Abstract
Bradycardia can be evoked by stimulation of both myelinated and non-myelinated vagal efferent fibres. The on-going activity and synaptic inputs to cardiac vagal preganglionic neurones with myelinated axons have been studied in detail, but there is little information regarding cardiac preganglionic neurones with non-myelinated axons. In the present study, the on-going discharge and afferent inputs to cardiac vagal efferents with non-myelinated axons were studied in anaesthetized rats and cats.
Extracellular recordings were made from vagal preganglionic neurones in the dorsal vagal motor nucleus (DVMN) of anaesthetized rats following electrical stimulation of the cervical vagus nerve. Based on calculated axonal conduction velocities, fifty-six neurones had non-myelinated axons. Sixteen of these C fibre neurones had on-going activity but this showed no relationship to central respiratory drive, lung inflation or the cardiac cycle. Activity of twenty-one of these fifty-six neurones was increased at short latency following right atrial injections of phenylbiguanide (PBG).
Eight presumed cardiac vagal preganglionic neurones were also recorded in the DVMN. Five of these were activated by PBG administration, and the one neurone with resting activity showed no indication of respiratory-related activity.
Finally, twenty-one single C fibres were recorded in peripheral branches of the cardiac vagus. They had a low rate of on-going activity, and in twelve fibres this on-going discharge was analysed in detail. On-going activity in seven of these fibres showed no relationship to central respiratory drive, lung inflation or the cardiac cycle, whereas the other five had prominent relationships with both central respiratory drive and lung inflation, and two of these also showed a relationship to heart period. PBG administration evoked an increased activity in fourteen of the fifteen fibres tested, and the latencies of nine of these responses (1.3 ± 0.5 s) were within the pulmonary circulation time.
In anaesthetized cats, extracellular recordings were made in the DVMN from thirty-three cardiac vagal preganglionic neurones with C fibre axons. The on-going activity of these neurones, when present, never exhibited an on-going- or reflexly induced respiratory rhythm, nor any indication of an input from the arterial baroreceptors. Right atrial injection of PBG evoked a short-latency response in four of the five spontaneously active neurones but was without effect on the eight neurones tested which did not exhibit on-going activity.
In conclusion, the majority of cardiac vagal preganglionic neurones located in the DVMN have C fibre axons, show no obvious input from central or peripheral respiratory- or cardiac-related inputs, but are activated by stimulation of pulmonary C fibre afferent fibres.
Neural coupling between the brainstem respiratory and cardiovascular control systems is thought to explain the phenomenon of respiratory sinus arrhythmia and respiratory modulation of reflexly evoked bradycardias (Jordan & Spyer, 1987; Richter & Spyer, 1990), and until recently it was considered that all cardioinhibitory reflexes were modulated in phase with the respiratory cycle (Jordan & Spyer, 1987). Bradycardias evoked by activation of arterial baroreceptors and chemoreceptors, or by nasopharyngeal or ocular stimulation, are all attenuated if the stimuli are given during a period of central inspiratory activity or lung inflation (Davidson, Goldner & McCloskey, 1976; Gandevia, McCloskey & Potter, 1978). In cats, this has been shown to be due, at least in part, to an inspiration-related inhibitory gating of the synaptic input to cardiac vagal preganglionic neurones (Gilbey, Jordan, Richter & Spyer, 1984). Since this inhibition is acting at the level of the preganglionic neurones, it follows that any cardioinhibitory response utilizing these neurones should be similarly modulated by respiration. However, in recent studies, the bradycardia evoked by stimulating pulmonary C fibre afferents was not influenced by either the phase of the central respiratory cycle or the degree of lung inflation (Daly & Kirkman, 1989; Daly, 1991). To account for this data, Daly (1991) proposed two populations of cardiac vagal preganglionic neurones, each with a chronotropic action but one lacking a modulating input from central inspiratory activity. There is anatomical evidence that at least two populations of preganglionic neurones project to the cardiac vagal branches of many species including rat, cat and dog (Nosaka, Yamamoto & Yasunga, 1979a; Bennett, Kidd, Latif & McWilliam, 1981; Kalia, 1981; Hopkins, 1987; Izzo, Deuchars & Spyer, 1993). One population, located in the ventrolateral nucleus ambiguus (NA), comprises neurones with small myelinated axons (B fibres) which have a powerful respiratory-related component to their on-going activity (McAllen & Spyer, 1978; Gilbey et al. 1984). The other group, located in the region of the dorsal vagal motor nucleus (DVMN), mainly have slowly conducting non-myelinated axons (C fibres; Ford, Bennett, Kidd & McWilliam, 1990), but there is little information regarding their synaptic inputs. Whilst there is good evidence that the B fibre group have a chronotropic action (McAllen & Spyer, 1978) little was known concerning the physiological role of the C fibre population. It was suggested that they may be involved in controlling coronary blood flow (Feigl, 1969) or have an inotropic action (Geis & Wurster, 1980) but neither function has been fully substantiated. However, there is now direct evidence demonstrating that selective stimulation of vagal C fibre efferents can evoke bradycardia in rats, rabbits and cats (Nosaka, Yasunaga & Kawano, 1979b; Woolley, McWilliam, Ford & Clarke, 1987; Jones, Wang & Jordan, 1995b). Thus, these neurones could, potentially, mediate the bradycardia evoked by pulmonary C fibre stimulation. The experiments described in the present study were designed to determine whether the properties of dorsal vagal preganglionic neurones were consistent with such a function. First, the on-going activity of vagal preganglionic neurones with non-myelinated axons was studied, particularly in relation to their possible inputs related to respiration and the cardiac cycle. Second, the effects of stimulating pulmonary C fibre afferents on these vagal efferents was also investigated.
Preliminary reports of this data have been published (Jones, Wang & Jordan, 1994, 1995a).
