Abstract
To examine the functional importance of the pre-Bötzinger complex for breathing we micro-injected, under in vivo conditions, the calcium channel blocker ω-conotoxin GVIA and the sodium channel blocker tetrodotoxin (TTX) into the ventrolateral medulla of adult cats, while monitoring respiratory rhythmic motor output in the phrenic nerve.
ω-Conotoxin GVIA caused a highly localized synaptic ablation by blocking presynaptic N-type calcium channels. When injecting 5–60 fmol ω-conotoxin GVIA unilaterally, the amplitude of phrenic nerve activity decreased bilaterally and sometimes disappeared, indicating central apnoea. These effects were reversible and could only be induced in a very localized area of the pre-Bötzinger complex. By injecting ω-conotoxin GVIA several times during an experiment and analysing the areas where injections affected respiratory activity, it was possible to map exactly the anatomical extent of the area critical for respiratory rhythm generation.
Following the precise localization of the pre-Bötzinger complex with ω-conotoxin GVIA, we injected TTX to induce an irreversible inactivation of this region. TTX injected unilaterally into the pre-Bötzinger complex irreversibly reduced the amplitude of phrenic nerve activity. Bilateral TTX injections eliminated respiratory rhythmic activity, causing a persistent central apnoea.
After bilateral lesioning of the pre-Bötzinger complex, it was still possible to induce gasping during hypoxia or asphyxia, indicating that respiration and gasping are generated by two different neuronal networks.
We propose that ω-conotoxin GVIA as employed in this study to investigate the functional role of the pre-Bötzinger complex can also be used as a general pharmacological approach to map other neuronal networks. We call this the ‘ω-conotoxin GVIA tracing’ method.
The respiratory system can generate two different kinds of ventilatory patterns. (1) Eupnoea: a ventilatory activity characterized by a slow, augmenting activation of respiratory muscles (Feldman, 1986; von Euler, 1986; Ezure, 1990; Bianchi, Denavit-Saubie & Champagnat, 1995; Richter, Ballanyi & Ramirez, 1996). This activity pattern is expressed under normal physiological conditions and will be called in the remainder of this study ‘respiration’ or ‘eupnoeic pattern’. (2) Gasping: a ventilatory pattern characterized by a rapid activation of inspiratory muscles, which is expressed under pathophysiological conditions such as hypoxia (St John, 1990). The two types of ventilatory activities differ in many aspects. For example, different respiratory disturbances have been associated with the different forms of ventilation. Thus, it has been proposed that the two motor patterns are generated by two independent rhythm-generating networks. A region in the lateral tegmental field of the medulla has been proposed as the site for the generation of gasping (St John, 1990; Fung, Wang & St John, 1994) and the pre-Bötzinger complex, located more ventral and lateral to this gasping centre, has been proposed as the site for the generation of the respiratory rhythm (Smith, Ellenberger, Ballanyi, Richter & Feldman, 1991; Funk, Smith & Feldman, 1993; Smith, Funk, Johnson & Feldman, 1995; Schwarzacher, Smith & Richter, 1995; Ramirez & Richter, 1996).
Both regions are located in relatively close vicinity within the medulla. Thus, there have been some discussions as to whether these regions do indeed constitute separate networks (Fung et al. 1994). In order to demonstrate the existence of two different networks, it was necessary to precisely localize and lesion one of these networks in order to examine whether the other network was still capable of generating the rhythmic activity. Previous in vitro studies in rats have precisely localized the pre-Bötzinger complex using chemical lesion experiments (Funk et al. 1993), but did not address the issue of gasping. In the study presented here we localized the pre-Bötzinger complex under in vivo conditions using a similar approach. The advantage of performing lesion experiments under in vivo conditions is the fact that it is possible to demonstrate that normal respiration in a functionally intact animal is generated by the pre-Bötzinger complex, an area which is different from the gasping centre. Similar experiments were performed in rats by Fung et al. (1994), who demonstrated that unilateral electrical lesions in a region which is close to the pre-Bötzinger complex can sometimes, but not always, eliminate respiration. Similarly Koshyia & Guyenet (1996) have demonstrated that injections of muscimol into the pre-Bötzinger complex can eliminate respiration in the rat in vivo. However, the study by Fung et al. (1994) may have affected axons en route from other regions, which makes these interpretations difficult, and the study by Koshyia & Guyenet (1996) aimed to investigate the interaction between sympathetic control and respiration, without considering the aspect of gasping.
Given the immense clinical and basic scientific interest in better defining the locations of the networks responsible for generating respiration and gasping, in this study we examined in further detail the functional role of the pre-Bötzinger complex in the process of rhythm generation for eupnoeic breathing by introducing a methodology which we call the ‘ω-conotoxin GVIA tracing’ method. Specific pharmacological disturbance of synaptic transmitter release in anatomically defined locations was used together with a recording from phrenic nerve activity to make an anatomically precise map of the pre-Bötzinger complex. The pharmacological agents of choice are the ω-toxins, a group of polypeptide ligands made either by predatory cone snails (ω-conotoxins) or by spiders (e.g. the ω-agatoxins), which are specifically targeted to presynaptic calcium channels. In this work we employed ω-conotoxin GVIA, previously shown to reduce action potential-elicited neurotransmitter release in a variety of synaptic systems (for a review, see Olivera, Miljanich, Ramachandran & Adams, 1994). In the mammalian central nervous system, it has become clear that ω-conotoxin GVIA is a specific antagonist of the N-type calcium channel, originally defined by Tsien and co-workers (Tsien, Lipscombe, Madison, Bley & Fox, 1988). ω-Conotoxin GVIA is a large peptide (27 amino acids) which binds N-channels with high affinity. Although, the precision of a pharmacological lesion depends on many factors, such as the injected volume or the size of the electrode tip used to inject a toxin, a large peptide has the advantage that the toxin remains more readily at the injection site without diffusing rapidly into other regions. We examined the effects of this ω-conotoxin on respiratory rhythm generation in the brainstem of the adult cat in vivo, and demonstrate that it is a potent pharmacological agent for disrupting respiratory rhythm in the very localized area of the pre-Bötzinger complex. We directly demonstrate, using the ω-conotoxin GVIA tracing methodology, that the neuronal network in the pre-Bötzinger complex is important for the generation of eupnoeic breathing patterns in adult mammals, and that both sides of the network are highly coupled. Having constructed a detailed map of the pre-Bötzinger complex using ω-conotoxin GVIA injections, we then injected small amounts of tetrodotoxin (TTX) into this highly restricted region, to permanently abolish respiratory rhythmic activity. Following bilateral injections of TTX into the pre-Bötzinger complex, respiration was always abolished without eliminating gasping. These experiments clearly demonstrate that the pre-Bötzinger complex is essential for the generation of the respiratory rhythm and that gasping is generated in a region outside the pre-Bötzinger complex.
