Abstract
Luminal membrane vesicles (LMV) were isolated from human and pig colonic tissues. They were characterized in terms of purity and ability to transport [14C]butyrate.
The activity of cysteine-sensitive alkaline phosphatase, and the abundance of villin, NHE2 and NHE3 proteins, markers of the colonic luminal membrane, were significantly enriched in the LMV compared with the original cellular homogenate. The LMV were free from contamination by other cellular organelles and basolateral membranes, as revealed by the negligible presence of either specific marker enzyme activity or characteristic immunogenic protein.
The transport of butyrate into the luminal membrane vesicles was enhanced 5-fold at pH 5.5 compared with pH 8.0. Butyrate transport was temperature dependent, and was stimulated in the presence of an outward-directed anion gradient in the order of butyrate > bicarbonate > propionate > chloride. Kinetic analysis of increasing substrate concentration showed saturation kinetics with an apparent Km value of 14.8 ± 3.6 mm and a Vmax of 54 ± 14 nmol min−1 (mg protein)−1.
Butyrate transport was significantly reduced in the presence of short chain fatty acids (SCFA), acetate, propionate and other monocarboxylates (pyruvate and l-lactate). Butyrate uptake was inhibited by several cysteine group modifying reagents such as p-chloromercuribenzosulphonic acid (pCMBS), p-chloromercuribenzoate (pCMB), mersalyl acid and HgCl2, but not by the stilbene anion exchange inhibitors, 4,4′-diisothiocyanostilbene-2,2′-disulphonate (DIDS) and 4,4′-dinitrostilbene-2,2′-disulphonate (SITS).
The described properties of butyrate transport across the luminal pole of the colon suggest the involvement of a carrier protein, in the form of a pH-activated anion exchange process. The transporter is distinct from the erythrocyte band-3 type anion exchanger and may belong to the monocarboxylate-type transport proteins (MCT1).
Short chain fatty acids (SCFA; acetate, propionate and butyrate) are the major anions in the colonic lumen. They are produced as a result of the fermentation of dietary fibre by microflora in the lumen of the large intestine (Cummings, 1984) and are rapidly absorbed (Ruppin, Bar-Meir, Soergel, Wood & Schmitt, 1980; Hatch, 1987; Bergman, 1990). Normal colonic epithelia derive 60–70% of their energy supply from SCFA, particularly butyrate (Scheppach et al. 1992). Butyrate induces cell differentiation, and regulates the growth and proliferation of normal colonic mucosa (Treem, Ahsan, Shoup & Hyams, 1994), and it reduces the growth rate of colorectal cancer cells in culture (Berry & Paraskeva, 1988). Despite the important role of SCFA in the maintenance of colonic health, the detailed molecular mechanism(s) by which SCFA interact with the colonic mucosa is not known. A number of studies have been performed in recent years aimed at identifying the mechanism of SCFA absorption across the colonic luminal membranes using either: (a) isolated membrane vesicles (Stein, Schröder, Milovic & Caspary, 1995; Harig, Ng, Dudeja, Brasitus & Ramaswamy, 1996) or (b) flux studies with whole epithelial preparations (Holtug, Rasmussen & Mortensen, 1992; Engelhardt, Gros, Burmester, Hansen, Becker & Rechkemmer, 1994) or (c) in vivo perfusion experiments (McNeil, Cummings & James, 1978; Holtug, Hove & Mortensen, 1995). Whilst the existence of a specific transport system has been implicated, there is no common consent regarding the mechanism(s) involved in the movement of butyrate across the colonic luminal membrane. Some workers have proposed an anion exchange process (Mascolo, Rajendran & Binder, 1991; Engelhardt et al. 1994), whilst others have suggested the absorption of the protonated acid across the intact epithelia as mechanisms for butyrate transport (Engelhardt, Burmester, Hansen, Becker & Rechkemmer, 1993; Sellin, DeSoignie & Burlingame, 1993).
In this paper we describe the properties of butyrate transport in purified and well characterized human and pig colonic luminal membrane vesicles (LMV). Membrane vesicles are particularly suitable for this study where the substrate, i.e. SCFA is rapidly metabolized by the colonic tissue. We report that the transport of butyrate across the luminal pole of human and pig colon is via a pH-activated anion exchange process. The elucidation of the mechanism of SCFA (butyrate) transport in the colon, and the information on the molecular structure of this transporter would facilitate the identification of the molecular and cellular mechanisms by which butyrate interacts with the colonic epithelia in healthy and diseased tissue.
METHODS
Materials: [U-14C]butyrate was purchased from ARC Inc. (St Louis, MO, USA; specific activity 55 mCi mmol−1 (2.04 GBq mmol−1)). Antibody to villin was purchased from The Binding Site (Birmingham, UK). Polyclonal antibodies against the NHE2 and NHE3 proteins were the kind gift of Dr M. Donowitz (Johns Hopkins University, Baltimore, MD, USA). Polyclonal antibody against lamb kidney Na+-K+-ATPase was kindly donated by Dr W. J. Ball (University of Cincinnati, Cincinnati, OH, USA). Antibody to erythrocyte band-3 was the gift of Dr R. Kopito (Stanford University, Palo Alto, CA, USA). All chemicals used were of highest analytical grade and either purchased from Sigma or Fisher Scientific (Loughborough, UK) unless otherwise noted.
Removal and storage of tissue
Pig colonic tissues were obtained from the abattoir within 10 min of slaughter of the animals. The tissue was divided into three parts, proximal, mid and distal. They were washed in ice cold 0.9% (w/v) NaCl (pH 7.0), and everted on a brass rod. They were blotted with a paper towel to remove excess mucus. The mucosa from each region was scraped off using a glass slide and the scrapings were immediately frozen in liquid N2. They were subsequently stored at −80°C until use. Pieces of histologically normal human proximal or distal colon were obtained from individuals in the course of surgery for conditions involving other parts of the gastrointestinal tract. Human colonic tissues were also obtained from three organ donors, none having a history of intestinal disease. After removal, tissues were divided into three parts, proximal, mid and distal. The tissues were immediately rinsed with 0.9% (w/v) NaCl (pH 7.0), wrapped in aluminium foil, rapidly frozen in liquid N2 and stored at −80°C until use. On the day of the experiment, the colonic tissue was thawed out in a hypotonic buffer containing protease inhibitors (100 mm mannitol, 2 mm Hepes-Tris (pH 7.1), 0.2 mm benzamidine, 0.2 mm phenylmethanesulphonic fluoride) and cut open longitudinally. The mucosa was scraped off and LMV were prepared as outlined below. Prior histological examination of the pig and human tissues showed that the colonocytes were intact and attached to the muscularis mucosa. Initial studies indicated that the mechanism and properties of butyrate transport were identical in proximal, mid and distal regions of either human or pig colon. Therefore, in the studies reported in this paper, mucosal scrapings from all three regions were pooled and used as the starting cellular homogenate. Pig colonic tissue has been used in experiments described throughout this paper. Human tissue has been used in a selective manner due to a limited supply. Tissues used in the experiments are indicated in the text.
For the work with the human tissue the approval of the Broadgreen Hospital NHS Trust, East Dyfed and South Sheffield Ethical Committees were obtained.
