Abstract
The fetus develops cardiovascular adaptations to protect vital organs in situations such as hypoxia and asphyxia. These include bradycardia, increased systemic blood pressure and redistribution of the cardiac output. The extent to which they involve maternal or placenta influences is not known. The objective of the present work was to study the cardiac output distribution in response to hypoxia in the chick embryo, which is independent of the mother.
Fertilized eggs were studied at three incubation times (10–13 days, 14–16 days and 17–19 days of a normal incubation time of 21 days). Eggs were placed in a Plexiglass box in which the oxygen concentration could be changed. Eggs were opened at the air cell and a chorioallantoic vein was catheterized. Cardiac output distribution was measured with 15 μm fluorescent microspheres injected during normoxia, during the last minute of a 5 min period of hypoxia and after 5 min of subsequent reoxygenation.
Hypoxia caused a redistribution of the cardiac output in favour of heart (+17 to +160 % of baseline) and brain (+21 to +57 % of baseline) at the expense of liver (−3 to −65 % of baseline), yolk-sac (−46 to −77 % of baseline) and carcass (−6 to −33 % of baseline).
The magnitude of the changes in cardiac output distribution to the heart, brain, liver and carcass in response to hypoxia increased with advancing incubation time.
The data demonstrate the development of a protective redistribution of the cardiac output in response to hypoxia in the chick embryo from day 10 of incubation.
Hypoxia produces a range of protective mechanisms in the mature fetus, in particular effects on the cardiovascular system (Hanson, 1988; Giussani, Spencer & Hanson, 1994).
The first component of the response to acute hypoxia involves a chemoreflex, mediated by the peripheral (particularly the carotid) arterial chemoreceptors: their activation initiates an increase in vagal efferent activity to the heart, which produces a bradycardia, and an increase in sympathetic activity, which produces a vasoconstriction in the carcass and a rise in systemic blood pressure. Endocrine mechanisms then contribute to the response, in particular the rise in fetal plasma vasopressin and catecholamines, which contribute to the bradycardia and the vasoconstriction, respectively (Rurak, 1978; Cohen, Piasecki, Cohn, Young & Jackson, 1984). In addition, the rise in blood pressure may contribute to the bradycardia via a baroreflex, but the fall in heart rate is only transient and β-adrenergic influences to the heart produce an increase in heart rate after a few minutes of hypoxia.
In contrast to the fall in blood flow to the carcass, blood flow to vital organs, particularly the brain, heart and adrenal glands, increases (Cohn, Sacks, Heymann & Rudolph, 1974; Peeters, Sheldon, Jones, Makowski & Meschia, 1979). This appears to be mediated predominantly by the release of local vasodilator substances such as nitric oxide (van Bel, Sola, Roman & Rudolph, 1995) and adenosine (Kurth & Wagerle, 1992).
The above description concentrates on responses initiated within the fetus. However, because the experimental production of fetal hypoxia usually necessitates making the mother (Boddy, Dawes, Fisher, Pinter & Robinson, 1974) or the placenta (Jensen, Roman & Rudolph, 1991) hypoxic, it is possible that a substantial component of the fetal responses are not initiated in the fetus itself. For example, hypoxia might produce a rise in maternal catecholamines (Boddy, Jones, Mantell, Ratcliffe & Robinson, 1974) which will easily cross the placenta. Substances such as prostaglandins (Thorburn, 1992) will also be produced by the placenta during hypoxia and can enter the fetal circulation to produce cardiovascular effects.
Therefore, we decided to study the response to hypoxia in the chick embryo, a developing animal that is independent of its mother in that gas exchange takes place by diffusion through micropores in the egg shell to the vascular bed in the chorioallantoic membrane (CAM), which can be seen as an equivalent to the placenta (Metcalfe & Stock, 1993). The chick embryo has the advantage that hypoxia can be simply induced by lowering the oxygen concentration of its environment, it has a short incubation time, is relatively resistant to infections, gives easy access to the arteries and veins of the CAM, and is cheap. Therefore, the chick embryo is an attractive animal model for perinatal cardiovascular research.
