Abstract
The effects of tetracaine were examined on rat ventricular myocytes. In both field-stimulated and voltage-clamped cells tetracaine (100–200 μm) produced an initial decrease of contraction before a recovery towards the control level. Removal of tetracaine produced a transient overshoot of contraction to levels greater than the control.
The transient decrease of contraction produced by tetracaine was accompanied by a small transient increase in the integral of the L-type Ca2+ current and a larger transient decrease of the Na+-Ca2+ exchange current on repolarization. These are attributed to decreased systolic release of Ca2+. On removal of tetracaine there was an increase of the Na+-Ca2+ exchange current. Before the addition of tetracaine, calculated Ca2+ influx and efflux across the sarcolemma were approximately equal. On adding tetracaine, efflux was transiently less than influx and, on removal of tetracaine, efflux was greater than influx.
These changes in Ca2+ fluxes result in an increase of cell Ca2+ during exposure to tetracaine. The calculated magnitude of this increase was equal to that measured directly by applying caffeine (20 mm) to release sarcoplasmic reticulum (SR) Ca2+ and integrating the resulting Na+-Ca2+ exchange current.
It is concluded that the effects of tetracaine can be accounted for by depression of calcium-induced Ca2+ release (CICR). The response is transient because the inhibition is compensated for by an increase of SR Ca2+ content such that there is no steady-state effect on the magnitude of the systolic Ca2+ transient. The consequences of this result for the effects of other modulators of CICR are discussed.
Calcium-induced calcium release (CICR) underlies excitation-contraction coupling in cardiac muscle. Calcium is released from the sarcoplasmic reticulum (SR) through a specialized release channel termed the ryanodine receptor (RyR). This receptor is activated during an action potential by calcium, which enters the cell through voltage-activated L-type calcium channels in the sarcolemma. Certain agents modify the sensitivity of the ryanodine receptor to calcium and as such are considered to be potential regulators of contraction. For example, both the compound cyclic ADP-ribose (Rakovic, Galione, Ashamu, Potter & Terrar, 1996) and phosphorylation of the ryanodine receptor (duBell, Lederer & Rogers, 1996) have been suggested to increase the gain of CICR and thence the magnitude of the systolic Ca2+ transient. Local anaesthetics such as procaine and tetracaine inhibit Ca2+ fluxes through the ryanodine receptor (Zahradníková & Palade, 1993; Györke, Lukyanenko & Györke, 1997) and suppress Ca2+ release and contraction in both cardiac and skeletal muscle (Almers & Best, 1976; Chapman & Leoty, 1981; Stephenson & Wendt, 1986; Klein, Simon & Schneider, 1992; Komai, Redon & Rusy, 1995). Tetracaine may therefore be a useful model compound for studying the effects of regulators of CICR. We have previously shown that tetracaine produces a transient suppression of spontaneous Ca2+ release from the SR (Overend, Eisner & O'Neill, 1997) (cf. Györke et al. 1997). This effect was attributed to the combination of (i) an inhibitory effect of tetracaine on CICR which is then gradually overcome by (ii) a subsequent increase of SR Ca2+ content. These results were shown to be consistent with inhibition of CICR. There was, however, no direct measurement of the gain of CICR. Furthermore there was no information about the effects of tetracaine on the normal, stimulated release of Ca2+ from the SR.
The related local anaesthetic, procaine, has been shown to directly inhibit SR calcium release (Zahradníková & Palade, 1993), and to increase SR calcium content (as assessed by rapid cooling contracture), an effect which has been attributed to a reduction in trans-sarcolemmal Ca2+ efflux (Komai et al. 1995). The initial aim of this paper was to obtain quantitative measurements of changes of CICR gain and SR Ca2+ content. Before this can be done, however, it is important to consider other possible actions of tetracaine which, for example, acts as a local anaesthetic by blocking sodium, calcium and potassium ion channels in nerve and muscle preparations at concentrations similar to those affecting the SR release channel (Hille, 1977; Chapman & Leoty, 1981; Carmeliet, Morad, Van der Heyden & Vereecke, 1986). Therefore, some of the inotropic effects of tetracaine could be attributable to effects on these sarcolemmal ionic fluxes. We present evidence suggesting that such a contribution is small, and that the transient nature of the effects of tetracaine on contraction can be attributed to depression of SR Ca2+ release and consequent changes of SR Ca2+ content. We conclude that agents which only affect CICR will only have transient effects on contraction.
METHODS
Experiments were carried out on cardiac myocytes isolated from rat ventricles using a collagenase and protease digestion protocol as previously described (Eisner, Nichols, O'Neill, Smith & Valdeolmillos, 1989). Rats were killed by stunning and cervical dislocation.
