Abstract
The biogenesis of secretory IgM occurs stepwise under stringent quality control, formation of μ2L2 preceding polymerization. How is efficiency of IgM secretion coupled to fidelity? We show here that ERp44, a soluble protein involved in thiol-mediated retention, interacts with ERGIC-53. Binding to this hexameric lectin contributes to ERp44 localization in the ER-golgi intermediate compartment. ERp44 and ERGIC-53 increase during B-lymphocyte differentiation, concomitantly with the onset of IgM polymerization. Both preferentially bind μ2L2 and higher order intermediates. Their overexpression or silencing in non-lymphoid cells promotes or decreases secretion of IgM polymers, respectively. In IgM-secreting B-lymphoma cells, μ chains interact first with BiP and later with ERp44 and ERGIC-53. Our findings suggest that ERGIC-53 provides a platform that receives μ2L2 subunits from the BiP-dependent checkpoint, assisting polymerization. In this process, ERp44 couples thiol-dependent assembly and quality control.
Keywords: endoplasmic reticulum, ERGIC, IgM, protein folding, quality control
Introduction
Secreted and membrane proteins are translocated into the endoplasmic reticulum (ER), where they fold and undergo several modifications (disulfide bond formation, N-glycan processing and subunit assembly) whose execution must be properly coordinated and timed. Quality control (QC) mechanisms are active at the ER–Golgi interface to ensure that non-native molecules are retained in, or retrieved to, the ER, and eventually dispatched to the cytosol for proteasomal degradation (Ellgaard and Helenius, 2003; Sitia and Braakman, 2003). QC recognizes different features, common to unfolded or not completely assembled molecules, such as immature N-glycans (Helenius and Aebi, 2004), hydrophobic patches (Blond-Elguindi et al, 1993) or exposed reactive cysteines (thiol-mediated retention; Sitia et al, 1990; Fra et al, 1993; Reddy et al, 1996). We first isolated ERp44 for its ability of covalently binding the oxidases Ero1α and Ero1β (Anelli et al, 2002), thus determining their intracellular localization (Otsu et al, 2006). ERp44 plays a crucial role in QC, mediating via its Cys29 (Anelli et al, 2003), the thiol-dependent retention of immunoglobulin (Ig) subunits (e.g., μ, L and J chains) and adiponectin (Wang et al, 2007). ERp44 also regulates the activity of the calcium channel IP3R1 (Higo et al, 2005), emerging as a possible integrator of redox and calcium homeostasis.
In addition to bulk flow (Wieland et al, 1987), the exit of proteins from the ER is regulated by intracellular lectins, which concentrate certain glycoproteins in forward transport vesicles (Hauri et al, 2002). Among these, ERGIC-53 is a hexameric transmembrane protein of the ER–Golgi intermediate compartment (ERGIC) (Schindler et al, 1993), which binds glycoproteins in the ER, transporting and releasing them in the Golgi (Hauri et al, 2002). ERGIC-53 shuttles between the ER and the Golgi because it can bind both COPI and COPII through its KKFF cytosolic terminus (Schindler et al, 1993; Vollenweider et al, 1998). Mutations in ERGIC-53 (also known as LMAN1) are responsible for most cases of combined deficiency of coagulation factor V and VIII (F5F8D), a recessive bleeding disorder caused by decreased blood levels of both clotting factors (Nichols et al, 1998; Neerman-Arbez et al, 1999). This implies a role for ERGIC-53 in factors V and VIII ER–Golgi transport (Vollenweider et al, 1998). Other F5F8D cases are linked to mutations in MCFD2 (Zhang et al, 2003), a small soluble protein mediating ERGIC-53 binding to factor VIII (Zhang et al, 2005). We recently demonstrated that ERGIC-53 binds mutant Ig-μ chains lacking the BiP-binding first constant domain (μΔCH1) (Mattioli et al, 2006). This interaction favors formation of detergent-insoluble μΔCH1 aggregates, an event that requires Cys575-dependent polymerization (Valetti et al, 1991).
IgM polymers are planar, multimeric proteins. They consist of 21 or 24 subunits, depending on whether they are secreted as J-chain containing ‘pentamers' ((μ2L2)5-J) or ‘hexamers' ((μ2L2)6) (where μ and L stand for IgM heavy and light chains, respectively). In secreted polymers, individual μ2L2 subunits (often called ‘monomers' in the immunological jargon) are assembled via disulfide bonds involving Cys575 in the C-terminal tailpiece of secretory μ (μs) chains (Davis et al, 1989b; Sitia et al, 1990). Cys575 acts also as a retention and degradation signal for unpolymerized secretory IgM (thiol-mediated retention; Fra et al, 1993; Reddy et al, 1996). In membrane μ (μm) chains, this 20-residue tailpiece is replaced by a longer hydrophobic segment, essential for membrane insertion and assembly with B-cell receptor signaling components.
The first assembly step (μ2L2 formation), common to both membrane and secreted IgM, is fast and efficient in both B and plasma cells (PCs): its fidelity is checked by BiP (see Hendershot and Sitia, 2005 and references therein). In contrast, IgM polymerization is slow and occurs only in PCs. As a result, most μs chains are degraded by proteasomes in B lymphocytes (Shachar et al, 1992), whereas μm chains that negotiated assembly into functional B-cell receptors are transported to the cell surface (Sitia et al, 1987; Hombach et al, 1988).
Therefore, secretory IgM biogenesis occurs in at least two sequential and independently regulated steps, μ-L assembly preceding polymerization (Hendershot and Sitia, 2005). We asked whether these two steps are executed in different subregions of the early secretory pathway, so as to optimize and couple assembly, QC and transport. We show that PDI and ERp44, two soluble proteins endowed with ER-localization motifs, are differentially distributed. Although PDI is localized in the ER, ERp44 accumulates in ERGIC and cis-Golgi vesicles. ERp44 and ERGIC-53 interact with each other and, together with MCFD2, are induced during B to PC differentiation. Both ERp44 and ERGIC-53 bind μ chains, preferentially if assembled with L chains. Their overexpression and silencing stimulate and decrease IgM polymerization in non-lymphoid cells, respectively. We propose that hexameric ERGIC-53 provides, with the help of MCFD2 and ERp44, a platform for IgM polymerization, receiving μ2L2 subunits that have already passed the BiP-dependent checkpoint. By spatially segregating the sequential execution of μ2L2 assembly and polymerization, IgM-secreting cells can couple assembly, transport and QC, ensuring efficiency and fidelity in the protein factory.
