Abstract
Stepping responses were studied in infants between the ages of 10 days and 10 months while they were supported to step on a slowly moving treadmill belt. Surface electromyography (EMG) from muscles in the lower limb, force exerted by the feet on the treadmill belt, and the motion of the lower limbs were recorded.
Two groups of infants were studied, those who had a small amount of daily practice in stepping and those who did not. Practice resulted in a dramatic increase in the incidence of stepping recorded in the laboratory, particularly for the periods between 1 and 6 months of age.
The majority of infants showed clear alternation between the flexor and extensor muscles during walking, regardless of age. Co-contraction between flexors and extensors, estimated by the overlap in area between rectified and smoothed EMG from a muscle pair, was greater for some muscle groups in the infant compared with the adult.
Practice resulted in a significantly lower co-contraction index for the tibialis anterior- quadriceps muscle pair. Practice did not affect the mean step cycle duration.
Infants of all ages could step at a range of treadmill speeds by adjusting their step cycle duration. The relationship between the treadmill speed and cycle duration was well fitted by a power function, similar to those reported for intact cats and adult humans. The change in step cycle duration resulted almost entirely from a change in the extensor burst duration, whereas the flexor burst duration remained constant.
Airstepping could be elicited in some infants. The cycle durations for airstepping were close to the shortest cycles recorded on the treadmill.
In conclusion, the system for generating rhythmic, alternating activity of the lower limbs for stepping is clearly developed by birth. The stepping is sustained and regular, particularly if stepping practice is incorporated briefly each day. The infant population provides a good subject pool for studying the afferent control of walking in the human, before cerebral influences are fully developed. The characteristics and maturity of the system remain to be determined.
The response of the walking human to specific, transient sensory disturbances is well documented (reviewed in Dietz 1986; Stein, Yang, Edamura & Capaday, 1991). Many of these responses in the human resemble those found in other mammals, such as the cat (reviewed in Rossignol, 1996). Interestingly, however, there are certain responses typically seen in reduced preparations of cats that are very weak in the adult human. For example, unloading the extensor muscles of the limb in the late stance phase is necessary for the initiation of the subsequent swing phase in the spinal or decerebrate cat (e.g. Duysens & Pearson, 1980; Conway, Hultborn & Kiehn, 1987; Gossard, Brownstone, Barajon & Hultborn, 1994). The magnitude of such responses was extremely weak in the intact human (Stephens & Yang, 1996a,b). Other conditions such as the importance of hip extension for the initiation of the swing phase (Grillner & Rossignol, 1978) are clearly not functioning in the same way in the intact human as in cat preparations, since humans can easily walk with large degrees of hip flexion such as are needed when walking in a crouched position through a low tunnel. The question remains, however, whether the underlying mechanisms for controlling walking are fundamentally different between these species, or whether the preparations studied (i.e. intact human versus spinal or decerebrate cats) can account for the differences. There is certainly some suggestion that the preparation can have a profound influence. The responses of load-sensitive receptors in the muscle, in particular, differ depending on whether the animal is spinalized, decerebrated (McCrea, Shefchyk, Stephens & Pearson, 1995) or intact (Whelan & Pearson, 1998). The reflex responses to force changes are clearest in immobilized spinal or decerebrate cats (Conway et al. 1987; Gossard et al. 1994; Guertin, Angel, Perreault & McCrea, 1995), less strong in walking decerebrate cats (Whelan, Hiebert & Pearson, 1995), and weakest in intact cats (Whelan & Pearson, 1998).
One of the ways to address the above question is to study walking in humans, in states comparable to the decerebrate or spinal cat. Adult humans are sometimes in such states, but when in those states, stepping behaviour is extremely rare (Calancie, Needham-Shropshire, Jacobs, Willer, Zych & Green, 1994; Hanna & Frank, 1995; Dietz, Colombo, Jensen & Baumgartner, 1995). Infants, on the other hand, exhibit a stepping response at birth (Peiper, 1929), and indeed much earlier in utero (de Vries, Visser & Prechtl, 1984). This stepping behaviour appears to be controlled largely by the spinal and brainstem circuitry, since anencephalic infants exhibit similar responses (Peiper, 1961). Thus, infants offer the opportunity for studying the response of humans to similar transient disturbances in sensory input during stepping, before the cerebrum exerts its full control. The response of infants to such disturbances may offer a glimpse at the underlying mechanisms for controlling human walking. This paper presents the development of feasible procedures to study the role of sensory input in stepping in the human infant.
In order to study how sensory input is controlled during infant stepping, regular, sustained stepping is needed. Minor perturbations can then be applied during a sequence of steps to determine the response to the disturbance (reviewed in Rossignol, 1996). Stepping can be elicited in infants at birth through to about 2 months of age. Ideally, this would be the best age to study stepping, because cerebral influences on stepping are presumably smallest at this time. In reality, infants at this age are rarely in an awake and alert state, optimal for eliciting stepping. After 2 months of age, stepping is difficult to obtain for a duration of about 4–6 months (McGraw, 1940). The ability to study stepping during this time (i.e. between 2 and 7 months of age) is clearly advantageous, because the infant is alert for longer periods of time in the day, and the cerebral influences probably remain small. Daily practice in stepping is known to prevent the disappearance of the stepping response and increase the number of steps obtained in the laboratory (Zelazo, Zelazo & Kolb, 1972). Thus, the first issue addressed in this paper is the effect of such practice. It is not clear whether daily practice would produce a sufficiently regular pattern of walking, and whether it would induce changes in the locomotor pattern itself. Thus, the walking patterns of two groups of infants were compared, those with practice and those without.
