Abstract
Despite a number of models of nerve injury, few studies have examined how peripheral nerve injury influences spinal somatosensory processing.
Ligation of two (L5-L6) of the three spinal nerves that form the sciatic nerve produces a partial denervation of the hindlimb. Following ligation, rats exhibited withdrawal responses to normally innocuous punctate mechanical and cooling stimuli (acetone) applied to the lesioned hindpaw. Such mechanical and cooling allodynia was not observed in sham-operated rats.
A significantly greater proportion of spinal neurones of ligated rats exhibited spontaneous activity at post-operative (PO) days 7–10 (P = 0.03) and 14–17 (P = 0.0001), compared with sham controls. The frequency of the spontaneous activity was significantly higher than that of the sham controls (P = 0.03 and P = 0.02 for days 7–10 and days 14–17, respectively).
At the earlier PO period, significantly (P = 0.02) more neurones of spinal nerve-ligated (SNL) rats responded to brush compared with the sham controls; at the later PO period the proportion of neurones of SNL rats responsive to prod was significantly (P = 0.007) reduced compared with the sham controls. The magnitude of the evoked neuronal response of SNL rats at PO days 7–10 was comparable to that of the sham controls. The magnitudes of brush- and prod-evoked neuronal responses of SNL rats were significantly smaller (P = 0.05 and P = 0.002, respectively) than the sham controls at PO days 14–17. In addition, neuronal responses of SNL rats to mechanical punctate stimuli and the C fibre-evoked neuronal responses were significantly reduced at the later PO period, compared with sham controls. Aβ-fibre-induced wind-up was not observed under any conditions.
These complex changes in neuronal responses are both time and modality dependent. The plasticity of some of the neuronal and behavioural responses following nerve injury was difficult to reconcile. We suggest that an interplay between pathological peripheral and central mechanisms may account for some of the changes that could contribute to allodynia and hyperalgesia.
Until recently, the mechanisms underlying clinical pain syndromes, where the pain is often severe and persistent, have relied on data from animal studies based on the application of acute stimuli. The symptoms of neuropathic pain arising from nerve injury, such as allodynia (touch-evoked pain), spontaneous pain, hyperalgesia (enhanced responses to a given noxious stimulus), sensory deficits with, in some cases, a sympathetic component, are not replicated in acute animal models. Early electrophysiological studies related to neuropathic pain used complete nerve section. More recent animal models are based on a restricted partial denervation of the hindlimb following sciatic nerve injury (reviewed by Zeltser & Seltzer, 1994). The behavioural consequences of these models mimic some aspects of the human symptoms listed above, although the extent and location of the injury differ. Two of the models involve the constriction of the sciatic nerve distal to the spinal cord: either a tight ligation of a portion of the sciatic nerve (Seltzer, Dubner & Shir, 1990), or the loose ligation of the entire nerve (Bennett & Xie, 1988). With both of these models there is a degree of subjectivity with regard to the tightness of the ligations (Bennett & Xie, 1988) and the proportion of the sciatic nerve that is tightly ligated (Seltzer et al. 1990). A number of studies have shown that there is an inherent variability with these approaches, since it is not possible to consistently injure the same ratio of myelinated and unmyelinated fibres in each animal, and the extent of the degeneration of different fibre types has been reported to be variable (Basbaum, Gautron, Jazat, Mayes & Guilbaud, 1991; Carlton, Dougherty, Pover & Coggeshall, 1991; Munger, Bennett & Kajander, 1992).
The third and more recently developed model uses tight ligation of two of the three spinal nerves of the sciatic nerve (Kim & Chung, 1992). Tight ligation of two of the three spinal nerves limits the problem of inter-rat variation of the extent of hindpaw deafferentation. The spinal cord is the primary relay site of somatosensory information into the central nervous system, and we have used this latter model to study the spinal electrophysiological consequences of nerve injury.
There is evidence that the aberrations in somatosensory processing, which follow partial nerve injury, are the culmination of a number of changes in the peripheral nervous system. Studies of peripheral nerve section (Wall & Devor, 1983; Burchiel, 1984), and partial denervation (Petersen, Zhang, Zhang & LaMotte, 1996; Study & Kral, 1996; Tal & Eliav, 1996), have shown that ectopic discharges are generated within the neuroma itself and the dorsal root ganglia (DRG); this activity may contribute to the physiological changes in the processing of somatosensory information. Furthermore, in the loose ligation model of nerve injury, a high level of spontaneous activity originating in the DRG (Kajander, Wakisaka & Bennett, 1992), which targets the spinal cord, has been shown to arise from injured A-fibres (Kajander & Bennett, 1992). Importantly, injured fibres and respective DRGs have been shown to contribute to behavioural responses, including allodynia, following L5-L6 spinal nerve ligation (Yoon, Heung & Chung, 1996). Single fibre recordings from the L4 dorsal root, following L5-L6 spinal nerve ligation, have identified a novel ‘modified rapidly adapting’ mechanoreceptor innervating the partially deafferented foot, thus suggesting changes in transduction processes following nerve injury (Na, Leem & Chung, 1993). In addition to changes in the electrical properties and transduction mechanisms of damaged peripheral fibres, a structural reorganization of large fibre (Aβ) termination at the level of the spinal cord has been demonstrated in this model (Lekan, Carlton & Coggeshall, 1996). Although there is clear evidence of multi-origin plasticity of the peripheral nervous system following nerve injury, there have been only a few studies of the impact of nerve injury on the corresponding dorsal horn neurones of the spinal cord of the rat and monkey (Palecek, Dougherty, Kim, Paleckova, Lekan, Chung, Carlton & Willis, 1992a; Palecek, Paleckova, Dougherty, Carlton & Willis, 1992b; Laird & Bennett, 1993; Leem, Park & Paik, 1995; Takaishi, Eisele & Carstens, 1996).
The aim of the present study was to fully characterize the on-going activity and evoked responses of spinal neurones in intact anaesthetized rats at different time points after nerve injury compared with sham-operated rats. We have used selective ligation of two (L5-L6) of the three spinal nerves (L4, L5, L6) that form the sciatic nerve. Selective spinal nerve ligation (SNL) produces a partial denervation of the hindpaw, which is easy to replicate and is associated with a highly reproducible cold and mechanical allodynia and thermal hyperalgesia, with a sympathetic component (Kim & Chung, 1992; Kim, Yoon & Chung, 1997).
Here we have replicated previous behavioural data, showing the development and maintenance of mechanical and cold allodynia 2–14 days post-spinal nerve ligation. Electrophysiological studies of the spinal neurones of these SNL rats over two post-operative periods (7–10 and 14–17 days) have then been performed.
The main findings of this study are that spinal neurones of SNL rats exhibit spontaneous activity, and that there is a temporal increase in this activity. In addition, we observed subtle changes in the proportion of neurones responding to different modalities of stimuli. These changes became more pronounced at later post-operative time points. Our findings also suggest that there are selective modifications in naturally and electrically evoked spinal neuronal responses. Overall, the changes in the spinal neurone responses following spinal nerve ligation had a time course of development similar to the behavioural changes associated with this model of neuropathic pain.
Part of this work has already been reported in abstract form (Chapman, Suzuki & Dickenson, 1997).
METHODS
Spinal nerve ligation
Experiments were carried out on sixty-five male Sprague-Dawley rats (University College animal house) weighing 140–170 g at the beginning of the study. Rats were housed, five per cage, under a 12 h day-12 h night cycle for at least 1 week prior to surgery. Rats were divided into an experimental group (ligation of the L5-L6 spinal nerves, n = 39) and a control group (sham operation for spinal nerve ligation, n = 26). All described procedures were approved by the Home Office (Project and Personal License) and follow the guidelines of the International Association for the Study of Pain (Zimmerman, 1983).
