Abstract
We examined the possibility of functional and molecular expression of volume-regulated Cl− channels in vascular smooth muscle using the whole-cell patch-clamp technique and quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) on cells from canine pulmonary and renal arteries.
Decreasing external osmolarity induced cell swelling, which was accompanied by activation of Cl−-dependent outward-rectifying membrane currents with an anion permeability sequence of SCN− > I− > Br− > Cl− > aspartate−. These currents were sensitive to block by DIDS, extracellular ATP and the antioestrogen compound tamoxifen.
Experiments were performed to determine whether the molecular form of the volume-regulated chloride channel (ClC-3) is expressed in pulmonary and renal arteries. Quantitative RT-PCR confirmed expression of ClC-3 in both types of smooth muscle. ClC-3 expression was 76.4% of β-actin in renal artery and 48.0% of β-actin in pulmonary artery.
We conclude that volume-regulated Cl− channels are expressed in vascular smooth muscle cells and exhibit functional properties similar to those found in other types of cells, presumably contributing to the regulation of cell volume, electrical activity and, possibly, myogenic tone.
Membrane stretch or increases in transmural pressure cause contraction of vascular smooth muscle cells, a response referred to as myogenic tone (Meininger & Davis, 1992). This response to mechanical force is accompanied by changes in the membrane conductance, the cytoskeleton and the activation or inactivation of a variety of second messenger systems (Osol, 1995). Early studies (Sparks, 1964; Uchida & Bohr, 1969; Harder, 1984) revealed that the myogenic response is associated with membrane depolarization and exhibits some dependence on extracellular Ca2+. Although modulation of large-conductance Ca2+-activated K+ channels (Brayden & Nelson, 1992) and stretch-activated non-selective cation channels (Davis, Donovitz & Hood, 1992) has been implicated in the myogenic response, the exact mechanisms responsible for the initial myogenic membrane depolarization are unknown.
It has recently been shown that pressure-induced depolarization and contraction of cerebral artery smooth muscle is inhibited by various Cl− channel blockers, suggesting that activation of Cl− channels may be responsible for myogenic membrane depolarization of these arteries (Nelson, Conway, Knot & Brayden, 1997). Although Ca2+-activated Cl− channels are known to be ubiquitous in smooth muscle (Large & Wang, 1996), the Cl− channels involved in the myogenic response were insensitive to niflumic acid, an effective blocker of Ca2+-activated Cl− channels, leading to speculation that these Cl− channels may be activated by membrane stretch, like those involved in cell volume regulation in many other tissues (Strange, Emma & Jackson, 1996). However, there is presently no direct evidence showing that volume-regulated Cl− channels are functionally or molecularly expressed in vascular smooth muscle.
In other tissues, these channels are known to possess many common biophysical and pharmacological properties, including activation by hypotonic solutions, outward rectification in symmetrical Cl− solutions, anion selectivity sequence of SCN− > I− > Br− > Cl− > F−, and sensitivity to block by hypertonic solutions, stilbene compounds, external nucleotides and the antioestrogen compound tamoxifen (Strange et al. 1996; Okada, 1997). Although three different proteins, P-glycoprotein (P-Gp; Valverde, Diaz, Sepúlveda, Gill, Hyde & Higgins, 1992), pIcln (Paulmichl, Li, Wickman, Ackerman, Peralta & Clapham, 1992) and ClC-2 (Gründer, Thiemann, Pusch & Jentsch, 1992), have been proposed as molecular candidates for volume-sensitive Cl− channels, recent data suggest that P-Gp and pIcln may instead be regulators of endogenous Cl− channels, and the properties of expressed ClC-2 channels fail to exhibit many of the characteristic properties of volume-sensitive Cl− currents (ICl,vol) found in native cells (Okada, 1997). We have recently identified another member of the ClC Cl− channel family, ClC-3, as the gene encoding ICl,vol in heart and many other mammalian cells (Duan, Winter, Cowley, Hume & Horowitz, 1997). We therefore tested for functional expression of ICl,vol in smooth muscle cells isolated from canine pulmonary and renal arteries using conventional patch-clamp techniques, and performed quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) to examine molecular expression of ClC-3.
