Abstract
The human Na+-glucose cotransporter (hSGLT1) was expressed in Xenopus laevis oocytes. The transport activity, given by the Na+ current, was monitored as a clamp current and the concomitant flux of water followed optically as the change in oocyte volume.
When glucose was added to the bathing solution there was an abrupt increase in clamp current and an immediate swelling of the oocyte. The transmembrane transport of two Na+ ions and one sugar molecule was coupled, within the protein itself, to the influx of 210 water molecules.
This stoichiometry was constant and independent of the external parameters: Na+ concentrations, sugar concentrations, transmembrane voltages, temperature and osmotic gradients.
The cotransport of water occurred in the presence of adverse osmotic gradients. In accordance with the Gibbs equation, energy was transferred within the protein from the downhill fluxes of Na+ and sugar to the uphill transport of water, indicative of secondary active transport of water.
Unstirred layer effects were ruled out on the basis of experiments on oocytes treated with gramicidin or other ionophores. Na+ currents maintained by ionophores did not lead to any initial water movements.
The finding of a molecular water pump allows for new models of cellular water transport which include coupling between ion and water fluxes at the protein level; the hSGLT1 could account for almost half the daily reuptake of water from the small intestine.
Water can cross cell membranes by three routes: the lipid bilayer, water channels or by a mechanism dependent on conformational changes in cotransport proteins (Zeuthen, 1991, 1994; Zeuthen, Hamann & La Cour, 1996; Loo, Zeuthen, Chandy & Wright, 1996; Zeuthen et al. 1997). It appears that the water fluxes maintained by the cotransport proteins are coupled in a constant ratio to the fluxes of the non-aqueous substrates. Furthermore, as shown in detail in epithelial cell layers, the water flux through the cotransport proteins can proceed uphill, against the direction of the water-chemical potential difference (Zeuthen, 1991, 1994; Zeuthen et al. 1996). Here we confirm and extend our previous observations that the Na+-glucose cotransporter expressed in Xenopus oocytes behaves as a water cotransporter (Loo et al. 1996; Zeuthen et al. 1997). In particular we demonstrate cotransport of water against an osmotic gradient, and we have eliminated potential artefacts due to unstirred layers, transport number and electrode effects (Reuss, 1996; Diamond, 1996). We conclude that the human Na+-glucose cotransporter has an inherent capacity for secondary active transport of water.
METHODS
The human Na+-glucose cotransporter hSGLT1 was expressed in Xenopus laevis oocytes as described previously (Hediger, Turk & Wright, 1989). Adult frogs were anaesthetized by immersion in 0.2 % Tricaine (3-aminobenzoic acid ethyl ester; Sigma) for 30 min. An insertion was made in the abdominal cavity from where an ovarian lobe was removed. After suture the animal was allowed to recover for at least 6 h in tap water. In the present study oocytes were incubated in Kulori medium (composition (mm): 90 NaCl, 1 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes; pH 7.4, 182 mosmol l−1) at 19°C for 3–7 days before experiments. Osmolarities of the test solutions were determined with an accuracy of 1 mosmol l−1. The experimental chamber was perfused with control solution (composition (mm): 90 NaCl, 20 mannitol, 2 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes; pH 7.4, 212 mosmol l−1). Isotonic test solutions were obtained by replacing mannitol by methyl-α-D-glucopyranoside (α-MDG; Sigma), a non-metabolizeable sugar that is transported by the hSGLT1 in the same way as glucose (Loo, Hazama, Supplisson, Turk & Wright, 1993). Since the osmotic coefficient for mannitol is about 3 % lower than that for α-MDG, minor adjustments of the mannitol contents of the test solutions were required to achieve osmolarities accurate to within 1 mosmol l−1. When the influence of unstirred layers and electrode artefacts were tested, the pore formers gramicidin D (150 to 300 nm) or nystatin (20 mg ml−1) were added to both bathing and test solutions. Connexin 50 expression in oocytes was as described in Zampighi, Kunig & Loo (1997).
