Abstract
Bicuculline-sensitive and strychnine-sensitive inhibitory postsynaptic currents (IPSCs) could be evoked in neurones of the rat substantia gelatinosa of the spinal trigeminal nucleus pars caudalis.
Spontaneous tetrodotoxin (TTX)-insensitive-mediated miniature IPSCs (mIPSCs) blocked by strychnine or bicuculline were also present in many neurones. The decay of the glycine receptor-mediated mIPSCs was fitted by a single exponential, whereas the decay of the GABAA receptor-mediated mIPSCs could in some instances be fitted by a single exponential, but in other instances required two exponentials.
An increase in baseline current noise developed during the course of the recording. This noise was abolished by strychnine (1 μm) but was insensitive to bicuculline (10 μm), TTX (0.5 μm), [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO, 1 μm) or baclofen (30 μm). The single-channel conductance underlying the noise was estimated to be 21 pS.
The μ-opioid agonist DAMGO (1–10 μm) reduced the amplitude of the evoked glycine receptor-mediated IPSC and the evoked GABAA receptor-mediated IPSC. The μ-opioid antagonist D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP, 1 μm) reversed the DAMGO inhibition.
The GABAB agonist baclofen (30 μm) reduced the amplitude of the evoked glycine receptor- mediated IPSC and the GABAA receptor-mediated IPSC. The inhibition was reversed by the selective GABAB antagonist 3-N[1-(S)-(3,4-dichlorophenyl) ethyl] amino-2-(S)-hydroxypropyl-P-benzyl-phosphinic acid (CGP 55845A, 1 μm).
Both DAMGO and baclofen reduced the frequency of glycine and GABAA receptor-mediated mIPSCs without affecting average amplitude, and increased the percentage of failures of the evoked glycine and GABAA receptor-mediated IPSCs, suggesting a presynaptic site of action.
The substantia gelatinosa (SG) of the spinal cord and the spinal trigeminal nucleus pars caudalis in the medulla is the central site of termination of most primary afferent fibres excited by painful stimuli (Light & Perl, 1979; Sugiura, Lee & Perl, 1986). Although some neurones in the SG send axons to more superficial or deeper areas, which contain neurones that project to rostral brain areas (Gobel, Falls, Bennett, Abdelmoumene, Hayashi & Humphrey, 1980; Light & Kavookjian, 1988), many SG neurones are thought to be interneurones with axonal arborizations that remain within the SG (Gobel, 1978; Bennett, Abdelmoumene, Hayashi & Dubner, 1980). These interneurones are thought to form a network that plays a role in modulating the primary afferent signals.
There is considerable evidence that inhibitory synaptic transmission mediated by glycine and GABA is involved in the SG neuronal network. Spontaneous synaptic potentials mediated by glycine and GABA are present in at least some neurones in the SG of the spinal trigeminal nucleus pars caudalis (Grudt & Williams, 1994) or spinal cord (Yoshimura & Nishi, 1995). Stimulation of primary afferent fibres in the dorsal root elicits polysynaptic inhibitory synaptic potentials mediated by glycine acting at glycine receptors and GABA acting at GABAA receptors in spinal SG neurones (Yoshimura & Nishi, 1995). Furthermore, application of glycine and GABAA antagonists results in the enhancement of the excitatory response recorded in SG neurones when primary afferent fibres are stimulated (Grudt & Williams, 1994). Consistent with these electrophysiological results, immunocytochemical studies have shown that the inhibitory transmitters glycine and GABA are present in many SG neurones (for review, see Todd & Spike, 1993).
Analgesia produced by the administration of opioids is thought to be mediated in part by an action in the SG (Duggan, Hall & Headley, 1977; Johnson & Duggan, 1981). The SG is probably also involved in the analgesic effects of endogenous opioids. Opioids acting at μ-opioid receptors, the opioid receptor type with the highest affinity for morphine-like analgesic drugs, have previously been shown to modulate the activity of SG neurones in several ways. They reduce excitatory amino acid release from primary afferents (Macdonald & Nelson, 1978; Jeftinija, 1988; Grudt & Williams, 1994) and hyperpolarize SG neurones (Yoshimura & North, 1983; Grudt & Williams, 1994). As yet, the actions of opioids on inhibitory synaptic transmission within the SG have not been examined. The purpose of the present study was to characterize inhibitory synaptic transmission in the SG and its modulation by μ-opioid and GABAB receptor activation. A preliminary account of these findings has been presented (Grudt & Henderson, 1996).
METHODS
Twenty-one- to 28-day-old Wistar rats of either sex were killed by cervical dislocation, and horizontal brain slices containing the spinal trigeminal nucleus pars caudalis were made as described previously (Grudt & Williams, 1994). A block of brainstem including the caudal part of the medulla was placed in a Vibratome (Oxford) containing oxygenated physiological saline at 4°C, and slices were cut at a setting of 275 μm. Two or three slices containing the SG of the spinal trigeminal nucleus were then stored in oxygenated physiological saline at room temperature. A single slice was placed in a tissue bath (volume of bath, 0.5 ml) mounted on the stage of a Zeiss Axioscope microscope and continually superfused with warmed (30°C) bicarbonate-buffered artificial cerebrospinal fluid (ACSF) at a rate of 2 ml min−1. The ACSF consisted of (mm): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.3 MgCl2, 2.4 CaCl2, 10 glucose and 26 NaHCO3, and was gassed with 95 % O2 and 5 % CO2.