METHODS
Anaesthesia
Fifteen cats of either sex were anaesthetized with sodium pentobarbitone (Nembutal; 40 mg kg−1, i.p.). Throughout the experiments, the level of anaesthesia was carefully evaluated at regular time intervals. Additional doses of anaesthetic (4–8 mg) were given intravenously if nociceptive mechanical stimuli elicited enhancement of phrenic nerve discharge, increase of respiratory frequency, increase in heart rate or a rise in arterial blood pressure. Salivation and mucus secretion were depressed by administering atropine sulphate (Braun, Melsungen, Germany) intravenously (0.1 mg kg−1), and dexamethasone (0.2 mg kg−1) was given for protection against oedema. If not otherwise indicated, all drugs were obtained from Sigma.
Surgical preparation
Both femoral veins and one femoral artery were cannulated for drug administration and monitoring the arterial blood pressure, respectively. Rectal temperature was maintained at 36.0-38.0 °C by external heating. A cannula inserted into the trachea was used to artificially ventilate the animal with oxygen-enriched air (60–70% O2). Asphyxia (hypercapnic hypoxia) was achieved by turning off the respirator. Hypoxia (normocapnic hypoxia) was produced by exchanging the oxygen-enriched gas mixture with one containing 5–10% O2 in N2. Animals were paralysed with gallamine triethiodide (Flaxedil; initial dose 10 mg kg−1, then 1–2 mg kg−1 hourly). The vagus nerves were severed to abolish the Hering-Breuer reflex. Respiratory-related movement artifacts while recording within the brainstem were reduced by a bilateral pneumothorax. The collapse of the lungs was prevented by a positive end expiratory pressure of 10–20 mmH2O.
Following occipital craniotomy, the caudal part of the cerebellum was carefully dissected free from connective tissue with the aid of a dissection microscope. The caudal end of the cerebellar vermis and caudal parts of one cerebellar hemisphere were gently lifted and fixed with a loop of lead wire in order to allow injections to be made through an electrode stereotactically positioned in the region of the pre-Bötzinger complex. Both phrenic nerves were exposed in the neck and cut distally. Their efferent discharge was recorded and amplified 10 000 times, filtered at 30–3000 Hz, rectified and integrated with a time constant of 50–100 ms. The integrated activity was used to assess the effect of ω-conotoxin GVIA injections on the central respiratory motor output.
Pressure injections into the pre-Bötzinger complex
Glass micropipettes (tip diameter, 10–25 μm) filled with either 5 μm ω-conotoxin GVIA (Olivera, McInstosh, Cruz, Luque & Gray, 1984) or 1 μm TTX were positioned in the general area of the nucleus ambiguus (Fig. 1B) by stereotactic measurements. The toxins were dissolved in artificial cerebrospinal fluid (CSF), containing (mm): 128 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 24 NaHCO3, 0.5 NaH2PO4, 30 d-glucose, and were stored as aliquots in the freezer (−20 °C) until needed. The toxins were injected by air pressure (100–300 kPa) using a Pico Spritzer (NPI Instruments). The injected amounts (25–50 nl) were calculated by measuring the movements of the fluid meniscus in the pipette with a microscope fitted with an ocular micrometer (accuracy, ±0.1 mm). For each injection, the position of the electrode was measured in relation to the obex, the mid-line and the dorsal surface. Control injections using the same type of electrode, but filled with artificial CSF had no effect on respiratory rhythmic activity.
Figure 1. ω-Conotoxin GVIA (30 fmol) causes central apnoea when injected into the pre-Bötzinger complex.
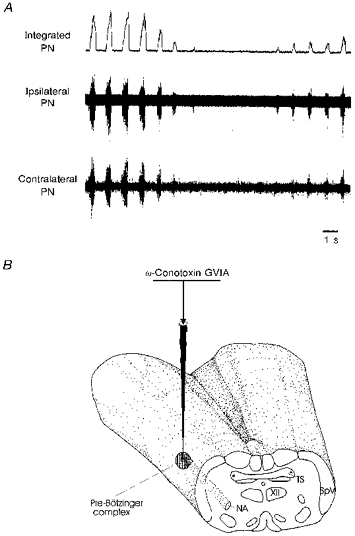
A, upper trace: integrated phrenic nerve activity. Middle trace: phrenic nerve activity recorded ipsilateral to the ω-conotoxin GVIA injection site. Lower trace: phrenic nerve activity recorded contralateral to the injection site. B, schematic illustration of the injection site. NA, nucleus ambiguus; PN, phrenic nerve; TS, solitary tract; SpV, spinal trigeminal nucleus; XII, hypoglossal nucleus.
Histology
At the end of the experiments (n = 15), similar glass micropipettes filled with either Pontamine Sky Blue (7 % in 3 m KCl; Molecular Probes) or BODIPY FL-strychnine (200 μm, Molecular Probes) were positioned at the same stereotactic location where toxins affected phrenic nerve discharge. The dyes were then injected with comparable pressures. Five minutes after dye injection, the animal was perfused transcardially with Ringer solution followed by 2.5% paraformaldehyde and 1.5% glutaraldehyde in phosphate buffer, pH 7.4, or killed in deep anaesthesia with an intravenous injection of a 3 m KCl solution. The brainstem was then removed and transferred into a fixative of 2.5% paraformaldehyde and 1.5% glutaraldehyde in phosphate buffer for 12–24 h. The fixed brainstem was mounted in the Horsley-Clarke coronal plane and serial 100 μm sections were cut using a vibratome. Sections were counterstained with Cresyl Violet, dehydrated, embedded and examined under a light microscope. Sections from brainstems labelled with fluorescent dyes were embedded in Histogel without counterstaining, examined with a fluorescence microscope (see Fig. 5D), and anatomically reconstructed with a camera lucida system (see Fig. 5B). At the end of an experiment, animals were killed with an overdose of anaesthetic.
