Abstract
We expressed the human eag-related gene (HERG), which is known to encode the delayed rectifier K+ current (IKr) in cardiac muscle, in Xenopus oocytes. Using a two-microelectrode voltage clamp technique, the effect of external Ca2+ and Mg2+ on the HERG current (IHERG) was investigated.
When [Ca2+]o was increased, the amplitude of outward IHERG elicited by depolarization decreased, and the rate of current onset slowed. The rate of current decay observed on repolarization was greatly accelerated. The threshold and fully activated potential of IHERG shifted to a more positive potential. On the other hand, the inactivation property represented by the negative slope of the I-V curve and the instantaneous conductance of IHERG were little affected by [Ca2+]o.
The effect of [Ca2+]o on IHERG can be interpreted using the channel blockade model. The blockade is voltage dependent; smaller dissociation constants (KM) at more negative potentials indicate that block is facilitated by hyperpolarization. KM changes e-fold for 14.5 mV and the fractional electrical distance of the binding site calculated from this value is 0.86.
Blockade by a low concentration of Ca2+ (0.5 mm) was inhibited by increasing [K+]o (from 2 to 20 mm), whereas blockade by a high concentration of Ca2+ (5 mm) was not affected by varying [K+]o, indicating that there is competition between permeating ions and blocking ions.
The effect of [Mg2+]o on IHERG was qualitatively similar to that of [Ca2+]o, but the potency was lower.
These results suggest that external Ca2+ and Mg2+ block the HERG channel in a voltage- and time-dependent manner, resulting in a voltage dependence which has been regarded as a property of the activation gate.
It is well known that intracellular Mg2+ and polyamines block the outward flow of K+ through inward rectifier K+ channels in a voltage-dependent manner (Matsuda, Saigusa & Irisawa, 1987; Lopatin, Makhina & Nichols, 1994): block is facilitated by depolarization, but is removed by hyperpolarization, resulting in an inward rectification. By this voltage-dependent block mechanism, inward rectifier K+ channels behave like voltage-dependent channels, in spite of the absence of a voltage-sensing S4 region in the channel proteins. A similar gating mechanism but operating in an opposite way was found in NMDA receptor channels (Nowak, Bregestovsk, Ascher, Herbet & Prochiantz, 1984), where external Mg2+ blocks the channels upon hyperpolarization, and in cardiac delayed rectifier K+ channels which carry the current IKr. Ho, Earm, Lee, Brown & Noble (1996) have shown that, in rabbit sino-atrial node cells, voltage dependence and the kinetics of IKr, which have been regarded as a result of voltage-dependent gating, are very sensitive to external Ca2+ and Mg2+ concentrations, and this effect can be explained by a voltage- and time-dependent block of IKr by external cations. This re-interpretation is functionally important, considering the significant role of this channel in repolarization and also in the pacemaker depolarization (Irisawa, Brown & Giles, 1993).
It has recently been shown that IKr is encoded by the HERG gene which belongs to the eag (ether-a-go-go)-like K+ channel family (Sanguinetti, Jiang, Curran & Keating, 1995; Trudeau, Warmke, Ganetzky & Robertson, 1995; Kiehn, Lacerda, Wible & Brown, 1996). The gating mechanism of HERG channels has generally been considered not to be different from that of other voltage-gated K+ channels, except for the fact that inactivation is very fast. The removal of inactivation on hyperpolarization is known to be responsible for inward rectification of the HERG channel (Smith, Baukrowitz & Yellen, 1996; Spector, Curran, Zou, Keating & Sanguinetti, 1996), as it is for the delayed rectifier K+ channel (Shibasaki, 1987). The activation mechanism has not yet been investigated in detail, however, and whether or not Ca2+ acts as a gating molecule as it does in the delayed rectifier K+ channel has not been determined. In the view of the fact that HERG channels, like other voltage-gated channels, possess a voltage-sensing S4 region, this question is of particular interest. In the present study, we have demonstrated that the HERG channel is blocked by external Ca2+ and Mg2+ in a voltage- and time-dependent manner, resulting in a voltage dependence which has been regarded as a property of the activation gate.
