Abstract
The noradrenaline (NA)-activated response was investigated in neurones acutely dissociated from the rat locus coeruleus (LC) using nystatin-perforated, conventional whole-cell and inside-out patch recording modes under current- and voltage-clamp conditions.
Under current-clamp conditions, NA hyperpolarized the LC neurones, abolishing the spontaneous action potentials. In voltage-clamp studies, NA induced an inwardly rectifying K+ current (INA) in a concentration-dependent manner with a half-maximum effective concentration of 2.2 × 10−7 m.
INA was mimicked by the α2-agonist UK14304 but was inhibited by either the α2B/α2C antagonist ARC239 or the α1- and α2B/α2C antagonist prazosin, suggesting the contribution of α2B/α2C adrenoceptors.
INA was inhibited by the intracellular application of GDPβS but fully activated by intracellular perfusion of GTPγS.
In the inside-out recording mode, the application of GTP to the cytoplasmic side of the patch membrane markedly enhanced the open probability of the NA-activated single channels which represented the inwardly rectifying properties.
These results indicate that the activation of α2B/α2C adrenoceptors coupled with GTP-binding protein directly activates the inwardly rectifying K+ currents in rat LC neurones, thus resulting in a decrease in the spontaneous firing activities.
The nucleus locus coeruleus (LC) contains the largest clusters of noradrenaline (NA)-containing neurones in the central nervous system (CNS) and also sends widespread projections throughout the CNS (Ungerstedt, 1971). The LC plays an important role in the control of cognitive and emotional processes including attention and anxiety (Foote, Bloom & Aston-Jones, 1983). To understand the noradrenergic function of the LC, it is important to elucidate how the adrenergic receptors regulate the excitability of the LC neurones through modulation of the ion channels as effectors.
The LC neurones have been reported to discharge spontaneous action potentials with a pacemaker-like regularity (Foote et al. 1983), and this spontaneous firing is reduced by the activation of α2-adrenoceptors (Williams & North, 1985). Experiments using LC slice preparations revealed that NA activates K+ channels coupled with G-proteins (North, Williams, Surprenant & Christie, 1987). However, the detailed properties of NA-activated K+ channel currents (INA) in the LC neurones examined in brain slice preparations still remain unclear, because of the complicated networks among neurones. To clarify the effect of NA on the LC neurones, it is therefore indispensable to use single neurones isolated from the surrounding neural structures. In the present study, we thus investigated the effects of NA on acutely dissociated rat LC neurones using the nystatin-perforated (Wakamori, Hidaka & Akaike, 1993; Akaike & Harata, 1994), conventional whole-cell and inside-out patch recording modes (Hamill, Marty, Neher, Sakmann & Sigworth, 1981). The use of these techniques on isolated LC neurones provides us with a number of advantages: (i) in isolated cells, the intrinsic properties of the LC neurones can be studied in the absence of synaptic inputs and transmitter uptake systems; (ii) the recording of either single channel or whole-cell currents can be made under good space-clamp conditions; and (iii) a rapid exchange of external solution surrounding a neurone can be obtained.
METHODS
Preparation
The neurones of the rat locus coeruleus were acutely dissociated as described previously (Nakagawa, Shirasaki, Wakamori, Fukuda & Akaike, 1990). Briefly, 2-week-old Wistar rats were decapitated under pentobarbitone anaesthesia (50 mg kg−1i.p.). The brain was quickly removed and was sliced at a thickness of 400 μm with a microslicer (DTK-1000; Dosaka, Kyoto, Japan). Following 30–60 min maintainance in the incubation medium saturated with 95 % O2 and 5 % CO2 at room temperature (21–23°C), the slices were treated with dispase (1700 picounits ml−1) at 31°C for 60 min. After the enzyme treatment, the slices were stored in the incubation medium for 1 h. Next, the region of the locus coeruleus was identified under a binocular microscope, and then micropunched out with an electrolytically polished injection needle. The micropunched-out pieces were mechanically triturated with fine glass pipettes in the standard external solution. After the dissociated neurones adhered to the bottom of the 35 mm culture dish (Primaria, Falcon, Lincoln Park, NJ, USA), electrophysiological recordings were made.
Electrical measurements
Electrical measurements were performed in the nystatin-perforated (Wakamori et al. 1993; Akaike & Harata, 1994), conventional whole-cell and inside-out patch recording modes (Hamill et al. 1981). The patch pipettes were made from borosilicate glass tubes (G-1.5; Narishige, Tokyo, Japan) using a vertical pipette puller (PB-7; Narishige). The resistance between the recording electrode filled with internal solution and the reference electrode in the standard external solution was 5–7 MΩ. In single channel experiments, the pipettes were coated with silicone (Shin-Etsu, Tokyo, Japan) near their tips to reduce the electrical capacitance.