Results
Endogenous ERp44 is enriched in the ERGIC and extends to cis-Golgi vesicles
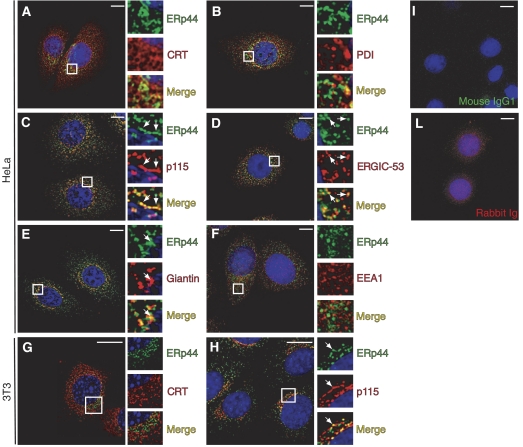
Using specific monoclonal antibodies, we found that only a small fraction of endogenous ERp44 co-distributes with the ER markers CRT and PDI (Figure 1A and B). Most of it accumulated distally, yielding intense co-staining with p115 and ERGIC-53 (panels C and D), and to a lesser extent with giantin (panel E). No colocalization at all was observed with EEA1, a marker of early endosomes, confirming the specificity of the immunofluorescence assays (panel F). Therefore, endogenous ERp44 is localized primarily in the ERGIC and cis-Golgi, and only to a minor extent in the ER of human HeLa, murine 3T3 (Figure 1G and H; Supplementary Figure S1) and primary B cells (Figure 7D).
Figure 1.
Endogenous ERp44 is primarily localized in the ERGIC. HeLa cells were co-stained with antibodies specific for ERp44 and markers of the ER (CRT, PDI), ERGIC (ERGIC-53, p115), Golgi (Giantin) and early endosomes (EEA1), as indicated (A–F). (G, H) 3T3 cells co-stained with antibodies specific for ERp44 and CRT or p115, respectively. Images were taken with a fluorescence microscope and analyzed with deconvolution techniques. For each panel, boxes show a higher magnification of the indicated area. Arrows indicate examples of colocalizing structures. Controls of HeLa cells stained with an unrelated mouse IgG1 antibody and a rabbit serum are shown (I, L, respectively). Endogenous ERp44 colocalizes mainly with ERGIC-53 and p115, although some overlapping signal can be observed also with CRT, PDI and giantin. No colocalization at all is observed with the early endosome marker EEA1. Bar=10 μm.
Figure 7.
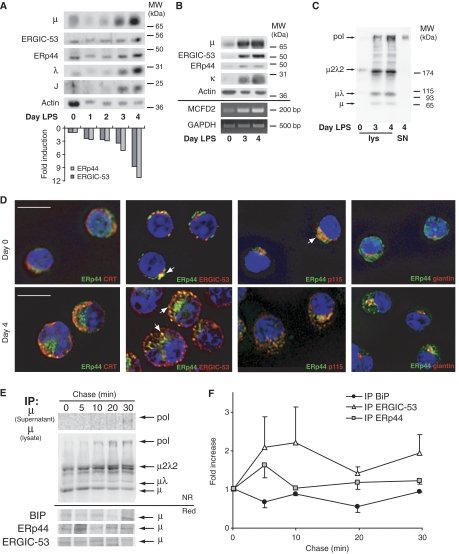
Localization and role of ERp44 and ERGIC-53 in IgM-secreting B cells. (A–C) Coordinated expression of ERp44 and ERGIC-53 in differentiating B cells, concomitant with the onset of IgM polymerization. I.29μ+ B-lymphoma cells (A) or primary murine splenocytes (B, C) were stimulated in vitro with LPS to induce plasmacytic differentiation. At the indicated days, aliquots were lysed and resolved electrophoretically under reducing (A, B) or NR (C) conditions. An aliquot of the secreted material at day 4 was loaded under NR conditions as a marker of polymers (SN, last lane on the right, panel C). Densitometric quantifications (A, lower panel) were normalized relative to the actin signal. Aliquots of primary B cells at days 0, 3 and 4 of differentiation were also subjected to RNA extraction, RT and PCR to amplify MCFD2 mRNA. GAPDH was used as a control for normalization. Note that ERGIC-53, ERp44 and MCFD2 are simultaneously upregulated during the last stages of B-cell differentiation, concomitantly with J-chain induction and the onset of IgM polymerization. (D) Murine splenocytes at days 0 and 4 of LPS stimulation were stained with the indicated antibodies; images were taken with a fluorescence microscope and analyzed with deconvolution techniques. Arrows indicate examples of colocalizing structures. Not only in B cells (day 0) but also in PCs (day 4 after LPS), ERp44 is located in the ERGIC compartment, showing intense colocalization with ERGIC-53 and p115. Bar=10 μm. (E, F) Dynamic interactions of nascent μ chains with BiP, ERp44 and ERGIC-53. Ramos cells were pulsed for 5 min with 35S-labeled methionine and cysteine, and chased for the indicated times. Culture SN and cell lys were IP with different antibodies as indicated on the left, and then resolved by SDS–PAGE under NR or reducing (red) conditions (E). After transfer to nitrocellulose, blots were subjected to autoradiography. Filters were then decorated with anti-μ antibodies, to verify the identity of the band co-IP with the different interactors (data not shown). Soon after the 5-min pulse, μ, μλ and μ2λ2 are already detectable in the lys. Polymers appear later, being easily detectable after 10 min of chase in the lys, and after 20–30 min in the SN. Densitometric quantifications of radioactive μ chains co-IP with BiP, ERp44 and ERGIC-53 were performed on reduced blots. Signals were normalized relative to a stable background, and data shown as ratio to the signal obtained at time 0 (average of three independent experiments, ±s.e.m.) (F). The level of labeled μ co-IP with BiP decreases immediately after the pulse and is reduced to one-half within 20 min. On the contrary, ERp44- and ERGIC-53-associated μ chains peak at the first chase points, just before IgM polymerization occurs.
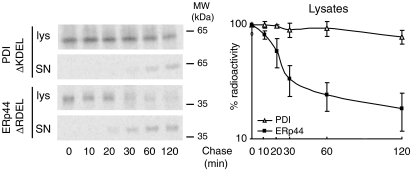
What mechanisms could localize ERp44 distally with respect to PDI? The proposal that soluble ER-resident proteins form a functional matrix that could restrict their exit to the Golgi (Munro and Pelham, 1986; Reddy et al, 1996), has been supported by the identification of complexes containing BiP, grp94 and PDI (Meunier et al, 2002). If the more distal localization of ERp44 reflected a lower affinity for such ER complexes, removal of the ER localization signal should allow ERp44 to be secreted more easily than other resident proteins. Accordingly, although the majority of PDIΔKDEL was still retained intracellularly within 2 h of chase, most labeled ERp44ΔRDEL was in the culture supernatant (Figure 2). The loss of some radioactive signal during the chase may reflect degradation, inaccessibility or post-translational cleavage of the N-terminal HA tag in late compartments of the exocytic route. In accord with its downstream localization, ERp44 could reach the extracellular space more rapidly than PDI. On the other hand, the observation that overexpressed ERp44 accumulates in the ER (Anelli et al, 2002) independently from the presence of an N-terminal tag (Supplementary Figure S1) suggests saturation of forward transport system(s).
Figure 2.
ERp44ΔRDEL is secreted more rapidly than PDIΔKDEL. HeLa cells transiently co-transfected with myc-PDIΔKDEL or HA-ERp44ΔRDEL were pulsed for 10 min with 35S-labeled methionine and cysteine and chased for the indicated times. Culture supernatants (SN) and cell lysates (lys) were IP with anti-myc or anti-HA (to isolate PDI and ERp44, respectively), resolved by reducing SDS–PAGE and subjected to autoradiography. Densitometric quantification of the disappearance of the two proteins from the lysates is shown on the right (level of radioactive signal present at each time chase with respect to time 0; average of two independent experiments quantified in duplicate, ±s.e.m.). Note that at the end of the chase almost all ERp44 is secreted, whereas a considerable amount of PDI is still present intracellularly.