Figure 5. Histological verification of ω-conotoxin GVIA injection sites.
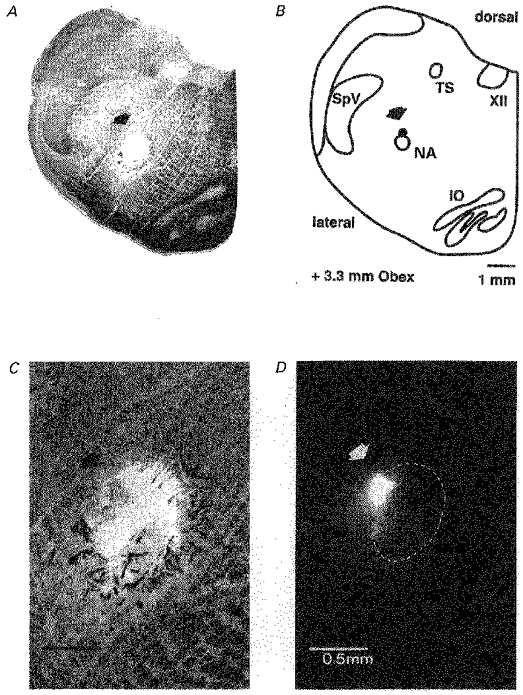
Photograph (A) and schematic diagram (B) of a transversal 100 μm section of the medulla containing the injection site within the pre-Bötzinger region (arrows). The filled circle in the reconstruction represents the position of the centre of the locus of the deposited dye. C and D, higher magnification of the injection site without and with fluorescence filtering, respectively. Positive fluorescence signal of injected BODIPY FL-strychnine is limited to an area of approximately 0.5 mm2. Dashed circle in D indicates the area of tissue damage due to numerous pressure injections performed during the experiment.
RESULTS
Effects of ω-conotoxin GVIA on respiratory rhythm
Pressure injections of ω-conotoxin GVIA into the pre-Bötzinger complex caused an immediate decrease in amplitude of phrenic nerve activity (Fig. 1A), while control injections (electrodes filled with artificial CSF) were ineffective. In less than 10% of the cases, ω-conotoxin GVIA injections were associated with blood pressure changes. In such cases, respiratory effects could be indirect and were therefore not evaluated further in this study. In those cases where no blood pressure changes occurred, the amplitude of phrenic nerve activity decreased on average by 22% (as evaluated for 46 ω-conotoxin GVIA injections in 7 animals). In many cases ω-conotoxin GVIA caused transient central apnoea, i.e. no respiratory activity was detectable on the phrenic nerve for several seconds, followed by a gradual recovery (Fig. 1A). On average, ω-conotoxin GVIA injected unilaterally significantly decreased the frequency of respiratory activity (8.9 ± 2 %, 51 injections in 7 animals). This effect was evaluated by averaging ten respiratory cycles before and during the effect of ω-conotoxin GVIA. In obtaining this average, we did not include the duration of the apnoea, but measured the frequency of respiratory activity following the period of apnoea. We also included the cases where ω-conotoxin GVIA evoked no apnoea. The effect on phrenic nerve activity was always bilateral. This is shown in Fig. 1, in which injection led to a complete lack of activity during the same interval, and a parallel time course of recovery on both sides (Fig. 1A).
Analysis of the effects of ω-conotoxin GVIA on phrenic nerve activity indicated that there was a significant decrease in the amplitude of activity, but that the burst duration was not significantly affected by applications of the toxin (−2% on average; n = 44 ω-conotoxin GVIA injections). The average burst obtained from twenty integrated phrenic nerve discharges before and following an ω-conotoxin GVIA injection revealed a similar picture: the burst duration was not affected but the amplitude was decreased (Fig. 2A). The typical augmenting shape of the phrenic burst was maintained.
Figure 2. Alterations of averaged integrated phrenic nerve activity following toxin injections into the left and subsequently the right pre-Bötzinger complex.
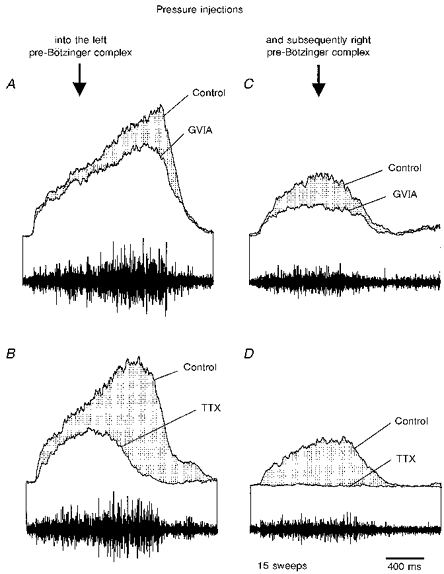
A, ω-conotoxin GVIA injection into the left pre-Bötzinger complex reduces the amplitude of averaged phrenic nerve activity. B, TTX injected into the left pre-Bötzinger complex reduces the amplitude and duration of phrenic nerve activity. C, ω-conotoxin GVIA injected into the right pre-Bötzinger complex after injecting TTX into the left pre-Bötzinger complex further reduces the amplitude of averaged phrenic nerve activity. D, TTX also injected into the right pre-Bötzinger complex eliminates phrenic nerve activity. The integrated phrenic nerve discharges shown in A-D were averaged from fifteen consecutive sweeps.