METHODS
Expression of HERG in oocytes
Complementary RNA of HERG was synthesized by in vitro transcription from 1 μg of linearized cDNA using T7 message machine kits (Ambion, Austin, TX, USA) and stored in 10 mm Tris-HCl (pH 7.4) at −80°C. Stage V-VI oocytes were surgically removed from female Xenopus laevis (Nasco, Modesto, CA, USA) anaesthetized with 0.17% tricaine methanesulphonate (Sigma). Following suture, frogs were allowed to recover in isolation in a tank. Theca and follicle layers were manually removed from the oocytes by using fine forceps. Oocytes were then injected with 40 nl of cRNA (0.1-0.5 μg μl−1). After injection, oocytes were maintained in modified Barth's solution containing (mm): 88 NaCl, 1 KCl, 0.4 CaCl2, 0.33 Ca(NO3)2, 1 MgSO4, 2.4 NaHCO3, 10 Hepes (pH 7.4), supplemented with 50 μg ml−1 gentamicin sulphate. Currents were studied 2–7 days after injection.
Two-microelectrode voltage clamp of oocytes
Normal Ringer solution contained (mm): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 Hepes (pH adjusted to 7.4 with NaOH). In experimental solutions for varying Ca2+, MgCl2 was omitted and the concentration of CaCl2 was varied as indicated. In experimental solutions for varying Mg2+, MgCl2 was varied as indicated and the concentration of CaCl2 was kept at 0.5 mm. To make a 20 mm K+ solution, the concentration of NaCl was reduced to 78 mm NaCl. Currents were measured at room temperature (21–23°C) with a two-electrode voltage clamp amplifier (Warner Instruments, Hamden, CT, USA). Electrodes were filled with 3 m KCl and had a resistance of 2–4 MΩ for voltage-recording electrodes and 0.6-1 MΩ for current-passing electrodes. Stimulation and data acquisition were controlled with Digidata and pCLAMP software (Axon Instruments). Unless otherwise stated, data were expressed as mean values ± s.d. (n = number of experiments).
RESULTS
HERG currents (IHERG) expressed in Xenopus oocytes were recorded and the effect of external Ca2+ concentration ([Ca2+]o) was investigated. As shown in Fig. 1A, depolarizing pulses from a holding potential of −60 mV induced activation of outward IHERG. In 0.5 mm [Ca2+]o with 2 mm [K+]o, the amplitude of outward currents measured at the end of a 5 s pulse (Iss) grew larger as the membrane was depolarized, reached its maximum at −40 mV, and then decreased progressively with further depolarization, resulting in a bell-shaped I-V relationship (Fig. 1B). The negative slope of the I-V curve is considered as a unique property of HERG channels distinct from other delayed rectifier K+ channels, and has been explained by assuming very fast (almost instantaneous) and voltage-dependent inactivation on depolarization (Smith et al. 1996; Spector et al. 1996).
Figure 1. Effect of external Ca2+ concentration on HERG currents elicited by depolarizing voltage pulses.
