Abstract
We have investigated the permeability-increasing effect of arachidonic acid on pial venular capillaries in vivo using the single microvessel occlusion technique.
Permeability to Lucifer Yellow dye (476 Da) increased dose dependently when arachidonic acid was applied focally to the abluminal surface of the vessels. This was completely reversible at all but the highest dose. The permeability increase was 1.65 × 10−6 ± 0.247 × 10−6 cm s−1 (mean ± s.e.m.) at 0.25 mm, 3.53 × 10−6 ± 0.872 × 10−6 cm s−1 at 0.5 mm, 12.61 × 10−6 ± 3.44 × 10−6 cm s−1 at 1 mm and 18.46 × 10−6 ± 5.90 × 10−6 cm s−1 at 2 mm arachidonic acid. There was a similar reversible dose-dependent permeability increase to eicosapentaenoic acid.
These permeability increases could be prevented by co-application of a mixture of the anti-oxidants superoxide dismutase and catalase (30 and 100 U ml−1), or by the iron chelator desferrioxamine (100 μm).
The permeability increases were also prevented by the cyclooxygenase and 5-lipoxygenase blockers indomethacin (100 μm) and nordihydroguariaretic acid (100 μm), respectively, when applied together, but not singly.
It was concluded that the permeability response to arachidonic acid depends on the formation of free radicals and subsequent lipid peroxidation.
Arachidonic acid has been suggested to play a part in the development of cerebral oedema (Chan & Fishman, 1984), and similar concentrations as those found in patients (Maier-Hauff et al. 1984), given by intracerebral infusion, increased the regional uptake of 14C-labelled aminoisobutyric acid (Ohnishi, Posner & Shapiro, 1992). Arachidonic acid could disrupt the blood-brain barrier by acting directly on the endothelium through membrane fluidity by the formation of eicosanoids or free radicals, or it could act indirectly through cerebral arteriolar dilatation resulting in venous capillary hypertension beyond physiological limits (Unterberg, Schmidt, Wahl, Ellis, Marmarou & Baethmann, 1991). A number of studies have indicated a role for oxygen free radicals in the cerebrovascular permeability-increasing effects of arachidonic acid. Thus albumin and sucrose transfer across cultured brain endothelial cells increased after exposure to arachidonic acid, and was prevented by different antioxidants (Shi, Cavitt & Audus, 1995). Increased albumin permeability following superfusion of arachidonic acid in the rat was prevented by a combination of catalase and superoxide dismutase (Wei, Ellison, Kontos & Povlishock, 1986).
We have used the single pial microvessel preparation to examine these issues. This preparation has the advantage that arachidonic acid can be applied focally to a 10-20 μm section of a single venular capillary under examination without affecting neighbouring tissues, so avoiding complications that may arise from changes in hydrostatic pressure. Some of the results of this work have been presented previously in a preliminary form (Easton & Fraser, 1994b).
METHODS
The methods have been fully described elsewhere (Easton & Fraser, 1994a) and the following is a brief summary. The experiments were performed on Wistar rats of both sexes aged 23–33 days and were within guidelines directed by The Animals (Scientific Procedures) Act 1986. The rats were anaesthetized with sodium pentobarbitone (60 mg kg−1i.p.), supplemented as necessary (assessed by the foot pinch reflex), and at the end of the experiment were killed by anaesthetic overdose followed by exsanguination. Cannulae were routinely inserted in the trachea and the common carotid artery. A section of the frontoparietal bones on the left side, between coronal and lambdoid sutures, was thinned with a dental drill. A metal ring (i.d., 7 mm; o.d., 13 mm) was glued onto the cranium surrounding the thinned bone with cyanoacrylate adhesive. The thinned cranial surface within the ring was superfused with artificial cerebrospinal fluid (ACSF) at 1–2 ml min−1 to ensure that a layer of fluid was present at all times. The thinned bone within the ring was then removed, carefully avoiding damage to the meninges and cerebral surface. The overlying meninges were removed to leave the pial microvessels free from a continuous layer of overlying tissue. The animal was placed on the stage of an epi-fluorescence microscope (ACM, Zeiss) and the cerebral surface viewed through a ×20 objective lens. Measurements were made by injecting small boluses (0.05–0.2 ml) of Lucifer Yellow (5 mm in 0.9 % NaCl) through the carotid cannula. The dye was trapped in a venular capillary with an occluding probe placed at one end of the vessel. The microscope image from a ca 10 μm section of the microvessel was relayed to a videodensitometer, the output of which was recorded on a chart recorder. The change in fluorescence in this section was measured over 1–2 min after which the probe was lifted and flow restored to the microvessel. The procedure of injecting dye and occluding the vessel was repeated for each permeability measurement.
