Abstract
The aim of this study was to characterize further the two main metabolic pathways of regulation of the Na+-Ca2+ exchanger in squid axons induced by its two naturally ocurring high-energy compounds: ATP and phosphoarginine (Pa). [Na+]o-dependent Ca2+ efflux (forward - exchange) and [Ca2+]o-dependent Ca2+ efflux (- exchange) were measured in internally dialysed squid axons at 16–17 °C.
Measurements of changes in the apparent affinity of the Na+-Ca2+ exchanger for transporting (, , , ) and regulatory () ions induced by ATP and Pa show marked differences for the two substrates: (i) ATP strongly alters the affinity for and , while Pa does not, and (ii) in the absence of , ATP has no stimulatiory effect; on the other hand, Pa causes a dramatic increase in - exchange with little activation of - exchange.
The MgATP analogue chromium-ATP (CrATP) completely inhibits MgATP stimulation of the Na+-Ca2+ exchanger. Nevertheless, even with the effects of the nucleotide blocked, Pa exhibits its usual activation of the [Na+]o-dependent Ca2+ efflux.
None of the classical serine-threonine-tyrosine kinase inhibitors, nor the PP1 and PP2 phosphatase inhibitors, affects either the ATP or the Pa effect. However, intracellular microinjections of an exogenous phosphatase (alkaline phosphatase) completely reverses the stimulation of the Na+-Ca2+ exchange induced by ATP and Pa.
Prolonged intracellular dialysis with highly permeable porous capillaries (18 kDa molecular weight cut-off), which normally induces a complete run-down of the MgATP effect, does not alter the Pa stimulation of the exchanger, even after 6 h of continuous dialysis.
We conclude that the ATP and Pa modulation of Na+-Ca2+ exchange in an invertebrate nerve fibre are two genuinely different mechanisms, which affect the carrier properties in very different ways. An interesting similarity between ATP and Pa is that a phosphorylation-dephosphorylation process seems to be a common feature of these two regulation modes.
The plasma membrane Na+-Ca2+ exchange is primarily responsible for Ca2+ extrusion in most cells, particularly during the rise in Ca2+ ([Ca2+]i) following activation of cell function. One of the key features of this countertransport system is that it is highly modulated by intracellular substrates including ATP, Ca2+, H+ and lipids (for references, see Hilgemann, Philipson & Vassort, 1996). In squid axons as well as in cardiac cells, the major up-regulation mechanism of the Na+-Ca2+ exchange involves intracellular MgATP. In both preparations, this nucleotide causes a strong stimulation of the exchange activity, including an increase in the affinity of the intracellular Ca2+ regulatory and Na+ transport sites (DiPolo, 1974; Blaustein, 1977; DiPolo & Beaugé, 1986; Berberian & Beaugé, 1996).
We have found recently in squid nerve fibres a novel form of up-regulation of the Na+-Ca2+ exchanger induced by a high-energy non-nucleotide phosphagen: phosphoarginine (Pa) (DiPolo & Beaugé, 1995), a compound that is normally present at millimolar concentrations in the cytosol of all invertebrates. The Pa stimulation: (i) occurs in the complete absence of ATP or ADP, (ii) is independent of and additive to the MgATP-stimulated exchange, (iii) is largely, but not absolutely dependent on Mg2+ ions and (iv) is fully and rapidly reversible with a Km of around 7.7 mm. The large magnitude of the stimulating effect of Pa, combined with its strong dependence on (DiPolo & Beaugé, 1995), makes this process suitable for extruding [Ca2+]i from regions in neurons where [Ca2+]i can reach very high levels (Llinas, Sugimory & Siver, 1994). The MgATP modulation of Na+-Ca2+ exchange has been characterized with respect to transport (, , , ), regulatory () and other ligand (,inorganic phosphate, vanadate) interactions (DiPolo & Beaugé, 1991). No such information is available, however, for the Pa effect. This is crucial information needed for the characterization of the two pathways involved in the metabolic regulation of this transport system.
In this study we have investigated the effect of Pa on the steady-state kinetic parameters of two partial reactions of the exchanger (- and - exchange) in relation to both transporting and regulatory species. We also determined whether the mechanism of Pa activation has similar characteristics to the phosphorylation-dephosphorylation process suggested for MgATP modulation of the Na+-Ca2+ exchanger (DiPolo & Beaugé, 1991).
METHODS
Experimental procedure
Experiments were carried out with two different squid species, Loligo pealei from the Marine Biological Laboratory, Woods Hole MA, USA, and Loligo plei from The Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela. The experimental procedure for internally dialysing squid axons has been described elsewhere (DiPolo, Bezanilla, Caputo & Rojas, 1985). Dialysis capillaries were of a new regenerated cellulose fibre with a molecular weight cut-off (MWCO) of 18000 Da (210 μm o.d.; 200 μm i.d.; No. 132225; Spectra/Porous Spectrum, Houston, TX, USA). We have compared the permeability of these capillaries with those of cellulose acetate used in previous work (180 μm; Fabric Research, MA, USA). For this, dialysis capillaries were perfused and superfused in the dialysis chamber with a Mops-Tris solution (0.56 m). 45Ca2+ was added to the internal perfusion medium while the external medium was collected every 3 min and measured. The measurements show that the regenerated cellulose fibres of 18000 Da MWCO have a permeability coefficient on average 4.2 times higher than the cellulose acetate capillaries. For intracellular microinjection a 50–75 μm glass capillary attached to a 1 μl syringe was positioned inside the axons at the beginning of the dialysis experiment. The left end of the axon was covered with mineral oil to avoid drying of the axon at the moment of injection. A total volume of 0.16 μl was injected over the entire dialysed region. This was performed by a slow mechanical withdrawal of the injector while maintaining the syringe plunger fixed. All microinjected and dialysis solutions were perfectly matched in osmolarity to avoid sudden changes in intracellular volume. We were particularly careful on this point since we have observed that small differences between intra- and extracellular osmolarities can induce large transient changes in Ca2+ efflux.