METHODS
Electrophysiology and solutions
Mongrel dogs of either sex were anaesthetized with sodium pentobarbitone (45 mg kg−1, i.v.). Segments of renal and pulmonary artery were removed, and the dogs were then killed by an overdose of pentobarbitone. Single smooth muscle cells were prepared from the pulmonary and renal arteries as previously described (Post, Gelband & Hume, 1995; Gelband & Hume, 1995). Dissociated cells were placed in a chamber on the stage of an inverted microscope (TMS, Nikon, Japan) and superfused with an external solution at 1.5-2.0 ml min−1. In some experiments, cell diameter was continuously monitored using a video edge detector (Crescent Electronics, Salt Lake City, UT, USA). The standard isotonic bath solution contained (mm): 107 N-methyl-d-glucamine (NMG), 107 HCl, 1.5 MgCl2, 2.5 MnCl2, 10 glucose, 70 d-mannitol and 10 Hepes (pH 7.4, 300 mosmol (kg H2O)−1). d-Mannitol was removed from this solution to make standard hypotonic solution (230 mosmol (kg H2O)−1) and 140 mm d-mannitol was included to make standard hypertonic solution (370 mosmol (kg H2O)−1). GdCl3 (0.05 mm) was routinely included in all bath solutions to prevent the possible contamination of membrane currents by activation of non-selective cation channels. The corresponding whole-cell pipette solution contained (mm): 95 CsCl, 20 TEACl, 5 ATP-Mg, 5 EGTA, 80 d-mannitol and 5 Hepes (pH 7.2, 300 mosmol (kg H2O)−1).
In experiments to test Cl− dependency, Cl− in the hypotonic solution was replaced with equimolar aspartate (aspartate−) to make solutions containing different [Cl−]. The corresponding pipette solution contained the following (mm): 90 NMG, 90 aspartic acid, 24 TEACl, 5 ATP-Mg, 5 EGTA, 76 d-mannitol and 5 Hepes (pH 7.2, 300 mosmol (kg H2O)−1). In experiments to test anion selectivity, the bath solution contained (mm): 115 NaX, 10 glucose and 10 Hepes (pH 7.3-7.4, 230–240 mosmol (kg H2O)−1) where X− denotes SCN−, I−, F−, Cl− or aspartate−. The corresponding pipette solution was the same solution containing 95 mm CsCl as described above.
Membrane currents were recorded using the whole-cell variant of the patch-clamp technique (Hamill, Marty, Neher, Sakmann & Sigworth, 1981). Patch pipettes were made from borosilicate glass capillaries and had a tip resistance of 2–5 MΩ. Ag-AgCl wires were immersed in the bath and pipette solutions and connected to a patch-clamp amplifier (Axopatch-1D, Axon Instruments). A 3 m KCl-agar salt bridge between the bath and the Ag-AgCl reference electrode was used to minimize changes in liquid junctional potential during some experiments. To obtain Cl− current-voltage relations, whole-cell currents were recorded during voltage pulses (150 ms) applied from the holding potential (usually 0 mV) or during a depolarizing voltage ramp applied at a rate of 25 mV s−1 following a 1 s voltage step to −100 mV. Changes in junctional potentials created between the pipette and the different bath solutions were corrected for. Experiments were performed at 21–23°C and currents were filtered at a frequency of 1 kHz and digitized on-line at 5 kHz using an IBM-AT compatible computer and pCLAMP 5.5 software (Axon Instruments). Data were expressed as means ± s.e.m. (n, number of observations). Statistical analysis was by Student's paired t test; a value of P < 0.05 was considered to be statistically significant.
