Abstract
We tested the hypothesis that activation of P2X receptors associated with vagal afferent nerves can evoke a Bezold-Jarisch (B-J) depressor reflex in anaesthetized rats.
Injection of αβ-methylene ATP (αβ-MeATP; 0.6-600 nmol i.v.) evoked a dose-dependent B-J reflex comprising bradycardia, hypotension and apnoea in rats anaesthetized with pentobarbitone. Apnoea was commonly preceded by hyperventilation. Bilateral vagotomy significantly reduced the bradycardia and most of the apnoeic response without affecting hyperventilation, and unmasked a vasopressor response. Hypotension and apnoea were subject to desensitization, and ATP was about 100 times less potent than αβ-MeATP in evoking the B-J reflex.
ED50 values for responses to αβ-MeATP were: bradycardia 14.6 ± 3.8 nmol; apnoea 47.1 ± 8.5 nmol; hyperventilation 23.3 ± 6.0 nmol, n = 14. The ED50 for apnoea was significantly greater than that for bradycardia or hyperventilation (P < 0.05). Atropine (2.8 μmol (kg body wt)−1 i.v.) antagonized the reflex bradycardia and hypotension.
The P2 antagonists suramin (14 μmol (kg body wt)−1 i.v.) and PPADS (17 μmol (kg body wt)−1 i.v.) antagonized the bradycardic and apnoeic components of the reflex response to αβ-MeATP, without reducing the vasopressor or hyperventilatory responses to the agonist.
Recordings from vagal afferents showed that pulmonary inflation receptors were activated by αβ-MeATP in 62% of units recorded (ED50 22 ± 5 nmol) and this was blocked by PPADS (17 μmol (kg body wt)−1 i.v.); unidentified vagal afferents were also activated.
αβ-MeATP activated carotid chemoreceptor afferents (ED50 23 ± 9 nmol), an action that was unaffected by PPADS or suramin.
The results support the hypothesis that P2X receptor subtypes for ATP are associated with specific sensory nerves that form part of the homeostatic mechanism for cardiovascular and respiratory regulation and these receptors therefore have physiological, pathological and therapeutic significance.
P2X purinoceptors are multimeric ATP-gated cation channels (see recent reviews by Humphrey et al. 1995; North, 1996; Buell, Collo & Rassendren, 1996) and seven separate P2X receptor subunits have been cloned (Valera et al. 1994; Suprenant, Rassendren, Kawashima, North & Buell, 1996). The distribution of these receptors has been studied by in situ hybridization, and in rat they are found in the brain and spinal cord, particularly within areas such as the substantia gelatinosa where primary afferent nerve fibres synapse (Collo et al. 1996). Autoradiographic studies have confirmed the presence of abundant P2X receptor binding sites in rat brain and spinal cord (Tuyau, Hansen, Dampney, Balcar & Bennett, 1997), and P2X receptors are also associated with sensory neurones in the periphery (Evans & Suprenant, 1996). For example, the nodose (vagal) ganglion expresses RNAs for six of the seven known P2X receptors, including the P2X3variant that appears only to be present in sensory ganglia (Kidd, Grahames, Simon, Michel, Barnard & Humphrey, 1995; Collo et al. 1996). P2X3 subunits have been detected immunohistochemically in small trigeminal nerve sensory fibres and endings in rat tooth pulp, and neurites of dissociated cultured neurones from the trigeminal ganglion were depolarized by pressure-ejected ATP (Cook, Vulchanova, Hargreaves, Elde & McCleskey, 1997).
Functional studies involving the vagus nerve have shown that intravenously administered ATP slows the cat heart via reflex actions involving vagal afferent and efferent nerve fibres (Emmelin & Feldberg, 1948), and ATP is known to activate a vagal reflex in dog and human (see Pelleg, Hurt & Michelson, 1990). More recently, Trezise, Kennedy & Humphrey (1993) and Trezise, Bell, Kennedy & Humphrey, (1994) demonstrated that activation of P2X receptors depolarizes nerve fibres in the rat isolated vagus. Whole-cell patch-clamp recordings from rat nodose neurones in culture confirmed that ATP depolarized neurones, as did the non-degradable agonist αβ-methylene ATP (αβ-MeATP; Khakh, Humphrey & Suprenant, 1995) which activates a subset of P2X receptors, including P2X1, P2X3 and P2X2-P2X3 heteromers (Buell, Collo & Rassendren, 1996). It has also been shown that ATP excites vagal C fibre nerve terminals in the dog lung, an effect that appeared to be mediated by P2X receptors because αβ-MeATP caused the same effect, which was antagonized by the P2 antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; Pelleg & Hurt, 1996).
The Bezold-Jarisch (B-J) reflex comprises a triad of apnoea, bradycardia and hypotension that was originally described as a cardiodepressor-vagal chemoreflex evoked via the stimulation of cardiac receptors by intravenously administered veratrine (Bezold & Hirt, 1867; Dawes & Comroe, 1954). ATP is reported to be one of the ‘detector substances’ that can also activate this complex reflex (Jarisch, 1941; Dawes & Comroe, 1954). The B-J reflex involves various cardiopulmonary sensory receptors: most of the cardiodepression and vasodepression results from activation of vagal afferents in the heart, particularly the left ventricle (coronary chemoreflex, Dawes & Comroe, 1954), whereas apnoea and about 10% of the cardiovascular depression arise from stimulation of vagal sensory receptors in the lungs, in cats and dogs anaesthetized with pentobarbitone (Dawes, 1947).
While studying the effects of purinoceptor agonists on nociceptor afferents in anaesthetized rats (Dowd, McQueen, Chessell & Humphrey, 1997), we observed that reflex bradycardia, hypotension and apnoea occurred in response to intra-arterial administration of αβ-MeATP. The reflex was obtained during some, but not all, of the experiments and it seemed to be influenced by the anaesthetic agent used. We have not found any reports concerning activation of the B-J reflex by ATP in rat, so the present investigation was undertaken to test the hypothesis that activation of P2X receptors associated with vagal afferents can evoke a B-J cardiorespiratory reflex in this species. We evoked cardiorespiratory reflexes by i.v. injection of αβ-MeATP and other less stable P2 purinoceptor agonists, including ATP. However, since ATP is rapidly degraded by nucleotide enzymes in vivo (Holton, 1959), it is of limited use in experiments because the cardiovascular and respiratory effects seen following its injection will result from mixed actions on P1 and P2 purinoceptors for adenosine and ATP respectively: adenosine is a major metabolite of ATP and it causes bradycardia, hypotension and hyperventilation (Drury & Szent-Györgi, 1929; Reid, Watt, Penny, Newby, Smith & Routledge, 1991). We determined which sensory nerves contribute to the reflex in animals anaesthetized with pentobarbitone by using a combination of selective denervation and electrophysiological recordings from afferent nerves. The antagonists suramin and PPADS were used in conjunction with the purinoceptor agonists to characterize the type of purinoceptor associated with the sensory nerves involved in evoking the B-J reflex. A preliminary account of the work has been published (McQueen, Moores, Dowd, Bond, Chessell & Humphrey, 1997).