The experimental chamber, the voltage clamp and the optical system for the volume measurements have been described in detail elsewhere (Parent, Supplison, Loo & Wright, 1992; Zeuthen et al. 1997). In short, the oocyte was impaled by two microelectrodes, one providing the clamp current (proportional to the Na+ current; Mackenzie, Loo & Wright, 1998), the other measuring the membrane potential. The experimental chamber had a volume of 30 μl and solution changes were 90 % complete in 5 s given a flow rate of 12 μl s−1. The solutions were fed into the chamber via a small mechanical valve, the dead space between the valve and the chamber being 5 μl. The oocyte was observed from below via an inverted microscope through a low power (× 3) long distance (5 mm) objective connected to a charge coupled device camera. To achieve a high stability of the oocyte image, the upper surface of the bathing solution was determined by the flat end of a Perspex rod. The rod also served as a light guide providing an illuminated background for the oocyte. Images were captured directly from the camera to the random access memory of a computer by means of a frame grabber which allowed 132 Mbyte s−1 data transfer rates (25 frames s−1, 768 × 576 pixels). Focusing was at the circumference of the oocyte and dark pixels were assigned as oocyte material, bright pixels as non-oocyte material. The oocyte was assumed to be spherical and the calculated volume was recorded on-line at a rate of one point per second. All numbers are given as means ±s.e.m. and n is the number of experiments in at least four oocytes
RESULTS
Isotonic conditions
The correspondence between current and water flow in the hSGLT1 is illustrated in Fig. 1A and B. Under clamped conditions the sugar α-MDG was added to the bathing solution where it isosmotically replaced mannitol. This elicited an abrupt increase in inward current and a linear increase in oocyte volume. The correspondence between the sugar-induced current (Is) and the inflow of water was shown by the fact that the integrated current (Qs) closely predicted the change in volume if it was assumed that a fixed number of water molecules were coupled to the influx of each Na+ ion. The average coupling ratio was 203 ± 20 water molecules per two Na+ ions and one sugar molecule or 19 ± 1 pl μC−1 (n= 16) for Is in the range 1320–2980 nA. Addition of phlorizin, a specific inhibitor of hSGLT1 (Hirayama, Loo & Wright, 1997), to the bathing solution (50–150 μm) abolished both Is and the volume increase.
Figure 1. Correspondence between sugar-induced current and water flow under isotonic conditions.
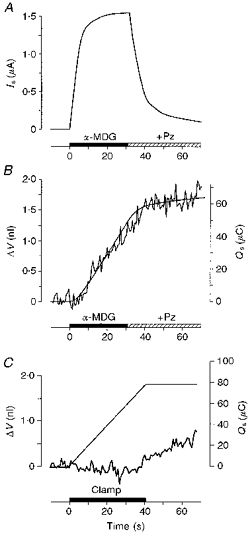
An hSGLT1-expressing oocyte was clamped continuously at a membrane potential of −50 mV by two-microelectrode voltage clamp. 10 mm of a transported sugar (α-MDG) was introduced abruptly into the bath (filled bar) where it replaced, isotonically, 10 mm mannitol. A, this caused an inward current (Is), which increased rapidly towards a maximum of 1540 nA (90 % complete in 13 s). B, the current was associated with a linear increase in oocyte volume (ΔV, jagged line) indicative of a constant rate of water influx of 36 pl s−1. The volume increase and the current were abolished by the addition of 50 μm phlorizin (+Pz). In the present experiment the integrated current (Qs, smooth line in B) describes the volume changes, if we assume that the entry of two Na+ ions and one sugar molecule couples directly to the entry of 249 water molecules. C, there was no initial change in oocyte volume when the inward Na+ current was mediated by the cation-selective gramicidin channel. An inward current of about 1900 nA was obtained (not shown) by changing the intracellular electrical potential from the resting potential (about −45 mV, not shown) to −100 mV by means of the voltage clamp (filled bar). The current, carried by Na+ ions, did not give rise to any immediate change in cell volume (jagged line in C) and the integrated current (smooth line) had no resemblance to the volume changes. The oocyte expressed hSGLT1 to the same degree as the one used in A and B, but the cotransporter was kept inactive since no sugar was present.
