−3× BW (r = 0.74; P < 0.001).
Differences exist between trabeculae from left and right ventricles. Firstly, the slopes of the relations between ATPase activity and between tension cost and body weight differed significantly between LV and RV (P < 0.05), although there was no difference in mean ATPase activity per volume and in tension cost between LV and RV (respectively, 0.13 ± 0.01 mmol l−1 s−1 (LV) and 0.11 ± 0.01 mmol l−1 s−1 (RV), and 3.0 ± 0.2 mmol kN−1 m−1 s−1 (LV) and 2.9 ± 0.1 mmol kN−1 m−1 s−1 (RV)). The differences in ATPase activity per volume and tension cost between young and mature guinea-pigs were highly significant (P < 0.001). These differences were significant in both left and right ventricular trabeculae (Table 1). The regression coefficients of the relations between force per cross-sectional area and body weight did not differ between LV and RV. However, there was a small but significant difference in the mean force per cross-sectional area between LV (43.8 ± 1.7 kN m−2) and RV (38.6 ± 1.6 kN m−2) (P < 0.05). This is mainly due to the difference between LV and RV in mature guinea-pigs. The mean force per cross-sectional area between young and mature guinea-pigs did not vary significantly, in both LVs and RVs (Table 1).
Table 1.
Differences between young and mature adult guinea-pigs
| Parameter | Young | Mature |
|---|---|---|
| ATPase activity per volume (mmol l−1 s−1) | ||
| LV + RV | 0.16 ± 0.01 (23) | 0.10 ± 0.01 (57)* |
| LV | 0.18 ± 0.02 (12) | 0.11 ± 0.01 (33)* |
| RV | 0.14 ± 0.01 (11) | 0.10 ± 0.01 (24)* |
| Force per cross-sectional area (kN m−2) | ||
| LV + RV | 38.3 ± 2.1 (23) | 42.9 ± 1.5 (57) |
| LV | 40.5 ± 3.1 (12) | 45.0 ± 2.0 (33) |
| RV | 35.9 ± 2.7 (11) | 39.9 ± 1.9 (24) |
| Tension cost (mmol kN−1 m−1 s−1) | ||
| LV + RV | 4.1 ± 0.2 (23) | 2.5 ± 0.1 (57)* |
| LV | 4.4 ± 0.4 (12) | 2.5 ± 0.1 (33)* |
| RV | 3.8 ± 0.2 (11) | 2.4 ± 0.1 (24)* |
| Heart weight per body weight (mg (100 g)−1) | 58.1 ± 0.9 (11) | 52.6 ± 1.6 (22)† |
ATPase activity per volume, force per cross-sectional area and tension cost are given for all trabeculae studied and for LV and RV trabeculae separately. Mean values are given ± s.e.m.
P < 0.001
P < 0.05, significantly different from young guinea-pigs. The number of observations is given in parentheses.
Age dependence of MHC composition
To see if the age-dependent changes in ATP consumption and tension cost are accompanied by a change in the myosin isoform pattern, we determined the MHC composition of the ventricular tissues by means of ELISA. Freeze-dried hearts were weighed first and total heart weights were normalized for body weight. These results are included in Table 1. The increase in body weight with age was larger than the increase in heart weight, resulting in a significantly lower heart weight to body weight ratio in mature guinea-pigs. Figure 6 illustrates the results obtained with ELISA. The mean percentage of α-MHC from all guinea-pig hearts studied did not differ between LV and RV (8.0 ± 1.4 and 9.3 ± 1.5 %, respectively). The fraction of α-MHC decreased with age, while the β-MHC fraction increased. The total (α+β)-MHC content in the ventricles, expressed relative to the total MHC content in the rat, did not change significantly (MHC ratio in young: 0.77 ± 0.05, and in mature: 0.85 ± 0.03). This suggests that the changes in α- and β-MHC are complementary (i.e. α-MHC is replaced by β-MHC). It is evident from Fig. 6 that there exists a strong correlation between the MHC composition and body weight (LV: r = 0.76, RV: r = 0.86). The α-MHC fraction decreased in the pooled data from 0.17 ± 0.02 (n = 24) in young to 0.04 ± 0.01 (n = 43) in mature guinea-pig hearts. Tension cost decreased significantly from 4.1 ± 0.2 mmol kN−1 m−1 s−1 in young to 2.5 ± 0.1 mmol kN−1 m−1 s−1 in mature adult guinea-pigs (P < 0.001; Fig. 7). The α-and β-MHC isoforms from guinea-pig could not be separated by SDS-PAGE, but the shift in MHC composition with age obtained in ELISA could be confirmed with Western immunoblotting (Fig. 2C).