DIDS and tamoxifen were freshly dissolved as stock solutions in DMSO, the final concentration of which was < 0.1%, which, by itself, did not affect Cl− currents. All chemicals were purchased from Sigma (USA).
Molecular techniques
Total RNA was prepared from vascular smooth muscle of pulmonary and renal arteries by use of the SNAP Total RNA Isolation Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. First-strand RNA (1 μg) was reverse transcribed by use of an oligo (dT)12-18 primer (500 μg μl−1). Specific ClC-3 primers were used that anneal to sequences near the carboxy terminus of the amino acid sequence forward (nucleotides 1891–1911) and reverse (nucleotides 2130–2150) (GA#U83464). The housekeeping gene control sequence β-actin was amplified in parallel with ClC-3 by using a forward primer that anneals at nucleotides 2508–2527 and a reverse primer that anneals at nucleotides 3110–3127 (GA#V01217), and was then compared with ClC-3 expression. Sense and antisense primers (20 μm) were combined with cDNA and 1 mm deoxyribonucleoside triphosphates (dNTPs), 40 mm Tris-HCl (pH 8.3), 100 mm KCl, 3 units of Taq (Promega), 1 Ampliwax Gem 100 (Perkin Elmer) and RNase-free water to a final volume of 50 μl. PCR was performed in a COY II Thermal Cycler under the following conditions: 32 cycles at 94°C, 1 min; 57°C, 30 s; 72°C, 1 min; and then incubated at 72°C, 10 min. PCR products were separated by 2% agarose gel electrophoresis. Quantitative PCR (Q-PCR) was performed by use of the PCR MIMIC Construction Kit (Clontech, Palo Alto, CA, USA) which is based upon a competitive PCR approach - non-homologous engineered DNA standards (referred to as PCR MIMICs) compete with target DNA for the same gene-specific primers. PCR MIMICs were constructed for ClC-3 and the control standard (β-actin), and competitive PCR was carried out by titration of sample cDNA with known amounts of the desired non-homologous PCR MIMIC constructs; serial dilutions of these constructs were added to PCR amplification reactions. Following PCR, products were separated by 2% agarose gel electrophoresis and quantified by use of Molecular Analyst (Bio-Rad, Hercules, CA, USA). Experiments utilizing PCR to determine ClC-3 expression in vascular smooth muscle cells were performed on pulmonary and renal arteries from at least five different animals. For Q-PCR, the concentration of the target DNA was normalized to β-actin expression. The exact number of independent samples utilized for these experiments was determined empirically by SigmaStat (Jandel Scientific, San Rafael, CA, USA), which indicates the number of samples that need to be tested in order to detect statistical differences. Student's t test was then used to detect significant differences among a number of means and significance was suggested by P values < 0.05.
RESULTS
Hypotonicity induces cell swelling and outwardly-rectifying membrane currents in pulmonary arterial smooth muscle cells
Figure 1A illustrates an experiment which shows the relationship betweeen changes in pulmonary arterial cell width and membrane current recorded at −100, 0 and +100 mV, before and during superfusion of the cell with the standard hypotonic (230 mosmol (kg H2O)−1) solution. Cell width started to increase within 1 min of the cell being exposed to the hypotonic solution and continued to increase during the subsequent 15 min of recording. Membrane currents at −100 and +100 mV were almost negligible in the isotonic solution, but began to increase following a delay of some 3–4 min after changing to the hypotonic solution. Figure 1B shows raw current traces evoked by step pulses, which developed during exposure to hypotonic solution and were completely abolished by a 10 min perfusion with a hypertonic solution.
Figure 1. Volume-regulated currents in canine pulmonary arterial smooth muscle cell.