Substrates and water were transported by the cotransporter in a ratio that was hypertonic relative to the bathing solution and the oocyte became slightly hypertonic (by about 0.3 to 0.5 mosmol l−1) following experiments such as those illustrated in Fig. 1A and B. After the return to control bathing solution this gave rise to a slow exponential osmotic influx of water lasting about 10 min with an average rate of 3 pl s−1.
Unstirred layer effects
Any secondary effects on the water flows originating from unstirred layer effects or electrode artefacts (Diamond, 1996) could be ruled out experimentally (Fig. 1C). The cation-selective ionophore gramicidin was inserted into the membrane of hSGLT1-expressing oocytes under conditions where the cotransporter was kept inactivated by the absence of sugar. When the clamp was turned on no initial swelling was observed (0.3 ± 0.5 pl s−1, n= 12), for inward clamp currents in the range 750–1900 nA; most probably these currents are carried by Na+. In terms of secondary effects, this situation is analogous to the situation where the current is mediated entirely by the sugar-activated hSGLT1 (Fig. 1A and B). The only difference is the molecular mechanism responsible for the Na+ transport. We must therefore conclude that it is molecular processes within the hSGLT1 itself that give rise to the water transport and not unstirred layer effects or electrode artefacts. It should be noted that the two situations are governed by the same passive water permeabilities given by the passive properties of the expressed hSGLT1 (see below) and the native oocyte membrane; the extra passive water permeabilty resulting from the gramicidin was insignificant (Zeuthen et al. 1997). The sugar that enters the oocyte in cotransport with Na+ will add to the intracellular osmolarity beyond that estimated from the Na+ currents by up to 50 %. However, initial osmotic swellings were absent for clamp currents far exceeding those used in cotransport experiments; inward Na+ currents of 500–4000 nA maintained by the cation channel Connexin 50 (White, Bruzzone, Goodenough & Paul, 1992; Zampighi et al. 1997) expressed in native oocytes did not cause any initial water fluxes for at least 20 s (n= 6). This margin makes it unlikely that the sugar transported in cotransport experiments (e.g. Fig. 1A) should cause any significant initial influx of water by simple osmosis. Unstirred layer effects have also been ruled out on the basis of theoretical considerations (Loo et al. 1996). Gramicidin-mediated currents maintained for longer periods led to small rates of swelling as predicted from simple osmometric behaviour. With currents of 1000 nA we observed swellings of 6 ± 3 pl s−1 (n= 12) after 50 s. Gramicidin and the anion ionophore nystatin tested in native oocytes gave results similar to those above (Zeuthen et al. 1997).
Uphill and downhill transport of water
The hSGLT1 was able to transport water uphill, against the water-chemical potential difference (Fig. 2A). If the bathing solution was made hypertonic by the addition of 10 mm of sugar, the induced current (Is) was associated with an influx of water. A measure of the osmotic component of the water flux generated by the 10 mosmol l−1 was obtained in the same oocyte by adding 10 mm mannitol in the absence of sugar. When the osmotic component of the water flux was subtracted from the total water flux the sugar-induced cotransport of water was obtained (Fig. 2B). This component of the water influx was closely linked to Is. If we assume that a fixed number of water molecules are transported for each two Na+ ions and one sugar molecule then the integrated current (Qs, Fig. 2B) corresponds to the cotransport component of the water inflow. On average the coupling ratio was 21 ± 2 pl μC−1 or 219 ± 23 water molecules per two Na+ (n= 20), with Is in the range 1440–3000 nA. Both the sugar-induced water flux and the osmotically induced flux through hSGLT1 were blocked by phlorizin. If the experimental changes of Fig. 2 were performed in the presence of 100 μm phlorizin, the oocyte shrank at a rate given by an osmotic water permeability (Lp) of 2.6 ± 0.7 × 10−6 cm s−1 (osmol l−1)−1 (n= 16), similar to the one we found for the native oocyte membrane (2.8 ± 1.0 × 10−6 cm s−1 osmol l−1)−1, n= 20) (Zhang & Verkman, 1991; Preston, Carroll, Guggino & Agre, 1992). Due to membrane foldings, Lp values expressed per apparent surface area result in values nine times higher (Zampighi et al. 1995).