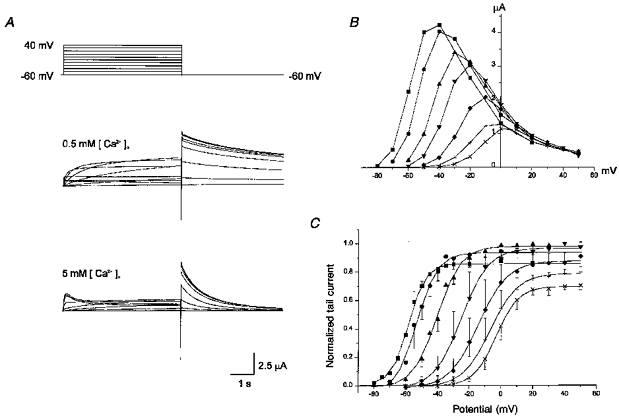
A, superimposed current traces elicited by depolarizing voltage pulses (5 s) in 10 mV steps (upper panel) from a holding potential of −60 mV in 0.5 mm (middle panel) and in 5 mm (lower panel) [Ca2+]o. [K+]o is 2 mm. B, plot of the steady-state current measured at the end of depolarizing pulses against the pulse potential in different external [Ca2+]o (obtained from the same cell shown in A). C, plot of the normalized tail current measured at its peak just after repolarization. The amplitude of the tail current was usually maximal at 0.5 mm [Ca2+]o and was taken as 1. Symbols with error bars represent mean ± s.d.: data obtained from 4 cells. Symbols in B and C: ▪, 0.1 mm; •, 0.2 mm; ▴, 0.5 mm; ▾, 1 mm; ♦, 2.5 mm; +, 5 mm; ×, 10 mm [Ca2+]. Lines in C are the fits to the Boltzmann equation, y = 1/{1 + exp((−V + V1/2)/dx)} (V1/2 from left to right, −58, −53, −40, −25, −14, −7, and −3 mV; dx from left to right, 5.3, 6.7, 7.6, 8.7, 9.2, 8.7, and 7.1 mV).
On returning to the holding potential of −60 mV, outward tail currents (Itail) developed. The amplitude of Itail was larger than that of Iss elicited by the preceding depolarizing pulse, reflecting the inwardly rectifying property of HERG channels. The amplitude of tail currents (Itail) increased progressively and reached its maximum at 0 mV in 0.5 mm [Ca2+]o. The Itail-V relationship was well fitted by a Boltzmann equation with V1/2 = −40 mV and dx = 7.6 mV (V1/2, membrane potential at half-maximal inactivation; dx, steepness of the curve; Fig. 1C). This curve has been considered to represent the voltage-dependent property of the activation gate of HERG channels. In other words, the development of IHERG on depolarization has been known as activation, and the decay of current on repolarization as deactivation.
When external Ca2+ was increased to 5 mm, the IHERG which developed on depolarization decreased significantly and the rate of current onset slowed (Fig. 1A, lower panel). By contrast, the amplitude of Itail decreased slightly and the decay rate of Itail was accelerated significantly. (Initial transient outward currents in the early part of depolarization were observed only in [Ca2+]o≥ 5 mm. Since they were not accompanied by outward tail currents when short pulses were applied, they were not considered as IHERG.) On the other hand, reducing [Ca2+]o to 0.1 mm induced changes in IHERG opposite to those induced by increasing [Ca2+]o. The decay of Itail became very slow and the holding current level at −60 mV shifted outward, indicating that at this potential, channels were already open. The holding potential was therefore changed to −80 mV in order to maintain the holding current near zero (data not shown).
The effect of various concentrations of [Ca2+]o on Iss and Itail is demonstrated in Fig. 1B and C. As [Ca2+]o was progressively increased, activation of outward current started at a more positive potential (shown in the Iss-V relationship as a rightward shift, Fig. 1B), and the amplitude of Iss decreased. The Itail-V curve, which represents the voltage dependence of conductance increase induced by depolarization, shifted progressively to the right (Fig. 1C). The steepness of the curve (dx) was also changed (dx = 5.3 mV in 0.1 mm, 8.7 mV in 5 mm), suggesting that the effect of Ca2+ is not a simple shift of voltage dependence. The amplitude of Itail also decreased progressively for [Ca2+]o > 0.5 mm. The negative slope of the Iss-V curve, which represents the inactivation property of IHERG (conductance decrease induced by depolarization), was, however, little affected by [Ca2+]o. In Fig. 2, the maximum conductance of HERG channels was obtained by applying two step pulses: a varying level of test pulses following the prepulse to +20 mV which is given to induce a full activation of IHERG. Test pulses induced a transient current increase before deactivation occurred (outward current for V > −80 mV, inward current for V < −80 mV). The amplitude of the currents was measured at its peak (Ipeak) and plotted against the membrane potential, showing a strong inward rectification which is typical of HERG channels (Fig. 2B). The Ipeak-V relationship and the reversal potential were hardly affected by [Ca2+]o.
Figure 2. Effect of [Ca2+]o on the inward rectification of the fully activated I-V relationship of HERG channels.