METHODS
Male Sprague-Dawley rats (250–500 g) bred at the animal facility of the Royal Free Hospital Medical School were anaesthetized with pentobarbitone sodium (Sagatal, 60 mg kg−1, i.p.), and cats (2–3.5 kg) of either sex were anaesthetized with a mixture of α-chloralose (Aldrich Chemicals, 50 mg kg−1) and urethane (Sigma, 0.5 g kg−1, i.p.). Rectal temperature was monitored and maintained at 37°C with a Harvard homeothermic blanket system. When surgical anaesthesia was established, the femoral vein and artery were cannulated for administration of drugs/fluids and for recording blood pressure using a Statham P23Db transducer (Statham Ltd, Puerto Rico), respectively. In the cats, the bladder was cannulated to prevent undue filling during the period of the experiment, avoiding reflex effects associated with bladder distension. A cervical tracheostomy was performed and the trachea cannulated just below the larynx. Tracheal pressure was measured with a Validyne transducer and amplified with a Buxco Pulmonary Mechanics Analyser (Model 6, Buxco Electronics Inc., Sharon, CT). A silicone cannula, prefilled with phenylbiguanide (PBG, Aldrich Chemicals; 200 μg ml−1 in cats and 400 μg ml−1 in rats), was advanced into the right atrium via the right external jugular vein. The ECG was recorded (Neurolog System, Digitimer) by leads attached to the chest and left hindpaw of the animal. The animals were placed in a stereotaxic frame and artificially ventilated with O2-enriched air and positive end expiratory pressure (1 cmH2O; Harvard rodent ventilator model 683). Tidal volumes and frequencies of ventilation were chosen which were appropriate for the particular species and were adjusted to maintain arterial blood PCO2 between 35 and 40 mmHg. All blood gas variables were measured using a Corning Blood Gas Analyser (Model 158). Tracheal pressure and end-tidal CO2 (ADC fast response CO2 analyser) were monitored continuously. The right phrenic nerve was dissected from a dorsolateral approach, cut peripherally and desheathed. The cut central end of the nerve was placed on bipolar silver wire recording electrodes. In three cats, the right carotid sinus nerve was also dissected free and placed intact on a pair of bipolar stimulating electrodes. Clamps applied to the vertebral spines at C7 and L2 or L3 were used to elevate and stabilize the animal. To expose the brainstem the nuchal muscles were removed, the occipital bone opened and the dura overlying the brainstem and cerebellum cut and reflected laterally. In some experiments the cerebellum was displaced rostrally with a small retractor to allow access to the region of the DVMN. The animals were then neuromuscularly blocked using gallamine triethiodide (Flaxedil, M&B Ltd; 5 mg kg−1) or vecuronium bromide (Norcuron, Organon Teknika; 200 μg kg−1). The level of anaesthesia during the period of neuromuscular blockade was monitored by noting the stability of the phrenic neurogram, the arterial blood pressure and heart rate, and the lack of cardiovascular changes to paw pinch. In addition, the animals were allowed to recover periodically from the effects of neuromuscular block. At the end of each experiment the animal was killed by overdose of the anaesthetic agent.
Preparation of the vagal branches
Cats
In the cats a thoracotomy was performed between the fourth and fifth ribs to gain access to the right cranial cardiac branch as described in full previously (McAllen & Spyer, 1976). The intact nerve was placed in a small electrode assembly in which fine silver wires (0.125 mm diameter) had been embedded 2 mm apart. This was attached to the underlying connective tissues by a small quantity of cyanoacrylate glue (R. S. Components, Corby, UK), insulated by application of petroleum jelly, and the thorax closed.
Rats
A gross neuroanatomical study was first performed on the cadavers of fifty rats to provide information on the projection and distribution of the cardiac vagal branches in this species. Dissection of the cardiac branch was performed in vitro with the heart and attached vagus placed in phosphate buffer solution and viewed with a binocular stereo-microscope (Nikon SMZ). A single cardiac branch leaves the thoracic vagus more rostral than in the cat, and in all rats examined it passed without bifurcation to a large cluster of ganglion cells at the posterior cavo-atrial junction of the heart. The ganglia were visualized by brief application of 1 % Neutral Red (BDH; Skoogh, Grillo & Nadel, 1983; Izzo & Jones, 1994). The origin of the branch is variable and occasionally stemmed from the recurrent laryngeal nerve. In other mammalian species this would be labelled the recurrent cardiac nerve (Randall & Armour, 1977); however, unlike other species, in rat there do not appear to be multiple branches to the heart from the right vagus.
In the brainstem-recording experiments, a lateral approach was used to visualize the vagal branches. To expose the right cardiac vagal branch, a portion of the second rib was removed, but unlike in cats, there was no need to ligate the azygos vein or perform a pulmonary lobectomy. Because of its high origin, it was possible to free 5–6 mm lengths of the nerve, which reduced the possibility of stimulus spread. Fine silver wires (0.125 mm diameter) soldered to fine insulated copper wire were used to stimulate the nerve, which was left intact during the experiment. The wires were secured at the thorax by suture and the assembly insulated by covering with a small amount of dental impression material (President, Coltene). The stimulus was 5 V or less, and, typically, cardiac arrest could be obtained by stimulating with 1 ms pulses at 1–2 V, 50 Hz. The mid-cervical vagus was also dissected free of the carotid sheath, cervical sympathetic trunk and aortic nerve and placed on a bipolar silver wire (0.5 mm diameter) stimulating electrode.
In the fibre recording experiments, a mid-line sternotomy was performed and the right cardiac vagal branch exposed after bilateral thymectomy. The cardiac branch, thoracic vagus and recurrent laryngeal nerves were cleared of connective tissue and sectioned peripherally. The vagus and attached cardiac branch were then transposed to a small Perspex recording chamber (1 cm × 2 cm × 0.5 cm) placed adjacent to the extrathoracic trachea and secured in position with dental impression material. The chamber was filled with phosphate-buffered saline (pH 7.4 at 20 °C). A stimulating electrode identical to the one used for the brainstem-recording experiments was placed on the mid-cervical vagus and insulated in position with the dental impression material. The cardiac branch was desheathed and split longitudinally. A suction electrode was manoeuvred into position using a three-dimensional micromanipulator and recordings made from fibres of the nerve. When on-going activity was recorded in a fibre, the cervical vagus was stimulated to determine the orthodromic latency and hence calculate the conduction velocity of the peripheral axon. To obtain an index of central respiratory drive, in some experiments a small balloon was placed between the liver and diaphragm to register diaphragmatic movements which were central in origin and not due to phasic movements of the lungs. In other experiments, the left phrenic nerve was dissected low in the neck, cut peripherally, and the central end desheathed and placed on bipolar recording electrodes.