Not much is known about the response of the chick embryo to hypoxia during the second half of its incubation period, although Tazawa (1981b) reported a decrease in heart rate and blood pressure in response to hypoxia in embryos at 14–16 days. In a previous study, we found that heart rate and blood flow to the CAM decreased in response to hypoxia in chick embryos at 9–16 days (van Golde, Mulder & Blanco, 1996a). This fall in blood pressure appears to contrast with the fetus, in which hypoxia produces an increase.
The aim of the present study was to examine whether the redistribution of the cardiac output (CO) in response to hypoxia in the chick embryo is similar to that found in the mammalian fetus and to examine whether the response to hypoxia changes during the development of the chick embryo. This would permit us to evaluate further the use of the chick embryo for perinatal cardiovascular research by using fluorescent microspheres.
METHODS
White Leghorn fertilized eggs were incubated in a commercial incubator at a temperature of 38°C and a humidity of 60 %. Eggs were rotated constantly to avoid adhesions between the embryo and its membranes (Tazawa, 1981a). For White Leghorn eggs, incubation time until hatching is 21 days. In the present study, we used chick embryos of 10–19 days incubation, corresponding to stages 36–45 of Hamburger & Hamilton (1951). To document the response to hypoxia, we used thirty-five chick embryos at three different incubation times: 10–13 days (n= 10), 14–16 days (n= 13), 17–19 days (n= 12).
Preparation
To determine the distribution of the CO, a chorioallantoic vein was catheterized for injections of fluorescent microspheres. Both catheterization of the chorioallantoic vein and the injection of fluorescent microspheres were performed in a clinical infant incubator, provided with a light microscope (WILD 3M; magnification, ×10). Temperature and humidity were maintained constant at 38°C and 60 %, respectively. Eggs were opened with an electric saw at the air cell and placed in a holder within a Plexiglass box. The oxygen concentration in the box was changed by supplying different mixtures of O2 and N2 at a constant flow of 5 l min−1. After opening the shell, the outer shell membrane became visible. Moistening this membrane with a 0.9 % NaCl solution made the vessels of the underlying CAM visible. Without damaging the chorioallantoic membrane vessels, the outer shell membrane was removed. A chorioallantoic vein was identified by its bright red colour and erythrocyte flow direction. The vein was lifted using sutures, opened with the tip of a 30 gauge needle and a polyethylene catheter, stretched by heat to a diameter of 100 μm, was inserted. The catheter, flushed with heparinized saline (10 i.u. ml−1), was connected to a 1.0 ml syringe and fixed to the egg shell with clay.
Protocol
For the measurement of CO distribution, fluorescent microspheres with a diameter of 15 μm were used (Fluospheres, Molecular Probes). Spheres were suspended in saline and 0.05 % Tween 80 (100000 spheres ml−1). Five minutes after catheterization, 0.2 ml (20000 spheres) of the suspension of orange fluorescent microspheres was injected. One minute later, the oxygen concentration was changed to 0.0 % by supplying 100 % N2 to the box. In the last minute of a 5 min period of hypoxia, 20000 yellow-green fluorescent microspheres were injected. Normoxia was re-established and after 5 min reoxygenation 20000 red fluorescent microspheres were injected. The chick embryos were decapitated immediately after the experiment without any prior manipulation and the CAM, brain, heart, lungs, intestine, liver and the yolk-sac were dissected.
Since we injected 0.6 ml fluid in each experiment, it could have affected the CO distribution in the embryo. Furthermore, the injected microspheres could have occluded part of a vascular bed, which might have effects on the distribution of the following microsphere injection. In order to document any effect of the volume and amount of microspheres injected, we carried out the same protocol as described above without changing the environmental oxygen supply in eleven chick embryos, in three incubation time groups: 10–13 days, n= 3; 14–16 days, n= 4; 17–19 days, n= 4.
In a further group of chick embryos at incubation time 13–15 days (n= 8), blood samples were obtained for blood gas analysis by puncturing the chorioallantoic artery. pH and PO2 values were determined after 2 and 5 min of hypoxia. Values were corrected for embryo temperature.