Preparation of colonic luminal membrane vesicles
The method previously described by Harig and colleagues (Harig, Dudeja, Knaup, Shoshara, Ramaswamy & Brasitus, 1990) was used with some modifications based on the technique described by Shirazi-Beechey et al. (1990). The mucosal scrapings from the entire colon were thawed in a buffer consisting of 100 mm mannitol, 2 mm Hepes-Tris (pH 7.1), 0.2 mm benzamidine and 0.2 mm phenylmethanesulphonic fluoride, at 1 g scrapings per 10 ml buffer. The scrapings were homogenized using a Polytron (Ystral Polytron, Scientific Instruments, Cambridge, UK) at setting 4 for 90 s and the homogenate was filtered through nylon gauze to remove fat and mucus. Magnesium chloride was added to give a final concentration of 10 mm and the suspension was stirred for 20 min, on ice. The suspension was centrifuged at 1500 g to remove large cell debris. The supernatant was filtered again through nylon gauze, to remove trace amounts of fat and mucus. The filtrate was centrifuged at 39 000 g for 25 min to sediment the luminal membranes. The pellet was resuspended in a buffer containing 100 mm mannitol, 20 mm Hepes-Tris (pH 7.5), 0.1 mm MgSO4, and homogenized using a hand held Potter/Elvejhem tissue homogenizer (Jencous Scientific, Bedfordshire, UK) with 40 strokes of a tight fitting Teflon pestle. The homogenate was centrifuged at 39 000 g for 35 min. The resulting pellet, including the outer fluffy layer, was resuspended in a minimal volume (300–600 μl) of a final buffer (given in figure legends) and homogenized by passing through a 27 gauge needle several times. The vesicles were stored in aliquots of 50–100 μl in liquid N2 until used.
Estimation of protein
Protein was determined by the ability to bind Coomassie Blue according to the BioRad assay technique. Bovine gamma globulin (30–150 μg) was used as a standard (Tarpey, Wood, Shirazi-Beechey & Beechey, 1995).
Assay of marker enzymes
All enzyme assays were conducted at 37°C. Succinate dehydrogenase, a marker for mitochondria, was assayed by the method of Pennington (1961). The activity of α-mannosidase, a marker for the Golgi apparatus, was determined by the method of Tulsiani, Opheim & Touster (1977). The activity of Tris-resistant α-glucosidase, a marker for endoplasmic reticulum, was assessed as outlined by Peters (1976) and Na+-K+-ATPase, an indicator for the basolateral membrane, was measured as described by Forbush (1983). For cysteine-sensitive alkaline phosphatase, a marker for luminal membranes, the method described by Brasitus & Keresztes (1984) was used.
Polyacrylamide gel electrophoresis and Western blotting
Gel electrophoresis and electrotransfer were carried out using the BioRad Mini Protean II gel apparatus (BioRad, Watford, UK) as described previously (Pinches, Gribble, Beechey, Ellis, Shaw & Shirazi-Beechey, 1993). Membrane proteins were separated by SDS-PAGE on 8% (w/v) polyacrylamide gels containing 0.1% (w/v) SDS, and were electrotransferred to nitrocellulose membranes. The non-specific protein-binding sites on the nitrocellulose membrane used for immunodetection of villin, NHE2 and NHE3 were blocked by immersion of the membrane in phosphate-buffered saline-tween (PBS-TM; PBS, 2.0% (w/v) non-fat dry milk (Oxoid); 0.05% (w/v) Tween 20) for 1 h. The nitrocellulose membranes were incubated with the respective primary antibodies for 2 h at a dilution of 1 : 2000 in PBS-TM. Immunodetection of Na+-K+-ATPase was carried out as described by Pinches et al. (1993), and for erythrocyte anion exchanger band-3 as reported by Thomas, Machen, Smolka, Baron & Kopito (1989). Secondary antibody conjugated to horseradish peroxidase raised in rabbit was used for immunodetection of NHE2, NHE3, band-3, and Na+-K+-ATPase; and raised in mouse (Dako AC, Denmark) for villin detection. Secondary antibody was used at a dilution of 1 : 2000 using the respective buffers. The immunoblots were developed by Enhanced Chemiluminescense (ECL) using a commercially available kit (Amersham International, UK) following the manufacturer's instructions. The abundance of each band was determined by scanning densitometry (Phoretix).
Butyrate uptake
Transport of butyrate was measured using a rapid filtration stop technique as described by Shirazi, Beechey & Butterworth (1981) with some modifications. Incubation buffer for uptake varied according to the conditions tested. The composition of buffers used is given in the figure legends. The stop solution consisted of 100 mm mannitol, 100 mm sodium gluconate, 20 mm Hepes-Tris (pH 7.5) and 1 mm sodium butyrate. For the kinetic analysis, butyrate concentrations in the incubation medium was varied from 0.5 to 50 mm. Osmolarity of the medium was adjusted by changing the concentration of mannitol in the medium.
RESULTS
Characterization of colonic LMV
The purity of the isolated colonic LMV were assessed using marker enzyme analysis and Western blotting.
Marker enzyme analysis
In order to determine any potential contamination of LMV by cellular organelles and basolateral membranes, the activity of marker enzymes, indicators of the endoplasmic reticulum, Golgi, mitochondria and the basolateral membrane were determined in LMV isolated from pig colon (see Table 1). In human colonic LMV, the activity of markers of the basolateral membrane and mitochondria was assessed. As shown in Table 1, the enrichment (1.5- to 2.6-fold) and recovery (2.7-4.2%) of these organelle markers in human and pig colonic LMV were low. Similarly, the enrichment (0.5%) and recovery (0.5%) of Na+-K+-ATPase, the classical marker of the basolateral membrane, were negligible in LMV isolated either from human or pig colon (see Table 1). In contrast, the activity of cysteine-sensitive alkaline phosphatase, a marker of the colonic luminal membrane (Vengesa & Hopfer, 1979; Harig et al. 1990) was enriched 10- to 17-fold with the recovery of 25% in the LMV isolated from pig and human colon compared with the original cellular homogenate.
Table 1.
Activities of marker enzymes in the luminal membrane vesicles isolated from pig and human colon
| Tissue source | Enzyme | Specific activity (nmol min−1 (mg protein)−1) | Enrichment1 | Recovery2 (%) |
|---|---|---|---|---|
| Pig | Cysteine-sensitive alkaline phosphatase | 11.0 ± 2 | 10.0 ± 3.0 | 22.0 |
| α-Mannosidase | 2.6 ± 0.6 | 1.5 ± 0.5 | 2.2 | |
| Tris-resistant α-glucosidase | 1.8 ± 0.3 | 2.2 ± 0.9 | 1.5 | |
| Succinate dehydrogenase | 4.7 ± 1 | 1.4 ± 2.1 | 4.2 | |
| Pig/human | Na+-K+-ATPase | 11.0 ± 2.5 | 0.5 ± 1.0 | 0.5 |
| Human | Cysteine-sensitive alkaline phosphatase | 6.7 ± 3.0 | 16.8 ± 3.0 | 24.5 |
| Succinate dehydrogenase | 1.1 ± 1 | 1.5 ± 0.1 | 2.7 |
LMV were isolated as described in Methods. All enzyme assays were carried out in triplicate and the activities are expressed as means ± s.e.m. for 3 experiments.