Recording electrodes in rats and cats
Extracellular recordings were made from neurones in the region of the dorsal vagal complex with glass microelectrodes using a preamplifier/current pump (Dagan model 2400). Bandpass filtering for the neural signals was at 0.1-3 kHz. Single-barrel microelectrodes were made from borosilicate glass (Clarke Electromedical, GC150F-10) pulled to a tip diameter of 1 μm. They were filled with Pontamine Sky Blue dye (BDH, 20 mg ml−1 in 0.5 m sodium acetate) for recording and subsequent staining. Five-barrelled microelectrodes (tip diameter, 3–5 μm) were made from the same glass. The recording barrel contained 4 m sodium chloride, and the other barrels contained Pontamine Sky Blue dye for marking and the glutamate receptor agonist d,l-homocysteic acid (DLH, 100 mm, pH 8.5). To record from the peripheral nerve fibres, the same glass was used to manufacture suction electrodes, but the tip was bumped back to a diameter of 30–50 μm and fire polished until the tip was 10–30 μm. These electrodes were backfilled with phosphate-buffered saline.
Reflex stimulation of brainstem neurones
The pulmonary chemoreflex was evoked by injecting a bolus of PBG into the right atrium. The injectate volume was between 10 and 50 μl (400 μg ml−1) in rats and 200 and 400 μl (200 μg ml−1) in cats. The small volumes used were chosen to minimize undue stretching of great vessels or cardiac chambers. The neuronal response was considered to be due to pulmonary C fibre stimulation if it occurred within 2 s in rats and 5 s in cats. Only a short time frame (1–2 s) was analysed after this latency, since PBG will gain access to the systemic circulation where it may also activate other reflexogenic areas (Daly & Kirkman, 1988; Presson, Todoran, De Witt, McMurtry & Wagner, 1997).
Data capture and analysis
Phrenic nerve activity and the ECG were amplified and filtered (0.5-5 kHz; Neurolog, NL104, NL125). Arterial blood pressure, heart rate, end-tidal CO2, tracheal pressure, ECG, phrenic nerve activity and neuronal activity were displayed on a computer using a 1401 interface and CHART software (CED) and stored on videotape using a digital data recorder (Instrutech VR-100A). In the fibre-recording experiments, on-line analysis of spike height and shape was made with spike template-matching software (SPIKE2, CED). Only discriminated units with a distinctive height and/or shape were included for analysis. Sampling rate was 15000 Hz and the window of data captured around the spike was between 2.5 and 10 ms. The success of the spike tracking was monitored off-line by superimposing spikes recorded at periodic intervals throughout the recording. This confirmed that spike shapes were identical and did not alter over time. The height occasionally declined slightly with time, probably because of breakdown of the seal. Spikes whose amplitude varied by >15 % were not accepted. Off-line analysis of the recorded data (phrenic-, tracheal pressure- and blood pressure-triggered correlations) was made using SPIKE2 software.
Histological localization of recording sites
Recording sites were marked by ionophoretic ejection of Pontamine Sky Blue (Hellon, 1971). Following the experiments, brainstems were fixed in 10 % formal saline, and serial frozen sections (80 μm) were cut and stained with Neutral Red. The marked recording sites were visualized and displayed on standard sections of brainstem taken from the stereotaxic atlases for rat (Paxinos & Watson, 1986) and cat (Berman, 1968).
Results are given as means ± s.e.m.
RESULTS
C fibre vagal efferents in the rat (brainstem recordings)
Standard criteria for antidromic activation following electrical stimulation of the mid-cervical vagus (<5 V, 1 ms pulses) were used to identify vagal preganglionic neurones. These included constant latency of the evoked response and collision of the evoked responses with appropriately timed on-going or evoked activity (see Fig. 1 in Wang, Jones, Ramage & Jordan, 1995a). Based on the calculated conduction velocities of their axons, fifty-six of the recorded neurones had non-myelinated axons (mean, 1.0 ± 0.04 m s−1; range, 0.6-1.7 m s−1) and all those histologically recovered (n = 16) were localized in the dorsal vagal motor nucleus (DVMN). Sixteen of these neurones with C fibre axons had on-going activity at rest (0.5-4 Hz). This activity showed no relationship to central respiratory drive (phrenic nerve discharge), lung inflation (tracheal pressure) or the cardiac cycle. Respiratory-related discharges could not be identified in the neurones even when their activity was increased by ionophoresis of the glutamate receptor agonist d,l-homocysteic acid, or when respiratory drive was augmented by hypoventilation (Fig. 1A). Right atrial injection of PBG (10–50 μl; 16–80 μg kg−1) increased activity in twenty-one of the fifty-six neurones with C fibre axons. Typically, the neurones responded within 2 s with a single burst of action potentials lasting between 0.4 and 3 s (Fig. 1B). A similar burst of action potentials could also be evoked in these neurones by hyperinflation of the lungs, which would also be expected to excite pulmonary C fibre afferents (Fig. 1C).
Figure 1. Rat: responses of vagal C fibre neurones.
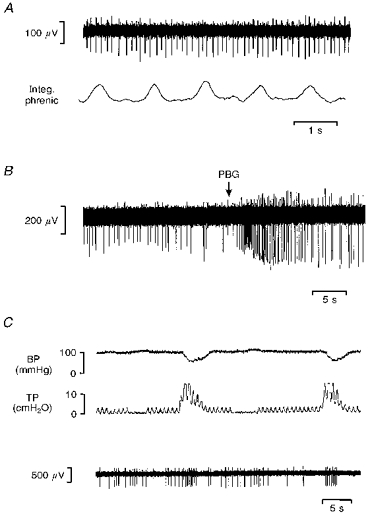
Multibarrelled electrode recording from two vagal neurones with C fibre axons antidromically activated by stimulation of cervical vagus nerve. A, lack of modulation of cell activity (top) when compared with integrated phrenic nerve discharge (Integ. phrenic) even though respiratory drive had been augmented by hypoventilation. B, injection of PBG (60 μg kg−1) into the right atrium at the point marked by the arrow. The glutamate receptor agonist d,l-homocysteic acid was applied by ionophoresis (20 nA) throughout the recordings. C, the responses of the same neurone to large lung inflations and deflations as indicated by the changes in tracheal pressure (TP). BP, blood pressure.
