Abstract
The interaction of Zn2+ and H+ ions with GABAA receptors was examined using Xenopus laevis oocytes expressing recombinant GABAA receptors composed of subunits selected from α1, β1, γ2S and δ types, and by using cultured rat cerebellar granule neurones.
The potency of Zn2+ as a non-competitive antagonist of GABA-activated responses on α1β1 receptors was reduced by lowering the external pH from 7.4 to 5.4, increasing the Zn2+ IC50 value from 1.2 to 58.3 μm. Zinc-induced inhibition was largely unaffected by alkaline pH up to pH 9.4.
For α1β1δ subunits, concentration-response curves for GABA were displaced laterally by Zn2+ in accordance with a novel mixed/competitive-type inhibition. The Zn2+ IC50 at pH 7.4 was 16.3 μm. Acidification of Ringer solution resulted in a reduced antagonism by Zn2+ (IC50, 49.0 μm) without affecting the type of inhibition. At pH 9.4, Zn2+ inhibition remained unaffected.
The addition of the γ2S subunit to the α1β1δ construct caused a marked reduction in the potency of Zn2+ (IC50, 615 μm), comparable to that observed with α1β1γ2S receptors (IC50 639 μm). GABA concentration-response curves were depressed in a mixed/non-competitive fashion.
In cultured cerebellar granule neurones, Zn2+ inhibited responses to GABA in a concentration-dependent manner. Lowering external pH from 7.4 to 6.4 increased the IC50 from 139 to 253 μm.
The type of inhibition exhibited by Zn2+ on cerebellar granule neurones, previously grown in high K+-containing culture media, was complex, with the GABA concentration-response curves shifting laterally with reduced slopes and similar maxima. The Zn2+-induced shift in the GABA EC50 values was reduced by lowering the external pH from 7.4 to 6.4.
The interaction of H+ and Zn2+ ions on GABAA receptors suggests that they share either a common regulatory pathway or coincident binding sites on the receptor protein. The apparent competitive mode of block induced by Zn2+ on α1β1δ receptors is shared by GABAA receptors on cerebellar granule neurones, which are known to express δ-subunit-containing receptors. This novel mechanism is masked when a γ2 subunit is incorporated into the receptor complex, revealing further diversity in the response of native GABAA receptors to endogenous cations.
The divalent cation Zn2+ is distributed widely throughout the central nervous system (CNS) and concentrated principally within neurones (Frederickson, 1989). Zinc-containing nerve fibres are mostly located in the telencephalon and Zn2+ ions have been located within synaptic vesicle-type structures in nerve terminals (Frederickson, 1989; Smart, Xie & Krishek, 1994). A number of different physiological roles for Zn2+ have been proposed, including: acting as a packaging or storage agent for neurotransmitters within synaptic vesicles (Frederickson, 1989); being released from nerve terminals following stimulation and interacting with postsynaptic excitatory and inhibitory amino acid receptors in the CNS (Peters, Koh & Choi, 1987; Westbrook & Mayer, 1987; Mayer & Vykicky, 1989; Mayer, Vyklicky & Westbrook, 1989; Smart & Constanti, 1990); exerting a neuromodulatory role, influencing γ-aminobutyric acidA (GABAA) receptor-mediated synaptic transmission in the hippocampus (Xie & Smart, 1991; Nakazawa, Inoue, Watano, Koizumi & Inoue, 1995); and a developmental role, influencing the structural and functional maturation of some classes of neurones, particularly in the cerebellum (Dvergsten, Fosmire, Ollerich & Sandstead, 1984).
An intriguing aspect of the functional role of Zn2+ in the CNS involves the interaction with the GABAA receptor. Zinc inhibits GABA-activated responses on a variety of neuronal preparations (Smart et al. 1994; Harrison & Gibbons, 1994); however, not all GABAA receptors, particularly those in adult brain slices, are sensitive to Zn2+ (Smart et al. 1994). A comparison of embryonic and adult rat sympathetic neurones revealed that Zn2+ inhibition of GABAA responses was correlated with neuronal age, with younger neurones being more sensitive to Zn2+ (Smart & Constanti, 1990; Smart, 1992). By utilizing recombinant GABAA receptors (see Sieghart, 1995 and Rabow, Russek & Farb, 1995, for review), later studies demonstrated that inhibition by Zn2+ was dependent on the receptor subunit composition, in particular the presence of the γ subunit (Draguhn, Verdoorn, Ewert, Seeburg & Sakmann, 1990; Smart, Moss Xie & Huganir, 1991). This principle may in part underlie the variation in Zn2+ sensitivity observed with native GABAA receptors (Smart et al. 1994).
In comparison with Zn2+, protons are naturally occurring in all tissues and thus constitute part of the physiological milieu to which receptors are normally exposed. Lowering of external pH can occur during a number of pathological processes, including ischaemic episodes, epileptiform activity and anoxia (Chesler, 1990; Chesler & Kaila, 1992) and is also, but less dramatically, a feature of normal synaptic transmission (Chen & Chesler, 1992) and activation of GABAA receptors per se (Kaila & Voipio, 1987; Kaila, 1994). By the nature of their size and charge, protons are capable of interacting with many types of voltage- and ligand-gated ion channels. GABA-activated responses mediated by invertebrate muscle (Takeuchi & Takeuchi, 1967; Smart & Constanti, 1982; Pasternack, Bountra, Voipio & Kaila, 1992) and vertebrate neuronal GABAA receptors (Groul, Barker, Huang, MacDonald & Smith, 1980; Gallagher, Nakamura & Shinnock-Gallagher, 1983; Smart, 1992; Kaila, 1994; Krishek, Amato, Connolly, Moss & Smart, 1996; Pasternack, Smirnov & Kaila, 1996) are sensitive to changes in the external pH. Furthermore, recombinant GABAA receptors are differentially modulated by external pH, depending on the subunit composition and may partly explain why native GABAA receptors exhibit variable sensitivities to protons (Krishek et al. 1996).
The potential for H+ and Zn2+ to interact at GABAA receptors was suggested by the sensitivity of the Zn2+ binding site on invertebrate muscle GABA receptors to external pH. Increasing the concentration of H+ enhanced the GABA-induced conductance, whilst the antagonistic effect of Zn2+ was markedly reduced, suggesting that Zn2+ and H+ competed for a similar site on this GABA receptor (Smart & Constanti, 1982). The present study investigates the extent of this phenomenon by studying the pH dependence of the Zn2+-induced inhibition of GABA-activated responses mediated by recombinant and native neuronal GABAA receptors. The results demonstrate that Zn2+ and H+ can interact at vertebrate GABAA receptors, and this interaction is dependent on the GABAA receptor subunit composition. Moreover, a novel modulatory role for Zn2+ was observed for GABAA receptors composed of α1β1δ subunits and native GABAA receptors in cerebellar granule neurones. These results lead to the possibility that both H+ and Zn2+ could be used as supplementary tools for probing the in vivo composition of native neuronal GABAA receptors, in addition to revealing a potential physiological mechanism that could regulate GABAA receptor function.