Enrichment is the ratio of the specific activity of the enzyme in the vesicle fraction over the specific activity present in the homogenate.
Recovery is the total activity of the enzyme in the vesicle fraction as a percentage of the total activity present in the homogenate.
Immunodetection of colonic luminal membrane markers
Antibodies to specific proteins characteristic of the luminal membrane of the colon were used to determine the enrichment of these markers in the vesicle fraction over the starting homogenate. Samples of colonic cellular homogenate and LMV were separated by SDS-PAGE and electrotransferred to nitrocellulose membranes. Villin is a major structural protein of the microvilli of small and large intestine with an apparent molecular mass of 95 kDa (Bretscher & Weber, 1979; Robine et al. 1985). A monoclonal antibody to villin cross-reacted specifically with a protein migrating at 95 kDa in pig and human colonic LMV (Fig. 1). The total abundance of villin in LMV isolated from either human or pig colon, as measured by scanning densitometry (Phoretix), was 15-fold over the levels determined in the homogenate.
Figure 1. Immunodetection of villin in pig and human colonic LMV.
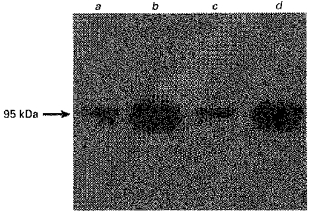
LMV were dissolved in sample buffer containing SDS. Samples (25 μg of protein each) were seperated by 8% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Immunoblotting was carried out as described in Methods. Lane a, pig colonic homogenate; lane b, pig colonic LMV; lane c, human colonic homogenate; lane d, human colonic LMV.
The Na+-H+ antiporter isoforms, NHE2 and NHE3, have been shown to be located on the luminal membrane of the absorptive epithelial cells of the colon and are appropriate indicators of the colonic luminal membrane (Hoogerwerf et al. 1996). Polyclonal antibodies to these two isoforms were used to further characterize the LMV used in these studies. Figure 2A and B, typical Western blots, show cross-reactions of the antibodies to NHE2 and NHE3 with proteins of 85 and 75 kDa molecular mass in human and pig colonic LMV. These are the reported molecular mass of NHE2 and NHE3, respectively (Hoogerwerf et al. 1996). The intensity of signals in LMV for NHE2 was 4-fold and for NHE3 6-fold over the levels detected in the original homogenate.
Figure 2. Immunodetection of NHE2 and NHE3 in pig and human colonic LMV.
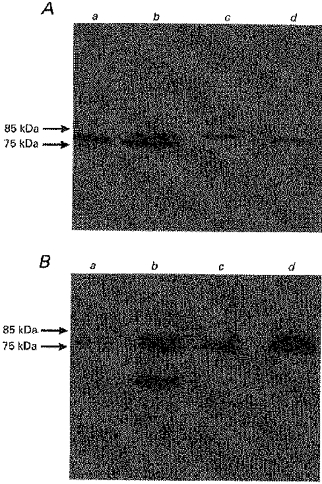
Colonic LMV and homogenate (25 μg of protein each) were separated by 8% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Immunoblotting was carried out as described in Methods. A, immunodetection of NHE2; B, immunodetection of NHE3. Lane a, pig colonic homogenate; lane b, pig colonic LMV; lane c, human colonic homogenate; lane d, human colonic LMV.
An antibody to Na+-K+-ATPase, an appropriate marker of the basolateral membrane, was used to determine any potential contamination of LMV isolated either from human or pig colon with basolateral membranes. Basolateral membrane vesicles (BLMV) were prepared from pig and human colonic tissues according to the procedure we described before (Pinches et al. 1993) and were used as a positive control. Therefore, by using two independent approaches: (1) determining the levels of activity of Na+-K+-ATPase in the LMV isolated from either human or pig colon (see Table 1) and (2) assessing the abundance of Na+-K+-ATPase in the same LMV fractions (Fig. 3), we have obtained very similar data confirming negligible presence of colonic BLMV in LMV fractions.
Table 2.
Effect of various intravesicular anions on butyrate transport
| Intravesicular anion | Uptake (pmol (mg protein)−1 (5 s)−1) |
|---|---|
| Sodium butyrate | 955 ± 90 |
| Sodium propionate | 755 ± 53 |
| Sodium acetate | 780 ± 35 |
| NaHCO3 | 943 ± 70 |
| NaCl | 615 ± 70 |
| Sodium gluconate | 360 ± 13 |
| No anion (mannitol) | 390 ± 120 |
LMV were preloaded with either 300 mm mannitol or with 100 mm mannitol and 100 mm of the following Na+ salts (butyrate, propionate, acetate, HCO3−, Cl−, gluconate), 20 mm Hepes-Tris (pH 7.5), and 0.1 mm MgSO4. Membrane vesicles (100 μg protein per assay) were incubated in the standard uptake medium containing 100 mm mannitol, 100 mm sodium gluconate, 20 mm Mes-Tris (pH 5.5), 0.1 mm MgSO4 and 1 mm [U-14C]butyrate. Uptake was measured at 37 °C for 5 s as described in Methods. The reaction was stopped by the addition of 1 ml of ice-cold stop buffer after 5 s. Values are presented as the means ± s.e.m. for three experiments.
Figure 3. Immunodetection of Na+-K+-ATPase.
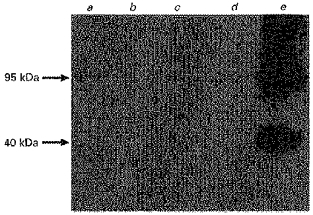
Pig and human colonic cellular homogenates, respective LMVs and pig colonic BLMV (control) were treated as described in Figure 1. Lane a, pig colonic cellular homogenate; lane b, pig colonic LMV; lane c, human colonic cellular homogenate; lane d, human colonic LMV; lane e, pig colonic BLMV. In BLMV (lane e), bands corresponding to 95 kDa, the α-subunit and 40 kDa, the β-subunit of Na+-K+-ATPase (Tarpley et al. 1995) are concentrated compared with the original cellular homogenate (lane a). In LMV, sample levels of Na+-K+-ATPase are negligible.
Characteristics of butyrate transport
Effect of the extravesicular medium pH on butyrate transport
Mascolo et al. (1991) and Harig et al. (1996), studying the mechanism of butyrate transport in rat and human colonic LMV, respectively, have shown that butyrate is transported via cellularly outward-directed gradients of either HCO3− or butyrate. It was shown by Mascolo et al. (1991) that the uptake of butyrate in exchange for HCO3− was identical in the presence or absence of constant CO2 tension. In addition, Harig et al. (1996) have demonstrated that the activity of the butyrate-HCO3− exchange was enhanced with reduction of the extravesicular medium pH from 7.5 to 5.5. We have extended these studies to assess the effect of varying the pH of the extravesicular medium on the initial rate of butyrate uptake in pig and human colonic LMV, in order to identify the optimum pH for the activity of the butyrate-anion exchanger. Furthermore, we have shown that the enhancement in the activity of the butyrate transporter at the acidic pH is not energized by the pH gradient (see below).