Figure 6. The α-MHC fraction of total MHC content plotted as a function of body weight.
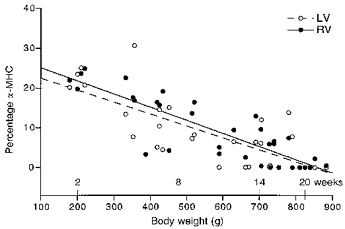
A negative correlation exists between the percentage of α-MHC and body weight. LV: percentage of α-MHC = (25.5 ± 2.8) - (3.0 ± 0.5) × 10−2× BW (r = 0.76; P < 0.001). RV: percentage of α-MHC = (28.4 ± 2.2) - (3.3 ± 0.4) × 10−2× BW (r = 0.86; P < 0.001).
Figure 7. Differences in tension cost (left) and percentage of α-MHC (right) between young (1- to 8-week-old) and mature (9- to 26-week-old) guinea-pigs.
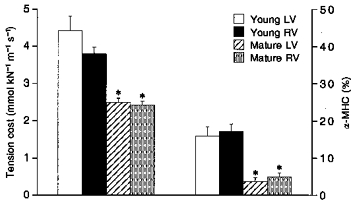
Mean values ± s.e.m.*P < 0.001, significantly different from young guinea-pigs (Student's t test). No significant differences were found between LV and RV.
Force and ATPase activity versus MHC composition
Because of this age-dependent shift in MHC composition we also studied isometric force per cross-sectional area and ATPase activity per volume as a function of MHC composition. A significant correlation was found between ATPase activity per volume and MHC composition (r = 0.60 for LV and 0.51 for RV; Fig. 8A), but not between isometric force per cross-sectional area and MHC composition (LV: r = 0.16, RV: r = 0.08; Fig. 8B). The slopes of the relations between ATPase activity and MHC composition for LV and RV were significantly different; however, the mean ATPase activity per volume was not significantly different between LV and RV. The ATPase activity per volume for the homodimers αα (V1) and ββ (V3) can be calculated from the overall regression lines between ATPase activity per volume and MHC composition for all trabeculae studied. From the intercepts of the regression lines it follows that ATPase activity per volume for V1 (0.39 ± 0.06 mmol l−1 s−1) was larger than that for V3 (0.10 ± 0.01 mmol l−1 s−1). Given the large number of data points (n = 63), the 95 % confidence limits of these estimates are given by 1.96 times the standard error of the estimate. Values obtained for LV and RV trabeculae separately are given in Table 2.
Figure 8. Dependence of ATP consumption, isometric force and tension cost on α-MHC.
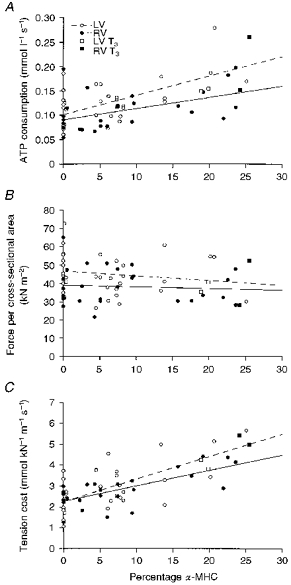
A, rate of ATP consumption per volume; B, isometric force per cross-sectional area; C, tension cost. %α, α-MHC expressed as a percentage of total MHC concentration; LV T3 and RV T3, respectively, left and right ventricular trabeculae from T3-treated guinea-pigs. Regression lines shown: LV: ATPase activity per volume = (0.10 ± 0.01) + (4.0 ± 0.9) × 10−3×%α (r = 0.60; P < 0.001), isometric force = (46.6 ± 2.4) - (0.26 ± 0.28) ×%α (r = 0.16), tension cost = (2.25 ± 0.19) + (0.11 ± 0.02) ×%α (r = 0.66; P < 0.001). RV: ATPase activity per volume = (0.9 ± 0.01) + (2.4 ± 0.8) × 10−3×%α (r = 0.51; P < 0.001), isometric force = (39.0 ± 2.6) - (0.09 ± 0.23) ×%α (r = 0.08), tension cost = (2.27 ± 0.15) + (0.07 ± 0.01) ×%α (r = 0.74; P < 0.001).