Microsphere distribution of measurement
Organs and the remaining carcass were digested in test tubes in a 2 m ethanol-KOH solution. The microspheres were isolated from the homogenate by centrifugation, a method shown to result in recovery of close to 100 % of the microspheres (van Oosterhout, Willigers, Reneman & Prinzen, 1995). The dye was extracted with 3 ml of 2-(2-ethoxyethoxy)ethylacetate and the fluorescence measured by fluorimetry using a LS-50B fluorospectrometer (Perkin Elmer). No correction for spectral overlap was used since the excitation and emission spectra of the three dyes were well separated. During fluorimetry, all samples had the same volume (3 ml). The fraction of CO that was directed to the tissue was expressed as the level of the fluorescence, corrected for background, of the sample divided by the sum of fluorescence of all tissues.
Analysis of data
All data were processed using SPSS software (SPSS Inc.). Since not all data obtained were normally distributed, we expressed it as median with interquartile range (IQR). For analysis of the changes in CO distribution in response to hypoxia, a non-parametric sample test (Wilcoxon ranked sign test) was applied. For analysis of changes during development, we used the Mann-Whitney U rank sum test. Significance was accepted by P < 0.05.
RESULTS
To study the possible effects of the amount of microspheres and the volume injected during the experiment, we determined the CO distribution after each of three injections of fluorescent microspheres without changing the environmental oxygen supply. We did not find any statistically significant difference between the CO distributions measured after each injection for each organ and each incubation time.
In normoxia a large fraction of the cardiac output was directed to the CAM (median range for the three groups: 40.5–55.6 %) with relatively small fractions directed to the heart (range: 1.7–4.0 %), lungs (range: 0.4–1.1 %), brain (range: 3.0–4.2 %), intestine (range: 1.9–3.6 %), and liver (range: 1.3–3.1 %; Fig. 1).
Figure 1. Cardiac output distribution for increasing incubation time.
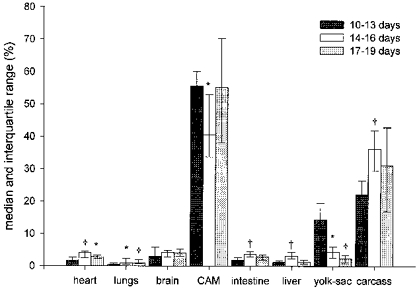
Bars represent median with interquartile range (p25-p75). *P < 0.05 compared with 10–13 days group; †P < 0.01 compared with 10–13 days group.
The exposure of the egg to 100 % N2 induced an isocapnic hypoxaemia with mean Pa,O2 values after 2 min of 9.3 mmHg (IQR: 6.4–11.2 mmHg) and after 5 min of 8.1 mmHg (IQR: 7.3–10.1 mmHg) with pH values of 7.43 (IQR: 7.41–7.45) and 7.41 (IQR: 7.41–7.43), respectively.
In response to hypoxia, we found a redistribution of the cardiac output in favour of heart, brain and CAM at the expense of intestine, liver, yolk-sac and carcass in all groups except in the 10–13 day group (Table 1 and Fig. 2). The fractions of CO directed to heart and brain in both the 14–16 and the 17–19 day groups increased significantly (P < 0.05). The fraction distributed to the CAM increased in the 14–16 day group, but not in the 17–19 day group. The fractions distributed to liver, yolk-sac and carcass decreased in the 14–16 day group in response to hypoxia. In the 17–19 day group, the fractions distributed to intestine, liver and carcass decreased. The changes observed in the organs of the youngest (10–13 days) group were statistically not significant and the fraction of the CO directed to the lungs did not change in any group.
Table 1.