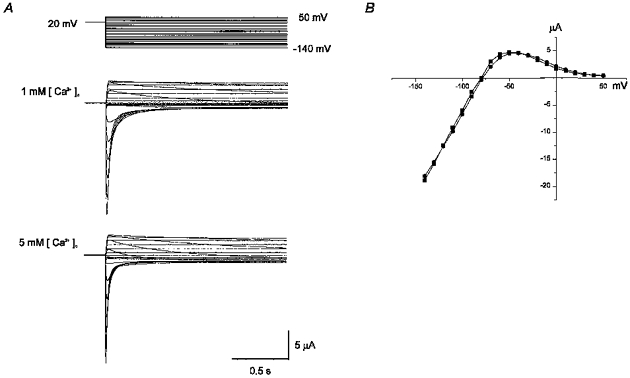
A, superimposed current traces elicited by various levels of test pulses ranging from −140 to +50 mV following the prepulse to +20 mV for 5 s (upper panel, front part of prepulse is not shown) in 1 mm (middle panel) and 5 mm (lower panel) [Ca2+]o. B, plot of the amplitude of peak currents measured at the beginning of the test pulse in 1 mm (•) and in 5 mm (▪) [Ca2+]o.
The above results show that external Ca2+ does not affect the inactivation property of IHERG, but affects only activation/deactivation. The effect is dose dependent: at higher [Ca2+]o, further depolarization is needed to activate the current and deactivation is facilitated. These effects are similar to those found in the rapidly activating delayed rectifier K+ current (IKr) of sinoatrial node cells of the rabbit heart as described by Ho et al. (1996). They interpreted this action of external Ca2+ as a voltage-dependent block on the basis of Woodhull's analysis (1973), and proposed a new interpretation for the IKr channel: activation/deactivation of the IKr channel is in fact controlled by voltage-dependent block/unblock by external Ca2+. It is still possible, however, that the action of Ca2+ is a result of a simple shift of voltage dependence of gating due to the surface charge effect. In order to test this possibility, we investigated the effect of [Ca2+]o on IHERG in different K+ concentrations where ionic strength was maintained constant (2 mm [K+]o-96 mm [Na+]o, 20 mm [K+]o-78 mm [Na+]o). If the effect of Ca2+ is mainly caused by the surface charge effect, Ca2+ should produce the same effect in 2 mm [K+]o and in 20 mm [K+]o, since the surface charge effect produced by changing [Ca2+]o is expected to be same in the two solutions. The result is demonstrated in Fig. 3.
Figure 3. The effect of [K+]o on the action of Ca2+ on HERG currents.
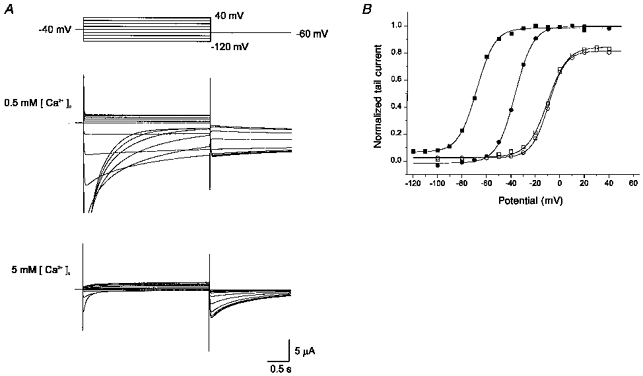
A, superimposed current traces elicited by depolarizing voltage pulses (3 s) in 10 mV steps (upper panel) from the holding potential of −40 mV in high-K+ solution (20 mm) with 0.5 mm (middle panel) and 5 mm (lower panel) [Ca2+]o. Some of the initial parts of the current in 0.5 mm [Ca2+]o are out of scale. Tail currents were recorded on repolarization to −60 mV. B, plot of the normalized tail current measured at its peak just after repolarization. The same experiment shown in A was performed in 2 mm [K+]o in the same cell and the data are shown in the plot. ▪, 0.5 mm [Ca2+]o-20 mm [K+]o; □, 5 mm [Ca2+]o-20 mm [K+]o; •, 0.5 mm [Ca2+]o-2 mm [K+]o; ○, 5 mm [Ca2+]o-20 mm [K+]o. Lines are the fits to the Boltzmann equation, y = 1/{1 + exp(-(V - V1/2)/dx)} (V1/2 from left to right, −68, −36, - 10, and −9 mV; dx from left to right, 7.3, 7.3, 8.1, and 6.8 mV). Same observation from three cells.