In order to determine the effect of the extravesicular medium pH on butyrate transport, either pig or human colonic LMV preloaded with a buffer containing 100 mm sodium butyrate, were incubated in isosmolar solutions of varying pH values (5.5-8.0). Figure 4 shows the initial rate (i.e. uptake measured at 5 s, see below) of butyrate uptake in response to changes in incubation media pH. In pig colonic LMV, there was a 5-fold enhancement of butyrate uptake at pH 5.5 compared with pH 8.0 in the presence of an outward-directed butyrate gradient. In human colonic LMV there was a 4-fold enhancement of butyrate uptake over the same pH values (data not shown). These results could imply that either butyrate transport is energized by the pH gradient, (pHout <pHin) or alternatively the acidic extravesicular pH is the optimum pH for butyrate transport. In order to distinguish between these two conditions, vesicles were loaded with a buffer containing 100 mm sodium butyrate either at pH 5.5 or 7.5. Both sets of vesicles were then incubated in a buffer containing sodium gluconate either with an imposition of a pH gradient, pHout <pHin (pHi, 7.5; pHo, 5.5) or in the absence of any pH gradients, pHout = pHin (pHi = pHo = 5.5). Gluconate was selected since it is known to be a membrane impermeable anion (Shirazi-Beechey et al. 1990). The results indicated that initial rates of uptake in conditions where the extravesicular pH was 5.5, irrespective of the intravesicular pH being either 5.5 or 7.5, were not significantly different. The rates of uptake were significantly lower when extra- and intravesicular pH were both 7.5. These findings support the conclusion that the acidic extravesicular pH and not the pH gradient is responsible for the stimulation of butyrate transport across the vesicular membrane.
Figure 4. Effect of extravesicular medium pH on butyrate uptake into human and pig colonic LMV.
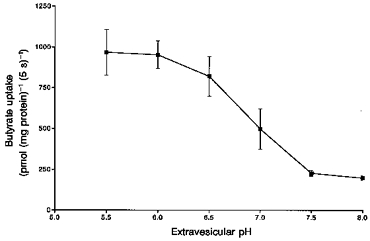
The LMV were loaded with the standard loading buffer containing 100 mm mannitol, 100 mm sodium butyrate, 0.1mm MgSO4 and 20 mm Hepes-Tris (pH 7.5). LMV (100 μg of protein per assay) were incubated in media containing 100 mm mannitol, 100 mm sodium gluconate, 0.1 mm MgSO4, 1 mm [U-14C]butyrate and 20 mm Hepes-Tris, for pH 7.0, 7.5 and 8.0 or Mes-Tris for pH 5.5, 6.0 and 6.5. Initial rate of butyrate uptake was measured as described in Methods, at 37 °C for 5 s. Values are presented as the means ± s.e.m. for three experiments.
The pKa for butyric acid is 4.8. Therefore at the standard assay condition, with an incubation medium of pH 5.5 containing 1 mm sodium butyrate, the concentration of anionic species in the medium amounts to 0.83 mm and that of butyric acid to 0.17 mm. In the intravesicular medium at pH 7.5, containing 100 mm sodium butyrate, the concentrations of butyrate and butyric acid amount to 99.8 and 0.2 mm, respectively. By reducing the intravesicular pH from 7.5 to 5.5, the concentration of butyric acid amounts to 17 mm. Therefore, if butyric acid was the transported species, the reduction in the intravesicular pH should lead to a reduction in transport. We have shown however, that modulation of the intravesicular pH did not have any effect on the initial rate of butyrate uptake.
The effect of various intravesicular anions on butyrate transport
We investigated the effect of the intravesicular anion on butyrate uptake, by loading the vesicles with either mannitol or various anions. The results indicated that an intravesicular anion is required for butyrate to be transported, as the initial rate of butyrate uptake was significantly decreased in mannitol-loaded vesicles compared with the vesicles loaded with anions. Table 2 summarizes the effect of different intravesicular anions on the initial rate (5 s uptake) of butyrate uptake. Initial rates of uptake were measured in the presence of an inwardly directed pH gradient (pHi, 7.5; pHo, 5.5), with either 300 mm mannitol or 100 mm butyrate, propionate, acetate, chloride, HCO3− or gluconate as the intravesicular anion. The results indicate that the initial rate of butyrate uptake under the conditions measured was highest in order of the intravesicular anion:
The rates of uptake were lower in mannitol- or gluconate-loaded vesicles (see Table 2). Our results indicate that butyrate transport in human and pig colon is via an anion exchanger, with a broad specificity for the exchanging anion. These are different to those reported by Mascolo et al. (1991) and Harig et al. (1996) who have shown that in rat and human colon, butyrate is only exchanged by either intravesicular HCO3− or butyrate. Harig et al. (1996) have extended their studies to investigate the mechanism for the transport of extravesicular chloride in exchange for intravesicular butyrate in human colonic luminal membrane vesicles. They have shown the absence of such an exchanger. In our studies lowering the concentration of the stimulating intravesicular anion from 100 to 25 mm reduced the rate of butyrate uptake to those equal to vesicles loaded with mannitol (data not shown). However, increasing the intravesicular anion concentration from 100 to 150 mm did not have any significant effect on the initial rates of butyrate uptake. The accumulated data indicate that an intravesicular anion and an anionic gradient (inside > outside) are required for butyrate to be transported.
The uptake of butyrate, in exchange with the intravesicular anion, was not affected by voltage clamping the membrane potential. Loading the vesicles with the loading buffer and high concentrations of K+ in the presence of the K+ ionophore valinomycin did not alter the initial rate of butyrate transport (data not shown). These results are in agreement with those obtained by Harig et al. (1996) and indicate an electroneutral anion exchange mechanism for butyrate uptake.
The transport of butyrate was not cation dependent as the replacement of Na+ with K+ or any other cations in the incubation media had no effect on the rate of butyrate uptake.
Time course of butyrate uptake
The time course of butyrate transport in the presence and absence of a pH gradient across the vesicle membrane was determined. LMV were preloaded with either 100 mm sodium butyrate, NaHCO3 or 300 mm mannitol buffered at pH 7.5. The incubation media contained 100 mm sodium gluconate buffered at either pH 5.5 (Fig. 5A) or pH 7.5 (Fig. 5B). In either the butyrate- or HCO3−-loaded vesicles and in the presence of a pH gradient (pHout < pHin) butyrate transport was stimulated. After 5 s, butyrate had accumulated in the vesicles 6- to 7-fold (overshoot) over the levels taken up at the equilibrium point, 30 min uptake (Fig. 5A). With the incubation medium of pH 7.5 the rates of uptake were low and no concentrative uptake of butyrate was observed (Fig. 5B). The rate of butyrate transport was linear over the initial 5 s period. Therefore, in the studies presented in this paper, 5 s uptake was employed.
Figure 5. Time course of butyrate uptake into pig colonic LMV in the presence and absence of an inward-directed pH gradient.