Cardiac output distribution in the chick embryo in normoxia, hypoxia and after 5 min of reoxygenation for three different incubation time groups
| 10–13 days (n = 10) | 14–16 days (n = 13) | 17–19 days (n = 12) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Reoxygen | Normoxia | Hypoxia | Reoxygen | Normoxia | Hypoxia | Reoxygen | |
| Heart | 1.73 | 2.44 | 4.34* | 4.04 | 5.72† | 5.56* | 2.83 | 6.95* | 3.45* |
| 1.42–2.73 | 1.97–2.77 | 2.45–5.21 | 2.77–4.54 | 4.45–7.07 | 4.57–6.50 | 2.38–3.32 | 4.40–10.94 | 2.49–7.66 | |
| Brain | 3.03 | 4.01 | 6.42 | 4.20 | 6.40 | 8.72† | 4.10 | 9.09† | 8.83† |
| 2.64–5.89 | 3.23–4.93 | 4.77–8.83 | 2.97–4.75 | 4.71–8.90 | 6.22–9.70 | 3.11–5.17 | 7.28–9.85 | 6.72–11.64 | |
| CAM | 55.56 | 64.48 | 48.33 | 40.45 | 56.72* | 38.90 | 55.18 | 56.65 | 56.16 |
| 49.04–60.00 | 50.97–71.19 | 37.54–58.72 | 33.85–52.86 | 42.89–64.27 | 29.13–47.01 | 38.04–70.16 | 40.12–75.78 | 34.96–65.03 | |
| Lungs | 0.41 | 0.78 | 0.79 | 0.89 | 1.51 | 1.07 | 1.05 | 1.38 | 0.94 |
| 0.35–0.88 | 0.60–1.33 | 0.64–1.13 | 0.59–2.31 | 0.97–2.73 | 0.54–2.23 | 0.72–1.96 | 0.51–1.66 | 0.45–1.25 | |
| Intestine | 1.92 | 2.85 | 3.47* | 3.64 | 2.96 | 5.56* | 2.92 | 1.36* | 3.25 |
| 1.26–2.75 | 1.64–3.21 | 1.94–4.74 | 3.07–4.44 | 1.91–5.02 | 3.77–7.96 | 2.00–3.42 | 0.76–2.11 | 2.07–6.94 | |
| Liver | 1.25 | 1.27 | 1.81* | 3.08 | 0.82† | 1.92* | 1.31 | 0.48* | 1.71 |
| 0.84–1.74 | 0.76–1.70 | 1.65–2.45 | 2.26–4.33 | 0.66–1.78 | 1.60–3.06 | 0.73–1.91 | 0.31–0.69 | 0.92–2.26 | |
| Yolk sac | 14.32 | 2.80 | 9.18 | 4.44 | 0.59† | 3.91 | 2.29 | 1.40 | 1.30† |
| 4.63–19.48 | 1.74–8.54 | 5.64–14.1 | 2.71–6.05 | 0.47–1.64 | 2.95–4.38 | 1.11–3.31 | 0.32–2.61 | 0.79–1.80 | |
| Carcass | 22.01 | 22.30 | 25.00 | 36.22 | 22.48† | 34.50 | 31.10 | 21.39† | 25.19 |
| 19.44–26.41 | 16.47–24.85 | 22.16–30.31 | 29.67–41.94 | 17.73–30.31 | 28.91–43.38 | 17.08–42.78 | 7.84–27.64 | 18.19–34.91 | |
Shown are median percentage of CO directed to each organ and interquartile range. Reoxygen, reoxygenation.
Significant difference compared with normoxia (P < 0.05)
significant difference compared with normoxia (P < 0.01).
Figure 2. Cardiac output distribution in the chick embryo in normoxia, hypoxia and after 5 min of reoxygenation for three different incubation time groups.
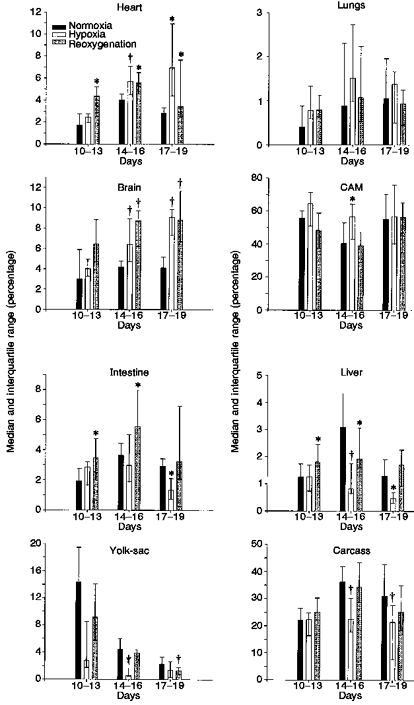
Bars represent median percentage of CO directed to each organ and interquartile range (p25-p75). * Significant difference compared with normoxia (P <0.05); † significant difference compared with normoxia (P <0.01).