The neurones were visualized with phase-contrast equipment on an inverted microscope (IMT-2; Olympus, Tokyo, Japan). The current and voltage were measured with a patch-clamp amplifier (CEZ-2400; Nihon Koden, Tokyo, Japan), monitored on both a storage oscilloscope (5100A; Iwatsu Electric, Tokyo, Japan) and a pen recorder (Recti-Horiz-8K; Nippondenki San-ei, Tokyo, Japan), and stored on magnetic tape with the use of a digital audiotape recorder (RD-130TE; TEAC, Tokyo, Japan). In single channel current recordings, data were filtered using a four-pole low-pass Bessel-type filter (FV-665; NF Electronic Instruments, Tokyo) with a -3 dB corner frequency of 1–2 kHz and sampled every 0.1-0.5 ms onto the hard disk of an IBM 386 computer (PS/V Entry; Nippon IBM, Tokyo, Japan) using pCLAMP software (version 6.0; Axon Instruments). Unitary current amplitudes were measured by forming histograms of the baseline and open-level datum points, and by fitting these histograms with Gaussian curves using a least-squares algorithm to find the area under each curve.
In the single channel current recordings, the inward currents are shown as downward deflections. The current-voltage (I-V) relationship was plotted against the inverted pipette potential (membrane potential, Vm). Neither the number of channels in the patch (N) nor the open probability of the individual channel (Po) could be determined. However, a variable NPo (the open probability of the patch) was determined by:
in which P(n) is the probability of the record staying at a level at which n channels open simultaneously (Friedrich, Paulmichl, Kolb & Lang, 1988).
Solutions
The ionic composition of the incubation medium was (mm): 124 NaCl, 5 KCl, 1.2 KH2PO4, 24 NaHCO3, 2.4 CaCl2, 1.3 MgSO4 and 10 glucose. The pH of the incubation medium was adjusted to 7.4 with 95 % O2 and 5 % CO2. The ionic composition of the standard external solution was (mm): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 Hepes. External solutions containing 10, 20 or 50 mm K+ were prepared by replacing NaCl with equimolar KCl. The pH of these external solutions was adjusted to 7.4 with tris (hydroxymethyl) aminomethane (Tris-OH). The ionic composition of the internal (patch pipette) solution for nystatin-perforated patch recordings was (mm): 20 NMG-methanesulphonate, 100 potassium methanesulphonate, 20 KCl, 5 MgCl2 and 10 Hepes. Nystatin was dissolved in methanol at 10 mg ml−1, and the stock solution was diluted with the internal solution just before use at a final concentration of 200 μg ml−1. The internal solution for conventional whole-cell patch recordings (Fig. 6) had the following composition (mm): 110 potassium methanesulphonate, 28 KCl, 5 Mg-ATP, 0.3 GTP (or GDPβS or GTPγS), 1 MgCl2, 5 EGTA and 10 Hepes. The pH of these internal solutions was adjusted to 7.2 with Tris-OH. The ionic composition of the patch pipette solution for single channel current recordings was (mm): 140 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 0.1 tetraethylammonium chloride (TEA-Cl), and 1 μm tetrodotoxin (TTX); the pH was adjusted to 7.4 with Tris-OH. The ionic composition of the intracellular solution for inside-out patch recordings was (mm): 106 potassium methanesulphonate, 40 NaCl, 2 Na2-ATP, 0.1 GTP (or GDP or GTPγS), 1 MgCl2, 5 EGTA and 5 Hepes; the pH was adjusted to 7.2 with Tris-OH.
Figure 6. Effects of GDPβS and GTPγS on INA.
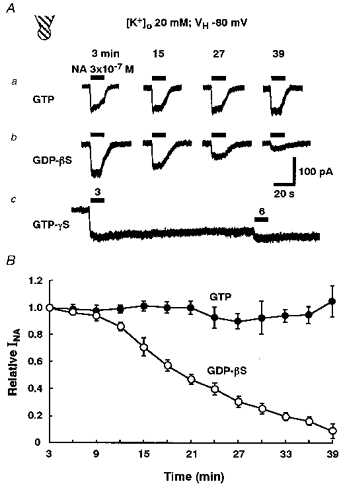
The neurones were intracellularly perfused with an internal solution containing each guanine nucleotide analogue by using the conventional whole-cell patch recording mode. All recordings were performed in external solution containing 20 mm K+ at a VH of −80 mV. A, representative recordings of INA after intracellular perfusion with internal solutions containing 0.3 mm GTP (a), 0.3 mm GDPβS (b) or 0.3 mm GTPγS (c). The horizontal bars indicate the period of 3 × 10−7 m NA application. The numbers above the horizontal bars show the time of intracellular dialysis. B, the relationship between the time of intracellular perfusion with GTP or GDPβS and the relative peak amplitude of INA. All responses were normalized to the peak amplitude of INA with intracellular perfusion for 3 min. Each point and the vertical bars represent the mean ±s.e.m. from 4 neurones.