C fibre cardiac vagal efferents in the rat (brainstem recordings)
Since the reponses of C fibre vagal efferents to PBG injections were heterogeneous, a further series of studies was performed to test whether specifically those non-myelinated vagally projecting neurones innervating the heart were also activated by stimulation of pulmonary C fibre afferents. An excitatory response was evoked in sixty-one neurones by electrical stimulation of the cardiac vagal branch in twenty-four rats (1–5 V, 1 ms). The majority (fifty-three) of these neurones received an excitatory orthodromic input. Thirteen of these neurones were histologically localized to the nucleus tractus solitarii (NTS). Another four neurones also received a long-latency (>50 ms) orthodromic excitatory input from the cardiac vagal branches. However, they were histologically localized in the ventrolateral DVMN and were presumed to be cardiac vagal preganglionic neurones. Finally, four neurones were positively identified as cardiac vagal preganglionic neurones by their antidromic activation from the cardiac branch of the vagus. Their axons had calculated conduction velocities (0.9-1.1 m s−1) within the C fibre range and all four were histologically localized in the DVMN. Of these eight neurones, all four neurones receiving C fibre orthodromic input and one of the two antidromically activated neurones tested were excited at short latency (<2 s) by right atrial injections of PBG (16–80 μg kg−1; Fig. 2A).
Figure 2. Rat: cardiac vagal C fibre neurone activity.
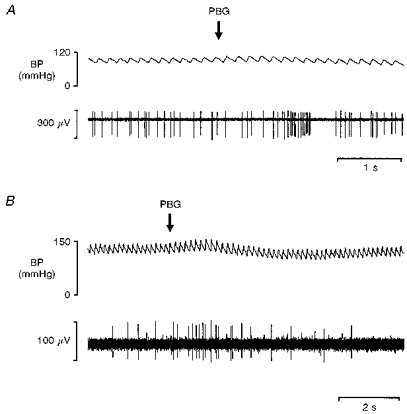
Traces show the simultaneously recorded activity of C fibre cardiac vagal preganglionic neurones (bottom traces) and blood pressure during the pulmonary chemoreflex. A, the neurone was recorded from the brainstem and PBG (40 μg kg−1) was injected into the right atrium at the point marked by the arrow. B, the efferent fibre was recorded from the cardiac branch of the vagus, and PGB (60 μg kg−1) was injected into the superior vena cava at the point marked by the arrow. Note the transient excitation of the neurones at a latency which is consistent with pulmonary C fibre activation.
Only one of the four antidromically activated neurones had on-going activity. This discharge showed no indication of respiratory modulation. The other three neurones had no on-going activity and when the cardiac branch was stimulated they exhibited evidence (initial segment-soma/dendritic (IS-SD) failure) that they were receiving a powerful inhibitory input from afferents in the cardiac branch (Fig. 8B; Wang, Ramage & Jordan, 1995b; Jordan & Wang, 1997).
Figure 8. Rat and cat: cardio-cardiac inhibition.

A, cat: the on-going firing pattern of a single cardiac C fibre efferent neurone. The spikes illustrated represent a digital spike processor output which has been edited to exclude the stimulus artefacts and antidromic spikes. The cardiac branch was stimulated during the ‘On’ period at 3 V, 1 ms, 1 Hz. B, rat: four oscilloscope sweeps showing the antidromic response pattern of a single cardiac vagal preganglionic neurone in the ventrolateral DVMN. At the points marked by the filled circles, the intact right cranial cardiac vagal branch was stimulated (4 V, 1 ms, 1 Hz), and the initial segment and somatodendritic components of the extracellularly recorded potential are clearly seen. The occasional absence of a full action potential makes these neurones difficult to locate and record. C, cat: the effect of paired pulse stimulation (100 ms interpulse interval; 7 V, 1 ms, 1 Hz) on the antidromic response of a single cardiac C fibre efferent (latency, 219 ms). The second spike occasionally fails to invade the soma/dendrites (SD) of the neurone leaving only the initial segment (IS) component of the extracellularly recorded potential. Even when the second spike does invade the soma there is still IS-SD delay apparent.
C fibre cardiac vagal efferents in rats (peripheral recordings)
Only eight presumed cardiac vagal preganglionic neurones had been recorded from the dorsal vagal nucleus of rats. Thus, in another series of experiments, recordings were made from efferent fibres in the peripheral cardiac vagus, identified on the basis of its anatomical position and cardiac arrest upon electrical stimulation, to enable the yield of cardiac vagal efferents to be increased. Orthodromic electrical stimulation of the mid-cervical vagus activated twenty-one single fibres with axons conducting in the range of C fibres (0.98 ± 0.4 m s−1). They had on-going activity at a rate of 1.9 ± 1.5 Hz. Single units were identified by characteristics of constant spike shape and amplitude (Figs 3, 4A and 5A). With the range of electrode tip diameters used in this study, electrical stimulation of the vagus often evoked activity in more than one recorded fibre. Only when the spike shape and/or amplitude of the active unit was sufficiently distinctive was it included for analysis (Fig. 3).
Figure 3. Rat: peripheral recording of cardiac vagal efferent fibre.
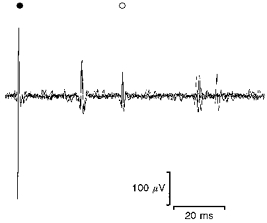
Three superimposed sweeps of a suction electrode recording in the rat. At the stimulus artefact (•) a single pulse was delivered to the cervical vagus (7 V, 0.1 ms, 1 Hz) and a recording obtained from a filament of the right cardiac vagal branch. Two C fibre units were evoked, the second of which (^) was spontaneously active and also received synaptic input of longer latency.
Figure 4. Rat: peripheral recording of cardiac vagal efferent fibre with no respiratory-related inputs.

A, four superimposed sweeps of the discriminated active unit. B-D, histograms of the activity of a cardiac vagal C fibre efferent triggered from: integrated phrenic nerve activity (B, Integ. phrenic; 20 ms bins, 131 sweeps), the femoral arterial blood pressure wave (C, BP; 5 ms bins, 1665 sweeps), and tracheal pressure (D, TP; 20 ms bins, 214 sweeps). Above each histogram is an average of the respective trigger input.