We have previously shown that under these conditions the rate of change of dye concentration in the measured section can be described by a single monoexponential, Ct = C0e-kt, where t is the time elapsed from the measurement of the initial dye concentration C0, and Ct is dye concentration at time t. The rate constant k is related to permeability P by k = 2P/r, where r is the vessel radius. For small molecules, such as Lucifer Yellow, this permeability coefficient is a good approximation of diffusive permeability without significant complication by convection (Easton & Fraser, 1994a). Substances were either applied in the superfusing solution, or by focal application through a micropipette with tips bevelled to 3–5 μm. Solutions of polyunsaturated fatty acids, mixed with lissamine-rhodamine dye so that they could be seen under the microscope, were backfilled into individual micropipettes positioned next to the microvessel, and pressure ejected.
Substances
ACSF was buffered to pH 7.40 ± 0.05 and contained (mm): 110.5 NaCl, 4.7 KCl, 2.5 CaCl2, 1.1 KH2PO4, 1.25 MgSO4.7H2O, 25 NaHCO3 and 15 Hepes. Hepes, Lucifer Yellow (CH, dilithium salt), lissamine-rhodamine, catalase, superoxide dismutase, indomethacin and nordihydroguariaretic acid were obtained from Sigma and desferrioxamine mesylate from CIBA-Geigy (Switzerland) as Desferal. Arachidonic, oleic and eicosopentaenoic acids were supplied by Cascade Biochem Ltd (Reading, UK) and were dissolved or provided as stock solutions in 100 % ethanol. Solutions were freshly prepared by removing stock solution and evaporating the ethanol with a stream of 100 % N2 gas, immediately dissolving the residue in 100 mm Na2HCO3. This was then diluted in ACSF before application to give a 1 mm final maximum Na2HCO3. All concentrations are expressed as final concentrations in the fluid in contact with the cerebral surface.
Statistics
The effects of treatments were subject to analysis of variance and the appropriate post tests. Non-parametric statistics were used when numbers were small or the distribution was apparently non-Gaussian.
RESULTS
The polyunsaturated fatty acids were applied focally to the abluminal surface of the single pial venular capillaries through a bevelled micropipette, since when 1 mm arachidonic acid was added to the whole exposed surface for as little as 1 min, oedema developed rapidly and the preparation became unusable. The preparation remained stable for 2–3 h, so that when a permeability increase was induced by polyunsaturated fatty acids, it returned to the baseline value within 5 min of the application (see Fig. 1A). Application of 1 mm oleic had no permeability-increasing effect. Rapid changes in permeability (as previously reported by Easton, Sarker & Fraser, 1997) were found to be related to the concentration of arachidonic acid (Fig. 1B and C). All subsequent values given in this paper refer to the first value obtained after the application of a substance.
Figure 1. The effects of focal application of long-chained fatty acids on pial venular permeability.