Solutions
The standard dialysis medium had the following composition (mm): 385 Tris-Mops (3-(N-morpholino) propanesulphonic acid), 45 NaCl, 5 MgCl2, 285 glycine and 1–3 Tris-EGTA (ethylene glycol-bis-β (aminoethyl ether)-N, N, N′, N′-tetraacetic acid). The pH was 7.3 and the temperature between 16 and 17°C. The osmolarity of all solutions was adjusted to 940 mosmol l−1. The estimation of [Ca2+] was made using the Maxchelator computer program (Chris Patton, Hopkins Marine Station, CA, USA) using the Harrison & Bers (1987) constants at high ionic strength, pH 7.4 and 17°C. The external sodium solution had the following composition (mm): 440 Na+, 0.5 Ca2+, 60 Mg2+ and 570 Cl− at pH 7.6. Removal of external sodium was compensated with Li+. In order to stop any endogenous production of ATP, and therefore control the [ATP] through internal dialysis, 1 mm NaCN was always present in the external solutions. Addition of ATP, GTPγS and p-nitrophenylphosphate (p-NPP) was done at a constant free [Mg2+]i. Before including [45Ca2+] in the internal medium, the axons were routinely dialysed for about 50–60 min with a standard dialysis medium containing 1 mm EGTA and free of calcium and ATP. In all experiments each axon served as its own control, since steady-state 45Ca2+ efflux was always measured before and after a given experimental condition. Phosphoarginine, alkaline phosphatase (type VII-NLA from bovine intestinal mucus), vanadate, p-NPP and GTPγS were purchased from Sigma. H7 (1-(5-isoquinolinylsulphonyl)-2-methyl piperazine), phorbol ester TPA (phorbol 12-myristate, 13 acetate), staurosporine, genistein, lavendustine A, tyrphostin, calmidazolium, okadaic acid and microcystin were purchased from LC Laboratories, MA, USA. Sangivamycin was a gift from Drug Synthesis, Division of Cancer Treatment, National Cancer Institute.
RESULTS
The effect of Pa and ATP on the apparent affinity of the Na+-Ca2+ exchanger for , , , and
Figure 1 summarizes the results of six experiments in which the steady-state -dependent Ca2+ efflux was determined as a function of [] in the presence and absence of Pa in axons completely depleted of ATP. It is clear that 5 mm Pa does not affected the apparent affinity, despite its great activating effect on the -dependent Ca2+ efflux. The calculated apparent affinity constant (, from the Hill equation) was 107 ± 18 mm with or without Pa. The lack of effect of Pa on the affinity of the exchanger towards is in marked contrast with the effect of internal ATP on the affinity for external Na+ (trans effect; see Fig. 1, interrupted line; Baker & Glitsch, 1973; DiPolo, 1974; Blaustein 1977). In order to investigate the possible effect of Pa on the interaction with the exchanger, the inhibition of the -dependent Ca2+ efflux by internal Na+ ions was explored in four experiments before and after the addition of 5 mm Pa to the dialysis medium. Figure 2 shows that the apparent inhibition constant (Ki) for increased slightly from 12.2 ± 0.6 to 20.9 ± 0.3 mm. Although statistically significant, this change is much smaller than the tenfold change induced by ATP (see Fig. 2, interrupted line; Requena, 1978).
Figure 1. The effect of Pa and ATP on the apparent affinity of the -dependent Ca2+ efflux (- exchange) for extracellular Na+.

Figure 2. The effect of Pa and ATP on the apparent affinities of the -dependent Ca2+ efflux (- exchange) for intracellular Na+.

Figure 3 is a representative experiment in which the activation of the forward Na+-Ca2+ exchange was measured in a single axon in the presence and absence of Pa. The axon was first dialysed without Pa and ATP, and the steady-state -dependent Ca2+ efflux was measured at different [Ca2+]i (0.3, 0.5, 0.8, 10, 50 and 200 μm). This protocol was repeated after addition of 5 mm Pa. Phosphoarginine causes a marked increase in the apparent affinity for . In this particular experiment, the decrease in the apparent affinity constant () went from 12.5 to 0.91 μm with a fitted Hill coefficient (nH) of 4.0. This Hill coefficient is in agreement with that reported previously (DiPolo & Beaugé, 1995), being almost three times higher than that determined for the activation of exchange in the presence of ATP (Blaustein, 1977). In an attempt to characterize the effect of Pa on all known regulatory and transport sites, we explored whether this phosphagen might affect the extracellular transport site. Figure 4 illustrates the results of five different experiments showing that Pa in the presence of does not significantly change the apparent affinity of the exchanger for this divalent cation, i.e. the apparent Km for is 0.7 ± 0.06 mm with or without Pa.
Figure 3. dependence of -dependent Ca2+ efflux (- exchange) in the presence and absence of Pa and ATP.