Table 2.
ATP consumption per volume and tension cost for V1 and V3
| Parameter | V1 (αα) | V3 (ββ) | Ratio |
|---|---|---|---|
| ATPase activity per volume (mmol l−1 s−1) | |||
| LV + RV (n = 63) | 0.39 ± 0.06 | 0.10 ± 0.01 | 3.9 |
| LV (n = 35) | 0.50 ± 0.09 | 0.10 ± 0.01 | 5.0 |
| RV (n = 28) | 0.33 ± 0.08 | 0.09 ± 0.01 | 3.7 |
| Tension cost (mmol kN−1 m−1 s−1) | |||
| LV + RV (n = 63) | 10.95 ± 1.17 | 2.28 ± 0.13 | 4.8 |
| LV (n = 35) | 12.99 ± 2.03 | 2.25 ± 0.19 | 5.8 |
| RV (n = 28) | 9.60 ± 1.21 | 2.27 ± 0.15 | 4.2 |
ATPase activity per volume and tension cost of the homodimers V1 (αα) and V3 (ββ), calculated from the regression lines between ATPase activity per volume and between tension cost and MHC composition. Intercept values of the different regression lines are given ± s.e.m.
To confirm that the age-dependent change in ATPase activity is caused by a change in the MHC composition, two mature guinea-pigs were subcutaneously injected daily with thyroid hormone (3,5,3′-triiodothyronine; T3, Sigma) for 1 week (0.1 mg (kg body weight)−1) to increase the fraction of α-MHC. The α-MHC percentage increased in T3-treated guinea-pigs to 22.1 %. Consequently, the ATPase activity per volume (0.18 ± 0.03 mmol l−1 s−1, n = 4) and tension cost (4.6 ± 0.4 mmol kN−1 m−1 s−1, n = 4) increased significantly compared with age-matched guinea-pigs.
The relation between tension cost and MHC composition is shown in Fig. 8C. From this figure it follows that the decrease in tension cost observed with age is correlated with a decrease in the fraction of α-MHC present in the heart ventricles. The slopes of the tension cost-MHC composition relations were significantly different between LV and RV; however, mean tension cost was not significantly different. Tension cost of V1 and V3 for LV and RV trabeculae separately are shown in Table 2. It follows, from the intercepts of the regression lines between tension cost and MHC composition for all trabeculae studied, that the tension cost of V1 is equal to 10.95 ± 1.17 mmol kN−1 m−1 s−1 and that of V3 amounts to 2.28 ± 0.13 mmol kN−1 m−1 s−1. The 95 % confidence limits of V1 and V3 were 10.95 ± 2.29 and 2.28 ± 0.24 mmol kN−1 m−1 s−1, respectively. Thus, the slow β-MHC isoform, which is predominantly present in mature guinea-pigs, is about 5 times more economical than the fast α-MHC isoform.
Calcium sensitivity versus MHC composition
We also performed regression analysis on the Hill parameters describing calcium sensitivity (nH and pK) and MHC composition. A significant correlation was obtained for the results from the first experimental group (including very young guinea-pigs; 1–2.5 weeks old), but not for the second experimental group (> 4 weeks old). Since the MHC composition varied substantially in the second group, it is concluded that the MHC composition cannot be a major determinant of the early change in calcium sensitivity observed in the first group.