The second issue addressed is whether the muscle activation patterns of infant stepping are regular and reproducible. Most earlier studies on supported stepping in infants reported that co-contraction was common (Forssberg & Wallberg, 1980; Berger, Altenmueller & Dietz, 1984; Forssberg, 1985, 1986; Thelen & Cooke, 1987), and that clear alternation of flexors and extensors was not present until much later in development, well after independent walking has been established (Berger et al. 1984; Leonard, Hirchfeld & Forssberg, 1991). Only one study reported clear reciprocally alternating contractions in supported walking (Okamoto & Goto, 1985). In re-examining this issue, our results agree with Okamoto & Goto (1985), in which alternation of flexor and extensor contraction was the norm in infants of all ages during supported stepping.
Finally, the responses to changes in sensory input induced by changes in treadmill speed or ground support were determined. Infants responded to changes in speed by modifying the extensor burst duration without much change in the flexor burst, as is the case in intact cats (Halbertsma, 1983) and adult humans (Grillner, Halbertsma, Nilsson & Thorstensson, 1979). When airstepping could be induced, the cycle durations were short, and the co-ordination of the two limbs less rigid than treadmill walking, as in spinal cats (Bradley & Smith, 1988).
METHODS
Subjects
A total of seventy-seven infants were studied; their ages ranged from 10 days to 10 months (see Table 1). Among these infants, thirty-eight performed some stepping. Stepping was defined as a minimum of four consecutive steps in one sequence. Infants were recruited through a number of different routes, including the maternity wards of four major hospitals, and the community health programme in Edmonton. Ethical approval was obtained from the appropriate facilities. The parent provided informed, written consent for the infant to participate in the study. Only healthy babies, born at or after 32 weeks gestation were included.
Table 1.
Number of infants in each experimental group
| Age (months) | 0-1 | 1-2 | 2-3 | 3-4 | 4-5 | 5-6 | 6-7 | 7-8 | 8-9 | 9-10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No practice | 14 | 7 | 6 | 5 | 3 | 6 | 7 | 7 | 2 | 1 | 58 |
| Practice | 0 | 2 | 4 | 3 | 3 | 1 | 4 | 1 | 0 | 1 | 19 |
The number of infants in each of the two groups, with no practice stepping and with practice stepping, are shown for the various age ranges. No infants were tested in the 0–1 and the 8–9 month age ranges who had practice stepping.
The infants were divided into two groups arbitrarily. The infants studied in the first 6 months of the project had no practice. Infants that were recruited subsequently were divided into two groups. Those who had parents that were already practicing stepping with them, or those who were recruited shortly after birth and had parents that were willing to institute daily practice were included in the group with practice (see details below). Those who were either recruited at a later age and the parents were not practicing stepping with them, or those recruited early and whose parents were not interested in practicing stepping were entered into the group without practice. The parents of the group without practice (58 in total) received no particular instructions except they were encouraged to bring their infant in for the experiment at an optimal time (i.e. about 1 h after a feed in very young infants, and during a time of day the child was normally alert for the older infants). The parents of the group with practice (19 in total) were given instructions on how to help their infant practice stepping. The method for eliciting the stepping response (Andre-Thomas & Autgarden 1966; Prechtl, 1977) was described to the parent(s) either in person or on the telephone. Parents were asked to elicit the response twice a day for a short time (about 1 min). Regular phone contact (every 2 weeks) was made with the parents to determine how the practice was progressing. The total duration of practice varied with the age of the infant at the time of testing. For fifteen of the nineteen infants, practice started the day we first made contact with the parents (on average, 3.5 weeks after birth), and was continued until the day of testing. The other four infants were recruited at an older age (on average, 4.5 months), whose parents were already practicing stepping with them. The same instructions were given to the parents, and they continued practicing until the day of testing. On average, 2.8 months elapsed between our first contact with the parents and the testing date. The total number of infants in the group with practice is considerably smaller than the group without practice. The numbers in the groups are uneven, because we wanted a similar number of subjects in each group that generated stepping, in order to compare the pattern of stepping (e.g. 21 without practice and 17 with practice).
Recording procedures
The recording session was typically 1 h long, and varied very little between infants of different age groups. Beckman surface electromyographic (EMG) electrodes were placed over four muscle groups, which could be the quadriceps (Quad) and tibialis anterior (TA) muscles on each leg, or the Quad, hamstrings (Ham), gastrocnemius-soleus (GS) and TA muscles of the left leg. Generally, miniature electrodes (2 mm recording diameter) were used for the lower leg and regular electrodes (7 mm recording diameter) were used for the thigh muscles, except for the very young infants, in which miniature electrodes were used on all muscles. The skin was cleaned with rubbing alcohol before application of electrodes. The electrode pairs were separated by approximately 1 cm. Adhesive skin markers were placed over the superior boarder of the iliac spine, the greater trochanter of the femur, the knee line, the lateral malleolus and the head of the fifth metatarsal of the left lower limb, for identification of landmarks on video. Depending on the size of the infant's foot, one or two force-sensitive resistors (Interlink Electronics, Camarillo, CA, USA), 2.5 cm in diameter were taped to the sole of the foot or shoe. For the purposes of other experimental manipulations not described here, a twin axis electrogoniometer (Penny and Giles, Biometrics Ltd, Blackwood Gwent, UK) was placed over the right hip joint in some infants.
When the sensors had been positioned, the infant was held over a slowly moving treadmill with his/her feet making contact with the treadmill belt. Young infants were held under their arms; some older infants (> 7 months old) preferred to be held by their hands. The belt speed was adjusted to a level that seemed optimal for eliciting stepping movements for each infant. A video camera recorded the motion of the left side of the body, and the analog data were recorded on VHS tape with the use of a PCM encoder (A.R. Vetter Co. Inc., Rebersburg, PA, USA). These two recording modes were synchronized by a pulse that generated a light signal on video and a pulse on analog tape at a rate of 1 Hz.