Electrophysiology
Voltage clamp control was achieved using the perforated patch technique (Horn & Marty, 1988) using amphotericin B. Due to the relatively high access resistance of the perforated patch (about 20 MΩ) we used the switch clamp facility of the Axoclamp-2A voltage clamp amplifier (Axon Instruments). Pipettes (1–3 MΩ in resistance) were filled with the following solution (mm): CsCH3O3S, 125; CsCl, 12; NaCl, 20; Hepes, 10; MgCl2, 5; Cs-EGTA, 0.1; titrated to pH 7.2 with CsOH. Amphotericin B was dissolved in DMSO and added to the filling solution to a final concentration of 240 μg ml−1, before use. Cells were bathed in a control solution of the following composition (mm): NaCl, 135; KCl, 4; Hepes, 10; glucose, 11; CaCl2, 2; MgCl2, 1; titrated to pH 7.4 with NaOH. To avoid interference from outward currents, all voltage clamp experiments were performed in the presence of 5 mm 4-aminopyridine and 0.1 mm BaCl2. Tetracaine was added where appropriate from a stock solution of 100 mm (in H2O), without osmotic correction. In some experiments (e.g. Fig. 1) voltage clamp was not used, and in this case 4-aminopyridine and BaCl2 were omitted. All experiments were carried out at 22°C.
Figure 1. The effects of tetracaine on contraction amplitude a nd systolic [Ca2+]i.

A, time course of cell shortening (top) and indo-1 ratio (R = F400/F500) (bottom). The cell was field stimulated at a frequency of 0.3 Hz. Tetracaine (100 μm) was applied for the period indicated by the bar. The indo-1 ratio was corrected for the intrinsic fluorescence of tetracaine (see Methods). B, averaged specimen (n = 5) Ca2+ transients taken from the periods (a-d) indicated in A.
Fluorescence measurements
In experiments designed to measure [Ca2+]i, cells were loaded with the acetoxymethyl ester form of indo-1 (Molecular Probes; 2.5 μm for 5 min, followed by at least 30 min for de-esterification) and placed in a bath on the stage of an inverted epifluorescence microscope (Nikon Diaphot TMD, Nikon, UK). Fluorescence was excited at 340 nm and measured at 400 and 500 nm. The ratio of the emitted fluorescence (400 nm/500 nm) was used as an index of [Ca2+]i. In other experiments cells were not loaded with the indicator. Fluorescence measurements in the presence of tetracaine were hampered by the fact that the drug itself fluoresces with an intensity which is considerably greater at 400 than at 500 nm. The intensity of this fluorescence is greater in the presence of a cell presumably because tetracaine (a weak base) is accumulated within the cytoplasm (which is acidic with respect to the extracellular fluid). The time course of the rise of this artifactual increase of fluorescence is complete within 2–3 s. We have therefore adjusted the indo-1 fluorescence traces (Fig. 1) by subtracting off a step increase of fluorescence at both 400 and 500 nm. This correction is fairly crude and the results should be interpreted accordingly. In particular, it is not possible to compare accurately the magnitude of a Ca2+ transient in tetracaine with one in control solution, although changes of fluorescence once tetracaine has been applied will be recorded faithfully.
In some experiments caffeine was used to release Ca2+ from the SR. As reported previously (Overend et al. 1997), in the presence of tetracaine, 10 mm caffeine often produces a series of oscillations of inward current, rather than a single release. This is similar to the effect of a lower concentration of caffeine in the absence of tetracaine and presumably reflects antagonism between tetracaine and caffeine (Almers & Best, 1976). For this reason 20 mm caffeine was used to measure SR calcium content. Under these conditions a single release of calcium resulted, and the accompanying Na+-Ca2+ exchange current could be measured easily.
Data were digitized using a Digidata 1200 series interface board (Axon Instruments) and analysed using software (ABFAN2) written by Dr A. W. Trafford (Department of Veterinary Preclinical Sciences, University of Liverpool).
Quantification of SR Ca2+ content and sarcolemmal Ca2+ fluxes
Inward Na+-Ca2+ exchange currents produced by the application of caffeine (20 mm) to voltage-clamped cells were integrated and converted to total cell calcium fluxes as described previously (Varro, Negretti, Hester & Eisner, 1993; Negretti, Varro & Eisner, 1995). Briefly, it is necessary to first correct for that fraction of the efflux which is not produced by Na+-Ca2+ exchange, and then relate the fluxes to cell volume. The volume was calculated from the cell membrane capacitance using the capacitance-to-volume ratio 6.76 pF pl−1 (Satoh, Delbridge, Blatter & Bers, 1996). It should be noted that, as in our previous work, the SR Ca2+ content is expressed with relation to cell (and not SR) volume. We have previously shown that 67% of Ca2+ efflux is generated by Na+-Ca2+ exchange (Negretti, O'Neill & Eisner, 1993). The remainder occurs via the sarcolemmal Ca2+-ATPase and mitochondrial sequestration. To take into account non-Na+-Ca2+ exchange efflux of Ca2+, current integrals were multiplied by a factor of 1.5. This correction was used for both caffeine- and repolarization (tail current)-activated current. The integrals of calcium currents were simply divided by a factor of 2 to compensate for the fact that each calcium ion carries two positive charges into the cell.
