Abstract
Combinations of cDNAs encoding mouse and chick nicotinic acetylcholine receptor (nAChR) subunits were transiently transfected into human BOSC 23 cells, and the expressed receptors were studied by simultaneously recording transmembrane currents and fluorescence transients using the whole-cell patch-clamp technique, and confocal microscopy with the Ca2+ indicator dye fluo-3.
The fractional Ca2+ current, Pf, of nAChRs was evaluated as the normalized ratio of nicotine-evoked fluorescence transient over total charge entering the cell (F/Q ratio). Mouse fetal muscle nAChR channels had a Pf,αβγδ value of 2.1 %. The substitution of the γ subunit with the ɛ subunit resulted in a 2-fold increase in Pf (4.2 %). The difference in Ca2+ permeability was confirmed by determination of Ca2+/Cs+ permeability ratios.
Among the chick neuronal nAChRs tested, Pf,α3β4 was 4.6 %, while Pf,α4β4 and Pf,α4β2 were 3.0 % and 2.9 %, respectively.
The amplitude of the current elicited by the activation of α3β4 nAChRs increased as the external Ca2+ concentration was raised from 2 to 110 mm, whereas currents flowing through all other nAChRs tested were reduced to various extents.
Our findings indicate that the adult-type muscle nAChR (αβɛδ) is more permeable to Ca2+ than the fetal-type (αβγδ), while ganglionic-like α3β4 nAChR is more permeable to Ca2+ than the examined α4-containing nAChRs. The functional significance is discussed.
Nicotinic acetylcholine receptors (nAChRs) belong to a superfamily of ligand-gated receptors. Muscle and neuronal nAChRs have differential subunit composition, functional profile and significance (Bertrand & Changeux, 1995). In vertebrates, all nAChRs are cation-selective channels with a Ca2+ permeability that varies significantly among the various receptor types (Vernino, Amador, Luetje, Patrick & Dani, 1992; Seguela, Wadiche, Dineley-Miller, Dani & Patrick, 1993; Vernino, Rogers, Radcliffe & Dani, 1994; Villarroel & Sakmann, 1996).
Ca2+ permeability of ion channels has been traditionally estimated as the relative Ca2+/Na+ permeability (PCa/PNa) calculated using a derived Goldman-Hodgkin-Katz (GHK) constant field equation (Lewis, 1979). With this approach, first applied in the frog endplate (Lewis, 1979; Adams, Dwyer & Hille, 1980) and then extended to fetal mouse muscle nAChRs of BC3H1 cells (Vernino et al. 1992) and to vertebrate neuronal nAChRs (Castro & Albuquerque, 1995; Nutter & Adams, 1995), the general view is that neuronal nAChRs have higher permeability to Ca2+ than muscle nAChRs (Vernino et al. 1992).
Recently, a direct model-independent approach relating whole-cell currents and fluorescence-based Ca2+ influx measurements has been used extensively to estimate the fractional Ca2+ current flowing through ligand-gated receptor channels (Schneggenburger, Zhou, Konnerth & Neher, 1993; Zhou & Neher, 1993; Vernino et al. 1994; Burnashev, Zhou, Neher & Sakmann, 1995). With this approach, the Ca2+ permeability of muscle and neuronal nAChRs has been estimated in BC3H1 cells (Pf = 2 %, Vernino et al. 1994), chromaffin cells (Pf = 2.5 %, Zhou & Neher, 1993; Pf = 4.1 %, Vernino et al. 1994; Pf = 4.4 %, Rogers & Dani, 1995) and cultured superior cervical ganglion cells (Pf = 4.7 %, Rogers & Dani, 1995).
Since native nerve cell systems express a diversity of nAChRs with unknown stoichiometry and uncertain subunit composition, it appears difficult to refer specific functional patterns to identified nAChR subunit compositions. The expression of homogeneous populations of neuronal and muscle nAChRs in heterologous expression systems provides a useful tool for associating receptor structure to its own functional profile, and avoids possible influences of the native cellular environment (Fucile, Mileo, Grassi, Salvatore, Alemà & Eusebi, 1996; Buisson, Gopalakrishnan, Arneric, Sullivan & Bertrand, 1997; Ragozzino et al. 1997). In this paper we compare the Ca2+ permeability of muscle (αβγδ, αβεδ) and neuronal (α3β4, α4β4 and α4β2) nAChRs reconstituted in human cells using a combination of confocal microscopy fluorescence measurements and patch-clamp recordings.
METHODS
cDNAs and expression vectors
The cDNAs encoding chick neuronal nAChR α3 (P09481), α4 (P09482), β2 (P09484) and β4 (P26153) subunits cloned in the SV40-based expression vector Flip (Nef, Oneyser, Alliod, Couturier & Ballivet, 1988; Couturier et al. 1990) were kindly provided by Dr Marc Ballivet (University of Geneva, Switzerland). Full-length cDNAs in the SV40-based pSM expression vector coding for α (P04756), β (P09690), γ (P04760), ε (P20782) and δ (P02716) subunits of mouse muscle nAChR were kindly provided by Dr J. Patrick (Baylor College of Medicine, Houston, TX, USA).