DISCUSSION
The main findings of this study are that in guinea-pig hearts there is a shift with age towards slow and more economical myosin isoforms. This replacement of fast α-MHCs by slow β-MHCs takes place at a moderate scale, but as β-MHCs are about 5 times more economical than α-MHCs, this causes appreciable changes in cardiac energetics. For guinea-pigs older than 4 weeks, the age-dependent shift in myosin heavy chain composition is not accompanied by a change in calcium sensitivity.
Comparison with previous studies
The mean maximum steady-state force at saturating Ca2+ concentration amounted to 41.5 ± 1.2 kN m−2. The mean rate of ATP consumption accompanying this level of steady-state force was 0.16 and 0.10 mmol l−1 s−1 in young and in mature hearts, respectively (Table 1). Assuming a myosin head concentration of 0.16 mm (Barsotti & Ferenczi, 1988) this would result in an ATP hydrolysis rate of 1.0 s−1 in young and 0.63 s−1 in mature hearts. If the difference in temperature during the experiments is taken into account, our values are similar to the value found by Barsotti & Ferenczi (1988) in guinea-pig at 12°C. However, the values in guinea-pig heart are about 4 times lower than those found in the rat (Ebus et al. 1994). Even taking into account that the rat hearts used in previous studies contained a mixture of both α- and β-MHC and that guinea-pigs might contain somewhat less myosin in comparison to rats (∼10 %, see Results), this difference cannot be due entirely to a difference in the myosin isoforms present. By measuring heat production and tension generation in tetanized papillary muscles, Gibbs & Loiselle (1978) found that rats used more energy to maintain tension than guinea-pigs and suggested that this was due to species differences. Our results extend these observations and suggest that corresponding MHC isoforms in rats and guinea-pigs are intrinsically different with respect to their ATPase activity. However, caution should be exerted in extrapolating these results to cardiac performance in vivo since the heart does not contract isovolumetrically nor is it maximally activated.
Age-dependent changes in MHC composition
Age-associated changes in the myosin composition have been found previously in other animal models. For instance, in the rat, an isomyosin shift towards fast MHCs was observed with age (Watras, 1981); the slow V3 disappeared from the ventricles after birth and was replaced by fast V1. Since the in vitro ATPase activity of V1 is higher than that of V3 (Pope et al. 1980), this shift correlated with an increase in the ATPase activity observed from birth to the adult stage. In rabbit hearts, V1 present in young animals is eventually completely replaced by V3 (Lompréet al. 1981). Our results indicate that a shift in the same direction occurs in the guinea-pig. This shift is associated with a decrease in the rate of ATP consumption, while isometric force per cross-sectional area remains unchanged, which consequently results in a functional change in the energetic cost of force maintenance.
It has been suggested that thyroid hormone mediates the shift in isomyosins found with ageing (Hoh et al. 1977). Indeed, thyroid hormone is involved in the expression of myosin isoforms (Martin, Pagani & Solaro, 1982; Effron, Bhatnagar, Spurgeon, Ruaño-Arroyo & Lakatta, 1987) and the implications for cardiac energetics are now well established (Leijendekker, van Hardeveld & Elzinga, 1987; Hasenfuss et al. 1991). Also, in our study, the T3 treatment changed the MHC composition in guinea-pig ventricles. The shift in MHC composition is such that it could explain quantitatively the change in economy.