ERp44 interacts with ERGIC-53
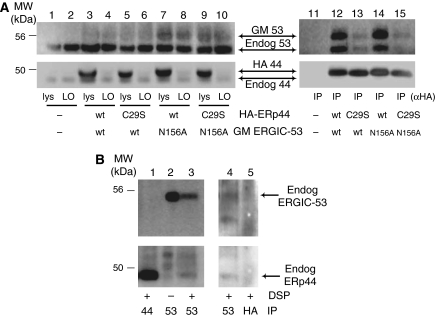
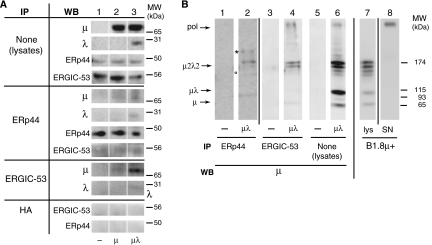
ERp44 localization could also reflect interactions with molecules favoring ER exit. Because both ERp44 and ERGIC-53 bind μΔCH1 chains (Anelli et al, 2003; Mattioli et al, 2006) and colocalize (Figure 1), we hypothesized that ERp44 interacts with ERGIC-53. HeLa cells were thus co-transfected with HA-ERp44 and a glycosylated myc-tagged ERGIC-53 (GM ERGIC-53; Figure 3A), and crosslinked with DSP. Anti-HA antibodies efficiently and specifically immunoprecipitated (IP) exogenous ERp44 (compare whole lysates (lys) and left overs (LO), lanes 1–10). Not only GM ERGIC-53 but also endogenous ERGIC-53 (recognizable by its higher mobility) was co-IP with ERp44 (lane 12). The absence of signal in mock-transfected cells (lane 11) confirmed the specificity of the observed co-immunoprecipitation (see also Figure 6 below for further controls). To investigate whether the interactions between ERp44 and ERGIC-53 depend on, or are stabilized by, cargo molecules, we analyzed the active site mutants HA-ERp44 C29S (Anelli et al, 2003) and GM ERGIC-53 N156A (Itin et al, 1996). The N156A mutation had only a slight effect on the interaction (lane 14), possibly because inactive molecules can form hetero-hexamers with wild-type (wt) endogenous ERGIC-53. In contrast, replacing Cys29 in ERp44 drastically decreased the levels of co-immunoprecipitation (lane 13). Nonetheless, some ERGIC-53 N156A molecules were co-IP with ERp44 C29S (lane 15), suggesting that the two proteins can establish direct interactions. The detection of endogenous ERp44–ERGIC-53 complexes (Figure 3B, lane 3) excluded that the observed interactions were due to overexpression or ectopic localization. Altogether, these findings indicated that ERp44 and ERGIC-53 interact with each other, and that the interaction could be stabilized by binding to common substrate(s).
Figure 3.
ERp44 interacts with ERGIC-53. (A) After crosslinking with DSP, aliquots (100 μg of protein) of the RIPA lysates from HeLa transfectants expressing various combinations of wt or mutant HA-ERp44 and GM ERGIC-53 as indicated were IP with anti-HA antibodies and resolved by reducing SDS–PAGE (lanes 11–15). Smaller aliquots of the lysates (30 μg), before (lys) and after IP (left overs (LO), lanes 1–10), were loaded to verify the levels of expression and immunoprecipitation efficiency. After transferring to nitrocellulose, blots were decorated with polyclonal anti-ERGIC-53 (upper panels) and anti-ERp44 (lower panels). Both antibodies recognize doublets: the upper band corresponds to the tagged exogenous molecules, and the faster migrating one to the endogenous ones. The diffuse appearance of exogenous GM ERGIC-53 is due to the presence of N-glycans. Note that anti-HA antibodies efficiently precipitate exogenous, but not endogenous ERp44 molecules (lanes 3–10). In cells overexpressing wt ERp44 (lanes 12 and 14), abundant exogenous and endogenous ERGIC-53 co-IP with ERp44. Co-immunoprecipitation is less efficient in cells expressing the inactive ERp44 C29S mutant (lanes 13 and 15). In contrast, mutation in ERGIC-53 active site (N156A) has only minor effects (lane 14). When inactive mutants of both ERp44 and ERGIC-53 (C29S and N156A, respectively) are coexpressed, some ERGIC-53 can still be co-IP with ERp44, suggesting the two molecules can directly, but transiently, interact. ERGIC-53 did not co-immunoprecipitate in non-transfected cells (lane 11). (B) Endogenous ERp44 and ERGIC-53 non-covalently interact. HeLa cells (300 μg), treated with or without DSP as indicated and lysed in RIPA buffer supplemented with 2 mM Ca2+, were IP with anti-ERGIC-53 (lanes 2 and 3), or anti-ERp44 monoclonal antibodies (lane 1), to confirm the identity of the bands co-IP by anti-ERGIC-53, and resolved by reducing SDS–PAGE. The nitrocellulose was sequentially decorated with rabbit anti-ERGIC-53 (upper panel) and anti-ERp44 antibodies (lower panel), as indicated. Endogenous ERp44 can be co-IP with endogenous ERGIC-53, the association being more evident after crosslinking. In a similar, independent experiment (right panel), anti-HA (lane 5) was used in parallel with anti-ERGIC-53 (lane 4), as a further specificity control for the ERp44 co-IP with anti-ERGIC-53. The blot was first probed with anti-ERp44 and then with anti-ERGIC-53. In this experiment, the ERp44-specific monoclonal antibody did not co-immunoprecipitate detectable ERGIC-53, possibly because of its lower efficiency in immunoprecipitation.
Figure 6.
ERp44 and ERGIC-53 preferentially interact with μ2λ2 or higher order assemblies. HeLa cells were transiently transfected with vectors driving the expression of μ chains, alone or in combination with λ, or empty vector as control. (A) Aliquots from lys (300 μg) were IP with anti-ERp44, anti-ERGIC-53 or anti-HA as a control, and resolved under reducing conditions. Aliquots (30 μg) of the lys before IP were analyzed to verify the levels of expression of the relevant proteins (upper four panels). After transfer to nitrocellulose, blots were decorated with the indicated antibodies (WB). Note that more μ can be co-IP with ERp44 and ERGIC-53 when λ chain is also present. (B) The anti-ERp44 (lanes 1 and 2) and anti-ERGIC-53 (lanes 3 and 4) immunoprecipitations obtained from HeLa cells transfected with or without μ+λ as described above, were resolved under non-reducing (NR) conditions and blots decorated with anti-μ antibodies. Aliquots (15 μg) from the corresponding lysates were also analyzed under NR conditions (lanes 5 and 6). All the lanes come from two twin gels run in parallel. Lysates and SN of B1.8μ+ hybridoma cells (lanes 7 and 8) are shown for comparison. The migration of polymers and other IgM assembly intermediates is indicated on the left-hand margin. Lanes are separated by white or black lines, as coming from the same or twin gels, respectively. The asterisk (*) and the circle (°) point to bands containing both μ and ERp44, and likely consisting of ERp44-μ2λ2 and ERp44-μλ mixed disulfides, respectively (see Supplementary Figure S6).