METHODS
Animals and anaesthesia
Experiments were licensed under UK Home Office regulations. Male Wistar rats ranging between 250 and 550 g in body weight were anaesthetized with either pentobarbitone (60 mg (kg body wt)−1i.p., mean weight 361 ± 13 g, n = 26, followed at hourly intervals with 6 mg i.v., if necessary - dependent on response to applying pressure to a limb joint and the basal heart rate and blood pressure (BP)) or urethane (single dose 0.6 ml (100 g body wt)−1i.p. of 25% w/v aqueous ethyl carbamate; mean weight 374 ± 37 g, n = 6). In three experiments the rat (mean weight 374 ± 25 g) was anaesthetized with trichloroethylene and the brain and spinal cord destroyed by pithing, as described by Gillespie & Muir (1967). A servo-controlled heating blanket (Harvard) maintained rectal temperature at 38°C.
Ventilation and blood pressure
The trachea was cannulated and connected to a pneumotachograph head linked to an electrospirometer (Mercury Electronics CS5) and a computerized recording system (MacLab-8 and Macintosh LC475 computer) for measuring and recording tracheal air flow, respiratory frequency, tidal volume and respiratory minute volume (RMV). The animals breathed room air spontaneously, apart from the pithed rats and the majority of those used for neural recordings, which were artificially ventilated and neuromuscularly blocked with gallamine (8 mg i.v. at hourly intervals, together with 6 mg pentobarbitone in the neural experiments). Both femoral arteries were cannulated, one catheter being used to record arterial blood pressure via a BP transducer (Bell & Howell) linked to the MacLab, the other being used for taking samples (0.2 ml) of arterial blood for measurement of arterial oxygen pressure (Pa,O2), and arterial carbon dioxide pressure (Pa,CO2) and pH (Ciba Corning 238 analyser) during the experiment. Heart rate was measured from the BP recording, with the time scale expanded to allow the number of pulses in a fixed period to be counted pre- and post-injection. The right external jugular vein was cannulated for intravenous administration of drugs.
Surgical procedures
In five experiments the vagi were sectioned at mid-cervical level, and in two cases the nodose ganglia were subsequently excised. The carotid sinus nerves (left and right side) were identified at their junction with the glossopharyngeal (IX cranial) trunk and both the left and right nerves were sectioned in four experiments. Denervation was confirmed by the abolition of the reflex hyperventilation that was evoked before denervation by the peripheral chemoreceptor stimulant sodium cyanide (2 μmol i.v.).
Neural recording from arterial chemoreceptors
The left carotid sinus nerve was sectioned at its junction with the glossopharyngeal trunk and the peripheral end desheathed. The nerve was immersed under paraffin oil and recordings of neural activity were made extracellularly from dissected nerve filaments using bipolar platinum-iridium electrodes connected to an amplifier (Neurolog NL104) and a computerized digital video recording system (Sony VCR EV-C500E; Dell 450/L PC). Individual action potentials from multi-unit (1–4) recordings were counted via a pulse height voltage discriminator (Digitimer D130) and quantified using the PC and computer software developed in-house. The contralateral carotid sinus nerve remained intact and the animals breathed room air in two experiments, in one of which the rat was anaesthetized with urethane. In two further experiments under pentobarbitone both carotid sinus nerves were cut and the rat was artificially ventilated and the neuromuscular system was blocked with gallamine.
Neural recording from vagal afferents
The right vagus nerve was sectioned at mid-cervical level and recordings of neural discharge (1–5 units) made from filaments dissected from the desheathed peripheral end in six rats, using the techniques described above. In some experiments gallamine was administered and the animals were artifically ventilated to prevent reflex cardiorespiratory changes from influencing the responses.
Drugs
The drugs used included: adenosine, adenosine 5′-triphosphate disodium (ATP), adenosine 5′-O-(3-thiophosphate) (ATPγS), αβ-MeATP (lithium salt) (all from Sigma); 2-methyl-5-hydroxytryptamine maleate (2-Me-5-HT) and suramin hexasodium (from RBI); MDL 72222 (a gift from Merrell Dow); trichloroethylene, atropine sulphate and sodium cyanide (from BDH); gallamine triethiodide (from May & Baker); PPADS (tetrasodium salt; from Tocris Cookson). Drugs were dissolved in saline (0.9% w/v aqueous sodium chloride) and injected in volumes of 0.1 ml, washed in with 0.2 ml saline. The control was 0.3 ml saline and all injections were completed within 2 s, with the exception of antagonists which were slowly injected over 15–30 s. Injections were i.v. (jugular) or i.a. (femoral catheter in some experiments) and the minimum interval between successive doses was 5 min. In order to minimize the number of animals used, more than one procedure was performed wherever this was feasible (in a total of eight experiments involving atropine or vagotomy after recovery from PPADS, cutting carotid sinus nerves after vagotomy or PPADS), but at least one experiment in each group was done without any pre-treatment as a check that pre-treatment had not influenced the responses obtained.
Data and analysis
Half-maximum response (ED50) values for bradycardia, apnoea and hyperventilation were calculated from individual log dose-response curves for the agonists before and after selective denervation or the administration of antagonist. As the concentration of the injected drug at the receptor site is not known, nor is it in equilibrium following a bolus injection, and because of difficulties in establishing a maximum response from receptors prone to desensitization, we minimized the number of doses used in vivo and the calculated ED50 was expressed as an apparent value, i.e. the best estimate. Results are presented as means ± s.e.m. Statistical analysis (generally ANOVA, Student's t test, or the non-parametric Mann-Whitney test (two-tailed) - when variances were significantly different) was used to determine whether differences between group means were statistically significant. The Null hypothesis was rejected at P ≤ 0.05.
RESULTS
Intravenous injection of αβ-MeATP (0.6-600 nmol) in animals anaesthetized with pentobarbitone evoked a rapid dose-dependent bradycardia, hypotension and apnoea, the latter preceded by a transient hyperventilation (Fig. 1A). In approximately 20% of experiments there was a delayed secondary hyperventilation after the apnoea. Repeating the doses at intervals of less than 5–10 min led to a decrease in magnitude (total loss after high doses) of the apnoea, bradycardia, and hypotension, but not the hyperventilatory component of the response. ATPγS (18–366 nmol) caused similar responses, although it was less potent than αβ-MeATP in evoking bradycardia. Although we did not study the nucleotide in detail, ATP in doses approximately 100-fold greater than those of αβ-MeATP also caused a slight hyperventilation, apnoea and bradycardia. Adenosine injected i.v. in high doses (4.7-11.2 μmol) resulted in a bradycardia without hyperventilation or apnoea, which unlike the bradycardia evoked by αβ-MeATP was slightly delayed in onset and unaffected by bilateral vagotomy or atropine. Similar cardiorespiratory effects to those of αβ-MeATP were evoked by the 5-HT3 agonist, 2-Me-5-HT (33–330 nmol).