In this preparation, the SG is an easily identifiable translucent area separated from the lateral surface of the medulla by the spinal trigeminal tract. Individual neurones were visualized using Nomarski optics, and whole-cell voltage-clamp recordings were made using 3–4 MΩ pipettes containing (mm): 135 CsCl, 5 EGTA, 10 Hepes, 0.1 CaCl2, 2 MgCl2, 0.5 NaGTP and 5 MgATP, with the pH adjusted to 7.3 with CsOH. Intracellular Cs+ was used to reduce activation of a potassium conductance by μ-opioid or GABAB receptor activation in the postsynaptic neurone (Grudt & Williams, 1994). Seals of 2–5 GΩ were obtained. Access resistance ranged from 5 to 20 MΩ and was checked every few minutes. If it changed by more than 15 %, the experiment was excluded. The currents were amplified using an Axopatch 200A amplifier (Axon Instruments), saved to digital audiotape (Biologic) and to a computer for subsequent analysis. Input resistance was estimated by measuring the current required to produce a 5 mV change in membrane potential.
Synaptic currents were evoked by square-wave constant-voltage pulses (0.1–0.3 ms duration) delivered at 0.2 Hz using a stimulating electrode which was an ACSF-filled glass pipette, slightly larger than those used for recording. The stimulating electrode was placed in the SG 20–40 μm rostral or caudal to the neurone from which the recording was being made. CNQX (10 μm) and in some experiments D-APV (30 μm) were present to block excitatory amino acid AMPA and NMDA receptors, respectively. Evoked and spontaneous current records were filtered at 2 kHz and acquired at 4–5 kHz. Spontaneous synaptic currents were analysed using pCLAMP or Axograph software (Axon Instruments). The frequency of spontaneous synaptic currents was determined by setting a detection threshold level above the current noise and manually accepting or rejecting events thus identified. The decay of each spontaneous IPSC was fitted using the Simplex or Chebyshev methods and the quality of the fit was determined by visual inspection. Similar results were obtained with the two methods. To quantify current noise, all point histograms were constructed from 5–10 s of current recording. The resulting histograms were fitted with one or two Gaussians using a least-squares fitting method.
Drugs were applied in known concentrations in the superfusing solution by switching from the control solution to one which differed only in its content of the drug. Drugs and their sources were: D-2-amino-5-phosphonovaleric acid (D-APV, gift from Tocris Cookson), 3-N[1-(S)-(3,4-dichlorophenyl) ethyl] amino-2-(S)-hydroxypropyl-P-benzyl-phosphinic acid (CGP 55845A, gift from Ciba-Geigy), D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP, Peninsula) and baclofen, bicuculline methiodide, 6-cyano-7-nitroqinoxaline-2,3-dione (CNQX), [D-Ala2, N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), strychnine hydrochloride and tetrodotoxin (TTX) (all from Sigma).
Results are given as means ± s.e.m.
RESULTS
Whole-cell recordings were made from 108 neurones. Neurones were held at -70 mV. The input resistance of the neurones ranged from 300 MΩ to 2.1 GΩ, with a mean of 1.1 ± 0.1 GΩ (n = 35). The whole-cell capacitance as determined from amplifier settings after cancelling transients was 12 ± 4 pF, with a range of 7–19 pF.
Stimulation within the SG elicits glycine and GABAA receptor-mediated IPSCs
In the presence of the excitatory amino acid antagonists CNQX (10 μm) and D-APV (30 μm), focal stimulation evoked a synaptic current that was abolished by TTX (0.5 μm, n = 4). TTX also blocked the fast inward somatic sodium current evoked by a step from -70 to -20 mV (n = 5, data not shown). In the presence of CNQX (10 μm) and D-APV (30 μm) plus the GABAA receptor antagonist bicuculline (10 μm), focal stimulation evoked a synaptic current in twenty-four of twenty-five neurones, which in all cases was blocked or greatly reduced (> 90 %) by the glycine receptor antagonist strychnine (1 μm; Fig. 1A and B), indicating that it was mediated by synaptically released glycine acting at a strychnine-sensitive glycine receptor. The amplitude of the evoked glycine receptor-mediated IPSC ranged from 30 to 800 pA, with a mean of 138 ± 38 pA. Since under physiological conditions (i.e. low intracellular Cl− rather than the high intracellular Cl− used in our experiments) activation of glycine receptors would cause an outward or inhibitory current, this synaptic current will be referred to as the glycine inhibitory postsynaptic current (IPSC).
Figure 1. Evoked glycine and GABAA receptor-mediated IPSCs.
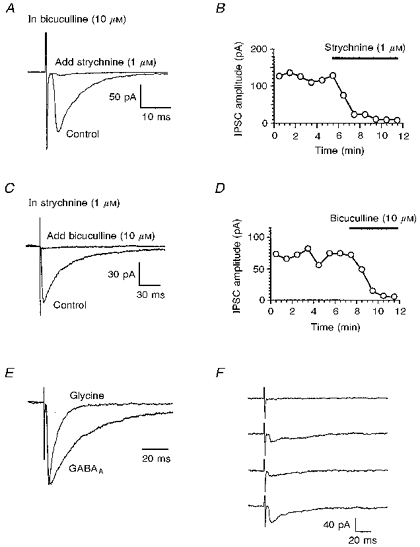
All experiments were performed in the presence of CNQX (10 μm) and D-APV (30 μm) to block excitatory synaptic currents. A and C, superimposed current traces (each an average of 12 evoked IPSCs) obtained before and after addition of either strychnine (A) or bicuculline (C). In these and other figures, the initial stimulus artifacts have been truncated. B and D, time course of the inhibition of IPSCs by the antagonists. Synaptic currents were evoked once every 5 s and 12 were averaged to obtain the data points shown. E, the glycine and GABAA receptor-mediated IPSCs shown in A and C have been scaled to the same amplitude to facilitate comparison. F, 4 consecutive individual GABAA receptor-mediated IPSCs showing variable amplitude and a failure (upper trace).