Figure 2. Uphill and downhill transport of water by the hSGLT1.
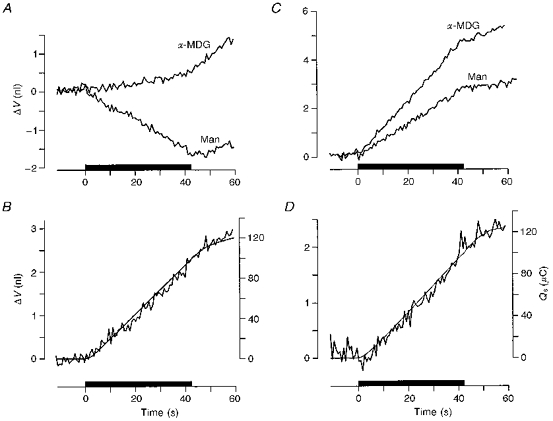
A and B, uphill transport. A, the oocyte was clamped to −50 mV and 10 mm of sugar was added to the bathing solution (filled bar) equivalent to an increase of the extracellular osmolarity of 10 mosmol l−1. This caused an inwardly directed current (not shown) and a linear increase in oocyte volume (ΔV, top curve, α-MDG). If the extracellular osmolarity was increased by 10 mosmol l−1 by the addition of 10 mm mannitol, the oocyte shrank as expected from a simple osmometer (bottom curve, Man). The difference between the two volume changes is given as the jagged line in B and represents cotransport of water mediated by the hSGLT1 under the hypertonic conditions. The integrated current (Qs, smooth line in B) describes the volume changes if it is assumed that the hSGLT1 cotransports 202 water molecules for each two Na+ ions and one sugar molecule. C and D, downhill transport of water by the hSGLT1. In C, 20 mm mannitol was replaced abruptly by 10 mm sugar (filled bar), a decrease of the extracellular osmolarity of 10 mosmol l−1. This caused an inwardly directed current (not shown) and a rapid linear increase in oocyte volume (top curve, α-MDG). If the extracellular osmolarity was decreased by 10 mosmol l−1 by removing mannitol, the oocyte swelled at a slower rate (bottom curve, Man). The difference between the two volume changes represents the cotransport of water mediated by the hSGLT1 under the hypotonic conditions (D, jagged line). The integrated current (Qs, smooth line in D) describes the volume changes if it is assumed that 195 water molecules enter coupled directly to the entry of two Na+ ions and one sugar molecule. Small swellings were observed after the bathing solution was changed to a sugar-free solution due to the finite washing out time for sugar.
The division of the hSGLT1-mediated water flux into a cotransport and an osmotic component was also possible under hypotonic conditions (Loo et al. 1996; Fig. 2C and D. In the presence of 100 μm phlorizin both components were inhibited and the oocyte swelled at a rate given by an Lp of 2.9 ± 1.4 × 10−6 cm s−1 (osmol l−1)−1 (n= 24), a value similar to the one for the native membrane of 2.3 ± 0.7 × 10−6 cm s−1 (osmol l−1)−1 (n= 23) (Zhang & Verkman, 1991; Preston et al. 1992; Zampighi et al. 1995; Loo et al. 1996).
Cotransport and osmotic components
The water fluxes through the hSGLT1 obtained under iso-, hypo- and hypertonic conditions are summarized in Fig. 3, normalized relative to an oocyte with an expression level giving a maximal sugar-induced current (Is) of 1000 nA; this corresponds roughly to 5 × 1010 copies of hSGLT1 (Loo et al. 1993). The contribution of the native oocyte membrane has been subtracted. In the presence of sugar, the water flux was given by the regression line JH2O (in pl s−1μA−1) = 20.7 + 1.51 Δπ (in mosmol l−1) (r= 0.93) which divides into two components, a constant contribution of 20.7 pl independent of the osmolarity and an osmotic contribution given by an Lp of 3.6 ± 0.2 × 10−6 cm s−1 (osmol l−1)−1. In the absence of sugar the flux of water was entirely osmotic; for the oocyte described above, the flux was described by the line JH2O= 1.45 Δπ (r= 0.96), equivalent to an Lp of 3.4 ± 0.1 × 10−6 cm s−1 (osmol l−1)−1. The similarity of the water permeabilities obtained in the presence and absence of sugar suggests that sugar does not induce any change in the passive pathway of the hSGLT1 and supports the formal separation of the water flux into a cotransport and an osmotic component. Furthermore, as the sugar-induced currents were independent of the external osmolarity (data not shown) the ratio between the number of cotransported water molecules and the number of Na+ ions, the coupling ratio, was independent of osmolarity and averaged 20 pl μC−1 or 222 water molecules per two Na+ ions.