ERGIC-53 modulates ERp44 localization in the early secretory pathway
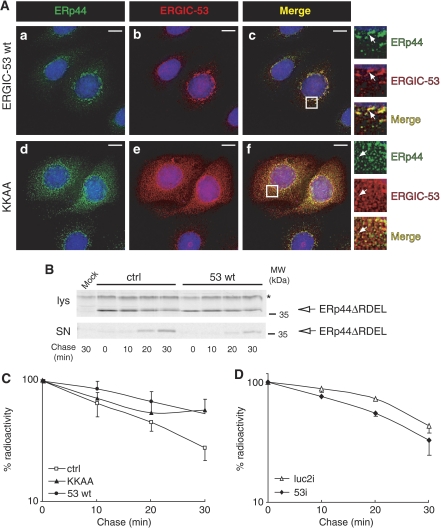
To demonstrate an active role of ERGIC-53 in determining ERp44 localization, we used an ERGIC-53 mutant (KKAA) that—owing to the replacement of the phenylalanines mediating COPII binding—is mainly localized in the ER. The KKAA mutant also recruits endogenous ERGIC-53, as well as its partner MCFD2, into the ER (Vollenweider et al, 1998; Nyfeler et al, 2006). If indeed ERp44 localization depended on interactions with ERGIC-53, endogenous ERp44 molecules would be enriched in the ER in the presence of KKAA. Accordingly, overexpression of ERGIC-53 KKAA by transient transfection (Figure 4A, panels d–f) or by tetracycline removal in a stable inducible cell line (Supplementary Figure S2, panel b) redistributed endogenous ERp44 in the ER, as indicated by its more diffuse pattern and its higher colocalization with CRT.
Figure 4.
ERGIC-53 mediates ERp44 localization in the early secretory pathway. (A) HeLa cells transiently transfected with vectors driving the expression of wt ERGIC-53 (a–c) or the ER-localized mutant ERGIC-53 KKAA (d–f) were co-stained with antibodies specific to ERp44 and ERGIC-53. Images were taken with a fluorescence microscope and analyzed with deconvolution techniques. In each panel, boxes show a higher magnification of the indicated areas. Arrows indicate examples of colocalizing structures. Mutant ERGIC-53 KKAA acts as a localization dominant negative and causes the ER accumulation of endogenous ERGIC-53 molecules as well (Vollenweider et al, 1998 and data not shown); note that endogenous ERp44 is in part delocalized with the mutant lectin, as demonstrated by the diffuse pattern shown in panel d and by the colocalization of the two molecules (f). However, some ERp44 is able to reach the ERGIC region (not shown). Bar=10 μm. (B, C) HeLa cells were transiently transfected with HA-ERp44ΔRDEL alone (ctrl, empty square) or with wt ERGIC-53 (53 wt, filled circles) or the ER-localized KKAA mutant (KKAA, filled triangles). Forty-eight hours after transfection, cells were pulse-labeled and chased for the indicated times as described in legend to Figure 2. Cell lys and SN were IP with anti-HA (to isolate ERp44), resolved by reducing SDS–PAGE and subjected to autoradiography (B). Panel C shows densitometric analyses of lys, performed as in legend to Figure 2 (average of four independent experiments, ±s.e.m.); signals were normalized relative to a stable background (see * in panel B). The overexpression of either wt or KKAA ERGIC-53 inhibits ERp44ΔRDEL secretion. (D) HeLa cells were subjected to RNAi for ERGIC-53 (53i, filled diamonds) or treated with control duplexes (luc2i, empty triangles), and then transiently transfected with HA-ERp44ΔRDEL, as indicated. Seventy-two hours from RNAi (48 h from transfection), cells were pulse-labeled, chased and analyzed as described for Figure 2. In the absence of ERGIC-53, ERp44ΔRDEL is secreted more rapidly (average of three independent experiments, ±s.e.m.). As a control of the efficiency of RNAi, see Figure 5B.
These findings suggested that ERGIC-53 can bind ERp44 and dictate its subcellular localization. Accordingly, ERGIC-53 silencing delocalized ERp44 in the ER (Supplementary Figure S3). Moreover, ERp44ΔRDEL remains longer intracellularly when ERGIC-53 (wt or the mutant KKAA) is overexpressed (Figure 4B and C); opposite effects are induced by ERGIC-53 silencing (Figure 4D). These effects were not due to stress induced by silencing or transfection, as no UPR activation was evident (Supplementary Figure S4).
ERp44 and ERGIC-53 stimulate IgM polymerization
Because ERGIC-53 forms hexamers (Neve et al, 2005; Nyfeler et al, 2006) and interacts with μΔCH1 (Mattioli et al, 2006), we reasoned that ERGIC-53 could promote IgM polymerization by concentrating μ2L2 subunits and possibly arranging them in a suitable orientation on the plane of the membrane. ERp44 could help this process as part of the polymerization complex, or indirectly by retaining unassembled subunits. To test this model, we tried to reconstitute the ‘polymerization machinery' in HeLa cells that are incompetent in IgM polymerization. Cells were transiently transfected with Ig μs and λ chains, alone or in various combinations of ERp44 and/or wt or mutant ERGIC-53. Secreted glycoproteins were concentrated by ConA precipitation, resolved on non-reducing SDS–PAGE and immunoblotted with anti-μ, to analyze secretion and polymerization efficiency. The latter was expressed as the percentage of secreted polymers with respect to total anti-μ-reactive species present in the supernatants. LDH release was monitored to exclude cell death or artifactual IgM release (Supplementary Figure S5).
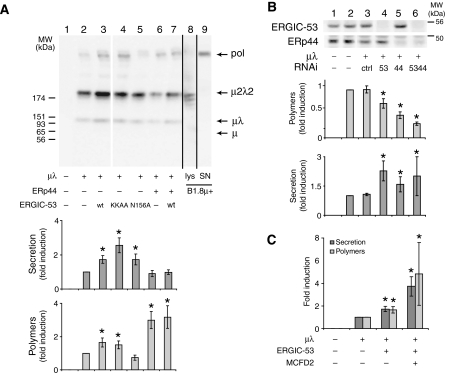
Owing to weak thiol-dependent retention (Anelli et al, 2003; Otsu et al, 2006), HeLa cells mainly secreted μ2λ2 subunits: μλ and polymers were released in smaller amounts (Figure 5A, lane 2). The overexpression of active ERGIC-53 (wt or KKAA mutant) significantly increased both secretion and polymerization (lanes 3 and 4). In contrast, the inactive N156A mutant stimulated secretion but inhibited polymer formation (lane 5). In agreement with its role in thiol-mediated retention, ERp44 overexpression (alone or in combination with ERGIC-53 wt, lanes 6 and 7, respectively) inhibited μ2λ2 secretion and increased polymerization, without affecting the overall secretion. Conversely, silencing either ERGIC-53 or ERp44 (Figure 5B, lanes 4 and 5) inhibited IgM polymerization, the effects being maximal when both proteins were downregulated (lane 6). Coexpression of MCFD2—a soluble protein necessary for ERGIC-53 interaction with factors V and VIII (Zhang et al, 2003)—further increased the promoting activity of ERGIC-53 on IgM polymerization (panel C). These results indicated that ERGIC-53, MCFD2 and ERp44 promote IgM polymerization in HeLa cells.