In the presence of excitatory amino acid antagonists and strychnine (1 μm), a synaptic current was evoked in forty-two of forty-four neurones, which was abolished or reduced by bicuculline (10 μm), indicating that it was mediated through GABAA receptors (Fig. 1C and D). Similar stimulus intensities were required to evoke glycine and GABAA receptor-mediated IPSCs. The mean amplitude of the evoked GABAA receptor-mediated IPSC was 76 ± 9 pA, with a range of 14–240 pA. The time course of evoked glycine receptor-mediated IPSCs was faster than that of evoked GABAA receptor-mediated IPSCs. To illustrate this, Fig. 1E shows representative examples of glycine and GABAA receptor-mediated IPSCs scaled to the same amplitude. We have not examined the kinetics of the evoked IPSCs in detail, since in many neurones they probably resulted from multiple presynaptic inputs of differing propagation times and synapsing on different parts of the cell. In the presence of bicuculline and strychnine, evoked responses were completely abolished in about half of the neurones. In the other neurones (e.g. Fig. 1A), there was a small residual synaptic current, but this was never larger than 10 pA. This response was not investigated further.
In individual neurones the amplitude of individual glycine or GABAA receptor-mediated IPSCs evoked by a constant intensity of stimulation applied at a frequency of 0.2 Hz was highly variable, and in some instances a stimulus occasionally failed to evoke an IPSC (Fig. 1F and Fig. 4). Such variability and the presence of failures have been reported in other brain regions (Rosenmund, Clements & Westbrook, 1993; Ulrich & Huguenard, 1995; Umemiya & Berger, 1995) and may be due to the failure of an action potential to elicit transmitter release upon depolarizing the terminal (Allen & Stevens, 1994).
Figure 4. μ-Opioid and GABAB receptor-mediated inhibition of evoked glycine receptor-mediated IPSCs.
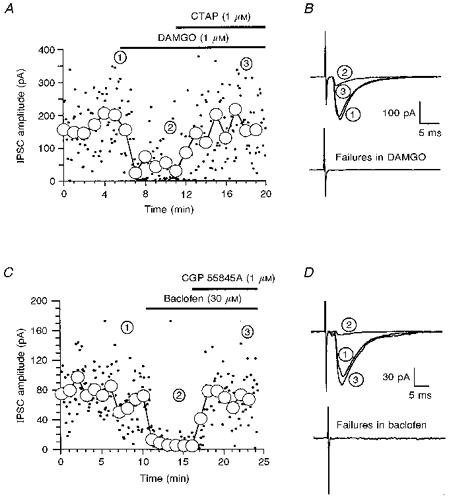
A and C, time course of the reduction of the amplitude of evoked glycine receptor-mediated IPSCs by the μ-opioid agonist DAMGO (A) or the GABAB agonist baclofen (C) and the reversal by antagonists. The circles represent the average of 12 sequential individual IPSCs, which were evoked once every 5 s. B and D, the upper traces are three superimposed recordings, each of which is the average of 12 sequentially evoked IPSCs. The numbers adjacent to the traces correspond to the time points in A. The lower traces are averages of 12 failures in the presence of DAMGO (B) or baclofen (D).
Spontaneous glycine and GABAA receptor-mediated IPSCs
In many neurones, spontaneous inhibitory synaptic currents were observed in the presence of the excitatory amino acid antagonist CNQX (10 μm) and TTX (0.5 μm). TTX-insensitive spontaneous synaptic currents are thought to result from transmitter release at a single site and are therefore referred to as miniature synaptic currents (mIPSCs). In many cases the frequency of mIPSCs was low (< 0.1 Hz). The selective glycine receptor antagonist strychnine (1 μm) blocked a proportion of the synaptic currents, indicating that they were glycine receptor-mediated mIPSCs, whilst others were blocked by the selective GABAA receptor antagonist bicuculline (10 μm). Glycine and GABAA receptor-mediated mIPSCs were often present in the same neurone. In general, GABAA receptor-mediated mIPSCs occurred at a higher frequency than glycine receptor-mediated mIPSCs. In the presence of CNQX, bicuculline and strychnine there were no detectable spontaneous synaptic currents.
The kinetics of mIPSCs were examined in neurones having a sufficiently high frequency of mIPSCs to permit analysis, and the data are summarized in Table 1. As with evoked IPSCs, the time course of glycine receptor-mediated mIPSCs was faster than that of GABAA receptor-mediated mIPSCs. The decay of glycine receptor-mediated mIPSCs (six neurones) was best fitted by a single exponential (Fig. 2A). The decay of GABAA receptor-mediated mIPSCs (five neurones) was more complicated; in some instances the decay could be fitted by a single exponential but in other instances fitting with two exponentials was required. Figure 2B shows a section of a current recording from a single cell in which GABAA receptor-mediated mIPSCs requiring either a single or a double exponential were observed. Examples of double and single exponential GABAA receptor-mediated mIPSCs are shown in Fig. 2C and D, respectively. In all five neurones examined, both single and double exponential decay GABAA mIPSCs were present. The percentage of GABAA receptor-mediated mIPSCs that could be fitted by a single exponential ranged from 37 to 65 %, with an average of 49 ± 5.2 % among the five neurones. In each neurone, the time constant of the single exponential decay was significantly different from either of the two time constants of the double exponential decay. For GABAA receptor-mediated mIPSCs with a double exponential decay, the average contribution of the fast component to the total amplitude of the mIPSC was between 50 and 60 % for all neurones, with an average across the five neurones of 54 ± 1.7 %.