The calcium sensitivity of force production and ATPase activity were highest in very young guinea-pigs (Fig. 4), while they remained the same after 4 weeks of age (> 300 g). These findings are in agreement with a previous study of force development in enzymatically digested cardiac myocytes (van der Velden, Strang, Stienen & Moss, 1997). Since the MHC composition still changes after this period (Fig. 6), calcium sensitivity of force production and ATPase activity do not seem to be influenced by the type of MHC isoform present. Bhatnagar, Walford, Beard, Humphreys & Lakatta (1984) did not find an altered force-pCa relation with ageing in rat papillary muscle. Reiser et al. (1994) did find a comparable change, of about 0.2–0.3 pCa units, in the calcium sensitivity of force production in rat hearts during development from neonatal to the adult stage, as we have found. Similar results were obtained in chicken hearts (Godt et al. 1990). It can be concluded that the decrease in calcium sensitivity stabilized quickly after birth. The changes found by Reiser et al. (1994) were ascribed to shifts in TnT and TnI isoforms during development from neonatal to adult stage. We did not find changes in the TnT and TnI composition between very young and mature guinea-pigs, both in SDS-PAGE and Western immunoblotting. Since only one TnI isoform was found it is possible that our TnI antibody was unable to detect different isoforms. Therefore a change in the TnI composition cannot be completely excluded. Other factors that might be involved in the change in calcium sensitivity of isometric force and ATPase activity could be the state of phophorylation of troponin isoforms (Solaro & Van Eyk, 1996; Li et al. 1997) and myosin light chain 2 (Morano, Rösch, Arner & Rüegg, 1990). An illustration that factors other than MHC composition are involved in the age-dependent change in calcium sensitivity of force development is given by Gibson, Wendt & Stephenson (1992). A full investigation of these factors in guinea-pig is beyond the scope of this study.
Model calculations and recent results of in vitro motility measurements (Alpert, Mulieri & Hasenfuss, 1992; VanBuren, Harris, Alpert & Warshaw, 1995) indicate that the average force per cross-bridge of purified V3 myosin is larger than that of V1 by a factor of 2–4. The force per cross-sectional area in young and mature guinea-pig hearts was the same (Table 1) and also no significant correlation was found between the force per cross-sectional area and MHC composition (Fig. 8B). However, the relatively large error in the estimation of the force per cross-sectional area may obscure possible differences in force per cross-bridge of pure α- and β-MHCs.
Implications for cardiac overload
The myosin composition not only changes during development and with age, but also under pathological conditions. During cardiac hypertrophy in rats, a shift from the fast isomyosin V1 to the slow isomyosin V3 occurs (Mercadier et al. 1981; Gorza et al. 1981). This change appears to be functional, because with cardiac overload more economical isoforms are expressed. It should be noted that in this respect the rat heart cannot be compared with the human heart, since V3 already predominates in the adult human ventricles. The guinea-pig heart, however, resembles the human heart more closely, since they both contain predominantly V3 during the adult stage.
The fraction of α-MHC decreased from 0.17 ± 0.02 in young to 0.04 ± 0.01 in mature adult guinea-pigs. This age-dependent shift in myosin isoform composition might interfere with changes found after cardiac overload. Malhotra, Siri & Aronson (1992) used an aortic banding technique to induce cardiac hypertrophy in guinea-pigs. A decreased myosin ATPase activity was found in the hypertrophied left heart ventricles. The animals used weighed 225–275 g at the time of the operation and when killed they weighed 300–900 g, which on the basis of our results implies that age-related changes might have interfered with their results. Malhotra et al. (1992) have already suggested that the decrease in ATPase activity could be the result of normal growth. Our results suggest that this is the case, and provide evidence that in long-term studies of cardiac overload age dependence of the myosin composition should be taken into account.
Molecular basis of changes in economy
The natural shift in MHC composition with age in guinea-pig hearts can also be used to explore the energetics of ‘pure’ isomyosins. In our experiments, isometric force and ATP consumption were measured, which have the advantage that they directly provide information on energy utilization and economy of contraction. Furthermore, the measurements at saturating Ca2+ concentrations reflect the maximal capacity of the contractile proteins and therefore are not influenced by factors that modulate activity. From the regression line describing the relation between tension cost and the α-MHC fraction of all trabeculae studied, it follows that the tension cost of pure α-isomyosin (V1) is 10.95 ± 1.17 mmol kN−1 m−1 s−1 and the tension cost of pure β-isomyosin (V3) is 2.28 ± 0.13 mmol kN−1 m−1 s−1. The slow β-MHC, which is predominantly present in mature guinea-pigs, is thus 5 times more economical than the fast α-MHC. This increase in economy is the result of the decrease in ATP consumption, since there is no significant change in isometric force per cross-sectional area with age.