Figure 1. Cardiorespiratory effects of αβ-MeATP.

Cardiorespiratory effects of a dose of αβ-MeATP (100 nmol i.v. at continuous vertical line) in a rat anaesthetized with pentobarbitone. In each panel the upper trace from the computerized chart recorder is arterial BP, and the lower trace is respiratory airflow (inspiration downwards, arbitrary units (a.u.), mV). Heart rate was measured by counting individual beats in the BP trace. During the control state (A) bradycardia, transient hyperventilation, vasodepression and apnoea were obtained in response to the rapid injection of αβ-MeATP. After cutting both vagus nerves at mid-cervical level (B) the bradycardia, hypotension, and apnoea were abolished, leaving hyperventilation and a vasopressor response to the same dose of agonist. Cutting both carotid sinus nerves (C) abolished hyperventilation without reducing the vasopressor response. The delayed arrhythmia seen in C differed from the reflex bradycardia observed in A, and was associated with the substantial rise in systemic blood pressure; the arrhythmia may result from activation of C fibre sympathetic cardiac afferents innervating the left ventricle (see Hainsworth, 1991).
Mean arterial BP was 106 ± 5 mmHg and respiratory minute volume 210 ± 27 ml min−1 in rats (n = 23) anaesthetized with pentobarbitone; arterial blood gases and pH did not differ significantly between the groups. In control animals (n = 23) mean pH was 7.44 ± 0.01, mean Pa,CO2 was 35.9 ± 1.4 mmHg and mean Pa,O2 was 71.8 ± 2.1 mmHg. Corresponding values for vagotomized rats (n = 10) were: 7.45 ± 0.02, 31.4 ± 2.0 and 75.3 ± 1.9; after cutting the carotid sinus nerves they were 7.39 ± 0.05, 34.8 ± 1.9 and 67.0 ± 6.2 (n = 5). Changes in reflex responses obtained after selective denervation were therefore not secondary to alterations in basal conditions, as indicated by the stable arterial blood gas tensions and pH.
In animals anaesthetized with urethane the reflex bradycardia, hypotension and apnoea in response to αβ-MeATP (20–600 nmol) was attenuated or absent in four of five experiments although hyperventilation and vasopressor responses were present in them all, so pentobarbitone was used for the majority of our experiments.
Reflex bradycardia and hypotension
Injection of αβ-MeATP in anaesthetized rats caused dose-dependent bradycardia within the first 5 s following i.v. injection which lasted for up to 5 s (Figs 1 and 4). ATP injected in high doses (2–5 μmol) caused bradycardia, but this response was longer lasting in comparison with that evoked by αβ-MeATP; adenosine in high doses also caused delayed long-lasting bradycardia. Experiments in pithed rats showed that i.v. αβ-MeATP did not cause bradycardia, whereas high doses of ATP and adenosine caused similar responses to those observed in intact anaesthetized animals, namely bradycardia and hypotension (see Fig. 2).
Figure 4. Summary of pooled ED50 data for brachycardia, apnoea and hyperventilation evoked by αβ-MeATP.
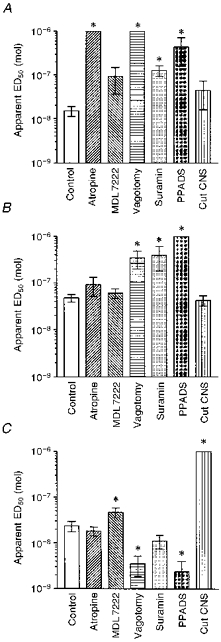
Mean apparent ED50 values ± s.e.m. for reflex changes evoked by i.v. αβ-MeATP before (Control, n = 14) and after various procedures in rats anaesthetized with pentobarbitone. A, bradycardia was significantly reduced by atropine (2.8 μmol (kg body wt)−1, n = 4), bilateral vagotomy (n = 3), suramin (14 μmol (kg body wt)−1, n = 3) and by PPADS (17 μmol (kg body wt)−1, n = 4), but unaffected by MDL 72222 (0.24 μmol (kg body wt)−1, n = 2) or cutting the sinus nerves (Cut CNS, n = 3). B, apnoea was unaffected by atropine (n = 4), MDL 72222 (n = 3), or cutting the carotid sinus nerves (n = 4), but was reduced after vagotomy (n = 5), suramin (n = 4) or PPADS (n = 4). C, hyperventilation was reduced by cutting the carotid sinus nerves (n = 4), and slightly but significantly by MDL 72222 (n = 4). The response was potentiated by vagotomy (n = 5) or PPADS (n = 4), and unaffected by atropine or suramin (both n = 4). *P < 0.05 versus control values (Mann-Whitney test).
Figure 2. Cardiovascular effects in an artifically ventilated pithed rat in which reflex effects were abolished.

Effects of αβ-MeATP (60 nmol i.v. at vertical line) (A) and adenosine (375 nmol i.v.) in an artificially ventilated pithed rat (B) in which reflex effects were abolished. The P2X receptor agonist gave a vasopressor response with no bradycardia, whereas adenosine caused bradycardia and hypotension.
There was a triphasic change in arterial BP following injection of αβ-MeATP, comprising a transient hypotension associated with the bradycardia, and then a more prolonged (1–2 min) vasopressor phase (see Fig. 1) and after higher doses (100 nmol or more), a longer lasting (up to 10 min) delayed-depressor phase was obtained; but high doses were not routinely tested because of concerns over receptor desensitization. ATPγS, ATP and adenosine (4.7-11.2 μmol) caused only hypotension, there being no vasopressor component; slight hypertension occurred after 2-Me-5-HT, which also caused transient vasodepression secondary to the bradycardia. Intra-arterial injection of αβ-MeATP also caused reflex cardiovascular effects, but had only about one-tenth the potency of the same dose injected i.v., indicating that the sensory receptors were located in or near the heart and lungs. In pithed rats αβ-MeATP caused only vasopressor responses, whereas injected ATPγS, ATP and adenosine were exclusively vasodepressor responses and lacked any vasopressor component (Fig. 2). Blood pressure responses were not studied in detail because the primary reflex vasodepression was entirely secondary to the bradycardia, but we noted that the vasopressor response to αβ-MeATP in anaesthetized rats was not significantly affected by suramin or PPADS (mean rise in BP evoked by 100 nmol αβ-MeATP before suramin (14 μmol (kg body wt)−1) was 26 ± 2 mmHg, and after was 25 ± 3 mmHg, n = 3; BP caused by 40 nmol αβ-MeATP before PPADS (17 μmol (kg body wt)−1) was 39 ± 15 mmHg, and after was 39 ± 8.5 mmHg, n = 6). The reflex bradycardia was attenuated when successive high doses of αβ-MeATP were injected at intervals of less than 10 min, with a tendency to generate bell-shaped log dose-response curves, and some reduction was evident even after delays of 10–15 min. It was therefore necessary to avoid using high doses of P2 receptor agonist, and to accept that it would not be possible to establish the true maximal responses. The bradycardia was calculated from log dose-response (Fig. 3) and expressed as an apparent ED50 for each experiment.