Table 1.
Kinetics of glycine and GABAA receptor-mediated mIPSCs
| Double exponential fit | ||||||
|---|---|---|---|---|---|---|
| n | Amplitude (pA) | Rise time (ms) | Single exponential fit decay τ (ms) | Fast component decay τ (ms) | Slow component decay τ (ms) | |
| Glycine | 6 | 69 ± 11 | 0.67 ± 0.13 | 3.9 ± 0.4 | — | — |
| GABAA | ||||||
| Single | 5 | 48 ± 7 | 2.4 ± 0.7 | 26 ± 5 | — | — |
| Double | 5 | 81 ± 14 | 1.9 ± 0.6 | — | 10 ± 3 | 69 ± 17 |
GABAA receptor-mediated mIPSCs have been divided into two groups depending on whether the decay of the mIPSC was best fitted by one or two exponentials. A small number of GABAA receptor-mediated mIPSCs not well fitted by either one or two exponentials have not been included in the analysis. The n values refer to the number of neurones, not the number of events analysed in each neurone, which ranged from 10 to 76. The data are given as means ± s.e.m., where s.e.m. is the error among the means of the individual neurones.
Figure 2. Kinetics of GABAA and glycine receptor-mediated mIPSCs.
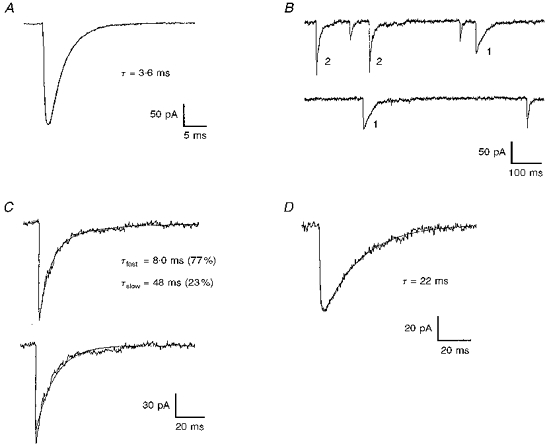
A, the decay of glycine receptor-mediated mIPSCs is fitted by a single exponential. B, a section of current trace in which GABAA receptor-mediated mIPSCs exhibit both single (labelled 1) and double (labelled 2) exponential decays. C, a GABAA receptor-mediated mIPSC which has a decay best fitted by a double exponential (top) but not by a single exponential (bottom). D, a GABAA receptor-mediated mIPSC which has a decay best fitted by a single exponential.
The amplitude of the GABAA mIPSCs shown in Table 1 are only for those IPSCs for which the IPSC was well fitted by either a single or double exponential. Since it was often difficult to determine whether the decay of a small mIPSC was better fitted by a single or double exponential, the amplitudes shown are artificially high. In a single neurone the average amplitude of all GABAA receptor-mediated mIPSCs irrespective of decay kinetics ranged from 28 ± 3 to 57 ± 7 pA (n≥ 44 for each neuron), with an average across five neurones of 41 ± 12 pA. The difference in amplitude of glycine and GABAA receptor-mediated mIPSCs (Table 1) is probably less than these results suggest, since there was often a significant amount of glycine noise (see below) which necessitated setting a higher peak detection threshold when measuring the amplitude of glycine receptor-mediated mIPSCs, and thus very small glycine receptor-mediated mIPSCs would have been excluded. Measuring the amplitude of all GABAA receptor-mediated mIPSCs using a threshold similar to that used for glycine receptor-mediated mIPSCs yielded an average amplitude in five neurones of 62 ± 6.8 pA, which is comparable to the average amplitude of glycine receptor-mediated mIPSCs, 69 ± 11 pA.
Glycine receptor-mediated noise
In addition to evoked and miniature glycine receptor-mediated IPSCs, glycine receptors are involved in an additional phenomenon in the SG. During the course of the recording a gradual thickening of the current trace was observed in almost all neurones (Fig. 3A). This progressive increase in ‘noise’ was accompanied by a negative shift in the holding current. The glycine receptor antagonist strychnine (1 μm) blocked both the noise and the shift in the holding current in all nineteen neurones to which it was applied, whilst CNQX (10 μm) and bicuculline (10 μm) were without effect in the same neurones. Therefore, in spite of the fact that in nearly all neurones it was possible to evoke a GABAA IPSC and to observe GABAA mIPSCs, the noise appears to be mediated entirely by activation of strychnine-sensitive glycine receptors. Since glycine receptor-mediated evoked and miniature IPSCs were present in most cells, this noise may be due to tonically released glycine.
Figure 3. Glycine noise.
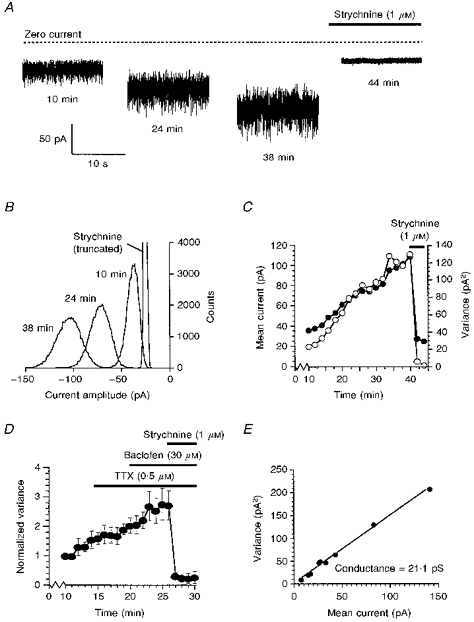
A, the increase in baseline noise which occurred during the recording was completely blocked by the glycine receptor antagonist strychnine. B, all-points histograms constructed from 5 s portions of the current traces shown in A. C, increase in mean current (•) and variance (○) over time. The mean current and variance were determined from the Gaussian distributions shown in B. In C and D, time is the time after initiation of whole-cell recording. D, TTX and the GABAB agonist baclofen had little or no effect on the glycine noise. The data points shown are the means of 3 cells. For each cell, the variance was normalized to the variance at 10 min. E, plot of variance against mean current amplitude from which the single-channel conductance underlying the glycine noise was estimated.