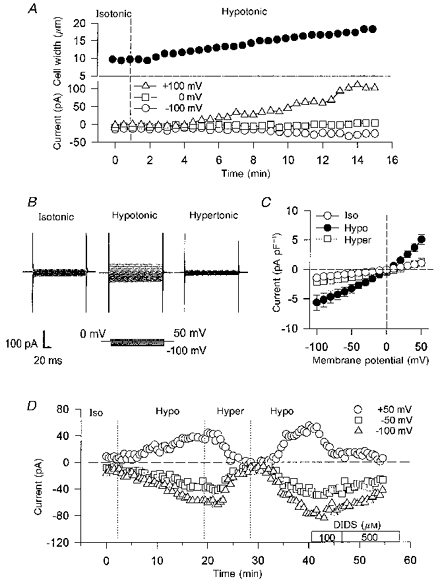
A, simultaneous measurement of cell width and the membrane currents induced by a hypotonic solution. B, raw membrane currents activated during 150 ms voltage steps from 0 mV to potentials ranging from −100 to 50 mV. The cell was first equilibrated with the isotonic solution, then exposed to the hypotonic solution. Thereafter, the hypertonic solution was applied. C, current-voltage relations for volume-regulated currents in the isotonic, hypotonic and hypertonic solutions with 115 mm [Cl−]o and 115 mm [Cl−]i (n = 4). D, time courses of membrane currents at +50, −50 and −100 mV in isotonic, hypotonic, hypertonic and hypotonic + DIDS (100 and 500 μm) solutions.
Figure 1C is a plot of the current-voltage relations obtained from several cells in solutions with different osmolarities. In these experiments, both bath and pipette solutions contained 115 mm Cl−, and the currents activated during exposure to hypotonic solution exhibited clear outward rectification; the mean difference current was −2.53 ± 0.70 pA pF−1 at −50 mV (n = 4) and 4.03 ± 0.85 pA pF−1 at 50 mV (n = 4). The reversal potential was approximately 0 mV, which is the predicted equilibrium potential of Cl− (0 mV). The hypotonically activated currents were reduced by subsequent exposure to hypertonic solutions at each membrane potential. Figure 1D shows that the hypotonically activated currents were reversed by exposure to hypertonic solutions, and could be reactivated by subsequent exposure to hypotonic solutions. Membrane currents activated during exposure to hypotonic solutions were also markedly inhibited by the stilbene compound, DIDS.
Cl− dependence and anion selectivity
In order to examine Cl− dependence, the reversal potentials (Vrev) for the volume-sensitive currents were measured using either voltage steps or ramps in hypotonic solutions containing six different concentrations of external Cl− ([Cl−]o), replaced with aspartate−. As shown in Fig. 2A, reducing [Cl−]o from 115 to 28 and 9 mm shifted Vrev rightward, indicating a strong Cl− dependence of the volume-sensitive conductance. The inset (Fig. 2A) shows the relationship between [Cl−]o and Vrev of the volume-sensitive conductance obtained from a number of pulmonary cells. The straight line represents a theoretical slope of 57 mV per 10-fold decrease in [Cl−]o, which is predicted from the Nernst equation assuming that Cl− is the only permeable ion. The slope of the relationship measured experimentally closely followed the predicted slope of 57 mV per 10-fold change in [Cl−]o for changes in [Cl−]o > 40 mm, but deviated from the predicted slope at [Cl−]o < 40 mm, suggesting that the replacement anion aspartate− may exhibit some limited permeability through these channels.
Figure 2. Effect of [Cl−]o, anion substitution, external ATP and tamoxifen on current-voltage relations for the volume-regulated currents elicited in canine pulmonary arterial smooth muscle cells by voltage ramps.