Statistics
All values are presented as means ± s.e.m. for n experiments. Significance was assessed using a paired t test.
RESULTS
Figure 1 shows the effect of tetracaine on the amplitude of contraction and associated calcium transients in field-stimulated rat ventricular myocytes. Application of 100 μm tetracaine transiently reduced the amplitude of contraction. Contraction then gradually recovered towards the control level over a period of 1–2 min, despite the maintained presence of the drug. The magnitude of peak contraction was also more variable in the presence of tetracaine. Removal of tetracaine was associated with a transient elevation of contraction amplitude above the control level, which subsequently recovered to the control level. Similar effects of tetracaine addition and removal were seen on the systolic Ca2+ transient record (Fig. 1A, lower trace). This suggests that changes in contraction amplitude are a consequence of changes in the Ca2+ transient magnitude, and do not reflect effects of the drug on the contractile apparatus. The amplitude of the Ca2+ transient does not recover to the control level, while the contraction appears to demonstrate recovery to the control level. We cannot exclude the possibility that the lack of complete recovery of the Ca2+ transient is due to errors in correction for the intrinsic fluorescence of tetracaine (see Methods). However, the changes in the amplitude of the Ca2+ transient which occur during the maintained presence of tetracaine, and the overshoot on removal of tetracaine must be due to changes of [Ca2+]i rather than artifactual changes of fluorescence.
It is likely that the effects of tetracaine are a result of reduced calcium-induced calcium release (CICR). However, tetracaine has well-documented effects on sodium and calcium currents across the sarcolemma (INa and ICa, respectively) (Hille, 1977; Carmeliet et al. 1986). The former will decrease excitability, and this effect can be eliminated by using voltage-clamped cells. The latter will directly decrease Ca2+ entry. The experiment illustrated in Fig. 2 shows a typical result in a voltage-clamped cell. The effects of tetracaine on contraction (Fig. 2A) are qualitatively similar to those seen in field-stimulated cells: tetracaine produces an initial decrease of contraction followed by recovery and then an overshoot on removal of tetracaine. The transient overshoot of contraction amplitude on removal of the drug, in this case, was accompanied by spontaneous oscillations, indicating some degree of calcium overload at this point in the experiment. Figure 2B shows specimen records of current and contraction. The immediate major depression of contraction (b) is accompanied by a modest decrease (to 83%) of the peak magnitude of the calcium current. This is associated with an increase in the integral of the Ca2+ current (see later). However, while contraction recovers towards control levels, there is no recovery of the calcium current (c). Similarly, the overshoot in contraction amplitude on removal of tetracaine (d) is not accompanied by an increase in peak calcium current above the control level. Changes in the peak amplitude of the calcium current, therefore, cannot explain the recovery of contraction amplitude during continued superfusion with tetracaine, nor the transient overshoot of contraction observed on its removal. On average, in twelve cells, the minimum level of contraction reached in 100 μm tetracaine was 40.0 ± 4.5 % of the control level and this recovered to 96.2 ± 2.6 % in the steady state. This level of contraction during steady-state exposure to tetracaine was not significantly different from that in control (P > 0.1). On removal of tetracaine the mean peak level of contraction reached was 170.8 ± 16 % of the control level.
Figure 2. The effects of tetracaine on contraction and membrane current in a voltage-clamped cell.

A, time course of changes of contraction. Tetracaine (100 μm) was applied for the period indicated by the bar. The membrane potential was held at −40 mV, and 200 ms duration depolarizing pulses to 0 mV were applied at a frequency of 0.5 Hz. B, specimen records of membrane current (top) and contraction (bottom) obtained at the times (a-d) indicated in A.
In an attempt to gauge to what extent inhibition of the L-type calcium current may account for changes in contraction, in Fig. 3 we compared the effect of tetracaine with that produced by deliberately reducing the calcium current, by reducing the size of the depolarizing step (from 40 to 30 mV). Reducing the size of the depolarization reduced the peak calcium current considerably more than did exposure to 100 μm tetracaine (here, to 54 % of the control peak ICa, cf. 83 % in tetracaine). However, the contraction elicited by the smaller pulse was only marginally reduced in magnitude, in contrast to the dramatic reduction of contraction amplitude observed during early exposure to tetracaine. This confirms that only a small proportion of the effects of tetracaine on contraction amplitude can be due to reduction of the L-type calcium current.
Figure 3. Comparison of the effects of tetracaine with those of decreasing the size of depolarization.