To quantify the glycine noise, the mean amplitude and variance of the current were determined. All-point distributions were constructed (Fig. 3B). Prior to the application of strychnine, the distribution at each time point was skewed towards negative amplitudes, and fitting required two Guassians. The skew of the distribution towards more negative amplitudes may be due to a small contribution from quantally released glycine, since spontaneous glycine receptor-mediated IPSCs were also present in many neurones. The mean current amplitude and variance associated with the noise was therefore determined from the larger component. After addition of strychnine (1 μm), the amplitude distribution could be well fitted by a single Gaussian, the mean current amplitude shifted towards zero and the variance was very much reduced (Fig. 3A-C).
As the example in Fig. 3C shows, the mean amplitude and variance of the current noise increased throughout the recording, showing no sign of levelling off even after 40 min. TTX (0.5 μm, n = 3) had little or no effect on the noise (Fig. 3D), indicating that action potential-dependent release of glycine did not significantly contribute to the noise.
It is unlikely that the noise is due to diffusion of Cl− from the pipette since the amplitude of evoked (e.g. Fig. 1B) and mIPSCs did not similarly increase. In four cells the amplitude of mIPSCs and noise were compared. During the first 10 min of recording the strychnine-sensitive variance of the current increased by 172 ± 10 %, while the glycine receptor-mediated mIPSC amplitude remained stable at 102 ± 14 % of control.
To determine if the noise was mediated by activation of glycine receptor/ion channels possessing properties similar to those characterized previously, the single-channel conductance underlying the noise was estimated. The mean current and variance induced by glycine receptor activation was determined by subtracting the mean current and variance in strychnine from the values just prior to the addition of strychnine. The unitary conductance as calculated from the ratio of the variance to the mean current was 21 pS (Fig. 3E). This is in the range of unitary conductances found from directly measuring single channels where openings of 19–104 pS have been reported (Hamill, Bormann & Sakmann, 1983; Takahashi & Momiyama, 1991; Kaneda, Farrant & Cull-Candy, 1995).
μ-Opioid or GABAB receptor activation inhibits glycine and GABAA IPSCs
μ-Opioid
In ten of eleven neurones the selective μ-opioid receptor agonist DAMGO (1 μm) reduced the amplitude of evoked glycine receptor-mediated IPSCs (Fig. 4A and B). In the other neurone there was no effect on the IPSC. The inhibition ranged from 23 to 83 %, with a mean of 53 ± 7 % (n = 10). The inhibition of the glycine receptor-mediated IPSC was rapid in onset and was completely reversed by the non-selective opioid antagonist naloxone (1–3 μm, n = 3) and by the selective μ-opioid antagonist CTAP (1 μm, n = 4). DAMGO (1 μm) also reduced the amplitude of evoked GABAA receptor-mediated IPSCs, but in only nine of sixteen neurones. The GABAA receptor-mediated IPSCs in the other seven neurones were unaffected by DAMGO. The inhibition ranged from 34 to 84 % with a mean of 49 ± 7 % (n = 9), and was reversed by CTAP (1 μm, n = 3). DAMGO at 10 μm produced no additional inhibition when applied after 1 μm DAMGO (n = 2 for glycine IPSCs and n = 3 for GABAA IPSCs), demonstrating that 1 μm was a maximally effective concentration of DAMGO.
As well as decreasing the amplitude of evoked IPSCs, DAMGO (1 μm) also increased the proportion of failures in neurones in which they were observed. Failures of evoked glycine receptor-mediated IPSCs increased from 12 ± 8 to 36 ± 12 % (n = 5, Fig. 4B) and GABAA IPSCs from 6 ± 8 to 32 ± 19 % (n = 4). An increase in failures is consistent with a presynaptic site of action of DAMGO. An additional method to discriminate between a pre- and postsynaptic site of action is to study mIPSCs. A decrease in the frequency of mIPSCs with no change in amplitude is most likely to indicate a presynaptic site of inhibition. Unfortunately, in most neurones the frequency of glycine and GABAA receptor-mediated mIPSCs was not high enough to allow examination of the effects of μ-opioid receptor activation on mIPSCs. However, in the two neurones in which we have studied DAMGO (1 μm), there was a reversible reduction in the frequency of glycine receptor-mediated mIPSCs by 63 and 35 %, whilst the amplitude remained constant at 101 and 99 % of control, respectively. The control frequencies of glycine receptor-mediated mIPSCs in these two neurones were 0.30 and 0.12 Hz. In contrast, application of DAMGO (1 μm, n = 5) for up to 10 min had no effect on the glycine receptor-mediated noise. DAMGO (1 μm) reversibly reduced the frequency of GABAA mIPSCs in two of four neurones by 47 and 36 %, while the amplitude was unaffected, being 107 and 104 % of control, respectively. In two other neurones neither the frequency nor the amplitude were affected by DAMGO. The control frequencies of GABAA receptor-mediated mIPSCs were 0.28 and 2.07 Hz in the two neurones affected by DAMGO, and 0.11 and 0.70 Hz in the two neurones unaffected by DAMGO.