Drugs
The drugs used in the present study included: dispase (Godo Shusei, Tokyo, Japan), nystatin, adrenaline, yohimbine, prazosin, quinine, N-ethylmaleimide (NEM), glycine, staurosporine, dibutyryl-cyclic AMP, H-89 (N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinoline sulphonamide HCl), guanosine 5′-triphosphate (GTP), guanosine 5′-diphosphate (GDP), guanosine 5′-O-2-thiodiphosphate (GDPβS), guanosine 5′-O-3-thiotriphosphate (GTPγS) (Sigma), noradrenaline (NA), 4-aminopyridine (4-AP), tetraethylammonium chloride (TEA-Cl) (Tokyo Kasei, Tokyo, Japan), UK14304 (5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline), PMA (phorbol-12-myristate-13-acetate; Research Biochemicals International), ARC239 (2-(2,4-(O-methoxyphenyl)-piperazin-1-yl)ethyl-4,4-dimethyl-1,3-(2H,4H)-isoquinolindione), ML-9 (1-(5-chloronaphthalene sulphonyl)homopiperazine; Tocris Cookson, UK), BaCl2 (Ishizu Seiyaku, Osaka, Japan), KN-62 (1-(N,O-bis[5-isoquinolinesulphonyl]-N-methyl-L-tyrosyl)-4-phenylpiperazine; Seikagaku Corp., Tokyo, Japan), genistein (Wako, Tokyo, Japan). The drugs insoluble in water were first dissolved in dimethylsulphoxide (DMSO) and were then further diluted in the external solution. The final concentrations of DMSO were always less than 0.1 %, at which concentration DMSO alone had no effect on the membrane potential or electrical activity. In the experiment to investigate the effect of K+ channel blockers on INA (Fig. 5), either quinine or BaCl2 was directly dissolved in the external solution. An external solution containing 4-AP or TEA was then prepared by replacing equimolar NaCl in the external solution with one of these agents.
Figure 5. Effects of K+ channel blockers on INA.
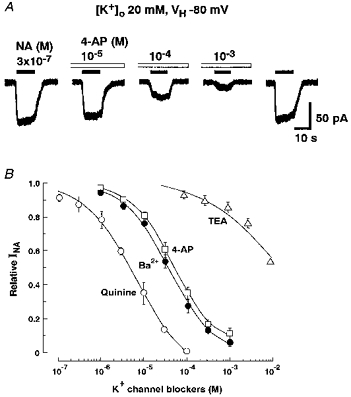
All recordings were performed in external solution containing 20 mm K+ at a VH of −80 mV. The neurones were pretreated for 1 min with each K+ channel blocker before the simultaneous application of 3 × 10−7 m NA. A, 4-aminopyridine (4-AP) inhibited INA in a concentration-dependent manner. B, the concentration-inhibition relationships for various K+ channel blockers on INA. All responses were normalized to the peak current induced by 3 × 10−7 m NA alone. Each point and the vertical bars represent the mean ±s.e.m. from 5 neurones.
The drugs were applied using a rapid application system termed the ‘Y-tube method’ (Murase, Randic, Shirasaki, Nakagawa & Akaike, 1990). With this technique, the external solution surrounding a neurone could be exchanged within 10–20 ms.
Statistical analysis
Each experimental value was presented as the mean ± standard error of the mean (s.e.m.) and was analysed by Student's paired t test. P values of less than 0.05 were considered significant.
For constructing the concentration-response curves, the data were fitted to a modified Michaelis-Menten equation using least-squares fitting: I= (ImaxCn)/(Cn+ EC50n), where I is the drug-induced current, Imax is the maximum response, C is the concentration of an agonist, EC50 is the concentration that evokes a half-maximum response and n is the Hill coefficient. For constructing concentration-inhibition curves, the data were fitted to the following equation using least-squares fitting: I= 1 − {Cn/(Cn+ IC50n)}, where I is the current amplitude normalized to the control response without an antagonist, C is the concentration of an antagonist, and IC50 is the concentration for half-inhibition.
RESULTS
NA responses
To characterize the NA responses, whole-cell current recordings were made on freshly dissociated rat LC neurones using the nystatin-perforated patch recording configuration. As shown in Fig. 1, the LC neurones showed spontaneous action potentials at frequencies between 1.5 and 3.5 Hz (2.7 ± 0.21 Hz, n= 8) under current-clamp conditions. The application of NA hyperpolarized the cell membrane and prevented the firing of action potentials. This effect was dependent upon the concentration of NA, and the membrane potential and firing rate both completely recovered after washing out the agonist.
Figure 1. Effect of NA on the membrane potential of an LC neurone.
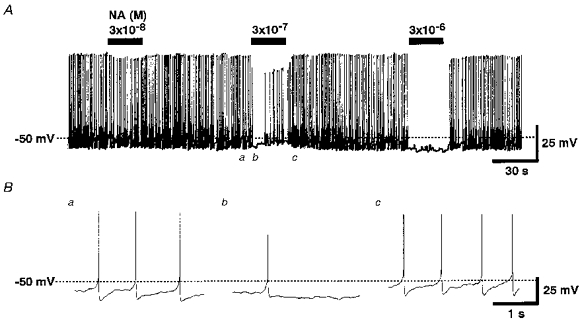
A, spontaneous action potentials recorded in the perforated patch recording configuration. NA (3 × 10−8, 3 × 10−7 and 3 × 10−6 m) was added to the external solution as indicated by the bars. The action potentials are not fully resolved because of the insufficiently high frequency response of the chart recorder. B, digitized potential traces at symbols a-c indicated in A.