Each trial typically lasted 1 or 2 min, after which the infants rested. During these rest periods, EMGs were often recorded, to determine whether clear and separate bursts of EMG could be obtained from each muscle group. This is important, as cross-talk between muscles is more likely to occur in infants than adults because of their small size. Recordings of spontaneous movements were made for about 2–5 min while the infant was lying down or being held, without being excessively active. We reasoned that if there was no cross-talk, then over this period of time, isolated activity from each of the four muscles should be visible. If isolated EMG activity could not be obtained from a muscle group, then the EMG data from that muscle were considered suspect for cross-talk, but the data were still used for subsequent calculations on co-contraction. Quiet periods during the sequence of recording also allowed us to estimate the noise level in each channel.
Data analysis
The video data were examined for sequences of sustained stepping. A hard copy of the raw data was printed on a chart recorder, and the sequences of data corresponding to good stepping (as identified on the video record) were selected for analysis. The EMG data were then full-wave rectified and low-pass filtered at 30 Hz, and converted from analog to digital form together with the data from the force sensitive resistors, goniometer and synchronization pulse at 250 Hz. The data from undisturbed walking steps were averaged with alignment to either: (1) the beginning of foot-floor contact, as indicated by the force-sensitive resistors, or (2) the onset of muscle activity in a muscle that showed a clear bursting pattern with each step (usually the TA). The duration of the step cycle and EMG bursts were calculated from these averages.
Co-contraction was calculated from the EMG in the step cycle after averaging across a number of undisturbed walking steps. The step cycles were all normalized to 100% in time before averaging, so that slight differences in step cycle duration did not result in blurring of the EMG. The area under the averaged EMG-time curve for one muscle in a step cycle was calculated after removal of a bias. The bias was defined as a 50–100 ms window with the lowest EMG amplitude in the average. The bias values were similar to the values obtained during times when the muscle was quiescent in the same subject, as recorded during the period of spontaneous activity. The area under the averaged EMG from a muscle was defined as 100%, and the amplitude at each point in time expressed as a percentage of this total. The index of co-contraction was defined as the overlap in area between any two muscle pairs. For example, at any point in time in the step cycle, if both EMGs from a pair of antagonist muscles were not zero, then the overlap at that point in time was defined by the EMG with the lower amplitude. The total amount of overlap in the step cycle is the sum of these individual overlap points. The maximum possible overlap was 100%. These co-contraction indices were compared with data obtained in adults from another study (Stephens & Yang, 1996a). The differences in the co-contraction index between infants and adults were compared using Student's t test.
The relationship between (1) cycle duration and treadmill speed, (2) EMG burst durations and treadmill speed or cycle duration, (3) cycle duration and age were quantified by fitting the data to regression lines. The relationships for (2) and (3) above were fitted with a straight line, and that for (1) was fitted with a power function of the form y = a/xb, where y is the cycle duration (in s) and x is the velocity (in m s−1) of the treadmill belt (Grillner et al. 1979; Halbertsma, 1983). For the straight-line relationships, the value of the slope was tested to determine if it was significantly different from zero using Student's t test (Pedhazur, 1982). For the power function, the goodness of fit was evaluated with an F ratio (Pedhazur, 1982).
Sequences of airstepping were identified from video, and the cycle durations were calculated either from the video record (i.e. from initial hip flexion to hip flexion again) or from the EMG record (i.e. the onset of TA activity). The cycle durations during airstepping were compared with the mean cycle duration during treadmill walking with Student's paired t test. The significance level was set at 0.05 for all statistical tests.
RESULTS
Practice increased the incidence of obtaining stepping
The incidence of stepping in the laboratory varied with age. Stepping was defined as four continuous, alternating steps on the treadmill. Incidence of stepping was defined as the number of infants in an age group that showed stepping at least once in the experiment. Without training, stepping was especially difficult to obtain in infants between the ages of 0 to 6 months (Fig. 1A). Training resulted in a dramatic increase in the incidence of stepping in these infants (Fig. 1B). By the age of about 7–8 months, most infants showed stepping on the treadmill without training. In Fig. 1B, an asterisk indicates that no data were collected for those age groups. Note that the 5–6 month age group with practice was based on one child only. Thus, that data point may not be reliable. Nevertheless, even if this data point were removed, the trend from all other age groups remains clear.
Figure 1. Incidence of stepping.
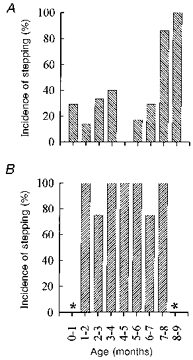
The incidence of obtaining sustained stepping (4 or more alternating steps) on the treadmill for 2 groups of infants: 58 who had no systematic practice stepping (A) and 19 who practiced each day (B). Practice resulted in a dramatic increase in the chance of obtaining stepping. The asterisk in B indicates no data were collected for those age groups. The number of infants in each group is shown in Table 1.
The mean number of stepping sequences also increased with training. Figure 2 shows the mean number of stepping sequences that contained five or more continuous, alternating steps per experimental session in infants who showed stepping. The number of such sequences also increased with training. Note that all infants tested between the ages of 4 and 5 months who had no practice stepping did not step in the laboratory (Fig. 2, ♦).
Figure 2. Effect of stepping practice.
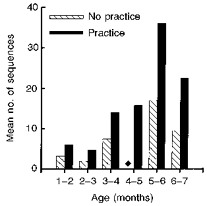
Stepping practice resulted in a substantial increase in the mean number of stepping sequences that contained 5 or more steps in a testing session in infants who showed stepping. Note that in the 4–5 months age group, no stepping could be elicited in any of the infants without practice (♦).
EMG patterns in stepping
Clear alternation of flexors and extensors could be obtained during stepping from infants of all ages. Figure 3 illustrates different muscle groups for three different infants, aged 2 to 7.2 months. The EMG pattern of walking averaged across a number of steps is shown on the right-hand side of Fig. 3. Note that Quad, Ham and GS muscles are co-active in the stance phase, and the TA is active in the swing phase (Fig. 3C). EMG bursts at the beginning of the stance phase were seen in some infants (Fig. 3B and C). These early bursts were most commonly seen in extensor muscles, but occasionally in flexor muscles too.