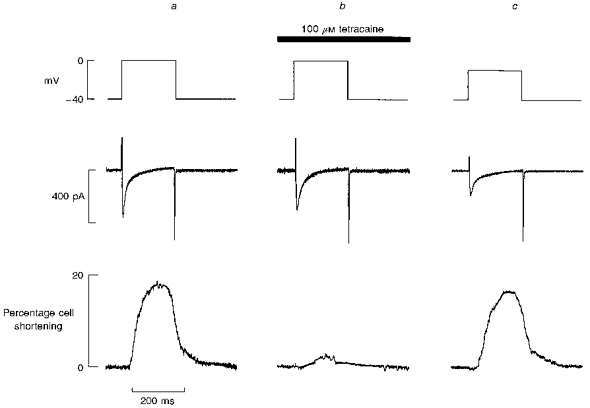
In all panels the traces show (from top to bottom): membrane potential, current, cell length. In a and b, the depolarizing pulse was to 0 mV. Panel a, control; b, after 4 s exposure to tetracaine (100 μm); c, in the absence of tetracaine (depolarization to −10 mV). Membrane potential was held at −40 mV and depolarizing pulses of 200 ms duration were applied to elicit contraction.
Measurement of Ca2+ flux balance in tetracaine
The transient elevation of contraction amplitude observed on removal of tetracaine may be indicative of an increase in the SR calcium load of the cells following exposure to tetracaine. This is further suggested by the presence of spontaneous oscillations in some cells immediately following removal of the drug (Fig. 2). Results presented later in this paper provide quantitative measurements of this increase of SR Ca2+ content. The aim of the series of experiments described below was to investigate the origin of this increase. In order to do this we have measured the sarcolemmal fluxes of calcium. As in our previous work (Negretti et al. 1995; Trafford, Díaz, Negretti & Eisner, 1997b), Ca2+ entry was measured by integrating the L-type Ca2+ current and Ca2+ efflux from the Na+-Ca2+ exchange current tail activated on repolarization (Fedida, Noble, Shimoni & Spindler, 1987; Bridge, Smolley & Spitzer, 1990). To facilitate the measurements, short (100 ms duration) pulses were used in order to minimize Ca2+ efflux during depolarization, when any Na+-Ca2+ exchange flux will be obscured by the L-type Ca2+ current. The contraction record in Fig. 4 again shows a transient reduction of amplitude followed by a recovery on application of tetracaine and overshoot on removal of tetracaine. Figure 4B illustrates sample current records. The integrated currents for this cell show that in control conditions (Fig. 4Ba) depolarization produces a gain of about 4 μmol (l cell volume)−1 Ca2+ via the L-type Ca2+ current. On repolarization there is a loss of calcium from the cell which is of the same magnitude as the initial gain. In other words, influx equals efflux. On average, in twelve cells, the Ca2+ entry during the Ca2+ current was 4.19 ± 0.43 μmol l−1 in comparison with average efflux of 4.55 ± 0.37 μmol l−1 (all fluxes expressed with respect to total cell volume). These are not significantly different (P > 0.10). The records illustrated in Fig. 4Bb show the early effects of tetracaine. Despite the reduction in the peak magnitude of the Ca2+ current, the integrated Ca2+ entry is greater (presumably due to reduced Ca2+-induced inactivation of the current, because of the smaller Ca2+ transient: Sipido, Callewaert & Carmeliet, 1995; Adachi-Akahane, Cleemann & Morad, 1996). The main effect of tetracaine on membrane current is, however, a decrease of the Na+-Ca2+ exchange current tail on repolarization due, presumably, to the decreased magnitude of the systolic Ca2+ transient. The net effect is that the cell has gained about 3 μmol Ca2+ l−1 at the end of the record shown. During the period of exposure to tetracaine, as the sizes of the systolic Ca2+ transient and contraction increase, so does that of the current tail on repolarization. Therefore, in the steady state in tetracaine (Fig. 4BC) Ca2+ entry on depolarization exactly balances the loss on repolarization, such that there is no net change of cell Ca2+. The mean data confirm that influx (4.00 ± 0.42 μmol l−1) and efflux (4.64 ± 0.38 μmol l−1) are not significantly different and, therefore, balance in tetracaine (P > 0.05) once a steady-state level of contraction is achieved. When tetracaine is removed there is a small decrease of the integrated calcium current. This is accompanied by a much larger increase of Ca2+ loss on repolarization due to the larger Ca2+ transient. On this pulse there is a net loss of cell calcium of almost 4 μmol l−1. Although not shown, as the contraction and systolic Ca2+ transient decrease towards control levels, the Ca2+ loss on repolarization decreases to control levels and Ca2+ flux balance is once again achieved. This post-tetracaine depletion of calcium presumably accounts for the gradual reduction of contraction towards the control steady-state level.
Figure 4. Transient loss of Ca2+ flux balance during application and removal of tetracaine.