When [K+]o was increased to 20 mm, the holding potential was changed to −40 mV in order to minimize the continuous current flow. But the tail current was recorded at −60 mV, as it was in 2 mm [K+]o, in order to compare the effect of Ca2+ in different [K+]o under the same conditions. Itail in 2 mm and in 20 mm [K+]o was measured and normalized values were plotted in Fig. 3B (using the same method as in Fig. 1C). In 5 mm Ca2+, Itail-V curves in 2 mm (○) and in 20 mm (□) [K+]o almost overlapped. When [Ca2+]o was reduced to 0.5 mm, the shift in the Itail-V curve was far more pronounced in 20 mm [K+]o (▪) than in 2 mm [K+]o (•): the voltage at which the HERG channel is half-open (V1/2) is −36 mV in 2 mm [K+]o, and −68 mV in 20 mm [K+]o. This discrepancy strongly contradicts the prediction based on the surface charge effect. A possible explanation can be found, if we suppose that the Itail-V relationship, which was formerly thought to represent steady-state activation, represents a steady-state block of IHERG by external Ca2+. If this is the case, the discrepancy implies that when the concentration of blocking ion (Ca2+) is low, a high concentration of permeating ion (K+) interferes with the blockade and the same degree of block is obtained at more negative voltages. It agrees well with the assumption that permeating ions (K+) compete with blocking ions (Ca2+) at the same site.
It was reported that external Mg2+ blocks IKr channels of SA node cells in the same way as external Ca2+ (Ho et al. 1996). We tested the effect of varying [Mg2+]o on IHERG and the result is demonstrated in Fig. 4. [Ca2+]o was kept constant at 0.5 mm to prevent the development of a leak conductance in oocytes in the absence of external Ca2+. The effect of increasing [Mg2+]o was similar to that of Ca2+. When [Mg2+]o was increased progressively, the IHERG which developed on depolarization started at a more positive potential and the amplitude of Iss decreased. The decay rate of Itail was accelerated significantly (Fig. 4A, lower panel), and the Itail-V curve shifted progressively to the right (Fig. 4C). The result, however, revealed that the effect of Mg2+ is less potent than Ca2+: addition of 10 mm Mg2+ shifted V1/2 by 20 mV, whereas addition of 4.5 mm Ca2+ shifted it by 33 mV.
Figure 4. Effect of [Mg2+]o on HERG currents elicited by depolarizing voltage pulses.
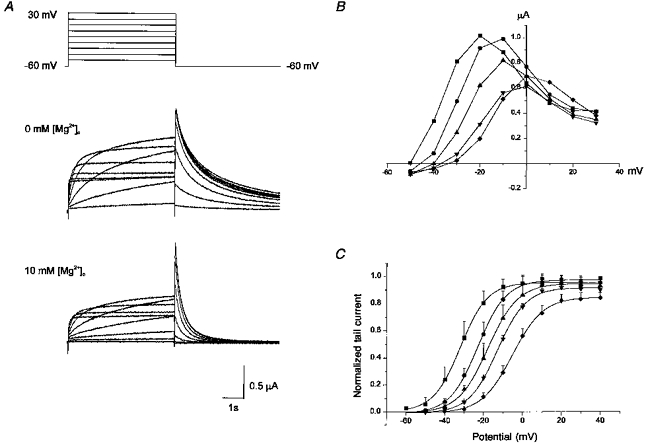
A, superimposed current traces elicited by depolarizing voltage pulses (5 s) in 10 mV steps (upper panel) from the holding potential of −60 mV in 0 mm (middle panel) and in 10 mm (lower panel) [Mg2+]o. [Ca2+]o was 0.5 mm and [K+]o was 2 mm. B, plot of the steady-state current measured at the end of depolarizing pulses against the pulse potential in varying external [Mg2+]o (obtained from the same cell shown in A). C, plot of the normalized tail current measured at its peak just after repolarization. Symbols with error bars represent mean ± s.d. Data obtained from 4 cells. Symbols in B and C: ▪, 0 mm; •, 2.5 mm; ▴, 5 mm; ▾, 10 mm; ♦, 20 mm [Mg2+]o. Lines in C are the fits to the Boltzmann equation, y = 1/{1 + exp((−V+V1/2)/dx)} (V1/2 from left to right, −32, −23, −17, −12, and −5 mV; dx from left to right, 7.0, 7.2, 7.7, 7.3, and 7.8 mV).