Figure 3. Electromyography and foot contact patterns during treadmill stepping.
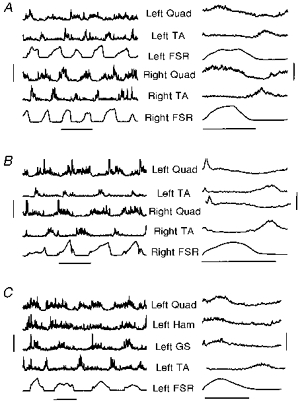
The EMGs from left and right limbs during treadmill stepping are shown for subjects B. P. and A. J. in A and B, while those from 4 muscles in the left limb are shown for subject C. D. in C. The ages of these subjects at the time of recording were 2, 7.2 and 3.3 months, and the treadmill speed was 0.10, 0.19 and 0.12 m s−1, respectively, for A, B and C. The vertical bars on the left indicate the scale for each of the EMG graphs, which is 200 μV for A, 120 μV for trace 4, and 100 μV for all other traces in B, and 60 μV for trace 2, 50 μV for trace 4, and 100 μV for traces 1 and 3 in C. The data from force sensors (FSR) are in arbitrary units. The traces on the right represent the means of a number of undisturbed steps (19 and 20 steps for the left and right legs for A, 62 and 72 steps for the left and right legs for B, and 25 steps for C). The EMG scale is indicated by the vertical bar on the right, which is 60 μV for trace 1 and 80 μV for all other traces in A, 30 μV for trace 2 and 70 μV for all other traces in B, and 20 μV for trace 2 and 40 μV for all other traces in C. The horizontal bars at the bottom of each section represent 1 s for each of the graphs.
The index of co-contraction averaged across all subjects who showed good EMG recordings from these muscle pairs is shown in Fig. 4. The mean index of co-contraction for the TA-Quad pair was not significantly different from adults, whereas that for the TA-GS pair was significantly higher in the infants. For an intuitive understanding of the co-contraction index, note that for the subjects shown in Fig. 3, the mean co-contraction index for the TA-Quad muscle pair was 47% for Fig. 3A, 31% for Fig. 3B, and 24% for Fig. 3C.
Figure 4. Index of co-contraction during stepping.
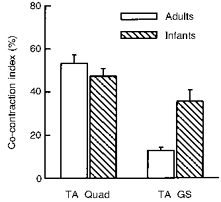
The index of co-contraction was not significantly different for the TA-Quad muscle pair for infants and adults, but the index was significantly higher for the infants in the TA-GS pair. These data represent pooled data from 19 infants and 7 adults for the TA-Quad muscle pair, and 12 infants and 10 adults for the TA-GS muscle pair.
Practice did not alter the stepping pattern appreciably. The step cycle duration was not significantly different for the two groups (mean ± s.d. was 1.92 ± 0.54 s with practice and 1.63 ± 0.53 s without practice). The co-contraction index for the TA-Quad group, however, was significantly lower in infants with practice (mean ± s.d. was 39 ± 14 s with practice, and 56 ± 14 s without practice). The combination of TA and GS recordings was not obtained in many infants. Thus, the co-contraction index was not calculated for this agonist-antagonist pair.
Infants adapted their stepping to the treadmill speed
The treadmill speed was systematically varied in nine subjects (ranging in age from 1.6 to 8.7 months). In these subjects, stepping could be obtained over a range of speeds, with the stepping becoming less consistent at the highest and lowest speeds. Data from an infant aged 1.6 months are shown in Fig. 5. The step cycle duration decreased with increasing treadmill speed (Fig. 5A). Moreover, the extensor burst duration is inversely correlated with the treadmill speed, and the flexor burst showed little change over the range of speeds examined (Fig. 5B). The slope of the regression line for the GS burst duration was significantly different from zero, whereas the one for the TA was not.
Figure 5. Step cycle and EMG burst durations at different speeds for one subject.
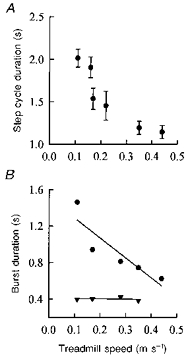
Subject I. R. was 1.6 months old at the time of testing. A, the step cycle duration decreases with increasing treadmill speed. B, this decrease results entirely from a decrease in the duration of the extensor (GS; •) burst (correlation coefficient, r = 0.89). The flexor (TA; ▾) burst in the swing phase remains unchanged for the different speeds (r = 0.22). Note that the flexor burst at the highest speed in this subject was not sufficiently clear to estimate the duration accurately.
Pooled across all thirty-eight subjects, the step cycle duration was strongly correlated with the speed of the treadmill (Fig. 6A). The data were fitted with a power function: y = a/xb. The best-fitting line yielded values for a and b of 0.65 and 0.55, respectively, values very similar to those reported by Halbertsma (1983) for the intact cat (a ranged from 0.495 to 0.608 and b from 0.54 to 0.65). The variance accounted for was 52%, the F ratio was highly significant (P < 0.05). A considerable amount of scatter is seen in the pooled data, indicating that at any one treadmill speed, different infants chose to step at very different rates, particularly at the slower treadmill speeds. Interestingly, when the extensor EMG burst durations were plotted against the step cycle duration (Fig. 6B), the relationship was highly linear regardless of treadmill speed. The slope of the relationship between burst and cycle duration was significantly different from zero for both the extensors and flexors.
Figure 6. Step cycle and EMG burst durations at different speeds for all subjects.