Figure 3. log dose-response curves showing the effects of αβ-MeATP.

Results from individual experiments illustrating the effects of αβ-MeATP on heart rate (A), apnoea (B) and hyperventilation (C), before (^) and after (•) the purinoceptor antagonist PPADS (17 μmol (kg body wt)−1 i.v.) which reduced the bradycardia and apnoea components of agonist-induced responses, whereas the hyperventilation component was unaffected or even enhanced by PPADS. Apparent ED50 values were estimated from the log dose-response plots.
Bradycardia evoked by αβ-MeATP was significantly attenuated by atropine (2.8 μmol (kg body wt)−1) and by bilateral vagotomy (Fig. 1B), as well as by the P2 purinoceptor antagonists suramin (14 μmol (kg body wt)−1 i.v.) and PPADS (17 μmol (kg body wt)−1 i.v., but not by 1.7 μmol (kg body wt)−1). Pooled ED50 data from all the experiments are shown in Fig. 4A. In some instances it was not possible to calculate the dose required to match the original ED50 for some responses after denervation or administration of an antagonist (e.g. after vagotomy the bradycardia was totally abolished, likewise after PPADS), so a minimum ED50 of 1 × 10−6 mol was entered to allow statistical analysis using non-parametric tests. The transient vasodepression which occurred at the same time as the bradycardia was absent when the reflex bradycardia was prevented, and PPADS also blocked the delayed prolonged hypotension previously evoked by αβ-MeATP. ATPγS was less effective than αβ-MeATP in evoking reflex bradycardia (ED50 78.1 ± 6.8 nmol, n = 4; P < 0.001 versus αβ-MeATP, t test). High doses of ATP or adenosine caused a bradycardia that was unaffected by vagotomy, atropine, suramin or PPADS. Antagonism caused by the single dose of PPADS (17 μmol (kg body wt)−1 i.v.) was short lasting (10–15 min) in some experiments, but longer in others (45 min) - even though the same batch of drug was used - so an additional dose of antagonist was administered when it was apparent that its effects were waning; suramin (14 μmol (kg body wt)−1) antagonism tended to last longer, up to about 60 min.
Activation of vagal 5-HT3 receptors has been shown to elicit similar reflexes to those described above (Fozard, 1984), so the possibility that endogenous 5-HT might be involved in the reflexes was tested. We used the 5-HT3 receptor agonist 2-Me-5-HT and found it gave responses similar to those caused by αβ-MeATP. The reflex bradycardia and apnoea were antagonized by MDL 72222, but not by the P2 antagonists suramin or PPADS (data not shown). The mean ED50 for bradycardia evoked by αβ-MeATP was not significantly affected by the 5-HT3 antagonist (see Fig. 4).
Respiratory effects
Injections of αβ-MeATP in rats anaesthetized with pentobarbitone evoked a complex dose-related change in ventilation, comprising a transient (2–3 breaths) hyperventilation of rapid onset (2–3 s post-injection) followed by dose-related apnoea lasting from 1–20 s (see Fig. 1 and 3C). In some experiments a 5–30 s period of delayed hyperventilation followed the apnoea, but as it usually coincided with delayed hypotension, we did not investigate it further during this study. When high doses of agonist were repeated at short (< 5 min) intervals, apnoea was attenuated, so 10–15 min were allowed to elapse between successive high doses. The hyperventilation was less prone to desensitization. Intra-arterial injections of αβ-MeATP (200 nmol) or 2-Me-5-HT (33 μmol) evoked smaller respiratory responses to those obtained when the drugs were given i.v. in the same animal, and the i.v. to i.a. equipotent molar ratio for αβ-MeATP was approximately 10 (n = 2). Reflex apnoea following i.v. αβ-MeATP was usually attenuated or absent when urethane was used as the anaesthetic, although dose-related increases in ventilation were obtained.
Apnoea
The ED50 for apnoea evoked by αβ-MeATP was calculated from individual log dose-response plots (Fig. 3B). By pooling data from different experiments in which various procedures were performed we established that neither atropine (2.8 μmol (kg body wt)−1 i.v.), MDL 72222 (0.24 μmol (kg body wt)−1 i.v.) nor cutting the carotid sinus nerves had any significant effect on the apnoea, as shown in Fig. 4B, although the dose of MDL 72222 used, abolished apnoea and bradycardia caused by 2-Me-5-HT. Bilateral vagotomy significantly reduced the apnoea (Fig. 1B), but did not eliminate it in all experiments - high doses of αβ-MeATP still elicited a reduced apnoeic response in some experiments post-vagotomy, an effect which was abolished by surgical removal of both nodose ganglia. The P2 purinoceptor antagonist suramin (14 μmol (kg body wt)−1 i.v.) significantly reduced the apnoeic response to αβ-MeATP (Fig. 5), and a similar effect was obtained with PPADS (17 μmol (kg body wt)−1 i.v.). Qualitatively similar respiratory responses were observed with ATPγS, and the compound did not differ significantly from αβ-MeATP in evoking apnoea (ED50 (1.2 ± 0.6) × 10−7 nmol). ATP (maximum dose 5.4 × 10−6 mol caused apnoea, with a slight hyperventilation. Adenosine in doses of up to 3.7 × 10−6 mol did not cause apnoea, but the nucleoside was similar to ATP in that it caused a slight hyperventilation.
Figure 5. Activation of pulmonary vagal afferents by αβ-MeATP.