The magnitude of the response expressed as a percentage of baseline level increased with increasing incubation time. We found a statistically significant (P < 0.05) increase in response between the 10–13 day group and the 17–19 day group for heart, brain, intestine, liver and carcass (Fig. 3).
Figure 3. Magnitude of response to hypoxia for increasing incubation time.
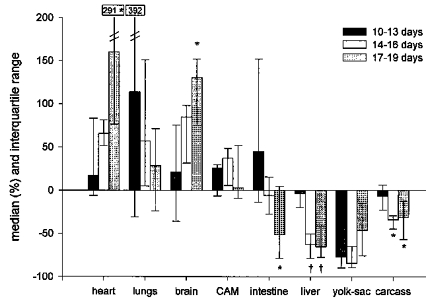
The bars represent the median change expressed as percentage of baseline level with the interquartile range (p25-p75). *P < 0.05 compared with 10–13 days group; †P <0.01 compared with 10–13 days group.
Five minutes after the start of reoxygenation, we found that CO distribution had still not returned to baseline level (Table 1 and Fig. 2). The fraction of CO directed to the heart was significantly greater than baseline level (P < 0.05) in all three groups. The same was found for the fractions to the brain in the 14–16 day and 17–19 day groups. In the organs in which flow had fallen in hypoxia, it increased during reoxygenation, even being significantly greater than control in the intestine in the 10–13 and 14–16 day groups and liver in the 10–13 day group. Flow remained below control in the yolk-sac in the 17–19 day group.
DISCUSSION
Response to hypoxia
In the present study, we found that there was a redistribution of CO in response to hypoxia in the chick embryo after 13 days of incubation. During hypoxia the fraction of CO directed to heart, brain and CAM increased at the expense of intestine, liver, yolk-sac and carcass. The increase in the fraction directed to the heart ranged from 17 to 160 %, to the brain from 21 to 130 % and to the CAM from 3 to 37 % of baseline level. The changes in the 10–13 day group were not statistically significant, but became larger and more significant in the older groups. This suggests that there is a development in the compensatory ability of the chick embryo to provide oxygen delivery to these vital organs under hypoxic conditions. The redistribution of the CO in response to hypoxia, favouring the heart and brain at the expense of intestine, liver and carcass is similar to that found in mammalian fetuses of the sheep (Jensen et al. 1991; Peeters et al. 1979), rhesus monkey (Jackson, Piasecki & Novy, 1987) and llama (Giussani et al. 1996). Since the chick embryo is independent of the mother, the similarity in response to hypoxia suggests that in the mammalian fetus such responses may occur independently of maternal or placental factors.
The magnitude of the response to hypoxia increased significantly (P < 0.05) as incubation time advanced. In the youngest (10–13 days) group, there was no change compared with baseline (P > 0.05). However, the magnitude of the response in the older groups increased significantly (P < 0.05). These data suggest a maturation of this protective reflex in the second half of the incubation time. Iwamoto, Kaufman, Keil & Rudolph (1989) have described a similar maturation in the response to hypoxia in the sheep fetus, comparing the response at 60 % of full gestation with that at 70 % full gestation and near term. In the fetus at 60 % of full gestation sheep there was no change in blood flow to the carcass in response to hypoxia, whereas in the fetus at 70 % of full gestation there was a decrease in blood flow to the carcass, suggesting that peripheral vasoconstrictor response has developed by this time (Iwamoto et al. 1989). Not much is known about the development of oxygen sensitive chemoreceptors, such as aortic and carotid bodies, during gestation, but it is reported that afferent chemoreceptor activity is present in fetal sheep at 90 days of gestation (60 % gestation; Blanco, Dawes, Hanson & McCooke, 1984). In the chick embryo, sympathetic nerve axons reach the heart at day 13 but they do not release sufficient neurotransmitter to change cardiac function until day 16 (Hu & Clark, 1989). Parasympathetic neurones reach the heart at day 13 but are also not functional until day 16 (Hu & Clark, 1989). However, adrenergic and cholinergic receptors have been identified in the myocardium and peripheral vascular beds as early as day 3 (Hu & Clark, 1989). Girard (1973) demonstrated an increasing blood pressure response to noradrenaline in the chick embryo from day 6 to 17. It is not yet clear how much hormonal and neural mechanisms contribute to the distribution of CO during hypoxia in the developing chick embryo. Further studies are being conducted to answer this question.