Expression of nAChR subunits in BOSC 23 cells
Transient transfections of the nAChR subunits were carried out in the retroviral packaging cell line BOSC 23, as previously described (Fucile et al. 1996; Ragozzino et al. 1997). Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco), supplemented with 10 % fetal calf serum (Hyclone, USA). Subunit cDNAs were added in equivalent amounts (1–2 μg each per 35 mm dish) as previously detailed (Fucile et al. 1996; Ragozzino et al. 1997). Between 8 and 12 h after the start of transfection, cells were washed twice and refed with DMEM containing 10 % fetal calf serum. Cells were used for electrophysiological examination 36–48 h after transfection.
Electrophysiological recordings
Recordings of ACh- and nicotine-induced currents (IACh and INic, respectively) were performed on transfected cells using the whole-cell configuration of the patch-clamp technique (Hamill, Marty, Neher, Sakmann & Sigworth, 1981). Agonists were applied by a gravity-driven perfusion system. Whole-cell recordings were performed using borosilicate glass patch pipettes (2–4 MΩ tip resistance with CsCl solution) connected to an Axopatch-200A amplifier (Axon Instruments). Membrane currents were digitized at 500 Hz and analysed with pCLAMP programs (Axon Instruments). The series resistance, estimated from slow transient cancellation, ranged from 6 to 12 MΩ and was compensated by 80–90 %. Unless otherwise indicated, cells were voltage clamped at −50 mV. To measure current reversal potentials (Vrev) in cells expressing muscle nAChRs, current-voltage relationships of nicotine-induced currents (INic-V relationships) were analysed. For this purpose, voltage ramps (from −50 to +30 mV; 0.5 s duration) were applied during nicotine superfusion, 5–10 s after the peak of the responses; current amplitude decay during ramp application was less than 5 %. Corresponding control ramps were subtracted. Steady-state INic-V relationships were also obtained by measuring the peak current amplitude at different holding potentials. Reversal potentials determined for INic by this method were similar to those obtained with ramps (not shown). Determinations of Vrev were not performed in cells expressing neuronal receptors, as currents flowing through these receptors showed a marked inward rectification (Mathie, Colquhoun & Cull-Candy, 1990). To calculate the relative Ca2+vs. Cs+ permeability (PCa/PCs) we used the following extended GHK equation (Lewis, 1979), considering only Cs+ and Ca2+, as the contribution of other ions should be negligible (see ‘Solutions’):
 |
(1) |
where R, T and F are standard thermodynamic parameters, and [Ca2+] and [Cs+] represent Ca2+ and Cs+ activities, respectively. The expression was solved for the shift in Vrev, i.e. ΔVrev = Vrev,20 - Vrev,1, where Vrev,1 is the reversal potential determined in an external solution with Ca2+ at 1 mm concentration ([Ca2+1]o) and Vrev,20 is the reversal potential determined in an external solution with Ca2+ at 20 mm concentration ([Ca2+20]o; see Castro & Albuquerque, 1995):
 |
(2) |
yielding:
 |
(3) |
With the PCa/PCs value obtained from eqn (3), we estimated the fractional Ca2+ current, Pf, using the following expression derived from the GHK equation, with zero internal Ca2+ and monovalent ion concentrations (see Schneggenburger et al. 1993):
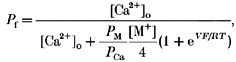 |
(4) |
where V is membrane potential, [M+] is the activity of monovalent ions in the external medium and PM/PCa is calculated considering PCs/PNa = 1.4 (Adams et al. 1980; Nutter & Adams, 1995) and PM≈PNa, as Na+ is (by far) the major cation of the external medium.
Fluorescence measurements
Fluorescence determinations were made using real time confocal laser microscopy (Odyssey, Noran Instruments, CA, USA). The unit was driven by Image-1 software (Universal Imaging Corporation, West Chester, PE, USA), equipped with an argon laser and interfaced to an upright microscope (Axioscope, Zeiss, Germany). An excitation wavelength of 488 nm was used and emission was monitored at 515 nm. The laser beam was set at 30 % intensity, with minimum power. Real time acquisition was performed at a video rate. Records of fluorescence signal and membrane currents were synchronized starting at the cell-attached configuration. Images were acquired and stored on video recorder (Sony VO 9600P) and analysed off-line. Measurements of fluorescence over time had an effective resolution of 20 Hz. The rise in cytosolic Ca2+ concentration induced by nicotine (used in place of ACh to prevent possible fluorescence signals due to the activation of muscarinic AChRs) was expressed as the ratio of fluorescence increase after treatment over basal fluorescence (ΔF/F0). Unless otherwise specified, nicotine was applied to transfected cells at 100 μm concentration. Fluorescence signals were calculated as means over square domains approximating cell shape, assuming a homogeneous receptor density. All determinations were performed with the confocal slit set at 100 μm, to detect fluorescence changes over the entire cell depth. Identical conditions of illumination and detection were maintained, taking care that the basal fluorescence of each cell was similar.