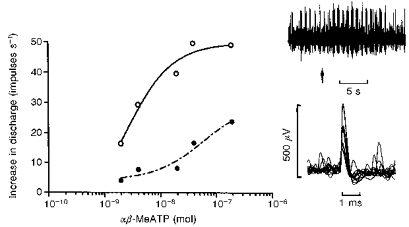
Multi-unit recording from vagal afferents which increased their discharge frequency without any rapid adaptation when the lungs were inflated, i.e. lung inflation receptors. Injection of the P2X agonist αβ-MeATP increased firing of the three units counted by the spike voltage discriminator (^, continuous line) and PPADS (17 μmol (kg body wt)−1 i.v.) antagonized the response (•, dashed line). The upper neurogram shows the neural discharge before and after a control i.v. injection of 2 nmol αβ-MeATP at arrow - note that bursts of activity occurred during inspiration. The lower inset shows a sample of superimposed action potentials (3) triggered by the injection of αβ-MeATP. The number of action potentials triggered during the 5 s period following onset of the response was plotted as the increase above basal values which averaged 14.1 ± 1.4 impulses s−1 before, and 16.3 ± 1.5 impulses s−1 after PPADS. The respiratory pump was set to a stroke volume of 3 ml with a frequency of 84 strokes min−1.
Hyperventilation
Increased ventilation within the first 5 s after an injection of αβ-MeATP was a prominent feature of the response to the P2 agonist and was caused by an increase in depth and frequency of respiration (see Fig. 1). This response was generally followed by a period of apnoea (see above), depending on the dose of agonist administered: low doses usually only evoked hyperventilation. Injection of 2-Me-5-HT also evoked an increase in RMV. Delayed hyperventilation was observed following αβ-MeATP, but this effect commenced 8–15 s after the injection, following a period of apnoea, and may have been secondary to the cessation of breathing or the hypotension. Cutting the carotid sinus nerves, thereby denervating the carotid bodies - confirmed by the lack of hyperventilation previously evoked by sodium cyanide (2 μmol i.v.) - significantly reduced the rapid-onset hyperventilation (n = 4), see Fig. 1C. Since the hyperventilatory response was substantially reduced by cutting the carotid sinus nerves it was not feasible to determine a post-denervation ED50 for αβ-MeATP, so responses were expressed as being > 1 × 10−6 mol.
The primary increase in ventilation was measured during the 6 s period immediately following the injection and the mean apparent ED50 determined from individual log dose-response plots. The pooled data illustrated in Fig. 4C showed that suramin and PPADS tended to potentiate the reflex hyperventilation evoked by αβ-MeATP, and vagotomy also increased the response. There was a slight but statistically significant decrease in responsiveness to αβ-MeATP following the 5-HT3 receptor antagonist MDL 72222; atropine had no effect. Qualitatively similar responses were observed with ATPγS, and its ED50 (2.8 × 10−8 mol, n = 4) was not different from that of αβ-MeATP; vagotomy also potentiated the increase in ventilation obtained with ATPγS. As described above, ATP caused a slight dose-related increase in ventilation when injected at a high dose of 5.5 × 10−6 mol (i.v.), but there was also a delayed slight hyperventilation accompanying the profound hypotension which was unaffected by cutting the carotid sinus nerves. Adenosine lacked any fast onset stimulant action on ventilation when given in doses up to 3.7 × 10−6 mol i.v., but did cause a slight delayed hyperventilation that persisted after denervation of the carotid bodies.
ED50 values
Analysis of the mean ED50 values for αβ-MeATP-induced bradycardia, apnoea and hyperventilation (see Fig. 4) showed that they differed significantly (ANOVA P = 0.003). Bonferroni's multiple comparison test indicated that the control ED50 value for apnoea was significantly higher than that for bradycardia (P < 0.01) and hyperventilation (P < 0.05), whereas those for bradycardia and hyperventilation did not differ from each other.
Neural recordings
Vagal afferents
Recordings were obtained mainly from vagal pulmonary inflation (slowly adapting stretch) receptors, whose discharge was in phase with inspiration and related to the stroke volume of the ventilator. Activity was also recorded from some spontaneously active vagal afferents which did not respond to pulmonary inflation, deflation, sodium cyanide, or to changes in mean BP. Overall, twenty-four multi-unit recordings were made and 62% of these (mainly pulmonary inflation receptors, but also unidentified vagal afferents) were rapidly (within 2–3 s of injection) excited by i.v. αβ-MeATP (see Fig. 5), and also by 2-Me-5-HT, whereas 38% of the afferents were unresponsive to these drugs, including four recordings (17% of total population) that responded to lung inflation. The increase in discharge lasted from 2–10 s, the longer duration being associated with high doses. The mean ED50 for αβ-MeATP-induced excitation was (2.2 ± 0.5) × 10−8 mol (n = 4) before, and (1.4 ± 0.2) × 10−7 mol (n = 3) after PPADS (17 μmol (kg body wt)−1; P < 0.05, paired t test n = 3). Vagal activation by 2-Me-5-HT (a single dose of 33 μmol was unaffected by PPADS, although it was abolished by the 5-HT3 antagonist MDL 72222 (0.24 μmol (kg body wt)−1) which did not significantly affect the excitatory response to αβ-MeATP. The ED50 for αβ-MeATP-induced apnoea or bradycardia did not differ significantly from the vagal neural ED50.
In one experiment under urethane anaesthesia two separate multi-unit recordings of vagal sensory discharge were made. In one of these recordings the afferents were unaffected by αβ-MeATP, but were strongly excited by 2-Me-5-HT (33 μmol), whereas in the other they were activated by the purinoceptor agonist within 2 s of injection and the excitation lasted for 3–10 s. The ED50 for αβ-MeATP was 1.5 × 10−7 mol, and in this recording the afferents showed only a very weak excitatory response to 2-Me-5-HT, either before or after excitation by αβ-MeATP.
Carotid body chemoreceptors
Recordings from the peripheral end of sectioned carotid sinus nerves in four rats demonstrated that αβ-MeATP injected i.v. caused a rapid dose-related increase in chemosensory discharge, as illustrated in Fig. 6. Intra-arterial injection of αβ-MeATP also increased chemosensory discharge, but with only one-tenth of the potency of the same dose injected i.v.; high doses of ATP ((1.8-5.5) × 10−6 mol) were also chemo-excitatory. The mean apparent ED50 for chemo-excitation evoked by αβ-MeATP was (2.3 ± 0.9) × 10−8 mol (n = 4), which was not significantly different from (2.3 ± 0.6) × 10−8 mol (n = 14), obtained for hyperventilation. Neither suramin (14 μmol (kg body wt)−1) nor PPADS (17 μmol (kg body wt)−1) antagonized the chemo-excitant action of αβ-MeATP, nor did they affect the chemo-excitation evoked by sodium cyanide or asphyxia (stopping the respiratory pump for 30 s).
Figure 6. Activation of carotid body chemoreceptors by αβ-MeATP and ATP, and the effect of P2 purinoceptor antagonist PPADS.