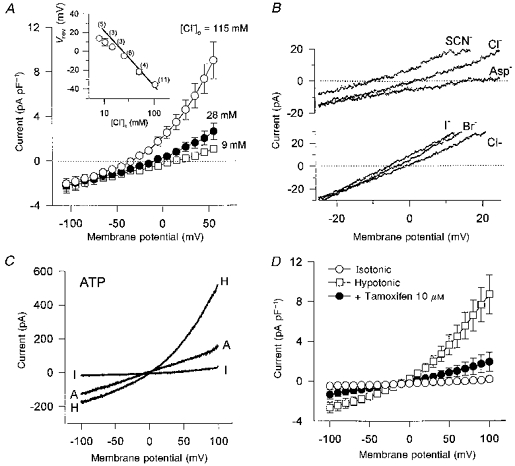
In A, the pipette solution contained 24 mm Cl− (n = 5). Inset, relation between the reversal potential (Vrev) and [Cl−]o. Each circle indicates the mean value of Vrev with ±s.e.m. from n observations (indicated in parentheses). The straight line indicates the theoretical slope of 57 mV per 10-fold decrease in [Cl−]o, which is predicted from the Nernst equation. In B, NaCl (115 mm) in the bath solution was replaced entirely by the same concentration of NaI, NaBr, NaSCN or sodium aspartate. The pipette solution contained 115 mm Cl−. C, typical currents obtained with voltage ramps in the isotonic solution (I), hypotonic solution (H) and hypotonic solution including ATP (A, 10 mm). D, effect of tamoxifen (10 μm) on hypotonic-induced membrane currents (n = 4).
Relative anion selectivity was determined by total replacement of Cl− with other anions in the hypotonic solutions. Figure 2B shows typical hypotonically activated membrane currents elicited in a pulmonary cell by voltage ramps applied in the presence of different extracellular anions. I− appeared to be slightly more permeable than Br−, which was more permeable than Cl− in this example. Likewise, SCN− appeared to be more permeable than Cl−, which was more permeable than aspartate−. The mean data accumulated from a group of cells gave Vrev values (in mV) of −8.29 ± 0.92 (n = 7), −4.67 ± 1.49 (n = 12), −3.30 ± 1.16 (n = 10), 0.46 ± 1.01 (n = 13) and 12.40 ± 1.69 (n = 5) for SCN−, I−, Br−, Cl− and aspartate−, respectively. Accordingly, the relative permeability for each anion (X−) to Cl− was estimated using the Goldman-Hodgkin-Katz equation. The sequence of was SCN− (1.36 ± 0.06) > I− (1.19 ± 0.06) > Br− (1.09 ± 0.04) > Cl− (1.00) > aspartate− (0.63 ± 0.05). These data indicate that the membrane currents activated by hypotonic cell swelling in pulmonary arterial smooth muscle cells can be identified as ICl,vol.
Pharmacology
The effects of DIDS, the antioestrogen compound tamoxifen, and external ATP were examined on ICl,vol in canine pulmonary smooth muscle cells using both voltage steps and voltage ramps (Figs 1D, 2C and D). DIDS (100 μm), ATP (10 mm) and tamoxifen (10 μm) inhibited ICl,vol, especially the outward currents, at positive potentials. At 100 mV, DIDS, ATP and tamoxifen significantly inhibited ICl,vol by 78.7 ± 17.1% (P < 0.05, n = 5), 62.4 ± 6.5% (P < 0.05, n = 4) and 79.0 ± 11.5% (P < 0.05, n = 4), respectively, whereas less potent effects of these compounds were observed at −100 mV (25.8 ± 14.8% (n.s., n = 5), 4.6 ± 11.1% (n.s., n = 4) and 53.2 ± 27.3% (n.s., n = 4), respectively).