To determine if DAMGO selectively inhibited either the single or double exponential decay GABAA receptor-mediated mIPSCs, the kinetics of mIPSCs in DAMGO were examined. In the two neurones in which the GABAA receptor-mediated mIPSCs were reduced by DAMGO, the percentage of mIPSCs with a single exponential decay was 31 and 69 % (n = 13 for both neurones) in control and 31 and 50 % (n = 13 and 12), respectively, in DAMGO. Therefore, DAMGO does not selectively reduce the frequency of either single or double exponential decay mIPSCs.
GABAB
The GABAB receptor agonist baclofen (3–30 μm) reduced the amplitude of evoked glycine receptor-mediated IPSCs (Fig. 4C and D) and GABAA receptor-mediated IPSCs. Inhibition by baclofen occurred in all neurones tested and at 30 μm was nearly complete, reducing the amplitude of glycine receptor-mediated IPSCs by 95 ± 1 % (n = 5) and GABAA IPSCs by 89 ± 3 % (n = 6). The selective GABAB antagonist CGP 55845A (1 μm) reversed the inhibition by baclofen (3–30 μm) of both glycine (n = 8) and GABAA (n = 9) receptor-mediated IPSCs.
The effect of baclofen on failures was determined using data obtained from neurones exposed to 3 μm baclofen, because the large inhibition caused by 30 μm would have made it difficult to differentiate between failures and low-amplitude successes. Baclofen (3 μm) reduced the amplitude of glycine IPSCs by 65 ± 10 % (n = 3) and GABAA IPSCs by 77 ± 1 % (n = 4). As with the μ-opioid inhibition of the evoked IPSCs, inhibition by baclofen was associated with an increase in the percentage of failures in neurones in which they were present. Failures of glycine receptor-mediated IPSCs increased from 5 ± 7 to 34 ± 16 % (n = 3), while failures of GABAA receptor-mediated IPSCs increased from 2 ± 4 to 37 ± 10 % (n = 4), consistent with the inhibition being presynaptic. Baclofen also reduced the frequency of mIPSCs without affecting their amplitude. In three neurones baclofen (30 μm) reversibly reduced the frequency of glycine receptor-mediated mIPSCs by 64 ± 16 %, whilst the amplitude remained constant at 99 ± 10 % of control. The control frequencies of glycine receptor-mediated mIPSCs in these three neurones ranged from 0.071 to 0.72 Hz. In contrast, the GABAB agonist baclofen (30 μm, n = 7; Fig. 3D) had no effect on the glycine receptor-mediated noise. In four other neurones baclofen (30 μm) reversibly reduced the frequency of GABAA mIPSCs by 68 ± 4 %, whilst the amplitude was unchanged at 105 ± 14 % of control. In these four neurones, the control frequencies of GABAA receptor-mediated mIPSCs ranged from 0.23 to 0.81 Hz. The reduction in frequency of mIPSCs with no change in amplitude probably indicates that baclofen is acting presynaptically.
As with DAMGO, baclofen did not selectively inhibit either the single or double exponential decay GABAA receptor-mediated mIPSCs. In the four neurones in which the frequency of GABAA receptor-mediated mIPSCs was reduced by baclofen, the percentage of mIPSCs with a single exponential decay was 53 ± 11.6 % (n = 10–17 for each neurone) in control and 54 ± 10.8 % (n = 10 or 11 for each neurone) in the presence of baclofen.
DISCUSSION
It has been shown previously that stimulating primary afferent fibres produces excitatory amino acid-mediated synaptic potentials in most SG neurones (Schneider & Perl, 1988; Grudt & Williams, 1994). There is also substantial evidence that GABA and glycine mediate synaptic transmission in the SG (Todd & Spike, 1993; Grudt & Williams, 1994; Yoshimura & Nishi, 1995). The present study extends those findings by demonstrating that virtually all SG neurones receive an input from both glycine-containing and GABA-containing afferents. What is unclear is whether the GABA and glycine inputs are separate or whether GABA and glycine are cotransmitters released from the same nerve terminals. In the spinal cord many presynaptic boutons appear to contain both glycine and GABA (Todd & Spike, 1993). In the presence of excitatory amino acid, glycine and GABAA antagonists there was very little or no evoked current remaining and miniature synaptic events were absent, demonstrating that there are no other major fast synaptic transmitters present in the SG.
GABAA receptor-mediated mIPSCs with single and double exponential decays were present in the same neurone. The main difference between GABAA receptor-mediated mIPSCs in various neuronal preparations is the time constant of decay of the synaptic current. The decay of the GABAA receptor-mediated mIPSC has been observed to be either monophasic (Ropert, Miles & Korn, 1990; Otis & Moody, 1992; Puia, Costa & Vicini, 1994) or biphasic (Edwards, Konnerth & Sakmann, 1990; Puia et al. 1994). In the SG it is unlikely that cable filtering along the dendritic tree is responsible for the slow component of decay of the GABAA receptor-mediated mIPSC, because no mIPSCs were recorded that had only the slow phase of decay; those with a slow decay component also exhibited a fast decay component. Furthermore, the rise time of the biphasic mIPSCs was as fast as that of the fast monophasic mIPSCs. The decay of the IPSC could be determined by (i) rapid removal of the transmitter, (ii) desensitization of the receptor or (iii) the kinetics of channel relaxation. For (ii) and (iii) the subunit composition of the GABAA receptor/ion channel complex may be of critical importance.