Figure 5. Rat: peripheral recording of cardiac vagal efferent fibre with respiratory-related inputs.

A, four superimposed sweeps of the discriminated active unit. B-D, histograms of the activity of a cardiac vagal C fibre efferent triggered from: integrated phrenic nerve activity (B; 20 ms bins, 115 sweeps), the femoral arterial blood pressure wave (C; 5 ms bins, 1693 sweeps), and tracheal pressure (D; 20 ms bins, 337 sweeps). Above each histogram is an average of the respective trigger input.
Respiratory-related activity could not be induced in any of six fibres studied during short periods of hypoventilation (5–10 s), even when the respiratory efforts of the animal were highly pronounced. In order to explore this further, the on-going discharge of another twelve of the twenty-one single fibres was analysed with respect to an index of central respiratory drive (integrated phrenic (six fibres) or diaphragmatic movement (six fibres)). The activity of seven of the twelve fibres showed no relationship to central respiratory drive, arterial blood pressure or lung inflation (Fig. 4). Therefore the activity profiles of these fibres mirror those preganglionic neurones recorded in the DVMN. In contrast, the activity of five of the twelve fibres showed prominent relationships to central respiratory drive and lung inflation (Fig. 5). In addition, two of these neurones also showed a relationship to arterial blood pressure (Fig. 5).
Eight of the twenty-one single cardiac C fibre efferents could be shown to receive vagal synaptic input (Fig. 3) at a latency ranging between 50 and 75 ms, indicating that the afferents were likely to be non-myelinated. Administration of PBG (10–60 μg kg−1) evoked an increase in activity of fourteen of the fifteen fibres tested (Fig. 2B); however, only nine of these fourteen were excited within the pulmonary circulation time (1.3 ± 0.5 s). Their basal discharge frequency in the control period averaged 1.6 ± 1.5 Hz and rose to a peak of 21 ± 5.6 Hz. None of the neurones tested changed their firing pattern when an equal volume of phosphate-buffered saline was injected. In addition, PBG application evoked a short-latency excitatory response in one of six multiunit recordings made, and a further three were activated at slightly longer latency (outside the pulmonary circulation time).
Cardiac vagal preganglionic neurones in the cat
The reflex studies which formed the basis of the hypothesis leading up to the present experiments were performed mainly on cats (Daly & Kirkman, 1988, 1989; Daly, 1993). Thus, the responses of non-myelinated cardiac vagal efferent neurones to stimulation of pulmonary C fibre afferents were studied in a group of cats. Thirty-three neurones were antidromically activated by electrical stimulation of the right cardiac vagal branches (Fig. 6A). Their axons had calculated conduction velocities (0.78 ± 0.03 m s−1) indicative of non-myelinated axons, and their cell bodies were localized histologically in the DVMN. Eleven of these neurones exhibited a regular low rate of on-going activity (0.3-1 Hz; Fig. 6B), whereas the other twenty-two were silent or fired infrequently. In addition to their antidromic response, two neurones also received a long-latency synaptic excitation from afferents in the cardiac branch (210–260 ms). In contrast, five neurones had properties (inhibition of on-going discharge and/or IS-SD failure; Fig. 8A and C) indicating that they also received an inhibitory synaptic input from afferents in the cardiac branch.
Figure 6. Cat: cardiac C fibre efferent in the DVMN.
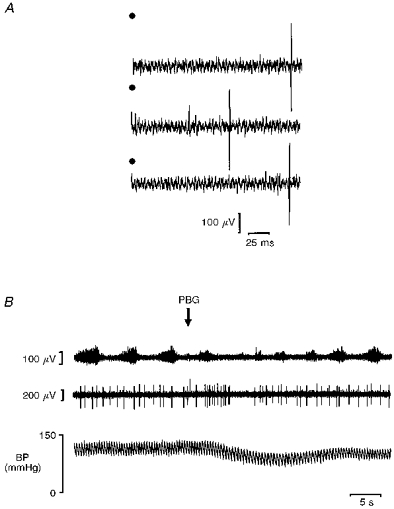
A, three traces showing a cardiac vagal preganglionic neurone antidromically activated (latency, 181 ms) by stimulating the right cranial cardiac branch in the cat (6 V, 1 ms, 1 Hz). The middle trace illustrates a spontaneous spike which causes cancellation of the antidromic spike. Stimulation at time zero is indicated by •. B, traces show the simultaneously recorded phrenic nerve activity (top), activity of a C fibre cardiac vagal preganglionic neurone (same neurone as illustrated in A, middle) and blood pressure (bottom) during the pulmonary chemoreflex (at the point marked by the arrow, 16 μg kg−1 PBG was injected into the right atrium). Note the transient excitation of the neurone at a latency which is consistent with pulmonary C fibre activation. This neurone had no input from the carotid sinus nerve. Note there is no apparent relationship between the activity of the neurone and the central respiratory activity.
Injection of PBG (20.6 ± 2 μg kg−1) into the right atrium evoked a short-latency (<5 s) increase in activity in four of the five spontaneously active C fibre cardiac vagal preganglionic neurones tested, but was without effect on the eight without on-going activity. In those cells exhibiting a response to PBG, discharge increased from 0.6 ± 0.1 Hz to a peak of 6.8 ± 1.1 Hz within 5 s, the burst of activity lasting 4 ± 0.4 s (Fig. 6B).
Recordings from only two of the eleven cardiac C fibre preganglionic neurones with on-going activity were stable enough over a long period to allow their activity profiles to be studied in detail. As in the central recordings in rats, this on-going activity showed no obvious relationship to either phrenic nerve activity (Fig. 7B) or tracheal pressure (Fig. 7C), suggesting that they receive little or no input from either the central respiratory network or lung inflation afferents, respectively. Nor was their activity related to the ECG or arterial blood pressure (Fig. 7A), indicating the absence of an input from the arterial baroreceptors. This latter inference was confirmed more directly in four neurones which were unaffected by electrical stimulation of the carotid sinus nerve, though in the same experiments this same stimulus did activate orthodromically seventeen neurones in the NTS with latencies of between 5 and 125 ms, suggesting that both the myelinated and non-myelinated carotid sinus nerve afferents had been activated. None of these NTS neurones received a C fibre input from stimulation of the cardiac branch, and of the eight neurones tested, seven were not influenced by PBG injections, suggesting that the NTS pathways for cardiac C fibre and pulmonary C fibre reflexes were separate from those mediating chemoreceptor and baroreceptor reflex pathways.