The procedure of ligation of the spinal nerves was performed as previously described by Kim & Chung (1992). Briefly, under halothane anaesthesia (1.5-2% in 66% N2O and 33% O2), the rat was placed in a prone position and the left paraspinal muscles were separated from the spinous processes at the L4-S2 level. Part of the L6 transverse process was carefully removed and the L4-L6 spinal nerves were identified. The L5-L6 spinal nerves were isolated and tightly ligated, distal to the dorsal root ganglion and proximal to the formation of the sciatic nerve, with 6–0 silk thread. Following complete haemostasis the wound was sutured. The total period of anaesthesia did not exceed 30 min. The procedure of the sham operation for spinal nerve ligation was identical to that of the experimental group, except that the spinal nerves were not ligated.
Behavioural studies
After surgery rats were maintained under the same conditions as during the pre-operative period. The posture and behaviour of the rats following recovery from the anaesthesia and during the first post-operative (PO) day were carefully monitored. Following spinal nerve ligation, rats sustained good health, including normal weight gain and a normal level of general activity and grooming. A mild deformity of the lesioned paw, with the toes being held together, was observed in the SNL rats but not in the sham-operated rats. None of the rats exhibited autotomy. From post-operative day 2 onwards, behavioural tests to assess sensitivity to mechanical and cooling stimuli were performed for up to 14 days. The weight gain and general behaviour of the rats were monitored throughout the PO period. In addition, observations of posture, foot position and growth of toe nails were made. For behavioural testing, rats were placed in transparent plastic cubicles on a mesh floored table, and a period of acclimatization was allowed prior to testing. The mechanical sensitivity of the ipsilateral and contralateral hindpaw was assessed by measuring the frequency of withdrawal of the foot to normally innocuous mechanical punctate stimuli. Stimuli were applied, from below, to the plantar surface of the foot with three different von Frey filaments (bending forces of 1, 5 and 9 g). Each trial consisted of the application of a single von Frey hair ten times, each for a period of 2–3 s. Testing with ascending consecutive von Frey hairs was separated by a period of at least 5 min. The occurrence of foot withdrawal for each trial was expressed as a percentage response frequency ((no. of foot withdrawals/10 (no. of applications)) × 100 =% response frequency). The sensitivity of the ipsilateral and contralateral hindpaw to cooling was assessed as previously described (Kim et al. 1997) by the application of a drop of acetone onto the plantar region of the foot. Although this stimulus may include both chemical and tactile components, it was used to ensure that these original behavioural reports could be replicated. In this case each trial consisted of five applications of acetone, each separated by a period of 5 min. The response frequency to acetone was calculated as the (no. of foot withdrawals/5 (no. of applications)) × 100.
Electrophysiological studies
Electrophysiological studies of experimental spinal nerve-ligated (SNL) and sham-operated rats were performed at two post-operative periods (PO days 7–10 and PO days 14–17). Methods were similar to those previously described (Dickenson & Sullivan, 1990). Rats were intact, anaesthesia was induced with 2–3% halothane in 66% N2O and 33% O2, and a tracheal cannula was inserted. Rats were placed in a stereotaxic frame to ensure stability during electrophysiological recordings. A laminectomy was performed, lumbar vertebrae L1-L3 were located and segments L4-L5 of the spinal cord were exposed. The cord was held rigid by clamps caudal and rostral to the exposed section. Upon completion of surgery the level of anaesthesia was reduced to 1.5-1.8% halothane, which produced a state of complete areflexia. It has previously been shown that at this depth of anaesthesia blood pressure is in the range of 100–140 mmHg and electrocorticograms exhibit regular slow waves, with neither parameter being overtly altered by noxious stimuli (Weil-Fugazza, Godefroy & Le Bars, 1984). This level of anaesthesia was maintained throughout experiments that lasted up to 9 h. During this time the core body temperature of the rat was monitored and maintained (36.5-37°C) by means of a heating blanket connected to a rectal thermal probe via an automatic feedback control unit. The rats breathed spontaneously throughout the experiment and therefore were able to regulate their acid-base balance. At the end of the experiment rats were killed with an overdose of halothane.
Extracellular recordings of convergent dorsal horn neurones, ipsilateral to spinal nerve ligation or sham procedure, were made with parylene-coated tungsten electrodes, which were descended through the cord by a SCAT microdrive. The depth of the neurone from the surface of the dorsal horn of the spinal cord was recorded. An average of three neurones per rat were characterized. Data were captured and analysed by a CED 1401 interface coupled to a Pentium computer with Spike2 software (rate and post-stimulus histogram functions).
The criteria for selection of a neurone was a Aβ-fibre-evoked response followed by a C fibre-evoked response to electrical stimulation. Any spontaneous activity of neurones was recorded over a period of at least 10 min or until response stabilization. Note that all evoked responses to natural stimuli were normalized by the subtraction of the spontaneous activity of the individual neurone. The response of the neurone to various natural stimuli (brush, prod (5 mm diameter; 4 N cm−2), mechanical punctate and thermal (heating and cooling)), of the most responsive part of the receptive field (preferred receptive field), were recorded. A large range of mechanical punctate stimuli (von Frey filaments) were employed, encompassing those considered under normal conditions to be innocuous (bending forces of 1, 5 and 9 g) and noxious (bending forces of 15, 20, 25 and 50 g). Thermal stimuli were applied as previously described (Bester, Menendez, Besson & Bernard, 1995) by a constant jet of water applied directly onto the receptive field (temperatures: 32, 37, 40, 42, 45 and 50°C). The neuronal response to cooling of the receptive field following the application of a drop of acetone was also recorded. All stimuli were applied for a period of 10 s and the frequency of the neuronal response to the applied stimuli was measured. Stimuli were applied at 5 min intervals.
The responses of neurones following transcutaneous electrical stimulation (2 ms wide pulses) of the centre of the receptive field were also recorded. All selected neurones had a clear short latency Aβ-fibre-evoked response followed by a C fibre-evoked response. Responses were elicited by a train of sixteen stimuli (frequency of 0.5 Hz) at three times the threshold for Aβ-fibres (stimulus range 0.14-0.2 mA) and then for C fibres (mean stimulus intensity 1.5 mA), and post-stimulus histograms were built. The Aβ-fibre-evoked responses were taken as the action potentials recorded 0–20 ms after the electrical stimulus, and C fibre-evoked responses were taken as the action potentials recorded 90–300 ms after the electrical stimulus. The remaining neuronal response (300–800 ms post-stimulus), occurring as the neurones exhibited a hyperexcitability to the repetitive stimulation, was taken as the post-discharge/afterdischarge of the neurone. Wind-up (Mendell, 1966) was calculated as the difference between the total number of action potentials at C fibre latencies (90–800 ms) produced by the train of sixteen stimuli and the baseline. The baseline, the non-potentiated response, was calculated as the number of action potentials produced by the first stimulation (initial baseline response) multiplied by the total number of stimuli (16). The mean depths of the neurones of spinal nerve-ligated rats and sham-operated rats studied at PO days 7–10 were 595 ± 36 and 653 ± 54 μm, respectively. The mean depths of the neurones of spinal nerve-ligated rats and sham-operated rats studied at PO days 14–17 were 688 ± 28 and 690 ± 38 μm, respectively.
Data are presented as means ± standard error of mean (s.e.m.) unless stated otherwise. Statistical analysis was performed with a one-way analysis of variance (ANOVA) with Fisher's protected least significant difference test and χ2 analysis, and levels of significance were taken as P < 0.05 and P < 0.001. Linear relationships were determined with a simple regression.