Figure 2A shows the typical NA (10−6 M)-induced currents (INA) in normal external solution containing 5 mm K+ at various holding potentials (VH) under voltage-clamp conditions. During the application of NA, the membrane conductance was increased (not shown). The reversal potential (ENA) of INA estimated from the I-V relationship was −80.0 ± 0.23 mV (n= 5) in normal external solution containing 5 mm K+ (Fig. 2B). This value was almost identical to the theoretical K+ equilibrium potential (−80.5 mV) calculated from the Nernst equation, at the given K+ concentration of the external and pipette solutions. As the extracellular K+ concentration ([K+]o) was increased to 10, 20 and 50 mm, the ENA shifted to −61.0 ± 0.54 mV (n= 5), −43.3 ± 1.54 mV (n= 5) and −21.3 ± 1.09 mV (n= 5), respectively. The slope of the ENA values for a 10-fold change in [K+]o was 60 mV (Fig. 2B, inset). This finding thus indicates INA to be selectively carried by K+.
Figure 2. Activation of the inwardly rectifying K+ current by NA.
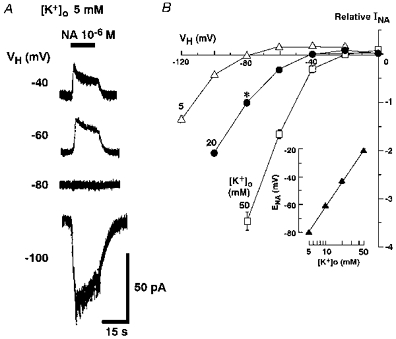
A, NA (10−6 m)-induced currents at various holding potentials (VH) in the same neurone perfused with external solution containing 5 mm K+. B, the current–voltage (I–V) relationship of NA-induced responses in external solution with three different K+ concentrations (5, 20 or 50 mm). The intracellular K+ concentration ([K+]i) was 120 mm throughout the experiments. All responses were normalized to the peak current induced by NA in external solution containing 20 mm K+ at a VH of −80 mV (*). The I–V curves of the NA-induced responses showed inward rectification. Each point and the vertical bars represent the mean ±s.e.m. from 5 neurones. The inset shows the relationship between the external K+ concentration ([K+]o) and the reversal potential (ENA) of NA-induced current. Each ENA was estimated based on the I–V relationship.
The I–V relationship of INA exhibited a unique inward rectification (Fig. 2). The inward rectification became prominent when the external solution contained K+ ions of high concentration. Therefore, to record INA with a relatively high signal/noise ratio, all subsequent whole-cell current recordings were made in an external solution containing 20 mm K+.
Effects of adrenoceptor agonists and antagonists
To clarify the subtypes of adrenoceptors participating in the induction of INA, the effects of adrenoceptor agonists and antagonists were examined. Figure 3A shows the currents induced by NA at various concentrations in external solution containing 20 mm K+ at a VH of −80 mV. The NA responses increased in a concentration-dependent manner. Adrenaline and a selective α2-adrenoceptor agonist, UK14304, mimicked the NA response. The peak amplitudes of the responses to these adrenoceptor agonists are plotted in Fig. 3B, in which the EC50 values and the Hill coefficients were 2.2 × 10−7 m and 0.88 for NA (n= 5), 4.0 × 10−8 m and 0.79 for adrenaline (n= 6), and 4.6 × 10−8 m and 0.95 for UK14304 (n= 6), respectively. The maximum currents induced by NA, adrenaline and UK14304 normalized to the peak amplitude of the 10−6 m NA-induced response were 1.29 ± 0.06 (n= 5), 1.03 ± 0.07 (n= 5) and 0.92 ± 0.02 (n= 5), respectively. The maximum responses induced by adrenaline and UK14304 were significantly smaller at P < 0.05 than those of NA. On the other hand, a partial agonist of the α2A-adrenoceptor, oxymethazoline, evoked no response, even at concentrations of up to 10−6 m (n= 4, data not shown).
Figure 3. Inward currents induced by adrenoceptor agonists.
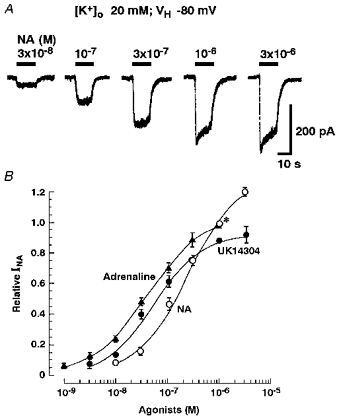
All recordings were performed in external solution containing 20 mm K+ at a VH of −80 mV. A, the NA-induced currents at various concentrations. B, the concentration-response relationship for various adrenoceptor agonists. All responses were normalized to the peak current induced by 10−6 m NA (*). Adrenaline and UK14304 mimicked the NA response. Each point and the vertical bars represent the mean ±s.e.m. from 5 neurones.
The LC neurones were perfused with external solution containing each NA antagonist for 15 s before simultaneous application of 10−6 m NA and one of the antagonists. A non-specific α2-adrenoceptor antagonist, yohimbine, reversibly inhibited INA in a concentration-dependent manner with an IC50 of 1.7 × 10−7 m (n= 5, Fig. 4A and B). An α1-blocker known to antagonize at the α2B/α2C receptor, prazosin, and an α2B/α2C antagonist, ARC239, also inhibited INA in a concentration-dependent fashion (Fig. 4B). The IC50 values for prazosin and ARC239 were estimated to be 3.6 × 10−6 m (n= 5) and 6.0 × 10−6 m (n= 5), respectively.
Figure 4. Effects of adrenoceptor antagonists on the NA-induced current (INA).