Figure 7. Cat: cardiac vagal C fibre neurone activity.
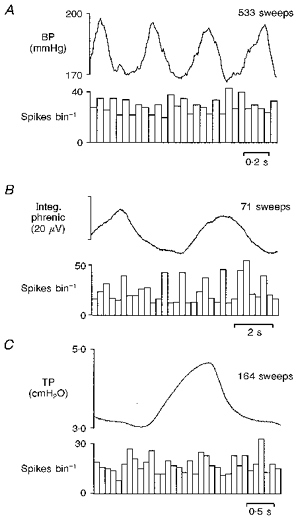
Histograms of the activity of a cardiac vagal C fibre preganglionic neurone triggered from: the femoral arterial blood pressure wave (A; 50 ms bin width), integrated phrenic nerve activity (B; 300 ms bin width) and tracheal pressure (C; 100 ms bin width). Above each histogram is an average of the respective trigger input.
DISCUSSION
The present experiments have demonstrated in both rats and cats that the on-going activity of the majority of C fibre vagal efferents located in the DVMN, including those projecting to the heart, shows no modulation in phase with either the respiratory, ventilatory or cardiac cycles. In addition, these neurones can be activated at short latency when pulmonary C fibre afferents are stimulated by injection of phenylbiguanide into the right atrium. The typical response of these efferents to this stimulus is a single, short-lasting burst of action potentials.
The initial experiments on rats clearly demonstrated that a large proportion of vagal efferent neurones in the DVMN with C fibre axons were activated at short latency by stimuli that would activate pulmonary C fibre afferents. In the subsequent experiments we tried to determine if these neurones projected to the heart. Due to the difficulty of these experiments, only four neurones were antidromically identified as projecting to the cardiac branch in the rat. In addition, another four neurones were recorded which were located in the same region and had similar properties to the antidromically identified neurones. They received an excitatory orthodromic input from the cardiac branch, and it is likely that these were also vagal preganglionic neurones but their antidromic spike had been cancelled by the evoked orthodromic spikes. This was reported to occur in about one-third of C fibre preganglionic neurones recorded in a previous study in rats (Nosaka, 1986). Taken together, five of the six presumed cardiac vagal preganglionic neurones tested were excited by pulmonary C fibre stimulation. Only one of the four antidromically activated cardiac preganglionic neurones had on-going activity, but this did not show any obvious respiratory-related discharge.
The size of the population of cardiac vagal efferents recorded in rats was increased by recording with suction electrodes from fibres in the cut end of the cardiac branch of the vagus. Both multiunit and single-unit activity were recorded. Although the technique has certain disadvantages (bias towards spontaneously active units and difficulty in performing a collision test), a major advantage is that the recording time of single fibres is greatly prolonged, and variations in blood pressure no longer pose a problem to recording stability. As with the brainstem recordings, almost all of the fibres tested were activated within the pulmonary circulation time by PBG injected into the right atrium. This was similar to the data obtained in the cats, where it was also demonstrated that the majority of spontaneously active non-myelinated cardiac vagal neurones recorded in the DVMN were activated by such PBG injections. These responses to PBG are likely to be due to activation of pulmonary C fibre afferent fibres, since the responses occurred within the pulmonary circulation times, control volume injections did not produce a response, and the excitation was mimicked by hyperinflation of the lungs. Responses of longer latency were discarded since the PBG would then have entered the systemic circulation where it could potentially activate several other groups of afferent fibres. The response of the C fibre preganglionic neurones to PBG administration was a brief, intense burst of activity (Figs 1B, 2A and 6B). This transient response was not because the cell recording had been lost, since it was possible to show that the antidromic response was still present following the PBG administration. In addition, the response was similar when recording from efferent fibres in the cardiac nerve (Fig. 2B), where problems of brainstem movement are overcome. Indeed, PBG has been reported to evoke a similar transient response in neurones in the NTS (Hines, Toney & Mifflin, 1994), which are presumably antecedent to those recorded in the present study.
In both cats and rats, cardiac vagal efferent neurones with C fibre axons, when active, had a low rate of activity. The activity of the majority of these neurones, whether recorded in the brainstem or from peripheral fibres, does not appear to be affected by inputs from the arterial baroreceptors. The on-going discharge showed no temporal relationship to the arterial pulse wave, and no response to carotid sinus nerve stimulation could be detected in any of the neurones tested, as demonstrated previously in cats (Ford et al. 1990) and rats (Nosaka, Yasunaga & Tamai, 1982). Nor did the discharge show any tendency to occur in one particular phase of the respiratory cycle (monitored as phrenic nerve activity). Even when respiratory drive was increased by hypoventilation, such that conditions were optimum, no respiratory rhythm was observed, suggesting that this group of preganglionic neurones are unaffected by central respiratory drive. This contrasts with the activity of cardiac vagal preganglionic neurones with myelinated axons, which invariably show both respiratory- and cardiac-related components to their discharge (McAllen & Spyer, 1978; Gilbey et al. 1984). In the present study, the activity recorded in a small group of peripheral C fibre axons also had respiratory-related modulation, and some, in addition, had a cardiac-related discharge (Fig. 5) giving an activity profile not dissimilar to that recorded centrally from preganglionic neurones with B fibre axons. The central origin of this group of neurones is not known. None of the C fibre efferent neurones recorded in the DVMN would fit into this group. However, Nosaka et al. (1982) reported a small group of vagal preganglionic neurones in the intermediate zone between the DVMN and NA which had slowly conducting axons, but their activity profiles were not defined. Alternatively, there are reports that the axons of some vagal afferent (Duclaux, Mei & Ranieri, 1976) and efferent fibres (Chase & Ranson, 1914) are myelinated centrally but lose their myelin peripherally. In the present fibre-recording experiments these would appear to be non-myelinated efferents, but in central recording they would have a faster calculated conduction velocity.