CO distribution after reoxygenation
The cardiac output distribution determined after 5 min of reoxygenation differs from baseline level in some tissues. The fractions of the CO directed to heart, brain, intestine and liver were above baseline and those to CAM, yolk-sac and carcass were below baseline. In a previous study, we reported an overshoot in CAM blood flow during reoxygenation, after a 5 min period of hypoxia (van Golde et al. 1996a). This suggests that in the reoxygenation phase, CO increases and blood flow to heart, brain, intestine and liver increase, providing more oxygen and nutrients to these tissues than in normoxia before hypoxia. The effect may be the result of circulating catecholamines and local factors such as nitric oxide and adenosine released during the hypoxia period and the immediate posthypoxic period. Recently, a similar response was reported in the sheep fetus at 75 % of gestation 1 h after an 8 h period of hypoxia (Richardson, Korkola, Asano, Challis, Polk & Fraser 1996), in that heart rate and carcass blood flow were increased compared with baseline. They did not find increased blood flow to the brain. Interestingly, we observed changes in CO distribution in the reoxygenation phase in the youngest (10–13 day) group, whereas we did not observe a response to hypoxia in this group. An explanation might be that the vasodilatory properties of the different vascular beds develop at an earlier stage than the vasoconstrictory properties.
Baseline measurements
Under normoxic conditions, the distribution of CO in the chick embryo is comparable with that reported in the sheep fetus. Two vascular beds received a major percentage of the combined cardiac output: the CAM and yolk-sac, responsible for gas exchange and nutrient delivery to the chick embryo, respectively, and therefore comparable with the placenta in the mammalian fetus (Metcalfe & Stock, 1993). Relatively small fractions are distributed to heart, brain, lungs and abdominal organs. This distribution of the cardiac output is similar to the data we previously found in a larger group of chick embryos (Mulder, van Golde, Prinzen & Blanco, 1997). A similar distribution was reported in the late gestation sheep fetus by Jensen et al. (1991).
The chick embryo as a model
In this study we found that in the second half of its incubation time, the chick embryo is a feasible animal for perinatal cardiovascular research in that determining CO distribution in normoxia, hypoxia and after reoxygenation using fluorescent microspheres is possible.
Fluorescent microspheres were introduced and validated for the study of CO, CO distribution and organ blood flow and their accuracy is similar to that of the radioactive microsphere method. They have the advantage that they do not require radioactivity (Glenny, Bernard & Brinkley, 1993; van Oosterhout et al. 1995) but a slight disadvantage is that the dye concentration cannot be measured directly. Instead, microspheres have to be isolated from the tissues by digestion. This was successful in all chick embryo tissues in the present and previous study (Mulder et al. 1997) as for many organs of adult animals (van Oosterhout et al. 1995). The microsphere method would permit determination of absolute organ blood flow data if an arterial reference blood sample could be obtained simultaneously with the microsphere injection. This was not possible due to the difficult access to the chick embryo and the limited blood volume; therefore we are unable to present actual organ blood flow rates.
In order to document any effect of the microsphere and volume injection itself we examined eleven chick embryos, in which we injected the same three dyes of microspheres in the same volume, without inducing hypoxia. Since no significant change was observed we conclude that the injection of fluorescent microspheres and the volume injected did not affect the measurement of the CO per se.