The figure represents the result of a single experiment in which the apparent affinity of the - exchange for was measured before (•) and after (^) the addition of 5 mm Pa to the dialysis medium. The lines through the points were fitted to the Hill equation (K½= 0.91 ± 0.12 μm with Pa, and 12.5 ± 0.5 μm without Pa. The Hill coefficient is 4.0 and 1.9 with and without Pa, respectively.
Figure 4. The effect of Pa on the apparent affinity of the -dependent Ca2+ efflux (- exchange) for .

Axons were dialysed in the presence (^) and in the absence (•) of Pa. The line through the points is the best fit to a Michaelis-Menten function (K½= 0.7 ± 0.06 mm). Each symbol represents a different axon. - exchange was measured in the presence of a saturating [Li+]o (440 mm). Note the absence of effect of Pa on the apparent affinity of the external site of the exchanger.
It is well known that the -dependent Ca2+ influx (reverse mode) and the -dependent Ca2+ efflux (homologous Ca2+ exchange mode) are activated by external monovalent cations (Baker & McNaughton, 1978). Therefore, we examined whether Pa and/or ATP induced a ‘trans’ effect on the affinity of the external monovalent cation site. In these experiments, ions were substituted by variable combinations of plus , and the steady-state -dependent Ca2+ efflux measured as a function of [Li+]o. Figure 5A and B shows that neither Pa nor ATP have any effect on the apparent affinity of that site for . The apparent Km for the monovalent cation site was 100 ± 9 and 124 ± 15 mm in the presence of Pa and ATP, respectively.
Figure 5. The effect of Pa and ATP on the apparent affinity of the external monovalent cation site during - exchange.
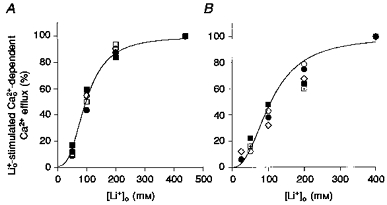
A, activation of Ca2+ efflux by in the presence (^) and absence (•) of Pa. B, activation of Ca2+ efflux by in the presence (^) and absence (•) of ATP. Each symbol represent a different axon. The lines through the points are the best fits to a Hill equation (K½ = 100 ± 9 mm, n = 2.78 ± 0.03 in the presence of Pa, and K½ = 124 ± 15 mm, n = 1.8 ± 0.02 in the presence of ATP.
Pa promotes a large activation of exchange at low and induces a preferential stimulation of the over the - exchange mode
One of the striking characteristics of the ATP modulation of Na+-Ca2+ exchange in squid axons is that at low the nucleotide has little or no effect (DiPolo & Beaugé, 1984). This feature is illustrated in Fig. 6 in an axon containing a non-saturating [Ca2+]i (0.82 μm). Without ATP and in the presence of 50 mm , Ca2+ efflux is low (about 25 fmol cm−2 s−1). Removing causes a large activation in the Ca2+ efflux to about 700 fmol cm−2 s−1, which is totally dependent on the presence of ; this indicates that the increment is the consequence of a release of inhibition of the exchanger. Addition of ATP in the absence of increases the efflux of Ca2+ by 275 fmol cm−2 s−1; this stimulation is independent of external Na+ and is abolished by 100 μm vanadate, thus reflecting only the activity of the Ca2+ pump.
Figure 6. The effect of ATP on Ca2+ efflux in the absence of .

Unless otherwise stated, all concentrations are given as millimolar. The arrows indicate the removal or addition of compounds to the dialysis medium. •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . The concentration of vanadate (Van) was 100 μm. Axon diameter, 600 μm. Temperature, 16.5 °C. Note the absence of ATP stimulation of -dependent Ca2+ efflux in the absence of .
A comparable experiment, using Pa, is depicted in Fig. 7, where both -dependent () and -dependent (-) fluxes were explored. Without Pa and ATP, a Ca2+ efflux of 36 fmol cm−2 s−1 in the presence of 50 mm is increased to about 470 fmol cm−2 s−1 when is set to 5 mm. This increment is totally inhibited upon replacement of by . When 1 mm is included in the Li+ medium, a Ca2+ efflux of about 350 fmol cm−2 s−1 through the - exchange mode is observed. Returning to the -containing -free bathing solution brings Ca2+ efflux back to its original levels. This experiment shows that in the absence of high-energy substrates and - are very similar in magnitude. Addition of 5 mm Pa while maintaining a low [Na+]i (5 mm) causes a dramatic (6-fold) increase in Ca2+ efflux. This flux occurs through the exchange mode since replacement of by reduces the efflux to background levels (5–6 fmol cm−2 s−1). On the contrary, 1 mm in the presence of activates a - exchange component only twofold.
Figure 7. The effect of Pa on - and - exchange at low .
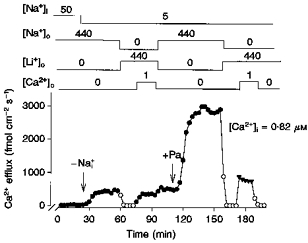
Unless otherwise stated, all concentrations are given as millimolar. The arrows indicate the removal or addition of compounds to the dialysis medium. •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . Note that in the absence of and Pa, the magnitude of the - exchange (Ca2+ efflux in the presence of ) is not different from that of the - exchange (Ca2+ efflux in the presence of ). Also, in the presence of Pa there is a large activation of - exchange with small activation of - exchange. Axon diameter, 510 μm. Temperature, 17.5 °C.