RESULTS
Behavioural studies
Behavioural testing showed a progressive development of evoked allodynia of the lesioned hindpaw of the SNL rats. Allodynia was apparent by the presence of a withdrawal response, and in some cases licking, of the lesioned hindpaw following stimulation with von Frey filaments, which are normally considered to be innocuous (1–9 g). Cooling of the lesioned hindlimb of SNL rats, by application of acetone, also evoked a withdrawal response. Both mechanical and cooling allodynia was observed as early as PO day 2. Allodynia reached a maximum at PO days 9–12 and was still maintained at PO day 14 (Fig. 1A). Neither mechanical stimulation (von Frey filament, 9 g) nor cooling with acetone of the contralateral hindpaw resulted in withdrawal responses (Fig. 1A). Sham-operated rats did not exhibit mechanical or cooling allodynia either ispilaterally or contralaterally (Fig. 1B).
Figure 1. The temporal development of mechanical and cooling allodynia of the hindpaw of rats with spinal nerve ligation but not sham-operated rats.
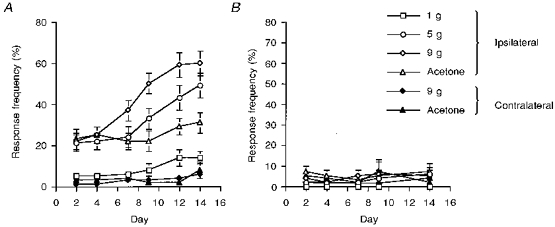
A, following spinal nerve ligation, rats exhibited brisk withdrawal responses of the ipsilateral (open symbols) hindpaw to stimulation with mechanical punctate stimuli in the innocuous range (bending weights, 1–9 g) and application of acetone. This mechanical and cooling allodynia developed progressively over a 2-week period. Stimulation of the contralateral (filled symbols) hindpaw with mechanical punctate stimulation (bending weight, 9 g) or acetone did not evoke any behavioural responses. The data, obtained from 39 rats with spinal nerve ligation, are presented as the mean response frequencies of withdrawal to the stimuli ±s.e.m. B, following sham surgery, rats did not exhibit withdrawal responses of the ipsilateral (open symbols) or contralateral (filled symbols) hindpaw to stimulation with mechanical punctate stimuli in the innocuous range (bending weights, 1–9 g) or application of acetone. The data, obtained from 26 rats with spinal nerve ligation, are presented as the mean response frequencies of withdrawal to the stimuli ±s.e.m.
Rats (SNL and sham) used in the above behavioural assessment of the manifestation of allodynia were subsequently used for electrophysiological studies of the spinal neuronal responses at one of the two designated PO periods (PO days 7–10 and PO days 14–17). The earlier PO period was chosen on the basis that at this stage allodynia increases in a linear fashion, presumably reflecting physiological changes associated with the nerve injury. During the later PO period, the level of allodynia, in particular to 9 g stimulation, had begun to plateau (Fig. 1A), indicating a stabilization, but maintenance, of the altered physiological state.
Electrophysiological studies Spinal nerve ligation at post-operative days 7–10
A total of forty-five ipsilateral dorsal horn neurones were characterized in sixteen SNL rats during PO days 7–10 (mean rat weight, 199 ± 4 g). Of the characterized neurones, 49% had a low level of on-going spontaneous activity (mean firing of 1.1 ± 0.25 Hz). Ninety-five per cent of the neurones responded to brush (mean firing of 17 ± 3 Hz) and prod (mean firing of 24 ± 3 Hz) of the receptive field of the neurone. Application of acetone directly onto receptive fields of neurones evoked a response (3 ± 1 Hz) in 66% of the studied neurones.
The response of the neurones to localized punctate mechanical stimuli of the receptive field with a range of von Frey filaments was studied. Ninety-eight per cent of the neurones responded to mechanical stimulation (mean threshold of 7.4 ± 1 g). The mean frequency of neuronal firing increased in a linear manner in response to stimulation with incrementally increasing strengths of von Frey filaments over the 1–25 g range. However, the stimulus response curve plateaued with the highest strength von Frey filament (50 g, Fig. 2). Ninety-six per cent of the neurones studied responded to thermal stimulation of the receptive field. The mean frequency of neuronal firing to thermal stimuli in the 32–42°C range did not increase with increased temperatures of stimulation. However, following thermal stimulation over the 42–50°C temperature range, the mean frequency of neuronal firing increased in a linear manner (Fig. 3A).
Figure 2. Responses of spinal neurones of spinal nerve-ligated and sham-operated rats to mechanical punctate stimulation of the peripheral receptive field at PO days 7–10.
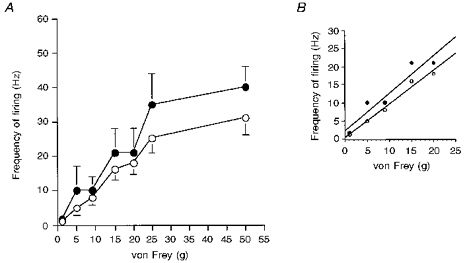
A, the mean frequency of firing of neurones of spinal nerve-ligated rats (^) and sham-operated rats (•) to a range of mechanical punctate stimuli at PO days 7–10. There were not dramatic differences between the responses of the two groups of neurones to the range of stimuli. However, there was a tendency for the evoked responses to the two higher von Frey bending weights of the neurones of the spinal nerve-ligated rats to be smaller than the responses of the sham-operated rats. B, the responses of both groups of neurones to the innocuous range of stimuli (1–9 g) were similar. The correlation coefficients for spinal nerve-ligated rats and sham-operated rats over this range were y = 0.9x+ 0.3, r2 = 0.98 and y = 1.0x+ 2.4, r2 = 0.91, respectively. The data are presented as the mean frequencies of the evoked firing (Hz) ±s.e.m.
Figure 3. Responses of spinal neurones of spinal nerve-ligated and sham-operated rats to thermal stimulation of the peripheral receptive field at PO days 7–10 and PO days 14–17.
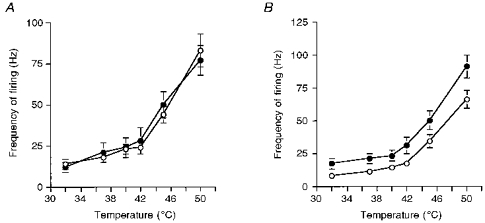
A, the mean frequency of firing of neurones of spinal nerve-ligated rats (^) and sham-operated rats (•) to a range of thermal stimuli at PO days 7–10. The responses of the neurones of the two groups were very similar. Neuronal responses increased incrementally with higher temperatures of thermal stimuli (42–50 °C). B, the mean frequency of firing of neurones of spinal nerve-ligated rats (^) and sham-operated rats (•) to a range of thermal stimuli at PO days 14–17. The responses of the neurones of the two groups were similar. Neuronal responses increased incrementally with higher temperatures of thermal stimuli (42–50 °C). There was a shift in the stimulus response function of the neurones of spinal nerve-ligated rats compared with sham-operated rats. Data are presented as the mean frequencies of the evoked firing (Hz) ±s.e.m.
The latencies and thresholds of neuronal responses following electrical Aβ-fibre and C fibre stimulation was also measured (Table 1). The mean electrically evoked responses of the neuronal population following a train of sixteen stimuli (0.5 Hz) at three times the threshold for Aβ-fibres and C fibres were measured (Table 2). The neuronal population exhibited enhanced responses, termed wind-up, and afterdischarge (Table 2) following constant intensity repetitive electrical stimulation at three times the strength for C fibres, but not Aβ-fibres.