DISCUSSION
The action of external Ca2+ and Mg2+ on the HERG channel presented in this paper is similar to that on the rapidly activating delayed rectifier K+ current (IKr) of sinoatrial node cells of the rabbit heart (Ho et al. 1996). In the present study, we not only confirmed that their action is common to both IKr and IHERG, but also discovered several important features which were not easily tested in native IKr: competition with K+ and a flow-independent effect.
The gating characteristics of the HERG channel are depicted as follows:
Two different approaches are possible for modelling the effect of Ca2+ on the HERG channel presented in the present paper. The first approach is to regard the action of Ca2+ as a modifier of intrinsic voltage-dependent gating (see also Discussion in Ho et al. 1996). This has been widely investigated and it is well known that various divalent cations can modify gating of the voltage-dependent channels either by screening the surface charge of the membrane (Green & Andersen, 1991; Hille, 1992), or by direct modulation (Armstrong & Lopez-Barnes, 1987; Spires & Begenisich, 1992, 1994). This approach requires the presence of intrinsic voltage-dependent gating which persists even in a Ca2+-free solution. However, it has not been possible to determine the intrinsic gating properties experimentally, since in Ca2+-free or low-Ca2+ solutions (less than 0.1 mm), a non-specific leak conductance developed progressively, which prevented further study. The disappearance of channel properties, including gating and selectivity, in Ca2+-free solution has also been observed in studies on the delayed rectifier K+ channel in squid giant axon (Armstrong & Lopez-Barnes, 1987), and it was suggested that Ca2+ ions act as a cofactor for channel gating. It is, however, not certain whether this phenomenon implies that the HERG channel also requires Ca2+ ions to preserve its properties. On the other hand, the increase of monovalent cationic conductance in divalent cation-free solutions has been observed in control oocytes (Arellano, Woodward & Miledi, 1995), suggesting that it may be related to a native property of oocytes.
Another approach is to interpret the action of Ca2+ independently of intrinsic gating. In cardiac SA node cells, IKr was recorded in Ca2+-free solutions and the intrinsic gating properties were described (Ho et al. 1996). However, the effect of Ca2+ and Mg2+ was not satisfactorily explained by a simple shift of the voltage dependence of gating. A significant difference in the steepness of the activation curve (dx = 4.2 mV in Ca2+-free and 9.1 mV in 5 mm Ca2+) was observed, suggesting that the Ca2+-dependent process is fundamentally different from intrinsic voltage-dependent gating. This tendency was also observed in the present paper. Ho et al. (1996) interpreted the action of external Ca2+ and Mg2+ on IKr as a voltage-dependent block, on the basis of Woodhull's analysis (1973), and successfully modelled the effect of Ca2+ and Mg2+ on IKr. Since the effects on IHERG presented in the present paper are similar to those on IKr, we applied the same model to the HERG channel for quantitative analysis. The Ca2+-dependent process can be added to Scheme 1:
Scheme 2.
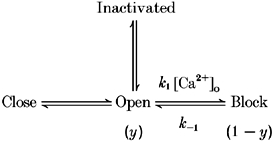
Scheme 1.