Since during activation of Ca2+ efflux by Pa the rate of - is much slower than that of the exchange mode (Fig. 7), it should be expected that in the presence of , a high [Ca2+]o should inhibit -dependent Ca2+ efflux. Figure 8 shows this particular point. As seen before (Fig. 7) during - exchange (presence of and ), 5 mm Pa increased Ca2+ efflux by a factor of two (1750 fmol cm−2 s−1). On the other hand, under exchange (presence of , absence of ) Pa activates -dependent Ca2+ efflux by a factor of six (5680 fmol cm−2 s−1). While keeping constant, the addition of 3 mm not only failed to activate further, but induced a significant inhibition in the -dependent Ca2+ efflux. Table 1 summarizes the results of several experiments in which the effect of Pa and ATP on both and - exchange components were explored at low and high concentrations. It is clear that at low [Na+]i (5 mm), the MgATP effect is rather small (only a 30 % increase), compared with a more than 800 % increase induced by Pa on the -dependent component of the Ca2+ efflux. At higher [Na+]i (50 mm), both substrates markedly activated the -dependent Ca2+ efflux. As mentioned above, most of the activation of the exchanger by Pa and ATP occurs on the partial reaction.
Figure 8. The effect of high [Ca2+]o on - and - exchange in the presence of Pa.

Unless otherwise stated, all concentrations are given as millimolar. The arrows indicate the removal or addition of compounds to the dialysis medium. •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . Note the small magnitude of the - exchange with 2 mm compared with the large increase in - exchange component and the inhibition of - exchange component induced by 3 mm . Axon diameter, 495 μm. Temperature, 17 °C.
Table 1.
The effect of Pa and ATP on - and - exchange at low and high internal Na+
| -dependent Ca2+ efflux (-) | -dependent Ca2+ efflux (-) | |||
|---|---|---|---|---|
|
|
|
|||
| Ratio | Ratio | |||
| [Na+]i (mm) | +Pa/–Pa | +ATP/–ATP | +Pa/–Pa | +ATP/–ATP |
| 0-5 | 8.5 ± 1.2 (4) | 1.3 ± 0.3 (3) | 2.0 ± 0.3 (4) | 1.6 ± 0.54 (6) |
| 50 | 5.7 ± 0.8 (22) | 5.5 ± 0.85 (15) | 1.9 ± 0.5 (4) | 2.2 ± 0.83 (6) |
[Ca2+]i, 0.82 μm; ATP, 2 mm; Pa, 5 mm. The data are given as the means ± s.e.m. The values in parentheses represent different experiments collected at the Marine Biological Laboratory, Woods Hole, MA, USA between 1994 and 1996.
Effect of phosphatase and kinase activators and inhibitors on the Pa and ATP stimulation of Na+-Ca2+ exchange
There is much evidence that a phosphorylation process is involved in the MgATP stimulation of Na+-Ca2+ exchange in squid axons (DiPolo & Beaugé, 1991). We have reported that the MgATP analogue CrATP, which is a substrate and powerful inhibitor of most kinases, completely blocks the MgATP stimulation of Na+-Ca2+ exchange without affecting the Na+-Ca2+ exchange per se (normal Vmax at saturating in the presence of CrATP; DiPolo & Beaugé, 1993). Based on this information we decided to explore whether this analogue also affected the Pa activation of the exchanger. In the experiment illustrated in Fig. 9, after the routine predialysis period (see Methods), the axon was dialysed with the radioactive medium containing 100 μm vanadate to block the Ca2+ pump component of the Ca2+ efflux. Addition of 2 mm MgATP caused an increase in Ca2+ efflux, which was totally dependent on extracellular Na+. After readdition of ions and stabilization of Ca2+ efflux to a level of about 300 fmol cm−2 s−1, the inclusion of 3 mm CrATP in the dialysis medium at a constant [MgATP] progressively inhibited all MgATP stimulation. In contrast, 5 mm Pa in the presence of both MgATP and CrATP caused the usual large activation of the -dependent Ca2+ efflux, thus indicating that CrATP blocks the stimulation induced by MgATP without affecting the Pa stimulation. Figure 10A and B shows three experiments in which the kinase inhibitors staurosporine and genistein completely failed to affect Pa activation of Na+-Ca2+ exchange. Similarly, staurosporine at concentrations up to 100 μm did not affect the MgATP stimulation of the -dependent Ca2+ efflux (experiments not shown). These compounds act as competitive inhibitors with respect to ATP; therefore, the inhibitor concentrations used in these experiments (between 100 and 200 μm, which is several orders of magnitude higher than the IC50; see also Fig. 13) were high enough to ensure that these negative results are genuine.
Figure 9. The effect of CrATP on the MgATP and Pa stimulation of -dependent Ca2+ efflux (- exchange).
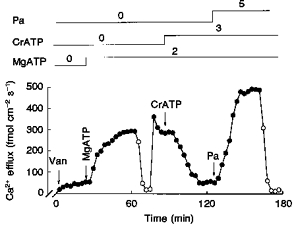
All concentrations are given as millimolar. The arrows indicate the removal or addition of compounds to the dialysis medium. •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . Note the large activation of -dependent Ca2+ efflux induced by MgATP in the presence of vanadate (Van, 100 μm) and its complete inhibition by CrATP. Note also that Pa still activates in the presence of CrATP. Axon diameter, 650 μm. Temperature, 17 °C.