Figure 3. Active, sugar-induced (α-MDG) and osmotic, mannitol-induced (Man) water fluxes mediated by hSGLT1 as a function of the transmembrane osmotic difference Δπ.
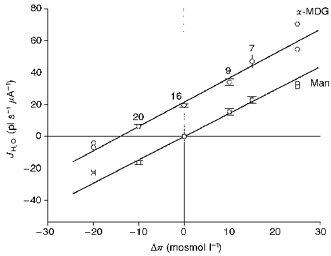
Data were obtained as described in Figs 1 and 2, and were performed at a clamp potential of −50 mV, and Na+ concentration of 90 mm. The fraction of the water flux going through the native oocyte membrane has been subtracted. The water fluxes through the hSGLT1 were normalized relative to the sugar-induced current obtained at isotonic conditions with 10 mm sugar. The number of observations at each point are indicated; values at -20 and +25 mosmol l−1 are single observations. The slope of the two lines represents the passive water permeability of the hSGLT1. In addition, sugar induces a constant contribution to the total water flux, as evidenced by the constant vertical displacement of the two lines in agreement with the prediction of the Gibbs equation (see text). Thus the vertical displacement of the two curves gives the coupling ratio of the hSGLT1 in picolitres of water transported per microcoulomb.
Dependence on external parameters
The molecular mechanism responsible for the sugar-induced cotransport of water had a high Arrhenius activation energy (Ea) in contrast to the osmotic water flux which had a low Ea. Experiments such as those in Fig. 1 performed at 16, 22, 26 or 31°C, gave Ea= 26 ± 0.3 kcal mol−1 for the water cotransport and 25 ± 0.3 kcal mol−1 for the sugar-induced current (n > 5 at each temperature), analogous to rabbit SGLT1 (Loo et al. 1996). It follows that the coupling ratio was independent of temperature. The equality and high value of the activation energies support the notion that the sugar-induced current and the cotransport of water are tightly linked by a process that involves conformational changes in the protein. Ea for the osmotic water flux through the hSGLT1 was 4.2 ± 0.6 kcal mol−1 (n > 6 at each temperature), similar to that of water channels (Preston et al. 1992).
The cotransport and the osmotic component of the water flux were also distinguishable on account of their properties in the presence of small hydrophilic osmolytes. The osmotic pathway through the hSGLT1 was permeable to formamide (MW = 45). In paired experiments, 10 mm formamide added to the control solution induced a shrinkage of 21 ± 2 pl s−1 (n= 7) while additions of 10 mm mannitol (MW = 182) gave 33 ± 4 pl s−1. In respect of the native membrane, formamide and mannitol behaved similarly: in the presence of 100 μm phlorizin, formamide induced a shrinkage of 14 ± 1 pl s−1, equal to that obtained with mannitol (14 ± 2 pl s−1). With this value subtracted, the reflection coefficient σ of formamide for the osmotic pathway of the hSGLT1 can be calculated as (21 - 14)/ (33 - 14) = 0.37, similar to the value suggested for water channels in kidney proximal tubules (Gutiérrez, Gonzalez, Echevarria, Hernandez & Whittembury, 1995). The sugar-induced cotransport, however, was insensitive to the molecular size of the osmolyte. Experiments similar to those in Fig. 2A and B, but with 10 mm formamide replacing 10 mm mannitol, gave rates of cotransport of water that were 0.94 ± 0.07 times (n= 7) that obtained in the presence of mannitol only.