The ω-conotoxin GVIA tracing method as an anatomical probe
The major finding of this study was that ω-conotoxin GVIA effects were extremely localized. Thus, we have used ω-conotoxin GVIA injections to accurately determine stereotactically the precise anatomical region of the pre-Bötzinger complex of the brainstem which is involved in respiratory rhythm generation. For a histological characterization we also injected dyes into the region where respiration was affected.
Stereotactic co-ordinates of the pre-Bötzinger complex
ω-Conotoxin GVIA was injected at quantities of 5–60 fmol at a variety of loci in the area of the nucleus ambiguus. These quantities of ω-conotoxin GVIA were applied successively while moving at a defined co-ordinate with respect to the obex in 200–500 μm steps from dorsal (starting at a depth of 3 mm) to ventral (maximal depth, 5.5 mm). We waited for at least 1–2 min between two successive injections if there was no obvious effect on respiratory activity. However, if there was an effect on respiratory activity, we waited for a longer period (up to 5 min) to make sure that control conditions were reached (judged by the amplitude and frequency of integrated phrenic nerve discharge, which was printed on-line on a thermoarray recorder as a hard copy). An example is shown in Fig. 3. In this case the same quantity of ω-conotoxin GVIA was applied at different depths at a co-ordinate of 3.3 mm rostral to obex, 3.6 mm lateral to mid-line (Fig. 3A). ω-Conotoxin GVIA injections had, at the depths of 4.0 and 5.0-5.5 mm, no or little effect (average reduction, < 10%, evaluated for 10 consecutive cycles), but at 4.3 and 4.5 mm had clear effects on the amplitude of phrenic nerve activity (average reduction, > 20% (see above), or apnoea). Thus, each ω-conotoxin GVIA injection could simply be scored on its amplitude effects (Fig. 3B). Since all ω-conotoxin GVIA effects reversed quickly (see Fig. 3), several ω-conotoxin GVIA injections could be performed in one individual cat to precisely map the anatomical region of the pre-Bötzinger complex. A series of 130 anatomically defined ω-conotoxin GVIA injections into seven animals was evaluated for the absence (average reduction of integrated phrenic nerve amplitude in response to ω-conotoxin GVIA injections, < 10%) or presence of respiratory effects (average reduction of integrated phrenic nerve amplitude in response to ω-conotoxin GVIA injections, > 20%, or apnoea). Note in Fig. 4 that the respiratory effects were restricted to a narrow area within the transverse (Fig. 4A) and horizontal planes (Fig. 4B) of the nucleus ambiguus. Our data revealed an area not larger than 0.6 mm3 (3.0-3.6 mm rostral of the obex, 3.0-4.2 mm lateral of the mid-line, 3.8-4.7 mm from the dorsal surface, Fig. 4C). These regions correspond to the anatomical co-ordinates of the pre-Bötzinger complex as established by intracellular recordings from respiratory neurons in the adult cat (Schwarzacher et al. 1995).
Figure 3. Mapping of the pre-Bötzinger complex.
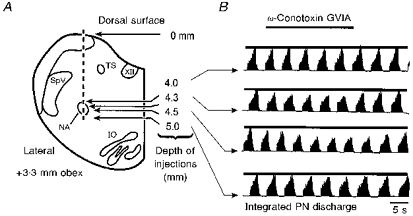
A, schematic representation of the experimental procedure to map the pre-Bötzinger complex. The electrode was penetrated into the brainstem as indicated by the vertical dashed line. ω-Conotoxin GVIA was injected at the depths indicated by the four arrows. B, recordings of integrated phrenic nerve discharge during pressure injection of 10 fmol ω-conotoxin GVIA at different depths, as indicated in A. Note that effects were limited to a dorsoventral extension of less than 1 mm. IO, inferior olive.
Figure 4. Stereotactic co-ordinates of the pre-Bötzinger complex.
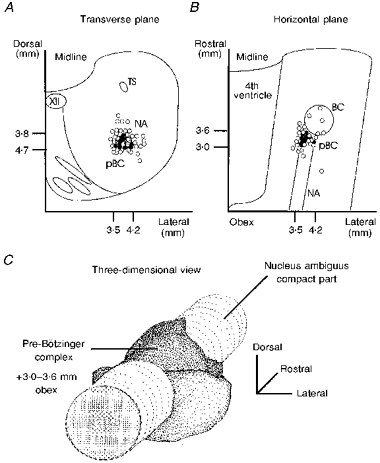
Positions of ω-conotoxin GVIA injection sites with or without effect on the respiratory rhythm from five successful experiments are superimposed on a transverse (A) and horizontal (B) plane according to stereotactic measurements. ○, ω-conotoxin GVIA injections with no detectable effect. •, ω-conotoxin GVIA probes which caused a clear decrease in burst amplitude. C, outlines of the effective areas (darker planes indicate the areas covered by effective ω-conotoxin GVIA injection sites) in a three-dimensional view are shown in relation to the column of the compact part of the ambigual nucleus. Effective ω-conotoxin GVIA injection sites were limited to an area 3.0-3.6 mm rostral to the obex, 3.5-4.2 mm lateral to the mid-line, and 3.8-4.7 mm ventral to the dorsal surface. BC, Bötzinger complex; pBC, pre-Bötzinger complex.
Histological landmarks of the pre-Bötzinger complex
We also confirmed the localization histologically by injecting Pontamine Sky Blue (n = 11). These injections resulted in labelling of areas up to 2–3 mm in diameter. This relatively large area stained by Pontamine Sky Blue was due to the diffusion of the dye, which was not stopped by the fixation: blue labelling of the ventral surface, which was not present 15 min after injection, appeared 2 h after fixation. Nevertheless, in five cats it was possible to confine the centre of the injection to the area of the pre-Bötzinger complex. Further histological confirmation of the pre-Bötzinger complex was obtained by injecting the fluorescent dye BODIPY-strychnine, which binds specifically to glycine receptors that are densely distributed throughout the medullary reticular formation. BODIPY-strychnine injections resulted in a positive fluorescent signal restricted to an area of approximately 0.5 mm2 (Fig. 5, n = 4). The pre-Bötzinger complex was defined histologically as the region of the rostral nucleus ambiguus and ventrolateral reticular formation between the retrofacial nucleus, rostrally and the lateral reticular nucleus, caudally. This region in the transverse plane is co-extensive with the rostral compact and semi-compact divisions of the nucleus ambiguus (i.e. the retrofacial nucleus). It is situated at the same rostrocaudal level as the rostral pole of the hypoglossal nucleus, the intramedullary course of the most rostral hypoglossal rootlets, and the largest ventrolateral extent of the principal nucleus of the inferior olive (for details see Schwarzacher et al. 1995).