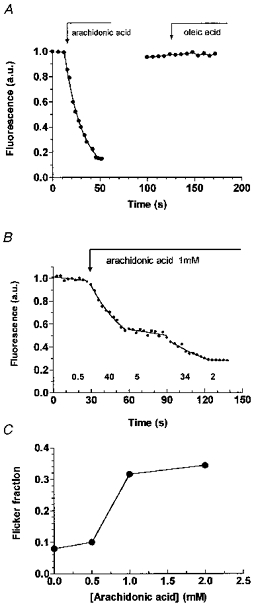
A, arachidonic acid (1 μm) ejected from a 4 μm-tip micropipette placed adjacent to the occluded segment of a 11 μm diameter venular capillary resulted in a rapid loss of fluorescence, indicating a large increase in permeability. An occlusion on the same vessel 50 s later showed that the original permeability had been restored, and oleic acid at the same concentration produced no change in permeability. B, an example in which the permeability varied (flickered) during arachidonic acid application. The vessel was 11 μm in diameter, and the numbers below the curve refer to the measured PLY. C, the number of times a flickering permeability response was obtained expressed as a fraction of the total number of applications at that arachidonic acid concentration. These responses were seen in 2/25 applications with 0.25 mm arachidonic acid, 1/10 with 0.5 mm, 12/38 with 1.0 mm and 11/21 with 2 mm. This trend, was significant at P < 0.01 (χ2 test for trend, 6.674, d.f. = 1).
Dose-response relationship to arachidonic and eicosapentaenoic acids
The resulting changes in permeability to Lucifer Yellow (PLY) in response to arachidonic acid application are plotted in Fig. 2A as mean values compared with control means before application and mean PLY after application. The permeability increases for each concentration were 1.65 × 10−6 ± 0.247 × 10−6 cm s−1 (mean ± s.e.m.) at 0.25 mm (range, 0.45 × 10−6-4.37 × 10−6 cm s−1), 3.53 × 10−6 ± 0.872 × 10−6 cm s−1 at 0.5 mm (range, 1.05 × 10−6- 10.79 × 10−6 cm s−1), 12.61 × 10−6 ± 3.44 × 10−6 cm s−1 at 1 mm (range, 0.75 × 10−6-63.51 × 10−6 cm s−1), and 18.46 × 10−6 ± 5.90 × 10−6 cm s−1 at 2 mm arachidonic acid (range, 0.54 × 10−6-77.21 × 10−6 cm s−1). PLY after removal of arachidonic acid was reversed within 2 min at 0.25, 0.5 and 1 mm, but with 2 mm it remained elevated to at least double the control value in 7/14 of the microvessels.
Figure 2. The dose-response relationship between focally applied polyunsaturated fatty acids and acute increases in permeability.
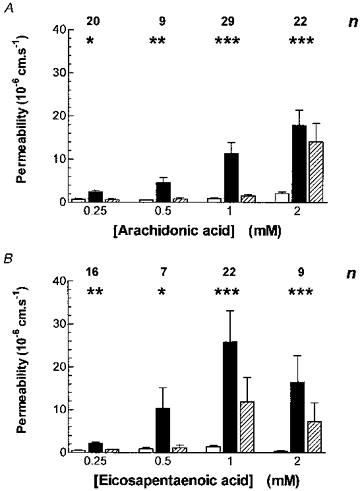
A, arachidonic acid applied to a total of 40 venular capillaries (diameter: range, 5–23 μm; mean ± s.d., 10.9 ± 3.8 μm) from a total of 24 rats. The numbers above the groups of bars denote the number of vessels used for each concentration of arachidonic acid. In this and subsequent bar charts open bars denote the controls, filled bars long-chained fatty acid application and hatched bars the recovery measurement. Two-way analysis of variance showed that permeability increases were significant for arachidonic acid application and concentration (P < 0.001 for both; F = 9.38, d.f. = 2, 153 and F = 7.30, d.f. = 3, 153, respectively). The post test for linear trend was also significant (P < 0.0001). The asterisks denote the significance of Student's paired t tests between the control and arachidonic acid (*P < 0.05, **P < 0.01 and ***P < 0.001). B, a similar bar chart showing the dose-response relationship between focal applications of eicosapentaenoic acid (EPA) and acute increases in pial venular capillary permeability. A total of 27 venular capillaries (diameter: range, 6–19 μm; mean ± s.d., 11.4 ± 3.67 μm) from a total of 18 rats. EPA application also caused a significant permeability increase (two-way analysis of variance P = 0.0015, F = 6.845, d.f. = 2, 120), and the post test for linear trends was significant at P < 0.05. The asterisks denote the significance of paired t tests between the control and EPA.