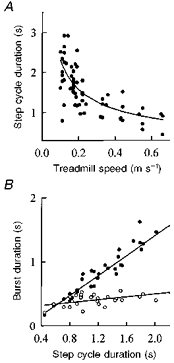
A, pooled data from 38 subjects shows that the step cycle duration varies systematically with treadmill speed. Note that 9 out of the 38 subjects were tested at a number of speeds, and thus contributed more than one data point to this figure. The relationship is fitted with a power function y = a/xb, where a = 0.65, b = 0.55. B, EMG burst durations for the gastrocnemius-soleus (GS; •) and tibialis anterior (TA; ^) muscles for the 9 subjects tested at a number of speeds indicates that the change in step cycle duration results almost entirely from a change in the extensor burst duration. The correlation coefficient, r, was 0.94 for the extensors and 0.53 for the flexors.
The data from the nine infants who stepped at a variety of speeds were used to determine whether age had a systematic effect on the preferred treadmill speed. We were confident that the entire range of speeds at which an infant could step were tested in most of the subjects in this group. The percentage of time in which stepping was observed at a particular treadmill speed was calculated. A minimum length of 20 s was deemed necessary to obtain a good estimate. Treadmill speeds at which less than 20 s of stepping was attempted were not used. One subject's data were not included for that reason. The speed with the highest percentage stepping time was considered representative of the infant's preferred speed. This preferred speed was plotted against age in Fig. 7A. Clearly, younger infants preferred a slower speed than older infants. Interestingly, at an intermediate speed where data were collected for most infants in this group (6 out of 9), the step cycle durations were similar for infants of all ages (Fig. 7B).
Figure 7. Preferred treadmill speed and step cycle duration for infants of different ages.
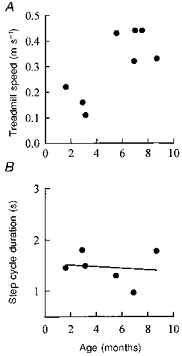
A, the treadmill speed at which the highest percentage of stepping was seen is shown for 8 of the 9 infants as a function of the infant's age. One subject did not have sufficiently long trials to make an accurate estimate. B, 6 of these 9 subjects stepped at an intermediate treadmill speed of 0.2 m s−1. At this constant speed, infants of all ages showed similar step cycle durations, r = 0.13, P > 0.05.
Airstepping
Airstepping was not easy to obtain. In the nine subjects in whom some airsteps (1–6 airsteps in different subjects) were obtained, the duration of the step cycle during the airstepping was significantly shorter than the mean cycle duration during treadmill walking (Fig. 8B). The EMG pattern during airstepping was usually less regular than treadmill walking, and the overlap or co-contraction between flexors and extensors was greater. Electromyographic data during airstepping from one subject is shown in Fig. 8A for two airsteps (left), and for a mean of four airsteps (right). Data from treadmill walking for the same subject are shown in Fig. 4B. Qualitatively, the relationship between the two limbs was less co-ordinated during airstepping than treadmill walking in all subjects. Reciprocally alternating steps were observed, as were single-sided stepping and synchronous stepping of both limbs.
Figure 8. EMG and cycle durations for airstepping.
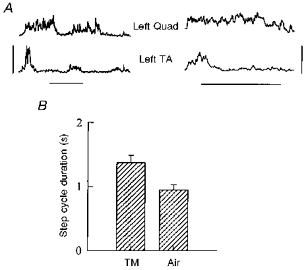
A, rectified and smoothed EMG data from subject A. J. during 2 airsteps is shown on the left. The mean over 4 steps in the same subject is shown on the right. The vertical bar represents 70 μV for the traces on the left and 40 μV for the traces on the right. The horizontal bars represent 0.5 s. B, the cycle duration pooled across 9 subjects shows that the airsteps were significantly shorter in duration than the treadmill steps.
DISCUSSION
The primary new findings in this paper are as follows. (1) Training dramatically increases the incidence of obtaining stepping, while having no significant effect on the cycle duration, and a minor effect on the EMG pattern of stepping. (2) The EMG from the leg muscles shows alternation between flexors and extensors in infants of all ages during supported treadmill stepping. (3) Infants adapt their stepping to the presence of ground support and to the treadmill speed. These adaptations suggest a rather mature rhythm-generating system.
Stepping practice makes it feasible to study infant stepping
Regular stepping with consistent EMG patterns can be obtained for short periods in infants. Daily practice enhances the success of obtaining stepping in the laboratory, consistent with earlier findings (Zelazo et al. 1972). This is important for future studies, as sustained, regular stepping for a short period is essential to study how the subject responds to disturbances. Practice thus makes it feasible to study how reflexes are controlled in slightly older infants (i.e. between 2 and 7 months). Stepping practice did not appear to alter the basic characteristics of the walking pattern, namely the optimal treadmill speed for stepping and the cycle duration. The EMG patterns remained qualitatively similar to age-matched infants that had not practiced stepping, but the co-contraction indices were significantly lower in infants who had practiced. Thus, the small amount of practice may have enhanced the expression of a more regular and reciprocally alternating stepping pattern.
Characteristics of infant stepping
Young infants stepped better at slower speeds. Why this was so, and why younger infants were unable to keep up with the faster treadmill speeds is unclear. It may be that the slower contraction speed of muscle in the young (Close, 1964), incomplete myelination and the small diameter of both peripheral and central nerves (e.g. Yakovlev & Lecours, 1967; Gutrecht & Dyck, 1970) which together result in slower conduction velocities made it impossible for the younger infants to keep up with the faster speeds. Slower locomotor rhythms are also seen in neonatal rats (Bekoff & Trainer, 1979: swimming; Westerga & Gramsbergen, 1993: walking) and cats (Bradley & Smith, 1988: walking). At a particular treadmill speed, the step cycle duration was not systematically different for infants of different ages except at the very slowest speed (not shown). Thus, other as yet unidentified factors must be more important in determining the duration of the step cycle. One possibility is the amount of weight borne by the legs (see below on airstepping), but this will require a more detailed recording of the forces under the feet.