Bilateral, chemical lesion of the respiratory rhythm generator with TTX following its anatomical localization with ω-conotoxin GVIA
One advantage of ω-conotoxin GVIA was its transient and immediate effect on respiratory rhythm generation, which enabled us to identify precisely the anatomical localization of the areas important for respiratory rhythm generation. However, to indicate that this area is indeed essential for respiratory rhythm generation, a permanent elimination of the respiratory rhythm would be necessary. Thus, following the ω-conotoxin GVIA injections to identify the pre-Bötzinger complex, TTX was injected into the same location. TTX injected in quantities of 5–10 fmol had irreversible effects on respiration.
A representative experiment is shown in Fig. 6. The pre-Bötzinger complex was localized using ω-conotoxin GVIA injections, which led immediately to a reversible central apnoea and a characteristic decrease in the amplitude of subsequent phrenic nerve discharges (not shown). The ω-conotoxin GVIA-containing electrode was then replaced with one containing TTX, which was positioned at the same stereotactic location. A single TTX injection induced a dramatic, irreversible effect on respiratory activity. The amplitude was still reduced 15 min following the injection; note that the frequency of phrenic nerve discharge was also enhanced (Fig. 6A, right panel). We assume that TTX, in contrast to the ω-conotoxin, also affected axons running through the pre-Bötzinger complex. Thus, it was to be expected that the TTX effects would be different from those induced by the ω-conotoxin GVIA. As seen in the average burst, the effects of TTX (Fig. 2B) on the shape of phrenic nerve discharge were more pronounced than those induced by ω-conotoxin GVIA (Fig. 2A). The amplitude of integrated phrenic nerve discharge was more reduced than following the ω-conotoxin GVIA injection. The initial portion of the TTX-affected burst was still augmenting, but the decay started earlier, resulting in a significant shortening of the burst (Fig. 2B). Following the TTX-induced irreversible knock-out of the left pre-Bötzinger complex, we localized the pre-Bötzinger complex of the right side using the ω-conotoxin GVIA tracing method. Injections of ω-conotoxin GVIA into the right pre-Bötzinger complex further reduced the amplitude of phrenic nerve discharge, and induced irregularities in the rhythmic phrenic nerve discharge (Fig. 6B). The reduction in the amplitude of the phrenic nerve discharge was also demonstrated as an average (Fig. 2C). In all five experiments using this protocol, respiratory activity was also reversibly affected by ω-conotoxin GVIA injections. Irregularities in rhythmic phrenic nerve activity were also induced by the TTX injection, which led within 2–4 min to an irreversible elimination of respiratory rhythmic activity (Fig. 6C, lower panel). In all five experiments, bilateral lesioning of the pre-Bötzinger complex with TTX resulted in a central apnoea, which was definitely irreversible since respiratory rhythmic activity did not return even when waiting for more than 1 h. Even during asphyxia, which leads to hypercapnic conditions, we never observed the generation of an augmenting burst in the phrenic nerve. The only ‘structured’ activity was gasping, which had a motor pattern clearly different from normal respiration. Thus, we conclude that following bilateral lesioning of the pre-Bötzinger complex, respiration was abolished and not generated in a subthreshold manner. This is also indicated by the fact that bilateral injections affected not only the amplitude of phrenic nerve activity, but also the rhythm itself (Fig. 6B and C).
Figure 6. Effect of toxin injections into the left and subsequently right pre-Bötzinger complexes.
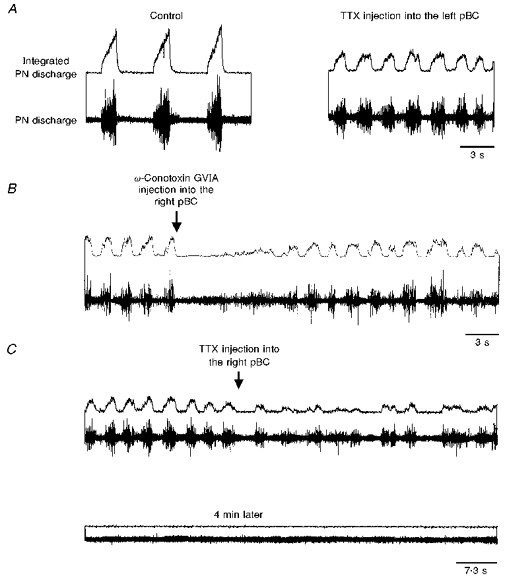
Upper traces, integrated phrenic nerve discharge; lower traces, phrenic nerve discharge. A, left panel, control; right panel, phrenic nerve discharge following TTX injection into the left pre-Bötzinger complex. B, after injecting TTX into the left pre-Bötzinger complex, ω-conotoxin injected into the right pre-Bötzinger complex caused central apnoea which was followed by irregular phrenic nerve discharge. C, TTX, injected into the same location as in B, caused severe irregularities in phrenic nerve activity (upper panel) which led within 4 min to a terminal apnoea (lower panel).
Induction of gasping following bilateral TTX lesioning of the pre-Bötzinger complex
Asphyxia or hypoxic conditions were introduced between 20 and 30 min following bilateral TTX lesioning of the pre-Bötzinger complex. In the absence of respiratory activity (Fig. 7B) hypoxia and asphyxia still induced a tonic activation of the phrenic nerve, which in five out of six experiments was followed by a transient series of three to five gasp-like discharges (Fig. 7C). We never observed more than five gasps. This effect was reversible. After returning to control conditions for approximately 10 min, gasps could again be initiated by asphyxia or hypoxia. In contrast to the control, when the phrenic nerve was activated by a typical augmenting pattern during normal breathing (Fig. 7A), gasping was characterized by a very rapid activity onset (Fig. 7C). This is further demonstrated by averaging the shape of four integrated phrenic bursts during normal breathing before bilateral lesioning of the pre-Bötzinger complex (Fig. 7D), and during gasping following bilateral lesioning of the pre-Bötzinger complex (Fig. 7E).