During incubation the concentrations in the air cell of the egg of both oxygen and carbon dioxide change. Oxygen concentration falls from 21 % at day 4 to 12 % at day 20 and the carbon dioxide concentration increases from 0.6 % at day 4 to 6 % at day 20 (Romijn & Roos, 1938). Therefore, the opening of the air cell of the egg could have had consequences on the embryonic blood gases, influencing CO distribution. However, in a previous study we could not demonstrate any change in arterial PO2, PCO2 and pH resulting from the opening of the air cell (van Golde, Mulder, van Straaten & Blanco, 1996b).
Conclusions
We showed that the chick embryo shows a redistribution of the CO in response to hypoxia and subsequent reoxygenation. Hypoxia favours blood flow to heart and brain at the expense of intestine, liver, yolk-sac and carcass. The observed redistribution of the CO in reponse to hypoxia is not significant at 10–13 day incubation time but develops during the last third of incubation. In the reoxygenation phase CO is redistributed in favour of heart, brain, intestine and liver compared with baseline measurements, a response which is already present at 10–13 days of incubation.
The present study provides a feasible animal model for hypoxia studies in perinatal cardiovascular research, due to its simplicity and the independence of the chick embryo from maternal influences. This model opens new possibilities for future studies that will help us to understand the consequences of hypoxia and asphyxia and their underlying mechanisms during fetal cardiovascular development.
Acknowledgments
We wish to thank the staff of the laboratories of Anatomy-Embryology and Physiology for their assistance in the study.
References
- Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. Journal of Physiology. 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Fisher R, Pinter S, Robinson JS. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. Journal of Physiology. 1974;243:599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K, Jones CT, Mantell C, Ratcliffe JG, Robinson JS. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology. 1974;94:588–591. doi: 10.1210/endo-94-2-588. [DOI] [PubMed] [Google Scholar]
- Cohen WR, Piasecki GJ, Cohn HE, Young JB, Jackson BT. Adrenal secretion of catecholamines during hypoxemia in fetal lambs. Endocrinology. 1984;114:383–390. doi: 10.1210/endo-114-2-383. [DOI] [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. American Journal of Gynaecology and Obstetrics. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- Girard H. Adrenergic sensitivity of circulation in the chick embryo. American Journal of Physiology. 1973;224:461–469. doi: 10.1152/ajplegacy.1973.224.2.461. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JAD, Hanson MA. Fetal cardiovascular reflex responses to hypoxaemia. Fetal and Maternal Medicine Review. 1994;6:17–37. [Google Scholar]
- Giussani DA, Riquelme RA, Moraga FA, McGarrigle HH, Gaete CR, Sanhueza EM, Hanson MA, Llanos AJ. Chemoreflex and endocrine components of cardiovascular responses to acute hypoxemia in the llama fetus. American Journal of Physiology. 1996;271:R73–83. doi: 10.1152/ajpregu.1996.271.1.R73. [DOI] [PubMed] [Google Scholar]
- Glenny RW, Bernard S, Brinkley M. Validation of fluorescent-labeled microspheres for measurement of regional organ perfusion. Journal of Applied Physiology. 1993;74:2585–2597. doi: 10.1152/jappl.1993.74.5.2585. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Hanson MA. The importance of baro- and chemoreflexes in the control of the fetal cardiovascular system. Journal of Developmental Physiology. 1988;10:491–511. [PubMed] [Google Scholar]
- Hu N, Clark EB. Hemodynamics of the stage 12 to stage 29 chick embryo. Circulation Research. 1989;65:1665–1670. doi: 10.1161/01.res.65.6.1665. [DOI] [PubMed] [Google Scholar]