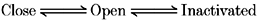
Since the intrinsic gating (open-close, open-inactivated) in the absence of Ca2+ was not determined in the present experiment, we only dealt with the condition in which the conversion between open and inactivated and open and closed states can be neglected. We analysed Itail at a fixed potential (−60 mV), so that the inactivation parameter was constant. From the results obtained at the lowest [Ca2+]o used (0.1 mm, Fig. 1C; Itail reaches it maximum at −60 mV), we can assume that the fraction of closed states is negligible for V > −60 mV. Thus, over the voltage range V > −60 mV, the Ca2+-dependent block process is a major factor only in determining the fraction of conducting channels. The amplitude of Itail thus represents the fraction of conducting channels (y) at the end of the test pulse. The effect of [Ca2+]o on y at various membrane potentials was obtained by dividing Itail by the maximum Itail (which indicates when y = 1). The dose-response relationship between y and [Ca2+]o was fitted by the following equation:
Dissociation constants (KM) at −50, −40, −30, −20, −10, 0, 10 and 20 mV were 0.2, 0.4, 0.8, 1.4, 3.0, 6.7, 13.8 and 26.6 mm, respectively. Hill coefficients, n, were between 0.8 and 1.4. This value is compatible with previous results (Ho et al. 1996) showing that the number of binding sites for Ca2+ in IKr channel is one. The decrease in KM following hyperpolarization (e-fold change of 14.5 mV) shows that blockade is facilitated by hyperpolarization. From the voltage dependence of the dissociation constants, the fractional electrical distance (δ) was calculated to be 0.86. The effect of [Mg2+]o was subject to same analysis. The result shows that Mg2+ is less potent than Ca2+, and the KM value for Mg2+ at 0 mV is 24.8 mm (6.7 mm for Ca2+). KM is also voltage dependent (e-fold change of 16.3 mV) and the fractional electrical distance (δ) is calculated to be 0.67.
This analysis supports the hypothesis that external Ca2+ and Mg2+ block the HERG channel in a voltage- and time-dependent manner, and these properties have been regarded as properties of the activation gate. The idea is related to the gating particle theory, originally suggested by Frankenhaeuser & Hodgkin (1957) to explain the decrease in membrane conductance induced by increased [Ca2+]o. The gating particle theory supposes that Ca2+ ions are an essential component in voltage-dependent gating, acting as a voltage sensor or charged gating particles for the channel.
Although we have tested the channel blockade model as the most likely mechanism for the action of external Ca2+ and Mg2+, there are several points both in the present study and in the previous study on IKr (Ho et al. 1996) which do not agree with this model. The decrease in maximum conductance at positive potentials (Figs 1C and 4C) and the slowing of current onset with increasing [Ca2+]o and [Mg2+]o are not easily explained by a simple block model. In order to reproduce the decrease in maximum conductance, it may be necessary to introduce another blocked state which is voltage independent. The slowing of current onset with increasing [Ca2+]o and [Mg2+]o does not conform to a simple block model, in which the unbinding rate constant (k-1) does not depend on the concentration of blocking ions. This result may possibly be explained by introducing multiple blocked states (B1, B2 and B3), since the rate of current onset depends not only on k-1 but also on the fraction of channels in each state. If a higher concentration of blocking ions increases the fraction of B3 over B1, current onset will be slowed. The presence of multiple non-conducting states is also supported by a recent study by Wang, Liu, Morales, Strauss & Rasmusson (1997). They observed that the time course of the onset of IHERG on depolarization is sigmoidal, and suggested that at least three closed states (C1, C2 and C3) were required to reproduce the sigmoidal time course. Another interpretation is, however, still possible, namely that Ca2+ and Mg2+ modify the activation gate in such a way that they accelerate channel closure and slow channel opening.
In spite of such disagreement, the results in Fig. 3 showing the dependence of the Ca2+ effect on [K+]o can only be explained by the channel blockade model. Various K+ channels are known to be affected by [K+]o and the effect of [K+]o on HERG channels has already been examined (Baukrowitz & Yellen, 1995; Wang, Morales, Liu, Strauss & Rasmusson, 1996; Yang, Snyders & Roden, 1997). However, the effect of [K+]o in relation to varying [Ca2+]o has never been tested. The results shown in Fig. 3 clearly demonstrate the competition between K+ and Ca2+. This feature can be found when permeating ions and blocking ions bind to the same site. The other theories, such as the surface charge and gating modifier theories, do not predict such interaction. Details of the interaction between the two ions need to be investigated further.