The idea that free radicals were responsible for the permeability increases was tested by applying eicosapentaenoic acid (EPA), which, like arachidonic acid, is metabolized by cyclooxygenase and 5-lipoxygenase to produce free radicals (Alexander-North, North, Kiminyo, Buettner & Spector, 1994), but unlike arachidonic acid, EPA metabolites have little permeability-increasing effects (Sardesai, 1992). Figure 2B shows the effect of EPA application on PLY. The permeability increases for each concentration were 1.63 × 10−6 ± 0.305 × 10−6 cm s−1 (mean ± s.e.m.) at 0.25 mm (range, 0.36 × 10−6- 4.39 × 10−6 cm s−1), 6.32 × 10−6 ± 1.698 × 10−6 cm s−1 at 0.5 mm (range, 1.82 × 10−6-35.55 × 10−6 cm s−1), 26.53 × 10−6 ± 7.702 × 10−6 cm s−1 at 1 mm (range, 1.05 × 10−6-127 × 10−6 cm s−1) and 16.57 × 10−6 ± 9.304 × 10−6 cm s−1 at 2 mm EPA (range, 1.13 × 10−6-62.3 × 10−6 cm s−1). Very like arachidonic acid, there was a dose-dependent increase in PLY compared with controls, and the effect of the two lower doses of EPA was fully reversible, while in 7/20 microvessels at 1 mm, and in 4/6 microvessels at 2 mm, PLY remained elevated to at least double the control value.
The effect of free radical scavengers
Three concentrations of catalase (100, 200 and 500 U ml−1) were applied alone and combined with 1 mm arachidonic acid and had no effect on the induced permeability increase (see Fig. 3A). Similarly, superoxide dismutase (SOD; 60 U ml−1) had no effect. The combination of catalase (100 U ml−1) and SOD (60 and 30 U ml−1) blocked the arachidonic acid induced permeability increase, but catalase (100 U ml−1) alone and SOD (10 U ml−1) alone were ineffective. The 100:60 combination was effective at preventing the PLY responses to concentrations of arachidonic acid (Fig. 3B) and EPA (Fig. 3C) from 0.25 to 2 mm. The involvement of free radicals in the signalling of permeability increase was further tested by using the iron chelator desferrioxamine, which when included in the superfusing solution reduced the PLY responses to arachidonic acid (Fig. 3D) and to EPA (Fig. 3E).
Figure 3. The effects of free-radical scavengers on the permeability response to polyunsaturated fatty acids.
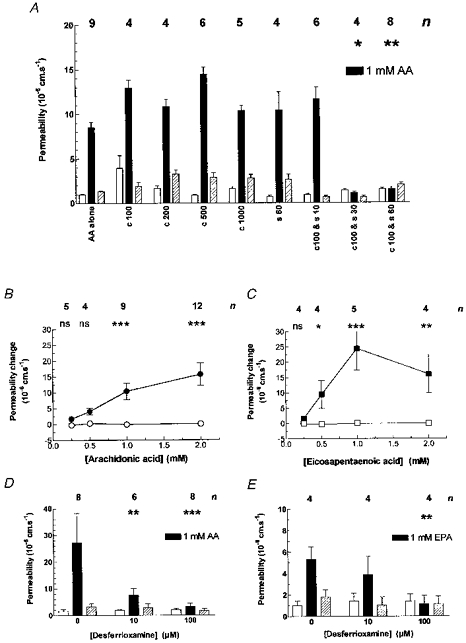
A, the permeability-increasing effect of arachidonic acid can be blocked by a combination of the free radical scavenger superoxide dismutase (SOD) with catalase. Different combinations of catalase and SOD (c and s, the concentrations specified in U ml−1) were added to the superfusing solution while 1 mm arachidonic acid was focally applied. Each set of bars shows a treatment on (n) microvessels (total 24 from 11 rats; diameter: range, 4–21 μm; mean ± s.d., 9.7 ± 4.8 μm). Two-way analysis of variance showed that there were significant effects with P < 0.0001 (F = 370.4, d.f. = 2, 110) for 1 mm arachidonic acid application and P < 0.0001 (F = 22.69, d.f. = 8, 110) for the free radical scavengers. Bonferroni's multiple comparison test between control and arachidonic acid permeabilities were significant at least at the P < 0.05 level for all groups except c100-s30 and c100-s60, and these two responses were significantly different from those of the arachidonic