The coupling ratio for the hSGLT1 was not only independent of external osmolarities, temperatures and size of osmolytes as shown above, but also of membrane potential, Na+ and sugar concentrations (Fig. 4). The mean of all experiments was 210 ± 4 (n= 180 in 30 oocytes).
Figure 4. Cotransport of water as function of the Na+ current when this is altered by varying the membrane potential (A), the external Na+ concentration (B; substituting with choline) and the external sugar concentration (C; substituting with mannitol).
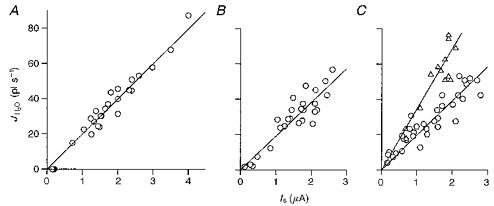
In A the clamp voltage was varied over the range +20 to −100 mV at external Na+ concentrations of 90 mm and sugar concentrations of 10 mm; the relation between the water flux and clamp currents was linear, given by JH2O (pl s−1) = 20.0 ± 0.42 Is (μA) (r= 0.99), indicative of a coupling ratio of 215 water molecules per 2 Na+. In B Na+ concentrations were varied over the range 1–90 mm at a clamp potential of −50 mV; the sugar concentration was 10 mm. JH2O (pl s−1) = 19.4 ± 0.7 Is (μA) (r= 0.91), indicative of a coupling ratio of 208 water molecules per 2 Na+. In C sugar concentrations were varied between 0.1 and 20 mm, at 90 mm Na+ and a clamp potential of −50 mV. JH2O (pl s−1) = 19.4 ± 0.7 Is (μA) (r= 0.92), indicative of a coupling ratio of 209 water molecules per 2 Na+. For comparison we show an example of data obtained with an oocyte expressing rabbit sglt1 (▵) (Zeuthen et al. 1997). This protein has a larger coupling ratio than the hSGLT1: 390 water molecules per 2 Na+, in the present example 323 water molecules per 2 Na+ and 1 glucose; JH2O (pl s−1) = 33.2 ± 1.4 Is (μA) (r= 0.88).
DISCUSSION
Cotransport adds a novel property to transmembrane water transport: it can proceed uphill, against the water chemical potential difference. The strict coupling of the fluxes within the protein provides an efficient mechanism for transforming the free energy contained in the transmembrane electrochemical Na+ gradient (and glucose chemical gradient) into an uphill flow of water. From Gibbs equation, equilibrium will prevail when:
where ‘o’ defines the outside and ‘i’ the inside compartment, [W] is the water concentration (proportional to exp(-osmolarity/nw)), n is the coupling ratio (= 210), nw is the molarity of water (= 55 m), and Ψ the electrical potential (Zeuthen, 1996). If the extracellular Na+ concentration is ten times higher than the inside, glucose concentrations ([G]) the same and the membrane potential −50 mV, then it can be calculated that the inward water flux would proceed in spite of an adverse osmotic difference of up to about 1700 mosmol l−1; if the intracellular concentration of glucose was ten times higher than on the outside, the value would be 1100 mosmol l−1. It follows that the cotransport is able to overcome, and is quite insensitive to, the small osmotic differences that may occur physiologically (see Fig. 3).
The precise molecular mechanism underlying secondary active water transport is as yet unknown. It is, however, well established that a significant number of water molecules are bound and released by enzymatic proteins during conformational changes. This applies both to aqueous enzymes (Colombo, Rau Parsegian, 1992; Rand, Fuller, Butko, Francis & Nicholls, 1993; Quin, Haser & Payan, 1995) as well as to membrane-bound proteins (Zimmerberg & Parsegian, 1986; Kornblatt & Hoa, 1990); values in the range of 10–1200 water molecules are reported. We suggest that similar effects take place in membrane proteins of the cotransport type: water is taken up together with the other substrates on one side and released together with these at the other side of the membrane.