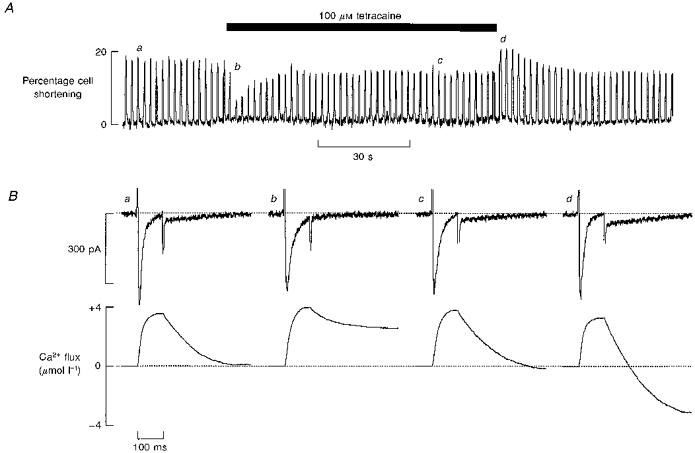
A, time course of the effects on contraction of applying tetracaine for the period shown by the bar. B, specimen records of membrane current (top) and cumulative integral (bottom). Membrane potential was held at −40 mV and depolarizing pulses of 100 ms duration were applied at 0.5 Hz. The Ca2+ flux traces show the cumulative integral of the calcium current (initial upward deflection) followed by a downward deflection due to the Ca2+ efflux. The records were obtained at the times (a-d) shown in A. (See Methods for calculation of sacrolemmal Ca2+ movements.) The vertical positions of the current traces have been aligned to facilitate comparison. Tetracaine produced an outward shift of holding current of 7 pA and this has been removed to facilitate comparison between records.
The net changes of calcium illustrated in Fig. 4 take place over each cycle of contraction and relaxation. It appears that exposure to tetracaine causes a net increase of calcium by the cell, and this is balanced by a loss of calcium from the cell on its removal. The total amount of calcium gained and lost in this way can therefore be calculated by summing the net calcium flux for each cycle. An example of this type of calculation is illustrated in Fig. 5. This calculation was performed using the same data as in Fig. 4. The bar illustrates the period of tetracaine superfusion. The upper panels represent the values of Ca2+ influx and efflux, respectively, calculated from the integrals of Ca2+ current and tail current, associated with each cycle of contraction. The bottom panel illustrates the cumulative difference between influx and efflux, i.e. the total amount of calcium gained or lost by the cell since the start of the record. The data presented earlier show that, on average, in either control or tetracaine, in the steady state Ca2+ influx and efflux are equal. However, any small differences will add up with this cumulative method. We have therefore calculated the mean steady-state values of calcium influx and efflux for control, tetracaine and recontrol. These steady-state values were subtracted from the integral of each pulse in the appropriate solution. As shown in Fig. 4, application of tetracaine is associated with a reduction in calcium efflux from the cell, and a transient elevation of calcium entry above the control level, probably as a consequence of reduced calcium-induced inactivation of ICa. Both these effects will contribute to increasing the calcium content of the cell. As the cell gains calcium, contraction recovers towards the control level, and with it efflux from the cell, activated by the increasing magnitude of associated Ca2+ transients. On removal of tetracaine there is a transient elevation of the efflux integral, producing a net loss of calcium from the cell. There is also a significant undershoot of calcium entry into the cell, which will further contribute to reducing cell calcium content. The net calcium loss on removal of tetracaine calculated in this way (35.5 ± 3.3 μmol l−1, n = 11) is similar to (P > 0.05) the calculated amount of calcium gained in the presence of tetracaine (33.7 ± 3.1 μmol l−1, n = 11).
Figure 5. Net accumulation of calcium during exposure to and removal of tetracaine.

The traces show (from top to bottom): calculated Ca2+ entry via the L-type Ca2+ current, Ca2+ efflux on repolarization (calculated as shown in Fig. 4), cumulative change of cell Ca2+ content. Calcium content (presumably SR) is expressed per unit total cell volume.
In the steady state, the flux of calcium into the cell in Fig. 4 is minimally affected by 100 μm tetracaine. This is reflected in the fact that contraction amplitude recovers towards the control level in the steady state (where influx and efflux are balanced). Inhibition of the calcium current may, however, contribute to any observed reduction in the steady-state amplitude of contraction below that observed under control conditions, during prolonged exposure to tetracaine. This effect is more obvious in Fig. 6, which illustrates the effect of 200 μm tetracaine on contraction and associated sarcolemmal currents and calcium fluxes. Changes in contraction amplitude during exposure to 200 μm tetracaine follow a similar, although somewhat exaggerated, pattern as observed with lower concentrations of the drug. Exposure to 200 μm tetracaine (indicated by the bar) eventually produces a new steady-state level of contraction, which is below the control level. In this case, recovery time is also considerably prolonged. The associated ICa (Fig. 6B, top panel) is inhibited to a greater extent by tetracaine, thereby reducing calcium entry into the cell more significantly. In this case, the calcium current integral was reduced to 62.1 % of the mean control value by exposure to 200 μm tetracaine. In the same cell a reduction in the magnitude of ICa to 82.6 % of the mean control value was observed during exposure to 100 μm tetracaine. The decrease in the Ca2+ current integral during exposure to 200 μm tetracaine was reflected by a reduction in the steady-state amplitude of contraction to 54.8 % of the control level, in comparison with 85.5 % during exposure to 100 μm tetracaine.