Figure 5.
ERp44, ERGIC-53 and MCFD2 modulate IgM polymerization in HeLa cells. (A) ERp44 and ERGIC-53 overexpression promotes IgM polymerization in HeLa cells. HeLa cells transiently transfected with various combinations of vectors driving the expression of μ, λ, ERp44, ERGIC-53 (wt or mutants) as indicated, were washed and cultured for 4 h. Aliquots of the lysates were analyzed by Western blotting to monitor the expression levels of the different transgenes (data not shown). Secreted IgM were concentrated with ConA sepharose, resolved under non-reducing conditions, blotted and decorated with anti-μ antibodies (lanes 1–7). A white line separates lanes 3 and 4, which were juxtaposed from the same gel. Lanes 8 and 9 (coming from other gels run in parallel) show the lysates and supernatants from an IgM-producing murine hybridoma (B1.8 μ+). Arrows point to IgM polymers (pol), μ2λ2 and μλ subunits, and free μ. Lower panels show the quantification of total and polymeric secreted IgM. Data are expressed as fold increase with respect to cells expressing μ and λ alone. Total IgM secretion was calculated as the amount of IgM secreted in 4 h relative to the intracellular pool in each transfectant. The efficiency of polymerization was calculated as the percentage of polymers with respect to the total signal obtained with anti-μ in secreted material. Average of five independent experiments like the one shown in the top panel, ±s.e.m.; *P<0.05 t-test. Note that ERp44 and ERGIC-53 overexpression (both wt and KKAA mutant) increases IgM polymerization. (B) ERp44 and ERGIC-53 silencing inhibits IgM polymerization in HeLa cells. RNAi was performed in HeLa cells with control duplexes (ctrl) or duplexes specific to ERp44 or/and ERGIC-53, as indicated. The day after, cells were transfected with plasmids driving the expression of μ and λ, or with empty vector as a control. Seventy-two hours from RNAi, cells were washed and cultured for 4 h. To check for the efficiency of ERp44 and ERGIC-53 silencing, 15 μg of proteins per lane were loaded on reducing SDS–PAGE, and nitrocellulose was then probed with antibodies against endogenous ERp44 and ERGIC-53, as indicated. After 72 h of silencing, about 15% of ERGIC-53 and 25% of ERp44 were detectable in the lys (lanes 4–6). Secreted IgM were treated as described in panel A and WB data analyzed as described above. The lower panels show quantification of total and polymeric IgM secreted by HeLa cells treated as indicated. Data are expressed as fold increase or decrease with respect to cells expressing μ and λ, and not subjected to silencing. Both ERp44 and ERGIC-53 silencing inhibit IgM polymerization (lanes 4 and 5), their effects being additive (lane 6). Average of three independent experiments, ±s.e.m.; *P<0.05 t-test. (C) MCFD2 synergizes with ERGIC-53 in promoting IgM polymerization and secretion. HeLa cells expressing μ, λ and ERGIC-53 were transiently transfected with or without MCFD2 as indicated and processed as described for panel A. Average of two independent experiments, quantified in duplicate ±s.e.m.; *P<0.05 t-test.
ERGIC-53 interacts preferentially with partially assembled IgM subunits
To optimize the multistep processes of polymerization and QC of the IgM factory, subunits that have completed assembly with L chains (i.e., μ2λ2) should preferentially reach the polymerization machinery. By binding to the unpaired first constant (CH1) domain (Hendershot and Sitia, 2005), BiP might prevent unassembled μ and μ2 to engage in futile polymerization efforts. Therefore, ERGIC-53 should bind better to μ2L2 or higher order assemblies. Accordingly, more μ was co-IP by anti-ERGIC-53 or anti-ERp44 when also λ was present (compare lanes 2 and 3; Figure 6A), despite similar amounts were detected in the lysates before immunoprecipitation (upper panels). Noteworthy, some endogenous ERGIC-53 was also co-IP with ERp44 in these conditions. The interaction, detectable without previous crosslinking and in the presence of a mild detergent, was specific, as no ERGIC-53 could be precipitated by anti-HA (Figure 6A, bottom panel). To analyze better ERp44- and ERGIC-53-binding preferences, IP samples were also resolved under non-reducing conditions (Figure 6B). In cells expressing both μ and λ, mainly μ2λ2 and higher order assemblies were captured by anti-ERGIC-53, despite μ, μ2 and particularly μλ intermediates were easily detectable in the lysates (compare lanes 4 and 6). Two main μ-containing complexes were detected in the anti-ERp44 immunoprecipitations: μ2λ2 and a slower migrating species (indicated by an asterisk), which contained μ2λ2–ERp44 mixed disulfides (Supplementary Figure S6). Some covalent μλ–ERp44 complexes were detected (lane 2, see ° and Supplementary Figure S6). Little if any μλ was isolated with ERGIC-53 (lane 4), despite the prevalence of these species in the lysates (lane 6).
These results indicated that ERp44 preferentially binds to unassembled μλ and μ2λ2, whereas ERGIC-53 interacts with μ2λ2 and higher order assemblies.
ERGIC-53 and ERp44 in B-cell differentiation
If ERp44, ERGIC-53 and MCFD2 were involved in IgM polymerization, their levels should increase during B-cell differentiation. This was clearly the case in both LPS-stimulated murine I.29μ+ B-lymphoma cells (van Anken et al, 2003; Romijn et al, 2005) and primary splenocytes (Figure 7A and B, respectively). ERGIC-53, ERp44 and MCFD2 sharply increased at days 3 and 4 of differentiation, when polymerization ensues (Figure 7C). Real-time PCR analyses of LPS-stimulated primary splenocytes confirmed that ERp44, ERGIC-53 and MCFD2 mRNAs accumulated concomitantly with the increase in Ig-μ transcripts (Supplementary Figure S7). Despite its abundance in activated splenocytes, little ERp44 localized in the ER (Figure 7D), being instead enriched in perinuclear and peripheral vesicles, containing giantin and ERGIC-53, respectively.
The above findings are consistent with ERp44 and ERGIC-53 assisting IgM polymerization also in B cells. To further confirm this, we sought to verify if the distal distribution of ERp44 with respect to ER markers in B cells (Figure 7D, and our unpublished results) corresponded to delayed interactions of newly made μ chains with ERp44 (and ERGIC-53) as they assemble into secretion competent polymers. Therefore, we investigated the dynamic associations of μ chains with BiP, ERp44 and ERGIC-53 performing pulse–chase assays in the human Burkitt lymphoma line Ramos (Figure 7E and F). In these cells, polymers were first detected intracellularly after 10 min of chase, and later in the supernatants. The levels of μ co-immunoprecipitation by anti-BiP antibodies decreased during the first 20 min of chase. The increase observed at the last point of chase (30 min) may reflect the onset of degradation of unpolymerized subunits as often observed in B-cell lines (Amitay et al, 1991). In contrast, the interactions with ERp44 and ERGIC-53 increased after the pulse, preceding the detection of intracellular polymers. These data are consistent with μ chains establishing sequential interactions with different chaperones during the stepwise IgM polymerization in a B-cell line.