acid alone group at P < 0.05 and P < 0.01. B, the effects of antioxidants on permeability increases induced by arachidonic and eicosapentaenoic acids. Arachidonic acid was applied at increasing concentrations in the presence of a combination of 100 U ml−1 catalase and 60 U ml−1 SOD (○) to 12 venular capillaries (diameter: range, 7–23 μm; mean ± s.d., 11.0 ± 6.28 μm) from a total of 10 rats when there was no discernible permeability change. The data are plotted as increased permeability for each observation and are compared with that derived from Fig. 1 for arachidonic acid alone (•) for comparison; n refers only to the treated (○) group. The differences between these responses were significant (P < 0.0001; Kruskal-Wallis statistic, 86.13 for 8 groups) and the asterisks above the individual concentrations denote the level of significance as assessed from Dunn's multiple comparison post test. C, eicosapentaenoic acid was examined in a similar way. It was applied in the presence of the scavengers (□) to 10 venular capillaries (diameter: range, 9–19 μm; mean ± s.d., 12.7 ± 3.52) from a total of 4 rats, and compared with EPA in the absence of the scavengers (▪). The differences in the responses was statistically significant (P < 0.0001; Kruskal-Wallis statistic, 53.46 for 8 groups). D, the iron chelator desferrioxamine also prevented the permeability increase due to arachidonic acid (AA) when included in the superfusing solution. Each set of bars shows the permeability before, during and after adding arachidonic acid with different concentrations of desferrioxamine on (n) microvessels (total 15 from 4 rats; diameter: range, 8–24 μm; mean ± s.d., 12.3 ± 5.23 μm). Two-way analysis of variance showed that there were significant effects with P < 0.005 (F = 6.18, d.f. = 2, 57) for 1 mm arachidonic acid application and P < 0.05 (F = 3.22, d.f. = 2, 57) for the different concentrations of desferrioxamine. Bonferroni's multiple comparison test showed that the decreased response to arachidonic acid was significant (P < 0.01 for 10 μm, and P < 0.001 for 100 μm). E, broadly similar results were obtained with 1 mm eicosapentaenoic acid (EPA). Four microvessels were tested from 4 rats (diameter: range, 6–24 μm; mean ± s.d., 11.4 ± 5.74 μm), with two-way analysis of variance giving P < 0.05 (F = 27.63, d.f. = 2, 27) for EPA but not significant for the different concentrations of desferrioxamine. Bonferroni's multiple comparison test showed that the decreased response to EPA was significant (P < 0.05) for 100 U ml−1 desferrioxamine.
The effect of cyclooxygenase and 5-lipoxygenase inhibitors
The cyclooxygenase inhibitor indomethacin (100 μm) and the 5-lipoxygenase inhibitor nordihydroguariaretic acid (NDGA, 100 μm) were used alone or in combination to determine their effect on the action of focally applied 1 mm arachidonic acid and EPA. Figure 4 shows that there was no effect on the permeability response when indomethacin or NDGA were applied alone, but when the inhibitors were applied together, the increase to both arachidonic acid and EPA was blocked.
Figure 4. The effects of cyclooxygenase and lipoxygenase blockade on the permeability response to fatty acids.