Figure 10. The effect of phosphatase and kinase inhibitors on the Pa and ATP stimulation of -dependent Ca2+ efflux (- exchange).
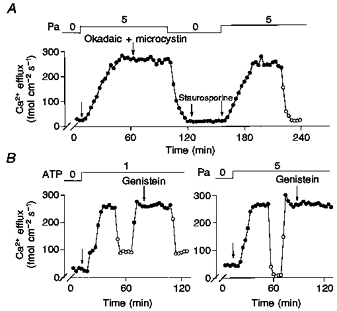
All concentrations are given as millimolar. The arrows indicate the removal or addition of compounds to the dialysis medium. •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . Note the lack of effect of any of these inhibitors on Na+-Ca2+ exchange. Okadaic acid + microcystin, 5 μm; staurosporine, 100 μm; genistein, 100 μm. When added, the inhibitors were always present until the end of the experiment. Axon diameters were: A, 550 μm; B, 525 μm (left) and 495 μm (right). Temperature, 17 °C.
Figure 13. The effects of several kinase and phosphatase inhibitors, activators and substrates on the Pa and ATP stimulation of - exchange.
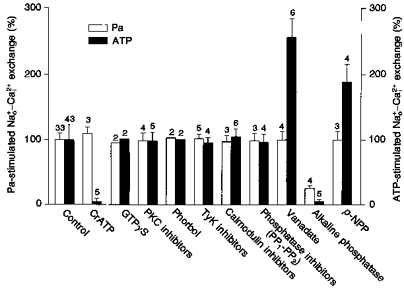
The abscissa shows the experimental conditions: control (100 %) for the Pa and ATP effect was taken from the mean of 33 (412 ± 39 fmol cm−2 s−1) and 43 (322 ± 38 fmol cm−2 s−1) different axons, respectively; CrATP (3 mm); GTPγS (100 μm); PKC inhibitors (staurosporine, 100 μm; H7, 20–30 μm, sangivamycin, 100–200 μm); PKC activators (phorbol ester TPA, 1 μm); TyK inhibitors (genistein, 100–200 μm; lavendustine, 100 μm; tyrphostin, 20 μm); calmodulin inhibitor (calmidazolium, 50 μm); phosphatase inhibitors (okadaic acid and microcystine, 1–2 μm); vanadate (100 μm); alkaline phosphatase injection (9–15 units in 0.16 μl); phosphatase substrate (p-NPP, 5 mm). In the control experiments the absolute values for the Pa and ATP stimulation (100 %) were 254 and 355 fmol cm−2 s−1, respectively. The error bars indicate s.e.m. The numbers above the bars represent different experiments. The [ATP] and [Pa] were 2 and 5 mm, respectively. The [Ca2+]i in these experiments was 0.82 μm. The mean temperature was 17 °C.
The effects of exogenous phosphatases, phosphatase inhibitors and phosphatase substrates on the ATP and Pa effects were also explored. Figure 11A shows that microinjection of alkaline phosphatase (9 units in a total volume of 0.16 μl) causes, after a delay of about 15 min, a progressive inhibition of Pa stimulation. Although substantial, this inhibition was not complete, since removal of Pa from the dialysis medium causes a further reduction in Ca2+ efflux. In three different experiments the mean inhibition was 77 ± 11 %. Another important observation in Fig. 11A is that addition of ATP increased Ca2+ efflux, but flux was totally inhibited by vanadate; this indicates that alkaline phosphatase also inhibited MgATP stimulation of Na+-Ca2+ exchange. This latter result is confirmed in Fig. 11B, where the injection of alkaline phosphatase almost completely inhibits (93 ± 5 %; n = 6) the ATP-stimulated -dependent Ca2+ efflux. Denaturation of the alkaline phosphatase by heat (20 min at 80°C) prevents the inactivation of the MgATP effect (experiments not shown). Inhibition of both ATP- and Pa-dependent processes by the exogenous phosphatase is not due to an ATPase activity or dephosphorylation of Pa, because incubating both substrates with the phosphatase at room temperature for more than 1 h does not induce more than 2 % hydrolysis of ATP or Pa (experiments not shown).
Figure 11. The effect of alkaline phosphatase injection on the Pa and ATP stimulation of -dependent Ca2+ efflux (- exchange).
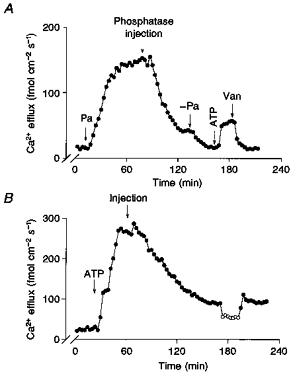
A and B: •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . The arrows indicate the removal or addition of Pa, ATP or vanadate (Van), or the intracellular injection of alkaline phosphatase. Nine units of alkaline phosphatase were injected into each axon in a total volume of 0.16 μl. Note that both Pa and ATP stimulation of -dependent Ca2+ efflux are inhibited by the exogenous phosphatase. The inhibition is complete for the case of ATP and only partial in the case of Pa. Axon diameter: A, 575 μm; B, 600 μm. Temperature, 17 °C.