All recordings were performed in an external solution containing 20 mm K+ at a VH of −80 mV. The neurones were pretreated for 15 s with each adrenoceptor antagonist before the simultaneous application of 10−6 m NA. A, yohimbine inhibited INA in a concentration-dependent manner. B, the concentration-inhibition relationships for various NA antagonists on INA. All responses were normalized to the peak current induced by 10−6 m NA alone. Each point and the vertical bars represent the mean ±s.e.m. from 5 neurones.
Effects of K+ channel blockers on INA
To elucidate the pharmacological profile of the NA-gated K+ channel, the effects of various K+ channel blockers on INA were tested at a VH of −80 mV. The neurones were pretreated for 1 min with external solution containing various K+ channel blockers. Then 3 × 10−7 m NA and one of the blockers were applied simultaneously. Among the K+ channel blockers tested, quinine, Ba2+ and 4-AP reversibly inhibited INA in a concentration-dependent manner. The IC50 values for quinine, Ba2+ and 4-AP were 6.0 × 10−6 m (n= 5), 3.5 × 10−5 m (n= 5) and 4.3 × 10−5 m (n= 5), respectively (Fig. 5A and B). TEA partially blocked INA at high concentrations over 10−4 m (Fig. 5B, n= 5).
Contribution of G-protein
To test the involvement of G-protein in the NA response, the effects of intracellular perfusion with non-hydrolysable guanine nucleotide analogues (GDPβS and GTPγS) were examined using the conventional whole-cell patch recording mode. When the neurones were intracellularly perfused with an internal solution containing 0.3 mm GTP, the application of 3 × 10−7 m NA at 3 min intervals evoked an almost constant INA over 39 min (Fig. 6Aa and B). On the other hand, the intracellular application of GDPβS (0.3 mm) caused a ‘run-down’ of INA; INA declined to 9.2 ± 5.4 % (n= 4) of the initial current after 39 min dialysis (Fig. 6Ab and B). In the neurones intracellularly perfused with 0.3 mm GTPγS, the first brief application of NA resulted in an irreversible and continuous full activation of the K+ current. Therefore, the second and successive applications of NA at 3 min intervals produced scarcely any additional inward current (Fig. 6Ac).
It has been reported that a sulfhydryl alkylating agent, N-ethylmaleimide (NEM), uncouples the receptors from pertussis toxin (islet activating protein; IAP)-sensitive G-protein (Gi and/or Go) without either disrupting agonist receptor binding (Kitamura & Nomura, 1987) or blocking the IAP-insensitive signalling pathways (Shapiro, Wollmuth & Hille, 1994). Therefore, the effect of NEM on INA was examined. After exposing the neurone to external solution containing 5 × 10−6 m NEM for 2 min, INA declined significantly (Fig. 7A and B, n= 7). This inhibitory effect of NEM on INA persisted for more than 10 min after the wash-out of NEM (not shown). In contrast, the inward current induced by 3 × 10−5 m glycine was not affected by NEM treatment (Fig. 7).
Figure 7. Effect of NEM on INA.
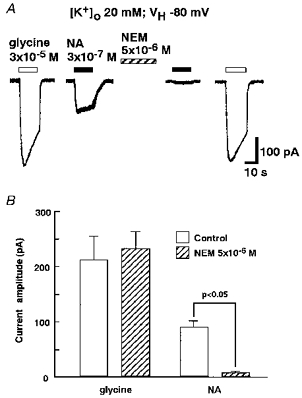
All recordings were performed in external solution containing 20 mm K+ at a VH of −80 mV. A, INA induced by 3 × 10−7 m NA was suppressed after the perfusion of external solution containing 5 × 10−6 m N-ethylmaleimide (NEM) for 2 min, whereas the 3 × 10−5 m glycine-induced response was not. B, the current amplitudes of NA- and glycine-induced responses before and after 2 min of NEM treatment were compared. Each column and the vertical bars represent the mean ±s.e.m. from 7 neurones.
It is well known that G-proteins are coupled to various intracellular signal transduction pathways. α2-Adrenoceptors have been shown to inhibit the activation of adenylate cyclase and thus reduce the levels of cAMP in NG108 cells (Sabol & Nirenberg, 1979) and HT29 human colonic adenocarcinoma cells (Bylund & Ray-Prenger, 1989). However, pretreatment (30 min) with a membrane-permeant cAMP, dibutyryl-cAMP (3 × 10−4 M, n= 6), had no effect on INA. To study the possibility of other types of second messenger-mediated activation of the K+ channel, several compounds acting on intracellular signalling pathways were examined. As a result, staurosporine (10−6 M, n= 3), H-89 (10−6 M, n= 3), genistein (10−5 M, n= 3), KN-62 (10−5 M, n= 3) and PMA (10−7 M, n= 3) were also found to have no effect on INA.