Transfected cells were incubated at 37°C with the membrane-permeant Ca2+ indicator fluo-3 acetoxymethyl ester (fluo-3 AM, 5 μm) for 45 min in serum-free DMEM, then washed extensively with standard medium. nAChR-expressing cells were identified as those responding with a fluorescence transient to a preliminary nicotine application. Nicotine-responsive cells were then patch clamped using electrodes filled with fluo-3 (cell impermeant; 250 μm, unless otherwise specified). After rupture of the patch membrane and the establishment of the whole-cell configuration, fluo-3 diffused from the patch pipette into the cell, leading to a slow increase in the basal fluorescence that reached a stable level (F0) within 5–6 min. Only cells that exhibited stable F0 during experiments were considered for the analysis. Nicotine-evoked fluorescence transients were probably evoked by Ca2+ entry through nAChRs as neither caffeine (5 mm) nor cell depolarization elicited fluorescence transients in fluo-3-loaded cells (not shown). Cells that lacked fluorescence recovery to F0 after nicotine washout were discarded.
The approach used for the determination of fractional Ca2+ current, Pf, of nAChR subtypes is exemplified in Fig. 1 for a cell transfected with αβɛδ subunit cDNAs. Pf was evaluated as the point ratio of fluorescence increase (F) over the total charge (Q) entering the cell during first 1.0-1.5 s of INic time course (F/Q = (ΔF/F0)/∫Idt). Each point was obtained by the simultaneous determination of fluorescence and charge every 50 ms during nicotine application. To determine the value of Pf, the F/Q ratio obtained in standard medium (containing 2 mm Ca2+, see ‘Solutions’) was normalized to the F/Q ratio in isotonic 110 mm Ca2+ medium (Fig. 1A and B):
Figure 1. Determination of fractional Ca2+ current activated by nicotine.

A, fluorescence transients (top) and whole-cell currents (bottom) evoked by nicotine applications (filled bars) in a cell expressing αβεδ nAChRs exposed to the indicated Ca2+ concentrations. Filled area represents current integral used to calculate F/Q ratio. B, F/Q relationship of INic obtained by fitting the single F/Q values (1 every 50 ms) of ΔF/F and current integral, Q, during nicotine application. F/Q ratio of INic at 2 mm Ca2+ (F/Q2, ▪) is normalized to that at 110 mm Ca2+ (F/Q110, ○) to determine Pf (5.2 % in this case). C, the same F/Q110 as in B, illustrated up to larger values of Q and on an expanded horizontal scale. Note the saturation of the F/Q110 relation with the increase of current integral. D, relationship between F/Q110 and the peak amplitude of INic (at 110 mm Ca2+). Each point represents the F/Q calibration of a single cell. Note the decrease in F/Q110 ratio with the increase in current size. The dotted line at 150 pA represents the upper limit value of INic in isotonic Ca2+ accepted for F/Q110 determination. E, measurement of Pf for fetal muscle nAChRs, using different fluo-3 concentrations in the recording pipette (means ± s.e.m., n = 6). Values obtained by cell-by-cell calibration, □; Pf values obtained at 250 μm fluo-3, normalizing mean F/Q values at 2 mm to those at 110 mm Ca2+ (n = 13), ▪.
Because of the saturation of dye, spectral changes at high intracellular Ca2+ concentrations (Neher, 1995), F/Q ratios in 110 mm Ca2+ were linear only for small Q values (Fig. 1C). Dye saturation could develop both for large currents and sustained nAChR activation in isotonic Ca2+ medium. The consequences of such saturation are illustrated in Fig. 1D, showing that F/Q determination in 110 mm Ca2+ is influenced by the amplitude of INic. Thus, to avoid errors due to dye saturation, currents recorded in isotonic Ca2+ were considered up to an amplitude of 150 pA (see Fig. 1D) and care was taken to compare fluorescence transients of similar amplitude in standard and isotonic Ca2+ medium. Moreover, cells that exhibited fluorescence changes upon shifting from standard to isotonic Ca2+ medium were not considered. Pf was measured by normalizing the determinations of F/Q in 2 mm Ca2+ to the mean F/Q ratio in isotonic 110 mm Ca2+ medium from all cells (I≤ 150 pA). When Pf was measured using a cell-by-cell calibration method (Vernino et al. 1994), mean values differed by less than 0.5 % from those determined under identical conditions (250 μm fluo-3), using the mean F/Q values obtained in standard vs. isotonic Ca2+ medium (Fig. 1E). To ensure that the determination of fractional Ca2+ currents was not affected by the competition of the dye with endogenous buffers for Ca2+ binding (see Neher, 1995), Pf for fetal muscle nAChRs was determined at different dye concentrations. The results obtained at 250 μm and 500 μm fluo-3 did not differ significantly (Fig. 1E), suggesting that at these dye concentrations most of the Ca2+ entering the cell binds to fluo-3. With all the above mentioned precautions, we determined bona fide the Ca2+ permeability through nAChR channels.