The change in ATP consumption reflects a change in cross-bridge kinetics. In a two-state model of cross-bridge action (Huxley, 1957), in which the rate of detachment of force-generating cross-bridges was rate limiting, the decrease in the rate of ATP turnover is explained by a decrease in the apparent detachment rate. The decrease in the rate-limiting transition in such a model would imply a minor increase in force per cross-sectional area, which is compatible with our results.
Concluding remarks
The human ventricular myocardium predominantly contains β-MHC, and therefore it is unlikely that a shift in the MHC composition is responsible for all observed mechanical and energetic changes which occur during hypertrophy and heart failure. Changes in other contractile proteins might alter the contractile performance of hypertrophied and failing human myocardium (Hirzel, Tuchschmid, Schneider, Krayenbuehl & Schaub, 1985; Henkel, VandeBerg, Shade, Leger & Walsh, 1989; Anderson, Malouf, Oakeley, Pagani & Allen, 1991). However, in the overloaded human ventricles, the small amount of V1 which is normally present seems to be lost (Mercadier et al. 1983). The individual variability in the fraction of α-MHC present in human ventricular myocardium, together with the steep relation between MHC isoforms and tension cost found in this study, might be partly responsible for the conflicting findings on human heart myosin ATPase activity (Mercadier et al. 1983; Alousi et al. 1990) in the literature, because even a minor decrease in the fraction of α-MHC might have a considerable influence on cardiac energetics.
Acknowledgments
The support of the Dutch Heart Foundation (grant 93.067) is gratefully acknowledged. We thank I. A. van Graas and M. van der Bijl for expert technical assistance and Dr J. Graham (Madison, WI, USA) for expert advice on the SDS-PAGE. We thank Dr P. P. de Tombe for providing us with tissue from hypothyroid and euthyroid rat hearts.
References
- Alousi AA, Grant AM, Etzler JR, Cofer BR, Van der Bel-Kahn J, Melvin D. Reduced cardiac myofibrillar Mg-ATPase activity without changes in myosin isoenzymes in patients with end-stage heart failure. Molecular and Cellular Biochemistry. 1990;96:79–88. doi: 10.1007/BF00228455. [DOI] [PubMed] [Google Scholar]
- Alpert NR, Gordon MS. Myofibrillar adenosine triphosphatase activity in congestive heart failure. American Journal of Physiology. 1962;202:940–946. doi: 10.1152/ajplegacy.1962.202.5.940. [DOI] [PubMed] [Google Scholar]
- Alpert NR, Mulieri LA. Increased myothermal economy of isometric force generation in compensated cardiac hypertrophy induced by pulmonary artery constriction in the rabbit. Circulation Research. 1982;50:491–500. doi: 10.1161/01.res.50.4.491. [DOI] [PubMed] [Google Scholar]
- Alpert NR, Mulieri LA, Hasenfuss G. Myocardial chemo-mechanical energy transduction. In: Fozzard HA, editor. The Heart and Cardiovascular System. New York: Raven Press; 1992. pp. 111–128. [Google Scholar]
- Anderson PAW, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans - A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circulation Research. 1991;69:1226–1233. doi: 10.1161/01.res.69.5.1226. [DOI] [PubMed] [Google Scholar]
- Barsotti RJ, Ferenczi MA. Kinetics of ATP hydrolysis and tension production in skinned cardiac muscle of the guinea pig. Journal of Biological Chemistry. 1988;263:16750–16756. [PubMed] [Google Scholar]
- Bhatnagar GM, Walford GD, Beard ES, Humphreys S, Lakatta EG. ATPase activity and force production in myofibrils and twitch characteristics in intact muscle from neonatal, adult and senescent rat myocardium. Journal of Molecular and Cellular Cardiology. 1984;16:203–218. doi: 10.1016/s0022-2828(84)80587-8. [DOI] [PubMed] [Google Scholar]