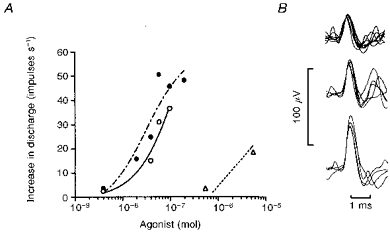
Multi-unit recording from carotid chemoreceptor afferent fibres in an artificially ventilated rat anaesthetized with pentobarbitone. A, all three afferents counted by the voltage discriminator were activated by αβ-MeATP (^, continuous line), and PPADS (17 μmol (kg body wt)−1 i.v.) did not antagonize the response (•, dashed line). Responses evoked by ATP (▵, dotted line) in the control state are also shown. B, three individual action potentials (successive triggered oscilloscope sweeps superimposed) sampled during their activation by αβ-MeATP. The increase in total discharge above pre-injection basal frequency was plotted in A, and basal discharge from these afferents during ventilation with room air averaged 9.3 impulses s−1 before and 9.9 impulses s−1 after PPADS.
Fast onset chemo-excitation was also observed under urethane anaesthesia in the one experiment performed (ED50 for αβ-MeATP 1.8 × 10−8 mol before and 4 × 10−8 mol after suramin (14 mol (kg body wt)−1); ED50 for ATPγS was 3.7 × 10−8 mol before suramin).
DISCUSSION
The results obtained using αβ-MeATP, an agonist which is relatively selective for particular subtypes of P2X receptor, together with subsidiary evidence from the rather weak and non-selective P2 receptor antagonists, PPADS and suramin (see Introduction), support our working hypothesis that activation of vagal P2X receptors in anaesthetized rats evokes a B-J reflex. Carotid body arterial chemoreceptors are also excited by P2X receptor agonists, causing reflex hyperventilation, so the overall cardiorespiratory response to i.v. injection of a P2X agonist such as αβ-MeATP in anaesthetized rats is quite complex, and will be considered in relation to the individual sensory elements involved. ATP had similar effects to those evoked by αβ-MeATP, but we did not study the nucleotide in detail because it is rapidly inactivated by ecto-nucleotidases and it was not feasible to use selective ATPase inhibitors in vivo.
Anaesthetic
The vagal reflexes bradycardia and apnoea were seldom obtained under urethane anaesthesia, whereas both reflexes were invariably present during pentobarbitone anaesthesia. Other authors have noted that reflex responses to ATP are affected by the anaesthetic (e.g. Emmelin & Feldberg, 1948; comparing chloralose with decerebration in cats), and this could be due to block of central reflex pathways, or perhaps to some interaction of the anaesthetic agent with P2X receptors in the periphery. Neural recording from vagal afferents indicated a reduced responsiveness to αβ-MeATP in one experiment under urethane, but more detailed studies would be required to establish the extent to which peripheral, as opposed to central, actions of the anaesthetic agent reduce responsiveness to P2X receptor agonists. We focused on studying the cardiorespiratory reflexes under pentobarbitone anaesthesia.
Vagal afferents
The rapid onset bradycardia and apnoea evoked by αβ-MeATP was abolished by atropine or bilateral vagotomy, indicating that it was a vago-vagal reflex. In some experiments some reduction in ventilation persisted after vagotomy, but this could be eliminated by surgical removal of both nodose ganglia, suggesting that the P2X agonist, particularly in high doses, can activate purinoceptors located in the sensory ganglion (see Khakh et al. 1995) as well as those at the peripheral nerve terminals. The antagonists PPADS and suramin were both capable of antagonizing the bradycardia and apnoea induced by αβ-MeATP. Desensitization to the reflex changes tended to occur when injections of the agonist were repeated at intervals of less than 10 min. Experiments in pithed rats, where reflexes were abolished by destruction of the brain and spinal cord, showed that only vasopressor effects, without bradycardia or vasodepression, were observed in response to αβ-MeATP. This confirms that there is no direct cardiac action of the agonist. In contrast, ATP and adenosine caused bradycardia and hypotension which was still present after vagotomy in anaesthetized animals, and was also obtained in pithed rats. ATP is known to be predominantly vasodepressor, and αβ-MeATP primarily vasopressor in rats, either when anaesthetized (Cox & Smits, 1996) or pithed (Schlicker, Urbanek & Gothert, 1989). Thus αβ-MeATP causes a vagally mediated reflex fall in BP, but the drug also raises BP by mechanisms that do not involve vagal afferents. We found that, in doses which antagonized the reflex bradycardia and apnoea, neither PPADS nor suramin reduced the vasopressor response to αβ-MeATP in anaesthetized animals. We did not study the antagonists in pithed rat, a preparation in which Schlicker et al. (1989) reported that PPADS antagonized the vasopressor response to αβ-MeATP. Further information on the vascular aspects of the actions of ATP can be obtained from a recent review (Rongen, Floras, Lenders & Smits, 1997).
Neural recordings confirmed what the reflex studies had suggested, namely that vagal afferents, particularly those associated with pulmonary function were activated by αβ-MeATP, and the mean ED50 value obtained for neural activation did not differ significantly from those for reflex bradycardia or apnoea. Not all vagal afferent fibres were excited by the P2X agonist, and it was not feasible in this study to establish whether cardiac as well as pulmonary vagal sensory nerves contribute to the reflex. More complex experiments involving local application of drugs and selective denervation of particular cardiac or pulmonary vagal branches would be necessary. Other workers have demonstrated that ventricular receptors, particularly those in the left ventricle, are activated by veratrine and are mainly responsible for the B-J reflex caused by this drug in cat (see Paintal, 1955). It has also been shown that vagal C fibre afferents from the lungs (Hurt, Wang, Xu, Strerious & Pelleg, 1994; Pelleg & Hurt, 1996) and the left ventricle (Taneyama, Benson, Hild & Goto, 1997) in dog can be activated by ATP, probably via P2X receptors (Pelleg & Hurt, 1996), and as reviewed in Introduction, rat nodose neurones and vagal afferent fibres possess P2X receptors. Ventricular afferents which travel to the spinal cord via sympathetic nerve tracts (see Hainsworth, 1991) may be affected by P2X agonists and so contribute to some of the cardiovascular changes observed, particularly following vagotomy and cutting the carotid sinus nerves (e.g. Fig. 1), but this possibility was not investigated during the present study. Thus, it is probable that cardiopulmonary vagal afferents in rats are similar to those in dog in having P2X receptors closely associated with at least part of the sensory fibre population, and the question of whether these purinoceptors have a discrete physiological role in activating or modulating input from particular sensory nerves is intriguing.
The reflexes evoked by αβ-MeATP were not secondary to release of endogenous 5-HT because the B-J reflex evoked by 2-Me-5-HT was antagonized by the 5-HT3 antagonist MDL 72222, which had no significant effect on the responses to the P2X agonist, and PPADS antagonized vagal responses to αβ-MeATP without affecting those to the 5-HT3 receptor agonist. This evidence confirmed that cardiorespiratory reflexes evoked by αβ-MeATP resulted from selective actions at P2X receptors associated with vagal afferents.