- Iwamoto HS, Kaufman T, Keil LC, Rudolph AM. Responses to acute hypoxemia in fetal sheep at 0.6-0.7 gestation. American Journal of Physiology. 1989;256:H613–620. doi: 10.1152/ajpheart.1989.256.3.H613. [DOI] [PubMed] [Google Scholar]
- Jackson BT, Piasecki GJ, Novy MJ. Fetal responses to altered maternal oxygenation in rhesus monkey. American Journal of Physiology. 1987;252:R94–101. doi: 10.1152/ajpregu.1987.252.1.R94. [DOI] [PubMed] [Google Scholar]
- Jensen A, Roman C, Rudolph AM. Effects of reducing uterine blood flow on fetal blood flow distribution and oxygen delivery. Journal of Developmental Physiology. 1991;15:309–323. [PubMed] [Google Scholar]
- Kurth CD, Wagerle LC. Cerebrovascular reactivity to adenosine analogues on 0.6-0.7 gestation and near-term fetal sheep. American Journal of Physiology. 1992;262:H1338–1342. doi: 10.1152/ajpheart.1992.262.5.H1338. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Stock MK. Current topic: oxygen exchange in the chorioallantoic membrane, avian homologue of the mammalian placenta. Placenta. 1993;14:605–613. doi: 10.1016/s0143-4004(05)80378-9. [DOI] [PubMed] [Google Scholar]
- Mulder ALM, van Golde JC, Prinzen FW, Blanco CE. Cardiac output distribution in the chick embryo stage 36 to 45. Cardiovascular Research. 1997;34:525–528. doi: 10.1016/s0008-6363(97)00065-5. 10.1016/S0008-6363(97)00065-5. [DOI] [PubMed] [Google Scholar]
- Peeters LLH, Sheldon RE, Jones MDJ, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. American Journal of Obstetrics and Gynecology. 1979;135:637–646. doi: 10.1016/s0002-9378(16)32989-1. [DOI] [PubMed] [Google Scholar]
- Richardson B, Korkola S, Asano H, Challis J, Polk D, Fraser M. Regional blood flow and the endocrine response to sustained hypoxemia in the preterm ovine fetus. Pediatric Research. 1996;40:337–343. doi: 10.1203/00006450-199608000-00024. [DOI] [PubMed] [Google Scholar]
- Romijn C, Roos J. The air space of the hen's egg and its changes during the period of incubation. Journal of Physiology. 1938;94:365–379. doi: 10.1113/jphysiol.1938.sp003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rurak DW. Plasma vasopressin levels during hypoxaemia and the cardiovascular effects of exogenous vasopressin in foetal and adult sheep. Journal of Physiology. 1978;277:341–357. doi: 10.1113/jphysiol.1978.sp012275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa H. Adverse effect of failure to turn the avian egg on the embryo oxygen exchange. Respiratory Physiology. 1981a;41:137–142. doi: 10.1016/0034-5687(80)90047-x. 10.1016/0034-5687(80)90047-X. [DOI] [PubMed] [Google Scholar]
- Tazawa H. Effect of O2 and CO2 in N2, He and SF6 on chick embryo blood pressure and heart rate. Journal of Applied Physiology. 1981b;51:1017–1022. doi: 10.1152/jappl.1981.51.4.1017. [DOI] [PubMed] [Google Scholar]
- van Bel F, Sola A, Roman C, Rudolph AM. Role of nitric oxide in the regulation of the cerebral circulation in the lamb fetus during normoxemia and hypoxemia. Biology of the Neonate. 1995;68:200–210. doi: 10.1159/000244238. [DOI] [PubMed] [Google Scholar]
- van Golde J, Mulder T, Blanco CE. Changes in mean chorioallantoic blood flow and heart rate elicited by hypoxia in the developing chick embryo. Pediatric Research. 1996a;39:69A. doi: 10.1203/00006450-199709000-00008. [DOI] [PubMed] [Google Scholar]
- van Golde J, Mulder T, van Straaten H, Blanco CE. The chorioallantoic artery blood flow of the chick embryo from stage 34 to 43. Pediatric Research. 1996b;40:867–871. doi: 10.1203/00006450-199612000-00016. [DOI] [PubMed] [Google Scholar]
- van Oosterhout MFM, Willigers HMM, Reneman RS, Prinzen FW. Fluorescent microspheres to measure organ perfusion: validation of a simplified sample processing technique. American Journal of Physiology. 1995;269:H725–733. doi: 10.1152/ajpheart.1995.269.2.H725. [DOI] [PubMed] [Google Scholar]