The GABAA receptor/ion channel is thought to be a hetero-pentameric complex. A large number of GABAA receptor subunit subtypes have been cloned (McKernan & Whiting, 1996), giving rise to the possibility of a large number of different subunit combinations that may result in subtly different receptor/ion channel properties. Numerous GABAA receptor subtypes have been found in the SG (Persohn, Malherbe & Richards, 1991; Bohlhalter, Weinmann, Mohler & Fritschy, 1996) and thus subunit composition differences may underlie the different kinetics of the GABAA receptor-mediated mIPSCs. If this is so, it seems unlikely that the receptor underlying the single exponential decay mIPSC observed in this study is also responsible for a component of the double exponential mIPSC, because the time constant of the single exponential decay was different from either of the time constants for the double exponential decay. In hippocampal CA1 pyramidal neurones single and double exponential decay-evoked GABAA receptor-mediated IPSCs are initiated on different parts of the neurone and have been hypothesized to represent different GABAA receptor subunit subtypes (Pearce, 1993). If indeed it is the case that different receptor populations underlie the single and double exponential decay, then the finding that both kinds of mIPSCs are present in a single SG neurone suggests that there must be selective targeting of receptor subtypes to different synapses, as has been suggested to occur in hippocampal CA1 neurones (Pearce, 1993). Spatial segregation of GABAergic inputs suggests that different inputs may have different functional roles (Nicoll, 1994). If the single and double exponential decay GABAA receptor-mediated mIPSCs are mediated by different synapses in the SG, there is no evidence for differential regulation of GABA release at these synapses by activation of μ-opioid and GABAB receptors, since agonists at those receptors decreased the frequency of both single and double exponential decay mIPSCs.
We have observed a TTX-insensitive baseline current noise resulting from activation of glycine receptors, which increased with time during the period of recording. This noise was insensitive to μ-opioid and GABAB receptor agonists, which reduced the frequency of glycine receptor-mediated mIPSCs. This indicates that the noise is not due to small, unresolved mIPSCs. One possible explanation for this phenomenon is that the amount of tonically released glycine is in fact constant over time, but the increase in noise results from the slow dialysis of chloride ions from the recording pipette to the distal dendrites where the tonically released glycine is exerting its effect. This, however, seems unlikely because the amplitudes of spontaneous and evoked glycine and GABAA receptor-mediated IPSCs that could be observed on top of the noise, did not exhibit a similar increase with time. Another possibility is that the increasing background noise reflects a deterioration of the slice with time, resulting in either an increase in tonic glycine release or a decrease in re-uptake of glycine with time. Again this seems unlikely because the characteristics of the evoked and spontaneous glycine receptor-mediated IPSCs did not show any changes with time. In addition, we only observed a background noise mediated through glycine receptors; there was no background current noise mediated by activation of GABAA receptors, in spite of the fact that evoked and spontaneous GABAA receptor-mediated IPSCs were commonly observed. A tonic, TTX-insensitive current mediated through GABAA receptors has been observed in cerebellar granule neurones (Kaneda et al. 1995; Wall & Usowicz, 1997). This did not appear to be due to reversal of the GABA re-uptake transporter. Furthermore, the tonic GABAA current did not result from a reduced uptake of spontaneously released transmitter. The GABAA noise in cerebellar granule neurones increases during development. It will be interesting to see if the glycine noise observed in the SG is also under developmental regulation.
Previously it has been shown that activation of μ-opioid (Macdonald & Nelson, 1978; Jeftinija, 1988; Grudt & Williams, 1994) and GABAB (Kangrga, Jiang & Randic, 1991; Blake, Cao, Headley, Collingridge, Brugger & Evans, 1993) receptors decreases excitatory transmitter release from primary afferent fibres. In the present paper we now show that μ-opioid as well as GABAB receptor activation also decreases the release of the inhibitory transmitters GABA and glycine from terminals in this region. Although the SG is known to play a role in modulating pain-related primary afferent input, the circuitry involved is not understood. It is therefore difficult to postulate with any degree of certainty how inhibition of inhibitory synaptic transmission by μ-opioid agonists relates to the spinal analgesia produced by opioids.
Opioids and GABAB receptor agonists hyperpolarize most SG neurones (Yoshimura & North, 1983; Kangrga et al. 1991; Grudt & Williams, 1994). Since many SG neurones are interneurones, some of which contain GABA or glycine, it is likely that glycine and GABA-containing neurones are hyperpolarized by these drugs. Such a hyperpolarization would be expected to cause a reduction in evoked GABA and glycine IPSCs by decreasing the likelihood that a given stimulus would elicit an action potential. However, the finding that μ-opioid and GABAA agonists reduce the frequency of action potential-independent mIPSCs in addition to evoked IPSCs, indicates that receptors for these drugs are located on terminals in the SG in addition to their probable presence on neuronal cell bodies.
μ-Opioid and GABAB inhibition of mIPSCs was associated with a decrease in the frequency with no change in average amplitude. Such an effect has been interpreted to indicate a presynaptic action. Recently, however, evidence has begun to accumulate that when long-term potentiation is induced a cluster of receptors mediating a synaptic current can become active (Isaac, Nicoll & Melenka, 1995; Liao, Hessler & Malinow, 1995). It is conceivable that modulation by μ-opioid and GABAB agonists could be due to a postsynaptic effect whereby a cluster of receptors are inactivated. This would also result in a decrease in frequency with no change in average amplitude and an increase in failures of evoked IPSCs. While presynaptic inhibition of transmitter release still seems the simplest explanation for a selective effect on miniature frequency, a postsynaptic action cannot be definitively ruled out.