The number of cardiac preganglionic neurones with C fibre axons recorded from the medulla in the present study is not large. Several problems account for this. In any study of non-myelinated fibres, the long conduction time increases the probability that either a spontaneous or synaptically evoked action potential will collide with the antidromic spike and prevent its positive identification. This is a particular problem if the neurone receives a synaptic input from the nerve being stimulated (Nosaka, 1986; Jones et al. 1995b). Indeed, in the fibre recordings it was clear that many of the spontaneously active cells received excitatory vagal synaptic input (Fig. 3). Additionally, as demonstrated in Fig. 8, these neurones often received an inhibitory input from cardiac branch afferents. This was often powerful enough to prevent somatic invasion of the antidromic spike (Ford et al. 1990; Wang et al. 1995b; Jordan & Wang, 1997). In the present recordings this was seen as a reduction in the on-going activity of the neurones when the antidromic stimulus was applied, and as IS-SD failure (Gustafsson & Lipski, 1980; Nosaka et al. 1982). This cardio-cardiac inhibitory effect was common in both species. In both extracellular and intracellular recordings in rats, this vago-vagal inhibition of DVMN cells was reduced selectively by application of the GABAA receptor antagonist bicuculline, but not by the glycine receptor antagonist strychnine (Wang et al. 1995b; Jordan & Wang, 1997).
Although the results demonstrate that C fibre vagal preganglionic neurones projecting to the cardiac branch are excited by PBG administration, this does not necessarily mean that these neurones have a cardioinhibitory function. It has previously been argued that B fibre cardiac preganglionic neurones have all the properties expected of a cardioinhibitory neurone (McAllen & Spyer, 1976, 1978). However, there has also been the suggestion, at least in rats and rabbits, that stimulation of C fibre efferents will also slow the heart (Nosaka et al. 1979b; Woolley et al. 1987). Recently, it was demonstrated that slowing of the heart rate can indeed be produced by selective stimulation of C fibre vagal efferent fibres in cats, rats and rabbits (Jones et al. 1995) so it is not unreasonable to suggest that at least some of the bradycardia evoked by pulmonary C fibre stimulation is mediated by this group of neurones. However, since the bradycardia evoked by selective stimulation of non-myelinated fibres is smaller than that evoked by stimulation of myelinated fibres (Jones et al. 1995), it is unlikely that activation of C fibre efferents can account for all of the bradycardia evoked when pulmonary C fibre afferents are stimulated. It is also possible that some of the cardiac-projecting neurones recorded here could be mediating other cardiac actions such as dromotropic effects or alterations in coronary flow. Indeed, selective stimulation of vagal C fibre efferent fibres does slow atrio-ventricular conduction in rabbits (Garcia, Wang & Jordan, 1997).
Many reflex falls in heart rate are mediated by cardioinhibitory neurones in the nucleus ambiguus which have a pronounced respiratory-related component in their discharge. The hypothesis put forward by Daly (1991) argued that pulmonary C fibres mediate their reflex chronotropic effects via another, as yet unidentified, group of vagal cardioinhibitory neurones which lack modulation from central respiratory activity. The data reported here provide evidence that such a group of neurones exists, that they are located in the region of the DVMN, and that some of this group are indeed activated by pulmonary C fibres in rat and cat. However, the data cannot fully justify the hypothesis as originally proposed (Daly, 1991), since the cardiac changes evoked during the pulmonary chemoreflex outlast the increase in discharge of the DVMN neurones. This, combined with previous observations that cardiac C fibre efferents alone are not potent enough to evoke cardiac arrest (Jones et al. 1995b) has led to a new hypothesis whereby the preganglionic projections of both the NA and DVMN preganglionic neurones converge on common target neurones in the cardiac vagal ganglia, and it is this final integration which determines the prevailing level of sinus arrhythmia.
Acknowledgments
This work was generously supported by project grants from the Medical Research Council, The Wellcome Trust and the British Heart Foundation.
References
- Bennett JA, Kidd C, Latif AB, McWilliam PN. A horseradish peroxidase study of vagal motoneurones with axons in cardiac and pulmonary branches of the cat and dog. Quarterly Journal of Experimental Physiology. 1981;66:145–154. doi: 10.1113/expphysiol.1981.sp002541. [DOI] [PubMed] [Google Scholar]
- Berman AL. The Brainstem of the Cat. Madison: University of Wisconsin; 1968. [Google Scholar]
- Chase MR, Ranson SW. The structure of the roots, trunk and branches of the vagus nerve. Journal of Comparative Neurology. 1914;24:31–60. [Google Scholar]
- Daly M, de Burgh Some reflex cardioinhibitory responses in the cat and their modulation by central inspiratory neuronal activity. Journal of Physiology. 1991;439:559–577. doi: 10.1113/jphysiol.1991.sp018682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M, de Burgh . Carotid chemoreceptor reflex cardioinhibitory responses: comparison of their modulation by central inspiratory neuronal activity and activity of pulmonary stretch afferents. In: Data PG, Acker H, Lahiri S, editors. Neurobiology and Cell Physiology of Chemoreception. New York: Plenum Press; 1993. pp. 333–343. [DOI] [PubMed] [Google Scholar]
- Daly M, de Burgh Kirkman E. Cardiovascular responses to stimulation of pulmonary C fibres in the cat: their modulation by changes in respiration. Journal of Physiology. 1988;402:43–63. doi: 10.1113/jphysiol.1988.sp017193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M, de Burgh Kirkman E. Differential modulation by pulmonary stretch afferents of some reflex cardioinhibitory responses in the cat. Journal of Physiology. 1989;417:323–341. doi: 10.1113/jphysiol.1989.sp017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson NS, Goldner S, McCloskey DI. Respiratory modulations of baroreceptor and chemoreceptor reflexes affecting heart rate and cardiac vagal efferent activity. Journal of Physiology. 1976;259:523–530. doi: 10.1113/jphysiol.1976.sp011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclaux R, Mei N, Ranieri F. Conduction velocity along vagal dendrites: a new type of fibre. Journal of Physiology. 1976;260:487–495. doi: 10.1113/jphysiol.1976.sp011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl EO. Parasympathetic control of coronary blood flow in dogs. Circulation Research. 1969;5:509–519. doi: 10.1161/01.res.25.5.509. [DOI] [PubMed] [Google Scholar]