Characterization of the NA-induced single channel current
To investigate the single channel properties of INA, single channel current recording using the inside-out patch recording mode was performed. In patches filled with external solution without NA, no single channel activities were observed under our experimental conditions. When the patch pipette was filled with solution containing 10−7 m NA and the cytoplasmic side of the inside-out patch was superfused with bathing solution containing 0.1 mm GDP, infrequent openings of single channels were observed at a membrane potential of −60 mV (NPo; 0.010 ± 0.002, n= 6, Fig. 8). Perfusion of the cytoplasmic side with 0.1 mm GTP instead of GDP markedly enhanced the NA-gated single channel activity (NPo; 0.046 ± 0.008, n= 6, Fig. 8), thus suggesting the involvement of G-protein in the NA response. Figure 9A shows the NA-operated single channel current recordings at various membrane potentials, when the cytoplasmic side was bathed with internal solution containing 0.1 mm GTP. The amplitude histograms were obtained at each membrane potential. An example of the amplitude histogram of channel opening at −60 mV is shown in the inset of Fig. 9B. The mean amplitudes from five neurones were used to plot the I-V relationship (Fig. 9B). The I-V relationship represented an inwardly rectifying property of the single channel currents. The single channel conductance in the inward direction was 34.6 ± 0.7 pS (n= 5).
Figure 8. Effect of GDP-GTP exchange on the NA-induced single channel current.
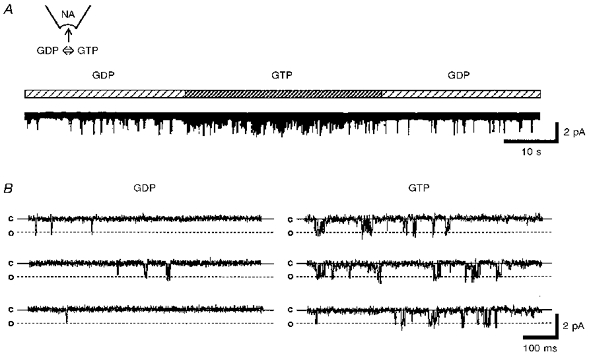
The inside-out patch recording mode was used to record the NA-induced single channel current. The patch pipette contained 10−7 m NA. The membrane potential (Vm) was held to −60 mV. A, 0.1 mm GDP and GTP were added to the bath solution as indicated by the horizontal bar above the record. B, digitized current traces obtained from A. The continuous lines show the closed level (c) and the dashed lines show the open level (o).
Figure 9. The I-V relationship of the NA-induced single channel current.
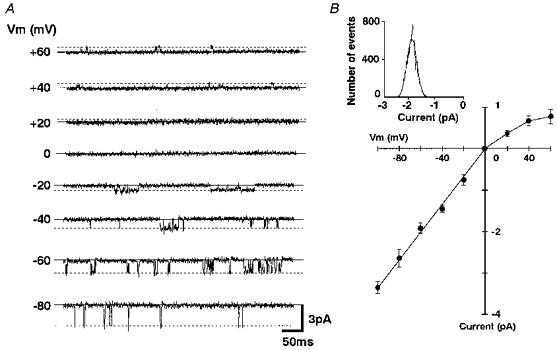
A, the NA-induced single channel current recordings obtained at various Vm values. The patch pipette contained 10−7 m NA. The continuous lines show the closed level and the dashed lines show the open level. B, I-V relationship of the NA-induced single channel currents. Each point and the vertical bars show the mean ±s.e.m. from 5 neurones. The inset shows an amplitude histogram of the currents recorded at −60 mV. The channel openings were fitted to the Gaussian curve with a peak value of −1.92 pA.
DISCUSSION
In the present study, we investigated the effect of NA on acutely dissociated rat LC neurones. Under current-clamp conditions, the dissociated LC neurones exhibited spontaneous pacemaker activity (Fig. 1) similar to that described in intracellular recordings from LC noradrenergic neurones in slice preparations (Williams, North, Shefner, Nishi & Egan, 1984). Since dissociated neurones are devoid of the afferent inputs that modulate the ionic conductances, the spontaneous firing of the action potentials observed in the present dissociated LC neurones might represent an intrinsic property of these LC neurones. In addition, the application of NA also produced hyperpolarization and reduced the firing frequency, thus indicating that some NA autoreceptors are located on the cell body and/or its proximal dendrites. These results also correlate with findings on rat LC slice preparations (Williams, Henderson & North, 1985) and cultured rat LC neurones (Masuko, Nakajima, Nakajima & Yamaguchi, 1986).
The present voltage-clamp studies showed that NA elicited inwardly rectifying K+ currents (Fig. 2) which were dependent on intracellular GTP acting via G-proteins (Figs 6 and 8). G-protein-activated inwardly rectifying K+ (GIRK) channels have been reported to be membrane proteins that conduct K+ currents near the resting membrane potential, and they are also important in controlling cell excitability (Wickman & Clapham, 1995). The GIRK channels have been shown to reflect the actions of G-protein coupled with the receptors for transmitters including acetylcholine, dopamine, 5-HT, GABA, somatostatin and noradrenaline (Inoue, Nakajima & Nakajima, 1988; Williams, Colmers & Pan, 1988; Penington, Kelly & Fox, 1993a; Sodickson & Bean, 1996; Katayama, Yakushiji & Akaike, 1997). So far, at least four members of the GIRK family of cDNAs, GIRK1, GIRK2, GIRK3 and GIRK4, are expressed in the CNS neurones (Duprat et al. 1995; Spauschus, Lentes, Wischmeyer, Dißmann, Karschin & Karschin, 1996). GIRK channels are tetramers and hence could exist as homo- or heteromeric complexes. In heterogenous expression systems, heteromeric channels, including GIRK1/GIRK2, GIRK1/GIRK3, GIRK1/GIRK4 and GIRK2/GIRK4, exhibit a strong inward rectification property when compared with homomeric channels (Duprat et al. 1995; Kofuji, Davidson & Lester, 1995; Lesage et al. 1995; Spauschus et al. 1996). However, it is not known which subunits of the GIRK channels are expressed and form the functional channels in the rat LC neurones. The subunit composition of the GIRK channels regulated by NA in rat LC neurones should thus be the target of further study.