ICl,vol in renal arterial smooth muscle cells
In order to examine whether other types of vascular smooth muscle cells might also express ICl,vol, we examined the effects of standard hypotonic solutions on membrane currents in canine renal arterial smooth muscle cells. As shown in Fig. 3A, similar volume-regulated currents were evoked by hypotonic solutions in these cells, and were completely abolished by a 10 min perfusion with a standard hypertonic solution. The hypotonically activated currents also exhibited outward rectification, with a Vrev near 0 mV, the predicted equilibrium potential for Cl− (0 mV) (Fig. 3B). The mean difference currents obtained (between isotonic and hypotonic solutions) were −0.29 ± 0.14 pA pF−1 at −50 mV (n = 3) and 1.05 ± 0.47 pA pF−1 at 50 mV (n = 3). In hypertonic solutions, the currents were reduced at each membrane potential. Figure 3C shows the Cl− dependency of the hypotonically activated currents in renal arterial myocytes. Reducing [Cl−]o from 115 to 55 and then to 11.5 mm shifted Vrev in a positive direction, suggesting a strong dependence on the Cl− gradient. The inset shows the relationship between [Cl−]o and Vrev of the volume-sensitive conductance in renal cells. The relationship closely followed the theoretical slope predicted from the Nernst equation (continuous line) assuming Cl− as the only permeant ion. Figure 3D shows the sensitivity of ICl,vol in renal arterial cells to DIDS. In the presence of 100 μm DIDS, ICl,vol at 100 mV was inhibited by 69.4 ± 6.2% (P < 0.05, n = 4), whereas block at −100 mV was less (47.1 ± 22.9%).
Figure 3. Volume-regulated Cl− currents in canine renal arterial smooth muscle cells.
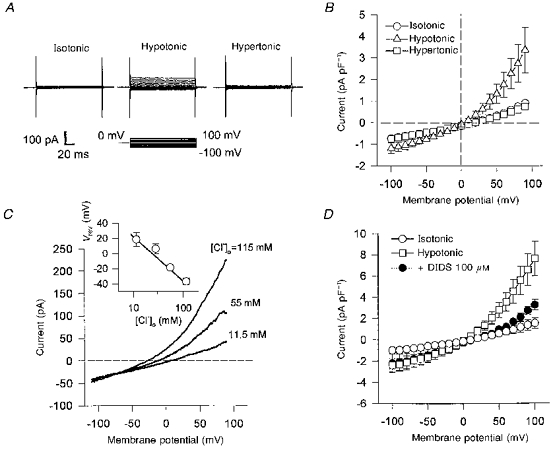
A, representative membrane currents in isotonic, hypotonic and hypertonic solutions. The currents were activated during 150 ms voltage steps from 0 mV to potentials ranging from −100 to 100 mV. B, current-voltage relations for volume-regulated currents in the isotonic, hypotonic and hypertonic solutions with 115 mm [Cl−]o and 115 mm [Cl−]i (n = 3). C, representative membrane currents elicited by voltage ramps with different [Cl−]o; the pipette solution contained 24 mm Cl−. Inset, relation between Vrev and [Cl−]o. The straight line represents the theoretical slope of 57 mV per 10-fold change in [Cl−]o predicted from the Nernst equation. D, effect of DIDS (100 μm) on hypotonically induced Cl− currents (n = 4).
A comparison of current densities of ICl,vol activated by the standard hypotonic solutions (230 mosmol (kg H2O)−1) in canine renal and pulmonary arterial cells revealed no significant differences between the two cell types. ICl,vol was 2.47 ± 1.0 pA pF−1 (n = 8) and 2.98 ± 0.3 pA pF−1 (n = 24) at 50 mV in canine renal and pulmonary arterial smooth muscle cells, respectively.
Molecular expression of ClC-3 in canine renal and pulmonary arteries
ClC-3 has recently been identified as the gene encoding ICl,vol in heart (Duan et al. 1997) and has been shown to be expressed in a wide variety of other tissues, including kidney, brain, lung and adrenal gland (Kawasaki et al. 1994). Whether ClC-3 is expressed in smooth muscle has not yet been examined. We used quantitative RT-PCR to test for molecular expression of ClC-3 in canine pulmonary and renal smooth muscle. Primers were designed to be specific for ClC-3 and do not cross-hybridize with other members of the ClC gene family. The competitive ‘mimic’ strategy of quantitative PCR was employed (Ariazi & Gould, 1996). As shown in Fig. 4, quantitative RT-PCR detected significant levels of ClC-3 transcriptional expression from pulmonary and renal arteries which were significantly different from each other (P < 0.05, n = 3). A representative gel used in digital analysis and comparison of mimic and ClC-3 specific amplification is shown in Fig. 4A. Digital analysis and comparison of mimic and ClC-3 specific amplification products was performed on the 10−4 dilution of mimic DNA and repeated on three independently generated samples. ClC-3 expression was 76.4% of β-actin in renal artery and 48.0% of β-actin in pulmonary artery.