Solutions
The standard external medium used for the estimation of Ca2+ permeability contained (mm): NaCl, 140; KCl, 2.5; CaCl2, 2; MgCl2, 2; Hepes–NaOH, 10; and glucose, 10 (pH 7.3). F/Q ratio calibrations were performed in a modified external medium containing (mm): CaCl2, 110; and Hepes–Ca(OH)2, 10 (pH 7.3; isotonic Ca2+ medium). Cells were internally dialysed with a solution containing (mm): the nAChR pore-impermeant N-methyl-d-glucamine (NMG) cation, 140; Hepes, 10; and fluo-3, 0.25, adjusted to pH 7.3 with HCl (Mulle, Choquet, Korn & Changeux, 1992a; Vernino et al. 1994). In some of the F/Q determinations, NMG-based internal solution was replaced with the following solution (mm): CsCl, 150; Hepes–CsOH, 10; and fluo-3, 0.25 (pH 7.3). For Vrev shift determinations, cells were equilibrated in the following media (mm): CsCl2, 150; Hepes–CsOH, 10; and CaCl2, 1; or CsCl, 150; Hepes–CsOH, 10; and CaCl2, 20 (pH 7.3). Internal solution had the following composition (mm): CsCl, 145; Hepes–CsOH, 10; and EGTA, 10 (pH 7.3). The effect of external Ca2+ on IACh amplitude was investigated by superfusing the cell with a NaCl-based medium (standard medium) containing increasing Ca2+ concentrations from 0.1 to 110 mm. Final osmolarity was kept at 300 mosmol l−1 by reducing the concentration of NaCl. In these experiments the internal solution was the following (mm): KCl, 140; MgCl2, 2; CaCl2, 1; Hepes–KOH, 10; EGTA, 11; and Mg-ATP, 2 (pH 7.3).
RESULTS
Ca2+ dependence of agonist-activated currents
In human cells transfected with cDNAs encoding α, β, γ and δ subunits of the nAChR equilibrated in standard medium, nicotine elicited inward currents (INic) with peak amplitudes ranging between 0.7 and 8 nA, which were reduced to 5 ± 1 % (n = 11) when cells were bathed in isotonic (110 mm) Ca2+ medium (Fig. 2A). Similarly, in cells transfected with cDNAs encoding the adult form of nAChR (αβεδ), INic was 0.5–3.5 nA in standard medium and decreased to 17 ± 2 % in isotonic Ca2+ (n = 17).
Figure 2. Dependence of agonist-activated current amplitude on external [Ca2+].

A, comparison of INic amplitude recorded in transiently transfected BOSC 23 cells in the presence of 2 mm and 110 mm Ca2+. Top, examples of currents elicited by nicotine in the presence of 2 and 110 mm Ca2+ in muscle (fetal and adult) and neuronal (α3β4 and α4β4) nAChR-expressing cells. Thick lines represent Ca2+ concentration in the external medium; bars represent nicotine application. Bottom, mean peak amplitude (±s.e.m.) of INic at 110 mm Ca2+ (▪), normalized to INic at 2 mm Ca2+ (□). B, dependence of IACh amplitude on external Ca2+ in transiently transfected human cells expressing α3β4 (•) and α4β2 (□) nAChRs. Peak amplitude of IACh is normalized to IACh at 2 mm Ca2+ in the same cell. ACh concentration, 100 μm (α3β4) and 20 μm (α4β2). Data represent means ±s.e.m. of 4–6 determinations. Traces show examples of IACh in α4β2- and α3β4-transfected cells in the presence of 0.1, 2 and 20 mm Ca2+ in the external medium. Bars represent ACh applications.
The neuronal nAChRs tested showed different dependence of INic amplitude on external calcium. In α4β2-transfected cells, INic ranged from 0.1 to 2 nA in standard medium and was reduced to 43 ± 8 % (n = 6) on changing the bathing solution to isotonic Ca2+. Similarly, in α4β4-transfected cells, INic ranged between 0.2 and 3.5 nA in standard solution and decreased to 64 ± 2 % (n = 6) in isotonic Ca2+. In contrast, in α3β4-transfected cells INic, ranging between 0.1 and 1.2 nA in standard solution, increased to 230 ± 17 % (n = 18) in isotonic Ca2+. Figure 2A illustrates the dependence on external Ca2+ concentration of INic flowing through cells expressing various nAChR subtypes.
We also investigated the dependence on external Ca2+ concentration of the current activated by ACh (IACh) in cells expressing neuronal nAChRs. The peak amplitude of IACh gradually increased in α3β4-transfected cells as [Ca2+]o was raised in the range 0.1–110 mm, indicating that the unusual Ca2+ dependence of nicotinic currents in α3β4-transfected cells does not depend on the agonist. In α4β2-transfected cells, IACh peak amplitude was not affected by decreasing the external Ca2+ concentration to 0.1 mm, but was reduced by raising [Ca2+]o to 5, 20 and 110 mm (Fig. 2B).