It has been shown that IAP-sensitive G-proteins (Gi and/or Go) couple to the α2-adrenoceptors. Pretreatment with IAP eliminated the α2-adrenoceptor-mediated hyperpolarization in rat LC neurones (Aghajanian & Wang, 1986). In the present study, NEM, a sulfhydryl alkylating agent, attenuated INA. By alkylating the cysteine residues in Gi and/or Go, NEM uncouples the receptors from IAP-sensitive G-proteins. Although NEM non-selectively alkylates various targets, the present results support the involvement of Gi and/or Go types of G-protein in the NA-mediated responses.
In the present study, an α2-adrenoceptor agonist, UK14304, mimicked the NA responses in a concentration-dependent manner (Fig. 3), thus suggesting the contribution of the α2-adrenoceptor. The inhibition of INA by a non-selective α2-antagonist, yohimbine, also lends support to this hypothesis (Fig. 4). Recently, α2-adrenoceptors have been classified into three subtypes, α2A, α2B and α2C (Bylund, 1992). In the present study, a partial agonist of the α2A-adrenoceptor, oxymethazoline, evoked no INA-like response. On the other hand, both an α1- and α2B/α2C antagonist, prazosin, and an α2B/α2C antagonist, ARC239, inhibited INA in a concentration-dependent manner (Fig. 4). These findings indicate that the α2B/α2C adrenoceptors are involved in the activation of INA. There have been several studies showing that K+ channels in both central and peripheral neurones can be mediated by α2-adrenoceptors (North, 1988). However, the pharmacological information provided by these studies was insufficient to characterize the receptor subtypes. As a result, the present observations thus provide the first evidence for the involvement of pharmacologically identifiable α2B/α2C receptors in the inwardly rectifying K+ channel activation in rat LC neurones.
The activation of α2-adrenoceptors leads to the inhibition of adenylate cyclase and the attenuation of cAMP production in NG108 cells and HT29 human colonic adenocarcinoma cells. (Sabol & Nirenberg, 1979; Bylund & Ray-Prenger, 1989). However, in rat LC neurones, the application of dibutyryl-cAMP had no effect on INA. In addition, other second messenger modulators including staurosporine, H-89, genistein, KN-62 and PMA did not affect INA. At present, it thus seems most likely that NA-activated K+ channels in rat LC neurones do not operate via cytoplasmic diffusible second messengers. This is also supported by the observation that NA could activate K+ channels even under inside-out patch recording conditions (Figs 8 and 9).
In the inside-out patch recording mode, the exchange of GDP to GTP in the bathing solution superfusing the cytoplasmic side of the patch enhanced the NA-gated single channel activities (Fig. 8). This finding is also consistent with our results obtained from conventional whole-cell current recordings (Fig. 6). Penington, Kelly & Fox (1993b) observed four conductance levels in 5-HT-activated single inwardly rectifying K+ channels recorded from acutely dissociated adult rat dorsal raphe neurones using the cell-attached and outside-out patch recording modes. On the other hand, we observed only one conductance level in this study, which was the same one as that observed in single inwardly rectifying K+ channel studies using rat LC and hippocampal neurones (Miyake, Christie & North, 1989; Oh, Ho & Kim, 1995; Grigg, Kozasa, Nakajima & Nakajima, 1996). The single channel conductance in the inward direction (Vm < 0 mV) was 34.6 ± 0.7 pS (Fig. 9), a value close to that of the inwardly rectifying K+ single channel observed previously in cultured LC neurones and dissociated hippocampal neurones (Oh et al. 1995; Grigg et al. 1996). Among the cloned GIRK channels expressed in Xenopus oocytes, the single channel conductance of homomeric channels was 27–31 pS, and that of heteromeric channels was 36–38 pS (Duprat et al. 1995; Lesage et al. 1995; Spauschus et al. 1996; Velimirovic, Gordon, Lim, Navarro & Clapham, 1996). Our findings using single channel recording thus suggest that the NA-activated K+ channels in rat LC neurones might therefore be heteromeric channels which consist of members of the GIRK family.
Acknowledgments
This work was supported by Grants-in Aid for Scientific Research (No. 07407002) to N. Akaike from the Ministry of Education, Science and Culture of Japan.