Figure 7. Induction of gasping following bilateral lesioning of the pre-Bötzinger complex.
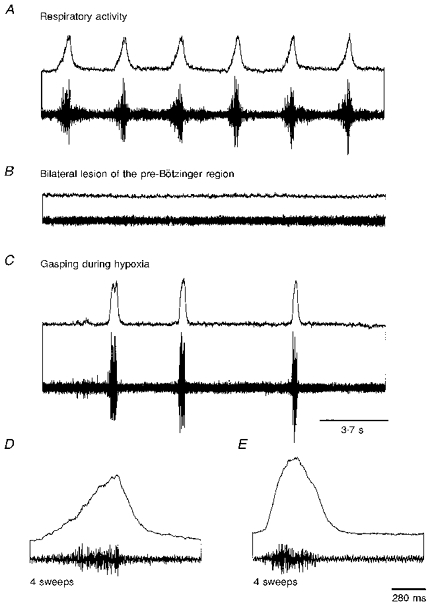
Respiratory activity (A) was eliminated following bilateral lesioning of the pre-Bötzinger complex with TTX (B). In the absence of respiratory activity, gasping was still elicited during hypoxia (C). The shape of the averaged phrenic nerve discharge as recorded during control conditions (D) was clearly different from the shape of phrenic nerve discharge (E) recorded during hypoxia in the absence of normal respiratory activity.
DISCUSSION
In this study we have demonstrated that the injection of the N-type calcium channel inhibitor ω-conotoxin GVIA, and the sodium channel inhibitor TTX, has dramatic effects on the centrally generated respiratory rhythm when injected locally into the pre-Bötzinger complex of adult cats. A sufficient dose of ω-conotoxin GVIA (ranging between 5 and 60 fmol) can immediately cause central apnoea for several seconds, as was manifest in the complete absence of respiratory rhythmic activity in the phrenic nerve. With an ω-conotoxin GVIA injection (5–60 fmol), the next bursts after toxin application had significantly decreased amplitudes, but the bursts returned to their normal amplitude some seconds after the ω-conotoxin GVIA injection. TTX injections also reduced the amplitude of phrenic nerve discharge. However, in contrast to ω-conotoxin GVIA, TTX affected respiratory activity irreversibly, which enabled us to permanently eliminate respiratory activity by lesioning both sides of the pre-Bötzinger complex. Respiratory activity did not recover even during asphyxia, which involves not only low oxygen but also high CO2 levels.
All of the injection loci from which respiratory rhythm was clearly affected are in the ventral medullary area, which corresponds to the pre-Bötzinger complex as first defined in neonatal rats (Smith et al. 1991). Bilateral TTX injections into this area resulting in irreversible apnoea indicate that the pre-Bötzinger complex is necessary for the generation of the respiratory rhythm. This result was not unexpected and further confirmed the finding obtained for rats in vitro (Smith et al. 1991; Funk et al. 1993). A detailed anatomical characterization of the pre-Bötzinger complex was obtained by Funk et al. (1993) using, in principle, a similar approach. In their study (Funk et al. 1993), injections of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 300–400 fmol) affected the respiratory motor output in a very limited area within the pre-Bötzinger complex and, as shown here, small antagonist injections were ineffective if injected only 200 μm away from the locus of the maximum response. In this in vitro study (Funk et al. 1993), unilateral lesions in the pre-Bötzinger complex affected the amplitude and decreased the frequency of the respiratory rhythmic motor output. This is consistent with our observations in the cat in vivo. As also described in the in vitro preparation (Funk et al. 1993), in the cat in vivo, the effect on the amplitude was more pronounced than the effect on the frequency of respiratory activity. Our injection sites were primarily localized in the dorsal zone of the pre-Bötzinger complex, which in vitro affected mainly the amplitude of the respiratory output (Funk et al. 1993). Thus, it is conceivable that the frequency effects would have been more pronounced if our injections were localized, on average, more ventrally in a zone which, under in vitro conditions, affected respiratory frequency more consistently (Funk et al. 1993). Although the map given in Fig. 4 coincides with the sites where most respiratory neurons were recorded under in vivo conditions (Schwarzacher et al. 1995) it cannot be excluded that the pre-Bötzinger complex extends more ventrally than suggested by our mapping study.
The finding that injections could become ineffective if injected only 200–300 μm away from the locus of the maximum response suggests a limited spread of the toxin. The fact that the ω-conotoxin GVIA toxin-induced apnoea had a very rapid onset and brief duration further indicates that the toxin effect must have been induced before significant diffusion would have occurred. As also discussed previously (McCrimmon, Feldman & Speck, 1986), the concentration of an injectate decreases very rapidly from the site of injection. Thus, the finding of a very localized effect was also theoretically not unexpected. The regions of the pre-Bötzinger complex established stereotactically clearly overlapped with the histologically determined regions as established by dye injection, but the area determined by dye injections was larger. Again this was not unexpected and could be explained by prolonged diffusion. The dye continued to diffuse even after fixation, while the stereotactic determinants depended on immediate toxin effects which occurred before significant diffusion could have occurred. In our experiments, the pipette used for injecting ω-conotoxin GVIA was removed and replaced with a pipette containing the dye. This could result in tissue distortion, which would affect the precision of our histologically determined area. However, possible distortions would have been already detectable in the stereotactically determined region of the pre-Bötzinger complex, and this was clearly not the case, since the map in Fig. 4 is based on multiple injections sites that were determined in different cats using numerous pipettes. If distortions had affected our results we would not see such a discrete and easily comparable localization of reactive sites obtained from different experiments.