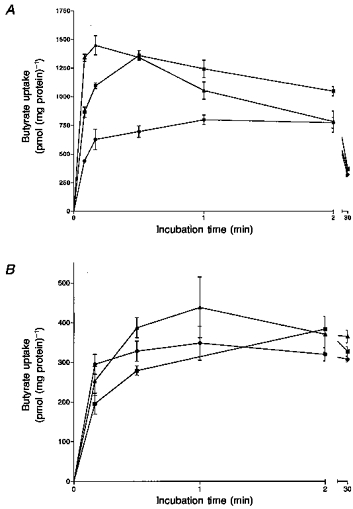
LMV were loaded with either 300 mm mannitol (•) or 100 mm mannitol and 100 mm of either sodium butyrate (▴) or NaHCO3 (▪), 20 mm Hepes-Tris (pH 7.5) and 0.1 MgSO4. LMV (100 μg protein per assay) were incubated in media containing 100 mm mannitol, 100 mm sodium gluconate, 20 mm Mes-Tris (pH 5.5; A) or 20 mm Hepes-Tris (pH 5.5; B), 0.1 mm MgSO4 and 1 mm [U-14C]butyrate. Uptake was measured at 37 °C as described. Values are presented as the means ± s.e.m. for three experiments.
Transport into intravesicular space
Uptake of radiolabelled butyrate could represent either binding to the vesicular membrane or transport into the intravesicular space. In order to determine whether uptake of butyrate was into the intravesicular space, we altered the osmolarity of the incubation medium from 300 to 700 mosmol l−1 by increasing the concentration of mannitol. It has been established that the amount of solute at the equilibrium point should be directly proportional to the intravesicular volume (Hopfer & Sigrist-Nelson, 1974). The rates of butyrate uptake measured at the equilibrium point (30 min) decreased proportionally to changes in the intravesicular volume (Fig. 6). Extrapolation of the straight line (r2 = 0.92) to infinity (Fig. 6) indicated that butyrate was not binding to the vesicular membrane.
Figure 6. Effect of osmolarity of the external medium on butyrate uptake by pig colonic LMV.
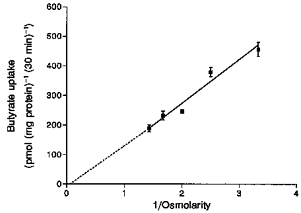
LMV were loaded with the standard loading buffer described in Fig. 4. LMV (100 μg protein per assay) were incubated for 30 min at 37 °C in media containing 100 mm sodium gluconate, 20 mm Mes-Tris (pH 5.5), 0.1 mm MgSO4, 1 mm [U-14C]butyrate and mannitol in concentrations to give the indicated osmolarity. Values are presented as the means ± s.e.m. for three experiments. Correlation coefficient r2 = 0.92.
Kinetics of butyrate transport
The initial rate of butyrate uptake was measured with increasing extravesicular butyrate concentrations (1–50 mm), in the presence (pHo 5.5) and the absence (pHo 7.5) of an inward-directed pH gradient. The uptake was measured in LMV isolated either from human or pig colon and preloaded with 100 mm HCO3−. Bicarbonate-loaded vesicles were used in order to eliminate any potential alterations in the butyrate concentration of the incubation media by the vesicle suspension buffer. Bicarbonate is a suitable replacement for butyrate as the intravesicular anion, as the rates of butyrate uptake with either the butyrate- or HCO3−-loaded vesicles were similar (see Table 2). The rates were calculated as the difference between the uptake at pHo = 5.5 to that at pHo = 7.5 in order to obtain rates for the pH-stimulated butyrate uptake. The uptake was saturable and conformed to Michaelis-Menten kinetics (Fig. 7). The Km and Vmax values for butyrate transport were calculated by linear regression analysis of the Eadie-Hofstee plot. Km was determined to be 14.8 ± 3.6 mm and Vmax 54 ± 14 nmol min−1 (mg protein)−1. Linear regression analysis produced a straight line (r2 = 0.95) indicating a single transport system for butyrate in the LMV.
Figure 7. Effect of increasing butyrate concentrations on butyrate uptake by pig colonic LMV.
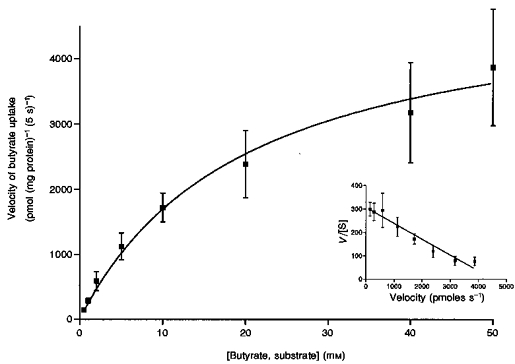
Initial rates of butyrate transport with increasing butyrate concentrations over the range 0.5 to 50 mm were determined. LMV were loaded with 100 mm mannitol, 100 mm NaHCO3, 20 mm Hepes-Tris (pH 7.5), and 0.1 mm MgSO4. LMV (100 μg protein per assay) were incubated in media containing 100 mm mannitol, 0.1 mm MgSO4 either 20 mm Mes-Tris (pH 5.5) or 20 mm Hepes-Tris (pH 7.5), and varying concentrations of sodium gluconate and sodium butyrate (to maintain a constant media osmolarity and Na+ concentration) and constant tracer amounts of [U-14C]butyrate. Uptake was measured at 37 °C for 5 s. Initial rates of pH-activated butyrate uptake were determined by subtracting the uptake in the absence of a pH gradient from those with an inwardly directed pH gradient. The data are presented as a Michaelis-Menten curve and (inset) as Eadie-Hofstee plot of V/[S] against V, whereV is the velocity and [S] the concentration of the substrate. Values are presented as the means ±s.e.m. for three experiments.
Investigation into the presence of a band-3 type protein in the colonic luminal membrane
Since our data indicated that the mechanism for the transport of butyrate is via a pH-activated anion exchange process, we set out to investigate whether the butyrate transport protein had any structural similarity to the 97 kDa, erythrocyte anion exchanger, band-3 protein. The colonic LMV were screened with an antibody to human erythrocyte band-3 protein. Human erythrocyte ghosts were used as a control. The antibody cross-reacted specifically with a protein in human erythrocyte ghosts with an apparent molecular mass of 97 kDa. There was no cross-reaction with any proteins in the colonic LMV (Fig. 8).
Figure 8. Investigation into the presence of a band-3 homologue in pig and human colonic LMV.
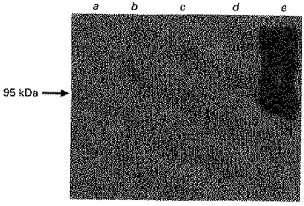
Samples (25 μg of protein each) were treated as outlined before (Fig. 1). Human erythrocyte ghosts were used as a control. Lane a, pig colonic homogenate; lane b, pig colonic LMV; lane c, human colonic homogenate; lane d, human colonic LMV; lane e, human erythrocyte ghosts.
The effect of structural analogues of butyrate on butyrate uptake
The major SCFA present in the lumen of the colon are acetate, propionate and butyrate (Cummings, 1984). The effect of acetate, propionate and other monocarboxylates present in the incubation media at a concentration of 20 mm on the initial rate of uptake of 1 mm butyrate was determined. As can be seen in Fig. 9, acetate and propionate inhibited butyrate uptake by 40%. These results are in agreement with earlier reports (Mascolo et al. 1991; Harig et al. 1996). In contrast to these reports however, we have shown that pyruvate, l-lactate and α-ketobutyrate reduced the rate of butyrate transport by > 50%. The inhibition of butyrate uptake by acetate and propionate was of a competitive nature. Pyruvate and l-lactate are known to be transported into the cell by a monocarboxylate transporter (MCT1) via a H+-symport mechanism (Poole & Halestrap, 1993). The results of the data presented in Fig. 9 suggest that pyruvate and l-lactate could either compete with the binding site for butyrate on the transporter or alternatively share a similar type of transporter as butyrate. If butyrate is transported by a MCT1 type transporter in the colon, then there are distinct mechanistic differences to that reported for MCT1, as the mechanism for the transport of butyrate does not appear to be by a H+-symport system.