Cotransport of water may be relevant for several biological mechanisms. (i) In epithelial transport it is well established that the Na+ transport is the primary point of investment of metabolic energy. However, present models have difficulties in explaining how the large, and even uphill, water transports across epithelia such as kidney proximal tubules or small intestine are coupled to the Na+ transport (Zeuthen, 1996). The coupling of water and ion fluxes in membrane proteins could be an important building block for such models. We suggest that Na+-glucose-H2O cotransport takes place at the apical membrane. In that connection it is interesting to note the high degree of formal similarity between the water transport through the hSGLT1 (Fig. 3) and through the intact small intestine (Parsons & Wingate, 1961; Zeuthen & Stein, 1994) as functions of external osmolarity with and without sugar. The K+-Cl−-H2O cotransporter has been suggested to participate in the water transport at the serosal membrane (Zeuthen, 1994). In leaky epithelia fluid transport is isotonic with plasma, whereas the cotransport of Na+ and water demonstrated here is hypertonic. Most probably, the passive osmotic permeability of the hSGLT1 (for the rabbit SGLT1 see Zampighi et al. 1995; Loike et al. 1996; Loo et al. 1996; Zeuthen et al. 1997) and of other membrane proteins (i.e. aquaporins) play an important role in the final achievement of isotonic transport. (ii) In whole-body water homeostasis the re-uptake of water in the small intestine (9 l per day) and the kidney proximal tubule (180 l per day) are of vital importance. In the human small intestine the water cotransport described here could account for the re-uptake of 4 l of water each day given a daily uptake of 1 m of glucose. It will be important to confirm preliminary observations that brush-border Na+-amino-acid cotransporters also cotransport water (Zeuthen, 1995); this would contribute to the re-uptake of water. Finally, we have suggested a role for water cotransport in the re-uptake of neurotransmitters (Loo et al. 1996), the transport of amino-acids in plants (Loo et al. 1996) and neuro-retinal adhesion (Zeuthen et al. 1996).
Acknowledgments
We are grateful for the technical assistance of S. Christoffersen, B. Deublein, I. Kjeldsen and T. Soland. The volume recording system was designed in collaboration with B. Belhage and G. Larsen (Bio-Science). This study was supported by the Danish Research Council, Novo Nordisk and US Grants NIH NS 25554 and NSF MCB 9420599.
References
- Colombo MF, Rau DC, Parsegian VA. Protein solvation in allosteric regulation: A water effect on hemoglobin. Science. 1992;256:655–659. doi: 10.1126/science.1585178. [DOI] [PubMed] [Google Scholar]
- Diamond JM. Wet transport proteins. Nature. 1996;384:611–612. doi: 10.1038/384611a0. 10.1038/384611a0. [DOI] [PubMed] [Google Scholar]
- Gutiérrez AM, Gonzalez E, Echevarria M, Hernandez CS, Whittembury G. The proximal straight tubule (PST) basolateral cell membrane water channel: selectivity characteristics. Journal of Membrane Biology. 1995;143:189–197. doi: 10.1007/BF00233447. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Turk E, Wright EM. Homology of the human intestinal Na+/glucose and Escherichia coli Na+/proline cotransporters. Proceedings of the National Academy of Sciences of the USA. 1989;86:5748–5752. doi: 10.1073/pnas.86.15.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama BA, Loo DDF, Wright EM. Cation effects on protein conformation and transport in the Na+/glucose cotransporter. Journal of Biological Chemistry. 1997;272:2110–2115. doi: 10.1074/jbc.272.4.2110. 10.1074/jbc.272.4.2110. [DOI] [PubMed] [Google Scholar]
- Kornblatt JA, Hoa GHB. A nontraditional role for water in the cytochrome c oxidase reaction. Biochemistry. 1990;29:9370–9376. doi: 10.1021/bi00492a010. [DOI] [PubMed] [Google Scholar]
- Loike JD, Hickman S, Kuang K, Xu M, Cao L, Vera JC, Silverstein SC, Fischbarg J. Sodium-glucose cotransporters display sodium- and phlorizin-dependent water permeability. American Journal of Physiology. 1996;271:C1774–1779. doi: 10.1152/ajpcell.1996.271.5.C1774. [DOI] [PubMed] [Google Scholar]