Ca2+ permeability of muscle nicotinic acetylcholine receptors
In αβγδ-transfected cells, nicotine activated inward currents (see above, Figs 2 and 3) and fluorescence transients displaying similar amplitudes in standard medium and isotonic Ca2+ (ΔF/F0 ranging between 0.1 and 2). Fluorescence transients had much slower rising phases and recoveries compared with INic (Fig. 3A). With the experimental protocol described in Fig. 1, we measured the Pf of αβγδ nAChR (Pf,αβγδ; Fig. 3A and B), which was 2.1 ± 0.3 % (mean ± s.e.m., n = 13), in excellent agreement with the value measured in BC3H1 cells (Vernino et al. 1994).
Figure 3. Fractional calcium current of fetal and adult muscle nAChRs.

A, fluorescence transients (top) and whole-cell currents (bottom) evoked by 100 μm nicotine in a cell expressing αβγδ nAChRs exposed to external medium at the indicated Ca2+ concentrations. Downward and upward arrows near to fluorescence traces represent nicotine application and washout, respectively. B, F/Q ratios at 2 mm Ca2+ (▪) and 110 mm Ca2+ (○). Pf = 1.1 %. (Same cell as A.) C and D, determination of Pf in a cell expressing adult αβεδ nAChRs. Traces and symbols as in A and B. In this cell, Pf = 6.6 %.
In cells transfected with cDNAs encoding αβεδ nAChRs, nicotine elicited current and fluorescence responses (Figs 1–3) distributed over a wide range of amplitudes, and the calculated Pf,αβεδ was 4.2 ± 1.0 % (n = 13), a value significantly higher than Pf,αβγδ (Student's t test, P < 0.05). Similar results were obtained by measuring the shift of the reversal potential (Vrev) of INic in Cs+-based solutions containing 1 and 20 mm Ca2+. In αβγδ-transfected cells (Fig. 4A) the shift in Vrev was 1.1 ± 0.2 mV (mean ± s.e.m., n = 5), while in αβεδ-transfected cells (Fig. 4B) it was 3.2 ± 0.7 mV (n = 7). The calculated values of PCa/PCs were 0.27 for αβγδ nAChRs and 0.9 for αβεδ nAChRs, yielding a Pf value (calculated with eqn (4)) of 1.35 % for αβγδ nAChRs and 4.4 % for αβεδ nAChRs.
Figure 4. Shift in Vrev in muscle nAChRs caused by changing external [Ca2+].

A, I–V relationship of the response evoked by nicotine (100 μm) application in the presence of 1 mm (•) and 20 mm (□) Ca2+ in a cell expressing αβγδ nAChRs. Inset, shift of Vrev of 0.8 mV; arrows indicate reversal potentials. Note the reduced amplitude of INic in 20 mm Ca2+. B, I–V relationship of INic in a cell expressing αβεδ nAChRs in 1 mm (•) and 20 mm (□) Ca2+. Note the larger Vrev shift (2.8 mV) in the inset. The experimental protocol is represented above the current traces (voltage ramps from −50 to +30 mV in 0.5 s).
Substituting the impermeant NMG-based internal solution with the permeant one led to Pf,αβγδ = 1.1 ± 0.1 (n = 5) and Pf,αβεδ = 3.8 ± 0.7 (n = 5) for fetal and adult nAChRs, respectively; values that are in excellent agreement with those estimated in similar conditions from the permeability ratios. The percentage of current carried by calcium did not show significant voltage dependence when cell holding potential was varied from −30 to −70 mV. Conversely, Pf increased when currents were recorded holding cells at a membrane potential of −10 mV, since close to the reversal potential the net current became smaller (Vernino et al. 1994). At −10 mV, Pf,αβγδ was 2.5 ± 0.2 (n = 4) and Pf,αβεδ was 6.2 ± 0.8 (n = 4). Considered together, our findings show that the replacement of the γ with the ε subunit causes an increase in Ca2+ current through reconstituted nAChRs, in agreement with analogous determinations in muscle cells (Villarroel & Sakmann, 1996), and with the fluorescence increase determined here.