Table 1.
A comparison of the mean latencies and thresholds of electrical C fibre- and Aβ-fibre- evoked responses of spinal neurones of spinal nerve-ligated and sham-operated rats at the two post-operative periods
| Post-operative day 7–10 | Post-operative day 14–17 | |||
|---|---|---|---|---|
| Spinal nerve ligation | Sham-operated | Spinal nerve ligation | Sham-operated | |
| C fibre latency (ms) | 188 ± 6 | 188 ± 7 | 195 ± 8 | 192 ± 7 |
| C fibre threshold (mA) | 1.5 ± 0.1 | 1.5 ± 0.09 | 1.5 ± 0.09 | 1.5 ± 0.08 |
| Aβ-fibre latency (ms) | 10 ± 0.5 | 8.6 ± 0.5 | 9 ± 0.5 | 8 ± 0.4 |
| Aβ-fibre threshold (mA) | 0.2 ± 0.05 | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.05 |
Table 2.
A comparison of the mean electrically evoked responses of spinal neurones of spinal nerve-ligated and sham-operated rats at the two post-operative periods
| Post-operative day 7–10 | Post-operative day 14–17 | |||
|---|---|---|---|---|
| Spinal nerve ligation | Sham-operated | Spinal nerve ligation | Sham-operated | |
| C fibre | 258 ± 21 | 272 ± 38 | 228 ± 16*** | 339 ± 24 |
| Aβ-fibre | 92 ± 6 | 84 ± 6 | 79 ± 5 | 105 ± 6 |
| Afterdischarge | 215 ± 26 | 199 ± 32 | 215 ± 22 | 274 ± 38 |
| Wind-up | 199 ± 26 | 252 ± 46 | 199 ± 30 | 302 ± 45 |
Mean C fibre-evoked neuronal responses of SNL rats at PO days 14–17 were significantly smaller than the evoked neuronal responses of sham-operated rats
P < 0.0001. Values are presented as mean action potentials ± s.e.m.
A total of twenty-three ipsilateral dorsal horn neurones were characterized in seven sham-operated rats during PO days 7–10 (mean rat weight, 212 ± 7 g). Twenty per cent of the neurones exhibited low levels of spontaneous activity (mean firing of 0.14 ± 0.12 Hz). Seventy-five per cent of the neurones responded to brush of the receptive field (mean firing of 27 ± 6 Hz) and 95% of the neurones responded to prod (mean firing of 40 ± 10 Hz). Application of acetone directly onto receptive fields of neurones evoked firing (7 ± 2 Hz) in 41% of the studied neurones.
Eighty-seven per cent of the neurones responded to localized punctate mechanical stimullation of the receptive field (mean threshold of 7.3 ± 2 g). The stimulus response function of these neurones to incrementally increasing strengths of von Frey filaments was similar to that observed in the SNL rats at this time point (Fig. 2). The rate of firing of the neurones increased with increasing intensities of von Frey stimuli over the 1–25 g range, which then plateaued between 25 and 50 g (Fig. 2).
Eighty-seven per cent of the neurones responded to thermal stimulation of the receptive field. Overall, the thermally evoked neuronal responses of the sham rats were identical to those observed in SNL rats at this time point (Fig. 3A). Again at the lower end of the temperature range studied (32–42°C), the mean frequency of neuronal firing did not increase incrementally with increasing temperatures. However, the mean frequency of neuronal firing increased in a linear manner with stimulation over the 42–50°C temperature range.
The latencies and thresholds of these neurones to electrical Aβ-fibre and C fibre stimulation were also studied (Table 1). Again, the mean electrically evoked responses of the neuronal population following stimulation at three times the threshold for Aβ-fibres and C fibres were measured (Table 2).
A comparison of neuronal responses of SNL and sham-operated rats at post-operative days 7–10
A significantly higher percentage of neurones of the SNL rats (50%) compared with sham-operated rats (20%) exhibited spontaneous activity (P = 0.03), the frequency of which was significantly higher in SNL rats compared with sham-operated rats (P = 0.03). A higher proportion of neurones responded to the various stimuli (brush, acetone, thermal and mechanical punctate) in the SNL rats, compared with the sham-operated rats, although a significant increase was only observed for brush (P = 0.02, Table 3). The magnitude of neuronal responses of the SNL rats to brush, prod and acetone were comparable to evoked neuronal responses of sham-operated rats (Table 4).
Table 3.
A comparison of the neuronal populations responding to various types of natural stimuli at the two post-operative periods
| Post-operative day 7–10 | Post-operative day 14–17 | |||
|---|---|---|---|---|
| Spinal nerve ligation | Sham-operated | Spinal nerve ligation | Sham-operated | |
| Brush | 95%* | 78% | 60% | 60% |
| Prod | 100% | 95% | 78%* | 100% |
| Acetone | 66% | 40% | 26% | 26% |
| Mechanical punctate | 98% | 87% | 85% | 91% |
| Thermal | 95% | 87% | 91% | 97% |
A higher proportion of neurones of SNL rats at PO days 7–10 responded to brush stimuli compared with the responsive neuronal population of sham-operated rats at this time point
P = 0.02. A smaller proportion of neurones of SNL rats at PO days 14–17 responded to prod stimuli compared with the responsive neuronal population of sham-operated rats at this time point
P = 0.007.
Table 4.
A comparison of the mean naturally evoked responses of neurones of spinal nerve-ligated and sham-operated rats at the two post-operative periods
| Post-operative day 7–10 | Post-operative day 14–17 | |||
|---|---|---|---|---|
| Spinal nerve ligation | Sham-operated | Spinal nerve ligation | Sham-operated | |
| Brush | 17 ± 3 | 27 ± 6 | 19 ± 3* | 28 ± 4 |
| Prod | 24 ± 3 | 40 ± 10 | 32 ± 4* | 50 ± 7 |
| Acetone | 3 ± 0.8 | 7 ± 2 | 6 ± 3 | 4 ± 1 |
At PO days 14–17 the mean brush-
P = 0.05 and prod-evoked (*P = 0.002) neuronal responses were significantly smaller than the evoked neuronal responses of sham-operated rats at this time point. Values are presented as mean frequencies of firing (Hz) ± s.e.m.
The thresholds and latencies of the spinal neurones of the SNL and sham-operated rats to electrical stimulation were similar (Table 1), as was the magnitude of the electrically evoked responses of the two groups of neurones (Table 2). The mechanical stimuli response functions of the neurones of the SNL and sham-operated rats were similar over the innocuous and noxious range of mechanical stimuli employed (Fig. 2). The thresholds of neurones of the SNL and sham-operated rats to mechanical stimuli were comparable, although 16% of neurones of the SNL but only 4% in sham-operated rats had a threshold of 9 g. The thermal stimuli response function of the neurones of the SNL and sham-operated rats were comparable (Fig. 3A).
Spinal nerve ligation at post-operative days 14–17
A total of sixty-six ipsilateral dorsal horn neurones were characterized in twenty-three SNL rats during PO days 14–17 (mean rat weight, 272 ± 6 g). Fifty-four per cent of the neurones had spontaneous activity (mean firing of 4.3 ± 1.2 Hz, Fig. 4A). Sixty per cent of the neuronal population responded to brush (mean firing of 19 ± 3 Hz) and 78% of the neurones responded to prod (mean firing of 32 ± 4 Hz, Fig. 4A) of the receptive field. Twenty-six per cent of the neuronal population responded to application of acetone with a mean firing rate of 6 ± 3 Hz.