The demonstration that respiratory activity can be eliminated by lesioning the pre-Bötzinger complex is consistent with other in vivo studies. The study by Koshyia & Guyenet (1996) examined the effect of respiratory activity on the sympathetic control system by eliminating respiratory activity with selective lesioning of the pre-Bötzinger complex. Although, the study by Fung et al. (1994) was focused on the localization of the gasping centre, this study also demonstrated that normal respiration is not an elaboration of gasping, but that it is possible to eliminate one rhythmic activity while maintaining the generation of the other activity. The elimination of gasping was achieved by lesions of the lateral tegmental field of the medulla, which is localized more medially and dorsally then the pre-Bötzinger complex (Fung et al. 1994).
In the study presented here, TTX was injected after precisely localizing the pre-Bötzinger complex with ω-conotoxin GVIA. In a wide variety of biochemical and physiological studies, it has been well established that ω-conotoxin GVIA is a high affinity antagonist of mostly presynaptic voltage-sensitive calcium channels, and therefore significantly affects synaptic transmission (Olivera et al. 1994). Therefore, contrary to TTX, ω-conotoxin GVIA does not abolish action potential conduction. In our ω-conotoxin GVIA experiments, pressure injection of this polypeptide ligand led to a highly localized and immediate effect on respiratory activity which reversed rapidly. The reversal was surprising, since electrophysiological experiments on neurons in regions other than the pre-Bötzinger complex indicate that ω-conotoxin GVIA is a very slowly dissociating antagonist, leading to a sustained block of synaptic transmission (Olivera et al. 1994). At least three explanations are conceivable. (1) It is known that in various neuronal systems ω-conotoxin GVIA does not block the entire pre-synaptic calcium current (Umemiya & Berger, 1994; Borst & Sakmann, 1996). Thus, we have probably only significantly disturbed, but not completely eliminated synaptic transmission. Therefore, the remaining synaptic interaction within this network could account for the reversibility of the ω-conotoxin GVIA effect. (2) The peptide could bind reversibly to N-type calcium channels of the pre-Bötzinger complex, but irreversibly in other regions of the nervous system. (3) The peptide could be endogenously inactivated. Given that these experiments were performed under in vivo conditions, it could well be that an inactivation is much faster than expected from an in vitro experiment.
The technique of blocking neuronal communication by drug injection is not new, and small molecule inhibitors such as kainic acid or, as in this study, TTX have frequently been used to study network properties in vivo (Coyle, Molliver & Kuhar, 1978; Berger & Cooney, 1982; Bianchi & Barillot, 1982; McCrimmon et al. 1986; Fung et al. 1994; Koshyia & Guyenet, 1996) and in vitro (Funk et al. 1993). Although under in vitro conditions, blockade of synaptic communication was reversible within approximately 5 min (Funk et al. 1993), even local injections of these drugs at a very specific locus often resulted in drastic changes in the motor output, which, under in vivo conditions, did not reverse within hours (see for example TTX effect in Fig. 6). Thus, as demonstrated in this study, mapping of a localized kernel would have been difficult using agents, such as TTX, which affected respiratory activity irreversibly and which presumably affected not only synaptic communication within the pre-Bötzinger complex, but also axons that are running through this area. Consequently, the TTX effects were also partly different to the effects evoked by ω-conotoxin GVIA alone. As in the case of TTX, long-lasting effects have been observed for kainic acid. Injected in femtomolar concentrations into the ventrolateral medulla, kainic acid specifically induced very localized, but often very long-lasting effects (McCrimmon et al. 1986). This is also consistent with our own experience when injecting other substances, such as strychnine, bicuculline, diltiazem, TEA or glutamate (authors’ unpublished observations). Thus, the use of ω-conotoxin GVIA has important advantages over previously used drugs, and the ω-conotoxin GVIA tracing method may, therefore, also be useful for studying other neuronal networks. In this study we have been able to show that a combination of electrophysiological recording with the anatomically precise application of the high affinity polypeptide ligand ω-conotoxin GVIA can be used to identify localized neuronal regions important for the function of a particular neuronal circuit. In combination with another toxin (TTX) this map can then be used to irreversibly lesion this area. However, it must be emphasized that the combination of these different toxins needs to be evaluated carefully. In this study, the irreversible cessation of respiratory activity could be due to the fact that TTX affected fibres en route from areas distant to the sites determined by the ω-conotoxin GVIA. However, given that ω-conotoxin GVIA could also induce, at least transiently, apneusis indicates that the cessation of respiratory rhythmic activity was not only due to areas distant from the pre-Bötzinger complex, but also to the essential role of the pre-Bötzinger complex in respiratory control. Whether, in fact, the resulting cessation of respiratory activity is indicative of a perturbation of the respiratory rhythm-generating mechanism is not conclusive in itself. A cessation of respiratory rhythmic activity, a decrease in the amplitude of phrenic nerve activity or a decrease in the frequency of rhythmic activity could also be due to an elimination of a tonic excitatory drive to the pre-Bötzinger complex. The most obvious effects on rhythm-generating mechanisms were observed in this study during bilateral lesioning of the pre-Bötzinger complex. ω-Conotoxin GVIA injected into one pre-Bötzinger complex evoked an irregular rhythmic activity when the other pre-Bötzinger complex was already lesioned (Fig. 6B). The TTX effect on rhythmicity was even more dramatic (Fig. 6C), which, as discussed above, could be due to additional effects from fibres en passant. Thus, unilateral lesions of the pre-Bötzinger complex do not sufficiently disturb the respiratory rhythm itself, suggesting that one pre-Bötzinger complex is sufficient for the generation of the respiratory rhythm.