A question which is functionally very important but which could not be investigated in detail in cardiac IKr is whether the effect of external cations depends on the direction of current flow. It is well known that voltage-dependent blockade of inward rectifier K+ channels by internal Mg2+ depends on EK (reversal potential for K+) since the blockade is flow dependent; Mg2+ blocks only outward currents (Matsuda et al. 1987). If this is also true for Ca2+ block of IKr, the results obtained from inward IKr in high-K+ solution cannot be extrapolated to normal conditions under which IKr flows outwards. It is not easy, however, to investigate IKr in normal K+ solution, since outward IKr is very small due to its inward rectifying property. Furthermore, it is difficult to isolate IKr from other outward currents in cardiac myocytes. Ho et al. (1996) therefore recorded IKr in symmetrical K+ solutions as an inward current. This study involving the expression of HERG allows these limits to be overcome, and we have found that outward IHERG is controlled by external Ca2+ and Mg2+ in the same way as inward IKr.
Acknowledgments
This work was supported by research grants from the Korea Science and Engineering Foundation (1997), Seoul National University Hospital (1997) and the Biotech 2000 Program from the Ministry of Science and Technology. We would like to thank Dr Gea-Ny Tseng of Columbia University for providing the HERG gene and for helpful discussion.
References
- Arellano RO, Woodward RM, Miledi R. A monovalent cationic conductance that is blocked by extracellular divalent cations in Xenopus oocytes. Journal of Physiology. 1995;484:593–604. doi: 10.1113/jphysiol.1995.sp020689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Lopez-Barnes J. External calcium ions are required for potassium channel gating in squid neurons. Science. 1987;236:712–714. doi: 10.1126/science.2437654. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: A tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin AL. The action of calcium on the electrical properties of squid axons. Journal of Physiology. 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WN, Andersen OS. Surface charges and ion channel function. Annual Review of Physiology. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associate Inc.; 1992. [Google Scholar]
- Ho WK, Earm YE, Lee SH, Brown HF, Noble D. Voltage- and time-dependent block of delayed rectifier K+ current in rabbit sino-atrial node cells by external Ca2+ and Mg2+ Journal of Physiology. 1996;494:727–742. doi: 10.1113/jphysiol.1996.sp021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiological Reviews. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Kiehn J, Lacerda AE, Wible B, Brown AM. Molecular physiology and pharmacology of HERG: Single-channel current and block by dofetilide. Circulation. 1996;94:2572–2579. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. Journal of Physiology. 1987;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the Ikr channel. Journal of General Physiology. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires S, Begenisich T. Chemical properties of the divalent cation binding site on potassium channels. Journal of General Physiology. 1992;100:181–193. doi: 10.1085/jgp.100.2.181. 10.1085/jgp.100.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires S, Begenisich T. Modulation of potassium channel gating by external divalent cations. Journal of General Physiology. 1994;104:675–692. doi: 10.1085/jgp.104.4.675. 10.1085/jgp.104.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. Journal of Physiology. 1997;502:45–60. doi: 10.1111/j.1469-7793.1997.045bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Morales MJ, Liu S, Strauss HC, Rasmusson RL. Time, voltage and ionic concentration dependence of rectification of h-erg expressed in Xenopus oocytes. FEBS Letters. 1996;389:167–173. doi: 10.1016/0014-5793(96)00570-4. 10.1016/0014-5793(96)00570-4. [DOI] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. Journal of General Physiology. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Snyders DJ, Roden DM. Rapid inactivation determines the rectification and [K+]o dependence of the rapid component of the delayed rectifier K+ current in cardiac cells. Circulation Research. 1977;80:783–789. doi: 10.1161/01.res.80.6.782. [DOI] [PubMed] [Google Scholar]