Figure 4. The individual response pattern of a neurone of a spinal nerve-ligated rat at PO days 14–17.
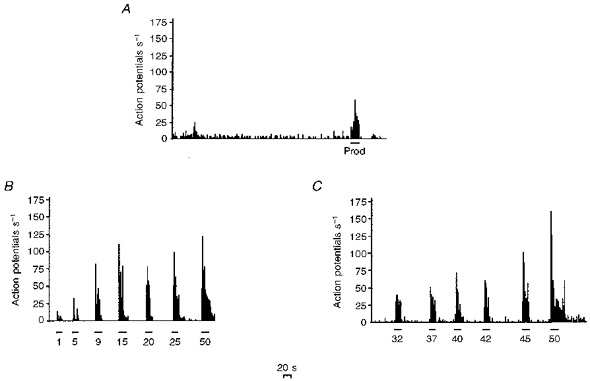
A, an example of the on-going spontaneous activity of a single neurone; the response of this neurone to prod stimulation is indicated and the period of stimulation is shown by the horizontal bar. B, the response of this neurone to graded mechanical punctate stimuli. The strengths of the stimuli, in grams, are indicated on the x-axis and the duration of stimulation is shown by the horizontal bars. C, the response of this neurone to graded thermal stimuli. The temperatures of the stimuli (°C) are indicated on the x-axis and the duration of stimulation is shown by the horizontal bars. In all cases the x-axis represents time in seconds (the scale is indicated), and the y-axis represents action potentials per second.
Eighty-five per cent of the neurones responded to mechanical punctate stimulation of the receptive field with a range of von Frey filaments (mean threshold of 7 ± 0.8 g). The mean firing rate of the neurones increased in a linear manner with incrementally increasing strengths of von Frey filaments over the 1–20 g range (Figs 4B and 5). With higher strength von Frey filaments, the mean neuronal response plateaued (Figs 4B and 5).
Figure 5. Responses of spinal neurones of spinal nerve-ligated and sham-operated rats to mechanical punctate stimulation of the peripheral receptive field at PO days 14–17.
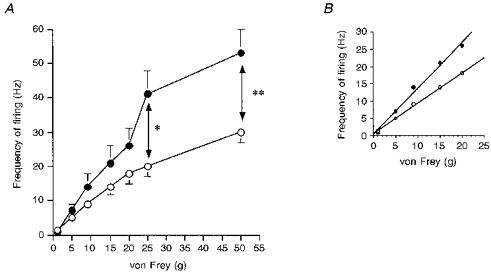
A, the mean frequency of firing of neurones of spinal nerve-ligated rats (^) and sham-operated rats (•) to a range of mechanical punctate stimuli at PO days 14–17. Overall, there was a shift in the stimulus response function of the neuronal responses of the spinal nerve-ligated rats compared with the sham-operated rats. The mean responses of the neurones of the spinal nerve-ligated rats to mechanical stimuli in the noxious range (25 and 50 g) were significantly smaller than the responses evoked in sham-operated rats. B, the slopes of the stimulus response functions of the two groups of spinal neurones to the innocuous range of stimuli (1–9 g) were different. However, the correlation coefficients for the spinal nerve-ligated rats and sham-operated rats indicated that both response functions were linear (y= 0.88x+ 0.65, r2= 0.99 and y= 1.33x+ 0.4, r2= 0.988). The data are presented as the mean frequency of the evoked firing (Hz) ± s.e.m.; levels of significance: * P < 0.05, **P < 0.001.
Ninety-one per cent of the neuronal population responded to thermal stimuli; over the lower range of temperatures studied (32–42°C), the rate of neuronal firing did not increase with increased temperatures of stimulation (Figs 3B and 4C). However, the stimulus response curve was linear over the temperature range of 42–50°C (Figs 3B and 4C).
The latencies and thresholds of these neurones to electrical Aβ-fibre and C fibre stimulation was also studied (Table 1). The mean electrically evoked responses of the neuronal population at three times the threshold for Aβ-fibres and C fibres were measured (Table 2).
A total of thirty-five ipsilateral dorsal horn neurones were characterized in nineteen sham-operated rats during PO days 14–17 (mean rat weight, 233 ± 3 g). Eight per cent of the neurones had spontaneous activity (mean firing of 1 ± 0.1 Hz). Sixty per cent of the neuronal population responded to brush (mean firing of 28 ± 4 Hz) and all of the neurones responded to prod (mean firing of 50 ± 7 Hz). Twenty-six per cent of the neurones responded to application of acetone (mean firing of 4 ± 1 Hz).
Ninety-one per cent of the neurones responded to mechanical punctate stimuli (mean threshold of 8 ± 1.7 g). The mean firing rate of the neurones increased with incrementally increasing strengths of von Frey filaments over the 1–50 g range studied (Fig. 5).
Ninety-seven per cent of the neurones responded to thermal stimulation of the receptive field. Over the lower range of temperatures studied (32–42°C), the rate of neuronal firing did not greatly increase with increased temperatures of stimulation (Fig. 3B). However, the stimulus response curve was linear over the temperature range of 42–50°C (Fig. 3B).
The latencies and thresholds of these neurones to electrical Aβ-fibre and C fibre stimulation were also measured (Table 1). Again, the mean electrically evoked responses at three times the threshold for Aβ-fibres and C fibres were measured (Table 2).
A comparison of neuronal responses of SNL and sham-operated rats at post-operative days 14–17
A significantly higher (P < 0.0001) percentage of neurones of the SNL rats (> 50%) compared with sham-operated rats (< 10%) exhibited spontaneous activity, the frequency of which was significantly elevated in SNL rats compared with sham-operated rats (P = 0.02). At this time point there was a significant decrease in the proportion of neurones of the SNL rats that responded to prod stimuli, compared with sham-operated rats (P = 0.007, Table 3). Brush- and prod-evoked neuronal responses of SNL rats were significantly smaller than the evoked responses of the sham-operated rats (P = 0.05 and P = 0.002, respectively; Table 4).
The mean latency and thresholds of the electrically evoked responses were similar for the SNL and sham-operated rats (Table 1). The magnitude of the C fibre-evoked neuronal responses of SNL rats was significantly smaller than that of sham-operated rats (P < 0.0001, Table 2). The mechanical stimuli response function of the neurones of SNL rats was to the right of the response function of sham-operated rats (Fig. 5). Neuronal responses of SNL rats evoked by 25 and 50 g von Frey weights were significantly smaller than evoked neuronal responses of sham-operated rats (Fig. 5). The mean thresholds of the neurones of the SNL and sham-operated rats to mechanical stimuli were comparable. However, 23% of neurones in SNL rats and 9% in sham-operated rats had thresholds of 9 g. Note that a significantly (P = 0.03) higher proportion of neurones in SNL rats at PO days 14–17, compared with PO days 7–10, had a threshold of 9 g for mechanical punctate stimuli. The thermal stimuli response function of the neurones of SNL rats was to the right of the stimulus response function of the neurones of sham-operated rats (Fig. 3B).
DISCUSSION
In this study we have replicated previously published behavioural data showing that after L5-L6 spinal nerve ligation, the ipsilateral hindpaw develops an increased mechanical sensitivity and rats exhibit directed pain behaviour to normally innocuous stimuli, termed mechanical allodynia (Kim & Chung, 1992; Kim et al. 1997). In addition, as previously shown, application of acetone to the ispilateral hindpaw also evoked directed pain behaviour (cooling allodynia; Kim et al. 1997). The temporal development of mechanical and cooling allodynia demonstrated in our study was comparable to that observed in previous studies (Kim & Chung, 1992; Kim et al. 1997). As they have previously demonstrated, neither innocuous mechanical stimuli nor cooling stimuli evoked pain behaviour in the sham-operated rats.