References
- Aghajanian GK, Wang Y-Y. Pertussis toxin blocks the outward currents evoked by opiate and α2-agonists in locus coeruleus neurons. Brain Research. 1986;371:390–394. doi: 10.1016/0006-8993(86)90382-3. [DOI] [PubMed] [Google Scholar]
- Akaike N, Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Japanese The Journal of Physiology. 1994;44:433–473. doi: 10.2170/jjphysiol.44.433. [DOI] [PubMed] [Google Scholar]
- Bylund DB. Subtypes of α1- and α2-adrenergic receptors. FASEB Journal. 1992;6:832–839. doi: 10.1096/fasebj.6.3.1346768. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Ray-Prenger C. Alpha-2A and alpha-2B adrenergic receptor subtypes: attenuation of cyclic AMP production in cell lines containing only one receptor subtype. Journal of Pharmacology and Experimental Therapeutics. 1989;251:640–644. [PubMed] [Google Scholar]
- Duprat F, Lesage F, Guillemare E, Fink M, Hugnot J-P, Bigay J, Lazdunski M, Romey G, Barhanin J. Heterologous multimetric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochemical and Biophysical Research Communications. 1995;212:657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiological Reviews. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Friedrich F, Paulmichl M, Kolb HA, Lang F. Inward rectifier K channels in renal epithelioid cells (MDCK) activated by serotonin. Journal of Membrane Biology. 1988;106:149–155. doi: 10.1007/BF01871397. [DOI] [PubMed] [Google Scholar]
- Grigg JJ, Kozasa T, Nakajima Y, Nakajima S. Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurons. Journal of Neurophysiology. 1996;75:318–328. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nakajima S, Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. Journal of Physiology. 1988;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama J, Yakushiji T, Akaike N. Characterization of the K+ current mediated by 5-HT1A receptor in the acutely dissociated rat dorsal raphe neurons. Brain Research. 1997;745:283–292. doi: 10.1016/s0006-8993(96)01141-9. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Nomura Y. Uncoupling of rat cerebral cortical α2-adrenoceptors from GTP-binding proteins by N-ethylmaleimide. Journal of Neurochemistry. 1987;49:1894–1901. doi: 10.1111/j.1471-4159.1987.tb02452.x. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by Gβγ subunits and function as heteromultimers. Proceedings of the National Academy of Sciences of the USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. Journal of Biological Chemistry. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- Masuko S, Nakajima Y, Nakajima S, Yamaguchi K. Noradrenergic neurons from the locus coeruleus in dissociated cell culture: culture methods, morphology, and electrophysiology. Journal of Neuroscience. 1986;6:3229–3241. doi: 10.1523/JNEUROSCI.06-11-03229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M, Christie MJ, North RA. Single potassium channels opened by opioids in rat locus ceruleus neurons. Proceedings of the National Academy of Sciences of the USA. 1989;86:3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K, Randic M, Shirasaki T, Nakagawa T, Akaike N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Research. 1990;525:84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shirasaki T, Wakamori M, Fukuda A, Akaike N. Excitatory amino acid response in isolated nucleus solitarii neurons of rat. Neuroscience Research. 1990;8:114–123. doi: 10.1016/0168-0102(90)90063-k. [DOI] [PubMed] [Google Scholar]
- North RA. Drug receptors and the inhibition of nerve cells. British Journal of Pharmacology. 1988;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ. μ and δ receptors belong to a family of receptors that are coupled to potassium channels. Proceedings of the National Academy of Sciences of the USA. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh U, Ho Y-K, Kim D. Modulation of the serotonin-activated K+ channel by G protein subunits and nucleotides in rat hippocampal neurons. Journal of Membrane Biology. 1995;147:241–253. doi: 10.1007/BF00234522. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. Journal of Physiolgy. 1993a;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Unitary properties of potassium channels activated by 5-HT in acutely isolated rat dorsal raphe neurones. Journal of Physiology. 1993b;469:407–426. doi: 10.1113/jphysiol.1993.sp019820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol SL, Nirenberg M. Regulation of adenylate cyclase of neuroblastoma × glioma hybrid cells by α-adrenergic receptors. I. Inhibition of adenylate cyclase mediated by α receptors. Journal of Biological Chemistry. 1979;254:1913–1920. [PubMed] [Google Scholar]
- Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-protein is blocked by N-ethylmaleimide in rat sympathetic neurons. Journal of Neuroscience. 1994;14:7109–7116. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. Journal of Neuroscience. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spauschus A, Lentes K-U, Wischmeyer E, Dißmann E, Karschin C, Karchin A. A G-protein-activated inwardly rectifying K+ channel (GIRK4) from human hippocampus associates with other GIRK channels. Journal of Neuroscience. 1996;16:930–938. doi: 10.1523/JNEUROSCI.16-03-00930.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiologica Scandinavica. 1971;367(suppl.):1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Velimirovic BM, Gordon EA, Lim NF, Navarro B, Clapham DE. The K+ channel inward rectifier subunits form a channel similar to neuronal G protein-gated K+ channel. FEBS Letters. 1996;379:31–37. doi: 10.1016/0014-5793(95)01465-9. [DOI] [PubMed] [Google Scholar]
- Wakamori M, Hidaka H, Akaike N. Hyperpolarizing muscarinic responses of freshly dissociated rat hippocampal CA1 neurones. Journal of Physiology. 1993;463:585–604. doi: 10.1113/jphysiol.1993.sp019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiological Reviews. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. Journal of Neuroscience. 1988;8:3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Henderson G, North RA. Characterization of α2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience. 1985;14:95–101. doi: 10.1016/0306-4522(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA. Catecholamine inhibition of calcium action potentials in rat locus coeruleus neurones. Neuroscience. 1985;14:103–109. doi: 10.1016/0306-4522(85)90167-8. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]