Discussion
The KDEL motif has long been thought as an ER localization device for soluble proteins. We show here a differential distribution of proteins endowed with such a motif, which reveals a further level of compartmentalization in the early secretory pathway: this could provide a novel mechanism that couples efficiency and fidelity in the IgM factory.
The peculiar subcellular distribution of ERp44
Our data show that, in both lymphoid and non-lymphoid cells, endogenous ERp44 is found downstream of traditional ER markers, concentrating in the ERGIC (Figure 1 and Supplementary Figure S1; Gilchrist et al, 2006; Wang et al, 2007). How is ERp44 localization determined? The concept that certain ER-resident proteins form complexes that assist the folding of incoming cargoes and impede faulty products to proceed further along the assembly line is gaining support (Meunier et al, 2002). Therefore, either for an unfolded substrate or a functional partner, interactions with large complexes can prevent forward transport and retain/concentrate the protein in an environment suitable for maturation or function. ERp44 is not part of these complexes (Meunier et al, 2002). Accordingly, when its ER retrieval motif is removed, ERp44 is secreted more rapidly and efficiently than the corresponding PDI mutant (Figure 2).
The ERp44–ERGIC-53 liaison
The localization of ERp44 also reflects its capability of interacting with ERGIC-53. Although most evident in transfectants overexpressing tagged molecules, co-immunoprecipitation can also be detected between the endogenous counterparts and without crosslinking (Figures 3B and 6A). Interestingly, the interaction is weaker between mutants in the active sites: the replacement of Cys29 in ERp44 (Anelli et al, 2003) has stronger effects than inactivating ERGIC-53 (Itin et al, 1996), perhaps because the N156A-ERGIC-53 mutant can form hetero-hexamers with endogenous wt molecules that retain some lectin activity. These observations indicate that ERp44 and ERGIC-53 establish weak reversible interactions, which can be stabilized by binding to common substrates.
Strong evidence for a physiological interaction between ERGIC-53 and ERp44 stems from delocalization assays. The ER-localized KKAA ERGIC-53 mutant recruited endogenous ERp44 in this compartment and influenced ERp44ΔRDEL release (Figure 4 and Supplementary Figure S2). Also the levels of wt ERGIC-53 correlated with ERp44ΔRDEL secretion (Figure 4) and endogenous ERp44 localization (Supplementary Figure S3). Besides excluding artifacts caused by cell lysis, crosslinking or immunoprecipitation, these results demonstrate that binding to ERGIC-53 occurs in physiological conditions and is important for ERp44 localization. Nonetheless, some ERp44 is found in the ERGIC of the KKAA transfectants (data not shown), which lack detectable ERGIC-53 in this compartment (Vollenweider et al, 1998 and our unpublished results). In the absence of forward-moving ERGIC-53, ERp44 could reach the ERGIC by bulk transport or, alternatively, interacting with additional escort proteins (Hauri et al, 2002).
Why does exogenous ERp44 accumulate in the ER? The possibility that overexpressed molecules fail to fold and become a substrate of ER-QC is unlikely, as suggested by the secretion of ERp44ΔRDEL. More likely, overexpression saturates the normal localization mechanisms (forward transport or perhaps net available space in the ERGIC).
A platform for IgM polymerization
The extraordinary efficiency and fidelity of antibody-secreting cells requires adequate production, QC and transport systems. Accordingly, B-cell differentiation entails dramatic structural and biochemical modifications, with the coordinated and sequential appearance of groups of functionally related proteins (van Anken et al, 2003; Romijn et al, 2005). The overlapping temporal and spatial distribution in differentiating B cells (Figure 7), their interactions (Figures 1, 2, 3 and 4) and their preferential binding to certain IgM subunits (Figure 6) suggest that ERp44 and ERGIC-53 concur in IgM polymerization. This hypothesis is confirmed by their ability to reconstitute polymerization when overexpressed in HeLa cells (Figure 5). Active ERGIC-53 promotes both polymerization and secretion, independently from its subcellular localization (wt or KKAA). Hexameric ERGIC-53 may provide a planar platform that concentrates μ2L2 subunits and favors their ordered assembly (Figure 8), avoiding formation of larger polymers (de Lalla et al, 1998). In support of this model, the inactive N156A mutant inhibits polymerization, likely because hetero-hexamers (with endogenous wt molecules) with reduced valency are formed. Moreover, MCFD2 synergizes with ERGIC-53 in promoting IgM polymerization (Figure 5B). Hence, although dispensable for Cathepsin Z and C binding (Nyfeler et al, 2006), MCFD2 seems to favor the interaction of ERGIC-53 with μ, as described for factor VIII (Zhang et al, 2005).
Figure 8.
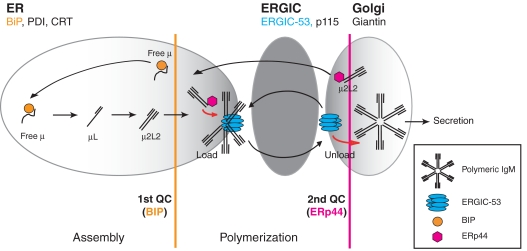
Schematic model of the IgM polymerization machinery. The IgM assembly line is schematized in its sequential arrangement. The distribution of ERp44 in the early secretory pathway (primarily in the ERGIC) is depicted as a gradient of gray. BiP-dependent control ensures that μ chains do not proceed to the polymerization machinery unless assembled with L chains (first QC step). ERGIC-53, a hexameric lectin acting in conjunction with MCFD2 (the latter is not shown) captures μ2L2 subunits, likely aligning them in a planar conformation, suitable for polymerization. ERp44 ensures that unpolymerized subunits are not secreted, retrieving them into the assembly line (second QC step). Because of its ability to bind IgM subunits and ERGIC-53, ERp44 may recruit and locally concentrate μ2L2, to stimulate further the polymerization machinery efficacy.
The role of ERp44
ERp44 could enhance IgM polymerization in several ways (Figure 8). First, the RDEL-dependent retention increases subunit concentration in an environment suitable for polymerization. Second, the ERp44Cys29–μCys575 mixed disulfides may provide oxidative equivalents necessary for polymer formation. Third, ERp44 could recruit Ero1α and β to the platform (Anelli et al, 2002; Otsu et al, 2006), thus providing additional oxidative power. Finally, the presence of some ERp44 downstream of the ERGIC provides a second checkpoint ensuring that only native polymers are secreted.
Sequential QC checkpoints in IgM polymerization
By concentrating distally with respect to PDI, ERp44 and ERGIC-53 receive IgM subunits that have completed the first step in the production line, that is the BiP-dependent μ2L2 assembly (Hendershot and Kearney, 1988; Sitia et al, 1990; Hendershot and Sitia, 2005). Indeed, more μ can reach ERp44 when also L chain is present, and μ2L2 and higher order assemblies preferentially associate with both ERGIC-53 and ERp44 in HeLa cells (Figure 6B and Supplementary Figure S6). The delayed interactions of nascent μ chain with ERp44 and ERGIC-53 with respect to BiP in B-lymphoma cells (Figure 7E and F) support the existence of sequential steps in IgM polymerization (Figure 8).