We have suggested that a phosphatase activity is involved in the MgATP modulation of the exchanger. It is known that inorganic phosphate (at a concentration that causes product inhibition of phosphatases; Beaugé, 1979) activates Na+-Ca2+ exchange only in the presence of MgATP (DiPolo & Beaugé, 1984). This indicates that an interplay of kinases and phosphatases is associated with the ATP stimulation. In assays of kinase activities in crude preparations a phosphatase substrate is frequently added to the media; this is done in order to competitively inhibit dephosphorylation by contaminant phosphatases, which would then act upon the exogenous substrate. One of the compounds used for this purpose is p-nitrophenylphosphate (p-NPP). Following this line of thought, we investigated whether the addition of the phosphatase substrate p-NPP had any effect on ATP and Pa stimulation of the exchanger. In Fig. 12A the axon was dialysed without ATP before the addition of Pa. From a steady state of about 25 fmol cm−2 s−1, 5 mm Pa induced a large increase in Ca2+ efflux to 280 fmol cm−2 s−1, and the incorporation of 5 mm p-NPP in the continuous presence of Pa had no effect. Note that at the end of the experiment, all Pa stimulation of Ca2+ efflux was totally dependent on . Figure 12B shows an experiment in which the effect of p-NPP was explored in the presence of ATP. Addition of ATP activates the Ca2+ efflux in two steps: a fast component that is close to 200 fmol cm−2 s−1, and a slow one that reached a level of about 410 fmol cm−2 s−1. The removal of reduces the Ca2+ efflux to the level of the fast (Ca2+ pump) component. Addition of 5 mm p-NPP in the presence of causes an increment in Ca2+ efflux from 415 to 612 fmol cm−2 s−1. Removal of brings the Ca2+ efflux to the levels of the fast component, thus indicating that p-NPP activates the Na+-Ca2+ exchange and not the Ca2+ pump.
Figure 12. The effect of p-nitrophenylphosphate (p-NPP) on the Pa and ATP stimulation of -dependent Ca2+ efflux (- exchange).

All concentrations are given as millimolar. The arrows indicate the removal or addition of compounds to the dialysis medium. •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . A, note the absence of effect of 5 mm p-NPP on the Pa stimulation of - exchange. B, note that p-NPP only activates the -dependent Ca2+ efflux in the presence of ATP (no activation of the Ca2+ pump component is observed). Axon diameter: A, 650 μm; B, 575 μm. Temperature, 16.5 °C.
Figure 13 illustrates a series of experiments where several protein kinases and phosphatase inhibitors, activators and substrates were tested on the Pa and ATP stimulation of -dependent Ca2+ efflux. The overall results can be summarized as follows: (i) inhibition of Pa and ATP stimulation was attained only with alkaline phosphatase injections; (ii) vanadate and p-NPP potentiated ATP stimulation and CrATP inhibited ATP stimulation, but all three agents were completely ineffective in the presence of Pa; (iii) all other compounds tested failed to show any affect on both ATP and Pa stimulation of the Na+-Ca2+ exchanger.
Pa stimulation of Na+-Ca2+ exchange does not run down after prolonged intracellular dialysis
We have recently shown that MgATP stimulation of Na+-Ca2+ exchange is lost after prolonged intracellular dialysis (Beaugé, Delgado, Rojas, Berberián & DiPolo, 1996; DiPolo, Berberián, Delgado, Rojas & Beaugé, 1997). On that basis we decided to explore whether a similar run-down occurs for the Pa modulation. Figure 14 shows an experiment in which the effects of ATP and Pa on Ca2+ efflux were tested after a prolonged intracellular dialysis. After more than 4 h of dialysis Ca2+ efflux remained low (about 36 fmol cm−2 s−1). Addition of 2 mm ATP caused an activation in the Ca2+ efflux to 110 fmol cm−2 s−1, being completely abolished by 100 μm vanadate, i.e. ATP stimulation occurs only through the Ca2+ pump. After removing ATP from the dialysis medium, addition of 5 mm Pa produced the usual activation in the -dependent Ca2+ efflux. The lack of wash-out effect on Pa, compared with that seen with ATP, was confirmed in five different axons.
Figure 14. The effect of prolonged dialysis on the ATP- and Pa-stimulated -dependent Ca2+ efflux.
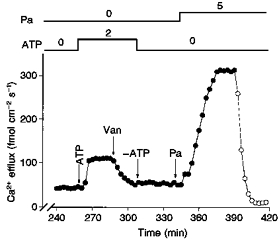
All concentrations are given as millimolar. The arrows indicate the removal or addition of compounds to the dialysis medium. •, Ca2+ efflux in the presence of . ^, Ca2+ efflux in the absence of . This axon was dialysed with a standard dialysis medium containing no ATP over 4.3 h prior to the addition of 2 mm ATP. Note the absence of ATP-dependent, -dependent Ca2+ efflux. Only the Ca2+ pump component continues to be inhibited by the addition of 100 μm vanadate (Van). Note that addition of 5 mm Pa (after 5.7 h) causes its usual activation of the -dependent Ca2+ efflux. Axon diameter, 550 μm. Temperature, 16 °C.