Figure 6. The effect of 200 μm tetracaine on contraction and current.

A, time course of cell shortening in response to electrical stimulation elicited by 200 ms depolarizing steps to 0 mV from a holding potential of −40 mV. Tetracaine (200 μm) was applied as indicated by the bar. B, specimen records of membrane current (top) and cumulative integral (bottom). The records were obtained at the times (a-d) shown in A. The records in a and b are the means of 10 and 5 pulses, respectively. Single pulses are shown in c and d.
Direct measurements of SR Ca2+ content
It is likely that the extra calcium gained by the cell during exposure to tetracaine is accommodated in the SR. We took this type of analysis one step further by comparing the effect of tetracaine on the SR calcium content more directly, by measuring the caffeine-induced Na+-Ca2+ exchange currents in stimulated myocytes under control conditions and during tetracaine superfusion. Figure 7A illustrates typical caffeine traces and their associated integrals. It is clear that exposure to tetracaine increases the size of the integral and therefore of the SR Ca2+ content. The histogram of Fig. 7B shows that, in six cells, the increase of SR Ca2+ content measured directly with this technique (32.6 ± 2.9 μmol l−1) is very similar to (although statistically different from, P < 0.05) that estimated from the integrated current records (30.3 ± 2.4 μmol l−1).
Figure 7. The gain of measured SR Ca2+ content matches that calculated from the sarcolemmal fluxes.
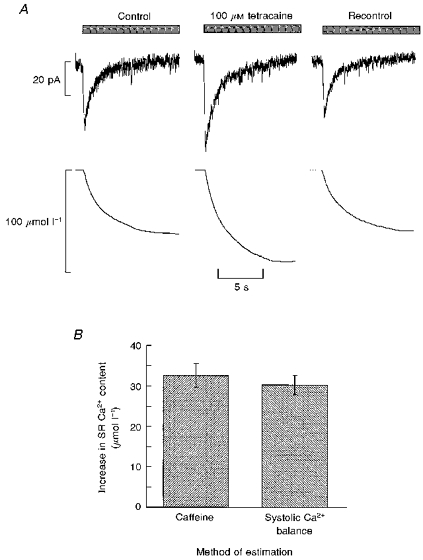
A, measurement of SR Ca2+ content. Caffeine (20 mm) was applied for the period indicated by the bars. Traces show current (top) and integrated current (bottom). From left to right: control; after 1.5 min exposure to tetracaine (100 μm); recontrol (1.5 min after removing tetracaine). B, histogram comparing the measured changes of SR Ca2+ (left) with those calculated as in Fig. 5 from the cumulative integrals (right).
DISCUSSION
The most striking feature of the results obtained in this study is the transient nature of the inotropic effects of tetracaine. Tetracaine transiently suppresses electrically stimulated contraction and systolic [Ca2+]i, and its removal is associated with a transient elevation of contraction and [Ca2+]i amplitude above the control level. These experiments were designed to investigate the mechanism by which tetracaine achieves these inotropic effects in rat ventricular myocytes. We have shown that the transient effects of tetracaine addition and removal cannot be accounted for by changes of ICa, but are likely to be due to a combination of inhibition of CICR and increased SR Ca2+ content.
During normal excitation-contraction coupling, calcium ions are released from the SR by calcium-induced release, triggered by calcium entry through voltage-gated sarcolemmal Ca2+ channels. The concentrations of tetracaine used here decrease calcium entry slightly, by direct inhibition of the L-type calcium current (Chapman & Leoty, 1981; Carmeliet et al. 1986). However, we have shown that this effect cannot account for the transient nature of the depression of contraction, and the effects of tetracaine are therefore likely to reflect, predominantly, a decrease in the ability of ICa to stimulate calcium release from the SR. Qualitatively, the biphasic nature of the effects of tetracaine is similar (but in the opposite direction) to that found for low concentrations of caffeine (O'Neill & Eisner, 1990; Györke & Palade, 1992). Caffeine produces a transient increase of systolic contraction and [Ca2+]i followed by a decay back to control levels. On removal of caffeine, there is an undershoot of the magnitude, before recovery back to control levels. The transient increase on application of caffeine was explained as resulting from a stimulation of CICR (O'Neill & Eisner, 1990). It is only transient because the increased release decreases the SR Ca2+ content. This hypothesis has recently received quantitative support (Trafford et al. 1998).