Clear alternation between flexors and extensors was seen during walking in the majority of infants, in agreement with Okamoto & Goto (1985). This was true regardless of the infant's age. Since previous reports suggested co-contraction was prevalent, but did not quantify the degree of co-contraction (Berger et al. 1984; Forssberg, 1985, 1986; Thelen & Cooke, 1987; Leonard et al. 1991), we felt that such quantification would be useful. Quantification of the overlap between flexor and extensor activity indicated that larger amounts of co-contraction exist during stepping in the infant than in the adult for the muscles about the ankle (Fig. 4). Tonic activity of the flexors, as reported by others (Forssberg, 1985, 1986; Leonard et al. 1991), however, was rarely seen. A number of factors could account for the greater degree of co-contraction seen at the ankle in the infants. (1) The immaturity of the system may be important. (2) A second factor may be cross-talk, which remained suspect in two infants for the GS-TA muscle pair, and three infants for the TA-Quad muscle pair, as judged from the lack of independent activity between the two muscle groups throughout the experiment. The co-contraction index reported here, thus represents an overestimate. (3) The control of posture and equilibrium could also have lead to more co-contraction in the older infants, who supported a larger amount of their own weight, particularly in those who were held by their hands only (see also Okamoto & Kumamoto, 1972; Berger et al. 1984; Okamoto & Goto, 1985).
Independent walking requires the ability to generate rhythmic stepping movements of the lower limbs, in addition to the control of postural and volitional aspects of stepping. The stepping behaviour reported here reflects primarily the generation of rhythmic movements. This aspect of the behaviour appears well developed at birth. Indeed, the circuitry for controlling stepping is assembled very early in embryonic life. Many vertebrates including tadpoles (Stehouwer & Farel, 1985), chicks (Narayana & Hamburger, 1971; O'Donovan, Sernagor, Sholomenko, Ho, Antal & Yee, 1992), rats (Kudo, Ozaki & Yamada, 1991; Greer, Smith & Feldman, 1992; Robinson & Smotherman, 1992) and cats (Graham Brown, 1915; Windle & Griffen, 1931) show spontaneous or induced stepping movements with clear alternating flexor and extensor activity early in embryonic life (reviewed in Sillar, 1994). The ability to locomote soon after birth must have been an important evolutionary advantage in many animals. Alternating stepping-like movements are also seen very early in utero in humans, as early as 10 weeks gestation (de Vries et al. 1984). When an animal or human starts to develop the ability to walk independently, the added demands of postural control alter the muscle activation patterns initially, showing large degrees of co-contraction (Okamoto & Kumamoto, 1972; Berger et al. 1984; Okamoto & Goto, 1985; Bradley & Smith, 1988; Westerga & Gramsbergen, 1993). With practice, the co-contraction decreases, and the muscle activation pattern returns to a reciprocal pattern of firing again (Berger et al. 1984; Okamoto & Goto, 1985).
Flexor activity was usually large in amplitude and short in duration in the human infant whereas the extensor activity was generally more prolonged and of smaller amplitude (Fig. 3). Weaker extensor activity, particularly of the Quad muscle group, may have been because the infants were supported and required minimal extensor activity to remain upright. This may also have been the reason Thelen and colleagues reported minimal Quad activity (Thelen & Fisher, 1982). Interestingly, consistent and adult-like bursting also occurs earlier in the flexors than extensors during walking in neonatal rats (Westerga & Grambergen, 1993).
The EMG profiles from averaged data (Fig. 3, right) indicate that some of the characteristics of adult walking are not yet present. For example, the two-burst pattern in the TA muscle (one in the swing phase, and one at the transition from swing to stance), typical of adult plantigrade walking, is absent in these infants. Peculiarities of infant stepping, reported by others, were also noted. A burst of activity at the beginning of the stance phase, particularly in the GS muscle, but sometimes in other muscles as well, was common in infants, in agreement with others (Berger et al. 1984; Forssberg, 1985; Leonard et al. 1991). This is probably related to the hyperactive spinal reflexes at this time in life (e.g. Mayer & Mosser, 1973; Issler & Stephens, 1983; Myklebust, Gottlieb & Agarwal, 1986).
Infant stepping is responsive to sensory input
Infants clearly respond to a change in treadmill speed by adjusting their step cycle duration, and in particular the duration of the extensor burst. The nature of this relationship (Figs 5 and 6) is remarkably similar to that previously reported for spinal and intact cats (Forssberg, Grillner & Halbertsma, 1980; Halbertsma, 1983), and adult humans (Grillner et al. 1979). Indeed, the parameters estimated for the power function were very similar to those reported for cats (Halbertsma 1983). No values were reported for the coefficients fitted to the human adults (Grillner et al. 1979), so a comparison cannot be made.
The dependence of the step cycle duration on treadmill speed was seen in very young infants (see, for example, Fig. 5). This is contrary to other reports indicating the response to treadmill speed is not seen in the ‘first few months’ of life (Ulrich, Jensen & Thelen, 1991). Our data suggest that the way in which infants respond to changes in treadmill speed is remarkably similar to the adult.
Airstepping
Airstepping could occasionally be elicited, but, in general, the number of cycles that could be obtained at any one time was limited. Interlimb co-ordination during airstepping was sometimes alternating (as in treadmill walking), sometimes synchronous, and sometimes in one limb only. Weaker and more variable coupling between the limbs during airstepping has been reported for young kittens (Bradley & Smith, 1988). The step cycle duration in airstepping was short, close to that seen at the fastest speeds on the treadmill. These differences are consistent with the idea that the amount of load on the limb during the stance phase is positively related to the duration of the extensor burst (Duysens & Pearson, 1980; Conway et al. 1987; Pearson & Collins, 1993; Hiebert, Gorassini, Jiang, Prochazka & Pearson, 1994; Guertin et al. 1995; Whelan et al. 1995).