Here we present the first full electrophysiological characterization of the proportions of responsive neurones and the magnitude of neuronal responses at different time points following spinal nerve ligation (SNL) compared with sham-operated rats. All neurones responded to peripheral stimulation of the lesioned hindpaw innervated by the sciatic nerve. As spinal nerves L5 and L6 were ligated, inputs into these segments were attenuated, so that the majority of the responsive neurones were rostral to the ligated segments.
Comparison of behavioural and electrophysiological findings
A number of behavioural studies have shown that the spinal application of different drugs, given at various post-operative time points, can reduce the mechanical allodynia that develops following SNL (Bowersox, Gadbois, Singh, Pettus, Wang & Luther, 1996; Lee & Yaksh, 1996; Chaplan, Malmberg & Yaksh, 1997; Hwang & Yaksh, 1997) and thermal hyperalgesia (Wegert et al. 1997). These previous studies suggest that there is a contribution of an altered spinal processing of sensory information to the final allodynia and hyperalgesia behaviour observed in this model. Here we have shown that twice as many spinal neurones of SNL rats have spontaneous activity compared with sham-operated rats. The frequency of the spontaneous activity of the spinal neurones of SNL rats was far higher than that of spinal neurones of sham-operated rats. The proportion of neurones of SNL rats with spontaneous activity was similar for the early and later PO periods, yet the frequency of spontaneous activity was far higher at the later PO period. These findings are in keeping with previous studies of spinal nerve ligation showing that signals from the injured fibres, or the dorsal root ganglion, to the spinal cord are important in the development and maintenance of neuropathic behaviour (Sheen & Chung, 1993; Yoon et al. 1996). The spontaneous activity of the spinal neurones of the SNL rats was greater at later post-operative time points, thus paralleling the time course of the evoked behavioural allodynia. Although we did not assess the extent of the on-going pain behaviour following spinal nerve ligation, such behaviour has been described (Kim et al. 1997), and, as previously hypothesized (Yoon et al. 1996), this may be related to the increased spontaneous activity of spinal neurones of SNL rats that we report here. The origin of this spontaneous activity of the spinal neurones is unknown. However, studies of other models of nerve injury have shown the generation of ectopic activity at the injury site (Wall & Devor, 1983; Tal & Eliav, 1996) and dorsal root ganglia (Wall & Devor, 1983; Kajander et al. 1992; Petersen et al. 1996; Study & Kral, 1996). Thus it is probable that the spontaneous activity we describe here is secondary to the generation of ectopic activity at the injury site or within the dorsal root ganglion.
Behavioural studies have shown that there is a drop in the threshold of the ipsilateral hindpaw to mechanical stimuli and a decreased withdrawal latency to thermal stimuli following spinal nerve ligation (Kim & Chung, 1992; Wegert et al. 1997). We did not find evidence for a reduction in the threshold of the neurones to mechanical punctate stimuli. However, we found that the proportion of neurones that had a threshold of 9 g for mechanical punctate stimuli were higher in SNL rats at the later PO time point (days 14–17) compared with both day 7–10 and sham-operated rats. In our behavioural studies we found that 9 g stimulation of the ipsilateral hindpaw consistently evoked a high frequency of hindlimb withdrawal, indicative of pain, in SNL rats at PO days 14–17, but not the sham-operated rats.
Temporal changes in the evoked neuronal responses after spinal nerve ligation
We report here temporal changes in the proportion of neurones responsive to different stimuli. At the early PO time point (days 7–10) the proportion of neurones of SNL rats responding to brush and acetone was larger than the proportion of neurones of sham-operated rats. However, at this time point the mean evoked responses of the neurones to brush and acetone were smaller in SNL compared with sham-operated rats. Overall, the proportional decrease of the mean neuronal response was greater than the increased proportion of responsive neurones, suggesting that the final excitability of the system to these stimuli was reduced. This is further exemplified when considering the response of neurones to prod. Although at the early PO time point the proportion of neurones responding to prod was similar for SNL and sham-operated rats, the mean evoked response of the neurones of the SNL rats was nearly 50% smaller than that of sham-operated rats. At this PO time point, neuronal responses to innocuous mechanical punctate stimuli were comparable for both SNL and sham-operated rats. However, at the same time, the noxious mechanically evoked responses of the neurones of the SNL rats were lower than the sham-operated rats. At this stage in the development of the neuropathic state, the electrically evoked responses and graded thermally evoked responses were comparable for the SNL and sham-operated rats.
At later time points (PO days 14–17) there were concomitant reductions in the proportion of neurones responding to brush, prod and acetone, and the magnitude of the responses. In addition, there was a shift in the innocuous mechanical punctate stimuli response function of the neuronal responses of SNL rats compared with sham-operated rats. As can be seen in Fig. 5, there was a reduction in the slope of the stimulus-response relationship of the neurones of SNL rats compared with sham-operated rats. This would indicate that the gain of the coding of this innocuous stimulus is altered in such a way that a smaller increment intensity of the stimulus produces a greater proportional shift in activity. If sensory coding of the stimulus relies on relative changes in activity, this may be a potential basis for allodynia. However, it should be noted that the absolute magnitude of the responses to a given stimulus intensity was reduced. In addition, there were also differences in the responsiveness of neurones of SNL rats to noxious mechanical punctate stimuli, compared with sham-operated rats. At this later stage of the neuropathic state, the electrically evoked and noxious thermally evoked responses of the neurones were smaller than those in sham-operated rats. The thresholds of the spinal neurones to the electrical and mechanical stimuli were not modified at either PO time points compared with sham-operated rats.
Overall, we report here temporal, intensity-dependent changes in the mechanical stimulus response function of the spinal neurones of SNL rats compared with sham-operated rats. At the early post-operative time points, although there was no modification in the responses of neurones to low intensity stimuli (normally innocuous), there was a decline in the magnitude of the responses of neurones of SNL rats to high-intensity mechanical stimuli. At the later post-operative time points, there was a pronounced shift to the right in the entire mechanical stimulus response function of the spinal neurones of SNL rats compared with sham-operated rats. These modifications in the responses of the spinal neurones to mechanical stimuli may result from changes in mechanical transduction processes. However, since the thresholds to mechanical stimuli were not modified, this would seem unlikely.
Results from other models of sciatic nerve injury in the rat
As discussed earlier, although there are numerous models of nerve injury, there are few electrophysiological studies of the changes in the responsiveness of spinal neurones following nerve injury. The findings of our study are similar in some aspects to the reported changes in spinal neurone responses in two other models of peripheral nerve injury.
Following chronic constriction injury (CCI model) of the sciatic nerve, a high percentage of spinal neurones, many of which had absent somatic receptive fields (Laird & Bennett, 1993), exhibited abnormal levels of spontaneous activity (Palecek et al. 1992b; Laird & Bennett, 1993). In addition, spinal neurones had increased afterdischarges (Palecek et al. 1992b), and were sensitive to tapping of the nerve injury site (Laird & Bennett, 1993). The number of neurones sensitive to low-intensity mechanical stimuli (Laird & Bennett, 1993), and the magnitude of mechanically evoked responses of the neurones were also reduced (Palecek et al. 1992b) compared with sham-operated rats; we observed a similar pattern.