METHODS
Expression vector construction
Murine α1, β1, γ2S and δ subunit cDNAs were incorporated into the mammalian expression vector pGW1 (British Biotechnology) as described previously (Krishek, Xie, Blackstone, Huganir, Moss & Smart, 1994).
Cell preparation
Xenopus laevis oocytes
Xenopus laevis were anaesthetized by immersion in a 0.2% w/v solution of ethyl-m-aminobenzoate (Tricaine). Stage IV-VI Xenopus oocytes were surgically removed and microinjected with equimolar ratios of murine cDNAs corresponding to combinations of α1, β1, γ2S and δ GABAA receptor subunits and subsequently cultured in a modified Barth's medium (MBM) comprising (mm): 110 NaCl, 1 KCl, 2.4 NaHCO3, 7.5 Tris-HCl, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4; and 50 μg ml−1 gentamicin; pH 7.6 as previously described (Krishek et al. 1994). All the Xenopus oocyte experiments were performed at room temperature (18–23°C). Cells were used after 2 days and accepted for study if they possessed membrane potentials of −30 to −60 mV and input resistances of 1–5 MΩ.
Cerebellar granule neurones
Sprague-Dawley postnatal day 4 (P4) rats were killed by decapitation and the cerebella removed and placed in ice-cold Hanks’ Ca2+/Mg2+-free balanced salt solution (HBSS). Meninges were removed and the cerebella were placed in the plating medium MEM 10/10 containing: MEM Earle's salts, 10% v/v horse serum, 10% v/v fetal calf serum, 600 mg l−1 glucose, 2 mm glutamine and 100 u ml−1 penicillin G and 100 μg ml−1 streptomycin. The cerebella were subsequently taken up into a 5 ml syringe and expelled through a 210 μm Nylon mesh into a 10 ml centrifuge tube. After allowing the tissue to sediment for 5 min, the microexplants and dissociated cells were plated onto poly-l-lysine-coated glass coverslips and maintained at 37°C in a 95% air and 5% CO2 atmosphere. After 18 h in vitro, the cultures were treated with 10 μm cytosine arabinoside for 24 h, then the media replaced with MEM 10 supplemented with 20 mm KCl (total concentration 25.37 mm). MEM 10 medium had a similar composition to MEM 10/10 but lacked the fetal calf serum. All media was replenished on days 5 and 7 after plating. Neurones were used for experimentation between days 6 and 11 in vitro, and possessed membrane potentials of −60.5 ± 12.5 mV (mean ± s.e.m.).
Electrophysiology
Intracellular recording
Membrane currents were recorded from Xenopus laevis oocytes, retaining their follicular cell envelope, using a two-electrode voltage-clamp method in conjunction with an Axoclamp-2A amplifier. Oocytes were superfused at 8–10 ml min−1 (bath volume 0.5 ml) with an amphibian Ringer solution containing (mm): 110 NaCl, 2 KCl, 5 Hepes and 1.8 CaCl2; pH 7.4. The microelectrodes were filled with 3 m KCl (voltage) and 0.6 m K2SO4 (current) and possessed resistances of 5–10 MΩ and 1–2 MΩ, respectively. All data were recorded on a Gould 2200 ink-jet pen recorder.
Whole-cell recording
Experiments on the cerebellar granule neurones were performed using a List EPC5 amplifier in the whole-cell recording mode. Patch electrodes (1–7 MΩ) were filled with a solution containing (mm): 140 KCl, 2 MgCl2, 1 CaCl2, 10 Hepes, 11 EGTA and 2.5 adenosine triphosphate. The cells were viewed under Nomarski differential interference contrast optics (Nikon Optiphot-2) and continuously superfused with a Krebs solution containing (mm): 140 NaCl, 4.7 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 Hepes and 11 glucose. Membrane currents were filtered at 1.5 kHz (−3 dB, 8-pole Butterworth filter, 48 dB octave−1) and recorded on a Gould WindoGraf 40–8474-00 pen recorder.
Analysis of GABA-activated membrane conductance
GABA-induced membrane conductance (ΔGN) was calculated in Xenopus oocytes by subtraction from the resting membrane conductance and normalized to the response induced by 10 μm GABA at pH 7.4. In the absence and presence of GABA, membrane conductances were frequently determined by stepping the membrane potential from a holding potential range of −30 to −40 mV with a −10 mV voltage command step (1 s duration and 0.2 Hz frequency). These data were used to construct equilibrium concentration-response relationships for GABA. The data were fitted with a logistic state function of the form:
where ΔG and ΔGmax represent the normalized GABA-induced conductance at a given concentration and the maximum conductance induced by a saturating concentration of GABA, respectively. A represents the concentration of GABA and EC50 defines the concentration of GABA inducing 50% of the maximum response. nH is the Hill coefficient. A Levenberg-Marquardt non-linear least-squares fitting routine was used.
The Zn2+ concentration-inhibition curves were fitted with an antagonist inhibition model described by:
where ΔGN′ and ΔGN represent the normalized GABA-induced conductance at a given concentration in the presence and absence of antagonist, respectively. B represents the Zn2+ concentration and IC50 defines the concentration of Zn2+ producing a 50% inhibition of the GABA response.
Drugs and solutions
Drugs and solutions were bath-applied in the Xenopus oocyte experiments. For rapid application to the neurones, solutions were applied close to (100–200 μm) the cells using a Y-tube manufactured from glass electrode tubing. The Y-tube was positioned at the periphery of the × 40 water immersion objective. Solutions were driven through the Y-tube in response to a vacuum. Drug application was achieved by isolating the vacuum using a solenoid valve, thereby allowing solution to flow out of the Y-tube over the recorded cells by gravity. A complete exchange of solution around the cells was effected within 50–80 ms. Throughout, Zn2+ was pre-applied for a minimum of 1 min prior to GABA application in Zn2+-containing Ringer (oocytes) or Krebs (cerebellar granule neurones) solutions. The zwitterionic nature of the GABA molecule is not substantially affected over the range of external pH from 5.4 to 9.5 (Krishek et al. 1996). Similarly, it is unlikely that the inhibition caused by Zn2+ of GABA-activated responses is dependent upon complexation, since the slopes of the GABA concentration-response curves are not increased by Zn2+ (Smart & Constanti, 1982).