- De Groot IJM, Lamers WH, Moorman AFM. Isomyosin expression patterns during rat heart morphogenesis: an immuno-histochemical study. Anatomical Record. 1989;224:365–373. doi: 10.1002/ar.1092240305. [DOI] [PubMed] [Google Scholar]
- Ebrecht G, Rupp H, Jacob R. Alterations of mechanical parameters in chemically skinned preparations of rat myocardium as a function of isoenzyme pattern of myosin. Basic Research in Cardiology. 1982;77:220–234. doi: 10.1007/BF01908175. [DOI] [PubMed] [Google Scholar]
- Ebus JP, Stienen GJM, Elzinga G. Influence of phosphate and pH on myofibrillar ATPase activity and force in cardiac trabeculae from rat. The Journal of Physiology. 1994;476:501–516. doi: 10.1113/jphysiol.1994.sp020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effron MB, Bhatnagar GM, Spurgeon HA, Ruaño-Arroyo G, Lakatta EG. Changes in myosin isoenzymes, ATPase activity, and contraction duration in rat cardiac muscle with aging can be modulated by thyroxine. Circulation Research. 1987;60:238–245. doi: 10.1161/01.res.60.2.238. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. Journal of General Physiology. 1981;78:457–497. doi: 10.1085/jgp.78.5.457. 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C, Loiselle D. The energy output of tetanized cardiac muscle: species differences. Pflügers Archiv. 1978;373:31–38. doi: 10.1007/BF00581146. [DOI] [PubMed] [Google Scholar]
- Gibson LM, Wendt IR, Stephenson DG. Contractile activation properties of ventricular myocardium from hypothyroid, euthyroid and juvenile rats. Pflügers Archiv. 1992;422:16–23. doi: 10.1007/BF00381508. [DOI] [PubMed] [Google Scholar]
- Giulian GG, Moss RL, Greaser ML. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Analytical Biochemistry. 1983;129:277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Godt RE, Fogaça RT, Nosek TM. Changes in force and calcium sensitivity in the developing avian heart. Canadian The Journal of Physiology and Pharmacology. 1990;69:1692–1697. doi: 10.1139/y91-251. [DOI] [PubMed] [Google Scholar]
- Gorza L, Pauletto P, Pessina AC, Sartore S, Schiaffino S. Isomyosin distribution in normal and pressure-overloaded rat ventricular myocardium. Circulation Research. 1981;49:1003–1009. doi: 10.1161/01.res.49.4.1003. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Mulieri LA, Blanchard EM, Holubarsch C, Leavitt BJ, Ittleman F, Alpert NR. Energetics of isometric force development in control and volume-overload human myocardium: comparison with animal species. Circulation Research. 1991;68:836–846. doi: 10.1161/01.res.68.3.836. [DOI] [PubMed] [Google Scholar]
- Henkel RD, VandeBerg JL, Shade RE, Leger JJ, Walsh RA. Cardiac beta myosin heavy chain diversity in normal and chronically hypertensive baboons. Journal of Clinical Investigation. 1989;83:1487–1493. doi: 10.1172/JCI114042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirzel HO, Tuchschmid CR, Schneider J, Krayenbuehl HP, Schaub MC. Relationship between myosin isoenzyme composition, hemodynamics, and myocardial structure in various forms of human cardiac hypertrophy. Circulation Research. 1985;57:729–740. doi: 10.1161/01.res.57.5.729. [DOI] [PubMed] [Google Scholar]
- Hoh JFY, McGrath PA, Hale PT. Electrophoretic analysis of multiple forms of rat cardiac myosin: effect of hypophysectomy and thyroxine replacement. Journal of Molecular and Cellular Cardiology. 1977;10:1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Progress in Biophysics and Biophysical Chemistry. 1957;7:255–317. [PubMed] [Google Scholar]
- Leijendekker WJ, van Hardeveld C, Elzinga G. Heat production during contraction in skeletal muscle of hypothyroid mice. American Journal of Physiology. 1987;253:E214–220. doi: 10.1152/ajpendo.1987.253.2.E214. [DOI] [PubMed] [Google Scholar]