Arterial chemoreceptors
Chemoreflexes were rapidly activated by intravenous αβ-MeATP or, at 100 times the dose, ATP, and the mean ED50s for neural activation and reflex hyperventilation evoked by αβ-MeATP were not significantly different. Cardiorespiratory reflexes evoked in the anaesthetized rat were therefore not confined to excitation of vagal afferents. ATP excites carotid body arterial chemoreceptors in cat and dog (Jarisch, Landgren, Neil & Zotterman, 1952), and McQueen & Ribeiro (1983) proposed the presence of P2 purinoceptors in the cat carotid body based on results obtained using αβ-MeATP. Recent studies on cat carotid body chemoreceptors in vitro showed that ATP surface receptors are present that can be transiently activated by an infusion of ATP, although the receptors were subsequently desensitized (Spergel & Lahiri, 1993).
The respiratory reflex and neural data obtained during the present study strongly suggest that the rat carotid body contains P2X receptors which can activate arterial chemoreflexes. The rapid onset of chemo-excitation in response to P2X agonists shows that it is not secondary to vasoconstriction within the carotid body, since changes in discharge following injection of vasoconstrictors are sluggish, being much slower in onset (> 5 s) and tending to last longer - potent vasoconstrictors such as endothelin-1 only evoke small increases in rat carotid chemoreceptor discharge (McQueen, Dashwood, Cobb, Marr, Bond & Spyer, 1995) compared with those we obtained. The purinoceptors in the rat carotid body seem to be relatively insensitive to desensitization by αβ-MeATP, and neither PPADS nor suramin acted as antagonists in the doses used. This means that these carotid body purinoceptors differ from other αβ-MeATP-sensitive P2X receptors that are antagonized by these compounds. It is possible to speculate that the receptors comprise different subunits (e.g. P2X4, whose responsiveness to ATP or αβ-MeATP (weak agonist) is unaffected or potentiated by PPADS or suramin in recombinant expression of receptors in Xenopus oocytes in vitro (Bo, Zhang, Nassar, Burnstock & Schoepfer, 1995)), but it has yet to be established how the pharmacology of recombinant subunits correlates with that of the native receptors in vivo. Other actions of suramin or PPADS (e.g. inhibition of ecto-ATPase) may mask an antagonist action at P2 receptors in the carotid body, although this seems unlikely since the same argument would apply to the bradycardia and apnoea, which were antagonized. Also αβ-MeATP is relatively resistant to breakdown so should be unaffected by enzyme inhibition (see Humphrey et al. 1995). The apparent potentiation of the respiratory response to αβ-MeATP after PPADS or vagotomy is probably secondary to abolition or reduction in the apnoea which normally masks part of the hyperventilation, an interpretation which is supported by chemosensory discharge evoked by αβ-MeATP being largely unaffected by PPADS.
Our conclusions concerning P2X receptor subtypes located on a variety of sensory nerves being involved in triggering cardiorespiratory reflexes are based on indirect evidence obtained using agonists and relatively non-selective antagonists (Humphrey et al. 1995) in functional experiments in vivo. It is tempting to surmise that, because of their sensitivity to αβ-MeATP the receptors associated with vagal afferents must be of the P2X1, P2X2, P2X3 or P2X2/3 subtype(s), but this needs further investigation. Further information from studies involving, for example, selective P2X receptor antagonists or, more directly, receptor autoradiography and immunocytochemistry, would provide useful additional evidence concerning the presence of particular αβ-MeATP-sensitive P2X receptor subtypes in the heart, lungs, and carotid body.
Vagally mediated bradycardia and apnoea evoked during the B-J reflex, and also the carotid chemoreflex hyperventilation in vagotomized rats, could be utilized for convenient functional characterization of compounds affecting P2X receptors, in much the same way as this reflex was utilized in the development of 5-HT3 receptor antagonists (Fozard, 1984).
The physiological significance of the B-J reflex has been the subject of considerable debate during the 130 years since it was first described, and it is commonly regarded as being a pharmacological curiosity. However, the presence of fast ATP-gated cation channels on sensory terminals in vital structures (e.g. left coronary artery and/or ventricle; pulmonary system), together with the local presence of the endogenous ligand ATP, strongly suggests a functional signalling role for ATP (see Zimmermann, 1994) and these P2X receptors. Their presence in the cardiopulmonary system and the carotid body could have pathophysiological significance, particularly when hypoxaemia or tissue damage results in substantial release of ATP from cells and nerve terminals, such as during increased activity in adrenergic nerves (e.g. in stress) that leads to co-release of ATP with noradrenaline (see Burnstock, 1988). These conditions may be mimicked pharmacologically by a P2X agonist activating the B-J cardiac nociceptive reflex, even in the presence of an anaesthetic agent which will depress the reflex. Whether non-noxious low level neural activity in these sensory pathways is part of the interoceptive homeostatic mechanism regulating the cardiovascular (e.g. see Linden, 1973; Hainsworth, 1991) and respiratory systems physiologically, and whether particular peripheral sensory receptors possess sub-populations of specific P2X receptors that enable locally released ATP to influence their sensitivity and activity discretely, are intriguing questions that require investigation. P2X receptors in the heart and lungs are potential therapeutic targets for the treatment of cardiovascular and respiratory disorders.