RESULTS
Inhibitory effect of Zn2+ on α1β1 GABAA receptors: modulation by external pH
Zinc is a potent antagonist at cultured neuronal GABAA receptors (Westbrook & Mayer, 1987; Smart & Constanti, 1990) and also at invertebrate GABA receptors (Smart et al. 1994). Previous evidence suggests that the Zn2+ binding site on the lobster muscle GABA receptor involved at least one histidine residue that could also competitively bind H+. Accordingly, lowering the external pH reduced the effectiveness of Zn2+. If a similar binding site exists on selected vertebrate GABAA receptors, then it should be possible to regulate the Zn2+-induced antagonism by changing the pH of the external solution.
For recombinant α1β1 GABAA receptors expressed in Xenopus oocytes, the whole-cell membrane conductance activated by 10 μm GABA was inhibited by 51.07 ± 1.6% (mean ± s.e.m., n = 23 oocytes) with 1 μm Zn2+ at pH 7.4 (Fig. 1A). Lowering the pH of the Ringer solution over the range 7.4 to 5.4 resulted in a potentiation of the GABA-induced response; however, the antagonism induced by 1 μm Zn2+ was now effectively abolished (Fig. 1B). In contrast, increasing the external pH from 7.4 to 9.4 reduced the response to 10 μm GABA, but the antagonism induced by 1 μm Zn2+ was apparently unaffected (51.7 ± 3.25%; Fig. 1C).
Figure 1. Sensitivity of the Zn2+ inhibition of GABA-activated conductances in Xenopus oocytes expressing α1β1 GABAA receptors.
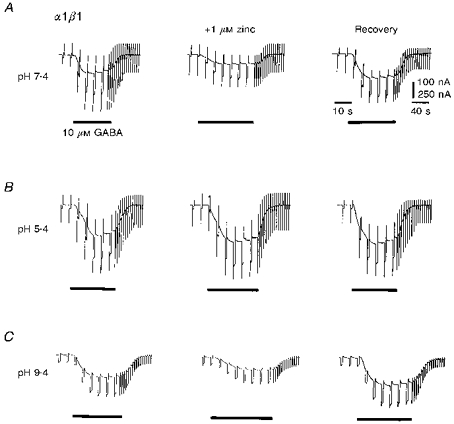
Membrane currents and conductance changes evoked by bath-applied 10 μm GABA in the absence and presence of 1 μm zinc at pH 7.4 (A), 5.4 (B) and 9.4 (C), respectively. The GABA-activated responses in all oocytes increased at low pH and decreased at high pH. Records are selected from two oocytes. Current calibration of 100 nA corresponds to A and B, and 250 nA corresponds to C. GABA was applied for the duration indicated by the horizontal bars. Recovery responses were obtained after 3 min in Ringer solution at the respective external pH values following exposure to Zn2+. Membrane conductance was ascertained by regularly applying hyperpolarizing voltage-clamp commands (−10 mV amplitude, 1 s duration, frequency 0.2 Hz) from the holding potential of −40 mV.
The ability of H+ to reduce the inhibitory potency of Zn2+ was examined by constructing concentration-inhibition curves for Zn2+ antagonizing the 10 μm GABA-activated response. At pH 7.4, the Zn2+ IC50 was 1.2 ± 0.4 μm (Fig. 2A, Table 1). Increasing the alkalinity of the Ringer solution to pH 9.4 produced little change in the inhibition curve or the IC50 (1.4 ± 0.5 μm; Fig. 2A; Table 1); however, increasing the H+ concentration 100-fold to pH 5.4, caused a parallel displacement in the inhibition curve and a substantial increase in the Zn2+ IC50 to 58.3 ± 9.4 μm (Fig. 2A; Table 1). For a complete block of the GABA-activated response, 1–2 mm Zn2+ was required at pH 5.4.
Figure 2. Concentration-response relationships for Zn2+ inhibition of GABA-activated conductances on α1β1 GABAA receptors.

A, left panel, normalized zinc concentration-inhibition curves were constructed for GABA at external pH 5.4, 7.4 and 9.4. For this and all subsequent zinc inhibition curves, the GABA-induced conductances were normalized to the response evoked by 10 μm GABA in the absence of zinc. All data were fitted according to the antagonist inhibition model (see Methods) and IC50 values determined (Table 1; n = 9). The points represent the means ± s.e.m. A, right panel and B, indicate GABA equilibrium concentration conductance curves constructed in the absence and presence of 1 μm zinc at pH 7.4 and 9.4, or 50 μm zinc at pH 5.4 and normalized to the response induced by 10 μm GABA in the absence of zinc at pH 7.4. The data were fitted with the logistic equation (see Methods; n = 18) and EC50 values and Hill coefficients determined (Tables 2 and 3).
Table 1.
Analysis of the Zn2+ inhibition plots for GABA in Xenopus laevis oocytes injected with murine GABAA receptor cDNAs
| IC50 (μm) | |||
|---|---|---|---|
| Subunit composition | pH 7.4 | pH 5.4 | pH 9.4 |
| α1β1 | 1.2 ± 0.4 | 58.3 ± 9.4 | 1.4 ± 0.5 |
| α1β1δ | 16.3 ± 3.5 | 49.0 ± 10.2 | 16.4 ± 4.5 |
| α1β1γ2S | 639.9 ± 34.9 | – | – |
| α1β1γ2Sδ | 615.4 ± 84.3 | – | – |
The IC50 values were obtained from fitting the antagonist inhibition model to the normalized curves (see Methods) for the inhibition of the response to 10 μm GABA. All values are means ± s.e.m. (n = 24).