Figure 4. Competitive PCR products resolved on 2% ethidium bromide agarose gels.
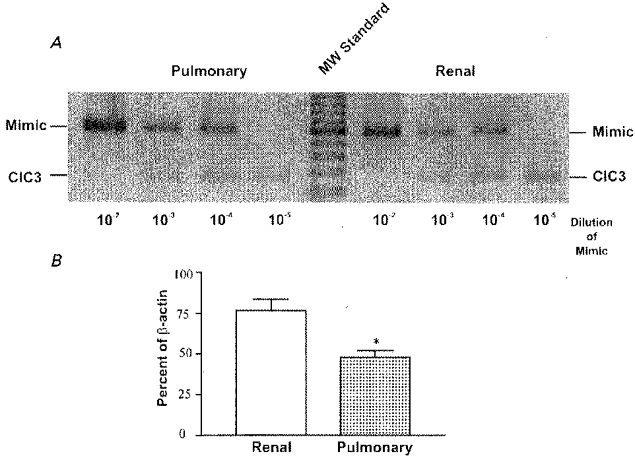
A representative gel of quantitative RT-PCR for ClC-3 in canine pulmonary and renal arteries is shown in A; 10-fold serial dilutions of mimic DNA were included in the PCR reactions while target cDNA (ClC-3) concentration remained constant. The actual concentrations of target cDNA were calculated and expressed as the percentage of β-actin RNA concentration (B). *Significant difference compared with ClC-3 transcripts detected in renal artery preparations, P < 0.05. Results are expressed as means ± s.e.m.
DISCUSSION
The present study provides evidence that volume-regulated Cl− channels functionally exist in canine pulmonary and renal arterial smooth muscle cells. Under conditions where Na+, K+, Ca2+ and non-specific cation currents were inhibited, cell swelling induced by exposure to hypotonic solutions was accompanied by an increase in membrane Cl− conductance. This hypotonically activated Cl− conductance exhibited outward rectification with a symmetrical Cl− gradient, a common biophysical characteristic of volume-regulated Cl− channels in many cells (Strange et al. 1996). The relative anion permeability sequence for ICl,vol of SCN− > I− > Br− > Cl− > aspartate−, and sensitivity to block by DIDS, ATP and tamoxifen are also consistent with properties previously characterized for ICl,vol in many native cells, as well as in the recently cloned volume-sensitive Cl− channel, ClC-3 (Kubo & Okada, 1992; Tseng, 1992; Vandenberg, Yoshida, Kirk & Powell, 1994; Ehring, Osipchuk & Cahalan, 1994; Jackson, Morrison & Strange, 1994; Yamazaki & Hume, 1997; Duan et al. 1997).
Consistent with our recent study in cardiac myocytes (Duan et al. 1997), the present molecular experiments demonstrate significant expression of the ClC-3 gene product in renal and pulmonary arteries, and previous reports have demonstrated widespread expression of this chloride channel in many tissues (Kawasaki et al. 1994). Although our estimates of membrane current densities for ICl,vol in canine renal and pulmonary arterial cells revealed no significant differences, quantitative RT-PCR revealed significantly higher levels of transcriptional expression of ClC-3 in renal compared with pulmonary smooth muscle. A number of factors might explain this apparent discrepancy. The molecular experiments provide information on mRNA expression levels, whereas the electrophysiological estimates only provide information on the density of functional channels. It is conceivable that the relatively mild stimulus (23% hypotonic) used to activate ICl,vol in the electrophysiological experiments was insufficient to reveal actual differences in channel density, or that the volume sensitivity of the channels in the two cell types may be different. While our previous report (Duan et al. 1997) leaves little doubt that ClC-3 encodes a volume-sensitive Cl− channel, and the present study clearly demonstrates ClC-3 expression in renal and pulmonary smooth muscle, further studies are required to unequivocally prove that ClC-3 alone is responsible for ICl,vol in these cells. It may be that this channel, in combination with another member of the ClC family, may form heterodimers (Forenz, Pusch & Jentsch, 1996), which may in fact encode ICl,vol in many native smooth muscle cells.