Ca2+ permeability of neuronal nAChRs
To investigate whether differences in Ca2+ permeability between muscle and neuronal nAChRs could be observed under our experimental conditions, the Ca2+ permeability of various combinations of neuronal nAChR subunits was examined. In cells transiently transfected with cDNAs encoding α3 and β4 nAChR subunits (Fig. 5A), nicotine elicited fluorescence transients that peaked to ΔF/F0 values ranging between 0.1 and 0.8 in standard solution and 0.5 and 2.0 in isotonic Ca2+ solution. Pf,α3β4 was 4.6 ± 0.7 % (n = 12), in agreement with values reported for neuronal AChRs in native systems which probably express α3β4 receptors (Vernino et al. 1994; Rogers & Dani, 1995). On the other hand, with the CsCl-based intracellular solution, the Pf,α3β4 value was 3.5 ± 1.1 (n = 5), which is significantly higher than Pf,αβγδ (Student's t test, P < 0.05) and comparable to Pf,αβεδ. Cells transfected with α4β2 or α4β4 cDNA pairs, upon stimulation with nicotine, displayed ΔF/F0 values ranging between 0.1 and 1.2 in standard medium, and 0.3 and 2.1 in isotonic Ca2+ solution (Fig. 5A). The simultaneous measurement of fluorescence and membrane currents gave slightly, but significantly, smaller Pf values: Pf,α4β2 = 2.9 ± 0.5 (n = 8) and Pf,α4β4 = 3.0 ± 0.3 (n = 10) (Student's t test, P < 0.05; see Fig. 5B), compared with Pf,α3β4.
Figure 5. Ca2+ permeability of neuronal α3β4, α4β2 and α4β4 nAChRs.

A, fluorescence transients (top), and whole-cell currents (bottom) induced by nicotine application (100 μm) in 2 mm Ca2+ medium to 3 cells expressing α3β4, α4β2 and α4β4 nAChRs, as indicated. Arrows as in Fig. 3. B, comparison of mean fractional Ca2+ currents in muscle and neuronal nAChRs at −50 mV membrane potential. Ordinate, percentage contribution of Ca2+ to the total charge carried by nicotine-activated currents. Data represent means ± s.e.m.
DISCUSSION
In this work, using confocal microscopy and the patch-clamp recording technique, we have shown that: (i) the muscle αβεδ nAChR is more permeable to Ca2+ than αβγδ nAChR; (ii) the neuronal ganglionic-like α3β4 nAChR is more Ca2+ permeable than α4β2 and α4β4 nAChRs, with the resulting sequence: α3β4 ≈ αβεδ > α4β4 ≈ α4β2 > αβγδ.
Dependence of whole-cell current amplitude on external Ca2+
It was found that INic is reduced in amplitude as external Ca2+ concentration is raised in cells expressing a variety of nAChRs, with the notable exception of α3β4 nAChR, for which INic is enhanced in isotonic Ca2+.
Whole-cell current amplitude may depend on several parameters, such as single channel conductance and kinetics, channel open probability and desensitization, which may be affected by Ca2+ (Bregestovski, Miledi & Parker, 1979; Mulle, Léna & Changeux, 1992b). The reduction of current amplitude in muscle nAChRs in isotonic Ca2+ is in agreement with the decreased channel conductance and lifetime observed at the neuromuscular junction (Bregestovski et al. 1979). Conversely, Ca2+ in the millimolar range has been shown to increase open probability and decrease single channel conductance in neuronal nAChRs, increasing the peak amplitude of agonist-evoked currents (Mulle et al. 1992b; Vernino et al. 1992), while higher Ca2+ concentrations are reported to reduce currents flowing through neuronal nAChRs (Zhou & Neher, 1993; Vernino et al. 1994; Nutter & Adams, 1995; Buisson et al. 1997). The Ca2+-dependent increase in agonist-induced current amplitude in α3β4-transfected cells might reflect the absence of Ca2+-dependent channel block, although the activation of a secondary conductance cannot be excluded. On the other hand, a similar failure of INic reduction in high Ca2+, observed in chick ciliary ganglion cells, was attributed to a high Ca2+ permeability of nAChRs combined with Ca2+-dependent changes in channel kinetics or open probability (Rathouz & Berg, 1994). This phenomenon may, however, have limited relevance in physiological conditions.
Ca2+ permeability of muscle nAChRs
Fluorescence determinations combined with whole-cell recordings reported here, showing that the fractional Ca2+ current of the muscle αβεδ nAChR is significantly higher than that of the fetal αβγδ nAChR, are in line with our determinations using the reversal potential shift procedure (see Methods), and with those reported elsewhere (Vernino et al. 1994; Villarroel & Sakmann, 1996). In particular, Pf values determined by fluorescence and electrophysiological methods using CsCl-based internal solution are in faithful agreement.
The difference in Ca2+ permeability adds up to the well-known functional differences in conductance and kinetics between the fetal and the adult forms of muscle nAChRs (Mishina et al. 1986). Postsynaptic Ca2+ elevation in muscle cells may take part in synaptic modulation at the neuromuscular junction (Cash, Dan, Poo & Zucker, 1996) and in the regulation of nAChR function (Miles, Audigier, Greengard & Huganir, 1994). Therefore, the higher Ca2+ permeability of adult muscle nAChRs may have physiological relevance in the regulation of neuromuscular synapse efficacy.
Ca2+ permeability of neuronal nAChRs
We have shown that the examined neuronal α3β4, α4β2 and α4β4 nAChRs are more Ca2+ permeable than fetal muscle nAChR. Of the neuronal nAChRs considered, the Ca2+ permeability of α3β4 nAChR is highest and compares to that of αβεδ nAChR.