Measurement of sarcolemmal fluxes and SR Ca2+ content during tetracaine application
In this paper we have examined the balance of fluxes across the membrane during the application of tetracaine. This was done by comparing the magnitude of Ca2+ entry during the depolarizing pulse via the L-type Ca2+ current with the Ca2+ efflux on repolarization. The initial effect of applying tetracaine on sarcolemmal fluxes is to decrease the Ca2+ efflux (as a result of the decreased Ca2+ transient) and to increase the Ca2+ entry into the cell. The latter effect is due to decreased inactivation of the Ca2+ current (presumably due to decreased calcium-induced inactivation) overcoming the small direct inhibitory effect of tetracaine on the Ca2+ current. This results in a net predicted increase of cell calcium on each stimulus in contrast to the balance under control conditions. The increase of SR Ca2+ content can be calculated from the net integral. The results (Fig. 5) show that there is a gradual increase of cell Ca2+ content on each beat in tetracaine until a new balance is achieved, when efflux again equals influx. This results from the increase of Ca2+ efflux due to the gradual increase of the systolic Ca2+ transient. On removal of tetracaine, the inhibitory effect on CICR is removed and the larger SR Ca2+ content produces a larger systolic Ca2+ release than in the control. Therefore the Ca2+ efflux from the cell is increased above control levels. This results in a decrease of SR Ca2+ content until the final steady state is reached.
The changes of SR Ca2+ content referred to above were calculated from the integrated current records. This method has the advantage that an estimate of change of SR content can be obtained after each pulse. The method is, however, rather indirect and, in particular, makes no allowance for changes of fluxes between pulses. That this is a valid method is shown by the fact that (i) under control conditions and in the steady state in tetracaine, influx and efflux balance and (ii) the estimate of SR Ca2+ gain from this method agrees quantitatively with that measured directly by applying 20 mm caffeine to release all the SR Ca2+ content. The measurement of increased SR Ca2+ content is also in agreement with qualitative results showing that the cooling contracture was increased by the related local anaesthetic procaine (Komai et al. 1995).
Changes of the gain of CICR
The present data show that tetracaine decreases the amount of Ca2+ release from the SR in response to a given size of Ca2+ current. In other words tetracaine decreases the gain of CICR. This effect is then overcome by an increase of SR Ca2+ content. The increase of SR Ca2+ release by an increase of SR Ca2+ content is in agreement with previous work (Bassani, Yuan & Bers, 1995; Lukyanenko, Györke & Györke, 1996; Györke et al. 1997). It is interesting to note that the fractional recovery of contraction is greater than that of the SR Ca2+ content. This presumably reflects the combination of (i) the steep relationship between [Ca2+]i and contraction and (ii) the fact that increasing SR Ca2+ content produces a fractionally larger increase of Ca2+ release (Bassani et al. 1995; Trafford et al. 1997b).
Implications for other work
The results of this paper have shown that tetracaine, despite having a maintained depressant effect on CICR, has only a transient effect on the magnitude of the systolic Ca2+ transient. This is consistent with the transient effects of caffeine on systolic Ca2+ (O'Neill & Eisner, 1990; Trafford, Díaz & Eisner, 1997a). The argument put forward to explain the effects of tetracaine should also apply to other compounds that inhibit Ca2+ release; as long as inhibition of Ca2+ release is not complete, the mechanism by which it occurs (i.e. direct blockade of the pore vs. effects on gating) should not influence the result. These observations have two consequences. (i) If one is looking to see whether a compound affects CICR, then the magnitude of the systolic Ca2+ transient or contraction is an inappropriate measure (at least in the steady state). Indeed if the compound acts more slowly than the time course of changing SR Ca2+ content, then no effect will be seen. To overcome this, it is necessary to measure SR Ca2+ content at the same time. Alternatively, one can make use of the fact that compounds which affect CICR (like tetracaine), although having no steady-state effect on the magnitude of the stimulated contraction, produce a steady-state effect on the frequency of spontaneous SR release (Györke et al. 1997; Overend et al. 1997). (ii) As discussed elsewhere (Trafford, Díaz & Eisner, 1998), maintained inotropic effects of substances such as cyclic ADP-ribose (Rakovic et al. 1996) or phosphorylation of the ryanodine receptor (duBell et al. 1996) cannot simply be attributed to effects on CICR.