This finding indicates that very close interactions exist between the left and right pre-Bötzinger complexes. It has been demonstrated for various mammals (e.g. monkeys and rabbits) that the left and right central medullary respiratory centres can still function when separated by mechanical lesioning along the mid-line of the brainstem (Gromysz & Karczewski, 1981, 1982). Such experiments were also performed in cats, but desynchronization was not possible (Eldridge & Paydarfar, 1989). Consistent with a strong bilateral coupling are anatomical tracing studies indicating that the pre-Bötzinger complex is an area in the ventrolateral medulla where extensive contralaterally projecting neurons are found (Ellenberger & Feldman, 1990; Smith et al. 1991). In our experiments, brief unilateral toxin injections either with ω-conotoxin GVIA or TTX resulted in bilateral effects. These bilateral effects were not due to the toxins diffusing to the contralateral side. (1) ω-Conotoxin GVIA injections that were only a few hundred micrometres away from the pre-Bötzinger complex did not evoke effects on the respiratory motor pattern. (2) Injections of the dye Pontamine Sky Blue did not cross the mid-line. Thus, the bilateral effects induced by unilateral toxin injections indicate that under in vivo conditions the networks located within the left and right pre-Bötzinger complexes are coupled by strong neuronal interactions. This was further indicated by the finding that the pattern of respiratory activity was severely affected following unilateral knockout of the pre-Bötzinger complex with TTX. A similar result was obtained under in vitro conditions. Funk et al. (1993) demonstrated that unilateral injections into the pre-Bötzinger complex had bilateral effects on the respiratory motor output. Accordingly, in current models of rhythm generation it has been proposed that bilateral excitatory synaptic coupling is necessary for bilateral synchronization of rhythmic activity (Smith et al. 1995).
Possibly the most important finding of this study was the demonstration that respiration and gasping are generated by different neuronal networks, since the respiratory network can be removed pharmacologically under in vivo conditions without also inactivating the gasping centre. This finding confirms previous experiments which have indicated that gasping can be selectively eliminated in a medullary area other than the pre-Bötzinger complex (Fung et al. 1995). This more anatomically oriented study, together with physiological evidence demonstrating that the motor pattern and the response properties to hypoxia are clearly different for respiration and gasping, all confirm the existence of two different neuronal networks, as has previously been postulated (St John, 1990). These two neuronal networks seem to be closely localized within the ventrolateral medulla.
Acknowledgments
We thank Mrs G. Thiele for expert assistance with the histology, and Mrs U. de Buhr and Mr W. Ochotzki for their help with the figures. Support by the DFG, SFB, NIH and NATO is greatly appreciated.
References
- Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. Journal of Applied Physiology. 1982;52:31–140. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Barillot JC. Respiratory neurons in the region of the retrofacial nucleus: Pontile, medullary, spinal and vagal projections. Neuroscience Letters. 1982;31:277–282. doi: 10.1016/0304-3940(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Molliver ME, Kuhar MJ. In situ injection of kainic acid: a new method for selectively lesioning neuronal cell bodies while sparing axons of passage. Journal of Comparative Neurology. 1978;180:301–324. doi: 10.1002/cne.901800208. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Paydarfar D. Desynchronized respiratory rhythms and their interactions in cats with split brain stems. Journal of Physiology. 1989;410:513–532. doi: 10.1113/jphysiol.1989.sp017547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Subnuclear organization of the lateral tegmental field of the rat. I: Nucleus ambiguus and ventral respiratory group. Journal of Comparative Neurology. 1990;294:202–211. doi: 10.1002/cne.902940205. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Progress in Neurobiology. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. 10.1016/0301-0082(90)90030-K. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Handbook of Physiology, section 1 The Nervous System, Intrinsic Regulatory Systems of the Brain. IV. Washington DC: American Physiological Society; 1986. Neurophysiology of breathing in mammals; pp. 463–454. [Google Scholar]
- Fung M-L, Wang W, St John WM. Medullary loci critical for expression of gasping in adult rats. Journal of Physiology. 1994;480:597–611. doi: 10.1113/jphysiol.1994.sp020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: Role of excitatory amino acids. Journal of Neurophysiology. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Gromysz H, Karczewski WA. Respiratory activity generated by a split-brain stem preparation of the rabbit. Acta Neurobiologica Experimentalis. 1981;41:237–242. [PubMed] [Google Scholar]
- Gromysz H, Karczewski WA. Phrenic motoneurone activity in split-brain stem cats and monkeys. Respiration Physiology. 1982;50:51–61. doi: 10.1016/0034-5687(82)90006-8. 10.1016/0034-5687(82)90006-8. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. Journal of Physiology. 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Feldman JL, Speck DF. Respiratory motoneuronal activity is altered by picomole injections of glutamate in the cat brainstem. Journal of Neuroscience. 1986;6:2384–2392. doi: 10.1523/JNEUROSCI.06-08-02384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM, McIntosh JM, Cruz LJ, Luque FA, Gray WR. Purification and sequence of a presynaptic peptide from C. geographus venom. Biochemistry. 1984;23:5087–6090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Miljanich G, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: The ω-conotoxins and ω-agatoxins. Annual Review of Biochemistry. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Current Opinion in Neurobiology. 1996;6:817–825. doi: 10.1016/s0959-4388(96)80033-x. 10.1016/S0959-4388(96)80033-X. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballanyi K, Ramirez JM. Respiratory rhythm generation. In: Miller AD, Bianchi AD, Bishop BP, editors. Neural Control of the Respiratory Muscles. Boca Raton, FL, USA: CRC Press; 1996. pp. 119–131. [Google Scholar]
- St John WM. Neurogenesis, control and functional significance of gasping. Journal of Applied Physiology. 1990;68:1305–1315. doi: 10.1152/jappl.1990.68.4.1305. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. The pre-Bötzinger complex in the cat. Journal of Neurophysiology. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger H, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Funk GD, Johnson SM, Feldman JL. Cellular and synaptic mechanisms generating respiratory rhythm: insights from in vitro and computational studies. In: Trouth CO, Millis R, Kiwull-Schone H, Schlaefke M, editors. Ventral Brainstem Mechanisms and Control of Respiration and Blood Pressure. New York: Marcel Dekker; 1995. pp. 463–496. [Google Scholar]
- Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends in Neurosciences. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Activation of A1 and A2 receptors differentially modulates calcium channels and glycinergic transmission in rat. Neuron. 1994;13:1443–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- von Euler C. Handbook of Physiology, section 2 The Respiratory System. Washington DC: American Physiological Society; 1986. Brain stem mechanisms for generation and control of breathing pattern; pp. 1–67. [Google Scholar]