DISCUSSION
This work constitutes a detailed study of the ATP and Pa modulation of Na+-Ca2+ exchange in squid axons. The marked differences between the effects of these two compounds on the steady-state ion dependency and ligand interactions demonstrate that they are indeed two different metabolic pathways in the regulation of the exchanger. Two of the crucial differences in support of our conclusion are: (i) the CrATP experiments, which demonstrate that even with the nucleotide effect blocked, Pa can induce its usual activation, and (ii) the long dialysis experiments, which show a clear run-down of the MgATP effect while the Pa stimulation remains unimpaired. This is in agreedment with our recent report that a dialysable soluble 13 kDa cytoplasmic protein is required for the MgATP stimulation of the Na+-Ca2+ exchanger (Beaugéet al. 1996; DiPolo et al. 1997). Furthermore, the lack of run-down of the Pa effect after prolonged dialysis is in agreement with our recent finding that the phosphagen can stimulate a Na+ gradient-dependent 45Ca2+ uptake in membrane vesicles from squid optic nerve without the presence of any cytosolic component (G. Berberián, R. DiPolo & L. Beaugé, unpublished observations).
An interesting finding of this study is the marked differences in the way in which ATP and Pa affect the interaction of the exchanger with Na+ and Ca2+ ions. The three major differences are related to , and . For , no effect of internal Pa on the affinity for external was found, in contrast to the well-known increase in the affinity induced by ATP (Baker & Glitsch, 1973; DiPolo, 1974; Blaustein, 1977). In relation to the interaction with the exchanger, the release of inhibition is much more pronounced with ATP than with Pa (Table 1). In fact, at low [Na+]i we detected a very small ATP stimulation of the Na+-Ca2+ exchange (30 %) compared with a dramatic activation by Pa (8.5-fold); this indicates that the ATP effect is associated with a release of inhibition on the transport site (Requena, 1978), while that of Pa is not. This is also in agreement with the fact that at high [Na+]i, the percentile activation of the by Pa and ATP are about the same (Table 1). Another similarity between these two processes is the increase in the apparent affinity for . Nevertheless, the dependency is significantly steeper for Pa than for ATP.
One unexpected finding of this study is that Pa affects the exchange much more than the - exchange mode. In fact, in the absence of Pa, both partial reactions have similar magnitudes compared with the preferential increase in the over the - exchange in the presence of Pa. This finding could be at least partially explained if, in the absence of Pa, the rate-limiting step resides in the efflux of Ca2+ while during Pa stimulation the influx of Ca2+ becomes rate limiting. In squid axons there is evidence that at alkaline pH (pH 8–9) there is a large increase in the and - partial reactions with small activation of the - exchange mode (DiPolo & Beaugé, 1987). Similarly, in isolated sarcolemmal vesicles the rate of Na+-Ca2+ exchange increases with increasing pH (deprotonation), while Ca2+-Ca2+ exchange decreases with increasing pH (Khananshvilli & Weil-Maslansky, 1994). Although attractive, at present we do not know whether the increase in the to - exchange ratio induced by Pa could be related to the mechanism of regulation of the Na+-Ca2+ exchanger by protons. Interestingly, both ATP and Pa activate Na+-Ca2+ exchange preferentially. This suggests that although these compounds seem to work through different metabolic pathways, the effect in terms of activation of partial reactions of the Na+-Ca2+ exchanger is similar for both.
In squid axons several experimental findings strongly support the view that the regulation of the Na+-Ca2+ exchange by MgATP involves a process of phosphorylation-dephosphorylation, either of the exchanger itself or of another regulatory structure (DiPolo & Beaugé, 1991). This includes the absolute requirement for Mg2+, activation by ATPγS, and activation by vanadate and inorganic phosphate. Another strong argument in favour of this hypothesis is that CrATP, a substrate of most kinases that acts as an end-product inhibitor, completely blocks the ATP effect, whereas Co (NH3)ATP, a poor substrate of most kinases (Dunaway-Mariano & Cleland, 1980), does not. In our study, all the commonly used protein kinase inhibitors and activators failed to induce any change either in the ATP or Pa stimulation, suggesting that whatever the mechanism of activation of the Na+-Ca2+ exchange by these two high energy compounds, it does involve the classical serine-threonine protein kinases. This is also the case for the commonly used phosphatase inhibitors of phosphatases (PP1 and PP2), which showed no effect on either pathway. Nevertheless, the MgATP effect is enhanced by two compounds that inhibit phosphatases: vanadate and p-NPP (see Table 1). An interesting finding is that an alkaline phosphatase, which has been reported to dephosphorylate mainly phosphotyrosine residues (Swarup, Cohen & Garbest, 1981) inhibits both the ATP and the Pa stimulation.
At present it is unknown whether the regulation of Na+-Ca2+ exchange by nucleotide and non-nucleotide high energy phosphate compounds is only present in invertebrates. In mammalian cardiac cells, the MgATP stimulation of Na+-Ca2+ exchange is related to the production of phosphatidylinositol 4, 5-bisphosphate (PIP2) through the inositol phosphate cascade (Hilgemann & Ball, 1996). In the invertebrate squid axon, the MgATP effect requires a soluble, low molecular weight cytoplasmic protein. It remains to be explored whether a similar phosphagen metabolic pathway is also involved in the regulation of the Na+-Ca2+ exchanger in vertebrates in which phosphocreatine would be the naturally occurring substrate.
Acknowledgments
We wish to thank the director and the staff of the Marine Biological Laboratory, Woods Hole, USA, and the staff of the Centro de Biofísica y Bioquímica at IVIC and Mochima, Venezuela. This work was supported by the USA National Science Foundation (IBN-9631107), the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICIT-Venezuela-S1-97001765), Fundacion Polar (Venezuela) the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-Argentina-4904/97), CONICOR (3511/95) and Fundación Andes (C-12777/9).