- Loo DDF, Hazama A, Supplisson S, Turk E, Wright EM. Relaxation kinetics of the Na+/glucose cotransporter. Proceedings of the National Academy of Sciences of the USA. 1993;90:5767–5771. doi: 10.1073/pnas.90.12.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo DDF, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proceedings of the National Academy of Sciences of the USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B, Loo DDF, Wright EM. Relationship between Na+/glucose cotransporter (SGLT1) currents and fluxes. Journal of Membrane Biology. 1998;162 doi: 10.1007/s002329900347. in the Press. [DOI] [PubMed] [Google Scholar]
- Parent L, Supplison S, Loo DDF, Wright EM. Electrogenic properties of the cloned Na+/glucose cotransporter. Journal of Membrane Biology. 1992;125:49–62. doi: 10.1007/BF00235797. [DOI] [PubMed] [Google Scholar]
- Parsons DS, Wingate DL. The effect of osmotic gradients on fluid transfer across rat intestine in vitro. Biochimica et Biophysica Acta. 1961;46:170–183. doi: 10.1016/0006-3002(61)90660-6. 10.1016/0006-3002(61)90660-6. [DOI] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–389. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Quin M, Haser R, Payan F. Carbohydrate binding sites in a pancreatic a-amylase-substrate complex, derived from X-ray structure analysis at 2.1 A resolution. Protein Science. 1995;4:747–755. doi: 10.1002/pro.5560040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand RP, Fuller NL, Butko P, Francis G, Nicholls P. Measured change in protein solvation with substrate binding and turnover. Biochemistry. 1993;32:5925–5929. doi: 10.1021/bi00074a001. [DOI] [PubMed] [Google Scholar]
- Reuss L. ‘Active’ water transport? Journal of Physiology. 1996;497:1. doi: 10.1113/jphysiol.1996.sp021743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Molecular Biology of the Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Kreman M, Boorer KJ, Loo DDF, Bezanilla F, Chandy G, Hall JE, Wright EM. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. Journal of Membrane Biology. 1995;148:65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Kunig N, Loo DDF. Structure and function of cell-to-cell channels purified from the lens and of hemichannels expressed in oocytes. In: Latorre R, Saez JC, editors. From Ion Channels to Cell-to-Cell Conversations. Plenum Press; 1997. pp. 309–321. [Google Scholar]
- Zeuthen T. Secondary active transport of water across ventricular cell membrane of choroid plexus epithelium of Necturus maculosus. Journal of Physiology. 1991;444:153–173. doi: 10.1113/jphysiol.1991.sp018871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Cotransport of K+, Cl− and H2O by membrane proteins from choroid plexus epithelium of Necturus maculosus. Journal of Physiology. 1994;478:203–219. doi: 10.1113/jphysiol.1994.sp020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Molecular mechanisms for passive and active transport of water. International Review of Cytology. 1995;160:99–161. doi: 10.1016/s0074-7696(08)61554-5. [DOI] [PubMed] [Google Scholar]
- Zeuthen T. Molecular Mechanisms of Water Transport. Texas: R. G. Landes Company; 1996. p. 170. [Google Scholar]
- Zeuthen T, Hamann S, La Cour M. Cotransport of H+, lactate and H2O by membrane proteins in retinal pigment epithelium of bullfrog. Journal of Physiology. 1996;497:3–17. doi: 10.1113/jphysiol.1996.sp021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T, Meinild A-K, Klaerke D, Wright EM, Belhage B, Loo DDF, Litman T. Water transport by the Na+/glucose cotransporter under isotonic conditions. Biology of the Cell. 1997;89:307–312. doi: 10.1016/s0248-4900(97)83383-7. 10.1016/S0248-4900(97)83383-7. [DOI] [PubMed] [Google Scholar]
- Zeuthen T, Stein WD. Cotransport of salt and water in membrane proteins: Membrane proteins as osmotic engines. Journal of Membrane Biology. 1994;137:179–195. doi: 10.1007/BF00232587. [DOI] [PubMed] [Google Scholar]
- Zhang R, Verkman AS. Water and urea transport in Xenopus oocyte: expression of mRNA from toad urinary bladder. American Journal of Physiology. 1991;260:C26–34. doi: 10.1152/ajpcell.1991.260.1.C26. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Parsegian VA. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986;323:36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]