Only subtle and late changes in spinal neuronal responses have been reported after ligation of one-third of the sciatic nerve (Seltzer model of neuropathy); these minor changes had a different temporal profile from the associated behavioural allodynia (Takaishi et al. 1996). The authors also reported the presence of neurones with absent mechanical receptive fields and neurones with enlarged mechanical receptive fields at PO week 16 (Takaishi et al. 1996). In addition, there were no changes in the thresholds of neurones to thermal stimuli, or the magnitude of the responses to graded thermal stimuli (Takaishi et al. 1996).
Comparisons with an electrophysiological study of the effects of spinal nerve ligation in the monkey
A previous study has characterized the responses of spinothalamic tract (STT) neurones of the monkey following ligation of spinal nerve L7. Monkeys exhibited mechanical allodynia and STT neurones had spontaneous activity, which was greater than that of the neurones of the sham-operated side, or normal monkeys (Palecek et al. 1992a). Overall, the thresholds of STT neurones to thermal and mechanical stimuli were decreased and the magnitude of the evoked responses of neurones rostral to the ligated segment were increased, compared with the contralateral sham-operated side. In contrast, neurones in the ligated segment had low responsiveness (Palecek et al. 1992a). The present study and the previous studies in the rat also reported increased spontaneous activity of spinal neurones. The findings of this primate study of increased rostral neuronal responses differs from both our present study and the other spinal electrophysiology studies in rodents with nerve injury, where less robust sensory responses have been reported. It is feasible that contralateral changes following spinal nerve ligation could modify the contralateral sham controls in the primate study. However, the contralateral responses were similar to those seen in unoperated monkeys. This discrepancy between the studies is even more surprising since the primate showed similar behavioural responses to the rats. Species differences or the selective sampling of STT neurones in defined segments versus the sampling of a broad range of spinal neurones may also explain the disparity in the findings.
Changes in neuronal responses contributing to plasticity following neuropathy
Overall, complex changes were observed in the pattern of responses of the same neurones of SNL rats to different peripheral stimuli. These changes varied depending on the modality of stimulus applied, and involved both innocuous and noxious intensities of stimulation. Consequently, this differential plasticity of the sensory neurones must arise from predominantly peripheral alterations in A- and C fibre function, since any overall change in spinal neuronal function would be expected to produce modality-independent alterations. It is not possible to ascertain whether the observed plasticity after nerve injury results from acquired de novo neuronal responses or changes in the existing response profiles of the neurones. As previously discussed, there are a number of alterations in peripheral nerve function in this model. A common factor in all models of nerve injury is a reduction in peripheral drive, accompanied by aberrant activity generated in the neuroma and/or DRG. Following spinal nerve ligation, we observed reduced neuronal responses in keeping with a previous report of reduced responses in the ligated segment in the monkey (Palecek et al. 1992a). Since this latter study also reported enhanced neuronal responses in segments rostral to the ligated segment, it would be of interest to compare relative neuronal activites over a number of defined segments. In addition to peripheral factors, we cannot rule out discrete changes in the intrinsic spinal pharmacology or physiology that could selectively influence different modalities of afferent activity, mechanisms of which may differ between models.
It has been proposed (Woolf & Doubell, 1994), that reorganization of afferent termination sites of Aβ-fibres in the dorsal horn could result in these low-threshold inputs activating projection neurones, thus targeting inappropriate higher brain centres. Clinically, it is clear that allodynia is mediated by low-threshold myelinated fibres, and in order for these normally innocuous inputs to be perceived as painful, abnormal central processing of these inputs would have to occur (see references in Torebjork, Wahren, Wallin, Hallin & Koltzenburg, 1995). A possible central basis for some aspects of central hypersensitivity is wind-up, which could relate to allodynia and hyperalgesia. Wind-up is an N-methyl-D-aspartate (NMDA) receptor-mediated increase in response to a constant stimulus, normally only elicited by high-threshold fibre stimulation. Although the high level of on-going spontaneous activity that follows SNL indicates a global increase in spinal excitability, the post-discharge and wind-up of the C fibre-mediated responses of the neurones of SNL rats were no different from the sham-operated rats at any PO time point. It has been reported that following peripheral inflammation, repeated Aβ-fibre stimulation and low-threshold natural stimuli can now produce wind-up (Woolf & Doubell, 1994). We never observed wind-up of neuronal responses following repetitive stimulation at Aβ-fibre intensities at any time point following spinal nerve ligation. However, mechanical allodynia associated with SNL is attenuated by spinal NMDA receptor antagonists (Chaplan et al. 1997; Wegert et al. 1997). Although we see no evidence for Aβ-fibre-induced wind-up, the arrival of low-threshold inputs onto altered spinal circuitry could account for these findings. Furthermore, following peripheral inflammation, a novel NMDA receptor mediation of low-threshold evoked activity and spontaneous activity was seen (Chapman & Dickenson, 1994). This body of physiological data provides a basis for future electrophysiological studies of the possible pharmacological substrates contributing to the symptoms of neuropathic pain.
Similar to our finding of less robust spinal sensory responses following neuropathy, positron emission tomography (PET) scans have revealed decreased contralateral thalamic activity in patients with chronic neuropathic pain (Di Piero et al. 1991; Iadarola et al. 1995). However, in contrast with these clinical findings, electrophysiological studies have demonstrated enhanced evoked responses of neurones in the ventrobasal thalamus complex and somatosensory cortex in CCI rats (see references in Guilbaud, 1991). These authors also provided evidence for marked reorganization of neurones in these areas, but as yet there have been no studies at these levels in the SNL model of neuropathy.
Our electrophysiological studies have shown that the responses of spinal neurones following SNL are modified compared with sham-operated rats. The temporal profile of these changes is similar to the profile of the development of mechanical and cooling allodynia. The problem of reconciliation of the behavioural and electrophysiological data that we report in this model, in common with that described in other models (Palecek et al. 1992b; Laird & Bennett, 1993), exemplifies the difficulty in understanding the relationship between the multiple foci plasticity of the somatosensory system and chronic pain states that follow nerve injury. Effectively, we have shown that the changes in neuronal responses that follow spinal nerve ligation are sensory deficits to stimuli in both the innocuous and noxious range. However, these same rats exhibited behavioural allodynia just prior to the electrophysiological studies and there was evidence for an increased level of spinal excitability, as indicated by high levels of spontaneous activity. The altered stimulus-response relationships we have reported may be a basis for the allodynias and thermal hyperalgesia reported in this model. Thus the concomitant presence of enhancements and reductions of neuronal activity are reminiscent of clinical descriptions of neuropathic pain (see Introduction), where pain and hypoaesthesia can coexist. The potential contribution of de novo neural responses to the behavioural alterations following this form of nerve injury, although difficult to assess, should be considered. The problem in understanding the causal basis of the behavioural and clinical symptoms is not surprising when considering the multiplicity of the factors, acting at many levels of the nervous system, that can potentially contribute to the consequences of nerve injury.
Acknowledgments
This study was funded by Glaxo-Wellcome, The Wellcome Trust and EEC Biomed II. R. S. has an ORS award.