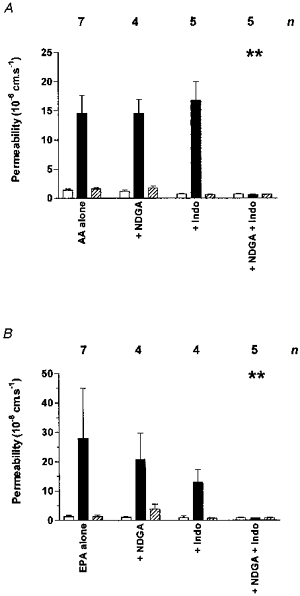
A, 1 mm arachidonic acid (AA) was applied to venular capillaries (17 from 10 rats; diameter: range, 5–15 μm; mean ± s.d., 9.9 ± 3.14 μm) in the presence of indomethacin (Indo) and nordihydroguariaretic acid (NDGA). The differences between these responses were significant (P < 0.001; Kruskal-Wallis statistic, 37.4 for 12 groups). Only the combined cyclooxygenase and lipoxygenase blockade completely prevented the permeability response (P < 0.01; Dunn's multiple comparison post test). B, when 1 mm eicosapentaenoic acid (EPA) was applied (12 from 6 rats; diameter: range, 5–13 μm; mean ± s.d., 10.2 ± 3.50 μm) again the overall difference in response was significant (P < 0.001; Kruskal-Wallis statistic, 33.8 for 12 groups), and only combined co-application of indomethacin and nordihydroguariaretic acid blocked the permeability response (P < 0.01; Dunn's multiple comparison post test).
DISCUSSION
This study shows that arachidonic acid increases permeability acutely in single cerebral microvessels by generation of oxygen free radicals in the course of its metabolism via the cyclooxygenase and lipoxygenase pathways. We found that focally administered arachidonic acid dose-dependently and reversibly increased pial venular capillary permeability to Lucifer Yellow, and that these increases could be very large, consistent with the formation of cerebral oedema. Previously, a high permeability state that had been induced by craniotomy (Easton et al. 1997), and in which free radical generation had been implicated, showed a characteristic flickering, not seen at lower permeabilities. As arachidonic acid, in the present series of experiments, also often resulted in a flickering permeability response, not limited to high permeabilities, the idea of free radical formation was raised. The arachidonic acid concentration used here is similar to that found in cerebral oedema fluid (Maier-Hauff et al. 1984), and to the 2 mm that produced maximal albumin extravasation (Ohnishi et al. 1992), and cerebral oedema in the rat (Chan & Fishman, 1984).
The arachidonic acid induced permeability increase was blocked by the combination of superoxide dismutase (60 U ml−1) and catalase (100 U ml−1; Fig. 3). Presumably, prompt removal of hydrogen peroxide by catalase increases the rate at which SOD converts the superoxide anion to H2O2. These two antioxidants were also needed to block induced increases in albumin extravasation seen by Wei et al. (1986). Desferrioxamine also prevented the permeability response to arachidonic acid at modest concentrations (100 μm rather than > 1 mm), which relates to its ability to prevent lipid peroxidation rather than direct free radical formation (Caraceni, Vanthiel & Borle, 1995). A similar concentration of desferrioxamine was found to be effective in reducing ischaemic brain injury in neonatal rats (Palmer, Roberts & Bero, 1994).
Further evidence for the hypothesis that free radical generation is responsible for the permeability response was obtained using eicosapentaenoic acid (EPA), a major constituent of fish oils. This (n-3) polyunsaturated fatty acid is not interconverted with arachidonic acid (n-6) in mammals, but is metabolized by the same enzymes to form different products: arachidonic acid generates 2-series prostaglandins and 4-series leukotrienes whereas EPA generates 3-series prostaglandins and 5-series leukotrienes (Sardesai, 1992). In general the EPA metabolites are less biologically active than those of arachidonic acid, and its inclusion in the diet is thought to promote anti-inflammatory effects by competing with arachidonic acid (Simopoulos, 1991). Despite this, the same four doses of EPA produced similar dose-dependent permeability increases to arachidonic acid, and were also blocked by the same combination of catalase and superoxide dismutase (Fig. 3C). Similarly indomethacin and NDGA were only effective against EPA when applied in combination (Fig. 4).