References
- Baker PF, Glitsch HG. Does metabolic energy participate directly in the Na+-dependent extrusion of Ca2+ from squid axons? Journal of Physiology. 1973;233:44–46P. [PubMed] [Google Scholar]
- Baker PF, McNaughton PA. The influence of extracellular calcium binding on the calcium efflux from squid axons. Journal of Physiology. 1978;276:127–150. doi: 10.1113/jphysiol.1978.sp012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. Vanadate-potassium interactions in the inhibition of Na, K-ATPase. In: Skou J, Norby J, editors. Na, K-ATPase, Structure and Kinetics. London: Academic Press; 1979. pp. 373–387. [Google Scholar]
- Beaugé L, Delgado D, Rojas H, Berberian G, DiPolo R. A nerve cytosolic factor is required for MgATP stimulation of a Na+ gradient-dependent Ca2+ uptake in plasma membrane vesicles from squid optic nerve. Annals of the New York Academy of Sciences. 1996;779:208–216. doi: 10.1111/j.1749-6632.1996.tb44788.x. [DOI] [PubMed] [Google Scholar]
- Berberián G, Beaugé L. ATP stimulation of a Na+ gradient-dependent Ca2+ uptake in cardiac sarcolemmal vesicles. Annals of the New York Academy of Sciences. 1996;779:282–283. doi: 10.1111/j.1749-6632.1996.tb44795.x. [DOI] [PubMed] [Google Scholar]
- Blaustein MP. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophysical Journal. 1977;20:79–110. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R. Effect of ATP on the calcium efflux in dialyzed squid giant axons. Journal of General Physiology. 1974;64:503–517. doi: 10.1085/jgp.64.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Interaction of physiological ligands with the Ca2+ pump and the Na+-Ca2+ exchange in squid axons. Journal of General Physiology. 1984;84:895–914. doi: 10.1085/jgp.84.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Reverse Na+-Ca2+ exchange requires internal Ca2+ and/or ATP. Biochimica et Biophysica Acta. 1986;854:298–306. [Google Scholar]
- DiPolo R, Beaugé L. Characterization of the reverse Na+-Ca2+ exchange in squid axons and its modulation by and ATP. Journal of General Physiology. 1987;90:505–525. doi: 10.1085/jgp.90.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Regulation of the Na-Ca exchange. An overview. Annals of the New York Academy of Sciences. 1991;639:100–111. doi: 10.1111/j.1749-6632.1991.tb17294.x. [DOI] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Effects of some metal-ATP complexes on Na+-Ca2+ exchange in internally dialysed squid axons. Journal of Physiology. 1993;462:71–86. doi: 10.1113/jphysiol.1993.sp019544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Effects of vanadate on MgATP stimulation of Na-Ca exchange support kinase-phosphatase modulation in squid axons. American Journal of Physiology. 1994;266:C1382–1391. doi: 10.1152/ajpcell.1994.266.5.C1382. [DOI] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Phosphoarginine stimulation of Na+-Ca2+ exchange in squid axons-a new pathway for metabolic regulation. Journal of Physiology. 1995;487:57–66. doi: 10.1113/jphysiol.1995.sp020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Berberián G, Delgado D, Rojas H, Beaugé L. A novel 13 kDa cytoplasmic soluble protein is required for the nucleotide (MgATP) modulation of the Na/Ca exchange in squid nerve fibers. FEBS Letters. 1997;401:6–10. doi: 10.1016/S0014-5793(96)01416-0. [DOI] [PubMed] [Google Scholar]
- DiPolo R, Bezanilla F, Caputo C, Rojas H. Voltage dependence of the Na+-Ca2+ exchange in voltage-clamped, dialyzed squid axons. Journal of General Physiology. 1985;86:457–478. doi: 10.1085/jgp.86.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaway-Mariano D, Cleland WW. Investigations of substrate specificity and reaction mechanism of several kinases using chromium (III) adenosine 5-triphosphate and chromium (III) adenosine 5-diphosphate. Biochemistry. 1980;19:1506–1515. doi: 10.1021/bi00548a038. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Bers D. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochimica et Biophysica Acta. 1987;925:133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Hilgemann D, Ball R. Regulation of cardiac Na+-Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Hilgemann D, Philipson K, Vassort G. Sodium-calcium exchange. Annals of the New York Academy of Sciences. 1996;779 doi: 10.1111/j.1749-6632.1996.tb44784.x. [DOI] [PubMed] [Google Scholar]
- Khananshvilli D, Weil-Maslansky E. The cardiac Na+-Ca2+ exchanger: Relative rates of calcium and sodium movements and their modulation by protonation-deprotonation of the carrier. Biochemistry. 1994;33:312–319. doi: 10.1021/bi00167a041. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Sugimory M, Siver RB. Localization of calcium concentration microdomains at the active zone in the squid giant synapses. Advances in Second Messenger Phosphoprotein Research. 1994;29:113–137. doi: 10.1016/s1040-7952(06)80012-1. [DOI] [PubMed] [Google Scholar]
- Requena J. Calcium efflux from squid axons under constant sodium electrochemical gradient. Journal of General Physiology. 1978;72:443–470. doi: 10.1085/jgp.72.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup G, Cohen S, Garbest DL. Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatases. Journal of Biological Chemistry. 1981;256:8197–8201. [PubMed] [Google Scholar]