- Ford TW, Bennett JA, Kidd C, McWilliam PN. Neurones in the dorsal motor vagal nucleus of the cat with non-myelinated axons projecting to the heart and lungs. Experimental Physiology. 1990;75:459–473. doi: 10.1113/expphysiol.1990.sp003423. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI, Potter EK. Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. Journal of Physiology. 1978;276:383–394. doi: 10.1113/jphysiol.1978.sp012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Wang Y, Jordan D. Selective vagal C-fibre efferent stimulation evokes both chronotropic and dromotropic responses in anaesthetized rabbits. Journal of Physiology. 1997;504.P:208–209P. [Google Scholar]
- Geis GS, Wurster RD. Cardiac responses during stimulation of the dorsal motor nucleus and nucleus ambiguus in the cat. Circulation Research. 1980;46:606–611. doi: 10.1161/01.res.46.5.606. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. Journal of Physiology. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Lipski J. Effect of membrane polarization and synaptic activity on the timing of antidromic invasion. Brain Research. 1980;181:61–74. doi: 10.1016/0006-8993(80)91259-7. 10.1016/0006-8993(80)91259-7. [DOI] [PubMed] [Google Scholar]
- Hellon RF. The marking of electrode tip position in nervous tissue. Journal of Physiology. 1971;241:12P. [PubMed] [Google Scholar]
- Hines T, Toney GM, Mifflin SW. Responses of neurons in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circulation Research. 1994;74:1188–1196. doi: 10.1161/01.res.74.6.1188. [DOI] [PubMed] [Google Scholar]
- Hopkins DA. The dorsal motor nucleus of the vagus nerve and the nucleus ambiguus: structure and connections. In: Hainsworth R, McWilliam PN, Mary DASG, editors. Cardiogenic Reflexes. Oxford: Oxford University Press; 1987. pp. 185–203. [Google Scholar]
- Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurones in the rat: Immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. Journal of Comparative Neurology. 1993;327:572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Jones JFX. Identification of vagal efferent fibres and their putative target neurones in cardiac ganglia of the anaesthetized rat. Journal of Physiology. 1994;481.P:14P. [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Activity of cardiac vagal preganglionic neurones during the pulmonary chemoreflex in the anaesthetized cat. In: O'Regan RG, Nolan P, McQueen DS, Paterson DJ, editors. Arterial Chemoreception: Cell to System. New York: Plenum Press; 1994. pp. 301–303. [DOI] [PubMed] [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Dorsal medullary neurones activated by stimulation of the cardiac vagal branch of the anaesthetized rat, and their behaviour during the pulmonary chemoreflex. Journal of Physiology. 1995a;483.P:89P. [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Selective stimulation of cardiac vagal C fibres in anaesthetized cats, rats and rabbits. Journal of Physiology. 1995b;489:203–214. doi: 10.1113/jphysiol.1995.sp021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D, Spyer KM. Central neural mechanisms mediating respiratory-cardiovascular interactions. In: Taylor EW, editor. Neurobiology of the Cardiovascular System. Manchester: Manchester University Press; 1987. pp. 322–341. [Google Scholar]
- Jordan D, Wang Y. Involvement of GABAA receptors in the inhibition of dorsal vagal preganglionic neurones by afferents in anaesthetized rats: an in vivo intracellular study. Journal of Physiology. 1997;501.P:72–73P. [Google Scholar]
- Kalia M. Brain stem localization of vagal preganglionic neurons. Journal of the Autonomic Nervous System. 1981;3:451–481. doi: 10.1016/0165-1838(81)90081-3. 10.1016/0165-1838(81)90081-3. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. Journal of Physiology. 1976;258:187–204. doi: 10.1113/jphysiol.1976.sp011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. Journal of Physiology. 1978;282:365–374. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka S. Electrophysiologic identification of preganglionic neurons in rat dorsal motor nucleus and analysis of vagus afferent projections. Experimental Neurology. 1986;91:366–381. doi: 10.1016/0014-4886(86)90076-2. 10.1016/0014-4886(86)90076-2. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Yamamoto T, Yasunaga K. Localization of vagal cardioinhibitory preganglionic neurons within rat brain stem. Journal of Comparative Neurology. 1979a;186:79–92. doi: 10.1002/cne.901860106. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Yasunaga K, Kawano M. Vagus cardioinhibitory fibres in rats. Pflügers Archiv. 1979b;379:281–285. doi: 10.1007/BF00581432. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Yasunaga K, Tamai S. Vagal cardiac preganglionic neurones: distribution, cell types and reflex discharges. American Journal of Physiology. 1982;243:R92–98. doi: 10.1152/ajpregu.1982.243.1.R92. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Presson RG, Todoran TM, De Witt BJ, McMurtry IF, Wagner WW. Capillary recruitment and transit time in the rat lung. Journal of Applied Physiology. 1997;83:543–549. doi: 10.1152/jappl.1997.83.2.543. [DOI] [PubMed] [Google Scholar]
- Randall WC, Armour JA. Gross and microscopic anatomy of the cardiac innervation. In: Randall WC, editor. Neural Regulation of the Heart. New York: Oxford University Press; 1977. pp. 13–41. [Google Scholar]
- Richter DW, Spyer KM. Cardiorespiratory control. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 189–207. [Google Scholar]
- Skoogh BE, Grillo MA, Nadel JA. Neutral red stains ganglia in the vagal motor pathway to ferret trachea without affecting ganglionic transmission. Journal of Neuroscience Methods. 1983;8:33–39. doi: 10.1016/0165-0270(83)90049-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jones JFX, Ramage AG, Jordan D. Effects of 5-HT and 5-HT1A receptor agonists and antagonists on dorsal vagal preganglionic neurones in anaesthetized rats: an ionophoretic study. British Journal of Pharmacology. 1995a;116:2291–2297. doi: 10.1111/j.1476-5381.1995.tb15067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. The effects of bicuculline and strychnine on the activity of dorsal vagal preganglionic neurones that respond to vagus nerve stimulation: an in vivo ionophoretic study in anaesthetized rats. Journal of Physiology. 1995b;489.P:158P. [Google Scholar]
- Woolley DC, McWilliam PN, Ford TW, Clarke RW. The effect of selective electrical stimulation of non-myelinated vagal fibres on heart rate in the rabbit. Journal of the Autonomic Nervous System. 1987;21:215–221. doi: 10.1016/0165-1838(87)90024-5. [DOI] [PubMed] [Google Scholar]