Ca2+ permeability of neuronal nAChRs has been estimated in native preparations, namely chromaffin cells (Zhou & Neher, 1993; Vernino et al. 1994), PC12 cells (Sands & Barish, 1991), ganglionic nerve cells (Fieber & Adams, 1991; Trouslard, Marsh & Brown, 1993; Nutter & Adams, 1995; Rogers & Dani, 1995), hippocampal pyramidal neurones (Castro & Albuquerque, 1995), as well as in injected Xenopus oocytes (Seguela et al. 1993; Costa, Patrick & Dani, 1994). With a few exceptions (Zhou & Neher, 1993; Vernino et al. 1994; Rogers & Dani, 1995), these studies are based on the determination of biionic (Ca2+/Cs+) reversal potential shift. Our findings with α3β4 nAChRs are in agreement with those reported in chromaffin cells and sympathetic neurones (Vernino et al. 1994; Rogers & Dani, 1995), which express putative α3β4 nAChRs. Discrepancies with previous results obtained in injected Xenopus oocytes, suggesting a higher Ca2+ permeability for α4β2 nAChRs with respect to α3β4 nAChRs (see Role & Berg, 1996), can be explained by the use of different experimental approaches (F/Q vs. reversal potential determination; see Burnashev et al. 1995) or cell expression systems (Ragozzino et al. 1997; Sivilotti, McNeil, Lewis, Nassar, Schoepfer & Colquhoun, 1997).
The significance of Ca2+ influx through neuronal nAChRs is related to the functional role played by receptors in both the central and the peripheral nervous systems. Ca2+ influx through neuronal nAChRs may regulate ion channel conductances (Mulle et al. 1992a) and neurotransmitter release (Gray, Rajan, Radcliffe, Yakehiro & Dani, 1996; Léna & Changeux, 1997; Wonnacott, 1997). In the peripheral nervous system, nAChRs, namely α3β4-containing receptors, are responsible for excitatory synaptic transmission and may mediate localized changes in intracellular Ca2+ concentration (Rathouz & Berg, 1994). The recent proposal that, both in the peripheral and in the central nervous system, functional nAChRs may be formed by more than two different subunits (see Role & Berg, 1996), makes it worthwhile to extend the analysis of Ca2+ permeability at least to some of these putative triplet nAChR subtypes.
In conclusion, our findings support the view that the adult form of nAChR is more Ca2+ conductive than the embryonic form of nAChR, and are in agreement with the view that neuronal nicotinic receptors are more Ca2+ permeable than the fetal muscle receptor. Conversely, our results disagree with the notion that the adult form of muscle nAChR is less Ca2+ permeable than neuronal nicotinic receptors, namely the CNS-like α4β2 nAChR. Nevertheless, the fractional Ca2+ current is of the same order of magnitude in all nicotinic receptors here examined, but it appears considerably reduced compared with some GluR subtypes (Burnashev et al. 1995) or homomeric α7 nAChRs (Seguela et al. 1993; Delbono, Gopalkrishnan, Renganathan, Monteggia, Messi & Sullivan, 1997), suggesting a different functional significance. We speculate that the structural determinants for these differences in Ca2+ permeability reside in the M2 pore-lining region of nAChR subunits, since the major determinants for the conductance differences between fetal and adult muscle nAChRs are located in the M2 domain of γ and ε subunits (Herlitze, Villarroel, Witzemann, Koenen & Sakmann, 1996), and M2 mutations may alter the calcium permeability of nAChRs (Bertrand, Galzi, Devillers-Thiéry, Bertrand & Changeux, 1993).
Acknowledgments
The authors wish to thank Drs Piotr Bregestovski, Nail Burnashev and Francesca Grassi for critical reading of the manuscript and advice, and Dr Aldo Giovannelli for helpful discussions. This work was supported in part by a grant from Ministero Università Ricerca Scientifica e Tecnologica (to F. E.).