The effect of external pH on the steady-state inhibition of the GABA-induced conductance by Zn2+ was studied using GABA equilibrium concentration-response curves. To compare the inhibition induced by Zn2+ at each pH, equipotent Zn2+ concentrations approximating the IC50 values were selected for study. At pH 7.4, the GABA concentration-response curve was depressed at all GABA concentrations by 1 μm Zn2+, consistent with a non-competitive type of inhibition (Fig. 2A). The GABA EC50 and Hill coefficient were unaffected by Zn2+ (Tables 2 and 3). Lowering the external pH from 7.4 to 5.4 enhanced the GABA-induced conductance and laterally displaced the concentration-response curve with a corresponding reduction in the EC50 to 3.2 ± 0.18 μm. The inhibitory effect of Zn2+ was markedly reduced with 50 μm now required to produce an equivalent depression in the GABA concentration-response curve compared with 1 μm Zn2+ at pH 7.4 (Fig. 2B). The antagonism produced by 50 μm Zn2+ at pH 5.4 was of a mixed/non-competitive type with a depression in the maximum response and small lateral curve displacement. There was no change in the Hill coefficient, but the EC50 for GABA was increased to 7.4 ± 0.5 μm (Tables 2 and 3). In contrast, GABA concentration-response curves at pH 9.4 were depressed compared with those at pH 7.4. The inhibition by Zn2+ at GABA concentrations > 10 μm was unaffected by the increased external pH; however, at GABA concentrations < 10 μm, inhibition was reduced and eventually abolished at GABA concentrations < 2 μm (Fig. 2B). Unusually, the type of inhibition observed at pH 9.4 was consistent with uncompetitive antagonism, with the inhibition increasing with the GABA concentration (Fig. 2B). The Hill coefficient for the GABA concentration-response curves in the presence of 1 μm Zn2+ was unaffected, whereas the EC50 was reduced from 2.5 to 1 μm (Tables 2 and 3).
Table 2.
Analysis of the equilibrium concentration-response curves for GABA in the absence and presence of Zn2+ in Xenopus laevis oocytes injected with GABAA receptor cDNAs
| Hill coefficient | ||||||
|---|---|---|---|---|---|---|
| pH 7.4 | pH 5.4 | pH 9.4 | ||||
| Subunit composition | Control | +Zn2+ | Control | +Zn2+ | Control | +Zn2+ |
| α1β1 | 0.95 ± 0.09 | 1.2 ± 0.1(1)* | 1.1 ± 0.06 | 1.1 ± 0.07 (50) | 1.2 ± 0.14 | 1.2 ± 0.2 (1) |
| α1β1δ | 0.85 ± 0.05 | 0.8 ± 0.06 (10) | 0.86 ± 0.05 | 0.75 ± 0.03 (50)* | 0.85 ± 0.15 | 0.7 ± 0.12 (10) |
| 0.6 ± 0.03 (100)* | ||||||
| α1β1γ2S | 1.3 ± 0.4 | 1.2 ± 0.03 (5) | – | – | – | – |
| 1.5 ± 0.9 (300) | ||||||
| α1β1γ2Sδ | 1.4 ± 0.06 | 1.5 ± 0.07 (10) | – | – | – | – |
| 1.4 ± 0.1 (300) | ||||||
The Hill coefficient (nH) data were determined from fitting the logistic model to the normalized curves (see Methods) with all values given as means ± s.e.m. (n = 50). Numbers in parentheses indicate the Zn2+ concentration (μm)
represents a significant change from control, P < 0.05.
Table 3.
Analysis of the equilibrium concentration-response curves for GABA in the absence and presence of Zn2+ recorded from GABAA receptor constructs
| EC50 (μm) | ||||||
|---|---|---|---|---|---|---|
| pH 7.4 | pH 5.4 | pH 9.4 | ||||
| Subunit composition | Control | +Zn2+ | Control | +Zn2+ | Control | +Zn2+ |
| α1β1 | 5.87 ± 0.7 | 6.6 ± 0.5 (1) | 3.2 ± 0.18 | 7.4 ± 0.5 (50)* | 2.5 ± 0.3 | 1.0 ± 0.1 (1)* |
| α1β1δ | 4.9 ± 0.41 | 28.4 ± 3.5 (10)* | 4.9 ± 0.4 | 15.6 ± 1.2 (50)* | 4.3 ± 0.34 | 47.8 ± 5.8 (10)* |
| 361 ± 53.1 (100)* | ||||||
| α1β1γ2S | 9.7 ± 0.2 | 10.06 ± 0.21 (5) | – | – | – | – |
| 6 ± 0.3 (300)* | ||||||
| α1β1γ2Sδ | 27.7 ± 0.96 | 27.7 ± 0.95 (10) | – | – | – | – |
| 16.2 ± 1.02 (300)* | ||||||
The EC50 data were determined from fitting the logistic model to the normalized curves (see Methods) with all values given as means ± s.e.m. (n = 50). Numbers in parentheses indicate the Zn2+ concentration (μm)
represents a significant change from control, P < 0.05.
Expression of α1, β1 and δ GABAA receptor subunits: a novel mode of Zn2+ antagonism
The level of inhibition of GABA responses by Zn2+ is reduced by the presence of γ subunits and also affected by exchanging α1 for α2 or α3 subunits (Draguhn et al. 1990; Smart et al. 1991; White & Gurley, 1995). However, the influence of the δ subunit on Zn2+ inhibition has not been quantitatively established. Oocytes expressing α1, β1 and δ subunits were assumed to co-assemble into α1β1δ GABAA receptors since the latter exhibited a distinctive sensitivity to H+ (Krishek et al. 1996). Zinc inhibition curves for α1β1δ subunit receptors indicated that increasing the external pH from 7.4 to 9.4 had little effect on the antagonism of responses to 10 μm GABA (IC50 values were 16.3 ± 3.5 μm (pH 7.4) and 16.4 ± 4.5 μm (pH 9.4), n = 8; Fig. 3A; Table 1); however, increasing the H+ concentration (pH 5.4) caused the inhibition curve for Zn2+ to be laterally displaced with an increased IC50 of 49.0 ± 10.2 μm (n = 4; Fig. 3A; Table 1).
Figure 3. Modulation by external pH of Zn2+ inhibition of GABA-induced responses recorded from oocytes expressing GABAA receptor α1β1δ subunits.

A, left panel, zinc concentration-inhibition relationships constructed at external pH 5.4, 7.4 and 9.4, and fitted according to the antagonist inhibition model and IC50 values determined (Table 1; n = 9). A, right panel, GABA equilibrium concentration-response curves constructed in the absence and presence of 10 and 100 μm Zn2+ at pH 7.4. B, GABA equilibrium concentration-response curves constructed in the absence and presence of 50 μm zinc (left, pH 5.4) and 10 μm zinc (right, pH 9.4). The EC50 values and Hill coefficients for GABA were determined from the logistic model (Tables 2 and 3; n = 20).