- Li P, Hofmann PA, Li B, Malhotra A, Cheng W, Sonnenblick EH, Meggs LG, Anversa P. Myocardial infarction alters myofilament calcium sensitivity and mechanical behavior of myocytes. American Journal of Physiology. 1997;272:H360–370. doi: 10.1152/ajpheart.1997.272.1.H360. [DOI] [PubMed] [Google Scholar]
- Lompré AM, Mercadier JJ, Wisnewsky C, Bouveret P, Pantaloni C, d'Albis A, Schwartz K. Species- and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Developmental Biology. 1981;84:286–290. doi: 10.1016/0012-1606(81)90396-1. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Siri FM, Aronson R. Cardiac contractile proteins in hypertrophied and failing guinea pig heart. Cardiovascular Research. 1992;26:153–161. doi: 10.1093/cvr/26.2.153. [DOI] [PubMed] [Google Scholar]
- Martin AF, Pagani ED, Solaro RJ. Thyroxine-induced redistribution of isoenzymes of rabbit ventricular myosin. Circulation Research. 1982;50:117–124. doi: 10.1161/01.res.50.1.117. [DOI] [PubMed] [Google Scholar]
- Mercadier J, Bouveret P, Gorza L, Schiaffino S, Clark WA, Zak R, Swynghedauw B, Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circulation Research. 1983;53:52–62. doi: 10.1161/01.res.53.1.52. [DOI] [PubMed] [Google Scholar]
- Mercadier J, Lompré AM, Wisnewsky C, Samuel J, Bercovici J, Swynghedauw B, Schwartz K. Myosin isoenzymic changes in several models of rat cardiac hypertrophy. Circulation Research. 1981;49:525–532. doi: 10.1161/01.res.49.2.525. [DOI] [PubMed] [Google Scholar]
- Morano I, Rösch J, Arner A, Rüegg JC. Phosphorylation and thiophosphorylation by myosin light chain kinase: Different effects on mechanical properties of chemically skinned ventricular fibers from the pig. Journal of Molecular and Cellular Cardiology. 1990;22:805–813. doi: 10.1016/0022-2828(90)90091-f. [DOI] [PubMed] [Google Scholar]
- Pope B, Hoh JFY, Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Letters. 1980;118:205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Westfall MV, Schiaffino S, Solaro RJ. Tension production and thin-filament protein isoforms in developing rat myocardium. American Journal of Physiology. 1994;267:H1589–1596. doi: 10.1152/ajpheart.1994.267.4.H1589. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Lecarpentier Y, Martin JL, Lompré AM, Mercadier JJ, Swynghedauw B. Myosin isoenzymic distribution correlates with speed of myocardial contraction. Journal of Molecular and Cellular Cardiology. 1981;13:1071–1075. doi: 10.1016/0022-2828(81)90297-2. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. In: Wilson J, editor. The Principles and Practice of Statistics in Biological Research. San Francisco: W. H. Freeman and Company; 1981. pp. 454–509. [Google Scholar]
- Solaro RJ, Van Eyk J. Altered interactions among thin filament proteins modulate cardiac function. Journal of Molecular and Cellular Cardiology. 1996;28:217–230. doi: 10.1006/jmcc.1996.0021. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiological Reviews. 1986;66:710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- VanBuren P, Harris DE, Alpert NR, Warshaw DM. Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circulation Research. 1995;77:439–444. doi: 10.1161/01.res.77.2.439. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Strang KT, Stienen GJM, Moss RL. Age-dependent change in calcium sensitivity of isometric force development in isolated myocytes from guinea pig. Journal of Muscle Research and Cell Motility. 1997;18:225–226. Abstract. [Google Scholar]
- Ventura-Clapier R, Mekhfi H, Oliviero P, Swynghedauw B. Pressure overload changes cardiac skinned-fiber mechanics in rats, not in guinea pigs. American Journal of Physiology. 1988;254:H517–524. doi: 10.1152/ajpheart.1988.254.3.H517. [DOI] [PubMed] [Google Scholar]
- Watras J. Changes in rat cardiac myosin during development and in culture. Journal of Molecular and Cellular Cardiology. 1981;13:1011–1021. doi: 10.1016/0022-2828(81)90476-4. [DOI] [PubMed] [Google Scholar]