Both the cyclooxygenase and lipoxygenase pathways of polyunsaturated fatty acid metabolism result in free radical generation (Rice-Evans & Bruckdorfer, 1992), but some eicosanoids formed also have significant permeability-increasing effects, although the evidence that these are involved in acute cerebrovascular permeability increase is often contradictory (e.g. Suzuka, Mabe & Ito, 1994; Olesen & Crone, 1986). The 5-lipoxygenase products, leukotrienes C4, D4 and E4, have been implicated as a cause of inflammatory permeability increase (Henderson, 1994), but this may be mediated through increasing hydrostatic pressure (Unterberg et al. 1991). In the present experiments focal application avoids effects on resistance vessels. Some studies report that permeability increases were attenuated by lipoxygenase but not cyclooxygenase inhibitors (Black & Hoff, 1985), but several lipoxygenase inhibitors also have scavenger activity (Salmon & Garland, 1991). We found that NDGA or indomethacin alone had no effect on the permeability response to arachidonic acid or EPA, but in combination were effective against both (Fig. 4). This suggests free radical generation is the key event in the acute permeability response, since they will be generated by either cyclooxygenase or 5-lipoxygenase.
The foregoing argument indicates that free radicals were generated by intracellular enzymes, but the antioxidants we used probably remained entirely (in the case of SOD and catalase), or very largely (in the case of desferrioxamine), extracellular. This suggest that it is the free radicals that diffuse out of the cell that have the permeability-increasing effect. Additionally, desferrioxamine at the concentration used prevented lipid peroxidation, but had no effect on free radical generation (Caraceni et al. 1995). This evidence favours peroxidation by extracellular free radicals as the crucial step in the permeability response. We previously found that alkalization of the cell interior by ammonium chloride results in a large immediate flickering permeability increase (Easton et al. 1997), and this could underlie the way in which free radicals lead to the acute permeability response. It is possible that lipid peroxidation leads to activation of the Na+-H+ antiporter, and the resulting pH rise may be the signal that increases permeability.
Acknowledgments
This work was supported by The Wellcome Trust.
References
- Alexander-North LS, North JA, Kiminyo KP, Buettner GR, Spector AA. Polyunsaturated fatty acids increase lipid radical formation induced by oxidant stress in endothelial cells. Journal of Lipid Research. 1994;35:1773–1785. [PubMed] [Google Scholar]
- Black KL, Hoff JT. Leukotrienes increase blood-brain barrier permeability following intraparenchymal injections in rats. Annals of Neurology. 1985;18:349–351. doi: 10.1002/ana.410180313. [DOI] [PubMed] [Google Scholar]
- Caraceni P, Vanthiel DH, Borle AB. Dual effect of deferoxamine on free-radical formation and reoxygenation injury in isolated hepatocytes. American Journal of Physiology. 1995;32:G132–137. doi: 10.1152/ajpgi.1995.269.1.G132. [DOI] [PubMed] [Google Scholar]
- Chan PH, Fishman RA. The role of arachidonic acid in vasogenic brain edema. Federation Proceedings. 1984;43:210–213. [PubMed] [Google Scholar]
- Easton AS, Fraser PA. Variable restriction of albumin diffusion across inflamed cerebral microvessels of the anaesthetized rat. The Journal of Physiology. 1994a;475:147–157. doi: 10.1113/jphysiol.1994.sp020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton AS, Fraser PA. Evidence that arachidonic acid disruption of blood-brain barrier is mediated by free radicals in the anaesthetized rat. The Journal of Physiology. 1994b;475.P:71P. [Google Scholar]
- Easton AS, Sarker MH, Fraser PA. Two components of blood-brain barrier disruption in the rat. The Journal of Physiology. 1997;503:613–623. doi: 10.1111/j.1469-7793.1997.613bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WR., Jr The role of leukotrienes in inflammation. Annals of Internal Medicine. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- Maier-Hauff K, Lange M, Schürer L, Guggenbichler C, Vogt W, Jacob K, Baethmann A. Glutamate and free fatty acid concentrations in extracellular vasogenic edema fluid. In: Go KG, Baethmann A, editors. Recent Progress in the Study and Therapy of Brain Edema. New York: Plenum Press; 1984. pp. 183–192. [Google Scholar]