Summary
In summary, the gross EMG pattern from infant walking shows many of the characteristics of adult walking. Sensory input can clearly modify the stepping rhythm as seen with different treadmill speeds and ground contact conditions. Changes in the stepping rhythm are mostly a result of changes in the extensor burst duration. The results suggest that the circuitry generating the alternate stepping movements is well developed at birth. Much remains to be studied, however, before a definitive statement regarding the state of maturity of the stepping pattern can be made. Minimal daily practice greatly enhances the incidence of stepping recorded from the laboratory, and will be important for future studies on the reflex control of stepping in the infant.
Acknowledgments
This work was supported by a program project grant from the Medical Research Council of Canada to J. F. Y. M. J. S. was supported by a studentship from the Alberta Heritage Foundation for Medical Research. Special thanks are due to Charlotte Gorgichuk for assisting with recruitment of subjects, and to Anne Blank for assisting with early stages of this project. Drs D. Bennett, M. A. Gorassini, K. G. Pearson, A. Prochazka and R. B. Stein provided valuable comments on earlier versions of the manuscript. We are grateful to all the clinical facilities (University of Alberta Hospitals, Royal Alexandra Hospital, Caritas Health Group, Public Health Division of the Capital Health Authority) from whom we recruited subjects.
References
- Andre-Thomas, Autgarden S. Clinics in Developmental Medicine. Vol. 24. Lavenham, UK: Spastics Society Medical Education and Information Unit in association with W. Heinemann Medical Books, Lavenham Press; 1966. Locomotion from pre- to post-natal life: How the newborn begins to acquire psycho-sensory functions. [Google Scholar]
- Bekoff A, Trainer W. The development of interlimb co-ordination during swimming in postnatal rats. Journal of Experimental Biology. 1979;83:1–11. doi: 10.1242/jeb.83.1.1. [DOI] [PubMed] [Google Scholar]
- Berger W, Altenmueller E, Dietz V. Normal and impaired development of children's gait. Human Neurobiology. 1984;3:163–170. [PubMed] [Google Scholar]
- Bradley NS, Smith JL. Neuromuscular patterns of stereotypic hindlimb behaviours in the first two postnatal months. I. Stepping in normal kittens. Developmental Brain Research. 1988;38:37–52. doi: 10.1016/0165-3806(88)90084-3. [DOI] [PubMed] [Google Scholar]
- Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury: Evidence for a central rhythm generator for locomotion in man. Brain. 1994;117:1143–1159. doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat during development. Journal of Physiology. 1964;174:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- De Vries JIP, Visser GHA, Prechtl HFR. Fetal motility in the first half of pregnancy. In: Prechtl HFR, editor. Continuity of Neural Functions from Prenatal to Postnatal Life, Clinics in Developmental Medicine. Vol. 94. Oxford: Spastics International Medical Publications; 1984. pp. 46–64. [Google Scholar]
- Dietz V. Short and long latency proprioceptive reflexes during the standing and stepping of normal and hemiparetic human subjects. In: Grillner S, Stein PSG, Stuart DG, Forssberg H, Herman RM, editors. Neurobiology of Vertebrate Locomotion, Wenner-Gren International Symposium Series. Vol. 45. London: Macmillan; 1986. pp. 661–671. [Google Scholar]
- Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Annals of Neurology. 1995;37:574–582. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Research. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Experimental Brain Research. 1985;57:480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Development and integration of human locomotor functions. In: Goldberger ME, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord, Fida Research Series. III. New York: Liviana, Padova, Italy/Springer Verlag; 1986. pp. 53–63. [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiologica Scandinavica. 1980;108:269–281. doi: 10.1111/j.1748-1716.1980.tb06533.x. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Wallberg H. Infant locomotion: a preliminary movement and electromyographic study. In: Berg K, Eriksson BO, editors. Children and Exercise IX, International Series on Sport Sciences. Vol. 10. Baltimore, MD, USA: University Park Press; 1980. pp. 32–40. [Google Scholar]
- Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Experimental Brain Research. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Graham Brown T. On the activities of the central nervous system of the un-born foetus of the cat: with a discussion of the question whether progression (walking etc.) is a ‘learnt’ complex. Journal of Physiology. 1915;49:208–215. doi: 10.1113/jphysiol.1915.sp001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Respiratory and locomotor patterns generated in the fetal rat brain stem-spinal cord in vitro. Journal of Neurophysiology. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Grillner S, Halbertsma J, Nilsson J, Thorstensson A. The adaptation to speed in human locomotion. Brain Research. 1979;165:177–182. doi: 10.1016/0006-8993(79)90059-3. 10.1016/0006-8993(79)90059-3. [DOI] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Research. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. Journal of Physiology. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutrecht JA, Dyck PJ. Quantitative teased fibre and histological studies of human sural nerve during postnatal development. Journal of Comparative Neurology. 1970;138:117–130. doi: 10.1002/cne.901380109. [DOI] [PubMed] [Google Scholar]
- Halbertsma JM. The stride cycle of the cat: The modelling of locomotion by computerized analysis of automatic recordings. Acta Physiologica Scandinavica. 1983;(suppl. 521):8–75. [PubMed] [Google Scholar]
- Hanna JP, Frank JI. Automatic stepping in the pontomedullary stage of central herniation. Neurology. 1995;45:985–986. doi: 10.1212/wnl.45.5.985. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Gorassini MA, Jiang W, Prochazka A, Pearson KG. Corrective responses to loss of ground support during walking. II. Comparison of intact and chronic spinal cats. Journal of Neurophysiology. 1994;71:611–622. doi: 10.1152/jn.1994.71.2.611. [DOI] [PubMed] [Google Scholar]