This inhibitory profile of Zn2+ on α1β1δ constructs initially appeared similar to that observed with α1β1 subunit-containing receptors; however, analysis of GABA equilibrium concentration-response curves revealed that Zn2+ acted as an apparent competitive inhibitor on α1β1δ receptors, a feature not observed with αβ or αβγ GABAA receptor constructs (Fig. 3A). At pH 7.4, the GABA EC50 was increased by 10 μm Zn2+, consistent with lateral/competitive displacement of the GABA concentration-response curve (Tables 2 and 3). The Hill coefficient was unaltered by 10 μm Zn2+. Interestingly, at a higher Zn2+ concentration (100 μm) the GABA response curve was further displaced but with a reduced slope despite the maximum response remaining unaffected. This suggests that the mechanism of Zn2+ antagonism is not purely competitive (Fig. 3A).
On lowering the external pH from 7.4 to 5.4, the GABA response was enhanced as expected (Krishek et al. 1996) but to ensure an equipotent effect of Zn2+ at pH 5.4 compared with that observed at pH 7.4, 50 μm Zn2+ was now required. The inhibitory potency of Zn2+, whilst remaining mixed/competitive, was reduced approximately 5-fold as estimated from the displaced concentration-response curves (Fig. 3B). The Hill coefficient was reduced and the EC50 for GABA was markedly increased in the presence of Zn2+ (Fig. 3B; Tables 2 and 3). At pH 9.4, the antagonism induced by an equipotent concentration of Zn2+ (approximate IC50 concentration) was slightly increased from that observed at pH 7.4 (Fig. 3B), but still the mechanism of action was apparently mixed/competitive without changing the Hill coefficient whilst increasing the EC50 for GABA (Fig. 3B; Tables 2 and 3).
Influence of the γ2S subunit in determining the sensitivity of the GABAA receptor to inhibition by Zn2+
As the δ and γ2 subunits differentially influence the sensitivity of α1β1 subunit-containing GABAA receptors with respect to H+ and Zn2+, the effect of co-expressing these subunits, within the same hetero-oligomeric structure was assessed by adding γ2S to GABAA receptors composed of α1β1 and α1β1δ subunits. For both α1β1γ2S and α1β1γ2Sδ subunit combinations, Zn2+ inhibition curves revealed that the GABA-activated responses were only inhibited by relatively high Zn2+ concentrations (> 10–100 μm) with estimated IC50 values of 639.9 ± 34.9 μm (α1β1γ2S) and 615.4 ± 84.3 μm (α1β1γ2Sδ; Fig. 4A and B; Table 1), respectively. Complete inhibition of the 10 μm GABA responses were not achieved over the range of 0.1 μm to 1 mm Zn2+. Whether the δ subunit differentially influenced the level and type of inhibition by Zn2+ of GABA responses transduced by α1β1γ2S GABAA receptors was assessed using GABA concentration-response curves. Interestingly, for both α1β1γ2S and α1β1γ2Sδ receptor constructs, low concentrations of Zn2+ (5–10 μm) were essentially ineffective, but at a higher Zn2+ concentration (300 μm), the maximum conductance induced by saturating concentrations of GABA (500 μm) was reduced in a mixed/non-competitive manner, with little change in the Hill coefficients and moderate reductions in the GABA EC50 values (Fig. 4A and B; Tables 2 and 3).
Figure 4. Regulation of α1β1γ2S and α1β1γ2Sδ GABAA receptor function by Zn2+.

A and B, left panels, zinc concentration-inhibition relationships constructed for 10 μm GABA-activated responses recorded from α1β1γ2S and α1β1γ2Sδ GABAA receptors, respectively. All data were fitted according to the antagonist inhibition model and IC50 values determined (Table 1, n = 6). A and B, right panels, GABA equilibrium concentration-response curves in the absence and presence of 5 and 300 μm or 10 and 300 μm zinc recorded from oocytes expressing α1β1γ2S and α1β1γ2Sδ GABAA receptors, respectively. The EC50 values and Hill coefficients for GABA were determined from the logistic model (Tables 2 and 3, n = 12).
Cerebellar granule neurones: emulation of α1β1δ receptors with regard to Zn2+ antagonism
The shift from non-competitive to mixed/competitive inhibition of the GABA-induced conductance by Zn2+ on co-expressing the δ subunit with α1β1 subunit-containing GABAA receptors is intriguing. The δ subunit mRNA is expressed most prominently in the granule cell layer of the cerebellum (Laurie, Wisden & Seeburg, 1992) and could participate in forming αβδ complexes. These neurones were therefore selected to determine whether native GABAA receptors also exhibit a mixed/competitive Zn2+ block in accordance with α1β1δ receptors.
The whole-cell membrane current activated by 10 μm GABA was inhibited reversibly by 10–500 μm Zn2+ at pH 7.4. Lowering the pH of the Krebs solution resulted in a small alleviation of the Zn2+ block (Fig. 5A). The reduced inhibitory potency of Zn2+ at low pH was manifest from the concentration-inhibition curves for the antagonism of the 10 μm GABA-activated current. Acidification of the Krebs solution to pH 6.4 caused a small parallel displacement in the inhibition curve and increased the IC50 to 253.7 ± 33.9 μm from 139.0 ± 17.2 μm at pH 7.4 (Fig. 5B).
Figure 5. Modulation of GABA-activated currents in cultured cerebellar granule neurones by H+ and Zn2+ ions.
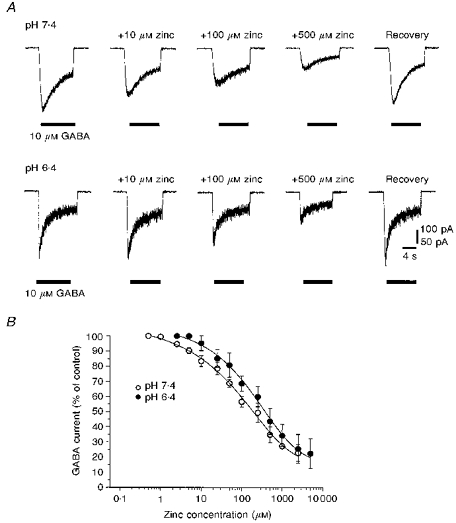
A, whole-cell membrane currents activated by 10 μm GABA before and after the application of 10–500 μm Zn2+ at external pH values of 7.4 (upper panel) and 6.4 (lower panel). Recovery responses were obtained in control Krebs solution 5 min after Zn2+ application at the respective external pH. Records are from two neurones. The 100 pA calibration bar applies to the upper records. The holding potential was −40 mV. B, zinc concentration-inhibition curves for the antagonism of the 10 μm GABA-activated response at external pH values of 7.4 (^) or 6.4 (•) constructed from fifteen cells. The curves were generated from the inhibition model (see Methods), but since the inhibition saturated at approximately 20% of the control GABA-activated response, a constant was incorporated and the IC50 values were determined.