References
- Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. Journal of General Physiology. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W, Best PM. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. Journal of Physiology. 1976;262:583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. American Journal of Physiology. 1995;268:C1313–1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bridge JH, Smolley JR, Spitzer KW. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science. 1990;248:376–378. doi: 10.1126/science.2158147. [DOI] [PubMed] [Google Scholar]
- Carmeliet E, Morad M, Van der Heyden G, Vereecke J. Electrophysiological effects of tetracaine in single guinea-pig ventricular myocytes. Journal of Physiology. 1986;376:143–161. doi: 10.1113/jphysiol.1986.sp016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RA, Leoty C. The effects of tetracaine on the membrane currents and contraction of frog atrial muscle. Journal of Physiology. 1981;317:475–486. doi: 10.1113/jphysiol.1981.sp013837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBell WH, Lederer WJ, Rogers TB. Dynamic modulation of excitation-contraction coupling by protein phosphatases in rat ventricular myocytes. Journal of Physiology. 1996;493:793–800. doi: 10.1113/jphysiol.1996.sp021423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Nichols CG, O'Neill SC, Smith GL, Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. Journal of Physiology. 1989;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Noble D, Shimoni Y, Spindler AJ. Inward current related to contraction in guinea-pig ventricular myocytes. Journal of Physiology. 1987;385:565–589. doi: 10.1113/jphysiol.1987.sp016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Lukyanenko V, Györke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular cells. Journal of Physiology. 1997;500:297–310. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Palade P. Calcium-induced calcium release in crayfish skeletal muscle. Journal of Physiology. 1992;457:195–210. doi: 10.1113/jphysiol.1992.sp019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: Hydrophilic and hydrophobic pathways for the drug-receptor reaction. Journal of General Physiology. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MG, Simon BJ, Schneider MF. Effects of procaine and caffeine on calcium release from the sarcoplasmic reticulum in frog skeletal muscle. Journal of Physiology. 1992;453:341–366. doi: 10.1113/jphysiol.1992.sp019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai H, Redon D, Rusy BF. Procaine enhancement of the rapid cooling contracture and inhibition of the decay of the potentiated state in rabbit papillary muscle. Journal of Molecular and Cellular Cardiology. 1995;27:2543–2550. doi: 10.1006/jmcc.1995.0041. 10.1006/jmcc.1995.0041. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I, Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflügers Archiv. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Negretti N, O'Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovascular Research. 1993;27:1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- Negretti N, Varro A, Eisner DA. Estimate of net calcium fluxes and sarcoplasmic reticulum calcium content during systole in rat ventricular myocytes. Journal of Physiology. 1995;486:581–591. doi: 10.1113/jphysiol.1995.sp020836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SC, Eisner DA. A mechanism for the effects of caffeine on Ca2+ release during diastole and systole in isolated rat ventricular myocytes. Journal of Physiology. 1990;430:519–536. doi: 10.1113/jphysiol.1990.sp018305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend CL, Eisner DA, O'Neill SC. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. Journal of Physiology. 1997;502:471–479. doi: 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakovic S, Galione A, Ashamu GA, Potter BVL, Terrar DA. A specific cyclic ADP-ribose antagonist inhibits cardiac excitation-contraction coupling. Current Biology. 1996;6:989–996. doi: 10.1016/s0960-9822(02)00643-7. [DOI] [PubMed] [Google Scholar]
- Satoh H, Delbridge LMD, Blatter LA, Bers DM. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: Species dependence and developmental effects. Biophysical Journal. 1996;70:1494–1504. doi: 10.1016/S0006-3495(96)79711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Callewaert G, Carmeliet E. Inhibition and rapid recovery of Ca2+ current during Ca2+ release from sarcoplasmic reticulum in guinea pig ventricular myocytes. Circulation Research. 1995;76:102–109. doi: 10.1161/01.res.76.1.102. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Wendt IR. Effects of procaine on calcium accumulation by the sarcoplasmic reticulum of mechanically disrupted rat cardiac muscle. Journal of Physiology. 1986;373:195–207. doi: 10.1113/jphysiol.1986.sp016042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford AW, Díaz ME, Eisner DA. Alteration of sarcolemmal and sarcoplasmic reticulum calcium fluxes in isolated rat ventricular myocytes produced by modulation of calcium-induced calcium release. Journal of Physiology. 1997a;501.P:133P. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford AW, Díaz ME, Eisner DA. Stimulation of Ca-induced Ca release only transiently increases the systolic Ca transient: measurements of Ca fluxes and s.r. Ca. Cardiovascular Research. 1998;37 doi: 10.1016/s0008-6363(97)00266-6. in the Press. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Díaz ME, Negretti N, Eisner DA. Enhanced calcium current and decreased calcium efflux restore sarcoplasmic reticulum Ca content following depletion. Circulation Research. 1997b;81:477–484. doi: 10.1161/01.res.81.4.477. [DOI] [PubMed] [Google Scholar]
- Varro A, Negretti N, Hester SB, Eisner DA. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflügers Archiv. 1993;423:158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- Zahradníková A, Palade P. Procaine effects on single sarcoplasmic reticulum Ca release channels. Biophysical Journal. 1993;64:991–1003. doi: 10.1016/S0006-3495(93)81465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