Clearly, inhibitory synaptic transmission is widespread in the substantia gelatinosa. Although it undoubtedly plays an important part in the physiology of this area, its exact role remains to be determined, as in fact does the nature of the neuronal circuitry of the SG.
Acknowledgments
We thank Dr B. Khakh, Dr M. M. Usowicz and Dr M. Wall for comments on the manuscript. T. J. G. was supportd by a National Research Service Award from the US National Institute on Drug Abuse (DA05534).
References
- Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proceedings of the National Academy of Sciences of the USA. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurones intracellularly stained with horseradish peroxidase. Journal of Comparative Neurology. 1980;194:809–827. doi: 10.1002/cne.901940407. [DOI] [PubMed] [Google Scholar]
- Blake JF, Cao CQ, Headley PM, Collingridge GL, Brugger F, Evans RH. Antagonism of baclofen-induced depression of whole-cell synaptic currents in spinal dorsal horn neurones by the potent GABAB antagonist CGP55845. Neuropharmacology. 1993;32:1437–1440. doi: 10.1016/0028-3908(93)90042-2. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy J-M. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. Journal of Neuroscience. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan AW, Hall JG, Headley PM. Suppression of transmission of nociceptive impulses by morphine: selective effects of morphine administered in the region of the substantia gelatinosa. British Journal of Pharmacology. 1977;61:65–76. [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch clamp study. The Journal of Physiology. 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel S. Golgi studies of the neurones in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis) Journal of Comparative Neurology. 1978;180:395–414. doi: 10.1002/cne.901800213. [DOI] [PubMed] [Google Scholar]
- Gobel S, Falls WM, Bennett GJ, Abdelmoumene M, Hayashi H, Humphrey E. An EM analysis of the synaptic connections of horse-radish peroxidase-filled stalked neurones and islet neurones in the substantia gelatinosa of the adult cat spinal cord. Journal of Comparative Neurology. 1980;194:781–807. doi: 10.1002/cne.901940406. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Henderson G. Inhibitory synaptic transmission in the substantia gelatinosa and its modulation by μ-opioid and GABAB receptor activation. Society for Neuroscience Abstracts. 1996;22:858. [Google Scholar]
- Grudt TJ, Williams JT. μ-Opioid agonists inhibit spinal trigeminal substantia gelatinosa neurones in guinea pig and rat. Journal of Neuroscience. 1994;14:1646–1654. doi: 10.1523/JNEUROSCI.14-03-01646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Bormann J, Sakmann B. Activation of multiple conductance state chloride channels in spinal neurones by glycine and GABA. Nature. 1983;305:805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Isaac JTR, Nicoll RA, Melenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Jeftinija S. Enkephalins modulate excitatory synaptic transmission in the superficial dorsal horn by acting at μ-opioid receptor sites. Brain Research. 1988;460:260–268. doi: 10.1016/0006-8993(88)90371-x. 10.1016/0006-8993(88)90371-X. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Duggan AW. Evidence that opiate receptors of the substantia gelatinosa contribute to the depression, by intravenous morphine, of the spinal transmission of impulses in unmyelinated afferents. Brain Research. 1981;207:223–228. doi: 10.1016/0006-8993(81)90698-3. 10.1016/0006-8993(81)90698-3. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-neurone and single-channel currents activated by GABA and glycine in granule neurones of the rat cerebellum. The Journal of Physiology. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangrga I, Jiang M, Randic M. Actions of (-)-baclofen on rat dorsal horn neurons. Brain Research. 1991;562:265–275. doi: 10.1016/0006-8993(91)90630-e. 10.1016/0006-8993(91)90630-E. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa neurones (lamina II) neurones with axons that terminate in deeper dorsal horn laminae (III-V) Journal of Comparative Neurology. 1988;267:172–189. doi: 10.1002/cne.902670203. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Re-examination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. Journal of Comparative Neurology. 1979;186:117–132. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Nelson PG. Specific-opiate-induced depression of transmitter release from dorsal root ganglion neurones in culture. Science. 1978;199:1449–1450. doi: 10.1126/science.204015. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends in Neurosciences. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. 10.1016/S0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. Neuroscience: Cajal's rational psychology. Nature. 1994;368:808–809. doi: 10.1038/368808a0. 10.1038/368808a0. [DOI] [PubMed] [Google Scholar]
- Otis TS, Moody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49:13–32. doi: 10.1016/0306-4522(92)90073-b. 10.1016/0306-4522(92)90073-B. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. 10.1016/0896-6273(93)90310-N. [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurones of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Ropert N, Miles R, Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. The Journal of Physiology. 1990;428:707–737. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Non-uniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–756. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Perl ER. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. Journal of Neuroscience. 1988;8:2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated C afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Single channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurones. Neuron. 1991;7:965–969. doi: 10.1016/0896-6273(91)90341-v. 10.1016/0896-6273(91)90341-V. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurones in laminae I-III of the mammalian spinal dorsal horn. Progress in Neurobiology. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. 10.1016/0301-0082(93)90045-T. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Huguenard JR. Purinergic inhibition of GABA and glutamate release in the thalamus: implications for thalamic network activity. Neuron. 1995;15:909–918. doi: 10.1016/0896-6273(95)90181-7. 10.1016/0896-6273(95)90181-7. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. Journal of Neuroscience. 1995;73:1192–1200. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. European Journal of Neuroscience. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in rat spinal cord in vitro. The Journal of Physiology. 1995;482:29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, North RA. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983;305:529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]