To determine the mode of Zn2+-induced inhibition in these cells the whole-cell GABA-induced current was measured in the absence and presence of Zn2+. At either pH 7.4 or 6.4, an approximate 10-fold increase in the GABA concentration was required in the presence of 300 μm Zn2+ to induce currents of similar magnitude to the controls (Figs 6A and 7A). In addition, GABA concentration-response curves revealed that the maximum normalized current (IN,max) induced by saturating concentrations of GABA was not significantly reduced by 300 μm Zn2+ (IN,max control, 2.1 ± 0.04; +Zn2+, 2.0 ± 0.06 at pH 7.4; and control, 1.7 ± 0.07; +Zn2+, 1.66 ± 0.05 at pH 6.4 (Figs 6B and 7B)); however, the GABA EC50 was increased by 300 μm Zn2+ from 10.76 ± 0.67 to 71.51 ± 10.11 μm (pH 7.4) and 18.05 ± 2.27 to 102.63 ± 12.20 μm (pH 6.4; Figs 6B and 7B). This lateral competitive-type shift in the control curve in the presence of Zn2+ with no apparent reduction in the maximum response was associated with a reduced Hill coefficient from 1.0 ± 0.05 to 0.7 ± 0.05 (pH 7.4) and 0.9 ± 0.06 to 0.7 ± 0.04 (pH 6.4), suggesting a more complex mode of antagonism for Zn2+ consistent with ‘mixed inhibition’ (Smart & Constanti, 1982).
Figure 6. Inhibition by Zn2+ of GABA-activated responses at pH 7.4 on cerebellar granule neurones.

A, whole-cell currents recorded at −40 mV holding potential for different concentrations of GABA (1 μm to 2.5 mm) in the absence (upper panel) and presence of 300 μm Zn2+ (lower panel). B, analysis of GABA concentration-response curves at pH 7.4 in the absence (^) and presence (•) of 300 μm Zn2+. The continuous line curve fit was generated by the agonist logistic model (see Methods). The dotted lines illustrate the corresponding curves for data obtained at pH 6.4 (see Fig. 7).
Figure 7. GABA-activated responses at pH 6.4 on cerebellar granule neurones: inhibition by Zn2+.

A, whole-cell currents recorded for 1 μm to 2.5 mm GABA in the absence (upper panel) and presence of 300 μm Zn2+ (lower panel). Holding potential, −40 mV. B, GABA concentration-response curves are plotted at pH 6.4 in the absence (^) and presence (•) of 300 μm Zn2+. The continuous line curve fit was provided by the logistic model (see Methods). For comparison, the dotted lines plot the corresponding curves for data obtained at pH 7.4 (see Fig. 6).
DISCUSSION
Zn2+-induced inhibition is dependent on the GABAA receptor subunit composition: role of α and γ subunits
Using recombinant GABAA receptors expressed in Xenopus oocytes, the potency of Zn2+ as a non-competitive antagonist on α1β1 subunit constructs was clearly reduced by the co-expression of the γ2S subunit in accordance with previous studies on human embryonic kidney cells (Draguhn et al. 1990; Smart et al. 1991). The addition of the γ2 subunit causes the most profound change in the sensitivity of the GABAA receptor to inhibition by Zn2+, although swapping other subunits in the ternary αβγ receptor complex can also be effective. Notably, exchanging α1 for either α2 or α3 subunits in the αxβ3γ2L (x = 1–6) receptor complex resulted in a 5.8- to 7.5-fold shift in the Zn2+ IC50 (White & Gurley, 1995). The relative insensitivity to Zn2+ of the γ subunit-containing receptors was demonstrated by the inability of Zn2+ (up to 100 μm) to completely block the responses to GABA, saturating at less than 50% inhibition. Hetero-oligomeric αxβ2γ2 GABAA receptors incorporating either the α4 or α6 subunits are also less sensitive to inhibition by Zn2+ compared with α1β1 constructs, but more sensitive when compared with α1β1γ2S receptors with IC50 values of 37 (α4β2γ2) and 150 μm (α6β2γ2; Knoflach et al. 1996). A similar shift to a lower Zn2+ sensitivity following the exchange of α subunits in the rat GABAA receptor complex has also been reported for α1β3γ2L (IC50, 245 μm) compared with α6β3γ2L (IC50, 47 μm) constructs (Saxena & Macdonald, 1996). The variability in the IC50 values reported for Zn2+ on recombinant GABAA receptors may be a consequence of changing the receptor subunit composition and species. However, the IC50 values alone cannot be used as the sole identifying criterion for the Zn2+ inhibition of GABAA receptors since it is clear that the apparent inhibitory efficacy of Zn2+ also varies, e.g. White & Gurley (1995) reported Zn2+ (10 μm) antagonism of responses to GABA that saturated at 10–25% inhibition on human α1β3γ2L constructs, despite the estimated IC50 of 1.2 μm from a shallow Zn2+ concentration-inhibition plot. This contrasts with the small enhancement of GABA-activated responses (17.6%) by 10 μm Zn2+ on rat α1β1γ2L subunits (Saxena & Macdonald, 1996).
The δ subunit confers a novel Zn2+-sensitive profile on the GABAA receptor
In comparison with the influence of the α and γ subunits in determining the degree of Zn2+ inhibition, the co-expression of the δ subunit resulted in a different type of antagonism of GABA-activated responses. The Zn2+ sensitivity of α1β1δ subunits (IC50 16.3 μm) was reduced compared with α1β1 (1.2 μm) receptors but considerably more sensitive than α1β1γ2S constructs (> 600 μm). This could be due to a changed quaternary structure of the receptor complex, which may reveal different binding sites for Zn2+ on the α1β1δ GABAA receptor, or alternatively, the affinity of Zn2+ for binding site(s) that are common to both α1β1 and α1β1δ constructs, may be reduced in the latter. Interestingly, the type of inhibition caused by Zn2+ changed from non-competitive, which is typical of αβ subunit receptors (Smart et al. 1994), to mixed/competitive. Competitive-type behaviour could be interpreted as both Zn2+ and GABA competing for the same binding site(s) within the α1β1δ GABAA receptor complex. This is considered unlikely since in functional competition studies, Zn2+ appeared to bind to a novel site on the GABAA receptor (Celentano, Gyenes, Gibbs & Farb, 1991; Smart, 1992). An alternative explanation would involve Zn2+ antagonizing via an allosteric interaction with the GABAA receptor affecting agonist binding and/or ion channel gating. Interestingly, for bullfrog dorsal root ganglion cells, the GABA-induced response was inhibited by high concentrations of Zn2+ (1–3 mm) in a competitive manner analogous to the Zn2+ inhibition seen in this study with α1β1δ GABAA receptors (Yakushiji, Tokutomi, Akaike & Carpenter, 1987). Whether these amphibian ganglia contain δ subunits remains to be seen, but using in situ hybridization it appears that the rodent counterparts probably do not (Persohn, Malherbe & Richards, 1991). GABA-activated currents recorded from rat α6 β3δ constructs expressed in L929 fibroblasts can also be inhibited by Zn2+ with an IC50 of 4.8 μm (Saxena & Macdonald, 1996). Responses to GABA were slow to recover from the effects of Zn2+, which was not observed in the present study using Zn2+ concentrations up to 2.5 mm with α1β1δ subunits.