Acknowledgments
The authors would like to thank Rebecca Walker for technical assistance with the molecular expression of ClC-3 experiments. This work waas supported by the Canadian MRC (D. D.) and NIH grant HL 49254 (J. R. H. and B. H.).
References
- Ariazi EA, Gould MN. Identifying differential gene expression in monoterpene-treated mammary carcinomas using subtractive display. Journal of Biological Chemistry. 1996;271:29286–29294. doi: 10.1074/jbc.271.46.29286. 10.1074/jbc.271.46.29286. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. American Journal of Physiology. 1992;262:C1083–1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Ehring GR, Osipchuk YV, Cahalan MD. Swelling-activated chloride channels in multidrug-sensitive and -resistant cells. Journal of General Physiology. 1994;104:1129–1161. doi: 10.1085/jgp.104.6.1129. 10.1085/jgp.104.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forenz C, Pusch M, Jentsch TJ. Heteromultimeric ClC chloride channels with novel properties. Proceedings of the National Academy of Sciences of the USA. 1996;93:13362–13366. doi: 10.1073/pnas.93.23.13362. 10.1073/pnas.93.23.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine renal artery: novel mechanism for agonist-induced membrane depolarization. Circulation Research. 1995;77:121–130. doi: 10.1161/01.res.77.1.121. [DOI] [PubMed] [Google Scholar]
- Gründer S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell free patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circulation Research. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. American Journal of Physiology. 1994;267:C1203–1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994;12:597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Kubo M, Okada Y. Volume-regulatory Cl− channel currents in cultured human epithelial cells. Journal of Physiology. 1992;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. American Journal of Physiology. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. American Journal of Physiology. 1992;263:H647–659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- Nelson M, Conway MA, Knot HJ, Brayden JE. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. Journal of Physiology. 1997;502:259–264. doi: 10.1111/j.1469-7793.1997.259bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Osol G. Mechanotransduction by vascular smooth muscle. Journal of Vacular Research. 1995;32:275–292. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- Paulmichl M, Li Y, Wickman K, Ackerman M, Peralta E, Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992;356:238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery: novel mechanism for hypoxic-induced membrane depolarization. Circulation Research. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- Sparks HV. Effect of quick stretch on isolated vascular smooth muscle. Circulation Research. 1964;14/15(suppl. 1):I254–260. [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Tseng G-N. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. American Journal of Physiology. 1992;262:C1056–1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]
- Uchida E, Bohr DF. Myogenic tone in isolated perfused resistance vessels from rats. American Journal of Physiology. 1969;216:1343–1350. doi: 10.1152/ajplegacy.1969.216.6.1343. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Diaz M, Sepúlveda FV, Gill DR, Hyde SC, Higgins CF. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992;355:830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Yoshida A, Kirk K, Powell T. Swelling-activated and isoprenaline-activated chloride currents in guinea pig cardiac myocytes have distinct electrophysiology and pharmacology. Journal of General Physiology. 1994;104:997–1017. doi: 10.1085/jgp.104.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J, Hume JR. Inhibitory effects of glibenclamide on cystic fibrosis transmembrane regulator, swelling-activated, and Ca2+-activated Cl− channels in mammalian cardiac myocytes. Circulation Research. 1997;81:101–109. doi: 10.1161/01.res.81.1.101. [DOI] [PubMed] [Google Scholar]