- Issler H, Stephens JA. The maturation of cutaneous reflexes studied in the upper limb in man. Journal of Physiology. 1983;335:643–654. doi: 10.1113/jphysiol.1983.sp014556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Ozaki S, Yamada T. Ontogeny of rhythmic activity in the spinal cord of the rat. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Tokyo: Japan Scientific Societies Press; 1991. pp. 3–20. [Google Scholar]
- Leonard CT, Hirshfeld H, Forssberg H. The development of independent walking in children with cerebral palsy. Developmental Medicine and Child Neurology. 1991;33:567–577. doi: 10.1111/j.1469-8749.1991.tb14926.x. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation during fictive locomotion in the cat. Journal of Physiology. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw MB. Neuromuscular development of the human infant as exemplified in the achievement of erect locomotion. Journal of Pediatrics. 1940;17:747–777. [Google Scholar]
- Mayer RF, Mosser RS. Maturation of human reflexes. Studies of electrically evoked reflexes in newborns, infants and children. In: Desmedt JE, editor. New Developments in Electromyography and Clinical Neurophysiology. Vol. 3. Basel: Karger; 1973. pp. 294–307. [Google Scholar]
- Myklebust BM, Gottlieb GL, Agarwal GC. Stretch reflexes of the normal infant. Developmental Medicine and Child Neurology. 1986;28:440–449. doi: 10.1111/j.1469-8749.1986.tb14281.x. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Hamburger V. Motility in chick embryos with substitution of lumbosacral by brachial and brachial by lumbosacral spinal cord segments. Journal of Experimental Zoology. 1971;178:415–432. doi: 10.1002/jez.1401780402. [DOI] [PubMed] [Google Scholar]
- O'Donovan M, Sernagor E, Sholomenko G, Ho S, Antal M, Yee W. Development of spinal motor networks in the chick embryo. Journal of Experimental Zoology. 1992;261:261–273. doi: 10.1002/jez.1402610306. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Goto Y. Primate Morphophysiology, Locomotor Analyses and Human Bipedalism. Tokyo: University of Tokyo Press; 1985. Human infant pre-independent and independent walking; pp. 25–45. [Google Scholar]
- Okamoto T, Kumamoto M. Electromyographic study of the learning process of walking in infants. Electromyography. 1972;12:149–158. [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. Journal of Neurophysiology. 1993;70:1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Pedhazur EJ. Multiple Regression in Behavioral Research. 2. New York: CBS College Publishing; 1982. [Google Scholar]
- Peiper A. Die schreitbewegungen der neugeborenen. Monatsschrift fur Kinderheilkunde. 1929;45:444. [Google Scholar]
- Peiper A. Cerebral Function in Infancy and Childhood. New York: Consultants Bureau; 1961. [Google Scholar]
- Prechtl H. The Neurological Examination of the Full-term Newborn Infant, Clinics in Developmental Medicine. 2. Vol. 63. London: Heinemann Medical Books for Spastics; 1977. p. 54. [Google Scholar]
- Robinson SR, Smotherman WP. Fundamental motor patterns of the mammalian fetus. Journal of Neurobiology. 1992;23:1574–1600. doi: 10.1002/neu.480231013. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology section 12, Exercise: Regulation and Integration of Multiple Systems. New York: John Wiley; 1996. pp. 173–216. [Google Scholar]
- Sillar KT. Synaptic specificity: development of locomotor rhythmicity. Current Opinion in Neurobiology. 1994;4:101–107. doi: 10.1016/0959-4388(94)90038-8. 10.1016/0959-4388(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Farel PB. Development of locomotor mechanisms in the frog. Journal of Neurophysiology. 1985;53:1453–1466. doi: 10.1152/jn.1985.53.6.1453. [DOI] [PubMed] [Google Scholar]
- Stein RB, Yang J, Edamura M, Capaday C. Reflex modulation during normal and pathological human locomotion. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Tokyo: Japan Scientific Societies Press; 1991. pp. 335–346. [Google Scholar]
- Stephens MJ, Yang JF. The effects of load on the human step cycle. Society for Neuroscience Abstracts. 1996a;22:726.8. [Google Scholar]
- Stephens MJ, Yang JF. Short latency, non-reciprocal group I inhibition is reduced during walking in humans. Brain Research. 1996b;743:24–31. doi: 10.1016/s0006-8993(96)00977-8. 10.1016/S0006-8993(96)00977-8. [DOI] [PubMed] [Google Scholar]
- Thelen E, Cooke DW. Relationship between newborn stepping and later walking: a new interpretation. Developmental Medicine and Child Neurology. 1987;29:380–393. doi: 10.1111/j.1469-8749.1987.tb02492.x. [DOI] [PubMed] [Google Scholar]
- Thelen E, Fisher DM. Newborn stepping: an explanation for a ‘disappearing’ reflex. Developmental Psychology. 1982;18:760–775. 10.1037//0012-1649.18.5.760. [Google Scholar]
- Ulrich BD, Jensen JL, Thelen E. Stability and variation in the development of infant stepping: implications for control. In: Patla AE, editor. Adaptability of Human Gait. Amsterdam: Elsevier/North-Holland; 1991. pp. 145–164. [Google Scholar]
- Westerga J, Grambergen A. Changes in the electromyogram of two major hindlimb muscles during locomotor development in the rat. Experimental Brain Research. 1993;92:479–488. doi: 10.1007/BF00229036. [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Hiebert GW, Pearson KG. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Experimental Brain Research. 1995;103:20–30. doi: 10.1007/BF00241961. [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Pearson KG. Comparison of the effects of stimulating extensor group I afferents on cycle period during walking in conscious and decerebrate cats. Experimental Brain Research. 1998 doi: 10.1007/s002210050239. in the Press. [DOI] [PubMed] [Google Scholar]
- Windle WF, Griffen AM. Observations on embryonic and fetal movements of the cat. Journal of Comparative Neurology. 1931;52:149–188. [Google Scholar]
- Yakovlev PI, Lecours A-R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Zelazo PR, Zelazo NA, Kolb S. ‘Walking’ in the newborn. Science. 1972;176:314–315. doi: 10.1126/science.176.4032.314. [DOI] [PubMed] [Google Scholar]