Figure 9. Effect of organic and inorganic anions of the extravesicular media on butyrate uptake.
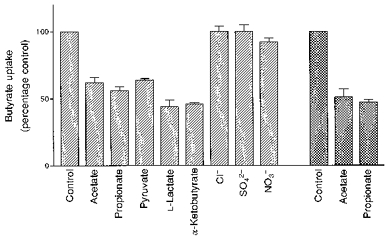
LMV, isolated from pig ( ) and human (
) and human ( ) colon, were preloaded with the standard loading buffer. LMV (100 μg protein per assay) were then incubated in media containing 100 mm mannitol, 80 mm sodium gluconate (60 mm sodium gluconate when Na2SO4 was present in the medium); 20 mm Mes-Tris (pH 5.5), 0.1 mm MgSO4, 1 mm [U-14C]butyrate and the following Na+ salts (20 mm final concentration) acetate, propionate, pyruvate, l-lactate, Cl−, NO3− and SO4−. Uptake was measured at 37 °C for 5 s as before. Values are presented as means ± s.e.m. for three experiments.
) colon, were preloaded with the standard loading buffer. LMV (100 μg protein per assay) were then incubated in media containing 100 mm mannitol, 80 mm sodium gluconate (60 mm sodium gluconate when Na2SO4 was present in the medium); 20 mm Mes-Tris (pH 5.5), 0.1 mm MgSO4, 1 mm [U-14C]butyrate and the following Na+ salts (20 mm final concentration) acetate, propionate, pyruvate, l-lactate, Cl−, NO3− and SO4−. Uptake was measured at 37 °C for 5 s as before. Values are presented as means ± s.e.m. for three experiments.
The inorganic anions, Cl−, NO3− and SO42-, when present in the incubation medium, had no effect on butyrate transport (Fig. 9).
Effect of various potential inhibitors on butyrate transport
Many anion exchange processes are inhibited by the classical anion exchange inhibitors, 4,4′-diisothiocyanostilbene-2,2′-disulphonate (DIDS) and 4,4′-dinitrostilbene-2,2′-disulphonate (SITS). These inhibitors were tested for their effects on butyrate transport. Incubation of the vesicles with DIDS and SITS (0.5 mm) had no inhibitory effect on butyrate transport (data not shown). Lack of inhibition of butyrate transport by DIDS and SITS has also been reported by other workers using flux studies (Mascolo et al. 1991; Stein et al. 1995: Harig et al. 1996).
Several transport proteins that have an accessible cysteine residue in the active site have been shown to be inhibited by mercury compounds, specific modifiers of SH-groups (Poole & Halestrap, 1993). The effect of several mercury compounds on butyrate uptake was determined and the results are summarized in Fig. 10. The compounds tested were pre-incubated with the membrane vesicles (100 μg protein per assay) for 30 min at 4°C. Control vesicles (100 μg protein per assay) were also maintained at 4°C for 30 min. Subsequently, the initial rates of 1 mm butyrate uptake were measured in the control and pre-treated vesicles. All tested mercurials inhibited the uptake of butyrate significantly (> 40%), with p-chloromercuribenzoate (pCMB) showing the greatest inhibition (60%). These results imply the involvement of SH-functional groups in the transport of butyrate across the membrane.
Figure 10. Effect of other potential inhibitors on butyrate transport into pig colonic LMV.
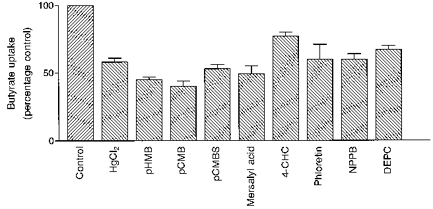
LMV, loaded with the standard loading buffer, were incubated for 30 min at 4 °C with indicated compounds. Mercurials, phloretin and NPPB were added to a volume of vesicles to give a final concentration of 0.5 mm, whilst the final concentrations of the DEPC and 4-CHC in LMV amounted to 1 and 5 mm, respectively. LMV (control) were incubated at 4 °C for 30 min. Butyrate uptake by pre-treated and control LMV was measured for 37 °C for 5 s with the uptake buffer having the standard composition described in Table 2. Values are presented as means of triplicates ±s.e.m.
The stimulation of butyrate transport at acidic pH could be related to the presence of protonated amino acid residues at active sites. In order to assess the participation of any reactive histidine residues in this stimulation, diethylpyrocarbonate (DEPC), a modifier of histidine residues, was used at 1 mm concentration in butyrate uptake studies. The transport of butyrate in the presence of DEPC was inhibited by 33% compared with the control (Fig. 10). This partial effect may indicate that reactive histidine residues are not easily accessible to DEPC or not directly involved in the translocation of butyrate.
Butyrate transport was shown to be inhibited by l-lactate and pyruvate (see above), substrates of MCT1. We therefore tested the effect of known inhibitors of this transport system on butyrate uptake into colonic LMV. α-Cyano-4-hydroxycinnamic acid (4-CHC), phloretin and 5-nitro-2-(3-phenylpropylamino)benzoate (NPPB) are potent inhibitors of MCT1 (Poole & Halestrap, 1993). Initial rates of butyrate uptake measured in the presence of 4-CHC (5 mm) was inhibited by only 20%. Phloretin (0.5 mm) reduced butyrate uptake by 40%. In the presence of NPPB (0.5 mm), butyrate uptake was reduced by 40%. Furthermore, investigating the transport of l-lactate into purified pig and human colonic LMV, we have recently shown that the mechanism for the transport of this monocarboxylate is similar to that reported for butyrate transport described in this paper. That is, when the extravesicular pH was 5.5 l-lactate transport was stimulated 6-fold compared with the value measured at pH 7.5. l-Lactate transport was additionally enhanced by an outward-directed anion gradient. Transport was also inhibited by SCFA (Ritzhaupt, Wood, Ellis, Hosie & Shirazi-Beechey, 1998). Additionally, using Western blot analysis and an antibody to human MCT1, we identified a specific protein with an apparent molecular mass of 40 kDa in human and pig colonic LMV. The abundance of the immunoreactive 40 kDa band was 30-fold enriched in LMV compared with the cellular homogenate, indicating unique localization to the luminal membrane (Ritzhaupt et al. 1998). These findings suggest that butyrate may be transported by a MCT1 type protein.