References
- Adams DJ, Dwyer TM, Hille B. The permeability of endplate channels to monovalent and divalent metal cations. Journal of General Physiology. 1980;75:493–510. doi: 10.1085/jgp.75.5.493. 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Changeux J-P. Nicotinic receptor: an allosteric protein specialized for intercellular communication. Seminars in the Neurosciences. 1995;7:75–90. [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiéry A, Bertrand S, Changeux J-P. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proceedings of the National Academy of Sciences of the USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski PD, Miledi R, Parker I. Calcium conductance of acetylcholine-induced endplate channels. Nature. 1979;279:638–639. doi: 10.1038/279638a0. [DOI] [PubMed] [Google Scholar]
- Buisson B, Gopalakrishnan M, Arneric SP, Sullivan JP, Bertrand D. Human α4β2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: a patch-clamp study. Journal of Neuroscience. 1996;16:7880–7891. doi: 10.1523/JNEUROSCI.16-24-07880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. Journal of Physiology. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S, Dan Y, Poo M, Zucker R. Postsynaptic elevation of calcium induces persistent depression of developing neuromuscular synapses. Neuron. 1996;16:745–754. doi: 10.1016/s0896-6273(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. α-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophysical Journal. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ACS, Patrick JW, Dani JA. Improved technique for studying ion channel expressed in Xenopus oocytes, including fast suppression. Biophysical Journal. 1994;67:395–401. doi: 10.1016/S0006-3495(94)80494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier S, Erkman L, Valera S, Rungger D, Bertrand S, Boulter J, Ballivet M, Bertrand D. α5, α3, and Non-α3. Three clustered avian genes encoding neuronal nicotinic acetylcholine receptor-related subunits. Journal of Biological Chemistry. 1990;265:17560–17567. [PubMed] [Google Scholar]
- Delbono O, Gopalakrishnan M, Renganathan M, Monteggia LM, Messi ML, Sullivan JP. Activation of the recombinant human α7 nicotinic acetylcholine receptor significantly raises intracellular free calcium. Journal of Pharmacology and Experimental Therapeutics. 1997;280:428–438. [PubMed] [Google Scholar]
- Fieber LA, Adams DJ. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. Journal of Physiology. 1991;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Mileo AM, Grassi F, Salvatore AM, Alemà S, Eusebi F. Identification of a determinant of acetylcholine receptor kinetics in the extracellular portion of the γ subunit. European Journal of Neuroscience. 1996;8:2564–2570. doi: 10.1111/j.1460-9568.1996.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Villarroel A, Witzemann V, Koenen M, Sakmann B. Structural determinants of channel conductance in fetal and adult rat muscle acetylcholine receptors. Journal of Physiology. 1996;492:775–787. doi: 10.1113/jphysiol.1996.sp021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léna C, Changeux J-P. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. Journal of Neuroscience. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. Journal of Physiology. 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Colquhoun D, Cull-Candy SG. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurons. Journal of Physiology. 1990;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K, Audigier SSM, Greengard P, Huganir R. Autoregulation of phosphorylation of the nicotinic acetylcholine receptor. Journal of Neuroscience. 1994;14:3271–3279. doi: 10.1523/JNEUROSCI.14-05-03271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Mulle C, Choquet D, Korn H, Changeux J-P. Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron. 1992a;8:135–143. doi: 10.1016/0896-6273(92)90115-t. [DOI] [PubMed] [Google Scholar]
- Mulle C, Léna C, Changeux J-P. Potentiation of nicotinic receptor response by external Calcium in rat central neurons. Neuron. 1992b;8:937–945. doi: 10.1016/0896-6273(92)90208-u. [DOI] [PubMed] [Google Scholar]
- Nef P, Oneyser C, Alliod C, Couturier S, Ballivet M. Genes expressed in the brain define three distinct neuronal nicotinic acetylcholine receptors. EMBO Journal. 1988;7:595–601. doi: 10.1002/j.1460-2075.1988.tb02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The use of Fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995;34:1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Nutter TJ, Adams DJ. Monovalent and divalent cation permeability and block of neuronal nicotinic receptor channels in rat parasympathetic ganglia. Journal of General Physiology. 1995;105:710–723. doi: 10.1085/jgp.105.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Fucile S, Giovannelli A, Grassi F, Mileo AM, Ballivet M, Alem S, Eusebi F. Functional properties of neuronal nicotinic acetylcholine receptor-channels expressed in transfected human cells. European Journal of Neuroscience. 1997;9:480–488. doi: 10.1111/j.1460-9568.1997.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Rathouz MM, Berg DK. Synaptic-type acetylcholine receptors raise intracellular calcium by two mechanisms. Journal of Neuroscience. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M, Dani JA. Comparison of quantitative calcium flux through NMDA, ATP and ACh receptor channels. Biophysical Journal. 1995;68:501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Sands SB, Barish ME. Calcium permeability of neuronal nicotinic acetylcholine receptor channels in PC12 cells. Brain Research. 1991;560:38–42. doi: 10.1016/0006-8993(91)91211-i. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. Journal of Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti LG, McNeil DK, Lewis TM, Nassar MA, Schoepfer R, Colquhoun D. Recombinant nicotinic recptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. Journal of Physiology. 1997;500:123–138. doi: 10.1113/jphysiol.1997.sp022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouslard J, Marsh SJ, Brown DA. Calcium entry through nicotinic receptor channels and calcium channels in cultured rat superior cervical ganglion cells. Journal of Physiology. 1993;468:53–71. doi: 10.1113/jphysiol.1993.sp019759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick JW, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Vernino S, Rogers M, Radcliffe KA, Dani JA. Quantitative measurements of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. Journal of Neuroscience. 1994;14:5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A, Sakmann B. Calcium permeability increase of endplate channels in rat muscle during postnatal development. Journal of Physiology. 1996;496:331–338. doi: 10.1113/jphysiol.1996.sp021688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends in Neurosciences. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflügers Archiv. 1993;425:511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]