References
- Basbaum AI, Gautron M, Jazat F, Mayes M, Guilbaud G. The spectrum of fiber loss in a model of neuropathic pain in the rat: an electron microscopic study. Pain. 1991;47:359–367. doi: 10.1016/0304-3959(91)90229-Q. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie Y-K. A peripheral mononeuropathy in rat produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–109. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bester H, Menendez L, Besson JM, Bernard JF. Spino (trigemino) parabrachiohypothalamic pathway: electrophysiological evidence for an involvement in pain processes. Journal of Neurophysiology. 1995;73:568–585. doi: 10.1152/jn.1995.73.2.568. [DOI] [PubMed] [Google Scholar]
- Bowersox SS, Gadbois T, Singh T, Pettus M, Wang Y-X, Luther RR. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. Journal of Pharmacology and Experimental Therapeutics. 1996;279:1243–1249. [PubMed] [Google Scholar]
- Burchiel KJ. Effects of electrical and mechanical stimulation on two foci of spontaneous activity which develop in primary afferent neurons after peripheral axotomy. Pain. 1984;18:249–265. doi: 10.1016/0304-3959(84)90820-0. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Dougherty PM, Pover CM, Coggeshall RE. Neuroma formation and numbers of axons in a rat model of experimental peripheral neuropathy. Neuroscience Letters. 1991;131:88–92. doi: 10.1016/0304-3940(91)90343-r. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. Journal of Pharmacology and Experimental Therapeutics. 1997;280:829–838. [PubMed] [Google Scholar]
- Chapman V, Dickenson AH. Enhanced responses of rat dorsal horn neurones after UV irradiation of the hindpaw; roles of the NMDA receptor. Neuroscience Letters. 1994;176:41–44. doi: 10.1016/0304-3940(94)90866-4. [DOI] [PubMed] [Google Scholar]
- Chapman V, Suzuki R, Dickenson AH. Carbamazepine and gabapentin modulate aberrant spinal neuronal responses after chung neuropathy. Society for Neuroscience Abstracts. 1997;23(2):1811. [Google Scholar]
- Dickenson AH, Sullivan AF. Differential effects of excitatory amino acid antagonists on dorsal horn nociceptive neurones in the rat. Brain Research. 1990;506:31–39. doi: 10.1016/0006-8993(90)91195-m. 10.1016/0006-8993(90)91195-M. [DOI] [PubMed] [Google Scholar]
- Di Piero V, Jones AKP, Iannotti F, Powell M, Perani D, Lenzi GL, Frackowiak RSJ. Chronic pain: a PET study of the central effects of percutaneous high cervical cordotomy. Pain. 1991;46:9–12. doi: 10.1016/0304-3959(91)90026-T. 10.1016/0304-3959(91)90026-T. [DOI] [PubMed] [Google Scholar]
- Guilbaud G. Neuronal responsivity at supra-spinal levels (ventraobasal thalamus complex and SM1 cortex) in a rat model of mononeuropathy. In: Besson JM, Guilbaud G, editors. Lesions of Primary Afferent Fibres as a Tool for the Study of Clinical Pain. Elsevier Science Publishers B.V; 1991. pp. 219–232. [Google Scholar]
- Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. 10.1016/S0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63:55–64. doi: 10.1016/0304-3959(95)00015-K. 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: A partial and differential deafferentation and spontaneous discharge in Aβ and Aδ primary afferent neurons. Journal of Neurophysiology. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neuroscience Letters. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Experimental Brain Research. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Laird JMA, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. Journal of Neurophysiology. 1993;69:1–14. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- Lee Y-W, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. Journal of Pharmacology and Experimental Therapeutics. 1996;277:1642–1648. [PubMed] [Google Scholar]
- Leem JW, Park ES, Paik KS. Electrophysiological evidence for the antinociceptive effect of transcutaneous electrical stimulation on mechanically evoked responsiveness of dorsal horn neurons in neuropathic rats. Neuroscience Letters. 1995;192:197–200. doi: 10.1016/0304-3940(95)11644-c. 10.1016/0304-3940(95)11644-C. [DOI] [PubMed] [Google Scholar]
- Lekan HA, Carlton SM, Coggeshall RE. Sprouting of Aβ fibers into lamina II of the rat dorsal horn in peripheral neuropathy. Neuroscience Letters. 1996;208:147–150. doi: 10.1016/0304-3940(96)12566-0. 10.1016/0304-3940(96)12566-0. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Experimental Neurology. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Munger BL, Bennett GJ, Kajander KC. An experimental painful peripheral neuropathy due to nerve constriction. Experimental Neurology. 1992;118:204–214. doi: 10.1016/0014-4886(92)90037-q. 10.1016/0014-4886(92)90037-Q. [DOI] [PubMed] [Google Scholar]
- Na HS, Leem JW, Chung JM. Abnormalities of mechanoreceptors in a rat model of neuropathic pain: possible involvement in mediating mechanical allodynia. Journal of Neurophysiology. 1993;70:522–528. doi: 10.1152/jn.1993.70.2.522. [DOI] [PubMed] [Google Scholar]
- Palecek J, Dougherty PM, Kim SH, Paleckova V, Lekan H, Chung JM, Carlton SM, Willis WD. Responses of spinothalamic tract neurons to mechanical and thermal stimuli in an experimental model of peripheral neuropathy in primates. Journal of Neurophysiology. 1992a;68:1951–1966. doi: 10.1152/jn.1992.68.6.1951. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Dougherty PM, Carlton SM, Willis WD. Responses of spinothalamic tract cells to mechanical and thermal stimulation of skin in rats with experimental peripheral neuropathy. Journal of Neurophysiology. 1992b;67:1562–1573. doi: 10.1152/jn.1992.67.6.1562. [DOI] [PubMed] [Google Scholar]
- Petersen M, Zhang J, Zhang JM, LaMotte RH. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–397. doi: 10.1016/0304-3959(96)03146-6. 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sheen K, Chung JM. Signs of neuropathic pain depend on signals from injured nerve fibres in a rat model. Brain Research. 1993;610:62–68. doi: 10.1016/0006-8993(93)91217-g. 10.1016/0006-8993(93)91217-G. [DOI] [PubMed] [Google Scholar]
- Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Eisele JH, Carstens E. Behavioral and electrophysiological assessment of hyperalgesia and changes in dorsal horn responses following partial sciatic nerve ligation in rats. Pain. 1996;66:297–306. doi: 10.1016/0304-3959(96)03023-0. 10.1016/0304-3959(96)03023-0. [DOI] [PubMed] [Google Scholar]
- Tal M, Eliav E. Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 1996;64:511–518. doi: 10.1016/0304-3959(95)00175-1. 10.1016/0304-3959(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Torebjork E, Wahren LK, Wallin G, Hallin R, Koltzenburg M. Noradrenaline-evoked pain and neuralgia. Pain. 1995;63:11–20. doi: 10.1016/0304-3959(95)00140-N. 10.1016/0304-3959(95)00140-N. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Wegert S, Ossipov MH, Nichols ML, Bian D, Vanderah TW, Malan TP, Porreca F., Jr Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve injured rats. Pain. 1997;71:57–64. doi: 10.1016/s0304-3959(97)03337-x. 10.1016/S0304-3959(97)03337-X. [DOI] [PubMed] [Google Scholar]
- Weil-Fugazza J, Godefroy F, Le Bars D. Increase in 5-HT synthesis in the dorsal part of the spinal cord, induced by a nociceptive stimulus: blockage by morphine. Brain Research. 1984;297:247–264. doi: 10.1016/0006-8993(84)90566-3. 10.1016/0006-8993(84)90566-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Doubell TP. The pathophysiology of chronic pain - increased sensitivity to low threshold Aβ-fibre inputs. Current Opinion in Neurobiology. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Yoon YW, Heung SN, Chung JM. Contributions of injured and intact afferents to neuropathic pain in an experimental rat model. Pain. 1996;64:27–36. doi: 10.1016/0304-3959(95)00096-8. 10.1016/0304-3959(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Zeltser R, Seltzer Z. A practical guide for the use of animal models in the study of neuropathic pain. In: Boivie J, Hansson P, Lindblom U, editors. Touch, Temperature and Pain in Health and Disease: Mechanisms and Assessments. Progress in Pain Research and Management. Vol. 3. Seattle, USA: IASP Press; 1994. pp. 295–338. [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