References
- Bezold von A, Hirt L. Über die physiologischen Wirkungendes des essigsauren Veratrins. Untersuchungen Physiologisches Laboratorium Würzburg. 1867;1:75–156. [Google Scholar]
- Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Letters. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors - an emerging channel family. European Journal of Neuroscience. 1996;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Sympathetic purinergic transmission in small blood vessels. Trends in Pharmacological Science. 1988;9:116–117. doi: 10.1016/0165-6147(88)90185-x. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlot-pich E, Neidhart S, Suprenant A, Buell G. The cloning of P2X(5) and P2X(6) receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves MN, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Cox BF, Smits GJ. Regional hemodynamic effects of purinergic P2 receptor subtype agonists in rats. Journal of Pharmacology and Experimental Therapeutics. 1996;277:1492–1500. [PubMed] [Google Scholar]
- Dawes GS. Studies on veratrum alkaloids. VII. Receptor areas in the coronary arteries and elsewhere as revealed by the use of veratridine. Journal of Pharmacology and Experimental Therapeutics. 1947;89:325–342. [PubMed] [Google Scholar]
- Dawes GS, Comroe JH., Jr Chemoreflexes from the heart and lungs. Physiological Reviews. 1954;34:167–201. doi: 10.1152/physrev.1954.34.2.167. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PPA. Activation by P2X purinoceptor agonists of sensory nerves innervating the rat knee joint. British Journal of Pharmacology. 1997;122:286P. [Google Scholar]
- Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. Journal of Physiology. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelin N, Feldberg W. Systemic effects of adenosine triphosphate. British Journal of Pharmacology. 1948;3:273–284. doi: 10.1111/j.1476-5381.1948.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Suprenant A. P2X receptors in autonomic and sensory neurons. Seminars in the Neurosciences. 1996;8:217–223. [Google Scholar]
- Fozard JR. MDL 72222, a potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn-Schmiedeberg's Archives of Pharmacology. 1984;326:36–44. doi: 10.1007/BF00518776. [DOI] [PubMed] [Google Scholar]
- Gillespie JS, Muir TC. A method of stimulating the complete sympathetic outflow from the spinal cord of the pithed rat. British Journal of Pharmacology. 1967;30:78–87. doi: 10.1111/j.1476-5381.1967.tb02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiological Reviews. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Holton P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. Journal of Physiology. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PPA, Buell G, Kennedy I, Khahk BJ, Michel AD, Suprenant A, Trezise DJ. New insights on P2X receptors. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;351:1–12. doi: 10.1007/BF00171316. [DOI] [PubMed] [Google Scholar]
- Hurt CM, Wang L, Xu J, Sterious W, Pelleg A. Electrophysiological-anatomic correlates of ATP-triggered vagal reflexes in dogs. 2. Vagal afferent traffic. American Journal of Physiology. 1994;267:H1093–1097. doi: 10.1152/ajpheart.1994.267.3.H1093. [DOI] [PubMed] [Google Scholar]
- Jarisch A. Kreislaufsteuerung durch das Herz. Klinische Wochenschrift. 1941;20:1045–1048. [Google Scholar]
- Jarisch A, Landgren S, Neil E, Zotterman G. Impulse activity in the carotid sinus nerve following intracarotid injection of potassium chloride, veratrine, sodium citrate, adenosine triphosphate and α-dinitrophenol. Acta Physiologica Scandinavica. 1952;25:195–211. doi: 10.1111/j.1748-1716.1952.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Khahk BS, Humphrey PPA, Suprenant A. Electrophysiological properties of P2X purinoceptors in rat superior cervical, nodose and guinea-pig celiac neurons. Journal of Physiology. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd EJ, Grahames CBA, Simon J, Michel AD, Barnard EA, Humphrey PPA. Localization of P2X purinoceptor transcripts in the rat nervous system. Molecular Pharmacology. 1995;48:569–573. [PubMed] [Google Scholar]
- Linden RJ. Function of cardiac receptors. Circulation. 1973;48:463–480. doi: 10.1161/01.cir.48.3.463. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Dashwood MR, Cobb VJ, Marr CG, Bond SM, Spyer KM. Endothelins and rat carotid body: autoradiographic and functional pharmacological studies. Journal of the Autonomic Nervous System. 1995;53:115–125. doi: 10.1016/0165-1838(94)00179-n. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Moores C, Dowd E, Bond SM, Chessell IP, Humphrey PPA. P2X receptor activation evokes a Bezold-Jarisch like reflex in anaesthetised rats. British Journal of Pharmacology. 1997;122:246P. [Google Scholar]
- McQueen DS, Ribeiro JA. On the specificity and type of receptor involved in carotid body chemoreceptor activation by adenosine in the cat. British Journal of Pharmacology. 1983;80:347–354. doi: 10.1111/j.1476-5381.1983.tb10040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. P2X purinoceptor plethora. Seminars in the Neurosciences. 1996;8:187–194. [Google Scholar]
- Paintal AS. A study of ventricular pressure receptors and their role in the Bezold effect. Quarterly Journal of Experimental Physiology. 1955;40:348–363. doi: 10.1113/expphysiol.1955.sp001135. [DOI] [PubMed] [Google Scholar]
- Pelleg A, Hurt CM. Mechanism of action of ATP on canine pulmonary vagal C-fiber nerve terminals. Journal of Physiology. 1996;490:265–275. doi: 10.1113/jphysiol.1996.sp021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleg A, Hurt CM, Michelson EL. Cardiac effects of adenosine and ATP. Annals of the New York Academy of Sciences. 1990;603:19–30. doi: 10.1111/j.1749-6632.1990.tb37658.x. [DOI] [PubMed] [Google Scholar]
- Reid PG, Watt AH, Penny WJ, Newby AC, Smith AP, Routledge PA. Plasma adenosine concentrations during adenosine-induced respiratory stimulation in man. European Journal of Clinical Pharmacology. 1991;40:175–180. doi: 10.1007/BF00280073. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Floras JS, Lenders JWM, Smits P. Cardiovascular pharmacology of purines. Clinical Science. 1997;92:13–24. doi: 10.1042/cs0920013. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Urbanek E, Gothert M. ATP, α,β-methylene ATP and suramin as tools for characterization of vascular P2X receptors in the pithed rat. Journal of Autonomic Pharmacology. 1989;9:357–366. doi: 10.1111/j.1474-8673.1989.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Spergel D, Lahiri S. Differential modulation by extracellular ATP of carotid chemosensory responses. Journal of Applied Physiology. 1993;74:3052–3056. doi: 10.1152/jappl.1993.74.6.3052. [DOI] [PubMed] [Google Scholar]
- Suprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Taneyama C, Benson KT, Hild PG, Goto H. Adenosine triphosphate attenuates renal sympathetic nerve activity through left ventricular chemosensitive receptors. Journal of Pharmacology and Experimental Therapeutics. 1997;280:570–575. [PubMed] [Google Scholar]
- Trezise DJ, Bell NJ, Kennedy I, Humphrey PPA. Effects of divalent cations on the potency of ATP and related agonists in the rat isolated vagus nerve - implications for P-2 purinoceptor classification. British Journal of Pharmacology. 1994;113:463–470. doi: 10.1111/j.1476-5381.1994.tb17012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise DJ, Kennedy I, Humphrey PPA. Characterization of purinoceptors mediating depolarisation of rat isolated vagus nerve. British Journal of Pharmacology. 1993;110:1055–1060. doi: 10.1111/j.1476-5381.1993.tb13920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyau M, Hansen MJ, Dampney RAL, Balcar VJ, Bennett MR. Autoradiography of [H-3]alpha, beta-methylene-ATP binding sites in medulla oblongata and spinal cord of the rat. Neurochemistry International. 1997;30:159–169. doi: 10.1016/s0197-0186(96)00062-9. [DOI] [PubMed] [Google Scholar]
- Valera A, Hussy N, Evans RJ, Adami N, North RA, Suprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends in Neurosciences. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]