The addition of the γ2S subunit to the construct α1β1δ, forming α1β1γ2Sδ, appeared to diminish the effectiveness of Zn2+, increasing the IC50 compared with that for α1β1 or α1β1δ constructs and causing inhibition to revert to non-competitive. The similarity in the Zn2+ IC50 values for the α1β1γ2S and α1β1γ2Sδ receptors could indicate a similar Zn2+ binding site(s) within these constructs; however, an alternative interpretation is that co-expression of α1, β1, γ2S and δ subunits only resulted in the formation of discrete α1β1γ2S and α1β1δ subunit receptors. This seems unlikely since as α1β1δ subunit assemblies are functionally expressed and exhibit distinctive properties (Saxena & Macdonald, 1994; Krishek et al. 1996), we would detect their greater sensitivity to Zn2+ in a mixed population of α1β1γ2S and α1β1δ receptors. Furthermore, expression of α1, β1, γ2S and δ subunits also results in receptors exhibiting distinctive single channel properties and sensitivity to H+ (Saxena & Macdonald, 1994; Krishek et al. 1996). Thus both of the δ subunit-containing receptors studied are likely to form functional receptor assemblies.
Modulation of the Zn2+-induced inhibition by H+ ions
The prospect of Zn2+ and H+ sharing binding sites on the GABAA receptor followed the marked reduction in Zn2+ antagonism on lowering the external Ringer solution pH around invertebrate muscle GABA receptors. Subsequent titration indicated that H+ probably binds to similar amino acids that co-ordinated the Zn2+ binding site (Smart & Constanti, 1982). Given the variety of recombinant GABAA receptor complexes that can be expressed and their differential sensitivity to H+ (Krishek et al. 1996), it was conceivable that H+ could interact with Zn2+ binding sites on various neuronal GABAA receptors. The results with α1β1 and α1β1δ receptor subunit combinations indicated that increasing the H+ concentration reduced the inhibitory potency of Zn2+, whereas increasing the external pH subtly changed the mode of antagonism exerted by Zn2+ on α1β1 receptors from non-competitive to uncompetitive. The Zn2+ binding site(s) might vary between different receptor complexes, but a parsimonious explanation is that Zn2+ binds to similar sites and exerts an allosteric effect, the nature and extent of which is dependent upon receptor subunit composition. In accordance with a similar binding site for Zn2+ on GABAA receptors, the α1β1γ2S construct, which is less sensitive to inhibition by Zn2+ (Draguhn et al. 1990; Smart et al. 1991) is also less sensitive to external pH (Krishek et al. 1996).
Interaction of Zn2+ and H+ ions at native GABAA receptors
The modulation of Zn2+ inhibition by H+ on recombinant GABAA receptors should also occur at selected neuronal GABAA receptors. Cerebellar granule neurones were chosen to investigate the H+-Zn2+ interaction since these cells express δ subunit mRNA (Laurie et al. 1992). The sensitivity of Zn2+ inhibition to pH on granule neurones was quite similar to the profile observed with α1β1δ constructs and unlike that seen with α1β1 or α1β1γ2S constructs. This might support the existence of αβδ subunit receptors, although granule neurones could express up to ten different receptor subunits (Wisden, Korpi & Bahn, 1996). An interesting difference between GABAA receptors on granule neurones and α1β1δ constructs, is that inhibition caused by Zn2+ on the latter proceeds to 100%, whilst for the former it saturates at 80%. The presence of saturation could be due to GABAA receptor heterogeneity and may be caused by αiβjγ2 or αiβjγ2δ subunit constructs (where i = 1, 6; j = 1–3), which have less sensitivity to Zn2+. The data are in accordance with granule neurones possessing a significant population of αiβjδ subunit receptors. Moreover, under the depolarizing culture conditions caused by supplementing the media with KCl, δ subunit mRNA is predicted to increase 10-fold in the first 4 days in vitro (Gault & Siegel, 1997). It is reasonable to expect some or all of the mRNA to be translated into δ subunit protein that is ultimately assembled into functional receptors. Evidence to support this notion comes from using cerebellar granule neurones maintained in a low K+-containing culture medium where GABA-activated currents are antagonized in a non-competitive manner by 30 μm Zn2+ (Kilic, Moran & Cherubini, 1993). This action of Zn2+ may be due to the non-depolarizing culture conditions (Kilic et al. 1993), where the δ subunit transcripts are expected to be insufficient to produce significant levels of δ subunit protein within the receptor complex (Gault & Siegel, 1997).
In comparison with the cerebellum, using cultured hippocampal neurones, the 5 μm GABA-induced conductance increase was reduced by decreasing the external pH from 8.4 to 7.4 (Pasternack et al. 1996). This profile was reversed in the presence of 50 μm Zn2+ such that low pH (7.4) now caused an enhanced GABA response compared with that at pH 8.4. One interpretation of this result is that on exposing hippocampal neurones to alkaline pH, the inhibitory effect of Zn2+ is enhanced. Thus acidifying the Krebs solution alleviated the Zn2+ block and apparently enhanced the GABA-activated current. This interaction was also studied for neuronal GABAA receptors in cultured rat sympathetic neurones (Smart, 1992) but external pH had little effect on the inhibitory action of Zn2+, possibly due to the expression of different isoforms of GABAA receptor not included in the present study. Overall, H+ and Zn2+ can clearly interact at some native GABAA receptors, but the full interpretation of these effects will only become apparent when the subunit compositions of individual native neuronal GABAA receptors are known.