DISCUSSION
Short chain fatty acids (acetate, propionate and butyrate) are produced in the lumen of the colon as a result of fermentation of dietary fibre by colonic microflora. SCFA play an important role in maintaining homeostasis in the colon. Butyrate is of special interest because it has been shown to promote growth and proliferation of normal colonic mucosa while surpressing the growth of cancer cells (Claussen, Bonnén & Mortensen, 1991). Identification of the properties of the transport pathway for butyrate in the colon would assist in elucidation of the mechanism by which butyrate interacts with the colonic epithelia in healthy and diseased tissues. The objective of the present study was to characterize and extend the available data on butyrate transport into LMV isolated from human and pig colon. SCFA are rapidly metabolized by the colonic tissue. Therefore colonic LMV provide a suitable system for the determination of the mechanism of transport of these solutes through the colonic luminal membrane domain.
In isolating LMV we used a modified technique. One essential modification was that MgCl2 was used instead of CaCl2 for the differential precipitation of plasma membranes. It has been shown that Ca2+ activates the membrane-bound phospholipases. This alters the membrane permeability leading to reduced rates of uptake (Sabolić & Burckhardt, 1982). The purity of isolated LMV were characterized extensively in our studies. Determination of the activity and abundance of marker enzymes, characteristic of organelle and basolateral membranes, showed the presence of negligible levels of endoplasmic reticulum, Golgi, mitochondrial and basolateral membranes in the LMV. Conversely the activity and abundance of several colonic luminal membrane markers were enriched in the membrane vesicles, indicating that the purified membrane vesicles originate from the colonic luminal membrane.
Our data on the mechanism by which butyrate is transported into the pig and human colonic LMV indicate that butyrate uptake is via a pH-activated, electroneutral anion exchange system. Butyrate uptake was stimulated significantly in the presence of an outward-directed anion gradient and inward-directed pH gradient. There is a broad range of anions that can exchange for butyrate. The stimulation of uptake by the extravesicular acidic pH was shown not to be due to H+ gradient-energized transport of butyrate. The more acidic extravesicular pH of 5.5 provides an optimal pH for the activity of the colonic butyrate transporter. Kinetic analysis of butyrate transport confirmed the presence of a single saturable transport protein.
Butyrate transport was not inhibited by DIDS and SITS. The butyrate transport protein does not appear to have any structural similarities to the erythrocyte Cl−-HCO3− exchange protein, band-3, as the antibody to the band-3 protein did not cross-react with any proteins in the colonic luminal membrane. SH-reactive compounds used in this study have been shown specifically to inhibit other transport systems such as the monocarboxylate transporter, MCT1 (Poole & Halestrap, 1993) but do not affect fluxes of sulphate and chloride (Deuticke, Rickert & Beyer, 1978). Butyrate transport was also significantly inhibited by SH-reactive compounds, indicating the involvement of SH-functional groups in the transport of butyrate across the membrane.
In agreement with data reported by Harig et al. (1996), butyrate transport was inhibited by several structural analogues such as acetate or propionate but not by chloride. However, we observed significant reduction of butyrate uptake in the presence of pyruvate, l-lactate and α-ketobutyrate. This could imply that these compounds either compete for the binding site with butyrate on the transporter or, alternatively, share the same transport protein as butyrate. Pyruvate and l-lactate are known to be transported through the apical plasma membrane of the small intestine (Tamai et al. 1995) and rat cardiac myocytes (Wang, Levi & Halestrap, 1996) by MCT1 via a H+-symport mechanism. 4-CHC, phloretin and NPPB are known to be potent inhibitors of MCT1. 4-CHC did not inhibit butyrate transporter significantly, whilst phloretin and NPPB reduced the rate of butyrate uptake significantly (40%).
The data presented in this paper indicate that the SCFA butyrate is transported from the lumen of human and pig colon across the luminal membrane into colonocytes by a pH-activated electroneutral anion exchange system. Whilst our data on the mechanism of butyrate transport in human and pig colon are similar to earlier studies conducted on rat and human colon (Mascolo et al. 1991; Harig et al. 1996), we further show that: (1) butyrate can exchange with a number of intravesicular anions, (2) butyrate transport is enhanced at low pH and not energized by the pH gradient and (3) the butyrate transport protein is not structurally similar to the erythrocyte anion exchanger, band-3. Furthermore, inhibition by both lactate and pyruvate and specific inhibitors of monocarboxylate transport proteins suggests that the colonic butyrate transporter may belong to the family of the MCT proteins. This conclusion can be supported by our recent findings that: (a) l-lactate is transported into human and pig LMV by a similar mechanism to that described for butyrate transport; (b) transport of l-lactate into LMV is inhibited by butyrate; and (c) the antibody to human MCT1 cross-reacts with a protein of 40 kDa exclusively present on the colonic luminal membrane (Ritzhaupt et al. 1998).
The information on the functional properties of the colonic butyrate transport described in this paper will assist the identification of the structure of the transporter. Work is in progress in our laboratory to characterize the molecular structure of the butyrate transporter. This information in turn would allow a better understanding of the molecular mechanisms by which butyrate affects the function of the colon in health and disease.
Acknowledgments
We are grateful to Drs W. J. Ball, M. Donowitz and R. Kopito for provision of the antibodies used in this study and to Dr J. Dyer for helpful comments and critical reading of the manuscript. Our thanks are due to Sister Joan Shaw of Broadgreen Hospital, Liverpool. S. P. Shirazi-Beechey is a Wellcome Trust Senior Lecturer. The financial support of Tenovus is gratefully acknowledged.