Several lines of evidence indicate that the ER can sustain IgM polymerization: (i) the presence of mannose 7–8 glycans in the conserved μs tailpiece indicates that polymerization makes these sugar moieties inaccessible to the Golgi enzymes (Davis et al, 1989a; Cals et al, 1996); (ii) myeloma cells lacking L chains form covalent polymers in which μ chains are bound to BiP (Bornemann et al, 1995); (iii) polymerization is still detectable when ER–Golgi transport is inhibited (Brewer et al, 1994); (iv) the ER-localized ERGIC-53 KKAA mutant also stimulates polymerization (Figure 5); and (v) expression of mutant μ chains (μΔCH1) in L chain producing cells causes the formation of detergent-insoluble polymers in dilated ER cisternae surrounded by ribosomes (Valetti et al, 1991). In the absence of L chains, however, μΔCH1 condensation, which requires Cys575-dependent polymerization, occurred in the ERGIC (Mattioli et al, 2006). All these data suggest that polymerization can occur in the ER. However, the subcellular localization and coordinated increase of ERGIC-53 and ERp44 during B-cell differentiation suggest that the spatial subdivision within the early secretory compartment is important to promote IgM biogenesis. By concentrating and orienting subunits, ERGIC-53 and possibly other molecules in B cells, could couple polymerization and forward transport, ERp44 bringing unpolymerized subunits back for another chance of polymerization or for degradation. In line with the two-step IgM QC (Figure 8), brefeldin-A inhibits the degradation of μ only when these are assembled with L chains (Elkabetz et al, 2003).
Changes in pH or Ca2+ concentration could mediate the detachment of completed polymers from ERGIC-53, as described for other substrates (Appenzeller-Herzog et al, 2004). However, the stimulatory effects of the ER-localized KKAA mutant suggest that the conformational changes that accompany polymerization could mediate the release, perhaps hiding the μ-chain ERGIC-53-binding sites. This important issue needs further investigation.
In view of their evolutionary conservation, ERp44 and ERGIC-53 could play a wider role in facilitating protein assembly and secretion, similar to the one we describe here for IgM.
Materials and methods
Pulse and chase, IP and Western blotting
Cells were incubated for 30 min in DMEM without methionine and cysteine, 1% dialysed FCS, pulsed with 35S-labeled amino acids (200 μCi/9 × 106 cells) and then washed and chased in complete medium for the indicated times. SN were harvested, cells treated with 10 mM NEM and lysed as described (Anelli et al, 2002). The IP material from cell lysates and SN was resolved on SDS–PAGE, and gels dried or transferred to nitrocellulose and visualized by autoradiography. Details of co-immunoprecipitation assays are described in Supplementary data. Western blot analyses were performed as described previously (Anelli et al, 2002). WB images were acquired with the Chemidoc-it Imaging System (UVP, Upland, CA) and processed with Adobe Photoshop 7.0 (Adobe Systems Inc.). In each panel, white lines indicate that lanes coming from a single gel were juxtaposed. Black lines separate lanes coming from different gels run under identical conditions.
Chemical crosslinking
Cells were washed and incubated for 30 min at 4°C with 1 mM DSP on a rocking platform. The reaction was quenched by rinsing the cells once with 20 mM Tris–HCl pH 7.4, followed by two incubations for 15 min at RT with the same buffer. Cells were then lysed in RIPA+10 mM NEM.
Immunofluorescence
Samples of stained HeLa or lymphoid cells (see Supplementary data for details) were analyzed on an Olympus inverted fluorescence microscope (model IX70) with DeltaVision RT Deconvolution System (Alembic, HSR, Milano). After deconvolution, images were processed with Adobe Photoshop 7.0 (Adobe Systems Inc.).
Transient transfection, RNAi and quantitative real-time PCR
Transient transfections were performed as described previously (Anelli et al, 2002). Lipofectamine and Plus reagent were used for 3T3 cells, as recommended by the supplier.
RNAi experiments were performed using Lipofectamine RNAiMAX reagent as recommended by the supplier. siRNA oligos were purchased from MWG-Biotech AG (Ebersberg, Germany); sequences are reported in Supplementary data.
Real-time PCR was performed using the Power SYBR® green PCR master mix (Applied Biosystems) in the Applied Biosytems 7900HT Real-Time PCR system following the manufacturer's instructions. Relative quantifications were carried out by the ΔΔCt method, using histone 3 (H3) as reference gene. Primer sequences are reported in Supplementary data.
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Christian Appenzeller, Ineke Braakman, Claudia Fazi, Hans-Peter Hauri, Ylva Lindqvist, Alexandre Mezghrani, Etienne Neve, Mieko Otsu, Ralph Petterson, Flora Peyvandi, Jakko Saraste, Cristina Scielzo, Marta Spreafico for providing helpful suggestions, discussions and essential reagents, Claudio Fagioli and Elena Pasqualetto for technical help, Ana Fella and Raffaella Brambati for secretarial assistance. We apologize with the many colleagues whose seminal papers could not be cited for space limitations. The financial support of Telethon—Italy (Grant no. GGP06155), Associazione Italiana per la Ricerca sul Cancro, and Cariplo (Project NOBEL) is gratefully acknowledged.