Physiological role(s) for Zn2+ and H+ ions interacting with GABA receptors
The ability of H+ and Zn2+ to interact at the GABAA receptor raises the prospect of regulation by endogenous ions. The hippocampus and neocortex are relatively rich in zinc-containing nerve fibres, with other areas, including the cerebellum, exhibiting weaker but detectable levels (Frederickson, 1989). Therefore, changes in external pH near where zinc-containing nerve fibres impinge on inhibitory synapses might be expected to have a regulatory role. In the cerebellum, the likely presence of δ subunit-containing GABAA receptors will influence the type of regulation by Zn2+, dependent upon accompanying α, β and/or γ subunits. For example, α1β1δ subunits are inhibited in a competitive manner and lowering the external pH causes a 5-fold change in sensitivity to Zn2+; however, for αβ constructs, the non-competitive inhibition and sensitivity to Zn2+ suggest that an equivalent acidification of extracellular pH would cause a profound 50-fold reduction in sensitivity to Zn2+. Thus the susceptibility of neuronal GABAA receptors to Zn2+ inhibition under circumstances of lowered pH will be dependent on receptor subunit composition. This type of endogenous regulation of GABAA receptors may have implications for neuronal excitability in both healthy and diseased neural tissue.
Acknowledgments
This work was supported by the Medical Research Council. B. J. K. is supported by a Maplethorpe Postdoctoral Fellowship of the University of London.
References
- Celentano JJ, Gyenes M, Gibbs TT, Farb DH. Negative modulation of the γ-aminobutyric acid responses by extracellular zinc. Molecular Pharmacology. 1991;40:766–773. [PubMed] [Google Scholar]
- Chen JCT, Chesler M. Extracellular alkaline shifts in rat hippocampal slice are mediated by NMDA and non-NMDA receptors. Journal of Neurophysiology. 1992;68:342–344. doi: 10.1152/jn.1992.68.1.342. [DOI] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Progress in Neurobiology. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in Neurosciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdoorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. 10.1016/0896-6273(90)90337-F. [DOI] [PubMed] [Google Scholar]
- Dvergsten CL, Fosmire GJ, Ollerich DA, Sandstead H. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. II. Impaired maturation of Purkinje cells. Developmental Brain Research. 1984;271:217–226. doi: 10.1016/0006-8993(83)90284-6. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. International Review in Neurobiology. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Nakamura J, Shinnock-Gallagher P. The effects of temperature, pH and Cl− pump inhibitors on GABA responses recorded from cat dorsal root ganglia. Brain Research. 1983;267:249–259. doi: 10.1016/0006-8993(83)90877-6. 10.1016/0006-8993(83)90877-6. [DOI] [PubMed] [Google Scholar]
- Gault LM, Siegel RE. Expression of the GABAA receptor δ subunit is selectively modulated by depolarisation in cultured rat cerebellar granule neurons. Journal of Neuroscience. 1997;17:2391–2399. doi: 10.1523/JNEUROSCI.17-07-02391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groul DL, Barker JL, Huang L-Y, MacDonald JF, Smith TG. Hydrogen ions have multiple effects on the excitability of cultured mammalian neurons. Brain Research. 1980;183:247–252. doi: 10.1016/0006-8993(80)90138-9. 10.1016/0006-8993(80)90138-9. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. 10.1016/0028-3908(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Progress in Neurobiology. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987;330:163–165. doi: 10.1038/330163a0. 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kilic G, Moran O, Cherubini E. Currents activated by GABA and their modulation by Zn2+ in cerebellar granule cells in culture. European Journal of Neuroscience. 1993;5:65–72. doi: 10.1111/j.1460-9568.1993.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acidA receptors α4β2γ2 and α6β2γ2. Molecular Pharmacology. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABAA receptor is associated with the receptor subunit composition. Journal of Physiology. 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. Journal of Neuroscience. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L. The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. Journal of Physiology. 1989;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L, Westbrook GL. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. Journal of Physiology. 1989;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K, Watano T, Koizumi S, Inoue K. Zinc potentiation of neurotransmission and inhibition of background cationic conductance in rat hippocampal neurons. Journal of Physiology. 1995;484:447–462. doi: 10.1113/jphysiol.1995.sp020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack M, Bountra C, Voipio J, Kaila K. Influence of extracellular and intracellular pH on GABA-gated chloride conductance in crayfish muscle fibres. Neuroscience. 1992;47:921–929. doi: 10.1016/0306-4522(92)90040-9. 10.1016/0306-4522(92)90040-9. [DOI] [PubMed] [Google Scholar]
- Pasternack M, Smirnov S, Kaila K. Proton modulation of functionally distinct GABAA receptors in acutely isolated pyramidal neurons of rat hippocampus. Neuropharmacology. 1996;35:1279–1288. doi: 10.1016/s0028-3908(96)00075-5. 10.1016/S0028-3908(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAA receptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42:497–507. doi: 10.1016/0306-4522(91)90392-2. 10.1016/0306-4522(91)90392-2. [DOI] [PubMed] [Google Scholar]
- Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-d-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: Recent advances in GABAA recepter research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: Role of the δ subunit. Journal of Neuroscience. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Molecular Pharmacology. 1996;49:567–579. [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacological Reviews. 1995;47:182–234. [PubMed] [Google Scholar]
- Smart TG. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. Journal of Physiology. 1992;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Constanti A. A novel effect of zinc on the lobster muscle GABA receptor. Proceedings of the Royal Society. 1982;B 215:327–341. doi: 10.1098/rspb.1982.0045. [DOI] [PubMed] [Google Scholar]
- Smart TG, Constanti A. Differential effect of zinc on the vertebrate GABAA-receptor complex. British Journal of Pharmacology. 1990;199:643–654. doi: 10.1111/j.1476-5381.1990.tb12984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. British Journal of Pharmacology. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Progress in Neurobiology. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Takeuchi N. Anion permeability of the inhibitory postsynaptic membrane of the crayfish neuromuscular junction. Journal of Physiology. 1967;191:575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonise NMDA and GABA responses of hippocampal neurones. Nature. 1987;328:640–643. doi: 10.1038/328640a0. 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- White G, Gurley DA. α subunits influence Zn block of γ2 containing GABAA receptor currents. NeuroReport. 1995;6:461–464. doi: 10.1097/00001756-199502000-00014. [DOI] [PubMed] [Google Scholar]
- Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmocology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. 10.1016/S0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- Xie X, Smart TG. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991;349:521–524. doi: 10.1038/349521a0. 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- Yakushiji T, Tokutomi N, Akaike N, Carpenter DO. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987;22:1123–1133. doi: 10.1016/0306-4522(87)92987-3. 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]