References
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Berry RD, Paraskeva C. Expression of a carcinoembryonic antigen by the adenoma and carcinoma derived epithelial cell lines: possible marker of tumour progression and modulation of expression by sodium butyrate. Carcinogenesis. 1988;9:447–450. doi: 10.1093/carcin/9.3.447. [DOI] [PubMed] [Google Scholar]
- Brasitus TA, Keresztes RS. Protein-lipid interactions in antipodal plasma membranes of rat colonocytes. Biochimica et Biophysica Acta. 1984;773:290–300. doi: 10.1016/0005-2736(84)90093-2. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proceedings of the National Academy of Sciences of the USA. 1979;76:2321–2325. doi: 10.1073/pnas.76.5.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen MR, Bonnén H, Mortensen PB. Colonic fermentation of dietary fibre to short-chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut. 1991;32:923–928. doi: 10.1136/gut.32.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH. Colonic absorption: The importance of SCFA in man. Scandinavian Journal of Gastroenterology. 1984;19:89–99. [PubMed] [Google Scholar]
- Deuticke B, Rickert I, Beyer E. Stereoselective, SH-dependent transfer of lactate in mammalian erythrocytes. Biochimica et Biophysica Acta. 1978;507:137–155. doi: 10.1016/0005-2736(78)90381-4. [DOI] [PubMed] [Google Scholar]
- Engelhardt WV, Burmester M, Hansen K, Becker G, Rechkemmer G. Effects of amiloride and ouabain on short-chain fatty acid transport in guinea-pig large intestine. Journal of Physiology. 1993;460:455–466. doi: 10.1113/jphysiol.1993.sp019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt Wv, Gros G, Burmester M, Hansen K, Becker G, Rechkemmer G. Functional role of bicarbonate in propionate transport across guinea-pig isolated caecum and proximal colon. Journal of Physiology. 1994;477:365–371. doi: 10.1113/jphysiol.1994.sp020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush B., III Assay of Na, K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Analytical Biochemistry. 1983;128:159–163. doi: 10.1016/0003-2697(83)90356-1. [DOI] [PubMed] [Google Scholar]
- Harig JM, Dudeja PK, Knaup SM, Shoshara J, Ramaswamy K, Brasitus TA. Apical membrane vesicles formed from organ donor colon demonstrate Na+ and H+ conductances and Na+/H+ exchange. Biochemical and Biophysical Research Communications. 1990;167:438–443. doi: 10.1016/0006-291x(90)92042-x. [DOI] [PubMed] [Google Scholar]
- Harig JM, Ng EK, Dudeja PK, Brasitus TA, Ramaswamy K. Transport of n-butyrate into human colonic luminal membrane vesicles. American Journal of Physiology. 1996;271:G415–422. doi: 10.1152/ajpgi.1996.271.3.G415. [DOI] [PubMed] [Google Scholar]
- Hatch M. Short chain fatty acid transport and its effects on ion transport by rabbit cecum. American Journal of Physiology. 1987;250:G469–474. doi: 10.1152/ajpgi.1987.253.2.G171. [DOI] [PubMed] [Google Scholar]
- Holtug K, Hove H, Mortensen PB. Stimulation of butyrate absorption in the human rectum in vivo. Scandinavian Journal of Gastroenterology. 1995;30:982–988. doi: 10.3109/00365529509096342. [DOI] [PubMed] [Google Scholar]
- Holtug K, Rasmussen HS, Mortensen PB. An in vitro study of short-chain fatty acid concentrations, production and absorption in pig (Sus scrofa) colon. Comparative Biochemistry Physiology A. 1992;103:189–197. doi: 10.1016/0300-9629(92)90262-o. 10.1016/0300-9629(92)90262-O. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CHC, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse C-H, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. American Journal of Physiology. 1996;270:G29–41. doi: 10.1152/ajpgi.1996.270.1.G29. [DOI] [PubMed] [Google Scholar]
- Hopfer U, Sigrist-Nelson K. Intestinal transport protein. Nature. 1974;252:422. doi: 10.1038/252422a0. [DOI] [PubMed] [Google Scholar]
- McNeil I, Cummings JH, James WP. Short chain fatty acid absorption by human large intestine. Gut. 1978;19:819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascolo N, Rajendran VM, Binder HJ. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991;101:331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Pennington RJ. Biochemistry of dystrophic muscle (mitochondrial succinate tetrazolium reductase and adenosine triphosphatase) Biochemical Journal. 1961;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TJ. Analytical subcellular fractionation of jejunal biopsy specimens: methodology and characterisation of the organelles in normal tissue. Clinical Science and Molecular Medicine. 1976;51:557–574. doi: 10.1042/cs0510557. [DOI] [PubMed] [Google Scholar]
- Pinches SA, Gribble SM, Beechey RB, Ellis A, Shaw JM, Shirazi-Beechey SP. Preparation and characterization of basolateral membrane vesicles from pig and human colonocytes: the mechanism of glucose transport. Biochemical Journal. 1993;294:529–534. doi: 10.1042/bj2940529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across the mammalian plasma membranes. American Journal of Physiology. 1993;264:C761–782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification of a monocarboxylate transporter isoform type 1 (MCT1) on the luminal membrane of human and pig colon. Biochemical Society Transactions. 1998;26:S120. doi: 10.1042/bst026s120. [DOI] [PubMed] [Google Scholar]
- Robine S, Huet C, Moll R, Sahuguillo-Merino C, Coudrier E, Zweibaum A, Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proceedings of the National Academy of Sciences of the USA. 1985;82:8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–1507. [PubMed] [Google Scholar]
- Sabolic I, Burckhardt G. Effect of the preparation method on Na+-H+ exchange and ion permeabilities in rat renal brush border membranes. Biochimica et Biophysica Acta. 1982;772:140–148. doi: 10.1016/0005-2736(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Scheppach W, Sommer H, Kirchner T, Paganelli G-M, Bartram P, Christi S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–56. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- Sellin JH, DeSoignie R, Burlingame S. Segmental differences in short-chain fatty acid transport in rabbit colon: Effect of pH and Na. Journal of Membrane Biology. 1993;136:147–158. doi: 10.1007/BF02505759. [DOI] [PubMed] [Google Scholar]
- Shirazi SP, Beechey RB, Butterworth PJ. The use of potent inhibitors of alkaline phosphatase to investigate the role of the enzyme in intestinal transport of inorganic phosphate. Biochemical Journal. 1981;194:803–809. doi: 10.1042/bj1940803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi-Beechey SP, Davies AG, Tebbutt K, Dyer J, Ellis A, Taylor CJ, Fairclough P, Beechey RB. Preparation and properties of brush-border membrane vesicles from human small intestine. Gastroenterology. 1990;98:1–12. doi: 10.1016/0016-5085(90)90288-c. [DOI] [PubMed] [Google Scholar]
- Stein J, Schröder O, Milovic V, Caspary WF. Mercaptopropionate inhibits butyrate uptake in isolated apical membrane vesicles of the rat distal colon. Gastroenterology. 1995;108:673–679. doi: 10.1016/0016-5085(95)90438-7. [DOI] [PubMed] [Google Scholar]
- Tamai I, Takanaga H, Maeda H, Sai Y, Ogihara T, Higashida H, Tsuji A. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochemical and Biophysical Research Communications. 1995;214:482–489. doi: 10.1006/bbrc.1995.2312. 10.1006/bbrc.1995.2312. [DOI] [PubMed] [Google Scholar]
- Tarpey PS, Wood IS, Shirazi-Beechey SP, Beechey RB. Amino acid sequence and the cellular location of the Na+-dependent d-glucose symporters (SGLT1) in the ovine enterocyte and the parotid acinar cell. Biochemical Journal. 1995;312:293–300. doi: 10.1042/bj3120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HA, Machen TE, Smolka A, Baron R, Kopito RR. Identification of a 185-kDa band 3-related polypeptide in oxyntic cells. American Journal of Physiology. 1989;257:C537–544. doi: 10.1152/ajpcell.1989.257.3.C537. [DOI] [PubMed] [Google Scholar]
- Treem WR, Ahsan N, Shoup M, Hyams JS. Fecal short-chain fatty acids in children with inflammatory bowel disease. Journal of Pedriatic Gastroenterology and Nutrition. 1994;18:159–164. doi: 10.1097/00005176-199402000-00007. [DOI] [PubMed] [Google Scholar]
- Tulsiani DRP, Opheim DJ, Touster O. Purification and characterisation of α-d-mannosidase from rat liver Golgi membranes. Journal of Biological Chemistry. 1977;252:3227–3233. [PubMed] [Google Scholar]
- Vengesa PB, Hopfer U. Cytochemical localization of alkaline phosphatase and Na+-pump sites in adult rat colon. Journal of Histochemistry and Cytochemistry. 1979;27:1231–1235. doi: 10.1177/27.9.39100. [DOI] [PubMed] [Google Scholar]
- Wang X, Levi AJ, Halestrap AP. Substrate and inhibitor specificities of the monocarboxylate transporters of single rat heart cells. American Journal of Physiology. 1996;270:H476–484. doi: 10.1152/ajpheart.1996.270.2.H476. [DOI] [PubMed] [Google Scholar]