References
- Amitay R, Bar-Nun S, Haimovich J, Rabinovich E, Shachar I (1991) Post-translational regulation of IgM expression in B lymphocytes. Selective nonlysosomal degradation of assembled secretory IgM is temperature-dependent and occurs prior to the trans-Golgi. J Biol Chem 266: 12568–12573 [PubMed] [Google Scholar]
- Anelli T, Alessio M, Bachi A, Bergamelli L, Bertoli G, Camerini S, Mezghrani A, Ruffato E, Simmen T, Sitia R (2003) Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J 22: 5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Alessio M, Mezghrani A, Simmen T, Talamo F, Bachi A, Sitia R (2002) ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family. EMBO J 21: 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Roche AC, Nufer O, Hauri HP (2004) pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. J Biol Chem 279: 12943–12950 [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJ (1993) Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75: 717–728 [DOI] [PubMed] [Google Scholar]
- Bornemann KD, Brewer JW, Beck-Engeser GB, Corley RB, Haas IG, Jack HM (1995) Roles of heavy and light chains in IgM polymerization. Proc Natl Acad Sci USA 92: 4912–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JW, Randall TD, Parkhouse RM, Corley RB (1994) Mechanism and subcellular localization of secretory IgM polymer assembly. J Biol Chem 269: 17338–17348 [PubMed] [Google Scholar]
- Cals MM, Guenzi S, Carelli S, Simmen T, Sparvoli A, Sitia R (1996) IgM polymerization inhibits the Golgi-mediated processing of the mu-chain carboxy-terminal glycans. Mol Immunol 33: 15–24 [DOI] [PubMed] [Google Scholar]
- Davis AC, Collins C, Shulman MJ (1989a) Differential glycosylation of polymeric and monomeric IgM. Mol Immunol 26: 147–152 [DOI] [PubMed] [Google Scholar]
- Davis AC, Roux KH, Pursey J, Shulman MJ (1989b) Intermolecular disulfide bonding in IgM: effects of replacing cysteine residues in the mu heavy chain. EMBO J 8: 2519–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lalla C, Fagioli C, Cessi FS, Smilovich D, Sitia R (1998) Biogenesis and function of IgM: the role of the conserved mu-chain tailpiece glycans. Mol Immunol 35: 837–845 [DOI] [PubMed] [Google Scholar]
- Elkabetz Y, Kerem A, Tencer L, Winitz D, Kopito RR, Bar-Nun S (2003) Immunoglobulin light chains dictate vesicular transport-dependent and -independent routes for IgM degradation by the ubiquitin-proteasome pathway. J Biol Chem 278: 18922–18929 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191 [DOI] [PubMed] [Google Scholar]
- Fra AM, Fagioli C, Finazzi D, Sitia R, Alberini CM (1993) Quality control of ER synthesized proteins: an exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO J 12: 4755–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ (2006) Quantitative proteomics analysis of the secretory pathway. Cell 127: 1265–1281 [DOI] [PubMed] [Google Scholar]
- Hauri HP, Nufer O, Breuza L, Tekaya HB, Liang L (2002) Lectins and protein traffic early in the secretory pathway. Biochem Soc Symp 69: 73–82 [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 73: 1019–1049 [DOI] [PubMed] [Google Scholar]
- Hendershot L, Sitia R (2005) Immunoglobulin assembly and secretion. In Molecular Biology of B Cells, Honjo T, Alt FW, Neuberger MS (eds), pp 261–273. Amsterdam NL: Elsevier Acad Press [Google Scholar]
- Hendershot LM, Kearney JF (1988) A role for human heavy chain binding protein in the developmental regulation of immunoglobin transport. Mol Immunol 25: 585–595 [DOI] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120: 85–98 [DOI] [PubMed] [Google Scholar]
- Hombach J, Leclercq L, Radbruch A, Rajewsky K, Reth M (1988) A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J 7: 3451–3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin C, Roche AC, Monsigny M, Hauri HP (1996) ERGIC-53 is a functional mannose-selective and calcium-dependent human homologue of leguminous lectins. Mol Biol Cell 7: 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli L, Anelli T, Fagioli C, Tacchetti C, Sitia R, Valetti C (2006) ER storage diseases: a role for ERGIC-53 in controlling the formation and shape of Russell bodies. J Cell Sci 119: 2532–2541 [DOI] [PubMed] [Google Scholar]
- Meunier L, Usherwood YK, Chung KT, Hendershot LM (2002) A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol Biol Cell 13: 4456–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HR (1986) An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46: 291–300 [DOI] [PubMed] [Google Scholar]
- Neerman-Arbez M, Johnson KM, Morris MA, McVey JH, Peyvandi F, Nichols WC, Ginsburg D, Rossier C, Antonarakis SE, Tuddenham EG (1999) Molecular analysis of the ERGIC-53 gene in 35 families with combined factor V-factor VIII deficiency. Blood 93: 2253–2260 [PubMed] [Google Scholar]
- Neve EP, Lahtinen U, Pettersson RF (2005) Oligomerization and interacellular localization of the glycoprotein receptor ERGIC-53 is independent of disulfide bonds. J Mol Biol 354: 556–568 [DOI] [PubMed] [Google Scholar]
- Nichols WC, Seligsohn U, Zivelin A, Terry VH, Hertel CE, Wheatley MA, Moussalli MJ, Hauri HP, Ciavarella N, Kaufman RJ, Ginsburg D (1998) Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell 93: 61–70 [DOI] [PubMed] [Google Scholar]
- Nyfeler B, Zhang B, Ginsburg D, Kaufman RJ, Hauri HP (2006) Cargo selectivity of the ERGIC-53/MCFD2 transport receptor complex. Traffic 7: 1473–1481 [DOI] [PubMed] [Google Scholar]
- Otsu M, Bertoli G, Fagioli C, Guerini-Rocco E, Nerini-Molteni S, Ruffato E, Sitia R (2006) Dynamic retention of Ero1alpha and Ero1beta in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid Redox Signal 8: 274–282 [DOI] [PubMed] [Google Scholar]
- Reddy P, Sparvoli A, Fagioli C, Fassina G, Sitia R (1996) Formation of reversible disulfide bonds with the protein matrix of the endoplasmic reticulum correlates with the retention of unassembled Ig light chains. EMBO J 15: 2077–2085 [PMC free article] [PubMed] [Google Scholar]
- Romijn EP, Christis C, Wieffer M, Gouw JW, Fullaondo A, van der Sluijs P, Braakman I, Heck AJ (2005) Expression clustering reveals detailed co-expression patterns of functionally related proteins during B cell differentiation: a proteomic study using a combination of one-dimensional gel electrophoresis, LC-MS/MS, and stable isotope labeling by amino acids in cell culture (SILAC). Mol Cell Proteomics 4: 1297–1310 [DOI] [PubMed] [Google Scholar]
- Schindler R, Itin C, Zerial M, Lottspeich F, Hauri HP (1993) ERGIC-53, a membrane protein of the ER-Golgi intermediate compartment, carries an ER retention motif. Eur J Cell Biol 61: 1–9 [PubMed] [Google Scholar]
- Shachar I, Amitay R, Rabinovich E, Haimovich J, Bar-Nun S (1992) Polymerization of secretory IgM in B lymphocytes is prevented by a prior targeting to a degradation pathway. J Biol Chem 267: 24241–24247 [PubMed] [Google Scholar]
- Sitia R, Braakman I (2003) Quality control in the endoplasmic reticulum protein factory. Nature 426: 891–894 [DOI] [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C (1990) Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell 60: 781–790 [DOI] [PubMed] [Google Scholar]
- Sitia R, Neuberger MS, Milstein C (1987) Regulation of membrane IgM expression in secretory B cells: translational and post-translational events. EMBO J 6: 3969–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C, Grossi CE, Milstein C, Sitia R (1991) Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J Cell Biol 115: 983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ (2003) Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18: 243–253 [DOI] [PubMed] [Google Scholar]
- Vollenweider F, Kappeler F, Itin C, Hauri HP (1998) Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of HeLa cells impairs the secretion of a lysosomal enzyme. J Cell Biol 142: 377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE (2007) Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 27: 3716–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland FT, Gleason ML, Serafini TA, Rothman JE (1987) The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50: 289–300 [DOI] [PubMed] [Google Scholar]
- Zhang B, Cunningham MA, Nichols WC, Bernat JA, Seligsohn U, Pipe SW, McVey JH, Schulte-Overberg U, de Bosch NB, Ruiz-Saez A, White GC, Tuddenham EG, Kaufman RJ, Ginsburg D (2003) Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet 34: 220–225 [DOI] [PubMed] [Google Scholar]
- Zhang B, Kaufman RJ, Ginsburg D (2005) LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J Biol Chem 280: 25881–25886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures